Abstract
We tested the hypothesis that five daily sessions of visual cortex transcranial random noise stimulation would improve contrast sensitivity, crowded and uncrowded visual acuity in adults with amblyopia. Nineteen adults with amblyopia (44.2 ± 14.9 years, 10 female) were randomly allocated to active or sham tRNS of the visual cortex (active, n = 9; sham, n = 10). Sixteen participants completed the study (n = 8 per group). tRNS was delivered for 25 min across five consecutive days. Monocular contrast sensitivity, uncrowded and crowded visual acuity were measured before, during, 5 min and 30 min post stimulation on each day. Active tRNS significantly improved contrast sensitivity and uncrowded visual acuity for both amblyopic and fellow eyes whereas sham stimulation had no effect. An analysis of the day by day effects revealed large within session improvements on day 1 for the active group that waned across subsequent days. No long-lasting (multi-day) improvements were observed for contrast sensitivity, however a long-lasting improvement in amblyopic eye uncrowded visual acuity was observed for the active group. This improvement remained at 28 day follow up. However, between-group differences in baseline uncrowded visual acuity complicate the interpretation of this effect. No effect of tRNS was observed for amblyopic eye crowded visual acuity. In agreement with previous non-invasive brain stimulation studies using different techniques, tRNS induced short-term contrast sensitivity improvements in adult amblyopic eyes, however, repeated sessions of tRNS did not lead to enhanced or long-lasting effects for the majority of outcome measures.
Subject terms: Sensory processing, Visual system, Striate cortex
Introduction
Amblyopia is a developmental disorder of the visual cortex, with a prevalence of approximately 1–5%1–3. Amblyopia causes a wide range of vision deficits4,5 including a monocular loss of high-contrast visual acuity6,7 that is particularly pronounced for crowded optotypes8,9, reduced contrast sensitivity in the affected eye6,10–12, and impaired or absent stereopsis13,14. Amblyopia is also associated with chronic suppression of the affected eye15,16 that may play a key role in the etiology of the disorder17.
Amblyopia involves abnormal processing within the primary and extrastriate visual cortex18 and therefore recovery from amblyopia requires a change in cortical function. Current amblyopia treatments achieve this by directly manipulating visual input to the brain. For example, the most common amblyopia treatment involves the provision of a clear retinal image in the amblyopic eye using refractive correction followed by occlusion of the non-amblyopic eye. This treatment improves amblyopic eye visual acuity, but has drawbacks in terms of compliance19 and reduced efficacy with increasing age20.
Transcranial electrical stimulation (tES) refers to a suite of non-invasive neuro-modulation techniques including transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS) and transcranial random noise stimulation (tRNS) that may enhance plasticity in targeted regions of the human brain21–23, including the visual cortex24–26. Currently, tES methods are being investigated as a potential neurorehabilitation tool for disorders including stroke27–30, chronic pain31,32 and tinnitus33,34 and there is growing interest in the use of tES and transorbital stimulation to treat disorders of vision (see35–38 for recent reviews).
Following early work that reported improved contrast sensitivity in adults with amblyopia after visual cortex repetitive transcranial magnetic stimulation39,40, a number of studies have investigated the application of anodal tDCS to amblyopia. A single session of anodal tDCS improves amblyopic eye contrast sensitivity41,42, increases visually evoked potential (VEP) amplitudes induced by stimuli presented to the amblyopic eye42, and balances the response to inputs from each eye within visual cortex41. Furthermore, anodal tDCS enhances the effect of perceptual learning (PL) in adults with amblyopia26 and recent studies have revealed that visual acuity, detection thresholds, and stereopsis improve in mature amblyopic rats following anodal tDCS43–45. One potential mechanism for anodal tDCS effects in adults with amblyopia is a reduction in GABA-mediated inhibition46 within the visual cortex. GABA has been associated with interocular suppression in strabismic cats47 and may act as a “break” on visual cortex plasticity48.
A recently developed tES technique, tRNS, involves an alternating current that randomly changes in frequency and amplitude49. tRNS may have larger effects on cortical activity than other tES protocols. For example, tRNS induced significantly greater improvements in tinnitus symptoms33 and larger increases in motor evoked potential amplitude (MEP)21 compared to either tDCS or tACS. Furthermore, visual cortex tRNS enhanced visual perceptual learning in adults with normal vision to a greater extent than anodal tDCS50,51 and the combination of tRNS and perceptual learning enhanced the transfer of learning to non-trained visual tasks in adults with amblyopia24. tRNS has also been reported to enhance visual perceptual learning in adults with cortical blindness51. Potential mechanisms for these effects include an acute enhancement of the signal-to-noise ratio within the visual cortex due to stochastic resonance52–54 and longer-lasting alterations in neural membrane function due to repetitive opening and closing of sodium channels55.
