Abstract
Muscle development requires myoblast differentiation and muscle fiber formation. Myod family inhibitor (Mdfi) inhibits myogenic regulatory factors in NIH3T3 cells, but how Mdfi regulates myoblast myogenic development is still unclear. In the present study, we constructed an Mdfi-overexpression (Mdfi-OE) C2C12 cell line by the CRISPR/Cas9 system and performed RNA-seq on Mdfi-OE and wild-type (WT) C2C12 cells. The RNA-seq results showed that the calcium signaling pathway was the most significant. We also established the regulatory networks of Mdfi-OE on C2C12 cell differentiation and muscle fiber type transformation and identified hub genes. Further, both RNA-seq and experimental verification demonstrated that Mdfi promoted C2C12 cell differentiation by upregulating the expression of Myod, Myog, and Myosin. We also found that the positive regulation of Mdfi on fast-to-slow-twitch muscle fiber transformation is mediated by Myod, Camk2b, and its downstream genes, such as Pgc1a, Pdk4, Cs, Cox4, Acadm, Acox1, Cycs, and Atp5a1. In conclusion, our results demonstrated that Mdfi promotes C2C12 cell differentiation and positively modulates fast-to-slow-twitch muscle fiber transformation. These findings further our understanding of the regulatory mechanisms of Mdfi in myogenic development and muscle fiber type transformation. Our results suggest potential therapeutic targets for muscle- and metabolic-related diseases.
Keywords: Mdfi, C2C12 cells, CRISPR/Cas9 system, RNA-seq, differentiation, muscle fiber type transformation
Introduction
Skeletal muscle accounts for about 45% of the human body weight (Turner and Badylak, 2012). Skeletal muscle development plays a crucial role in maintaining the muscle loss caused by disease, injury, and aging. Thus, it is of considerable significance to understand the mechanism of muscle myogenic development for preventing adverse effects on human health.
The skeletal muscle tissue maintains homeostasis through skeletal muscle satellites self-renewal and differentiation when suffered from pathological change or injury (Fry et al., 2015). When stimulated by injury or growth signals, skeletal muscle satellite cells are activated and proliferate to form myoblasts (Abou-Khalil et al., 2009). Subsequently, myoblasts exit the cell cycle, and the myogenic regulator factor (MRF) family, including myogenic factor 5 (Myf5), myogenic differentiation 1 (MyoD), myogenin (Myog), and myogenic factor 6 (Myf6), gradually expresses (Hernandez-Hernandez et al., 2017b). Myf5 is first expressed when satellite cells are activated. Previous studies have found that Myf5 transforms many non-muscle cells into muscle cells (Delfini et al., 2000). MyoD began to express at the proliferation phase of myoblasts and mainly maintains its early differentiation (Zammit et al., 2004). Myog is not expressed in the proliferation phase of myoblasts, but its expression is significantly upregulated when the cells entered the differentiation phase (Bentzinger et al., 2012). Myf6 mainly regulates the terminal differentiation of myoblasts and is highly expressed in mature muscle fibers (Bentzinger et al., 2012). As a result, these myoblasts undergo differentiation to either repair damaged muscle fibers or fuse into multi-nuclear myotubes to form new myofibers (Mashinchian et al., 2018). Myogenic differentiation is, therefore, an essential process in muscle development which determines myoblast fate, muscle formation, and regeneration. Elucidating the mechanism of muscle cell differentiation is critical for understanding skeletal muscle development.
Skeletal muscles are composed of multi-nucleated cells that have numerous myofibers (Bassel-Duby and Olson, 2006). Based on myosin heavy chains (MyHC), mature mammalian skeletal muscle fibers can be classified as type I (MyHC I), type IIa (MyHC IIa), type IIx (MyHC IIx), and type IIb (MyHC IIb) (Schiaffino and Reggiani, 2011). Slow-twitch muscle is mainly composed of type I and type IIa muscle fibers, which are rich in myoglobin and mitochondria, have a strong fatigue resistance. Type IIb muscle fibers mainly exist in fast-twitch muscles, displaying low myoglobin and mitochondrial content, and have weak resistance to fatigue resistance (Chen et al., 2018). Skeletal muscle fiber type transformation is directly correlated with some human muscular and metabolic diseases. For example, muscle atrophy leads to an increase in the proportion of slow-twitch muscle fibers and a decrease in the proportion of fast-twitch muscle fibers (Wang and Pessin, 2013). In the skeletal muscles of patients with type 2 diabetes, the proportion of slow oxidative fibers is decreased, which leads to a decrease in oxidative enzyme activity (Oberbach et al., 2006). Chronic liver disease (CLD) led to the transformation of muscle fibers from type IIb to type I (Aguirre et al., 2020). Chronic obstructive pulmonary disease (COPD) significantly increases the expression of tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK), then increases the proportion of type 1 muscle fibers and decreases the proportion of type 2 muscle fibers (Lu et al., 2017). Therefore, elucidating the mechanism of muscle fiber type transformation is essential for preventing adverse health effects.
Myod family inhibitor (Mdfi, also known as I-mfa) is a class of novel myogenic repressor. The yeast two-hybrid experiments showed that Mdfi inhibits the Myod family’s transactivation activity and leads to repressing the myogenic differentiation of NIH-3T3 cells (Chen et al., 1996). However, Huang et al. (2019) found that in chicken primary myoblasts overexpression of the MyoD family inhibitor domain-containing protein (MDFIC) promotes myotubes’ formation, while it shows opposite results after the knockdown of MDFIC (Huang et al., 2019). MDFIC shares a high degree homology C-terminal domain with Mdfi (Thebault and Mesnard, 2001). In addition, miR-27b inhibits Mdfi to regulate the development of pig muscle satellite cells (PSCs) in vitro (Hou et al., 2018). Conflicting reports on the function of Mdfi exist. We, therefore, explored the regulatory mechanisms of Mdfi in muscle development in the present study.
In this study, we constructed a Mdfi-overexpressing C2C12 cell line by the CRISPR/Cas9 system and performed RNA-seq on Mdfi overexpression (Mdfi-OE) and wild-type (WT) C2C12 cells. Real-time quantitative polymerase chain reaction (qPCR), Western blot, immunofluorescence, and RNA-seq analyses demonstrated that Mdfi promotes C2C12 cell differentiation by upregulating the expression of Myod and myogenin and positively modulates muscle fiber transformation, and successfully established the regulatory network. This study furthers our understanding of the regulatory mechanisms of Mdfi in myogenic differentiation and muscle fiber type transformation. Our results help develop new strategies for treating muscle- and metabolic-related diseases.
Materials and Methods
C2C12 Cell Culture, Transfection, and Differentiation
The C2C12 cell line (ATCC®, CRL-1772TM) used in this study was purchased from American Type Culture Collection (ATCC, VA, United States). The pX330-U6-Chimeric_BB-CBh-hSpCas9 (pX330, #42230) was purchased from Addgene (Cambridge, MA, United States). C2C12 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM)/High Glucose (Catalog No. SH30243.01, Hyclone, GE Healthcare Bio-Sciences, Pittsburgh, PA, United States) with 10% Fetal Bovine Serum (FBS) (Catalog No. FBS10099-141, Gibco, Grand Island, NY, United States). C2C12 cells were seeded in 6-well plates (2 × 105 cells per well). When plates reached 80–90% confluence, the cells were cultured by myogenic differentiation induction medium. C2C12 cells transfected with pX330, pX330-sgRNA plasmid, or co-transfected with pEGFP by Lipofectamine 2000 (Invitrogen, Carlsbad, CA, United States), according to the manufacturer’s instructions. The medium was replaced with fresh growth medium 6 h later.
Construction of a Mdfi-Overexpressing Cell Line by CRISPR/Cas9
We constructed a Mdfi-overexpressing C2C12 cell line by inserting a Mdfi transgene cassette into the genome ROSA26 locus using the CRISPR/Cas9 system. The Genome-CRISPRTM mouse ROSA26 safe harbor gene knock-in kit was purchased from GeneCopoeia Inc (Catalog No. SH-ROS-K200, GeneCopoeia Inc., Rockville, MD, United States). We transfected the MCP-ROSA26-CG01 vector into C2C12 cells with DC-DON-SH02, Mdfi donor, and DC-RFP-SH02. After transfection for 24 h, puromycin (2 μg/mL) was used to screen Mdfi-overexpressing monoclonal cells. After puromycin screening for 72 h, we obtained Mdfi-overexpressing monoclonal cells using limiting dilution assay.
RNA Extraction and qPCR Analysis
The methods used for the RNA extraction and PCR analysis have been described previously (Hou et al., 2017). Briefly, total RNAs were extracted from C2C12 cells using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. After DNase I (Takara Bio Inc., Japan) digestion, total RNAs (500 ng) were reverse transcribed to cDNA using PrimeScriptTM RT Master Mix (TaKaRa, Otsu, Shiga, Japan). SYBR Green Real-time PCR Master Mix reagents (Toyobo Co., Ltd., Osaka, Japan) were used for qPCR. The PCR reactions were carried out on a CFX96TM Optical Reaction Module (Bio-Rad, Hercules, CA, United States). The relative expression of mRNAs was normalized with β-actin levels using the ΔΔCt method. Primers used for qPCR are shown in Supplementary Table 1.
