Abstract
Immunoglobulin G (IgG) functionality can drastically change from anti- to proinflammatory by alterations in the IgG N-glycan patterns. Our previous studies have demonstrated that IgG N-glycans associated with the risk factors of dementia, such as aging, dyslipidemia, type 2 diabetes mellitus, hypertension, and ischemic stroke. Therefore, the aim is to investigate whether the effects of IgG N-glycan profiles on dementia exists in a Chinese Han population. A case–control study, including 81 patients with dementia, 81 age- and gender-matched controls with normal cognitive functioning (NC) and 108 non-matched controls with mild cognitive impairment (MCI) was performed. Plasma IgG N-glycans were separated by ultra-performance liquid chromatography. Fourteen glycan peaks reflecting decreased of sialylation and core fucosylation, and increased bisecting N-acetylglucosamine (GlcNAc) N-glycan structures were of statistically significant differences between dementia and NC groups after controlling for confounders (p < 0.05; q < 0.05). Similarly, the differences for these 14 initial glycans were statistically significant between AD and NC groups after adjusting for the effects of confounders (p < 0.05; q < 0.05). The area under the receiver operating curve (AUC) value of the model consisting of GP8, GP9, and GP14 was determined to distinguish dementia from NC group as 0.876 [95% confidence interval (CI): 0.815–0.923] and distinguish AD from NC group as 0.887 (95% CI: 0.819–0.936). Patients with dementia were of an elevated proinflammatory activity via the significant changes of IgG glycome. Therefore, IgG N-glycans might contribute to be potential novel biomarkers for the neurodegenerative process risk assessment of dementia.
Subject terms: Biomarkers, Inflammation, Risk factors
Introduction
Dementia is a major global challenge for health and social care in the 21st century. It occurs mainly in subjects older than 65 years, making them gradually lose their abilities and become more dependent1. Alzheimer disease (AD) is the most common form of dementia, accounting for approximately 60–80% of all types1. The concept of mild cognitive impairment (MCI) is as an intermediate state between normal cognition and dementia; nevertheless, the boundary between the MCI and dementia is gray2. Research has found that potentially modifiable risk factors of worldwide dementia, mainly vascular risk factors, account for 40% (hypertension, obesity, diabetes, later-life smoking, depression, physical inactivity, etc.)3,4. Besides, increasing age is also one of the strongest known risk factors for dementia5,6. During the process of senescence, cells secrete a large number of inflammatory factors and chemokines, such as interleukin-6 (IL-6), interleukin-1 beta (IL-1β), and tumor necrosis factor-alpha (TNF-α), which can produce chronic inflammatory responses5,7. Accumulating evidence has suggested that inflammatory responses make a valuable contribution towards the neurodegenerative cascades of dementia and MCI, and several markers were found to be able to accurately detect the disease severity and progression8–12. The mechanisms of dementia, however, are still largely unknown.
Glycosylation is a complex post-translational modification during gene expression involved in more than half of all mammalian proteins13. Glycan binding in functional proteins has a crucial role in the biological processes of molecular recognition and adhesion, cell signaling or immunological response14,15. Hence, molecular defects in glycan synthesis are recognized as direct causes of an increasing number of diseases16,17. Immunoglobulin G (IgG) exerts the role in the immune system the body18. In terms of its characteristic of structure with conserved N-glycosylation in Fc, so IgG is one of the mostly suitable choices for glycoproteins research19. The attachment of different glycans displays conformational flexibility and plasticity which can be dramatically influence on IgG effector functions15. In particular, the glycan portion of IgG critically affects immune effector functions mediated by the Fc receptor and complement C1q20. It modulates in relation to current physiological conditions, but can also change in an “on and off mode” during acute inflammation21. Hence, changes in IgG glycosylation not only participate in many kinds of inflammatory diseases22–25, but also the molecular mechanism may give rise to the promotion of inflammation21,26,27.
Considering the fact that the inflammatory role of IgG N-glycosylation and its association with the risk factors of dementia [such as aging, central obesity, dyslipidemia, type 2 diabetes mellitus (T2DM), hypertension, and ischemic stroke]28–34, we hypothesized that these associations might provide a possible explanation for the pathogenesis of dementia in a Chinese Han population. In addition, previous study provided the evidence in European ancestry individuals that a lower abundance of complex galactosylation and sialylation played a role in the development of AD35. Therefore, the aim is to explore whether the changes in IgG glycosylation can identify specific alterations for dementia progress in a Chinese Han population and reveal dementia potential pathogenesis. Meanwhile, we investigate the associations between IgG N-glycan profiles and inflammation factors including anti-inflammatory (IL-4 and IL-10) and proinflammatory factors [IL-1β, IL-6, CRP, TNF-α, and interferon-gamma (IFN-γ)], which may further explain the role of IgG glycosylation in dementia.
Results
Participants’ characteristics
In total, 81 patients with dementia, 81 age- and gender-matched participants with NC and 108 non-matched participants with MCI were included in the present study. The 81 dementia participants consisted of 47 (58.00%) AD, 18 (22.20%) vascular dementia (VaD), and 16 (19.80%) other type of dementia. The differences of demographic characteristics and inflammatory factors among three groups were presented in Table 1 and Fig. 1. The median ages for three groups were 82, 80, and 76 years, respectively. The number of females was generally higher than males for each group, but it was not statistically significant among three groups. The values of TC and LDL-C in the dementia group were significantly lower than these in the NC group (p < 0.017). In addition, age in the dementia group was significantly higher than that in the MCI group, whereas values of systolic blood pressure (SBP) and diastolic blood pressure (DBP) were lower than these in the MCI group (p < 0.017). The levels of SBP, DBP, TC, and LDL-C of MCI group were significantly higher than NC group (p < 0.017).
Table 1.
Characteristics of the study subjects.
| Parameters | NC (n = 81) | MCI (n = 108) | Dementia (n = 81) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | p | AD (n = 47) | p | VaD (n = 18) | p | OD (n = 16) | p | |||
| Gender (male/female) | 38/43 | 42/66 | 38/43 | 0.617 | 22/25 | 0.392 | 8/10 | 0.537 | 8/8 | 0.542 |
| Age, years | 80.00 (73.50–85.00) | 76.00 (73.00–81.00) | 82.00 (75.50–85.00)#& | <0.001 | 82.00 (77.00–82.00) | <0.001 | 79.50 (74.25–84.25) | 0.039 | 81.00 (74.00–84.75) | 0.039 |
| BMI, kg/m2 | 23.24 (20.96–25.62) | 23.81 (21.52–26.70) | 22.50 (20.84–24.45) | 0.072 | 22.46 (20.76–23.81) | 0.053 | 24.10 (20.89–26.96) | 0.580 | 22.65 (21.69–24.01) | 0.372 |
| Levels of education, n (%) | <0.001 | <0.001 | 0.001 | <0.001 | ||||||
| Illiteracy | 10 (12.80) | 45 (42.90) | 8 (10.70)#$ | 7 (15.20) | 0 (0.00) | 1 (6.30) | ||||
| Primary school | 30 (38.50) | 26 (24.80) | 20 (27.70) | 9 (19.60) | 9 (69.20) | 2 (12.50) | ||||