Within this single-blind, between subjects, randomized, sham-controlled study, we tested the hypothesis that five daily sessions of visual cortex tRNS alone would lead to improved amblyopic eye contrast sensitivity, along with improved crowded and uncrowded amblyopic eye visual acuity in adult participants.
Methods and materials
Participants
Amblyopia was defined as reduced best corrected visual acuity (> + 0.3 logMAR) in one eye in the absence of ocular pathology and at least a 0.2 logMAR acuity difference between the eyes. Anisometropia was defined as a difference in spherical equivalent between the two eyes of ≥ 0.50 Diopters (D), or a difference of astigmatism in any meridian ≥ 1.50 D56. Initial visual acuity was measured using an M&S logMAR chart. Inclusion criteria were: (i) presence of strabismic and/or anisometropic amblyopia; (ii) 0.0 logMAR visual acuity or better in the fellow eye (FE). Exclusion criteria57–60 were: (i) presence of a scalp skin condition that contraindicated tRNS; (ii) history of neurological or psychiatric disorders, such as seizures; (iii) current medication for the treatment of neurological or psychiatric disorders; (iv) a history of brain injury; (v) implanted medical devices. Potential participants were contacted following a search of the patient database at Dr. Teske and Associates. Interested participants completed telephone screening to determine eligibility. The experimental procedures were approved by the Ethics Review Board of the University of Waterloo, Canada and were consistent with the declaration of Helsinki. Written informed consent was obtained from all participants. All participants were remunerated for their time.
Procedure
A single-blind, sham controlled, between-subjects design was adopted. Randomization followed allocation concealment procedures and was conducted by an experimenter who was not involved in data collection or eligibility assessment using a random number generator. Randomization occurred after participants had met the eligibility criteria and completed study enrolment. Participants completed 5 consecutive daily stimulation sessions and a follow-up session 28 days after the final stimulation session. Outcome measures were completed by the participants using automated computer programs with no input from the experimenter. This procedure was designed to mitigate against experimenter bias.
Transcranial random noise stimulation
Subjects either received tRNS (2.0 mA, current density: 0.08 mA/cm, frequency range 0.1–640 Hz) or placebo (sham) stimulation of the primary visual cortex for 25 min over 5 consecutive days. Stimulation was delivered using a DC-stimulation MC device (Eldith, NeuroConn GmbH, Germany). We chose a relatively long and high intensity stimulation protocol because we planned to measure 6 outcomes (contrast sensitivity, crowded and uncrowded visual acuity for each eye) and therefore attempted to maximize the duration of the stimulation aftereffects61,62. There is no statistically significant difference between the effect of high frequency (101–640 Hz) and low frequency (0.1–100 Hz) visual cortex tRNS on visual perceptual learning enhancement50. Therefore, we chose to deliver the full frequency range. The stimulation was delivered via a pair of saline-soaked surface sponge electrodes (5 cm × 5 cm, 25 cm2) placed at Cz and Oz63, as determined by the international 10/20 electroencephalogram system. The AC current was initially ramped up to a maximum of 2 mA over 30 s and ramped down to 0 mA over 30 s at the end of the stimulation session. During sham stimulation, the 30 s ramp-up was immediately followed by the ramp-down out64. Our between subjects design and use of participants entirely naïve to non-invasive brain stimulation ensured that participants remained masked to their treatment allocation. Our application of tRNS conformed to tDCS safety guidelines57,65.
After the final tRNS session, participants were asked to rate the following sensations on a four-level scale (none, mild, moderate and severe): headache, neck pain, scalp pain, tingling, itching, burning sensation, sleepiness, trouble concentrating and acute mood change. Participants were also asked to rate whether any reported sensations were due to tES by selecting from the following options: no, remote chance, probable, definitely.