Immunofluorescent Assay
Wild-type and Mdfi-OE C2C12 cells were seeded in the 48-well at a density of 5 × 104/mL and maintained in the growth medium. When cells reached 90% confluence, we changed the growth medium to the differentiation medium (2% house serum) for induction differentiation. At differentiation for 1, 3, 5, and 7 days, we removed the old medium and washed the C2C12 cells three times by PBS. The C2C12 cells were fixed for 20 minutes by 80% acetone, permeabilized for 10 min by 0.5% TritonTM X-100 (Sigma-Aldrich, St. Louis, MO, United States). We used the BCA protein assay kit (Dingguo, China) to block for 1 h, followed by incubating the C2C12 cells with the primary myosin antibody for 1 h. We incubated the C2C12 cells with secondary antibody for 30 min. Finally, we incubated the C2C12 cells with DAPI. The myosin-positive C2C12 cells were observed and recorded using a Nikon TE2000-U inverted microscope (Nikon Instruments, Tokyo, Japan). More than six fields of view were captured in each cell well. The percentage of myosin-positive C2C12 cells, calculated as the number of nuclei present in multi-nucleic myotubes (myosin-positive and containing at least three nuclei) in comparison with the total number of nuclei. The percentage of MyHC-positive C2C12 cells was calculated as the number of nuclei present in multi-nucleic myotubes (MyHC I, MyHC IIa, and MyHC IIb -positive, and containing at least three nuclei) relative the total number of nuclei. Data were counted with Image J software (National Institutes of Health, Bethesda, MD, United States). They were analyzed with the GraphPad Prism (GraphPad Software, La Jolla, CA, United States). The data were expressed as the mean ± standard error of the mean (SEM) with SPSS software (SPSS, Inc., Chicago, IL, United States).
Immunoprecipitation
C2C12 cells were washed twice with precooled PBS, PBS was finally drained, and a 1mL precooled modified RIPA Buffer was added to the petri dish. The cell suspension was transferred to a 1.5 mL centrifuge tube at 4°C for 15 min. After standing, centrifuge at 14,000g for 15 min, and transfer the supernatant to a new centrifuge tube. The protein A agarose beads was washed twice with PBS and then prepared into 50% concentration with PBS. About 100 μL 50% protein A agarose beads was added to every 1 mL total protein and incubated at 4°C for 10 min. The supernatant was centrifuged at 4°C, 14,000 rpm for 15 min, and the supernatant was transferred to a new centrifuge tube to remove the protein A beads. The total protein was diluted at least 1:10 times, diluted to about 1 μg/μL with PBS, and incubated overnight at 4°C. An aliquot of 100 μL protein A agarose beads was then added into the centrifuge tube to capture the antigen-antibody complex. The mixture was shaken for 24 h at 4°C. After centrifugation at 14,000 rpm for 5 s, the agarose beads antigen-antibody complex was collected and the supernatant was removed. The agarose beads were washed with precooled NP-40 lysate three times. After washing, an aliquot of 60 mL of 2 × SDS-PAGE buffer was added in the centrifuge tube, mixed gently, add boiled with boiling water for 5 min. After centrifugation, the supernatant was transferred to a new centrifuge tube for subsequent electrophoresis and the remaining agarose beads were collected.
Luciferase Reporter Assay (Promoter Activity Detection)
The genomic DNA of C2C12 cells was isolated for the PCR amplification template. The promoter of the Camk2b gene was amplified by PCR using PrimerSTAR® (TaKaRa, Dalian, Liaoning, China). Then, we cloned the promoter of the Camk2b gene into the eukaryotic expression vector pGL3-Basic (named as pGL3-Basic-Camk2b). Similarly, we amplified the full length of the Myod gene and cloned it into the pcDNA3.1 plasmid (pcDNA3.1-Myod). According to Promega’s dual luciferase reporter assay kit (Promega, Madison, WI, United States), we transfected the pGL3-Basic, pGL3-Basic-Camk2b and pcDNA3.1, pGL3-Basic-Camk2b and pcDNA3.1-Myod into C2C12 cell by LipofectamineTM 3000 Transfection Reagent (Thermo Fisher Scientific, MA, United States). At last, we identified the double-luciferase activity by BioTek Synergy 2 multifunctional microplate reader (BioTek, Winooski, VT, United States). The ratio of the expression of firefly luciferase to renilla luciferase was the promoter activity. The PCR primer pairs were listed in Supplementary Table 1.
Chromatin Immunofluorescent Assay
The chromatin immunoprecipitation (ChIP)-IT® Express Magnetic ChIP kit & sonication shearing kit (Catalog number 53008) was purchased from Active Motif (Carlsbad, CA, United States). For the ChIP assay, the DNA was immunoprecipitated with the Myod or Camk2b antibody, and ChIP analysis was performed according to the manufacturer’s protocol. DNA samples prior to immunoprecipitation were used as a template for input control. Primers used for ChIP assay are shown in Supplementary Table 1. The antibodies used for ChIP assay are shown in Supplementary Table 1.
Western Blot Assay
Methods used for Western blot assay have been described previously (Hou et al., 2017). C2C12 cells lysed in RIPA buffer containing 1 μM phenylmethanesulfonyl fluoride (PMSF). About 30 μg protein lysates were separated using SDS-PAGE and then electroblotted onto polyvinylidene fluoride membranes (Bio-Rad). The membranes were blocked with 6% skim milk buffer for 2 h at room temperature and incubated with different diluted antibodies at 4°C overnight. Finally, the polyvinylidene fluoride membranes were incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 h. The antibodies used in this study are listed in Supplementary Table 2. The band intensities were quantified with Image J and normalized to those of β-actin. Data were analyzed using the GraphPad Prism and were expressed as change in fold relative to the control.
Mitochondrial DNA Copy Number
Total cellular DNA was extracted from WT and Mdfi-OE C2C12 cells with DNAzol reagent (Invitrogen). Mitochondrial 16S ribosomal RNA (mito-16sRNA) was used as the internal reference gene of mitochondrial DNA, and the hexokinase gene (Mito-HEXO) was used as the internal reference gene of nuclear DNA. The expression level of mito-16sRNA relative to Mito-HEXO was quantified, and the expression of mito-16sRNA reflected the copy number of mitochondrial DNA.
RNA-seq Analysis
Wild-type and Mdfi-OE C2C12 cell samples were sequenced using 150 bp paired-end mRNA sequencing methods based on the Illumina HiSeq platform (Caporaso et al., 2012). We used FastQC software to check Pass Filter Data quality. Cutadapt (version 1.9.1), a second-generation software of sequencing data quality statistics, was used to remove the adapter and low-quality sequences from the raw data and obtain clean data (clean reads). After quality control, clean reads were mapped to the mouse reference genome (Mus musculus 10.fa) using Hisat2 with one mismatch tolerance. We acquired expression levels based on the read counts using Subread (Liao et al., 2019). Differential expression analysis was performed on DESeq2 software. p < 0.05 and | log2FoldChange| > 1 was set as the cutoff criteria for differentially expressed analysis. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses of genes were conducted using KOBAS 3.0 (kobas.cbi.pku.edu.cn) (Xie et al., 2011). The KEGG database analysis was used to analyze the enriched pathways for the differentially expressed genes (DEGs). The Benjamini-Hochberg procedure was applied to control the false discovery rate (p < 0.05). String database v10.5 (string-db.org) was used to establish the protein–protein interactions (PPI) of DEGs between the Mdfi-OE and WT groups with the criterion of medium confidence (0.700) (Szklarczyk et al., 2019). Moreover, we applied Cytoscape 3.6 (Shannon et al., 2003) to visualize the network and highlight the most representative gene.
Statistical Analysis
All data are expressed as the mean ± SEM. There were three replicates in each group. The assumptions of normality of data and homogeneity of variances between the groups were analyzed by SPSS. The dual-luciferase reporter system data were analyzed by one-way ANOVA (SPSS 18.0, Chicago, IL, United States). Significant differences between the control and the treatment groups were determined using the Student’s t-test. We considered p < 0.05 to be statistically significant. ∗ is p < 0.05 and ∗∗ is p < 0.01.