| Middle school | 16 (20.50) | 12 (11.40) | 9 (12.00) | 3 (6.50) | 2 (15.40) | 4 (25.00) | ||||
| High school | 6 (7.70) | 13 (12.40) | 5 (6.70) | 4 (8.70) | 0 (0.00) | 1 (6.30) | ||||
| College or University or above | 16 (20.50) | 9 (8.60) | 33 (44.00) | 23 (50.00) | 2 (15.40) | 8 (50.00) | ||||
| Smoking, n (%) | 23 (28.40) | 28 (25.90) | 23 (29.90) | 0.832 | 10 (21.70) | 0.851 | 7 (46.70) | 0.250 | 6 (37.50) | 0.871 |
| Drinking, n (%) | 21 (25.90) | 28 (25.90) | 16 (19.80) | 0.554 | 7 (14.90) | 0.284 | 5 (27.80) | 0.986 | 4 (25.00) | 0.997 |
| Habit of salt intake | <0.001 | 0.180 | 0.143 | 0.199 | ||||||
| High | 23 (28.40) | 37 (34.30) | 8 (9.90)&$ | 4 (8.50) | 3 (16.70) | 1 (6.30) | ||||
| Normal | 20 (24.70) | 36 (33.30) | 58 (71.60) | 37 (78.70) | 7 (38.90) | 14 (87.50) | ||||
| Low | 38 (46.90) | 35 (32.40) | 15 (18.50) | 6 (12.80) | 8 (44.40) | 1 (6.30) | ||||
| Family history of dementia, n (%) | 2 (2.50) | 6 (5.60) | 7 (8.60) | 0.260 | 5 (10.60) | 0.150 | 1 (5.60) | 0.570 | 1 (6.30) | 0.552 |
| Hypertension, n (%) | 51 (63.00) | 67 (62.00) | 58 (71.60) | 0.347 | 32 (68.10) | 0.606 | 15 (83.30) | 0.209 | 11 (68.80) | 0.992 |
| Diabetes, n (%) | 18 (22.20) | 36 (33.30) | 28 (34.60) | 0.160 | 18 (38.30) | 0.113 | 7 (38.90) | 0.167 | 3 (18.80) | 0.170 |
| Depression, n (%) | 2 (2.50) | 2 (1.90) | 7 (8.60) | 0.084 | 4 (8.50) | 0.094 | 1 (5.60) | 0.639 | 2 (12.50) | 0.060 |
| Ischemic stroke, n (%) | 20 (24.70) | 30 (27.80) | 47 (58.00)&$ | <0.001 | 26 (55.30) | 0.002 | 13 (72.20) | <0.001 | 8 (50.00) | 0.037 |
| Malignant tumor, n (%) | 2 (2.50) | 1 (0.90) | 5 (6.20) | 0.132 | 3 (6.40) | 0.141 | 1 (5.60) | 0.379 | 1 (6.30) | 0.326 |
| Cardiovascular disease, n (%) | 22 (27.20) | 37 (34.30) | 27 (33.30) | 0.551 | 18 (38.30) | 0.385 | 4 (22.20) | 0.423 | 5 (31.30) | 0.582 |
| FBG, mmol/L | 5.09 (4.65–6.19) | 5.48 (4.73–6.66) | 5.10 (4.50–5.90) | 0.049 | 5.20 (4.50–5.90) | 0.165 | 4.15 (2.19–5.00) | 0.003 | 5.55 (4.80–5.88) | 0.219 |
| SBP, mmHg | 137.00 (125.00–150.00) | 148.50 (131.00–164.25) | 132.00 (124.00–144.50)#& | <0.001 | 134.00 (122.00–144.00) | <0.001 | 138.50 (123.00–161.00) | 0.007 | 132 (124.25–139.25) | <0.001 |
| DBP, mmHg | 75.00 (69.00–82.00) | 79.00 (72.75–86.25) | 72.00 (67.50–80.00)#& | <0.001 | 70.00 (66.00–78.00) | <0.001 | 79.00 (71.75–84.50) | 0.048 | 70.50 (66.25–78.00) | 0.004 |
| TC, mmol/L | 4.36 (3.80–5.01) | 4.96 (4.01–5.97) | 4.37 (3.59–5.58)# | 0.011 | 4.08 (3.56–5.58) | 0.005 | 5.32 (4.42–6.29) | 0.002 | 4.25 (3.43–5.37) | 0.007 |
| TG, mmol/L | 1.24 (0.89–2.10) | 1.25 (0.90–1.72) | 1.30 (0.98–2.32) | 0.470 | 1.20 (0.89–1.77) | 0.922 | 2.85 (1.31–3.96) | 0.002 | 1.21 (0.96–1.61) | 0.961 |
| HDL-C, mmol/L | 1.20 (0.96–1.45) | 1.22 (1.05–1.44) | 1.12 (0.93–1.31) | 0.086 | 1.12 (0.91–1.29) | 0.076 | 1.16 (0.79–1.96) | 0.758 | 1.12 (0.99–1.23) | 0.399 |
| LDL-C, mmol/L | 2.23 (1.72–2.61) | 2.65 (2.05–3.30) | 2.35 (1.78–3.19)# | 0.005 | 2.35 (1.80–3.31) | 0.004 | 1.71 (0.89–3.05) | 0.001 | 2.61 (2.09–3.39) | 0.001 |
Data were expressed median (P25 – P75), or n (%). NC normal cognitive functioning, MCI mild cognitive impairment, BMI body mass index, FBG fasting blood glucose, SBP systolic blood pressure, DBP diastolic blood pressure, TC total cholesterol, TG total triglycerides, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, AD Alzheimer’s disease, VaD vascular dementia, OD other type of dementia.
#p < 0.017, MCI group compared with NC group.
$p < 0.017, Dementia group compared with MCI group.
&p < 0.017, Dementia group compared with NC group.
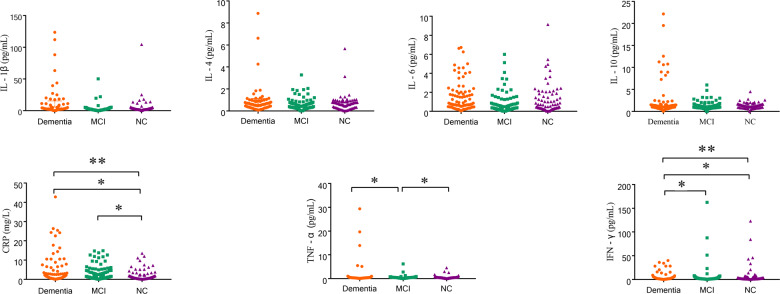
Fig. 1. The scatter plots of different levels of inflammatory factors among dementia, MCI, and NC groups.
The scatter plots show the different levels of anti-inflammatory and proinflammatory factors in dementia (orange), MCI (green), and NC (purple) groups. Anti-inflammatory factors include IL-4 and IL-10, while proinflammatory factors include IL-1β, IL-6, CRP, IFN-γ, and TNF-α. *There were significant differences between the two groups (p < 0.017); **There are significant differences for the trend tests (p < 0.05). NC normal cognitive functioning, MCI mild cognitive impairment, IL-4 interleukin-4, IL-10 interleukin-10, IL-1β interleukin-1 beta, CRP C-reactive protein, IFN-γ Interferon-gamma, TNF-α tumor necrosis factor alpha.
The levels of CRP, TNF-α, and IFN-γ were of significant differences among three groups, whereas the levels of IL-1β, IL-4, IL-6, and IL-10 were not of significant differences (p < 0.05) (Fig. 1).
IgG glycome compositions
Of the 22 initial glycans, 18 were significantly skewed (p < 0.05) (Supplementary Table 3). The comparisons of initial glycans compositions among dementia, MCI and NC groups were shown in Table 2, while these of derived traits were shown in Supplementary Table 4. After adjusting for BMI, levels of education, history of malignant tumor, habit of salt intake, ischemic stroke, diabetes, hypertension, and dyslipidemia in the logistic regression, 14 initial glycans (increased relative abundance of GP1, GP2, GP5, GP7, GP8, GP9, GP10, GP12, GP13, GP15, and GP22 as well as reduced relative abundance of GP14, GP16, and GP18) remained significantly different between dementia and NC groups (p < 0.05; q < 0.05) (Table 3 and Supplementary Fig. 1). Derived glycan traits were of significant differences in patients with dementia compared to NC group, primarily reflecting the decreased of sialylation and core fucosylation, as well as the increased bisecting N-acetylglucosamine (GlcNAc) N-glycan structures in dementia patients (Fig. 2 and Supplementary Table 5).
Table 2.
The levles of initial glycans from NC, MCI, and dementia patients.
| GPs | NC (n = 81) | MCI (n = 108) | Dementia (n = 81) | p* |
|---|---|---|---|---|
| Median (P25–P75) | Median (P25–P75) | Median (P25–P75) | ||
| GP1 | 0.05 (0.02–0.20) | 0.37 (0.16–0.60) | 0.33 (0.22–0.57)#$ | <0.001 |
| GP2 | 0.28 (0.10–0.45) | 0.37 (0.17–0.73) | 0.45 (0.24–0.78)#$ | 0.001 |
| GP4 | 42.02 (34.75–47.92) | 41.59 (34.85–47.32) | 39.16 (34.28–45.70)a | 0.575 |
| GP5 | 0.00 (0.00–0.02) | 0.05 (0.01–0.17) | 0.20 (0.07–0.36)#&$ | <0.001 |
| GP6 | 6.22 (4.76–7.78) | 6.23 (5.17–7.52) | 6.51 (5.21–7.73)a | 0.937 |
| GP7 | 0.01 (0.00–0.05) | 0.14 (0.05–0.27) | 0.15 (0.05–0.40)#$ | <0.001 |
| GP8 | 11.53 (10.35–12.87) | 12.35 (10.02–13.91) | 14.46 (13.11–15.57)#& | <0.001 |
| GP9 | 2.97 (1.72–4.64) | 3.35 (2.00–5.06) | 4.29 (3.29–5.58)#& | <0.001 |
| GP10 | 0.73 (0.52–1.13) | 1.11 (0.57–1.93) | 2.12 (1.19–2.87)#&$ | <0.001 |
| GP11 | 0.08 (0.06–0.23) | 0.31 (0.14–0.60) | 0.10 (0.05–0.31)&$ | <0.001 |
| GP12 | 0.14 (0.04–0.32) | 0.16 (0.08–0.31) | 0.32 (0.17–0.58)#& | <0.001 |
| GP13 | 0.14 (0.04–0.32) | 0.31 (0.15–0.76) | 0.36 (0.25–0.73)#$ | <0.001 |
| GP14 | 13.73 (11.43–17.54) | 13.02 (8.74–16.56) | 11.24 (7.89–14.26)a# | 0.001 |
| GP15 | 0.11 (0.06–0.18) | 0.21 (0.11–0.43) | 0.45 (0.23–0.68)#&$ | <0.001 |
| GP16 | 3.97 (2.96–4.64) | 3.66 (2.99–4.49) | 2.93 (2.34–3.46)a#& | <0.001 |
| GP17 | 0.83 (0.51–1.20) | 0.56 (0.34–0.90) | 0.75 (0.57–1.12)&$ | <0.001 |
| GP18 | 10.52 (7.73–13.29) | 9.27 (7.75–12.12) | 7.2 (5.89–9.06)#& | <0.001 |
| GP19 | 1.76 (1.28–2.24) | 1.86 (1.31–2.42) | 1.77 (1.29–2.12) | 0.429 |
| GP21 | 0.23 (0.17–0.31) | 0.18 (0.13–0.26) | 0.32 (0.23–0.53)#&$ | <0.001 |
| GP22 | 0.07 (0.46–0.14) | 0.14 (0.07–0.27) | 0.11 (0.08–0.24)#$ | <0.001 |
| GP23 | 0.67 (0.42–1.03) | 0.53 (0.34–0.82) | 0.84 (0.50–1.10)&$ | <0.001 |
| GP24 | 0.82 (0.59–1.33) | 0.83 (0.49–1.21) | 0.76 (0.47–1.05) | 0.449 |
NC normal cognitive functioning, MCI mild cognitive impairment.
