Abstract
Deficiency for telomerase results in transgenerational shortening of telomeres. However, telomeres have no known role in transgenerational epigenetic inheritance. C. elegans Protection Of Telomeres 1 (Pot1) proteins form foci at the telomeres of germ cells that disappear at fertilization and gradually accumulate during development. We find that gametes from mutants deficient for Pot1 proteins alter levels of telomeric foci for multiple generations. Gametes from pot-2 mutants give rise to progeny with abundant POT-1::mCherry and mNeonGreen::POT-2 foci throughout development, which persists for six generations. In contrast, gametes from pot-1 mutants or pot-1; pot-2 double mutants induce diminished Pot1 foci for several generations. Deficiency for MET-2, SET-25, or SET-32 methyltransferases, which promote heterochromatin formation, results in gametes that induce diminished Pot1 foci for several generations. We propose that C. elegans POT-1 may interact with H3K9 methyltransferases during pot-2 mutant gametogenesis to induce a persistent form of transgenerational epigenetic inheritance that causes constitutively high levels of heterochromatic Pot1 foci.
Subject terms: Epigenetics, Telomeres
Lister-Shimauchi et al. find that gametes from mutants that lack the genes encoding telomere binding proteins POT-1 or POT-2 affect the levels of POT1 telomere foci in progeny, in opposite ways, for multiple generations and that gametes deficient in heterochromatin formation reduce POT1 foci. These findings provide unexpected insights into an interface of telomere biology with transgenerational epigenetic inheritance.
Introduction
Most eukaryotic chromosome ends are composed of a tract of double-stranded tandem DNA repeats that terminates with a 3′ single-stranded overhang. Telomeric proteins prevent chromosome termini from being recognized as an aberrant form of DNA damage and protect the ends of linear chromosomes from nucleolytic attack. Telomeres normally erode as a consequence of incomplete DNA replication. However, telomere length is maintained by telomerase, which utilizes TElomerase Reverse Transcriptase (TERT) and telomerase RNA core subunits to add telomere repeats to chromosome termini. Telomere length is maintained across generations by high expression levels of TERT in germ cells1. Deficiency for telomerase in human families results in transgenerational shortening of telomeres, which causes an earlier age of onset of disease in successive generations2,3.
Ciliated protozoans that possess abundant telomeres enabled biochemical identification of single-stranded telomere binding proteins4–8, which were later shown to be functionally homologous to Pot1 proteins from Schizosaccharomyces pombe and humans9. Knockout of S. pombe Pot1 results in rapid telomere dysfunction9. Mammalian POT1 is part of a six subunit protein complex, termed shelterin, which interacts with telomeres to promote telomere stability and prevent chromosome termini from being recognized as DNA damage10,11.
Shelterin protects telomeres from being fused together by facilitating the formation of a T-loop at chromosome termini, where a strand invasion intermediate forms when the single-stranded 3′ telomeric overhang intercalates into a segment of double-stranded telomeric DNA12. Aside from 3′ single-stranded telomeric overhangs that are created in the context of telomere replication, POT1 may interact with single-stranded telomeric DNA that is displaced by the T-loop. In addition, a subset of nuclear POT1 may associate with shelterin complexes that interact with double-stranded telomeric DNA via the TRF1 and TRF2 Myb-domain proteins. That said, POT1 is 10-fold less abundant than some shelterin subunits and may therefore be enriched at segments of single-stranded telomeric DNA13.
Expression of POT1 in human cells results in telomere elongation14,15. Consistently, the shelterin subunits POT1 and TPP1 have been demonstrated to promote telomerase activity in vitro14,16,17. However, POT1 can also negatively regulate the activity of telomerase in vitro18, suggesting that POT1 may possess opposing telomeric functions.
The human genome contains a single POT1 gene that encodes a protein with two OB-fold domains, OB1 and OB2, which interact with single-stranded telomeric DNA. Mice, Arabidopsis, and Tetrahymena have multiple Pot1 genes that possess one or two OB-fold domains19–21. The nematode Caenorhabditis elegans possesses four Pot1 genes that encode single OB-fold domains: pot-1, pot-2, pot-3, and mrt-122. C. elegans POT-1 has an OB1 fold, whereas POT-2, POT-3, and MRT-1 each encode an OB2 fold23. Both POT-1 and POT-2 proteins can promote T-loop formation in vitro23. Mutation of either pot-1 or pot-2 leads to gradual lengthening of telomeres over ~16 generations, which depends on telomerase22. This phenotype is not exacerbated by simultaneous mutation of both pot-1 and pot-222, implying that these proteins may function together to repress telomerase. However, POT-1 and POT-2 proteins have distinct in vitro affinities for 5′ and 3′ ssDNA overhangs, respectively23. Furthermore, POT-1 but not POT-2 promotes tethering of telomeres to the nuclear periphery24, and C. elegans trt-1 telomerase mutants that are deficient for pot-2 but not pot-1 display an increased incidence of the telomerase-independent telomere maintenance pathway termed Alternative Lengthening of Telomeres (ALT)25,26. Together, these observations suggest that C. elegans POT-1 and POT-2 have distinct and common functions. A third C. elegans protein MRT-1 contains an OB2 fold that is required for telomerase activity in vivo27, consistent with the proposed role for mammalian POT-1 in telomerase function16,17.
Aside from shelterin, mammalian telomeric DNA interacts with histones that are enriched for H3K9 di- and trimethylation that promote genome silencing28. C. elegans telomeres also possess the H3K9 dimethyl histone silencing mark29, suggesting that heterochromatin may be a common theme of metazoan telomeres. Loss of heterochromatin in mouse primary cells deficient for the histone methyltransferases Suv39h1 and Suv39h2 is associated with telomere elongation28.
In this report, we uncover a novel connection between epigenetic inheritance and nuclear foci formed by telomere binding proteins. We find that gametes from mutants that lack C. elegans POT-1 or POT-2 single-stranded telomere binding proteins induce altered levels of telomeric foci for multiple generations. Histone H3 methyltransferases with known roles in transgenerational epigenetic inheritance also alter levels of telomeric foci. As regulation of telomeres and Pot1 have been tied to aging and cancer30,31, transgenerational epigenetic inheritance of Pot1 foci may be relevant to human health.