Visual function measurements
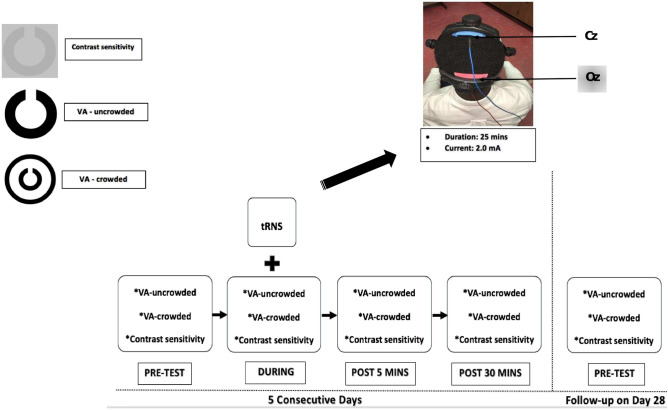
Monocular contrast sensitivity and visual acuity (both crowded and uncrowded) were measured for each eye before, during, 5 min post, and 30 min post stimulation on each stimulation day (Fig. 1). All measurements were made using Landolt-C optotypes presented using the Freiburg Vision Test (‘FrACT’)66,67 software package on a MacBook Pro (Version 10.13.6, 13-in, 2.7 GHz, 2560 × 1600). Gamma correction was conducted using a Spyder photometer and the FrACT software provided 10 bits of contrast resolution. The Landolt-C optotype was presented at 8 possible orientations and viewed from 3 m in a dark room. Participants identified the gap orientation using button presses. Trials were self-paced with a maximum display time of 30 s. A Bayesian adaptive (“Best PEST”) algorithm controlled optotype size for the crowded and uncrowded visual acuity threshold measurements and optotype contrast for the contrast sensitivity threshold measurement. Each threshold measurement lasted approximately 3 min. For the measurements made during stimulation, threshold measurement started 5 min into the stimulation. Landolt-C gap width was fixed at 30 arcmin for the non-amblyopic eye and 100 arcmin for the amblyopic eye during the contrast sensitivity measures. These parameters were based on pilot observations in individuals with moderate and severe amblyopia who could not resolve the 30 arcmin stimuli. Crowded optotypes were surrounded by a circle. Both the fellow eye and amblyopic eye were tested monocularly with the fellow eye tested first within each block. Uncrowded visual acuity was tested first within each block followed by crowded visual acuity and contrast sensitivity.
Figure 1.
Testing and stimulation protocol. Each measurement was recorded before stimulation (pre-test), during stimulation, 5 min after stimulation (Post 5 min) and 30 min after stimulation (Post 30 min) for 5 consecutive days. Baseline (pre-test) measurements were recorded again for each eye 28 days (Day 28) after the last stimulation session. Stimulation was delivered for 25 min at 2.0 mA. Active and reference electrodes were placed at Oz and Cz respectively. VA visual acuity.
Data analysis
Statistical analyses were performed in R (R Core Team, 2020)68 using the Bootstrap-Coupled Estimation package69. Visual acuities were recorded in logMAR units. Contrast sensitivity was recorded in log units. To test for tRNS effects within the 5 stimulation sessions, a mixed-effects analysis of variance (ANOVA) with a between-subjects factor of Group (active vs sham), a within-subjects factor of Day (day 1–5), and a within-subjects factor of Time (baseline, during, post 5 and post 30 min) was conducted for each measurement type for each eye separately. Planned pairwise comparisons (least significance difference test) between baseline and all other timepoints were examined for each day. In addition, to assess whether tRNS had cumulative or long-term effects on visual function, a mixed ANOVA with factors of Group (active vs sham) and Baseline (baseline day 1, baseline day 2, baseline day 3, baseline day 4, baseline day 5, baseline day 28) was conducted for each outcome measure for each eye. All ANOVA analyses reported passed Levene’s test for equality of variances (p > 0.05) and test of sphericity (p > 0.05).
Pairwise comparisons were conducted using the effect size Hedge’s g by bootstrap estimation (5000 bootstrap samples with replacement), with the 95% confidence interval around the g being bias-corrected and accelerated69. The permutation p values reported were calculated with 5000 reshuffles of the baseline and test labels performed for each permutation, with the P value indicating the likelihood of observing the mean difference, if the null hypothesis of zero difference is true, at an α of 0.05. Between group differences in the strength of any sensations induced by tRNS were assessed using the chi-squared test.