*p < 0.05 was considered statistically significant.
#p < 0.017, Dementia group compared with NC group.
$p < 0.017, MCI group compared with NC group.
&p < 0.017, Dementia group compared with MCI group.
aAnalysis of variance (ANOVA).
Table 3.
Associations of the normalized initial glycans.
| GPs | Dementia vs. NC | Dementia vs. MCI | MCI vs. NC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI)# | p-adjusted | q* | OR (95% CI)# | p-adjusted | q* | OR (95% CI)# | p-adjusted | q* | |
| GP1 | 6.78 (3.01–15.29) | 3.91E−06 | 1.72E−05 | 0.80 (0.54–1.18) | 2.59E−01 | 3.97E−01 | 9.53 (4.24–21.41) | 4.73E−08 | 1.04E−06 |
| GP2 | 1.64 (1.07–2.52) | 2.42E−02 | 3.80E−02 | 0.91 (0.62–1.34) | 6.19E−01 | 6.81E−01 | 1.96 (1.25–3.08) | 3.36E−03 | 8.21E−03 |
| GP4 | 0.84 (0.58–1.22) | 3.63E−01 | 4.70E−01 | 0.81 (0.55–1.21) | 3.01E−01 | 4.13E−01 | 1.12 (0.78–1.60) | 5.48E−01 | 5.74E−01 |
| GP5 | 32.78 (8.31–129.27) | 6.19E−07 | 4.50E−06 | 1.34 (0.85–2.11) | 2.03E−01 | 3.67E−01 | 8.16 (2.95–22.58) | 5.29E−05 | 2.91E−04 |
| GP6 | 1.16 (0.78–1.71) | 4.65E−01 | 5.11E−01 | 0.73 (0.49–1.09) | 1.27E−01 | 2.53E−01 | 1.29 (0.94–1.77) | 1.12E−01 | 1.36E−01 |
| GP7 | 4.71 (2.29–9.67) | 2.46E−05 | 6.77E−05 | 1.17 (0.79–1.73) | 4.25E−01 | 5.43E−01 | 6.58 (3.02–14.34) | 2.14E−06 | 2.35E−05 |
| GP8 | 6.02 (3.05–11.89) | 2.31E−07 | 3.10E−06 | 3.46 (2.02–5.94) | 6.58E−06 | 1.45E−04 | 1.03 (0.74–1.43) | 8.79E−01 | 8.79E−01 |
| GP9 | 2.06 (1.37–3.10) | 4.94E−04 | 1.09E−03 | 1.65 (1.12–2.44) | 1.14E−02 | 3.57E−02 | 1.37 (0.98–1.93) | 6.87E−02 | 8.89E−02 |
| GP10 | 4.87 (2.66–8.90) | 2.82E−07 | 3.10E−06 | 1.45 (1.00–2.10) | 4.94E−02 | 1.21E−01 | 2.51 (1.54–4.11) | 2.40E−04 | 1.06E−03 |
| GP11 | 1.34 (0.62–2.90) | 4.56E−01 | 5.11E−01 | 0.44 (0.25–0.77) | 3.77E−03 | 1.38E−02 | 4.37 (1.95–9.79) | 3.34E−04 | 1.22E−03 |
| GP12 | 2.24 (1.39–3.63) | 9.86E−04 | 1.97E−03 | 1.78 (1.09–2.88) | 2.02E−02 | 5.55E−02 | 1.41 (0.89–2.24) | 1.48E−01 | 1.71E−01 |
| GP13 | 6.65 (2.55–17.36) | 1.09E−04 | 2.66E−04 | 1.16 (0.80–1.68) | 4.45E−01 | 5.43E−01 | 4.89 (2.32–10.30) | 3.02E−05 | 2.21E−04 |
| GP14 | 0.49 (0.30–0.78) | 2.78E−03 | 5.10E−03 | 1.02 (0.69–1.50) | 9.26E−01 | 9.26E−01 | 0.62 (0.43–0.88) | 7.08E−03 | 1.56E−02 |
| GP15 | 8.10 (3.53–18.61) | 8.18E−07 | 4.50E−06 | 1.11 (0.78–1.57) | 5.80E−01 | 6.72E−01 | 3.28 (1.65–6.53) | 7.09E−04 | 2.23E−03 |
| GP16 | 0.34 (0.21–0.55) | 1.09E−05 | 4.00E−05 | 0.38 (0.23–0.63) | 1.74E−04 | 1.28E−03 | 0.70 (0.50–0.99) | 4.11E−02 | 6.45E−02 |
| GP17 | 0.96 (0.63–1.45) | 8.30E−01 | 8.33E−01 | 1.32 (0.85–2.05) | 2.17E−01 | 3.67E−01 | 0.63 (0.42–0.93) | 2.12E−02 | 3.58E−02 |
| GP18 | 0.36 (0.22–0.57) | 2.38E−05 | 6.77E−05 | 0.38 (0.22–0.66) | 5.89E−04 | 3.24E−03 | 0.65 (0.46–0.92) | 1.38E−02 | 2.52E−02 |
| GP19 | 0.68 (0.39–1.20) | 1.83E−01 | 2.52E−01 | 0.58 (0.30–1.15) | 1.19E−01 | 2.53E−01 | 0.85 (0.62–1.17) | 3.21E−01 | 3.53E−01 |
| GP21 | 1.60 (0.98–2.62) | 6.18E−02 | 9.06E−02 | 4.49 (2.15–9.38) | 6.49E−05 | 7.14E−04 | 0.58 (0.33–1.00) | 4.99E−02 | 7.32E−02 |
| GP22 | 3.59 (1.40–9.19) | 7.76E−03 | 1.31E−02 | 1.04 (0.69–1.56) | 8.53E−01 | 8.93E−01 | 4.60 (1.85–11.43) | 1.00E−03 | 2.75E−03 |
| GP23 | 0.87 (0.64–1.20) | 4.07E−01 | 4.98E−01 | 2.19 (1.31–3.64) | 2.62E−03 | 1.15E−02 | 0.57 (0.37–0.87) | 9.40E−03 | 1.88E−02 |
| GP24 | 1.04 (0.73–1.48) | 8.33E−01 | 8.33E−01 | 1.21 (0.86–1.70) | 2.71E−01 | 3.97E−01 | 0.69 (0.47–1.02) | 6.37E−02 | 8.75E−02 |
*q < 0.05: significant after correction for FDR (false discovery rate).
NC normal cognitive functioning, MCI mild cognitive impairment.
#Adjusting for the effects of age, sex, BMI, levels of education, history of malignant tumor, habit of salt intake, ischemic stroke, diabetes, hypertension and dyslipidemia (adjusting for the above effects other than age and sex for dementia vs. NC).
p < 0.05 was considered statistically significant.
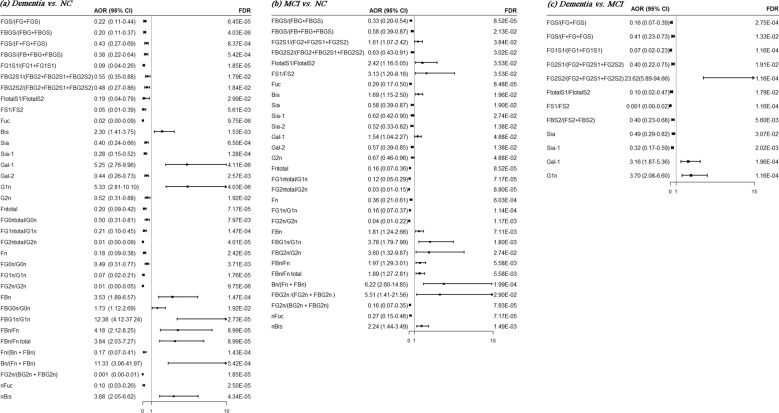
Fig. 2. Forest plots of the associations of the derived traits with MCI and dementia.