Results
The Expression Profile of Mdfi During C2C12 Cell Differentiation
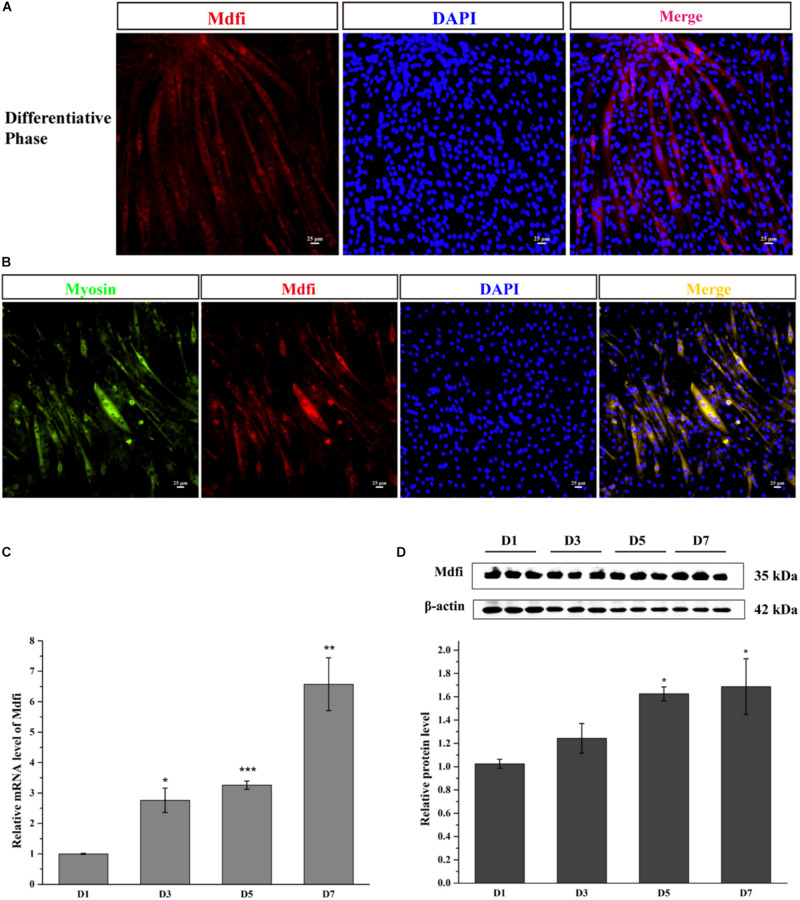
Myod family inhibitor is expressed mainly in the cytoplasm of C2C12 cells, which have been fused into myotube (Figure 1A). The Mdfi and myosin co-immunofluorescent assay results showed that Mdfi expressed mainly in the cytoplasm of myosin positive C2C12 cells (Figure 1B). Meanwhile, we identified the mRNA and protein level of Mdfi in the differentiation phase of C2C12 cells by qPCR and Western blot. The qPCR results showed that the mRNA level of Mdfi was increased at day 3 (p < 0.05), day 5 (p < 0.001), and day 7 (p < 0.01) compared to day 1 (Figure 1C). The Western blot results are consistent with the qPCR results. The protein level of Mdfi was higher on day 5 (p < 0.05) and day 7 (p < 0.05) than day 1 in the differentiation phase (Figure 1D). Although the protein level of Mdfi was not increased significantly at day 3 (p > 0.05) compared to day 1, there was still an increasing trend. These results indicated that the expression of Mdfi was significantly increased during myoblast differentiation.
FIGURE 1.
Mdfi-expression profile during C2C12 cell development. (A) Mdfi immunofluorescence of C2C12 cells in differentiative phase. (B) Mdfi and myosin co-immunofluorescence of C2C12 cells in differentiative phase. Myosin: green, a molecular marker of myogenesis; Mdfi: red; DAPI: blue, cell nuclei; Merge: C2C12 cells fused into multi-nucleic myotubes. (C) qPCR identified Mdfi mRNA level on days 1, 3, 5, and 7 after differentiation induction. (D) Western blot identified Mdfi protein level on days 1, 3, 5, and 7 after differentiation induction. *p < 0.05; **p < 0.01; ***p < 0.001. The results are presented as Mean ± SEM of three replicates for each group. Magnification 200×. The scale bar on the photomicrographs represents 25 μm.
Construction of a Mdfi-OE C2C12 Cell Line by CRISPR/Cas9
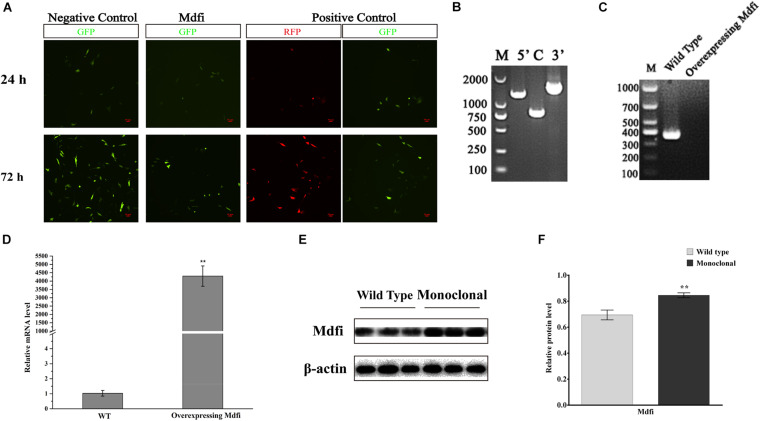
To explore the function of Mdfi in C2C12 myogenic development, we used the CRISPR/Cas9 system to construct a stable Mdfi-overexpressing (Mdfi-OE) C2C12 cell line. Immunofluorescent assay results showed that we have successfully inserted Mdfi into C2C12 cells at ROSA26 locus, which provided candidate Mdfi-OE monoclonal cells (Figure 2A). The PCR amplification results showed that the left and right homologous arms were successfully recombined, indicating the correct integration of the Mdfi donor at the ROSA26 locus (Figure 2B). Results of PCR amplification showed that the non-insertion fragment in the Mdfi-OE monoclonal cells could not be amplified (Figure 2C). These results indicated that monoclonal C2C12 cells represent a heterozygous C2C12 Mdfi-OE cell line. The qPCR results showed that the mRNA level of Mdfi was significantly upregulated compared with the WT (p < 0.01) (Figure 2D). The Western blot results showed that the Mdfi protein level of monoclonal cells was significantly increased compared to WT (p < 0.01), indicating that Mdfi successfully overexpressed in monoclonal cells (Figures 2E,F). These results indicated that we have successfully constructed a Mdfi-OE C2C12 cell line to explore the function of Mdfi in C2C12 myogenic development.
FIGURE 2.
Construction of a Mdfi-overexpressing C2C12 cell line. (A) Inverted fluorescence microscopy was used to investigate the expression of GFP and RFP protein in the C2C12 cell after puromycin screening for 24 and 72 h after transfected with Rosa26 + Donor, Rosa26 + Donor-Mdfi, and Rosa26+ Donor-RFP, respectively. (B) The homologous recombination status of left and right homologous arms and the insertion of the Mdfi gene in the monoclonal clone was confirmed. (C) Single or double allele insertion of Mdfi in monoclonal clone was confirmed through PCR amplification. (D) qPCR measured the mRNA level of Mdfi. (E) Western blot measured the protein level of Mdfi. (F) The relative protein levels obtained through Western blot band gray scanning analysis. ***p < 0.001. The results are presented as Mean ± SEM of three replicates for each group.
Characteristics of Differentiation and Muscle Fiber Types in C2C12 Cells With Mdfi-OE
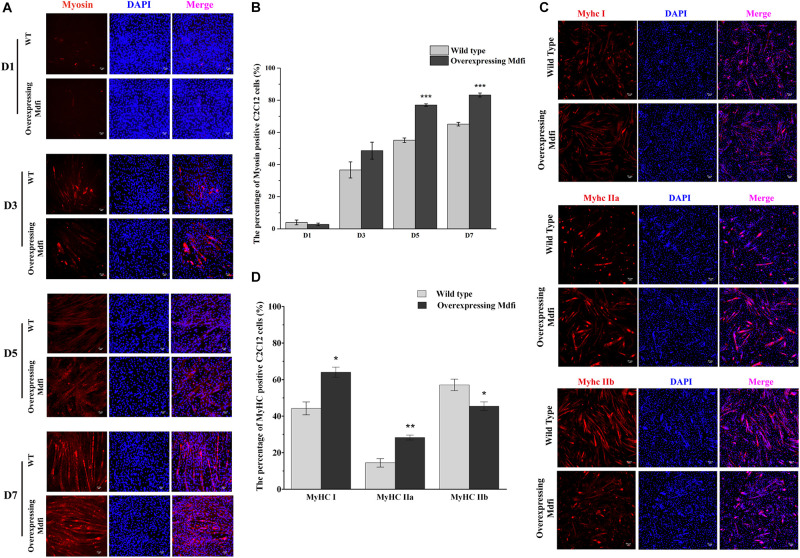
To further investigate the potential role of Mdfi in the differentiation of C2C12 cells, we induced the WT and Mdfi-OE C2C12 cells in the differentiated medium at D1, 3, 5, and 7. Under white light, we observed that Mdfi-OE C2C12 cells fused into thicker myotubes than the WT group (Supplementary Figure 1). Myosin immunofluorescent staining results showed that Mdfi significantly increased the percentage of the myosin-positive cells at D5 (p < 0.001) and D7 (p < 0.001) (Figures 3A,B). In addition, we performed immunofluorescent staining on different subtypes of MyHC. The results showed that overexpression of Mdfi significantly increased the percentage of MyHC I (p < 0.05) and MyHC IIa (p < 0.01) positive C2C12 cells (Figure 3C). In contrast, overexpression of Mdfi decreased the percentage of MyHC IIb (p < 0.05) positive C2C12 cells (Figures 3C,D).
FIGURE 3.
Characteristics of differentiation and muscle fiber types in C2C12 cells with Mdfi overexpression. (A) Representative images of differentiating wild-type and overexpressing Mdfi C2C12 cells on days 1, 3, 5, and 7 in white light or myosin immunofluorescent staining. Myosin: red, a molecular marker of myogenesis; DAPI: blue, cell nuclei; Merge: the C2C12 cells fused into primary myotubes are shown in the insets. (B) Quantitative diagram of the percentage of myosin-positive C2C12 cells. (C) MyHC I, MyHC IIa, and MyHC IIb immunofluorescence staining in WT and Mdfi-OE C2C12 cells, respectively. (D) Quantitative diagram of the percentage of MyHC I, MyHC IIa, and MyHC IIb-positive C2C12 cells. The results are presented as Mean ± SEM of three replicates for each group. Magnification 200×. The scale bar on the photomicrographs represents 25 μm.