Results
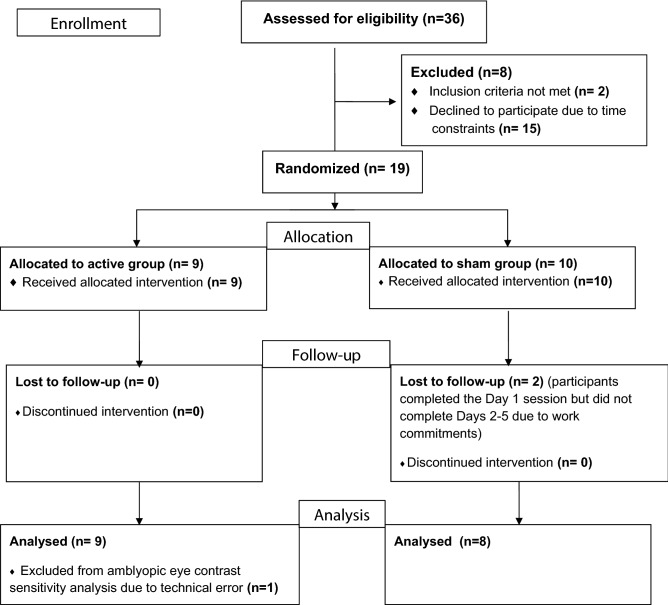
Nineteen healthy adults with amblyopia were recruited. Two participants in the sham group withdrew from the study after day 1 due to the time commitment required and were excluded from the analysis (Fig. 2). Clinical details of the remaining seventeen participants are provided in (Table 1). A technical error prevented an accurate amblyopic eye contrast sensitivity measurement on day 1 for one participant in the active group. This participant was excluded from the amblyopic eye contrast sensitivity analysis only (Fig. 2). There was no statistically significant group difference in age for the 17 participants that completed the study (Active group mean age = 38.7 years, SD = 13.6; Sham group mean age = 45.8, SD = 15.4; t15 = 1.0, p = 0.3). There were no adverse effects of tRNS, and there were no statistically significant between-group differences in the range or severity of subjective sensations reported (Table 2).
Figure 2.
CONSORT flow diagram for the study.
Table 1.
Participant details. M = male, F = female, patching = previous history of occlusion therapy, mixed = mixed amblyopia, aniso = anisometropic amblyopia, strab = strabismic amblyopia, stereoacuity is presented in seconds of arc, AME = amblyopic eye, FFE = fellow fixating eye, add = near power addition.
| Participant | Age/sex | Patching | Type of amblyopia | Stereoacuity | Visual acuity (logMAR) | Current refraction | |||
|---|---|---|---|---|---|---|---|---|---|
| AME | FFE | AME | FFE | ADD | |||||
| Active 1 | 27/M | Yes | Mixed | < 800 | + 1.40 | 0.00 | + 2.00 − 0.50 × 155 | + 0.50 DS | |
| Active 2 | 20/M | Yes | Aniso | 400 | + 0.50 | 0.00 | + 1.00 − 1.50 × 175 | Plano | |
| Active 3 | 37/M | Yes | Aniso | < 800 | + 0.30 | 0.00 | − 1.00 − 1.00 × 100 | − 0.25 − 2.50 × 090 | |
| Active 4 | 45/F | Yes | Aniso | 200 | + 0.30 | 0.00 | + 2.75 − 0.75 × 080 | + 2.00 − 0.25 × 159 | + 1.50 |
| Active 5 | 59/M | No | Aniso | < 800 | + 0.70 | 0.00 | + 8.25 DS | + 8.25 − 1.25 × 105 | + 2.50 |
| Active 6 | 41/F | Yes | Mixed | < 800 | + 0.50 | 0.00 | + 2.00 DS | + 0.25 − 3.75 × 179 | + 0.75 |
| Active 7 | 46/M | No | Aniso | < 800 | + 0.40 | 0.00 | + 0.75 DS | + 4.25 -0.50 × 165 | |
| Active 8 | 52/F | No | Aniso | 400 | + 0.70 | 0.00 | + 0.50 − 0.50 × 175 | + 2.00 − 0.50 × 075 | + 2.25 |
| Active 9* | 21/F | No | Aniso | 60 | + 0.50 | + 0.10 | + 0.50 − 4.00 × 180 | Plano | |
| Sham 1 | 51/F | Yes | Mixed | < 800 | + 0.30 | 0.00 | + 3.25 − 0.50 × 165 | Plano | |
| Sham 2 | 58/M | Yes | Aniso | 400 | + 0.30 | 0.00 | + 4.00 − 0.25 × 180 | + 1.50 − 0.50 × 180 | + 2.25 |
| Sham 3 | 52/F | No | Aniso | 200 | + 0.40 | 0.00 | − 2.50 − 0.50 × 155 | − 1.50 DS | + 2.75 |
| Sham 4 | 19/M | No | Aniso | < 800 | + 0.30 | 0.00 | + 2.25 DS | + 0.75 DS | |
| Sham 5 | 55/M | No | Aniso | < 800 | + 0.30 | 0.00 | + 1.75 − 0.75 × 085 | + 1.75 − 0.75 × 090 | + 2.00 |
| Sham 6 | 49/M | No | Strab | 100 | + 0.30 | 0.00 | + 1.75 DS | + 1.50 DS | |
| Sham 7 | 24/M | No | Mixed | < 800 | + 0.30 | 0.00 | + 8.25 − 2.00 × 160 | + 6.50 − 1.25 × 0.40 | |
| Sham 8 | 58/F | No | Strab | < 800 | + 0.50 | 0.00 | + 4.50 − 0.25 × 176 | + 3.50 − 0.50 × 165 | + 2.75 |
*Indicates the participant with missing data for the amblyopic eye contrast sensitivity measurement only.