Forest plots (a–c) show the odds ratios (ORs, black solid square) with a horizontal lines representing 95% confidence intervals (CIs) for derived traits with MCI and dementia. Each of statistically significant derived traits included study is represented by one row in the plots. The multiple logistic regression analysis was performed after adjusting for age, sex, BMI, levels of education, history of malignant tumor, habit of salt intake, ischemic stroke, diabetes, hypertension, and dyslipidemia (adjusting for the above effects other than age and sex for dementia vs. NC). a Dementia vs. NC, b MCI vs. NC, and c Dementia vs. MCI. NC normal cognitive functioning, AD Alzheimer’s disease.
Similarly, these 14 initial glycans were significantly different between AD and NC groups after adjusting for the effects of above confounders (p < 0.05; q < 0.05) (Supplementary Table 6 and Supplementary Fig. 1). In addition, 7 initial glycans (increased relative abundance of GP8, GP9, GP12, GP21, and GP23 as well as reduced relative abundance of GP16 and GP18) were of significant differences between dementia and MCI groups after adjusting these confounders (p < 0.05; q < 0.05) (Table 3). Twelve IgG glycans (increased relative abundance of GP1, GP2, GP5, GP7, GP10, GP11, GP13, GP15, and GP22 as well as reduced relative abundance of GP14, GP17, and GP23) were of significant differences between MCI and NC groups after adjusting for these confounders (p < 0.05; q < 0.05) (Table 3).
The dimension reduction and diagnostic values of IgG N-glycans
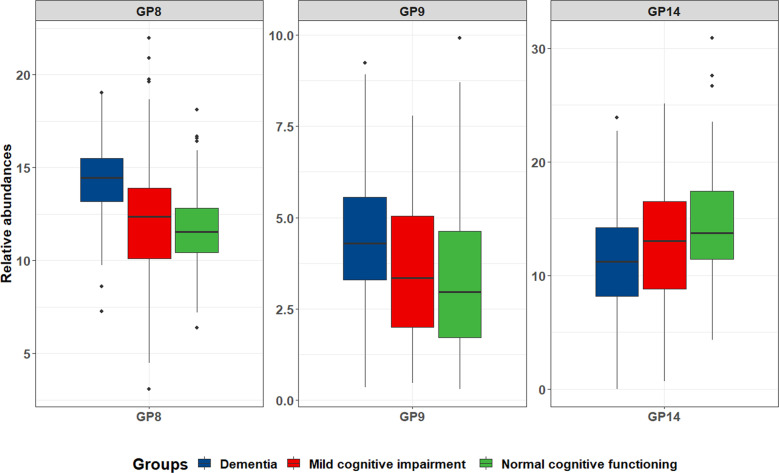
As shown in Fig. 3, the significant correlations among glycans indicated that multicollinearity might exist (p < 0.05). GP8, GP9, and GP14 were selected by intersection of dimension reduction methods [the Ridge and Stepwise (including the direction of Forward and Backward) based on logistic regression as well as Lasso regression], and thus were identified as a biomarker panel for the diagnosis of dementia (Supplementary Table 7). Figure 4 showed the distribution of these three glycans for dementia, MCI, and NC groups. Models were trained and evaluated using 5-fold cross-validation in which random forest classifier was applied. The AUC value of the model including GP8, GP9, and GP14 was determined to distinguish dementia from NC groups as 0.876 (95% confidence interval [CI]: 0.815–0.923). Furthermore, this model was also able to distinguish AD from NC groups with an AUC of 0.887 (95% CI: 0.819–0.936), to distinguish dementia from MCI groups with an AUC of 0.815 (95% CI: 0.752–0.868), to distinguish MCI from NC groups with an AUC of 0.640 (95% CI: 0.568–0.709), respectively (Fig. 5). This model was of high accuracy to predict by performing the random forest classifier for different train datasets [normalized mean square error (NMSE) = 1.131, mean squared error (MSE) = 0.124, and mean absolute error (MAE) = 0.264 for dementia vs. NC; NMSE = 0.976, MSE = 0.075, and MAE = 0.218 for AD vs. NC; NMSE = 3.373, MSE = 0.224, and MAE = 0.401 for dementia vs. MCI; NMSE = 7.322, MSE = 0.234, and MAE = 0.445 for MCI vs. NC] (Supplementary Table 8). In addition, the panels of biomarkers were selected by intersection of three methods to reduce dimension for AD vs. NC, dementia vs. MCI, MCI vs. NC, and AD vs. MCI, respectively (Supplementary Tables 9–12). The AUCs showed high accuracy for diagnostic models in every two groups (Supplementary Table 13 and Supplementary Fig. 2).
Fig. 3. The correlation coefficients in independent glycans.
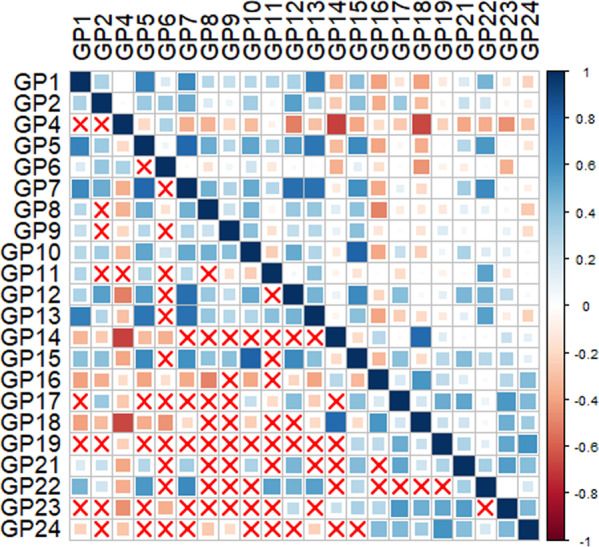
Statistically significant associations between two glycans were shown, p < 0.05, while the insignificant correlation coefficients were shown using symbol (“×”) in the boxes. The positive correlations are represented by blue color, while negative correlations are represented by red color.
Fig. 4. The distribution of a panel of biomarkers (GP8, GP9 and GP14) within the dementia, mild cognitive impairment (MCI), and normal cognitive functioning (NC) groups.
The box plots show the distribution of GP8, GP9, and GP14 in dementia (blue), MCI (red), and NC (green) groups. The following values correspond to center line, upper/lower bounds, and whisker max/min for dementia, MCI, and NC groups, respectively: GP8: center line (median): 14.46, 12.35, 11.53; lower/upper bounds (P25/P75): 13.11/15.57, 10.02/13.91, 10.35/12.87; whiskers max/min: 19.01/7.27, 21.96/3.09, 18.10/6.38; GP9: center line (median): 4.29, 3.35, 2.97; lower/upper bounds (P25/P75): 3.29/5.58, 2.00/5.06, 1.72/4.64; whiskers max/min: 9.23/0.36, 7.78/0.47, 9.91/0.31; GP14: center line (median): 11.24, 13.02, 13.73; lower/upper bounds (P25/P75): 7.89/14.26, 8.74/16.56, 11.43/17.54; whiskers max/min: 23.87/0.01, 25.14/0.69, 30.89/4.34.
Fig. 5. The ROC curves for the IgG N-glycans (GP8, GP9, and GP14) as diagnostic biomarkers by 5-fold cross-validation using the random forest classifier.
The ROC curves are represented by purple color lines, while 95% confidence intervals (CIs) are represented by blue dotted lines. a Dementia vs. NC, b AD vs. NC, c dementia vs. MCI, and d MCI vs. NC. NC normal cognitive functioning, MCI mild cognitive impairment, AD Alzheimer’s disease.
Correlation between IgG glycosylation and inflammation factors
The spearman correlation coefficients between N-glycans and inflammation factors were shown in Table 4. The anti-inflammatory (IL-4 and IL-10) state was positively correlated with GP8, GP10, and GP14 (p < 0.05). The proinflammation (CRP, TNF-α, and IFN-γ) factors were positively correlated with GP8 and GP10, whereas negatively correlated with GP16 (p < 0.05).
Table 4.