RNA-seq Analysis in Mdfi-OE vs WT C2C12 Cells
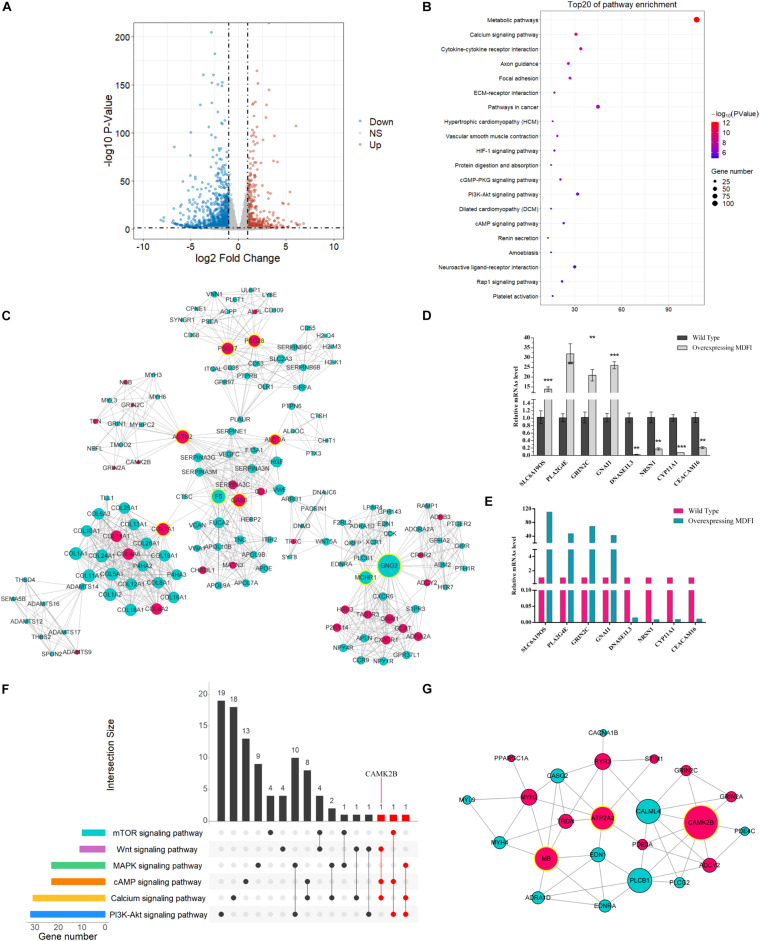
To further investigate the function of Mdfi in C2C12 myoblast differentiation, we performed RNA-Seq to profile genes expressed. The mRNA profiles were generated by sequencing six C2C12 cell samples (three each from Mdfi-OE and WT). Sample’s length, clean reads, Q30 quality scores, GC content, and unique mapped rate were summarized in Table 1. A total of 254 million clean reads were retrieved from the mRNA profiles. The length of the clean reads ranged from 147.69 to 148.30 nt. Q30 quality scores of the six samples were above 92%. The GC content of the samples ranged from 49 to 50%. The unique mapped rate was above 83%. Based on the criteria of p < 0.05 and | Log2FoldChange| > 1, we identified 1,522 DEGs between Mdfi-OE and WT groups (Supplementary Table 3). Among these DEGs, 434 genes were upregulated, and 1,088 genes were downregulated in Mdfi-OE vs. WT (Figure 4A). Mdfi was markedly upregulated by 64-fold. Based on the KEGG analysis, 94 pathways were significantly enriched with a p < 0.05 (Supplementary Table 4). The first 20 KEGG pathways were shown in Figure 4B. The “calcium signaling pathway” was the most enriched. Furthermore, we established a PPI network composed of 1,522 DEGs, 287 nodes, and 1,024 edges (Figure 4C). High degreed-nodes in the regulatory network are also known as hub genes. We identified nine hub genes in the gene network, including six upregulated genes and three downregulated genes. These genes all play the key roles in the differentiation of the C2C12 cells with overexpressing Mdfi, which warrants further investigations. To validate the results of RNA-seq, we used qPCR to validate changes in expression levels of C2C12 cells overexpressed with Mdfi with induced differentiation. The qPCR results for all the DEGs were consistent with the results of the RNA-seq data (p < 0.01) (Figures 4D,E).
TABLE 1.
Overview of mRNA sequencing data.
| Samples | ID | Length | Clean reads | Q30 (%) | GC (%) | Unique mapped reads (%) |
| Wild-Type 1 | DC1 | 148.29 | 42843674 | 92.47 | 50.40 | 50.40 |
| Wild-Type 2 | DC2 | 148.29 | 41911008 | 92.10 | 49.85 | 49.85 |
| Wild-Type 3 | DC3 | 148.30 | 47325970 | 92.39 | 49.69 | 49.69 |
| Mdfi-OE 1 | DE1 | 147.85 | 39635016 | 92.06 | 49.15 | 49.15 |
| Mdfi-OE 2 | DE2 | 147.69 | 40636938 | 92.11 | 49.11 | 49.11 |
| Mdfi-OE 3 | DE3 | 148.07 | 41973956 | 92.71 | 49.52 | 49.52 |
Samples: Wild-Type 1/2/3 represent normal C2C12 cells. Mdfi-OE 1/2/3 represent overexpression of Mdfi in C2C12 cells. ID represents sample serial numbers. Length represents the length of sequencing reads. Clean reads were done after removing impurity reads for raw reads. Q30% represents the percentage of bases with mass values greater than or equal to 30. GC Content is the G and C base content. Unique mapped reads are the percentage of each sample aligned to the mouse reference genome.
FIGURE 4.
RNA-seq analysis in Mdfi-OE vs. WT C2C12 cells. (A) The Volcano plot displays an overview of the DEGs. The X-axis represents the log-transformed P-value, and the Y-axis indicates the multiple of the DEGs. The gray dots represent DEGs that are not differentially expressed, the red dots represent the upregulated DEGs, and the blue dots represent the downregulated DEGs. |Log2FoldChange| > 1 and p < 0.05 were set as the criteria. (B) The first twenty pathways of the DEGs. The X-axis represents the number of DEGs enriched in the pathway. (C) PPI network in Mdfi-OE vs. WT. The network displays gene interactions. Nodes represent genes, and edges represent gene interactions. The upregulated genes are shown in red, downregulated genes are shown in green, and hub genes are shown in yellow. (D) qPCR validated of DEGs. (E) Expression levels of DEGs in RNA-seq. **p < 0.01, ***p < 0.001. (F) The upset plot of the intersection of each pathway. The transverse bar graph at the bottom left shows the number of genes enriched in each pathway. The black points in the dot matrix at the bottom left and in the bar graph at the top indicate genes included in the pathways. The bars above represent the number of genes corresponding to each intersection. The red bars represent the number of intersection genes enriched in three or more pathways (n ≥ 3). (G) The gene network displayed the gene interactions and was generated by Cytoscape (version 3.6). Nodes represent genes, and edges represent gene interactions. The upregulated genes are shown in red, downregulated genes are shown in green, and hub genes are shown in yellow.
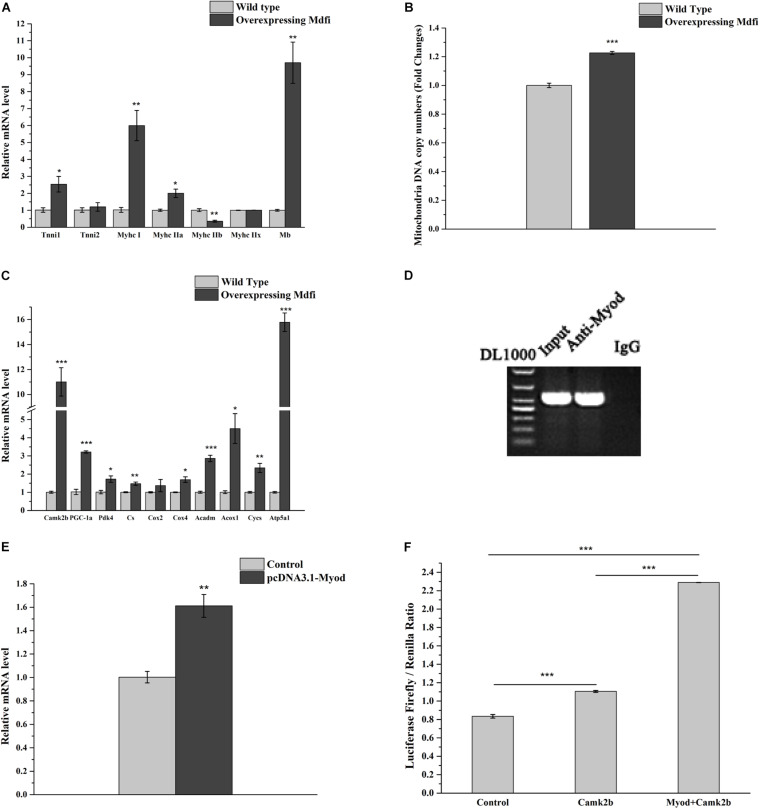
As expected, our RNA-seq analysis results showed that the IIa muscle fiber marker gene, myosin heavy chain 2 (Myh2) (about 1.33-fold), and myoglobin (Mb) (about 1.60-fold) were upregulated. In contrast, the IIb muscle fiber marker gene, myosin heavy chain 4 (Myh4) (about 2.46-fold), was downregulated. Furthermore, we identified six pathways involved in muscle fiber transformation in our RNA-seq results, such as “calcium signaling pathway,” “PI3K-Akt signaling pathway”, and “mTOR signaling pathway” (Table 2). In addition, we analyzed the intersection of genes in these pathways. The more pathways the gene is involved in, the more likely it is engaged in Mdfi regulating the transformation of muscle fiber types. As shown in the gene Venn diagram, calcium/calmodulin-dependent protein kinase II beta (Camk2b) was enriched in three pathways (Figure 4F). We then selected genes enriched in the above pathways to establish a regulatory network (Figure 4G); we found that most of the genes, especially Camk2b, ATPase sarcoplasmic/endoplasmic reticulum calcium ion transporting 2 (Atp2a2), and myoglobin (Mb), were related to calcium-induced muscle fiber type transformation. Particularly, Camk2b was the most degree hub gene in the gene network.