Results
Pot1 foci increase during embryonic development
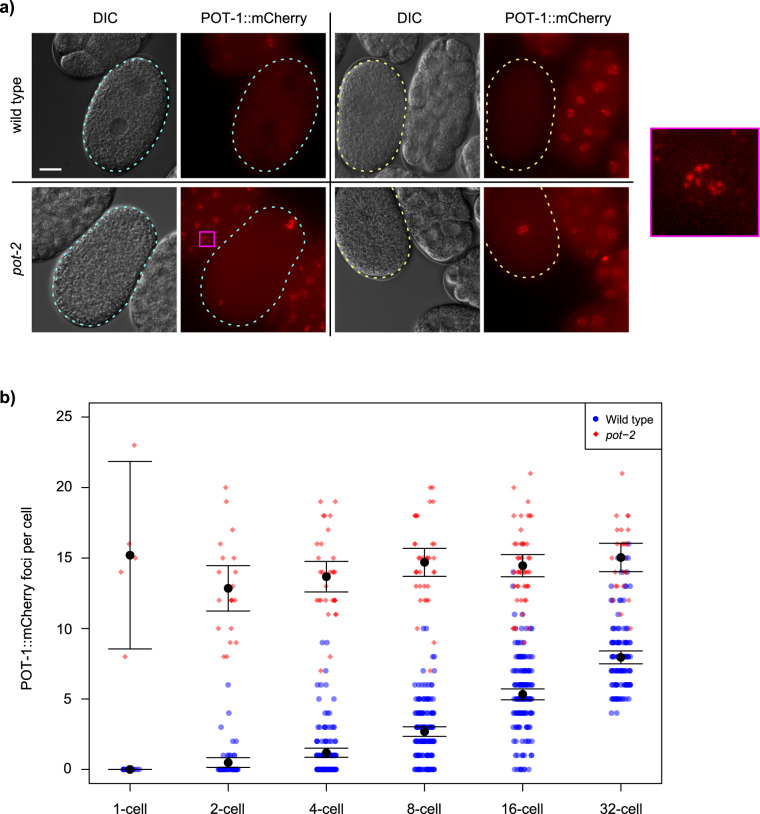
We previously created a single-copy transgene that expresses POT-1::mCherry and observed nuclear mCherry foci at telomeres of adult C. elegans germ cells as well as weak mCherry fluorescence throughout the nucleoplasm22. The specificity of POT-1::mCherry to telomeres of meiotic germ cells was supported by a predictable change in the number of POT-1::mCherry foci in response to an altered number of chromosomes22. We investigated the dynamics of POT-1 protein expression and observed strong POT-1::mCherry expression in mature sperm22. By contrast, the three most proximal (mature) oocytes displayed a reduced number of POT-1::mCherry foci (Supplementary Fig. 1a). Surprisingly, fertilization of oocytes resulted in 1-cell embryos that lacked POT-1 foci in both parental pronuclei and also in zygotic interphase nuclei (Fig. 1a). The number of POT-1::mCherry foci gradually increased during embryonic development to 8.0 ± 0.5 foci per nucleus in 32-cell embryos (Fig. 1b). We were able to quantify POT-1::mCherry foci in a small fraction of germ cells at L1–L4 larval stages and observed an abrupt transition where the number of foci doubled as L4 larvae matured into adults (Supplementary Fig. 1c, d). Therefore, POT-1 foci vanish in freshly fertilized embryos but accumulate during embryonic development and then undergo a pronounced transition at the onset of adulthood.
Fig. 1. Pot1 foci increase during early embryonic development.
a DIC and fluorescent images of 1-cell embryos before and after pronuclear fusion in either wild-type or pot-2 mutant strains. Cyan and yellow dashed lines indicate 1-cell embryos before and after pronuclear fusion, respectively. Scale bar is 10 µm. The portion of the image indicated by the magenta square is shown at 10x higher detail to the right. b POT-1::mCherry foci per nucleus of individual cells of embryos from 1- to 32-cell stage in wild-type (blue circles) and pot-2 mutants (red diamonds). The difference between wild-type and pot-2 mutants is significant at every stage (Wilcox p < 10−5). Error bars are 95% confidence intervals. Central black dots are means.
We previously showed that deficiency for pot-2 did not affect the abundance of POT-1::mCherry foci in meiotic cells of the adult germline22. Although wild-type animals had a diminished number of POT-1::mCherry foci in the most proximal oocytes (1.4 ± 1.3), pot-2 mutants had 11.4 ± 1.1 POT-1::mCherry foci per nucleus (Supplementary Fig. 1b, Wilcox p < 0.001). This corresponds to the number of chromosome ends expected for the 6 paired homologous chromosomes that are present in C. elegans oocytes. Abundant POT-1::mCherry foci were also observed in pronuclei and interphase nuclei of 1-cell pot-2 mutant embryos (15.2 ± 4.7 foci per nucleus) (Fig. 1a), suggesting that POT-2 normally dismantles POT-1 foci in 1-cell embryos. Mutation of pot-2 causes gradual telomere elongation over the course of 20 generations22, and the pot-2 mutant strain that we initially studied for expression of POT-1::mCherry foci had been cultured for more than 50 generations prior to analysis. We therefore utilized a strain in which the pot-2(tm1400) mutation had been outcrossed 14 times and possessed telomeres whose lengths were only modestly longer than those of wild-type (diagrammed in Supplementary Fig. 1e)22. Hermaphrodites from this outcrossed pot-2 mutant strain were crossed with males containing the pot-1::mCherry transgene, and freshly derived F3 pot-2 -/- mutant hermaphrodites were observed to possess high levels of POT-1::mCherry foci in all 1- and 2-cell F4 embryos (Supplementary Fig. 1f). Therefore, although telomeres of pot-2 mutant strains gradually lengthen over many generations of growth, acute loss of POT-2 induces abundant POT-1::mCherry foci in the interphase nuclei of early embryos.
Given the strong effect of deficiency for pot-2 on early embryonic POT-1::mCherry foci, we introduced an mNeonGreen tag at the endogenous pot-2 locus using CRISPR/Cas9-mediated genome modification32. We confirmed that this version of POT-2 with an N-terminal epitope tag was functional by examining the length of telomeric DNA of an mNeonGreen::pot-2 strain that had been grown for at least 50 generations. Southern blotting revealed telomeres of wild-type length, in contrast to the elongated telomeres of pot-2 mutants (Supplementary Fig. 2a). Cytoplasmic mNeonGreen::POT-2 was observed at all embryonic stages and in all adult germ cells (Supplementary Fig. 2b). Like POT-1::mCherry, mNeonGreen::POT-2 was strongly expressed in mature sperm, and embryonic mNeonGreen::POT-2 nuclear foci colocalized perfectly with POT-1::mCherry foci (Supplementary Fig. 2b). For simplicity, we hereafter refer to nuclear foci composed of C. elegans POT-1::mCherry or mNeonGreen::POT-2 proteins as ‘Pot1 foci’. mNeonGreen::POT-2 foci were absent from mature -1, -2, and -3 oocytes and from 1-cell interphase embryos and slowly reappeared during embryo development, similar to POT-1::mCherry foci (Supplementary Fig. 2b). However, ample cytoplasmic mNeonGreen::POT-2 levels did not qualitatively vary at these developmental stages (Supplementary Fig. 2b). Therefore, loss of nuclear mNeonGreen::POT-2 foci in 1- and 2-cell wild-type embryos is unlikely to be a consequence of transcriptional or translational regulation of the pot-2 locus.
Progeny of pot-1 and pot-2 mutants display altered Pot1 foci
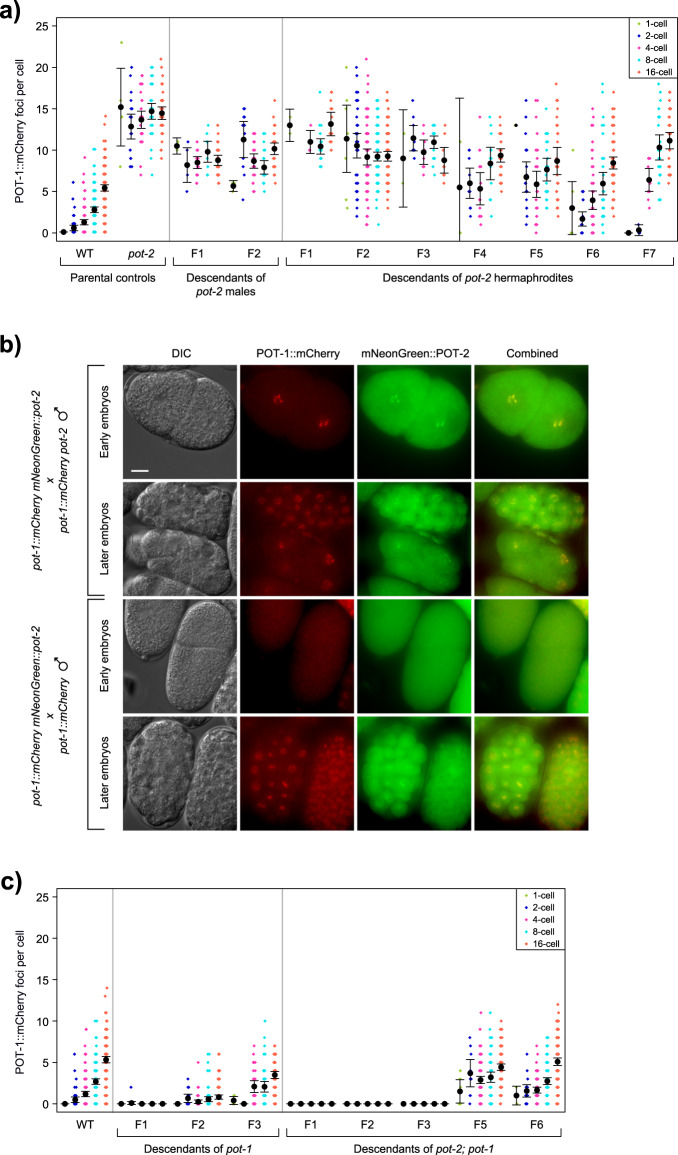
We asked if pot-2 deficiency in the oocytes of hermaphrodites would alter levels of Pot1 foci in progeny. When males expressing POT-1::mCherry were crossed with pot-2(tm1400) mutant hermaphrodites, abundant POT-1::mCherry foci were observed in 1- and 2-cell embryos of pot-2 heterozygous F1 cross-progeny (diagrammed in Supplementary Fig. 2c). This phenotype persisted for six generations, even for F2 lines that were homozygous wild-type for pot-2. The number of POT-1::mCherry foci per nucleus in early embryos gradually decreased over six generations but was still statistically increased at generation F7 in comparison to wild-type (Fig. 2a, ANOVA p < 2E-16). We confirmed this heritable effect using an independent loss-of-function pot-2 mutation, gk162073. When pot-2(gk162073) mutant hermaphrodites were crossed with wild-type males containing the pot-1::mCherry transgene, high levels of POT-1::mCherry foci were observed in all F2 embryos (Supplementary Fig. 2d, ANOVA p < 10E-14).