Correlation coefficient (rs) between normalized initial glycans and inflammation factors.
| Glycans | Anti-inflammatory factors | Proinflammatory factors | Inflammation | |||||
|---|---|---|---|---|---|---|---|---|
| IL-4 | IL-10 | CRP | TNF-α | IFN-γ | IL-1β | IL-6 | ||
| GP1 | −0.050 | 0.003 | 0.050 | 0.007 | −0.009 | −0.006 | 0.025 | / |
| GP2 | −0.053 | −0.026 | 0.069 | 0.069 | −0.055 | 0.007 | −0.058 | / |
| GP4 | −0.080 | −0.050 | 0.017 | 0.046 | 0.011 | 0.088 | −0.005 | / |
| GP5 | 0.004 | −0.056 | 0.073 | 0.053 | −0.017 | 0.053 | −0.013 | / |
| GP6 | −0.070 | 0.018 | −0.052 | 0.043 | 0.040 | −0.036 | 0.066 | / |
| GP7 | −0.007 | −0.017 | 0.016 | 0.016 | −0.042 | −0.007 | −0.072 | / |
| GP8 | −0.008 | 0.163* | 0.168** | 0.087 | 0.138* | 0.035 | 0.042 | +$ |
| GP9 | 0.046 | 0.036 | −0.023 | −0.009 | 0.005 | 0.041 | −0.040 | / |
| GP10 | −0.049 | 0.133* | 0.137* | 0.092 | 0.097 | 0.014 | 0.063 | +$ |
| GP11 | 0.038 | −0.061 | −0.010 | −0.031 | −0.047 | −0.012 | −0.015 | / |
| GP12 | −0.023 | 0.011 | −0.005 | 0.046 | −0.029 | 0.024 | −0.071 | / |
| GP13 | 0.045 | −0.014 | −0.012 | −0.042 | −0.119 | −0.051 | −0.063 | / |
| GP14 | 0.135* | 0.055 | −0.060 | −0.025 | 0.034 | −0.051 | 0.065 | +$ |
| GP15 | −0.021 | −0.013 | −0.016 | 0.013 | −0.064 | −0.003 | −0.024 | / |
| GP16 | 0.068 | −0.123 | −0.069 | −0.133* | −0.092 | −0.110 | −0.028 | −$ |
| GP17 | −0.057 | 0.002 | 0.011 | −0.076 | −0.072 | −0.015 | −0.092 | / |
| GP18 | 0.050 | −0.042 | −0.102 | −0.091 | −0.048 | −0.103 | −0.001 | / |
| GP19 | −0.014 | −0.060 | −0.056 | −0.063 | −0.099 | −0.065 | −0.082 | / |
| GP21 | −0.033 | −0.016 | 0.008 | −0.013 | 0.017 | 0.008 | −0.050 | / |
| GP22 | 0.006 | −0.053 | 0.000 | −0.038 | −0.070 | 0.005 | −0.065 | / |
| GP23 | −0.004 | −0.029 | 0.008 | −0.074 | −0.119 | −0.009 | −0.112 | / |
| GP24 | −0.019 | −0.045 | −0.064 | −0.059 | −0.009 | −0.068 | −0.047 | / |
IL-4 interleukin-4, IL-10 interleukin-10, CRP C-reactive protein, TNF-α tumor necrosis factor-alpha, IFN-γ interferon-gamma, IL-1β interleukin-1 beta, IL-6 interleukin-6.
*Correlation is significant at the 0.05 level.
**Correlation is significant at the 0.01 level.
$There is at least one significant inflammation factor.
/The association between glycans and inflammation remains unclear.
+The association between glycans and inflammation is positive.
−The association between glycans and inflammation is negative.
Discussion
In this study, we systematically investigated IgG N-glycan profiles in a case–control study including 81 dementia, 108 MCI, and 81 NC participants. To the best of our knowledge, the present study is the first study to assess the possible association between dementia and IgG glycosylation in Chinese Han population. The results demonstrated that 14 initial GPs reflecting the decreased of sialylation and core fucosylation, as well as the increased bisecting GlcNAc N-glycan structures were significantly associated with dementia after adjusting the effects of confounders. Diagnostic model including GP8, GP9, and GP14 was of promising capability to distinguish dementia from NC group with an AUC of 0.876 (95% CI: 0.815–0.923) and distinguish AD from NC group with an AUC of 0.887 (95% CI: 0.819–0.936) under 5-fold cross-validation random forest classifiers.
Dementia is a multifactorial disease driven by many diverse risk factors, including genetic variants, demographic risk factors (such as aging and gender), modifiable risk factors (central obesity, dyslipidemia, T2DM, hypertension, and ischemic stroke, etc.)36. Cumulative evidence indicates that variations in IgG Fc N-glycome play an important role in the anti-inflammatory or proinflammatory process37. Our previous studies have shown that the decreasing sialylation and the increasing bisecting GlcNAc are associated with risk factors of dementia, which are consistent with this study28,30–34,38–40. Therefore, aberrant glycosylation of IgG related to dementia implied a proinflammatory status in the participants with dementia in this study.
The associations between IgG Fc N-glycans and neurodegenerative diseases have been observed in previous studies35,41–43. Gizaw et al found that the fucose and bisecting-GlcNAc structures were significantly increased while disialylated N-glycans were decreased in AD patients’ serum when compared with the normal control group41. Maguire et al found that a significant decrease in soluble sialyltransferase activity in serum was reported in a study comparing 12 AD patients with 12 age-matched controls42. Lundström et al reported an increased FA2 (named GP4 in the present study) and a reduced FA2G1 (GP8 or GP9), FA2G2 (GP14), FA2G2S1 (GP18) of IgG1 in 31 patients with AD compared to 23 age-matched controls in European ancestry35. In addition, Russell et al found that the presence of Parkinson’s disease pathology in Caucasian population was predominantly explained with a reduced relative abundance of GP5, GP17, and an increased relative abundance of GP843. We also detected decreasing GP14 and GP18 for dementia patients in Chinese population in this study. However, other results are inconsistent with these studies. Glycans do not have a direct genetic template, thus glycan structures attached to proteins are determined by these complex dynamic interactions including genetic and epigenetic factors44,45. Therefore, the extensive differences in genetics or environmental exposures among these ethnic studies may partly explain the inconsistent associations between IgG Fc N-glycans and neurodegenerative diseases.
IgG mediates pro- and anti-inflammatory activities mainly through the engagement of its constant region with distinct Fc receptors46,47. Studies have shown that the decreasing sialylation and core fucosylation as well as increasing the bisecting GlcNAc contents within the Fc domain of IgG could activate its antibody-dependent cell mediated cytotoxicity (ADCC) by reducing inhibition to ligate Fcγ-RIIIa on natural killer (NK) cells, macrophage, and neutrophil, which can release proinflammatory factors (such as IL-1β, IL-6, CRP, TNF-α, and IFN-γ)17,48–51. Thus, it may have the ability to fine-tune FcγRIII-mediated from anti-inflammatory to proinflammatory effects20. These alterations in the IgG N-glycan patterns may provide a switch from innate anti-inflammatory activity in the steady state to adaptive proinflammatory effects upon antigenic challenge47. Consistent with these, the inverse correlation between sialylated (Sia), and core fucosylated IgG glycoforms (Fuc and nFuc) and dementia in the present study was somewhat expected. Increased bisecting GlcNAc N-glycan structure (Bis and nBis) was observed to be related with the state of dementia in the present study. In addition, our findings that the level of IgG sialylation (Sia), and core fucosylation (Fuc and nFuc) decreased in MCI, while bisecting GlcNAc (Bis and nBis) increased in MCI compared with NC group, suggesting that aberrant glycosylation of IgG might contribute to pathogenesis of dementia developed from MCI stage. These were further validated by the significant differences of glycans between dementia and MCI in the present study. Consequently, it could be hypothesized that these distinct differences in IgG glycosylation pattern in MCI might play a cascading role in the progression of dementia (Fig. 6).
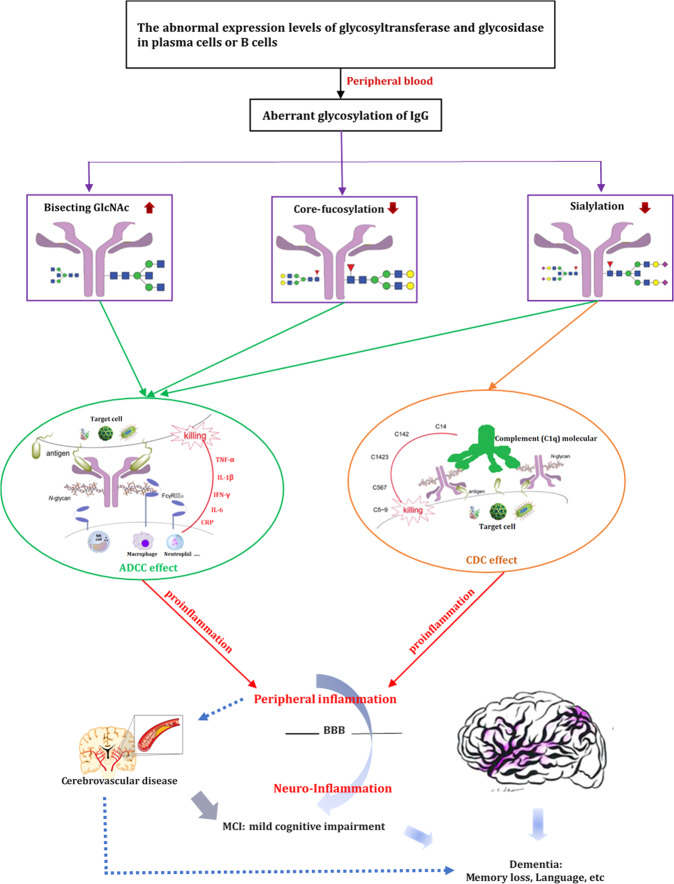
Fig. 6. The proposed roles of IgG N-glycans in the pathogenesis of dementia.