TABLE 2.
The significantly enriched pathways related to muscle fiber type transformation.
| Term | Count | p-Value |
| Calcium signaling pathway | 31 | 1.41E-11 |
| PI3K-Akt signaling pathway | 32 | 1.99E-06 |
| cAMP signaling pathway | 23 | 3.26E-06 |
| mTOR signaling pathway | 10 | 0.043912 |
| Wnt signaling pathway | 11 | 0.026485 |
| MAPK signaling pathway | 23 | 0.000322 |
Mdfi-OE Promoted C2C12 Cells Myogenic Differentiation
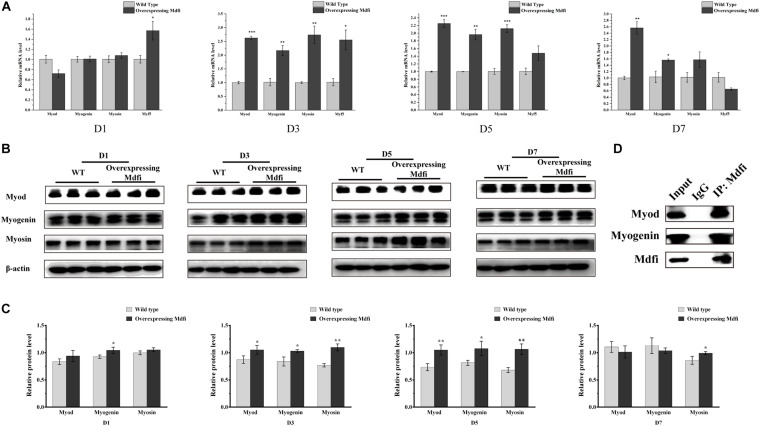
To further validate the regulatory mechanism of Mdfi on C2C12 cell differentiation, we performed additional experiments. The qPCR results showed that overexpression of Mdfi significantly increased the mRNA level of Myod (p > 0.001), Myog (p > 0.01), and Myosin (p > 0.001) mainly at D3 and D5 (Figure 5A). There was no significant difference in mRNA levels of Myod, Myog, and Myosin between Mdfi-OE and WT at D1 (Figure 5A). The Western blot results showed that Mdfi significantly increased the Myod protein level in D3 and D5, Myog protein level in D1, D3, and D5, and Myosin protein level in D3, D5, and D7 (Figures 5B,C). Although the Myod and Myosin protein level of Mdfi-OE was not increased significantly at D1 compared to WT, there was still an increasing trend (Figures 5B,C). There was no significant difference in protein levels of Myod and Myog between Mdfi-OE and WT at D7 (Figures 5B,C). Furthermore, the Co-IP results showed that Mdfi interacted with both Myod and Myog (Figure 5D). These results indicated that Mdfi promotes C2C12 myogenic differentiation by upregulating the expression of Myod, Myog, and Myosin.
FIGURE 5.
Mdfi promotes the differentiation of C2C12 cells. (A) qPCR detection of myogenic marker genes on days 1, 3, 5, and 7 in both WT and Mdfi-OE C2C12 cells. (B) Western blot identified the protein of myogenic marker genes on days 1, 3, 5, and 7 after differentiation induction in both WT and Mdfi-OE C2C12 cells. (C) The relative protein levels were obtained through Western blot band gray scanning analysis. (D) Co-IP identified the interaction of Mdfi with Myod and Myog, respectively. *p < 0.05; **p < 0.01; ***p < 0.001. The results are presented as Mean ± SEM of three replicates for each group.
Mdfi Promotes the Muscle Fiber Type From the Fast-Twitch Muscle Fiber to the Slow-Twitch Muscle Fiber
To further examine the regulation mechanism of Mdfi on the transformation of muscle fiber types, we performed additional experiments. The qPCR results showed that overexpression of Mdfi increased the mRNA level of Tnni1, MyHC I, MyHC IIa, and Mb but decreased the mRNA level of MyHC IIb (Figure 6A). Furthermore, we used qPCR to identify the mitochondrial DNA copy number, and the results showed that overexpression of Mdfi increased the mitochondrial DNA copy number in C2C12 cells (Figure 6B). We also detected the expression level of Camk2b and its downstream cellular energy metabolism and mitochondrial oxidative phosphorylation-related genes. The qPCR results showed that overexpression of Mdfi increased the mRNA level of Camk2b, PPARG coactivator 1 alpha (Pgc1a), pyruvate dehydrogenase kinase (Pdk4), citrate synthase (Cs), cytochrome c oxidase subunit 4 (Cox4), acyl-Coenzyme A dehydrogenase medium-chain (Acadm), acyl-Coenzyme A oxidase 1 (Acox1), cytochrome c (Cycs) and ATP synthase H+ transporting mitochondrial F1 complex alpha subunit 1 (Atp5a1) (Figure 6C). The Ch-IP results showed that Myod bound to the promoter region of Camk2b (Figure 6D). Overexpression of Myod increased the expression of Camk2b (Figure 6E). Dual-luciferase reporter system results showed that overexpressing Myod increased the promoter activity of Camk2b (Figure 6F). Combined with the above findings, Mdfi promoted the expression of Myod, thus upregulating the expression of Camk2b. Subsequently, Camk2b promoted the expression of downstream genes, such as Pgc1a, Pdk4, Cs, Cox4, Acadm, Acox1, Cycs, and Atp5a1. These results suggested that overexpression of Mdfi positively regulated the transformation of muscle fiber types from fast-twitch muscle to slow-twitch muscle.
FIGURE 6.
Mdfi positively regulated the transformation of muscle fiber types from fast-twitch muscle to slow-twitch muscle in C2C12 cells. (A) qPCR identified the mRNA level of marker genes of different muscle fiber types in WT and Mdfi-OE C2C12 cells, respectively. (B) qPCR identified the relative copy number of mitochondrial DNA in WT and Mdfi-OE C2C12 cells, respectively. (C) qPCR identified the mRNA levels of energy metabolism-related genes in WT and Mdfi-OE C2C12 cells, respectively. (D) ChIP assay identified the binding of Myod to the Camk2b promoter region. (E) qPCR detection of the mRNA level of the Camk2b gene in C2C12 cells after Myod overexpressing. (F) The dual-luciferase reporter system identified the promoter activity of Camk2b after Myod overexpressing. *p < 0.05; **p < 0.01; ***p < 0.001. The results are presented as Mean ± S.E.M. of three replicates for each group.
Discussion
In myoblast development, Mdfi blocked the myogenic differentiation of non-muscle-derived stem cells by inhibiting exogenous MRFs expression (Chen et al., 1996). However, Huang et al. (2019) found that MDFIC, which has the same domain as Mdfi, promoted the myogenic differentiation of muscle-derived stem cells. Its regulatory mechanism has not been studied in the muscle-derived stem cells. These opposite regulation functions of myogenic differentiation may be caused by the difference between species and cell types. Similarly, the function and regulatory mechanism of Mdfi on muscle fiber type transformation are also unclear. Therefore, we aimed to reveal the regulatory mechanism of Mdfi on myogenic cell differentiation and muscle fiber type transformation. In the present study, we successfully constructed a Mdfi-OE C2C12 cell line and found that overexpression of Mdfi promotes C2C12 cell differentiation and positively modulates fast-to-slow-twitch muscle fibers transformation.