Fig. 2. Pot gene mutations affect Pot1 foci in subsequent generations.
a POT-1::mCherry foci per cell in embryos from P0 parental controls and descendants of either pot-2 mutant males or hermaphrodites crossed to POT-1::mCherry worms. Dot color indicates stage of embryo. b F1 early- and late-stage embryos from crosses between pot-1::mCherry mNeonGreen::pot-2 hermaphrodites and either pot-1::mCherry pot-2 males or pot-1::mCherry males. Scale bar is 10 µm. c POT-1::mCherry foci counts per nucleus in embryos from parental controls and descendants of either pot-1 or pot-2; pot-1 mutant males crossed to POT-1::mCherry worms. Error bars are 95% confidence intervals.
We asked if the induction of POT-1::mCherry foci in the progeny of pot-2 mutant hermaphrodites was a maternal effect by crossing pot-2 mutant males with pot-1::mCherry hermaphrodites. This resulted in F1 cross-progeny with abundant POT-1::mCherry foci in all early-stage F2 embryos. This phenotype persisted for at least one additional generation (Fig. 2a). An independent cross of pot-2 mutant males expressing POT-1::mCherry with hermaphrodites expressing both POT-1::mCherry and mNeonGreen::POT-2 generated F1 cross-progeny with abundant POT-1::mCherry foci that colocalized with mNeonGreen::POT-2 foci throughout embryonic development, including in interphase 1-cell zygotes (Fig. 2b). In contrast, control crosses of pot-1::mCherry males with pot-1::mCherry mNeonGreen::pot-2 hermaphrodites yielded F1 cross-progeny with 1- and 2-cell F2 embryos that lacked Pot1 foci and later-stage embryos with wild-type levels of Pot1 foci (Fig. 2b). Together, these results indicate that gametes of pot-2 mutants transmit a form of nuclear inheritance that results in abundant telomeric foci composed of POT-1 and, somewhat paradoxically, POT-2 in early embryos for multiple generations.
‘Transgenerational inheritance’ refers to a phenotype that can be transmitted via an oocyte for at least three generations, whereas ‘intergenerational inheritance’ is transmitted for only one or two generations33. Deficiency for pot-2 creates gametes that transmit high levels of Pot1 foci for multiple generations. As growth of pot-1 and pot-2 mutants results in telomere elongation, we asked if deficiency for pot-1 would affect levels of Pot1 foci. We crossed males expressing POT-1::mCherry with pot-1 mutant hermaphrodites. We found that POT-1::mCherry foci were either absent or reduced in number at all stages of embryonic development for two generations of progeny, but levels of POT-1::mCherry foci reverted to wild-type at generation F3 (Fig. 2c, ANOVA p = 0.101). We confirmed this intergenerational effect using an independent loss-of-function allele of pot-1, gk177893, which induced similarly low levels of POT-1::mCherry foci in all F2 embryos (Supplementary Fig. 2d, ANOVA p < 10E-14). We generated an mNeonGreen::pot-2 strain that was homozygous mutant for pot-1 and found that mNeonGreen::POT-2 foci were absent from embryos and from most adult germ cell nuclei of this strain, although cytoplasmic mNeonGreen::POT-2 was clearly present (Supplementary Fig. 2e). As gametes derived from pot-1 or pot-2 mutants created progeny with low or high levels of Pot1 foci, respectively, we crossed pot-1::mCherry mNeonGreen::pot-2 males to pot-2; pot-1 double mutant hermaphrodites and found that F1, F2, and F3 embryos resembled those of pot-1 mutants, with few foci appearing during development (Fig. 2c). This occurred for descendants of F2 progeny that were homozygous wild-type for pot-1 and pot-2. However, when Pot1 foci appeared at generation F4, their levels were increased in comparison to wild-type and remained slightly elevated for F6 embryos, similar to later-generation progeny of gametes from pot-2 mutants (Fig. 2c, ANOVA p = 0.00332).
Deficiency for pot-2 promotes the telomerase-independent telomere maintenance pathway ALT22, which is active in ~10% of tumors26. We asked if ALT affects Pot1 foci by crossing pot-1::mCherry worms to trt-1 mutants that had been passaged for over 100 generations under crowded conditions in order to activate the ALT pathway25. F1 cross-progeny of ALT strains possessed F2 embryos with wild-type levels of POT-1 and POT-2 foci (Supplementary Fig. 2f), indicating that gametes from animals that maintain their telomeres via the ALT pathway do not create progeny with altered levels of Pot1 foci. We utilized progeny of these crosses to establish a stable trt-1 mutant strain that expressed POT-1::mCherry and maintained its telomeres by ALT. However, this ALT strain possessed wild-type levels of Pot1 foci (Supplementary Fig. 2g).
Epigenetic regulation of Pot1 foci by H3K9 methyltransferases
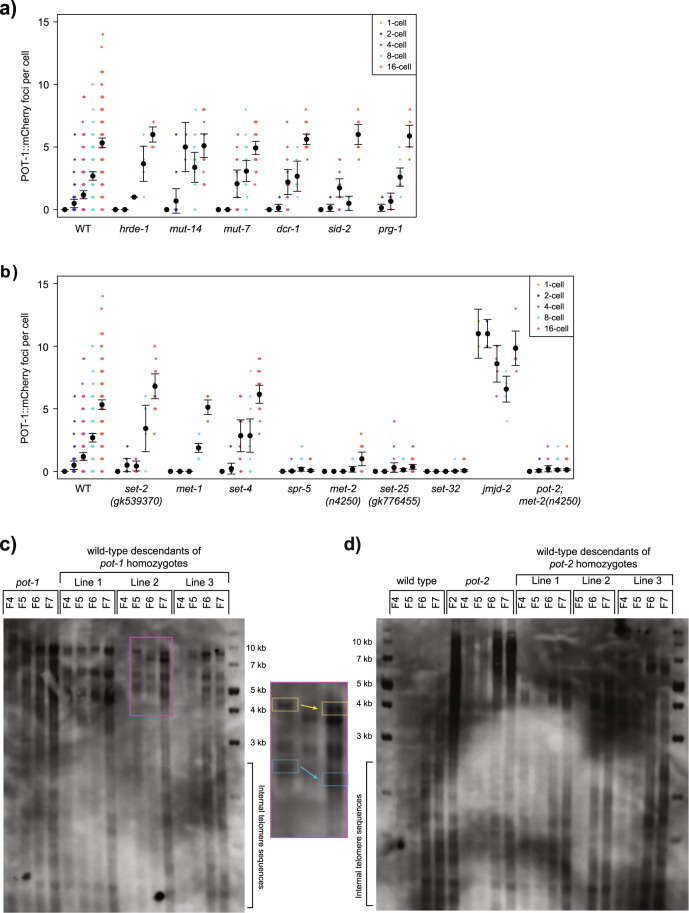
Gametes of pot-2 mutants alter levels of Pot1 foci for at least six generations, which is an example of transgenerational epigenetic inheritance that could be associated with factors that can modify gene expression for multiple generations in response to exogenous or endogenous cues, including small RNAs, histone modifications, or cytosine methylation33–37. We initially tested proteins that affect small RNAs that might guide histone modification at homologous genomic loci. We crossed pot-1::mCherry males with hermaphrodites deficient for proteins that promote various small RNA responses, mut-7, mut-14, dcr-1, hrde-1, prg-1, and sid-2. We did not observe significant changes to levels of Pot1 foci in F2 embryos from these crosses (Fig. 3a, ANOVA p > 0.05 for each).