The genetic or environmental factors may influence the abnormal expression of glycosyltransferase and glycosidase in plasma cells or B cells. The decreasing sialylation and core fucosylation as well as increasing the bisecting GlcNAc contents within the Fc domain of IgG could activate its antibody-dependent cell mediated cytotoxicity (ADCC) by reducing inhibition to ligate Fcγ-RIIIa on natural killer (NK) cells, macrophage, and neutrophil which could release proinflammatory factors (such as IL-1β, IL-6, CRP, TNF-α, and IFN-γ). Decreased IgG Fc sialylation could reduce its complement-dependent cytotoxicity (CDC) activity via C1q binding, which could lead to increase activation of the lectin-initiated complement pathway. These changes of the IgG N-glycan may make them possible from anti-inflammatory to proinflammatory effects upon antigenic challenge. The central nervous system and peripheral immune system exchanged in bi-directional ways. Activated peripheral immune cells that can penetrate the blood brain barrier (BBB) may regulate cognitive impairment, which include inhibiting angiogenic, neurotrophic, and neuroprotective mechanisms. Consequently, distinct differences in IgG glycosylation pattern in MCI might play a cascading role in the progression of dementia. Besides, persistent inflammation related to atherosclerosis also might provide a possible explanation for the pathogenesis of cerebrovascular disease (such as hypertension in midlife and diabetes), which could increase risk of MCI and dementia. Arrows indicate a contributory effect.
Our findings corroborate the results that peripheral inflammation correlates with dementia progress both prior to and following the onset of the disease52–55. Levels of proinflammation factors in serum were of significant differences among dementia, MCI and NC groups in the present study, which further validated possibly direct role of inflammation in the pathophysiology of MCI and dementia. According to the research findings, central nervous system and peripheral immune system exchanged in bi-directional ways56. Activated peripheral immune cells that can penetrate the blood brain barrier may regulate the pathogenesis of AD57,58. Inflammatory processes play an important role in cognitive impairment for the early phase of neurodegeneration which include inhibiting angiogenic, neurotrophic and neuroprotective mechanisms59. The high levels of proinflammatory factors (CRP, TNF-α, and IFN-γ) in the dementia group showed that inflammation might be one of the characteristics of dementia, which indicated that a chronic level of inflammation was a known risk factor for dementia60. We also found the anti-inflammatory (IL-10) state was positively correlated with GP8 and GP14, while the proinflammation (CRP and IFN-γ) factors were positively correlated with GP8. These findings indicated that significant changes of IgG glycosylation profiles might involve in the pathogenesis of dementia accompanying with inflammation response. The aberrant peripheral IgG glycome of individuals with dementia may reflect unknown modifications in the intermediate cellular environment or interactions of the biomolecules involved in B cell differentiation with the central nervous system at a known interaction site (Fig. 6). Besides, persistent inflammation related to atherosclerosis also might provide a possible explanation for the pathogenesis of cerebrovascular disease (such as hypertension and diabetes)61,62, implying an increased risk of MCI and dementia.
The study has several major strengths. First, to the best of our knowledge, this is the first study assessing IgG N-glycans levels in relation to the risk of dementia. Furthermore, we analyzed the association between IgG N-glycome and markers of inflammation including anti-inflammatory (IL-4 and IL-10) as well as proinflammatory factors (CRP, TNF-α, IFN-γ, IL-1β, and IL-6), contributing to explain the role of IgG glycosylation on dementia. However, This study has some limitations. First, because our study design was a case–control study, selection bias may still occur. In addition, it is very difficult to be warranted to infer causal relationships between the levels of IgG N-glycans and dementia. Second, all patients were on their dementia medications (donepezil, memantine, etc.), and it was not possible to distinguish the effects of dementia medications on the IgG glycome. Third, it is very hard to divide MCI into amnestic MCI (more likely due to AD) and non-amnestic groups based on available data in present study. Therefore, we failed to analyze and clarify why for some glycans there seem to be stepwise changes from NC to MCI to AD and for others MCI subjects have opposite trends from dementia and AD subjects. Lastly, because the sample size was not large enough, it was very hard to perform external cross-validation. Therefore, further external cross-validation validations for these novel biomarkers in cohort studies with large sample sizes and in multi-ethnic populations are also needed.
In conclusion, this study showed that individuals with dementia or on a pathway to dementia have an elevated proinflammatory activity in peripheral blood which manifests itself via the significant changes of IgG glycome profiles (reflecting the decreased of sialylation and core fucosylation, as well as the increased bisecting GlcNAc N-glycan structures). Aberrant IgG glycosylation might contribute to the pathogenesis of dementia because of the disequilibrium of anti- and proinflammatory status. Although it is yet to be determined whether the IgG glycosylation in blood plasma correlates with those in cerebrospinal fluid, it is evident that IgG N-glycans might contribute to the potential novel biomarkers for the neurodegenerative process risk assessment of dementia.
Methods
Participants
Participants were recruited from the Beijing Geriatric Hospital, the Emergency General Hospital, the Second Affiliated Hospital of Shandong First Medical University, the Tai’an City Central Hospital and the Jidong Oil-field Hospital of Chinese National Petroleum from May 2019 to January 2020. All participants were required to meet the following inclusion criteria: (1) Age 60 or above; (2) Chinese Han population; (3) signed informed consent prior to participation; and (4) blood sample available. Participants with the following diseases were excluded: rheumatoid arthritis, systemic lupus erythematosus and other rheumatoid immune disease. This study was approved by the Ethics Committee of the Capital Medical University, Beijing, China. The study was conducted according to the principles of the Declaration of Helsinki. Written informed consents were obtained from all participants before the study.
Diagnostic criteria
Participants were classified into three general categories: normal cognitive functioning (NC), MCI and dementia. The Chinese version of the Montreal Cognitive Assessment Basic (MoCA-BC) was used as a quick evaluation scale to screen for MCI and distinguish MCI individuals from NC elderly adults63. The optimal cut-off scores for MCI detection were determined according to the education level. For individuals with 6 or fewer years of education, the cut-off score was set as less than or equal to 19. For individuals with 7–12 years of education were set as less than or equal to 22. For individuals with more than 12 years of education were set as less than or equal to 24. Diagnostic criteria for dementia were based on the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders64,65. The diagnosis of AD was based on the National Institute of Neurological, Communicative Disorders and Stroke–Alzheimer Disease and Related Disorders Association (NINCDS-ADRDA)66.
Collection of blood sample and clinical traits
After overnight fasting, two tubes of blood samples (2 mL) were collected in the morning by venipuncture. The tube without containing ethylene diamine tetraacetic acid (EDTA) was used to separate serum to measure the blood biochemistry indexes and inflammatory factors. The other tube of the blood sample in vacuum negative pressure tubes containing EDTA was centrifuged at 4000 rpm for 10 min to separate plasma. The plasma was used to measure IgG glycans. Collected blood samples were processed within 8 h and stored at −80 °C until further measurement.
Information on demographic (age, sex, ethnicity, and levels of education) and clinical history were collected by a questionnaire. Weight and height measurements were conducted when participants had removed their shoes and other heavy objects from their pockets. The body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. After the participants resting at least 10 min in a sitting position, SBP (mmHg) and DBP (mmHg) were measured twice on the right arm by trained nurses using a standard mercury sphygmomanometer67. According to the guideline for the prevention and control of dyslipidemia of adults in China, the participants were grouped into dyslipidemia with total cholesterol (TC) ≥ 6.2 mmol/L, or triglycerides (TG) ≥ 2.3 mmol/L, or high-density lipoprotein cholesterol (HDL-C) < 1.0 mmol/L or low-density lipoprotein cholesterol (LDL-C) ≥ 4.1 mmol/L68.
Measurement of inflammatory factors and IgG N-glycans
The levels of inflammatory factors (IL-1β, IL-4, IL-6, IL-10, CRP, TNF-α, and IFN-γ) were measured using enzyme-linked immunosorbent assay (ELISA, R&D Systems, Bio-Techne China Co. Ltd., Beijing, China) according to the kits manufacturer’s instructions69. Take IL-6, for example. The standards, quality controls and serum samples were incubated in the micro-titration 96-well plate coated with polyclonal anti-human IL-6 antibody. Polyclonal anti-human IL-6 antibody labeled with horseradish peroxidase (HRP) was added to the wells and incubated with the immobilized antibody- IL-6 complex after a thorough wash. The HRP-conjugated antibody would react with the substrate and tetramethylbenzidine after another wash step. The reaction was stopped by acidic solution. Absorbance (optical density) was measured at 450 nm. The IL-6 concentration (mg/L) was interpolated from a four-parameter logistic 4-PL standard curve generated with ELISAcalc software. Each standard was estimated using the 4-PL curve-fit measurements.
The IgG was firstly isolated from human plasma as previously reported70. After washing and equilibrating protein G monolithic plates, 50 μL of plasma was diluted 10× with binding buffer (1× phosphate buffered saline, pH = 7.4) which was applied to the protein G plates, and then washed immediately. IgG was eluted with 1 mL of 0.1 M formic acid and neutralized with 1 M ammonium bicarbonate instantly. The following step was that IgG N-glycans were released and labeling was conducted. The released N-glycans were labeled with 2-aminobenzamide which was applied to make glycans visible by mixing with 2-aminobenzamide, dimethylsulfoxide, glacial acetic acid, and 2-picoline borane. In the end, IgG N-glycans were separated by hydrophilic interaction chromatography-UPLC into 24 IgG glycan peaks (GPs)70. The glycan structures of the most abundant glycans per peak were reported previously71.