Table 2.
Subjective experiences reported by participants after the day 5 active or sham tRNS session.
| Mild | Moderate | ||
|---|---|---|---|
| Headache | |||
| Active (N = 9) | 1 | 4 | |
| Sham (N = 8) | 2 | 0 | |
| Neck pain | |||
| Active (N = 9) | 2 | 0 | |
| Sham (N = 8) | 1 | 0 | |
| Scalp pain | |||
| Active (N = 9) | 1 | 0 | |
| Sham (N = 8) | 0 | 0 | |
| Tingling sensation | |||
| Active (N = 9) | 4 | 3 | |
| Sham (N = 8) | 6 | 1 | |
| Itching | |||
| Active (N = 9) | 4 | 2 | |
| Sham (N = 8) | 5 | 0 | |
| Burning sensation | |||
| Active (N = 9) | 2 | 3 | |
| Sham (N = 8) | 1 | 0 | |
| Sleepiness | |||
| Active (N = 9) | 2 | 1 | |
| Sham (N = 8) | 3 | 2 | |
| Trouble concentrating | |||
| Active (N = 9) | 0 | 0 | |
| Sham (N = 8) | 1 | 0 | |
| Acute mood change | |||
| Active (N = 9) | 0 | 0 | |
| Sham (N = 8) | 1 | 0 | |
| Others | Jaw stiffness | Neck tension | |
| Active (N = 9) | 1 | 1 | |
| Sham (N = 8) | 0 | 0 | |
| Any of the symptoms related to tES | Remote | Probable | Definite |
| Active (N = 9) | 0 | 3 | 6 |
| Sham (N = 8) | 2 | 0 | 6 |
No participants reported severe experiences. There were no statistically significant between group differences.
Contrast sensitivity
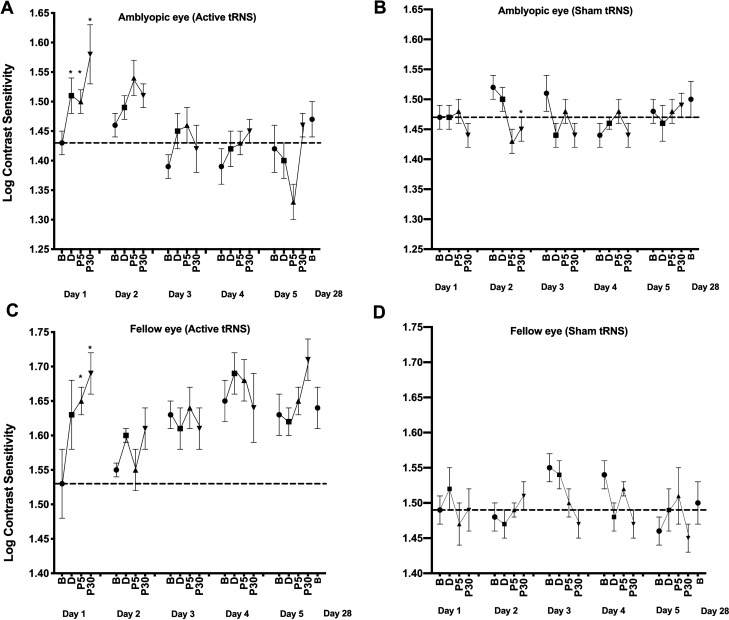
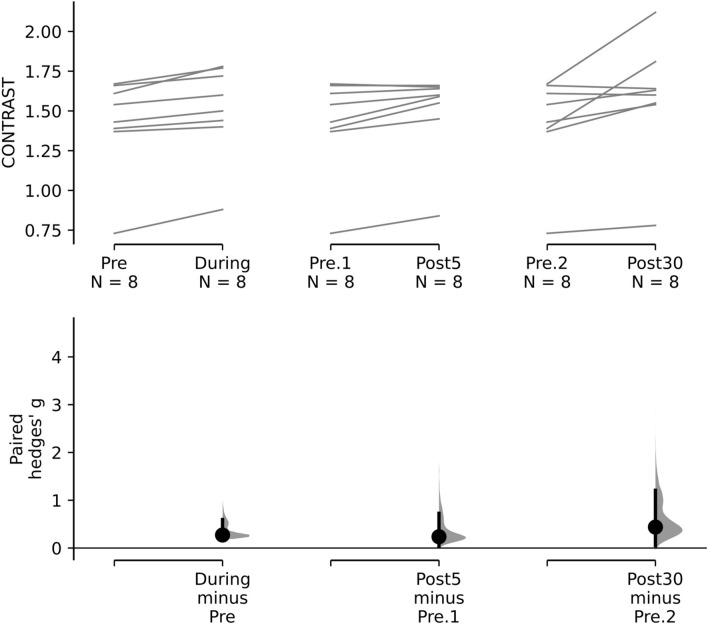
For the amblyopic eyes (Fig. 3—upper panel), there was a significant interaction between Group and Time, F3,42 = 3.584, p = 0.022, ηp2 = 0.216. No other omnibus main effects or interactions were significant. Planned pairwise comparisons between baseline and all other timepoints were examined for the active and sham groups for each day. During day 1, the active group exhibited a significant improvement in contrast sensitivity from baseline for all post-test measurements (during: g = 0.272 [0.195, 0.597], p = 0.01; post 5 min: g = 0.236 [0.039, 0.726], p = 0.035; and post 30 min: g = 0.438 [0.052, 1.207], p = 0.034: Fig. 4). No significant differences between baseline and any post-test were found for days 2–5. No significant differences between baseline and any post-test were found within the sham group for any day.
Figure 3.
The effects of tRNS on contrast sensitivity during each daily session and at the day 28 follow-up visit. Data are shown separately for the amblyopic (A and B) and fellow (C and D) eyes and for the active (A and C) and control (B and D) groups at baseline and during, 5 min (P5) and 30 min (P30) post tRNS. *Statistically significant difference from baseline (p < 0.05). Error bars show within-subject standard error of the mean (SEM). The dashed horizontal lines represent the mean before-stimulation threshold on day 1. Larger y-axis values indicate better contrast sensitivity.