Fig. 3. Pot1 foci number is epigenetically affected by genes involved in H3K9 methylation.
a, b Quantification of POT-1::mCherry foci in nuclei of F2 embryos derived from crosses of pot-1::mCherry males to hermaphrodites mutant for small RNA genes (a) or chromatin genes (b). c, d Southern blot using DNA from the genetically wild-type descendents of crosses between wild-type and pot-1 (c) or pot-2 (d) mutant worms. The portion of the pot-1 blot indicated by the magenta square is shown at higher detail to the right. Corresponding colored squares show telomere shortening across three generations. Error bars are 95% confidence intervals.
Although cytosine methylation does not occur in C. elegans38, telomeres in several species possess features of heterochromatin such as methylation of histone H3K9 or H3K2739–41. We therefore asked if Pot1 foci are affected by gametes from mutants with defects in genes that write or erase histone marks41. Levels of Pot1 foci were unaffected by gametes deficient for SET-2, which promotes transcription by methylating H3K442,43, for SET-4, an H4K20 methyltransferase44, or for MET-1, which promotes transcription by methylating H3K3645 (Fig. 3b, ANOVA p > 0.05 for each). However, a reduction of Pot1 foci was observed for the F2 embryos of hermaphrodites deficient for SPR-5, which demethylates H3K4me2 in a manner that allows for creation of the H3K9me3 genomic silencing mark (Fig. 3b, ANOVA p < 10−15)46. Consistently, dramatic reductions in Pot1 foci were observed for F2 progeny of animals deficient for independent alleles of the genome silencing proteins MET-2 and SET-25, which are H3K9 methyltransferases that act redundantly to promote localization of C. elegans heterochromatin domains to the nuclear periphery47(Fig. 3b and Supplementary Fig. 3a, ANOVA p < 10−8 for each). In contrast, an increase in Pot1 foci was observed for 1- and 2-cell F2 embryos of hermaphrodites deficient for JMJD-2, a demethylase that targets H3K9 or H3K36 (Fig. 3b, ANOVA p < 10−15)48,49. We also observed that deficiency for SET-32, an H3K23 methyltransferase that promotes transgenerational silencing responses to small RNAs, results in progeny with very low levels of Pot1 foci (Fig. 3b, ANOVA p < 10−8)47,50,51.
To further confirm our results, we analyzed a pot-1::mCherry strain that was homozygous mutant for the small RNA biogenesis factor mut-7 and found it had normal levels of Pot1 foci (Supplementary Fig. 3b), consistent with normal levels of Pot1 foci in the F2 progeny of mut-7 gametes (Fig. 3a). In contrast, we created pot-1::mCherry strains that were homozygous mutant for either met-2 or set-25 and observed dramatic reductions in levels of Pot1 foci (Supplementary Fig. 3b). We next asked if met-2 is epistatic to pot-2 by creating pot-2; met-2 double mutant hermaphrodites and observed reduced levels of Pot1 foci in F2 embryos created from crosses with pot-1::mCherry males (Fig. 3b, ANOVA p < 10−15).
As pot-1 or pot-2 mutants possess gametes that give rise to cross-progeny in which levels of Pot1 foci are altered for multiple generations, we asked if telomere length was perturbed in the progeny of pot-1 or pot-2 mutants. We crossed wild-type males to either control wild-type hermaphrodites or to well-outcrossed, early-generation pot-1 or pot-2 mutant hermaphrodites that possessed telomeres that are modestly longer than wild-type22. F2 cross-progeny that were wild-type for pot-1 or pot-2 were identified by PCR. Genomic DNA from the descendants of these genetically wild-type F2 was analyzed by Southern blotting using a probe for C. elegans telomere repeats (TTAGGC)n. We found that well-outcrossed pot-1 or pot-2 mutant lines had telomeres that were modestly elongated, but when either strain was crossed with wild-type and genetically wild-type F2s were isolated, descendants of gametes generated by either pot-1 or pot-2 mutants displayed telomeres that gradually shortened over the course of four generations (Fig. 3c, d). Therefore, gametes of pot-1 and pot-2 mutants that alter Pot1 focus levels for multiple generations do not induce the telomere lengthening phenotype that occurs in pot-1 or pot-2 mutants22. Similarly, telomere length was not consistently elongated in strains deficient for met-2, set-25, or set-32 H3 methyltransferases (Supplementary Fig. 3c), which like pot-1 mutants have gametes that create progeny with dramatic intergenerational reductions in levels of Pot1 foci.
Discussion
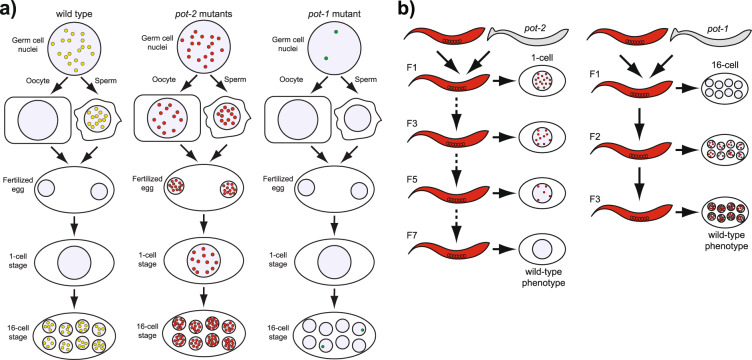
The stability of cytoplasmic mNeonGreen::POT-2 in conjunction with the absence of nuclear POT-2 foci in wild-type 1-cell embryos demonstrates that Pot1 foci are normally dismantled after fertilization (diagrammed in Fig. 4a). Pot1 foci are abundant in wild-type germ cells, decrease as oocytes mature, disappear from 1-cell zygotes, and gradually reappear during the initial cell cycles after fertilization. Although the physiological relevance of this temporal regulation is not clear, the dynamic regulation of telomeres during development is a common theme observed in distinct species. Telomere elongation occurs during blastulation in mouse embryos52, and telomerase activity spikes during blastulation in bovine embryos53. Similarly, components of the shelterin complex are temporally regulated during Xenopus development, such that high levels of transcription of telomerase and shelterin proteins were observed in blastula-stage embryos1,53,54.
Fig. 4. Models of Pot1 focus dynamics.
a Descriptive model of the developmental timing of Pot1 foci localization in wild type, pot-2 mutants, and pot-1 mutants. Red circles indicate POT-1::mCherry, green circles indicate mNeonGreen::POT-2, and yellow circles indicate colocalization of both transgenic proteins. b Descriptive model of the heritable phenotypes of progeny of gametes from pot-2 or pot-1 mutants.