All chromatograms were separated in the same manner into 24 peaks and the amount of glycans in each peak was expressed as a percentage of total integrated area as shown in Supplementary Table 1. The GP3 was excluded from all the calculations because it was eluted with contaminant in some samples that affected its value. In addition, the GP20 was also eliminated as its glycan structure has not been determined. The specific 54 derived traits representing the relative abundances of galactosylation, sialylation, bisecting N-acetylglucosamine (GlcNAc), and core fucosylation were calculated by the remaining 22 directly measured glycans72. In addition, Huff man et al. utilized another method to calculate overall aspects of derived traits73 (Supplementary Table 2). Normalization of UPLC data were detailed in previously published study70.
Statistical analyses
Demographic and clinical characteristics were represented as mean ± standard deviation for continuous variables underlying the normal distribution, otherwise the median (P25 − P75) was used. The differences of continuous variables among three groups were tested by one-way analysis of variance or the Kruskal–Wallis test. Categorical variables were represented as n (%), and the differences among the three groups were tested by chi-square test or Fisher exact test.
Spearman correlations were used to calculate the correlation coefficient (rs) among IgG glycans as well as between IgG glycans and inflammatory factors. The multiple logistic regression analysis was performed to identify each of 24 initial glycans related to dementia after adjusting the effects of confounders (including BMI, levels of education, history of malignant tumor, habit of salt intake, ischemic stroke, diabetes, hypertension and dyslipidemia). For the multiple corrections, the FDR (false discovery rate) was used based on the Benjamini–Hochberg procedure (q)74. Then the Ridge and Stepwise (including the direction of Forward and Backward) based on logistic regression as well as Lasso regression were performed to reduce dimension for significant glycans75–77. The intersection of the above three methods was identified as a panel of biomarkers for the diagnosis of dementia. Model performance was evaluated using the 5-fold cross-validation78. Models were implemented using the random forest which was an ensemble or collection of multiple decision tree models. This panel of biomarkers simultaneously was evaluated performance for other comparison groups (AD vs. NC, dementia vs. MCI, and MCI vs. NC, respectively). Classifier accuracy was measured by using NMSE, MSE, MAE, and the AUC with the receiver operating characteristic (ROC) curve.
Data analysis was performed using SPSS Statistics version 25.0 for Windows (IBM Corp., Armonk, NY, USA) and R version 3.4.3 (R Core Team, 2017). All reported p values were two-tailed. The q value is used to represent the p value after correction for multiple testing and hence q value < 0.05 and p < 0.05 were considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
The study was supported by grants from the National Key R&D Program of China (2017YFE0118800)—European Commission Horizon 2020 (779238-PRODEMOS), the National Natural Science Foundation of China (81872682 and 81773527), and the China-Australian Collaborative Grant (NSFC 81561128020–NHMRC APP1112767).
Author contributions
Y.W., W.W., B.S., and D.L. contributed for the study design. X.Z., H.Y., J.L., and Q.T. selected patients for the study and collected the clinical data. X.Z., X.M., J.Z., Y.L., J.S., and X.X. performed the glycans analysis. X.Z., Q.T., and H.H. performed the data analyses. X.Z., H.Y., and J.L. wrote the paper. All authors read and approved the final paper.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
The code generated in this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xiaoyu Zhang, Hui Yuan, Jihui Lyu.
Contributor Information
Wei Wang, Email: wei.wang@ecu.edu.au.
Youxin Wang, Email: wangy@ccmu.edu.cn.
Supplementary information
Supplementary information The online version contains supplementary material available at 10.1038/s41514-021-00055-w.
References
- 1.Prince, M. W. A., Guerchet, M., Ali, G. C., Wu, Y. T., Prina, M. World Alzheimer Report 2015—The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. (Alzheimer’s Disease International, London, 2015).
- 2.Tangalos EG, Petersen RC. Mild cognitive impairment in geriatrics. Clin. Geriatr. Med. 2018;34:563–589. doi: 10.1016/j.cger.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Liuzzo G. Atherosclerosis: an inflammatory disease. Rays. 2001;26:221–230. [PubMed] [Google Scholar]
- 4.Livingston G, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 5.Liu F, Wu S, Ren H, Gu J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat. Cell Biol. 2011;13:254–262. doi: 10.1038/ncb2167. [DOI] [PubMed] [Google Scholar]
- 6.Umeda-Kameyama Y, Akishita M. Age and sex: risk factors for dementia. Brain Nerve. 2016;68:713–718. doi: 10.11477/mf.1416200499. [DOI] [PubMed] [Google Scholar]
- 7.Helle, et al. Age-related inflammatory cytokines and disease - ScienceDirect. Immunol. Allergy Clin. North Am. 2003;23:15–39. doi: 10.1016/S0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 8.King E, et al. Peripheral inflammation in prodromal Alzheimer’s and Lewy body dementias. J. Neurol. Neurosurg. Psychiatry. 2018;89:339–345. doi: 10.1136/jnnp-2017-317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koyama A, et al. The role of peripheral inflammatory markers in dementia and Alzheimer’s disease: a meta-analysis. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013;68:433–440. doi: 10.1093/gerona/gls187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood H. Dementia: peripheral inflammation could be a prodromal indicator of dementia. Nat. Rev. Neurol. 2018;14:127. doi: 10.1038/nrneurol.2018.8. [DOI] [PubMed] [Google Scholar]
- 11.Darweesh SKL, et al. Inflammatory markers and the risk of dementia and Alzheimer’s disease: a meta-analysis. Alzheimer’s Dement. 2018;14:1450–1459. doi: 10.1016/j.jalz.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Shen XN, et al. Inflammatory markers in Alzheimer’s disease and mild cognitive impairment: a meta-analysis and systematic review of 170 studies. J. Neurol. Neurosurg. Psychiatry. 2019;90:590–598. doi: 10.1136/jnnp-2018-319148. [DOI] [PubMed] [Google Scholar]
- 13.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 14.Molinari M. N-glycan structure dictates extension of protein folding or onset of disposal. Nat. Chem. Biol. 2007;3:313–320. doi: 10.1038/nchembio880. [DOI] [PubMed] [Google Scholar]
- 15.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 17.Biermann MH, et al. Sweet but dangerous—the role of immunoglobulin G glycosylation in autoimmunity and inflammation. Lupus. 2016;25:934–942. doi: 10.1177/0961203316640368. [DOI] [PubMed] [Google Scholar]
- 18.Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291:2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 19.Krištić J, et al. Profiling and genetic control of the murine immunoglobulin G glycome. Nat. Chem. Biol. 2018;14:516–524. doi: 10.1038/s41589-018-0034-3. [DOI] [PubMed] [Google Scholar]
- 20.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front. Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novokmet M, et al. Changes in IgG and total plasma protein glycomes in acute systemic inflammation. Sci. Rep. 2014;4:4347. doi: 10.1038/srep04347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vuckovic F, et al. Association of systemic lupus erythematosus with decreased immunosuppressive potential of the IgG glycome. Arthritis Rheumatol. 2015;67:2978–2989. doi: 10.1002/art.39273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sebastian A, et al. Glycan biomarkers for rheumatoid arthritis and its remission status in Han Chinese patients. Omics. 2016;20:343–351. doi: 10.1089/omi.2016.0050. [DOI] [PubMed] [Google Scholar]
- 24.Barrios C, et al. Glycosylation profile of IgG in moderate kidney dysfunction. J. Am. Soc. Nephrol. 2016;27:933–941. doi: 10.1681/ASN.2015010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trbojevic Akmacic I, et al. Inflammatory bowel disease associates with proinflammatory potential of the immunoglobulin G glycome. Inflamm. Bowel Dis. 2015;21:1237–1247. doi: 10.1097/MIB.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Jong SE, et al. IgG1 Fc N-glycan galactosylation as a biomarker for immune activation. Sci. Rep. 2016;6:28207. doi: 10.1038/srep28207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plomp R, et al. Subclass-specific IgG glycosylation is associated with markers of inflammation and metabolic health. Sci. Rep. 2017;7:12325. doi: 10.1038/s41598-017-12495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu D, et al. Ischemic stroke is associated with the pro-inflammatory potential of N-glycosylated immunoglobulin G. J. Neuroinflamm. 2018;15:123. doi: 10.1186/s12974-018-1161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu JN, et al. The association between subclass-specific IgG Fc N-glycosylation profiles and hypertension in the Uygur, Kazak, Kirgiz, and Tajik populations. J. Hum. Hypertension. 2018;32:555–563. doi: 10.1038/s41371-018-0071-0. [DOI] [PubMed] [Google Scholar]