Mdfi-OE Promoted C2C12 Cells Myogenic Differentiation by Upregulating the Expression of Myod, Myogenin, and Myosin
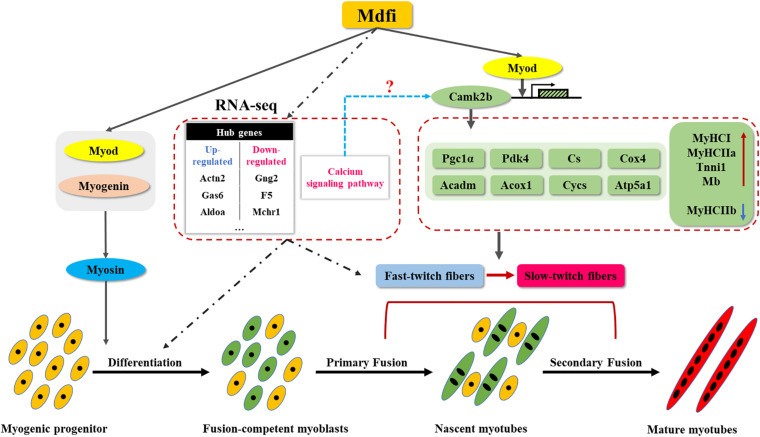
As myoblasts differentiated and fused into myotubes, myosin began to express (Crow and Stockdale, 1986; D’Albis et al., 1989). Therefore, myosin was considered as a marker of muscle differentiation and maturation in the study of muscle development (Pizon et al., 2005; Zhao et al., 2014). In the present study, we selected myosin as a marker of myoblast differentiation and the immunofluorescent staining of myosin showed that overexpression of Mdfi significantly increased the percentage of myosin-positive C2C12 cells. In the process of myogenic differentiation, the dynamically temporal and spatial expression of MRFs family proteins control the determination and differentiation of myoblasts (Hernandez-Hernandez et al., 2017a). Previous studies have shown that Di-(2-ethylhexyl)-phthalate (DEHP) inhibited C2C12 cell differentiation by repressing the expression of Myod and Myog, resulting in decreased myotube formation and MyHC expression (Chen et al., 2013). In our study, the qPCR and Western blot results showed that Mdfi-OE significantly increases the expression of Myod, Myog, and Myosin in C2C12 cells, mainly in the middle and late stages of differentiation (D3 and D5). Meanwhile, the expression of Mdfi is dynamic in the process of myogenic differentiation. Therefore, we speculate that Mdfi plays an important role in the middle and late stage of C2C12 cell differentiation. Furthermore, the Co-IP results showed that Mdfi interacted with both Myod and Myog. Therefore, we concluded that Mdfi regulates the differentiation and maturation of C2C12 cells by dynamically regulating Myod, Myog, and Myosin (Figure 7).
FIGURE 7.
Mdfi overexpression promoted C2C12 cell differentiation and positively modulated fast-to-slow-twitch muscle fibers transformation. We constructed a Mdfi-overexpressing C2C12 cell line by the CRISPR/Cas9 system. During C2C12 myogenic development, Mdfi promotes the differentiation of C2C12 cells by upregulating the expression of Myod, Myog, and Myosin. Mdfi positively modulated fast-to-slow-twitch muscle fibers transformation by upregulating the mRNA levels of Myod, Camk2b, and its downstream genes, such as Pgc1a, Pdk4, Cs, Cox4, Acadm, Acox1, Cycs, and Atp5a1. In addition, the calcium signaling pathway was the most significant in C2C12 cells with Mdfi-OE. We also systematically established regulatory networks and identified hub genes in them. Their regulation mechanism in Mdfi regulation of C2C12 cell differentiation and the muscle fiber type transformation warrants further investigations.
Pathway Enrichment and Gene Network Construction in RNA-seq Analysis
In our RNA-Seq analysis results, the “calcium signaling pathway” was the most enriched. The high intracellular calcium concentration induced myogenic differentiation and promoted the transformation of muscle fibers from fast-twitch to slow-twitch (Tu et al., 2016; Ravel-Chapuis et al., 2017). Numerous upregulated DEGs were enriched in the calcium signaling pathway, such as purinergic receptor P2X ligand-gated ion channel 5 (P2rx5), ryanodine receptor 3 (Ryr3), stromal interaction molecule 1 (Stim1), Atp2a2, and Camk2b, were involved in the process of myogenic differentiation. P2rx5, a ligand-gated ion channel, caused calcium to flow through the plasma membrane and promoted the terminal differentiation of myoblasts (Ryten et al., 2002). Ryr3 is a sarcoplasmic reticulum calcium release channel in skeletal muscle (Protasi et al., 2000). Stim1 is a sarcoplasmic reticulum membrane protein that can sense the change of calcium content. Overexpression of Stim1 promoted C2C12 cell differentiation and enhanced myotube formation, whereas Stim1-knockdown resulted in the opposite effects (Phuong et al., 2013). Atp2a2, also known as SERCA2, is a calcium transport ATPase, which functions as the reuptake of calcium from the cytoplasm to the sarcoplasmic reticulum (Harrer et al., 1995). Atp2a2 previously has been shown to express primarily in the slow-twitch skeletal muscle, and the knockdown of Atp2a2 in C2C12 cells inhibited the expression of slow-twitch muscle marker genes (Wei et al., 2015). Camk2b, a calcium-dependent kinase, promoted mitochondrial biogenesis and involved in muscle fiber type transformation (Al-Shanti and Stewart, 2009). These genes were significantly upregulated in our RNA-Seq results and involved in regulating the formation of muscle fibers through calcium-transport. This result suggests that Mdfi-OE may promote C2C12 cell differentiation by regulating the calcium signaling pathway.
We also successfully established a PPI network and identified nine hub genes. Six upregulated hub genes are actinin alpha 2 (Actn2), growth arrest-specific 6 (Gas6), pregnancy-specific glycoprotein family members (Psg17, Psg28), collagen type VII alpha 1 (Col7a1), and aldolase A fructose-bisphosphate (Aldoa). Actn2, a muscle-specific actin-binding protein, bound explicitly disintegrin and metallopeptidase domain 12 (Adam12) to promote myoblast fusion (Galliano et al., 2000). Gas6 was increasingly secreted during C2C12 cell differentiation, and it has the same expression trend during the regeneration of muscle injury (Chikazawa et al., 2020). Psg17 and Psg28 are the members of the pregnancy-specific glycoprotein family. Psg17 bound CD9 protein to regulate the pregnancy process of the mouse (Wynne et al., 2006). To our knowledge, there are no published reports on Psg28. Col7a1, one of the extracellular matrix components, had different expression patterns in various types of muscle fibers (Zhu et al., 2016). Aldoa interacts with RYR to promote calcium release from the sarcoplasmic reticulum into the cytoplasm (Kramerova et al., 2008). Meanwhile, three downregulated hub genes are G protein subunit gamma 2 (Gng2), coagulation factor V (F5), and melanin-concentrating hormone receptor 1 (Mchr1). Gng2 is a member of guanine nucleotide-binding protein (G protein), which plays an essential role in various transmembrane signaling systems, mainly by binding G Protein-Coupled Receptors (GPCR; Liang et al., 2016). F5 is a critical factor in the blood coagulation process, primarily promoting the conversion of prothrombin to thrombin (Rodgers et al., 1987). Mchr1 is a GPCR mainly expressed in the brain and also expressed in muscle tissue (Saito et al., 2013). Some of these hub genes are involved in muscle formation and regeneration of muscle injury, while others were novel genes not involved in muscle development. All the above hub genes are significantly changed in our RNA-Seq results. The role of these genes in the process of Mdfi regulating C2C12 cell differentiation warrants further investigations.
Mdfi-OE Positively Modulates Fast-to-Slow-Twitch Muscle Fibers Transformation
Skeletal muscle is a dynamically changing system with high plasticity. Skeletal muscle responds to external environmental stimuli, nutrient levels, mechanical training, and age, and adjusts the content of different MyHC subtypes in muscle fibers. In addition, the transformation of muscle fiber types occurs due to the changes in intracellular signal pathways caused by internal physiological changes, pathological stimulation, and stress (Grange et al., 2001; Pette, 2002). In the present study, we performed immunofluorescent staining to examine different subtypes of MyHC and found that overexpression of Mdfi increased the percentage of MyHC I and MyHC IIa positive C2C12 cells. In contrast, overexpression of Mdfi decreased the percentage of MyHC IIb positive C2C12 cells.
As expected, in the RNA-seq results, we found that Myh4, Myh2, and Mb expressed differently between WT and Mdfi-OE C2C12 cells. Moreover, the qPCR results showed that Mdfi overexpression increased the expression of MyHC I (Myh7), MyHC IIa (Myh2), Tnni 1, and Mb, while decreased the expression of MyHC IIb (Myh4). These qPCR results were consistent with the results of the RNA-seq data. This evidence indicated that the overexpression of Mdfi promotes the transformation of muscle fibers from type IIb to type IIa and type I. Furthermore, we explored the regulatory mechanism of Mdfi in regulating the transformation of muscle fiber types. In the qPCR results, we found that Camk2b was upregulated, enriched in three pathways related to the transformation of muscle fiber types, and identified as the hub genes in the network. The gene Camk2b, a downstream dependent kinase of the calcium signaling pathway, promoted mitochondrial biogenesis and participated in the transformation of muscle fiber types from fast-twitch muscle to slow-twitch muscle (Al-Shanti and Stewart, 2009). In mouse muscle development, Camk2 regulated the oxidative metabolism of mouse muscle mediating by AMPK signaling (Raney and Turcotte, 2008). Therefore, we examined the expression of Camk2b, and the qPCR results demonstrated that overexpression of Mdfi significantly increased its expression.