Gametes of pot-1 or pot-2 mutants altered levels of Pot1 foci for multiple generations (diagrammed in Fig. 4b). Wild-type POT-1 protein must be present in both parents in order for their gametes to create progeny with stable Pot1 foci. In contrast, wild-type POT-2 must be present in both parents in order for their gametes to create progeny with Pot1 foci that are dismantled in 1-cell embryos. Loss of POT-2 in either parent creates germ cells whose progeny contain high levels of embryonic Pot1 foci for six generations, even in the presence of wild-type POT-2. This represents one of the most persistent forms of transgenerational epigenetic inheritance ever documented33. However, telomere length was not markedly altered in the progeny of either pot-1 or pot-2 mutants. Therefore, although telomerase dysfunction in humans or mice causes transgenerational effects that are associated with inheritance of telomeres of altered lengths, we have identified a form of epigenetic inheritance that modulates the levels of Pot1 foci during development in a manner that is independent of and inconsequential to telomere length. It remains possible that high or low levels of Pot1 foci that are induced by gametes of pot-2 or pot-1 mutants, respectively, could affect additional functions of Pot1 that include repression of telomere recombination, repression of resection of the C-rich strand of the telomere, or repression of DNA damage checkpoint activation at telomeres19,55. Interestingly, a previous report found that mouse telomeres undergo increased levels of recombination and telomerase-independent extension during the initial divisions of parthenogenetically activated oocytes56. However, it is not clear if Pot1 proteins are relevant to this process.
C. elegans telomeres have been previously shown to localize to the nuclear periphery in a manner that is lost in pot-1 mutants but not in pot-2 mutants or in met-2; set-25 double mutants24. Localization of heterochromatic transgenes and H3K9me-rich chromosome arms to the nuclear periphery is promoted by either MET-2 or SET-25 alone, but is lost in met-2; set-25 double mutants47. In contrast, loss of either MET-2 or SET-25 alone led to dramatic reductions in Pot1 foci. Therefore, the mechanism by which telomeres and other heterochromatic segments of the genome localize within C. elegans nuclei is genetically distinct from the mechanism by which POT-1, POT-2, and H3K9 methyltransferases regulate levels of nuclear Pot1 foci. That said, it remains possible that gametes from mutants deficient for either pot-1 or pot-2 cause a heritable change in telomere positioning or mobility that contributes to or is associated with altered levels of Pot1 foci.
Telomeres can modulate a form of position effect variegation where genes adjacent to telomeres can be epigenetically silenced in a manner that is mitotically heritable and reversible. This epigenetic phenomenon, termed the telomere position effect57,58, could be related to the mechanism by which gametes of pot-1 or pot-2 mutants alter levels of Pot1 foci for several generations. The dominant ability of gametes from pot-1 or pot-2 mutants to alter telomeric foci when crossed with wild-type gametes resembles paramutation, where small RNAs produced from a silent locus can act in trans to induce permanent heritable silencing of a distinct locus in the genome59. A related silencing process, termed RNAi inheritance, occurs when exogenous small RNAs direct chromatin modifying proteins to induce transient transgenerational silencing of GFP transgenes35,36,60. Although we did not observe an effect of small RNA biogenesis factors on Pot1 foci, loss of two H3K9 methyltransferases, MET-2 and SET-25, or loss of the H3K23 methyltransferase SET-32 mimicked the low levels of Pot1 foci observed in response to deficiency for pot-1 until the F2 generation. We did not test for an effect of these methyltransferase mutations in later generations. However, previous reports have observed that the H3K9 methyltransferases MET-2 and SET-25 can mediate forms of transgenerational epigenetic inheritance where transgene silencing is modulated by environmental or endogenous cues61,62. Support for a role for histone methylation in creating heterochromatic Pot1 foci is provided by strains deficient for the H3K9 demethylase jmjd-249, which created gametes that induced high levels of Pot1 foci for multiple generations, similar to gametes of pot-2 mutants. As small RNA biogenesis factors did not influence Pot1 focus formation at telomeres, recruitment of histone H3 methyltransferases to telomeres might be carried out by a sequence-specific telomere binding protein like POT-1.
Telomeric repeats in C. elegans and humans are enriched in H3K9me229, while human subtelomeres are enriched in H3K9me363,64. We found that telomeric foci were perturbed by mutations in either met-2 or set-25, two histone methyltransferases that create H3K9me2 and H3K9me3, respectively47,65. In addition, the loss of the H3K4 demethylase spr-5 reduced telomeric Pot1 foci. While SPR-5 may demethylate H3K966, its main activity is demethylation of H3K4, which promotes H3K9 methylation46. Interestingly, the drop in Pot1 foci we observed during oogenesis and early embryonic development coincides with decreased levels of nuclear histone methylation. H3K9me2 is high in pachytene germ cells but is lost in mature oocytes at diakinesis65. Furthermore, H3K9me1, me2, and me3 levels were previously demonstrated to be low in 1-cell embryos and then to rapidly increase during early embryogenesis67. The developmental dynamics of global levels of H3K9 methylation could be related to the mechanism by which Pot1 focus formation is transiently eliminated in wild-type embryos.
Although the absence of MET-2 and SET-25 together abolishes all germline H3K9 methylation47, we found that these proteins are independently required to promote the stability of Pot1 foci. A third histone methyltransferase that is required for Pot1 focus formation, SET-32, methylates H3K23, which is a heterochromatic mark that has been recently identified in C. elegans51,68. SET-25 and SET-32 have previously been shown to respond to small RNAs by establishing silent chromosome domains that are then maintained for multiple generations69. Loss of MET-2 affects fertility in a manner that becomes apparent only after growth for multiple generations70, which suggests transmission of a heritable epigenetic factor by gametes. As the stability of Pot1 foci strongly depends on H3K9 and H3K23 methyltransferases that have previously been demonstrated to promote heterochromatin formation at internal segments of the genome, we suggest that Pot1 foci correspond to silent chromosome domains at chromosome termini.
We addressed high levels of Pot1 foci in the descendants of gametes from pot-2 mutants by examining the progeny of pot-2; pot-1 and pot-2; met-2 double mutants, which did not display Pot1 foci in embryos. This implies that the presence of POT-1 and H3K9 methylation at telomeres of gametes from pot-2 mutants triggers high levels of telomeric foci for multiple generations. The precise mechanistic relationship between POT-1, MET-2, SET-25, SET-32, and H3K9 or H3K23 methylation and between POT-2, JMJD-2, and H3K9 demethylation in the context of transgenerational epigenetic inheritance of Pot1 foci are intriguing questions that remain to be explored experimentally.
Jean Baptiste Lamarck and Charles Darwin postulated that environmental stimuli might induce the transgenerational epigenetic inheritance of traits that promote fitness in future generations71. Although we do not understand the functional significance of altered levels of Pot1 foci, previous analysis of the genomes of 152 wild C. elegans strains revealed that 12 possessed a putative inactivating mutation in pot-2 that was associated with long telomeres, suggesting that either POT-2 or telomere length could contribute to fitness in the wild72.
We conclude that Pot1 proteins regulate persistent forms of intergenerational and transgenerational epigenetic inheritance that influence developmental expression of Pot1 foci at telomeres. Regulation of telomeres has been tied to aging and cancer, and mutations of the Pot1 locus in human families are associated with an increase in the incidence of cancer30,31,73,74. If transgenerational epigenetic inheritance of Pot1 foci is relevant to human health, then Pot1 mutations segregating in human pedigrees might induce traits in future generations that could affect family members that lack the Pot1 mutation30.