- 30.Liu D, et al. The changes of immunoglobulin G N-glycosylation in blood lipids and dyslipidaemia. J. Transl. Med. 2018;16:235. doi: 10.1186/s12967-018-1616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu X, et al. Profiling IgG N-glycans as potential biomarker of chronological and biological ages: a community-based study in a Han Chinese population. Medicine. 2016;95:e4112. doi: 10.1097/MD.0000000000004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu D, et al. The association between normal BMI with central adiposity and proinflammatory potential immunoglobulin G N-glycosylation. Diabetes Metab. Syndr. Obes. 2019;12:2373–2385. doi: 10.2147/DMSO.S216318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, et al. Glycomics for type 2 diabetes biomarker discovery: promise of immunoglobulin G subclass-specific fragment crystallizable N-glycosylation in the Uyghur population. Omics. 2019;23:640–648. doi: 10.1089/omi.2019.0052. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, et al. The association between glycosylation of immunoglobulin G and hypertension: a multiple ethnic cross-sectional study. Medicine. 2016;95:e3379. doi: 10.1097/MD.0000000000003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundström SL, et al. Blood plasma IgG Fc glycans are significantly altered in Alzheimer’s disease and progressive mild cognitive impairment. J. Alzheimers Dis. 2014;38:567–579. doi: 10.3233/JAD-131088. [DOI] [PubMed] [Google Scholar]
- 36.Hugo J, Ganguli M. Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin. Geriatr. Med. 2014;30:421–442. doi: 10.1016/j.cger.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell, A., Adua, E., Ugrina, I., Laws, S. & Wang, W. Unravelling immunoglobulin G Fc N-glycosylation: a dynamic marker potentiating predictive, preventive and personalised medicine. Int. J. Mol. Sci.10.3390/ijms19020390 (2018). [DOI] [PMC free article] [PubMed]
- 38.Li X, et al. Type 2 diabetes mellitus is associated with the immunoglobulin G N-glycome through putative proinflammatory mechanisms in an Australian population. Omics. 2019;23:631–639. doi: 10.1089/omi.2019.0075. [DOI] [PubMed] [Google Scholar]
- 39.Gao Q, et al. immunoglobulin G N-Glycans as potential postgenomic biomarkers for hypertension in the kazakh population. Omics. 2017;21:380–389. doi: 10.1089/omi.2017.0044. [DOI] [PubMed] [Google Scholar]
- 40.Ge S, et al. Type 2 diabetes mellitus: integrative analysis of multiomics data for biomarker discovery. Omics. 2018;22:514–523. doi: 10.1089/omi.2018.0053. [DOI] [PubMed] [Google Scholar]
- 41.Gizaw ST, Ohashi T, Tanaka M, Hinou H, Nishimura S. Glycoblotting method allows for rapid and efficient glycome profiling of human Alzheimer’s disease brain, serum and cerebrospinal fluid towards potential biomarker discovery. Biochim. Biophys. Acta. 2016;1860:1716–1727. doi: 10.1016/j.bbagen.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Maguire TM, et al. A decrease in serum sialyltransferase levels in Alzheimer’s disease. Neurobiol. Aging. 1994;15:99–102. doi: 10.1016/0197-4580(94)90149-X. [DOI] [PubMed] [Google Scholar]
- 43.Russell AC, et al. The N-glycosylation of immunoglobulin G as a novel biomarker of Parkinson’s disease. Glycobiology. 2017;27:501–510. doi: 10.1093/glycob/cwx022. [DOI] [PubMed] [Google Scholar]
- 44.Kizuka, Y. & Taniguchi, N. Enzymes for N-glycan branching and their genetic and nongenetic regulation in cancer. Biomolecules10.3390/biom6020025 (2016). [DOI] [PMC free article] [PubMed]
- 45.Menni C, et al. Glycosylation of immunoglobulin g: role of genetic and epigenetic influences. PloS ONE. 2013;8:e82558. doi: 10.1371/journal.pone.0082558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bond A, Alavi A, Axford JS, Youinou P, Hay FC. The relationship between exposed galactose and N-acetylglucosamine residues on IgG in rheumatoid arthritis (RA), juvenile chronic arthritis (JCA) and Sjögren’s syndrome (SS) Clin. Exp. Immunol. 1996;105:99–103. doi: 10.1046/j.1365-2249.1996.d01-741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 48.Shields RL, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 49.Shinkawa T, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 2003;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 50.Hodoniczky J, Zheng YZ, James DC. Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol. Prog. 2005;21:1644–1652. doi: 10.1021/bp050228w. [DOI] [PubMed] [Google Scholar]
- 51.Zou G, et al. Chemoenzymatic synthesis and Fcγ receptor binding of homogeneous glycoforms of antibody Fc domain. Presence of a bisecting sugar moiety enhances the affinity of Fc to FcγIIIa receptor. J. Am. Chem. Soc. 2011;133:18975–18991. doi: 10.1021/ja208390n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Licastro F, et al. Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with Alzheimer’s disease: peripheral inflammation or signals from the brain? J. Neuroimmunol. 2000;103:97–102. doi: 10.1016/S0165-5728(99)00226-X. [DOI] [PubMed] [Google Scholar]
- 53.De Luigi A, et al. Peripheral inflammatory response in Alzheimer’s disease and multiinfarct dementia. Neurobiol. Dis. 2002;11:308–314. doi: 10.1006/nbdi.2002.0556. [DOI] [PubMed] [Google Scholar]
- 54.Holmes C, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73:768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 56.Holmes C, Butchart J. Systemic inflammation and Alzheimer’s disease. Biochem. Soc. Trans. 2011;39:898–901. doi: 10.1042/BST0390898. [DOI] [PubMed] [Google Scholar]
- 57.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav. Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Takeda S, Sato N, Morishita R. Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: relevance to pathogenesis and therapy. Front. Aging Neurosci. 2014;6:171. doi: 10.3389/fnagi.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ott BR, et al. Blood-cerebrospinal fluid barrier gradients in mild cognitive impairment and alzheimer’s disease: relationship to inflammatory cytokines and chemokines. Front. Aging Neurosci. 2018;10:245. doi: 10.3389/fnagi.2018.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akiyama H, et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agita A, Alsagaff MT. Inflammation, immunity, and hypertension. Acta Med. Indones. 2017;49:158–165. [PubMed] [Google Scholar]
- 62.Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Curr. Diabetes Rep. 2013;13:435–444. doi: 10.1007/s11892-013-0375-y. [DOI] [PubMed] [Google Scholar]
- 63.Chen KL, et al. Validation of the Chinese Version of Montreal Cognitive Assessment Basic for screening mild cognitive impairment. J. Am. Geriatrics Soc. 2016;64:e285–e290. doi: 10.1111/jgs.14530. [DOI] [PubMed] [Google Scholar]
- 64.Do, L. L. T. N. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). (Springer, USA, 2011).
- 65.Galvin JE, Sadowsky CH. Practical guidelines for the recognition and diagnosis of dementia. J. Am. Board Fam. Med. 2012;25:367–382. doi: 10.3122/jabfm.2012.03.100181. [DOI] [PubMed] [Google Scholar]
- 66.McKhann G, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- 67.Organization, W. H. A global brief on hypertension: silent killer, global public health crisis. World Health Day (2013).
- 68.Wang S, et al. Prevalence and associated factors of dyslipidemia in the adult Chinese population. PloS ONE. 2011;6:e17326. doi: 10.1371/journal.pone.0017326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang PH, et al. Pretreatment serum interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha levels predict the progression of colorectal cancer. Cancer Med. 2016;5:426–433. doi: 10.1002/cam4.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trbojevic-Akmacic I, Vilaj M, Lauc G. High-throughput analysis of immunoglobulin G glycosylation. Expert Rev. Proteom. 2016;13:523–534. doi: 10.1080/14789450.2016.1174584. [DOI] [PubMed] [Google Scholar]
- 71.Lemmers RFH, et al. IgG glycan patterns are associated with type 2 diabetes in independent European populations. Biochim. Biophys. Acta Gen. Subj. 2017;1861:2240–2249. doi: 10.1016/j.bbagen.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 72.Pucic M, et al. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol. Cell. Proteom. 2011;10:M111.010090. doi: 10.1074/mcp.M111.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huffman JE, et al. Comparative performance of four methods for high-throughput glycosylation analysis of immunoglobulin G in genetic and epidemiological research. Mol. Cell. Proteom. 2014;13:1598–1610. doi: 10.1074/mcp.M113.037465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful Approach to multiple testing. J. R. Stat. Soc. Ser. 1995;B 57:289–300. [Google Scholar]
- 75.Mcdonald GC. Ridge regression. Wiley Interdiscip. Rev. Comput. Stat. 2009;1:93–100. doi: 10.1002/wics.14. [DOI] [Google Scholar]
- 76.Hans C. Bayesian lasso regression. Biometrika. 2009;96:835–845. doi: 10.1093/biomet/asp047. [DOI] [Google Scholar]
- 77.Shacham M, Brauner N. Application of stepwise regression for dynamic parameter estimation. Comput. Chem. Eng. 2014;69:26–38. doi: 10.1016/j.compchemeng.2014.06.013. [DOI] [Google Scholar]
- 78.Grünauer A, Vincze M. Using dimension reduction to improve the classification of high-dimensional. Data. 2015;14:296. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The code generated in this study are available from the corresponding author upon reasonable request.