Figure 4.
Paired Hedges’ g for three comparisons (during stimulation, Post 5 min, Post 30 min) to pre-test contrast sensitivity are shown for the active group amblyopic eye contrast sensitivity data using a Cumming estimation plot. Raw contrast threshold data for each participant are plotted on the upper axes; each paired set of observations is connected by a line. On the lower axes, paired Hedges’s g is plotted as a bootstrap sampling distribution. Hedge’s g value is depicted as dots; 95% confidence intervals are indicated by the ends of the vertical error bars.
For the fellow eyes (Fig. 3—lower panel), there was a significant interaction between Group and Time, F3,45 = 3.303, p = 0.029, ηp2 = 0.191. No other omnibus main effects or interactions were significant. During day 1, the active group exhibited a significant improvement in contrast sensitivity from baseline for the post 5 min (g = 0.639 [0.127, 1.248], p = 0.033) and post 30 min (g = 0.846 [0.199, 1.661], p = 0.018) measurements. No significant differences between baseline and any post-test were found for days 2–5. No significant differences between baseline and any post-test were found within the sham group for any day.
Uncrowded visual acuity
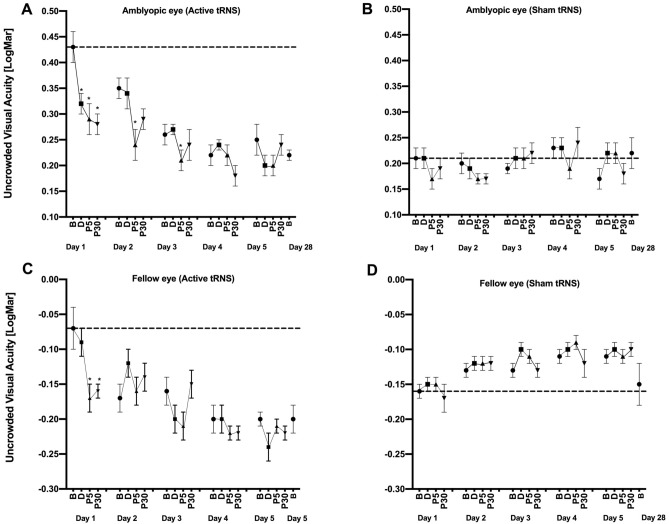
For the amblyopic eyes (Fig. 5—upper panel), there was a significant interaction between Group and Time, F3,45 = 3.325; p = 0.029, ηp2 = 0.192). No other omnibus main effects or interactions were significant. During day 1, the active group exhibited a significant improvement in uncrowded visual acuity from baseline for all post-test measurements (during: g = 0.224 [0.084, 0.575], p = 0.010; post 5: g = 0.281 [0.009, 0.640], p = 0.05; post 30: g = 0.307 [0.118, 0.795], p = 0.003). During days 2 and 3, the active group exhibited a significant difference between baseline and only the post 5 min measurement (day 2: g = 0.231 [0.091, 0.383], p = 0.015, day 3: g = 0.126 [0.003, 0.304], p = 0.038). No significant differences between baseline and any post-test were found during days 4 and 5. No significant difference between baseline and any post-test was found within the sham group for any day. By chance, there was a substantial difference in baseline uncrowded Landolt-C visual acuity between the active and sham group (compare the dashed lines in Fig. 5—upper panel).