Methods
Strains
All strains were cultured at 20 °C on Nematode Growth Medium (NGM) plates seeded with Escherichia coli OP50, unless otherwise indicated. Strains used include Bristol N2 wild-type, YA1197 ypSi2 (pdaz-1::pot-1::mCherry::ttb-2 3′UTR), VC20674 pot-1(gk177893), YA1022 pot-1(tm1620) III, YA997 trt-1(ok410) I, BR3417 spr-5(by134) I, YA1024 pot-2(tm1400) II, VC40055 pot-2(gk162073), YA1249 pot-2(ypSi3 [mNeonGreen::pot-2]) II, CB444 unc-52(e444) II, NL1820 mut-7(pk720) III, YY470 dcr-1(mg375) III, NL1838 mut-14(pk738) V, HC196 sid-2(qt42), YY538 hrde-1(tm1200), MT16973 met-1(n4589), MT14851 set-2(n4589), VC40257 set-2(gk539370), MT14911 set-4(n4600), MT17463 set-25(n5021), VC40568 set-25(gk697056), VC40718 set-25(gk776455), SX922 prg-1(n4357), MT13293 met-2(n4256), VC40243 met-2(gk531543), VC40995 met-2(gk919134), VC967 set-32(ok1457), and VC20722 jmjd-2(gk383243).
Passaging of the YA1197 strain in the presence of males for dozens of generations sometimes results in silencing of the transgene, despite ypSi2 being a single-copy insertion22. When transgene silencing occurred, we thawed a frozen stock of YA1197 hermaphrodites.
Generation of fresh pot-2 homozygous mutants from balanced stocks
The pot-2 locus and the pot-1::mCherry transgene both reside on Chromosome II. pot-2 is very close to unc-52, while pot-1::mCherry is very close to rol-6. We created heterozygous pot-2(tm1400) male stock by crossing pot-2(tm1400) hermaphrodites with an unc-52 − / + males, crossing single F1 males with unc-52 mutant hermaphrodites, choosing crosses that yielded Unc F1 males, and crossing their non-Unc male siblings with unc-52 hermaphrodites. The pot-2(tm1400) / unc-52 males were repeatedly crossed with unc-52 hermaphrodites. A rol-6 unc-52 double mutant strain was used to create pot-1::mCherry, unc-52 double mutants. pot-2(tm1400) / unc-52 males were crossed with rol-6, unc-52 double mutants, non-Unc F1 males were crossed with pot-1::mCherry, unc-52 hermaphrodites to create pot-2 − / + F1 hermaphrodites possessing a single copy of pot-1::mCherry (Supplementary Fig. 1e). pot-2 heterozygotes possessing two copies of pot-1::mCherry were obtained by selecting F2 worms that lacked Rol F3 but had some Unc F3 progeny. Most F3 embryos produced by these F2 worms did not display abundant early foci. However, a few, most likely the pot-2 homozygous mutants, did display early foci. We also studied F3 adult progeny that were pot-1::mCherry; pot-2 − / − homozygotes and found high levels of POT-1::mCherry foci in all 1- and 2-cell F4 embryos. pot-2 mutants that are created from well-outcrossed pot-2 mutant stocks have normal telomere lengths at generation F4, which slowly increase in length over the course of 16 generations22.
Generation of mNeonGreen::pot-2 transgenic strain
DNA constructs for the mNeonGreen::pot-2 transgene were generated based on the previously described SapTrap protocol75. CRISPR-based insertion into the genome was accomplished using previously described protocols32. Sanger sequencing of the mNeonGreen tag and neighboring genomic region confirmed the mNeonGreen insertion.
We generated epitope tags at the endogenous pot-1 locus through similar means. Though we were successful in generating both N- and C-terminal mKate tags of pot-1, we were unable to visualize the transgenes. This may be due to weak expression from the endogenous pot-1 locus, as expression of pot-2 is considerably higher than pot-1 based on RNAseq data from the modENCODE project76.
Generation of trt-1; pot-1::mCherry mNeonGreen::pot-2 ALT lines
trt-1 mutant worms were passaged for over 100 generations by chunking in order to obtain lines that maintained telomeres by ALT. Individual L4 ALT hermaphrodites were mated to males homozygous for pot-1::mCherry and mNeonGreen::pot-2. Singled F2 homozygotes containing both the fluorescent tags and the trt-1 mutation were identified by PCR. These lines were then passaged by chunking for another 20 generations to ensure continued activation of ALT.
Crosses
To perform crosses with strains containing pot-1::mCherry or mNeonGreen::pot-2, 24 males and 10 hermaphrodites were placed on a single NGM plate. Plates were incubated at 15 °C for 3 days to obtain F1 cross-progeny embryos from hermaphrodites that were all mated.
Imaging
Whole worms and embryos were mounted on 2% agarose pads and imaged live using a DeltaVision Elite microscope (Applied Precision) with a 60x Silicon oil objective lens. For germline imaging, immobilization was assisted by transferring worms into 5 µL drops of 0.2 mM levamisole on agarose pads. For embryo imaging, embryos were extruded from adults in M9 buffer and embryos were transferred onto agarose pads using a mouth pipet.
Statistics and reproducibility
Images were analyzed using ImageJ software. Foci were counted manually from fluorescent images, while embryo stage was determined from DIC images.
Statistical calculations were performed using R software. Focus numbers were compared using either the Wilcoxon rank-sum test or 2-way ANOVA, depending on the number of variables tested simultaneously. When making multiple simultaneous statistical comparisons, p-values were adjusted using the Holm-Bonferroni method. All error bars displayed represent 95% confidence intervals. Sample sizes for imaging experiments are listed in Supplementary Data 1.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank Felix Peng for initial observations of high levels of POT-1::mCherry foci in early embryos of pot-2 mutants. Dan Dickinson kindly provided plasmids used in the generation of the mNeonGreen::pot-2 targeting construct. We thank Bob Goldstein and Dan Dickinson for advice regarding CRISPR-mediated genome modifications. We are indebted to Amy Maddox for training on and access to her DeltaVision microscope. P.M. is the William Burwell Harrison Scholar and supported by NSF CAREER award #1652512. S.A. is supported by NIH grant R01GM135470.
Author contributions
E.L.S. and M.D. performed the experiments. E.L.S analyzed the data. P.M. and S.A. supervised the experiments. S.A. and E.L.S. wrote the manuscript with input from M.D. and P.M.
Data availability
Image datasets used for the generation of figure graphs can be found online at Dryad, 10.5061/dryad.mkkwh70xz77.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Evan H. Lister-Shimauchi, Michael Dinh.
Contributor Information
Evan H. Lister-Shimauchi, Email: evanhlister@gmail.com
Shawn Ahmed, Email: shawn@med.unc.edu.
Supplementary information
Supplementary information is available for this paper at 10.1038/s42003-020-01624-7.