Furthermore, we detected the expression level of downstream cellular energy metabolism and mitochondrial oxidative phosphorylation-related genes of Camk2b, such as Pgc1a, Pdk4, Cs, Cox4, Acadm, Acox1, Cycs, and Atp5a1. The knockin of Pgc1a in mice increased the distribution of red oxidized fibers and promoted the transformation of muscle fibers from type IIx to type I. Conversely, the knockout of Pgc1a in skeletal muscle induced the transformation of muscle fibers from type I and IIa to type IIx and IIb (Arany et al., 2007). Pdk4 was activated by the co-expression of Pgc1a and estrogen-related receptor alpha (Erra) and played an important role in glucose oxidative phosphorylation (Sugden and Holness, 2003). In rat liver mitochondria, the inhibition of Cox2 induced mitochondrial toxicity by inhibiting oxidative phosphorylation (Syed et al., 2016). During the development of porcine skeletal muscle, the addition of niacin supplementation promoted genes involved in mitochondrial fatty acid catabolism, citric acid cycle, and oxidative phosphorylation, such as Cact, Sdha, Cox4, and Cox6a1. Their expression induced the transformation of muscle fibers from type II to type I (Khan et al., 2013). The genes Acadm, Acox1, Cycs, and Atp5a1 were marker genes of mitochondrial fatty acid oxidation and oxidative phosphorylation in the muscle (Kitamura et al., 2020). The qPCR results showed an increase in expression of these genes. In addition, the Ch-IP results demonstrated that Myod binds to the promoter of Camk2b to regulate the transcriptional activation of Camk2b.
All the above results indicated that Mdfi promotes the transcriptional activation of Camk2b by binding to Myod, increasing the expression of downstream Pgc1a, Pdk4, Cs, Cox4, Acadm, Acox1, Cycs, and Atp5a1, and ultimately promoting the transformation of muscle fibers from type IIb to type IIa and type I (Figure 7). In addition, whether Mdfi activates Camk2b through the calcium signaling pathway to regulate muscle fiber type transformation needs to be further explored.
Conclusion
In conclusion, our findings have further characterized the regulatory function of Mdfi in C2C12 cell differentiation and muscle fiber type transformation. Mdfi-OE promoted the differentiation of C2C12 cells by upregulating the expression of Myod, Myog, and Myosin. Meanwhile, Mdfi-OE promoted the expression of Camk2b by binding to Myod. Camk2b, in turn, upregulated the expression of downstream genes, such as Pgc1a, Pdk4, Cs, Cox4, Acadm, Acox1, Cycs, and Atp5a1, and ultimately promoting the transformation of muscle fibers from the fast-twitch to the slow-twitch. In addition, combined with RNA-seq results, we found that the calcium signaling pathway was the most significant in C2C12 cells with Mdfi-OE. Its regulation mechanism in Mdfi regulation of C2C12 cell differentiation and the muscle fiber type transformation warrants further investigations. We also systematically established the regulatory networks of Mdfi-OE on C2C12 cell differentiation and muscle fiber type transformation and identified hub genes. These results led us to propose a regulatory mechanism model of how Mdfi regulates muscle development. Mdfi may be a therapeutic target for muscle- and metabolic-related diseases treatment.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: http://www.ncbi.nlm.nih.gov/bioproject/679162.
Author Contributions
CW, BH, and YJ designed the research. YJ and YZ performed the research and developed the methods. BH, ZN, and ZY analyzed the data. BH, QL, CH, and CW wrote the manuscript. All authors made contributions to this study and read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the South China Agricultural University Major Project for International Science and Technology Cooperation Cultivation (2019SCAUGH01), the Guangdong Provincial Key Area Research and Development Program (2018B020203002), the Provincial Agricultural Science Innovation and Promotion Project (2020KJ106), the China Postdoctoral Science Foundation (2018M640789), and the USDA (HAW05020-H).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.605875/full#supplementary-material
Cell fusion observation under white light.
All the primers used in this study.
Antibodies used in the study.
The DEGs in Mdfi-OE vs. WT.
The KEGG pathways were enriched by DEGs.
References
- Abou-Khalil R., Le Grand F., Pallafacchina G., Valable S., Authier F. J., Rudnicki M. A., et al. (2009). Autocrine and paracrine angiopoietin 1/Tie-2 signaling promotes muscle satellite cell self-renewal. Cell Stem Cell 5 298–309. 10.1016/j.stem.2009.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre F., Abrigo J., Gonzalez F., Gonzalez A., Simon F., Cabello-Verrugio C. (2020). Protective Effect of Angiotensin 1-7 on Sarcopenia Induced by Chronic Liver Disease in Mice. Int. J. Mol. Sci. 21:21113891. 10.3390/ijms21113891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shanti N., Stewart C. E. (2009). Ca2+/calmodulin-dependent transcriptional pathways: potential mediators of skeletal muscle growth and development. Biol. Rev. Camb. Philos. Soc. 84 637–652. 10.1111/j.1469-185X.2009.00090.x [DOI] [PubMed] [Google Scholar]
- Arany Z., Lebrasseur N., Morris C., Smith E., Yang W. L., Ma Y. H., et al. (2007). The transcriptional coactivator PGC-1 beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab. 5 35–46. 10.1016/j.cmet.2006.12.003 [DOI] [PubMed] [Google Scholar]
- Bassel-Duby R., Olson E. N. (2006). Signaling pathways in skeletal muscle remodeling. Ann. Rev. Biochem. 75 19–37. 10.1146/annurev.biochem.75.103004.142622 [DOI] [PubMed] [Google Scholar]
- Bentzinger C. F., Wang Y. X., Rudnicki M. A. (2012). Building muscle: molecular regulation of myogenesis. Cold Spr. Harb Perspect. Biol. 4:a008342. 10.1101/cshperspect.a008342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J. G., Lauber C. L., Walters W. A., Berg-Lyons D., Huntley J., Fierer N., et al. (2012). Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6 1621–1624. 10.1038/ismej.2012.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. M., Kraut N., Groudine M., Weintraub H. (1996). I-mf, a novel myogenic repressor, interacts with members of the MyoD family. Cell 86 731–741. 10.1016/s0092-8674(00)80148-8 [DOI] [PubMed] [Google Scholar]
- Chen S. S., Hung H. T., Chen T. J., Hung H. S., Wang D. C. (2013). Di-(2-ethylhexyl)-phthalate reduces MyoD and myogenin expression and inhibits myogenic differentiation in C2C12 cells. J. Toxicol. Sci. 38 783–791. [DOI] [PubMed] [Google Scholar]
- Chen X., Guo Y., Jia G., Liu G., Zhao H., Huang Z. (2018). Arginine promotes skeletal muscle fiber type transformation from fast-twitch to slow-twitch via Sirt1/AMPK pathway. J. Nutr. Biochem. 61 155–162. 10.1016/j.jnutbio.2018.08.007 [DOI] [PubMed] [Google Scholar]
- Chikazawa M., Shimizu M., Yamauchi Y., Sato R. (2020). Bridging molecules are secreted from the skeletal muscle and potentially regulate muscle differentiation. Biochem. Biophys. Res. Commun. 522 113–120. 10.1016/j.bbrc.2019.11.010 [DOI] [PubMed] [Google Scholar]
- Crow M. T., Stockdale F. E. (1986). Myosin expression and specialization among the earliest muscle fibers of the developing avian limb. Dev. Biol. 113 238–254. [DOI] [PubMed] [Google Scholar]
- D’Albis A., Couteaux R., Janmot C., Roulet A. (1989). Specific programs of myosin expression in the postnatal development of rat muscles. Eur. J. Biochem. 183 583–590. 10.1111/j.1432-1033.1989.tb21087.x [DOI] [PubMed] [Google Scholar]
- Delfini M. C., Hirsinger E., Pourquie O., Duprez D. (2000). Delta 1-activated notch inhibits muscle differentiation without affecting Myf5 and Pax3 expression in chick limb myogenesis. Development 127 5213–5224. [DOI] [PubMed] [Google Scholar]
- Fry C. S., Lee J. D., Mula J., Kirby T. J., Jackson J. R., Liu F., et al. (2015). Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat. Med. 21 76–80. 10.1038/nm.3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliano M. F., Huet C., Frygelius J., Polgren A., Wewer U. M., Engvall E. (2000). Binding of ADAM12, a marker of skeletal muscle regeneration, to the muscle-specific actin-binding protein, alpha -actinin-2, is required for myoblast fusion. J. Biol. Chem. 275 13933–13939. 10.1074/jbc.275.18.13933 [DOI] [PubMed] [Google Scholar]
- Grange R. W., Meeson A., Chin E., Lau K. S., Stull J. T., Shelton J. M., et al. (2001). Functional and molecular adaptations in skeletal muscle of myoglobin-mutant mice. Am. J. Phys. Cell Phys. 281 C1487–C1494. 10.1152/ajpcell.2001.281.5.C1487 [DOI] [PubMed] [Google Scholar]
- Harrer J. M., Ponniah S., Ferguson D. G., Kranias E. G. (1995). Expression of phospholamban in C2C12 cells and regulation of endogenous SERCA1 activity. Mol. Cell Biochem. 146 13–21. 10.1007/bf00926876 [DOI] [PubMed] [Google Scholar]