Figure 5.
The effects of tRNS on uncrowded visual acuity during each daily session and at the day 28 follow-up visit. Data are shown as in Fig. 3. Lower (smaller/more negative) y-axis values indicate better uncrowded visual acuity.
For the fellow eyes (Fig. 5—lower panel), there was a significant interaction between Group and Time, F3,45 = 3.504; p = 0.023, ηp2 = 0.200. No other omnibus main effects or interactions were significant. During day 1, the active group exhibited a significant improvement in contrast sensitivity from baseline for the post 5 (g = 0.817 [0.164, 1.75], p = 0.035) and post 30 (g = 0.774 [0.199, 1.54], p = 0.02) min measurements. No significant differences between baseline and any post-test were found for days 2–5. No significant differences between baseline and any post-test were found within the sham group for any day.
Crowded visual acuity
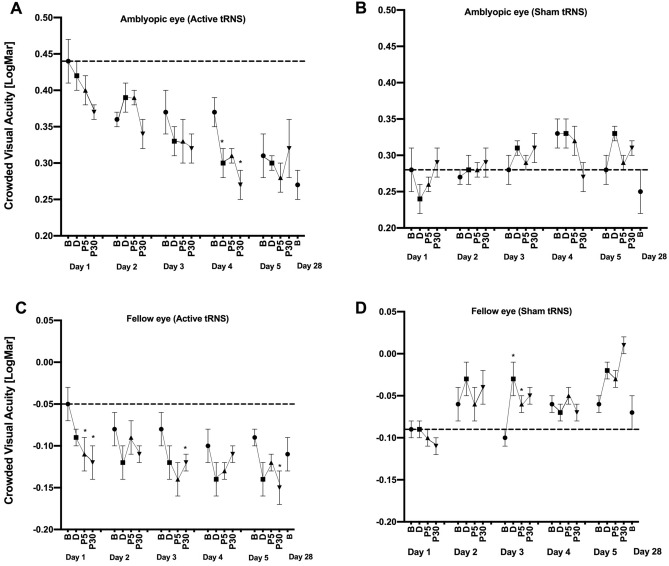
For the amblyopic eyes (Fig. 6—upper panel), there were no significant main effects or interactions (all p > 0.05). No significant changes from baseline were observed for any day for any group. As for uncrowded visual acuity, there was a substantial difference in baseline performance between the two groups that occurred by chance during randomization.
Figure 6.
The effects of tRNS on crowded visual acuity during each daily session and at the day 28 follow-up visit. Data are shown as in Fig. 3. Lower (smaller/more negative) y-axis values indicate better crowded visual acuity.
For the fellow eyes (Fig. 6—lower panel), there was a significant interaction between Group and Time, F3,45 = 5.733; p = 0.002, ηp2 = 0.291. No other omnibus main effects or interactions were significant. During day 1, the active group exhibited a significant improvement in crowded acuity from baseline for the post 5 (g = 0.404 [0.083, 0.9], p = 0.05) and post 30 min (g = 0.457 [0.09, 0.913], p = 0.039) measurements. During days 3 and 5, the active group exhibited a significant improvement in crowded acuity from baseline to post 5 min (g = 0.389 [0.065, 1.06], p = 0.007) and post 30 min (g = 0.721 [0.047, 1.4], p = 0.044) respectively. No significant differences between baseline and any post-test were found for days 2 and 4. No significant differences between baseline and any post-test were found for the sham group.