References
- 1.Pech MF, et al. High telomerase is a hallmark of undifferentiated spermatogonia and is required for maintenance of male germline stem cells. Genes Dev. 2015;29:2420–2434. doi: 10.1101/gad.271783.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armanios M, Blackburn EH. The telomere syndromes. Nat. Rev. Genet. 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roake CM, Artandi SE. Regulation of human telomerase in homeostasis and disease. Nat. Rev. Mol. Cell Biol. 2020;21:384–397. doi: 10.1038/s41580-020-0234-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottschling DE, Cech TR. Chromatin structure of the molecular ends of Oxytricha macronuclear DNA: phased nucleosomes and a telomeric complex. Cell. 1984;38:501–510. doi: 10.1016/0092-8674(84)90505-1. [DOI] [PubMed] [Google Scholar]
- 5.Gottschling DE, Zakian VA. Telomere proteins: specific recognition and protection of the natural termini of Oxytricha macronuclear DNA. Cell. 1986;47:195–205. doi: 10.1016/0092-8674(86)90442-3. [DOI] [PubMed] [Google Scholar]
- 6.Price CM, Cech TR. Telomeric DNA-protein interactions of Oxytricha macronuclear DNA. Genes Dev. 1987;1:783–793. doi: 10.1101/gad.1.8.783. [DOI] [PubMed] [Google Scholar]
- 7.Price CM. Telomere structure in Euplotes crassus: characterization of DNA-protein interactions and isolation of a telomere-binding protein. Mol. Cell. Biol. 1990;10:3421–3431. doi: 10.1128/MCB.10.7.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W, Skopp R, Scofield M, Price C. Euplotes crassus has genes encoding telomere-binding proteins and telomere-binding protein homologs. Nucleic Acids Res. 1992;20:6621–6629. doi: 10.1093/nar/20.24.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumann P, Cech TR. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science. 2001;292:1171–1175. doi: 10.1126/science.1060036. [DOI] [PubMed] [Google Scholar]
- 10.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 11.de Lange T. Shelterin-mediated telomere protection. Annu. Rev. Genet. 2018;52:223–247. doi: 10.1146/annurev-genet-032918-021921. [DOI] [PubMed] [Google Scholar]
- 12.Griffith JD, et al. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/S0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 13.Takai KK, Hooper S, Blackwood S, Gandhi R, de Lange T. In vivo stoichiometry of shelterin components. J. Biol. Chem. 2010;285:1457–1467. doi: 10.1074/jbc.M109.038026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colgin LM, Baran K, Baumann P, Cech TR, Reddel RR. Human POT1 facilitates telomere elongation by telomerase. Curr. Biol. 2003;13:942–946. doi: 10.1016/S0960-9822(03)00339-7. [DOI] [PubMed] [Google Scholar]
- 15.Loayza D, De Lange T. POT1 as a terminal transducer of TRF1 telomere length control. Nature. 2003;423:1013–1018. doi: 10.1038/nature01688. [DOI] [PubMed] [Google Scholar]
- 16.Wang F, et al. The POT1–TPP1 telomere complex is a telomerase processivity factor. Nature. 2007;445:506–510. doi: 10.1038/nature05454. [DOI] [PubMed] [Google Scholar]
- 17.Xin H, et al. TPP1 is a homologue of ciliate TEBP-β and interacts with POT1 to recruit telomerase. Nature. 2007;445:559–562. doi: 10.1038/nature05469. [DOI] [PubMed] [Google Scholar]
- 18.Kelleher C, Kurth I, Lingner J. Human protection of telomeres 1 (POT1) is a negative regulator of telomerase activity in vitro. Mol. Cell. Biol. 2005;25:808–818. doi: 10.1128/MCB.25.2.808-818.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hockemeyer D, Daniels J-P, Takai H, de Lange T. Recent expansion of the telomeric complex in rodents: two distinct POT1 proteins protect mouse telomeres. Cell. 2006;126:63–77. doi: 10.1016/j.cell.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 20.Shakirov EV, Surovtseva YV, Osbun N, Shippen DE. The Arabidopsis Pot1 and Pot2 proteins function in telomere length homeostasis and chromosome end protection. Mol. Cell. Biol. 2005;25:7725–7733. doi: 10.1128/MCB.25.17.7725-7733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacob NK, Lescasse R, Linger BR, Price CM. Tetrahymena POT1a regulates telomere length and prevents activation of a cell cycle checkpoint. Mol. Cell. Biol. 2007;27:1592–1601. doi: 10.1128/MCB.01975-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shtessel L, et al. Caenorhabditis elegans POT-1 and POT-2 repress telomere maintenance pathways. G3. 2013;3:305–313. doi: 10.1534/g3.112.004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raices M, et al. C. elegans telomeres contain G-strand and C-strand overhangs that are bound by distinct proteins. Cell. 2008;132:745–757. doi: 10.1016/j.cell.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira HC, Towbin BD, Jegou T, Gasser SM. The shelterin protein POT-1 anchors Caenorhabditis elegans telomeres through SUN-1 at the nuclear periphery. J. Cell Biol. 2013;203:727–735. doi: 10.1083/jcb.201307181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng C, Shtessel L, Brady MM, Ahmed S. Caenorhabditis elegans POT-2 telomere protein represses a mode of alternative lengthening of telomeres with normal telomere lengths. Proc. Natl Acad. Sci. 2012;109:7805–7810. doi: 10.1073/pnas.1119191109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickett HA, Reddel RR. Molecular mechanisms of activity and derepression of alternative lengthening of telomeres. Nat. Struct. Mol. Biol. 2015;22:875–880. doi: 10.1038/nsmb.3106. [DOI] [PubMed] [Google Scholar]
- 27.Meier B, et al. The MRT-1 nuclease is required for DNA crosslink repair and telomerase activity in vivo in Caenorhabditis elegans. EMBO J. 2009;28:3549–3563. doi: 10.1038/emboj.2009.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.García-Cao M, O’Sullivan R, Peters AHFM, Jenuwein T, Blasco MA. Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nat. Genet. 2004;36:94–99. doi: 10.1038/ng1278. [DOI] [PubMed] [Google Scholar]
- 29.McMurchy AN, et al. Correction: a team of heterochromatin factors collaborates with small RNA pathways to combat repetitive elements and germline stress. Elife. 2017;6:e32516. doi: 10.7554/eLife.32516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi J, et al. Rare missense variants in POT1 predispose to familial cutaneous malignant melanoma. Nat. Genet. 2014;46:482–486. doi: 10.1038/ng.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robles-Espinoza CD, et al. POT1 loss-of-function variants predispose to familial melanoma. Nat. Genet. 2014;46:478–481. doi: 10.1038/ng.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickinson DJ, Pani AM, Heppert JK, Higgins CD, Goldstein B. Streamlined genome engineering with a self-excising drug selection cassette. Genetics. 2015;200:1035–1049. doi: 10.1534/genetics.115.178335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez MF, Lehner B. Intergenerational and transgenerational epigenetic inheritance in animals. Nat. Cell Biol. 2019;21:143–151. doi: 10.1038/s41556-018-0242-9. [DOI] [PubMed] [Google Scholar]
- 34.Billi A.C., Fischer S.E.J. & Kim J.K. Endogenous RNAi pathways in C. elegans. WormBook, 1–49, 10.1895/wormbook.1.170.1 (2014). [DOI] [PMC free article] [PubMed]
- 35.Ashe A, et al. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell. 2012;150:88–99. doi: 10.1016/j.cell.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckley BA, et al. A nuclear Argonaute promotes multigenerational epigenetic inheritance and germline immortality. Nature. 2012;489:447–451. doi: 10.1038/nature11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greer EL, et al. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature. 2011;479:365–371. doi: 10.1038/nature10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson VJ, Johnson TE, Hammen RF. Caenorhabditis elegans DNA does not contain 5-methylcytosine at any time during development or aging. Nucleic Acids Res. 1986;14:6711–6719. doi: 10.1093/nar/14.16.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jamieson K, et al. Telomere repeats induce domains of H3K27 methylation in Neurospora. Elife. 2018;7:e31216. doi: 10.7554/eLife.31216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montero JJ, et al. TERRA recruitment of polycomb to telomeres is essential for histone trymethylation marks at telomeric heterochromatin. Nat. Commun. 2018;9:1548. doi: 10.1038/s41467-018-03916-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahringer J, Gasser SM. Repressive chromatin in Caenorhabditis elegans: establishment, composition, and function. Genetics. 2018;208:491–511. doi: 10.1534/genetics.117.300386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simonet T, Dulermo R, Schott S, Palladino F. Antagonistic functions of SET-2/SET1 and HPL/HP1 proteins in C. elegans development. Dev. Biol. 2007;312:367–383. doi: 10.1016/j.ydbio.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 43.Nottke AC, et al. SPR-5 is a histone H3K4 demethylase with a role in meiotic double-strand break repair. Proc. Natl Acad. Sci. USA. 2011;108:12805–12810. doi: 10.1073/pnas.1102298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vielle A, et al. H4K20me1 contributes to downregulation of X-linked genes for C. elegans dosage compensation. PLoS Genet. 2012;8:e1002933. doi: 10.1371/journal.pgen.1002933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersen EC, Horvitz HR. Two C. elegans histone methyltransferases repress lin-3 EGF transcription to inhibit vulval development. Development. 2007;134:2991–2999. doi: 10.1242/dev.009373. [DOI] [PubMed] [Google Scholar]