- Hernandez-Hernandez J. M., Garcia-Gonzalez E. G., Brun C. E., Rudnicki M. A. (2017a). The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 72 10–18. 10.1016/j.semcdb.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Hernandez M., Garcia-Gonzalez E. G., Brun C. E., Rudnicki M. A. (2017b). The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Sem.Cell Dev. Biol. 72 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L., Xu J., Jiao Y., Li H., Pan Z., Duan J., et al. (2018). MiR-27b Promotes Muscle Development by Inhibiting MDFI Expression. Cell Physiol. Biochem. 46 2271–2283. 10.1159/000489595 [DOI] [PubMed] [Google Scholar]
- Hou L., Xu J., Li H., Ou J., Jiao Y., Hu C., et al. (2017). MiR-34c represses muscle development by forming a regulatory loop with Notch1. Sci. Rep. 7:9346. 10.1038/s41598-017-09688-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Guo L., Zhao M., Zhang D., Xu H., Nie Q. (2019). The Inhibition on MDFIC and PI3K/AKT Pathway Caused by miR-146b-3p Triggers Suppression of Myoblast Proliferation and Differentiation and Promotion of Apoptosis. Cells 8:8070656. 10.3390/cells8070656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M., Ringseis R., Mooren F. C., Kruger K., Most E., Eder K. (2013). Niacin supplementation increases the number of oxidative type I fibers in skeletal muscle of growing pigs. BMC Vet. Res. 9:177. 10.1186/1746-6148-9-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Erlangga J. S., Tsukamoto S., Sakamoto Y., Mabashi-Asazuma H., Iida K. (2020). Daidzein promotes the expression of oxidative phosphorylation- and fatty acid oxidation-related genes via an estrogen-related receptor alpha pathway to decrease lipid accumulation in muscle cells. J. Nutr. Biochem. 77:108315. 10.1016/j.jnutbio.2019.108315 [DOI] [PubMed] [Google Scholar]
- Kramerova I., Kudryashova E., Wu B., Ottenheijm C., Granzier H., Spencer M. J. (2008). Novel role of calpain-3 in the triad-associated protein complex regulating calcium release in skeletal muscle. Hum. Mol. Genet. 17 3271–3280. 10.1093/hmg/ddn223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B., Li C., Zhao J. (2016). Identification of key pathways and genes in colorectal cancer using bioinformatics analysis. Med. Oncol. 33:111. 10.1007/s12032-016-0829-6 [DOI] [PubMed] [Google Scholar]
- Liao Y., Smyth G. K., Shi W. (2019). The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 47 e4710.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J. J., Wang Q., Xie L. H., Zhang Q., Sun S. H. (2017). Tumor necrosis factor-like weak inducer of apoptosis regulates quadriceps muscle atrophy and fiber-type alteration in a rat model of chronic obstructive pulmonary disease. Tob. Induc. Dis. 15:43. 10.1186/s12971-017-0148-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashinchian O., Pisconti A., Le Moal E., Bentzinger C. F. (2018). The Muscle Stem Cell Niche in Health and Disease. Curr. Top Dev. Biol. 126 23–65. 10.1016/bs.ctdb.2017.08.003 [DOI] [PubMed] [Google Scholar]
- Oberbach A., Bossenz Y., Lehmann S., Niebauer J., Adams V., Paschke R., et al. (2006). Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care 29 895–900. 10.2337/diacare.29.04.06.dc05-1854 [DOI] [PubMed] [Google Scholar]
- Pette D. (2002). The adaptive potential of skeletal muscle fibers. Can. J. Appl. Physiol. 27 423–448. [DOI] [PubMed] [Google Scholar]
- Phuong T. T., Yun Y. H., Kim S. J., Kang T. M. (2013). Positive feedback control between STIM1 and NFATc3 is required for C2C12 myoblast differentiation. Biochem. Biophys. Res. Commun. 430 722–728. 10.1016/j.bbrc.2012.11.082 [DOI] [PubMed] [Google Scholar]
- Pizon V., Gerbal F., Diaz C. C., Karsenti E. (2005). Microtubule-dependent transport and organization of sarcomeric myosin during skeletal muscle differentiation. EMBO J. 24 3781–3792. 10.1038/sj.emboj.7600842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protasi F., Takekura H., Wang Y., Chen S. R., Meissner G., Allen P. D., et al. (2000). RYR1 and RYR3 have different roles in the assembly of calcium release units of skeletal muscle. Biophys. J. 79 2494–2508. 10.1016/S0006-3495(00)76491-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raney M. A., Turcotte L. P. (2008). Evidence for the involvement of CaMKII and AMPK in Ca2+-dependent signaling pathways regulating FA uptake and oxidation in contracting rodent muscle. J. Appl. Physiol. 104 1366–1373. 10.1152/japplphysiol.01282.2007 [DOI] [PubMed] [Google Scholar]
- Ravel-Chapuis A., Belanger G., Cote J., Michel R. N., Jasmin B. J. (2017). Misregulation of calcium-handling proteins promotes hyperactivation of calcineurin-NFAT signaling in skeletal muscle of DM1 mice. Hum. Mol. Genet. 26 2192–2206. 10.1093/hmg/ddx109 [DOI] [PubMed] [Google Scholar]
- Rodgers G. M., Cong J. Y., Goll D. E., Kane W. H. (1987). Activation of coagulation factor V by calcium-dependent proteinase. Biochim. Biophys. Acta 929 263–270. 10.1016/0167-4889(87)90252-7 [DOI] [PubMed] [Google Scholar]
- Ryten M., Dunn P. M., Neary J. T., Burnstock G. (2002). ATP regulates the differentiation of mammalian skeletal muscle by activation of a P2X5 receptor on satellite cells. J. Cell Biol. 158 345–355. 10.1083/jcb.200202025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Hamamoto A., Kobayashi Y. (2013). Regulated Control of Melanin-Concentrating Hormone Receptor 1 through Posttranslational Modifications. Front. Endocrinol. 4:154. 10.3389/fendo.2013.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S., Reggiani C. (2011). Fiber types in mammalian skeletal muscles. Physiol. Rev. 91 1447–1531. 10.1152/physrev.00031.2010 [DOI] [PubMed] [Google Scholar]
- Shannon P., Markiel A., Ozier O., Baliga N. S., Wang J. T., Ramage D., et al. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13 2498–2504. 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden M. C., Holness M. J. (2003). Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am. J. Physiol. Endocrinol. Metab. 284 E855–E862. 10.1152/ajpendo.00526.2002 [DOI] [PubMed] [Google Scholar]
- Syed M., Skonberg C., Hansen S. H. (2016). Mitochondrial toxicity of selective COX-2 inhibitors via inhibition of oxidative phosphorylation (ATP synthesis) in rat liver mitochondria. Toxicol. Vitro 32 26–40. 10.1016/j.tiv.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Szklarczyk D., Gable A. L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., et al. (2019). STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47 D607–D613. 10.1093/nar/gky1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thebault S., Mesnard J. M. (2001). How the sequestration of a protein interferes with its mechanism of action: example of a new family of proteins characterized by a particular cysteine-rich carboxy-terminal domain involved in gene expression regulation. Curr. Protein Pept. Sci. 2 155–167. 10.2174/1389203013381143 [DOI] [PubMed] [Google Scholar]
- Tu M. K., Levin J. B., Hamilton A. M., Borodinsky L. N. (2016). Calcium signaling in skeletal muscle development, maintenance and regeneration. Cell Calcium 59 91–97. 10.1016/j.ceca.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N. J., Badylak S. F. (2012). Regeneration of skeletal muscle. Cell Tissue Res. 347 759–774. 10.1007/s00441-011-1185-7 [DOI] [PubMed] [Google Scholar]
- Wang Y., Pessin J. E. (2013). Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 16 243–250. 10.1097/MCO.0b013e328360272d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H., Li Z., Wang X., Wang J., Pang W., Yang G., et al. (2015). microRNA-151-3p regulates slow muscle gene expression by targeting ATP2a2 in skeletal muscle cells. J. Cell Physiol. 230 1003–1012. 10.1002/jcp.24793 [DOI] [PubMed] [Google Scholar]
- Wynne F., Ball M., McLellan A. S., Dockery P., Zimmermann W., Moore T. (2006). Mouse pregnancy-specific glycoproteins: tissue-specific expression and evidence of association with maternal vasculature. Reproduction 131 721–732. 10.1530/rep.1.00869 [DOI] [PubMed] [Google Scholar]
- Xie C., Mao X., Huang J., Ding Y., Wu J., Dong S., et al. (2011). KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 39 W316–W322. 10.1093/nar/gkr483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit P. S., Golding J. P., Nagata Y., Hudon V., Partridge T. A., Beauchamp J. R. (2004). Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J. Cell Biol. 166 347–357. 10.1083/jcb.200312007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Farruggio A. P., Bjornson C. R., Chavez C. L., Geisinger J. M., Neal T. L., et al. (2014). Recombinase-mediated reprogramming and dystrophin gene addition in mdx mouse induced pluripotent stem cells. PLoS One. 9:e96279. 10.1371/journal.pone.0096279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Shi X., Lu H., Xia B., Li Y., Li X., et al. (2016). RNA-seq transcriptome analysis of extensor digitorum longus and soleus muscles in large white pigs. Mol. Genet. Genomics 291 687–701. 10.1007/s00438-015-1138-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cell fusion observation under white light.
All the primers used in this study.
Antibodies used in the study.
The DEGs in Mdfi-OE vs. WT.
The KEGG pathways were enriched by DEGs.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: http://www.ncbi.nlm.nih.gov/bioproject/679162.