- 46.Greer EL, et al. A histone methylation network regulates transgenerational epigenetic memory in C. elegans. Cell Rep. 2014;7:113–126. doi: 10.1016/j.celrep.2014.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Towbin BD, et al. Step-wise methylation of histone H3K9 positions heterochromatin at the nuclear periphery. Cell. 2012;150:934–947. doi: 10.1016/j.cell.2012.06.051. [DOI] [PubMed] [Google Scholar]
- 48.Whetstine JR, et al. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell. 2006;125:467–481. doi: 10.1016/j.cell.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 49.Camacho J, et al. The memory of environmental chemical exposure in C. elegans is dependent on the Jumonji Demethylases jmjd-2 and jmjd-3/utx-1. Cell Rep. 2018;23:2392–2404. doi: 10.1016/j.celrep.2018.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalinava N, et al. Caenorhabditis elegans heterochromatin factor SET-32 plays an essential role in transgenerational establishment of nuclear RNAi-mediated epigenetic silencing. Cell Rep. 2018;25:2273–2284.e3. doi: 10.1016/j.celrep.2018.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz-Orbach L, et al. Caenorhabditis elegans nuclear RNAi factor SET-32 deposits the transgenerational histone modification, H3K23me3. Elife. 2020;9:e54309. doi: 10.7554/eLife.54309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schaetzlein S, et al. Telomere length is reset during early mammalian embryogenesis. Proc. Natl Acad. Sci. USA. 2004;101:8034–8038. doi: 10.1073/pnas.0402400101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Betts DH, Bordignon V, Smith L, Semple E, King WA. Telomerase activity in bovine blastocysts, fetal fibroblast and stem cell-like cell lines cultured under various conditions. Theriogenology. 1999;51:181. doi: 10.1016/S0093-691X(99)91740-5. [DOI] [Google Scholar]
- 54.Vizlin-Hodzic D, Ryme J, Simonsson S, Simonsson T. Developmental studies of Xenopus shelterin complexes: the message to reset telomere length is already present in the egg. FASEB J. 2009;23:2587–2594. doi: 10.1096/fj.09-129619. [DOI] [PubMed] [Google Scholar]
- 55.Wu L, et al. Pot1 deficiency initiates DNA damage checkpoint activation and aberrant homologous recombination at telomeres. Cell. 2006;126:49–62. doi: 10.1016/j.cell.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 56.Liu L, et al. Telomere lengthening early in development. Nat. Cell Biol. 2007;9:1436–1441. doi: 10.1038/ncb1664. [DOI] [PubMed] [Google Scholar]
- 57.Gottschling DE, Aparicio OM, Billington BL, Zakian VA. Position effect at S. cerevisiae telomeres: Reversible repression of Pol II transcription. Cell. 1990;63:751–762. doi: 10.1016/0092-8674(90)90141-Z. [DOI] [PubMed] [Google Scholar]
- 58.Tham W-H, Zakian VA. Transcriptional silencing at Saccharomyces telomeres: implications for other organisms. Oncogene. 2002;21:512–521. doi: 10.1038/sj.onc.1205078. [DOI] [PubMed] [Google Scholar]
- 59.Hollick JB. Paramutation and related phenomena in diverse species. Nat. Rev. Genet. 2017;18:5–23. doi: 10.1038/nrg.2016.115. [DOI] [PubMed] [Google Scholar]
- 60.Seth M, et al. The C. elegans CSR-1 argonaute pathway counteracts epigenetic silencing to promote germline gene expression. Dev. Cell. 2013;27:656–663. doi: 10.1016/j.devcel.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klosin A, Casas E, Hidalgo-Carcedo C, Vavouri T, Lehner B. Transgenerational transmission of environmental information in C. elegans. Science. 2017;356:320–323. doi: 10.1126/science.aah6412. [DOI] [PubMed] [Google Scholar]
- 62.Klosin A, et al. Impaired DNA replication derepresses chromatin and generates a transgenerationally inherited epigenetic memory. Sci. Adv. 2017;3:e1701143. doi: 10.1126/sciadv.1701143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thijssen PE, et al. Chromatin remodeling of human subtelomeres and TERRA promoters upon cellular senescence. Epigenetics. 2013;8:512–521. doi: 10.4161/epi.24450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arnoult N, Van Beneden A, Decottignies A. Telomere length regulates TERRA levels through increased trimethylation of telomeric H3K9 and HP1α. Nat. Struct. Mol. Biol. 2012;19:948–956. doi: 10.1038/nsmb.2364. [DOI] [PubMed] [Google Scholar]
- 65.Bessler JB, Andersen EC, Villeneuve AM. Differential localization and independent acquisition of the H3K9me2 and H3K9me3 chromatin modifications in the Caenorhabditis elegans adult germ line. PLoS Genet. 2010;6:e1000830. doi: 10.1371/journal.pgen.1000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Metzger E, et al. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437:436–439. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- 67.Mutlu B, et al. Regulated nuclear accumulation of a histone methyltransferase times the onset of heterochromatin formation in C. elegans embryos. Sci. Adv. 2018;4:eaat6224. doi: 10.1126/sciadv.aat6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vandamme J, et al. H3K23me2 is a new heterochromatic mark in Caenorhabditis elegans. Nucleic Acids Res. 2015;43:9694–9710. doi: 10.1093/nar/gkv1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woodhouse RM, Ashe A. Transgenerational epigenetic inheritance is revealed as a multi-step process by studies of the SET-domain proteins SET-25 and SET-32. Epigenet. Insights. 2019;12:251686571984421. doi: 10.1177/2516865719844214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lev I, et al. MET-2-dependent H3K9 methylation suppresses transgenerational small RNA inheritance. Curr. Biol. 2017;27:1138–1147. doi: 10.1016/j.cub.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 71.Skinner MK. Environmental epigenetics and a unified theory of the molecular aspects of evolution: a Neo-Lamarckian Concept that facilitates Neo-Darwinian evolution. Genome Biol. Evolution. 2015;7:1296–1302. doi: 10.1093/gbe/evv073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laricchia KM, Zdraljevic S, Cook DE, Andersen EC. Natural variation in the distribution and abundance of transposable elements across the Caenorhabditis elegans species. Mol. Biol. Evol. 2017;34:2187–2202. doi: 10.1093/molbev/msx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Calvete O, et al. The wide spectrum of POT1 gene variants correlates with multiple cancer types. Eur. J. Hum. Genet. 2017;25:1278–1281. doi: 10.1038/ejhg.2017.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karami S, et al. Telomere structure and maintenance gene variants and risk of five cancer types. Int. J. Cancer. 2016;139:2655–2670. doi: 10.1002/ijc.30288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwartz ML, Jorgensen EM. SapTrap, a Toolkit for high-throughput CRISPR/Cas9 gene modification in Caenorhabditis elegans. Genetics. 2016;202:1277–1288. doi: 10.1534/genetics.115.184275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gerstein MB, et al. Integrative analysis of the Caenorhabditis elegans genome by the modENCODE project. Science. 2010;330:1775–1787. doi: 10.1126/science.1196914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lister-Shimauchi, E. pot-1::mCherry transgene images from: Gametes deficient for Pot1 telomere binding proteins alter levels of telomeric foci for multiple generations, Dryad, Dataset, 10.5061/dryad.mkkwh70xz (2021). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Image datasets used for the generation of figure graphs can be found online at Dryad, 10.5061/dryad.mkkwh70xz77.