Cumulative and long-term effects of tRNS
For the amblyopic eyes, there was a significant interaction between Group and Baseline for uncrowded visual acuity (F5,65 = 3.372; p = 0.009, ηp2 = 0.206). Pairwise comparisons for the active group revealed a significant difference between the day 1 baseline and the day 3 (g = 0.372 [0.163, 0.771], p = 0.011), day 4 (g = 0.461 [0.243, 0.93], p < 0.003), day 5 (g = 0.369 [0.065, 0.809], p = 0.034), and day 28 (g = 0.454 [0.219, 1.03], p = 0.003) baselines. No pairwise comparisons were significant for the sham group. There were no significant interactions for amblyopic eye contrast sensitivity or crowded visual acuity measurements or for any of the fellow eye measurements.
Discussion
Our results partially supported our experimental hypothesis that five daily sessions of visual cortex tRNS would improve amblyopic eye contrast sensitivity as well as crowded and uncrowded visual acuity in adult patients. We observed tRNS-induced improvements in contrast sensitivity and uncrowded visual acuity relative to the sham group for both amblyopic and fellow eyes. Crowded visual acuity improved for the fellow but not the amblyopic eyes. Across all outcome measures, pairwise comparisons revealed that acute tRNS effects were statistically significant on day 1 but became non-significant for later sessions. Only amblyopic eye uncrowded visual acuity exhibited a lasting effect of tRNS at follow-up. Our discussion will focus primarily on the results for contrast sensitivity because initial baseline performance was matched between the groups. There were pronounced between-group baseline differences for amblyopic eye uncrowded and crowded visual acuity that occurred by chance during the randomization procedure (randomization occurred before baseline measures were conducted). The difference in baseline performance for the acuity outcome measures make it difficult to properly segregate tRNS effects from task learning effects. One reason for these baseline differences might be a difference in the proportion of patients with anisometropic amblyopia in active (78%) and sham (50%) groups. However, a much larger scale study will be required to determine whether amblyopia subtype influences the response to visual cortex tRNS.
tRNS-induced improvements in contrast sensitivity
Our observation that visual cortex tRNS improved amblyopic eye contrast sensitivity is consistent with a growing literature reporting improved contrast sensitivity, visual acuity, stereopsis, and an enhanced cortical response to amblyopic eye inputs following non-invasive visual cortex stimulation in adults with amblyopia26,39–42,70–72. A number of potential mechanisms have been proposed for tRNS effects. These include stochastic resonance and changes in the resting membrane potential22,52. Stochastic resonance refers to an improvement in signal to noise ratio when a certain amount of noise (in this case neural noise induced by tRNS) is added to non-linear systems54. A number of psychophysical studies have provided compelling evidence that stochastic resonance occurs during visual cortex tRNS24,73–75. It is possible that the during-stimulation improvements we observed on day 1 for amblyopic eye contrast sensitivity were due to stochastic resonance. However, tRNS aftereffects (i.e. effects that outlast the duration of stimulation) cannot easily be explained by stochastic resonance.
Terney et al., proposed that increased motor cortex excitability following tRNS is related to the activity of sodium channels within the neural membrane76. Specifically, they proposed that tRNS may cause repetitive membrane depolarization that is sufficient to repeatedly open sodium channels but sub-threshold for generating an action potential. These synchronized local depolarizations where further hypothesized to induce lasting long-term potentiation-like effects at the level of individual neurons. However, a subsequent study found that pharmacological manipulation of NDMA receptors had no effect on tRNS aftereffects, whereas the GABA agonist lorazepam and carbamazepine, a sodium channel blocker, attenuated tRNS aftereffects77. These results are not consistent with a mechanism related to long-term potentiation but do support the involvement of sodium channels. Alternative mechanisms for the effects of electrical stimulation have also been proposed including regional increases in cortical blood flow36, modified brain connectivity78 and changes in neurotransmitter concentration46,79. The precise underlying mechanism for the tRNS aftereffects we observed remains to be determined.
A previous study36 reporting improved amblyopic eye contrast sensitivity following both excitatory and inhibitory visual cortex rTMS proposed a mechanism linked to cortical homeostasis. According to this hypothesis, excitatory stimulation has a more pronounced effect on weakly activated/suppressed neural populations whereas inhibitory stimulation has a greater effect on strongly activated populations. Therefore, both excitatory and inhibitory stimulation are capable of restoring a level of homeostasis to the amblyopic visual cortex by reducing the difference in activation between amblyopic eye dominated neurons (weak activation/suppression) and fellow eye dominated neurons (strong activation)41. This, in turn, reduces suppression and/or the relative attenuation of amblyopic-eye-driven neural activity. It is plausible that the excitatory tRNS we employed in this study acts through a homeostatic mechanism.
We also observed improved fellow eye contrast sensitivity in the tRNS group relative to the sham group. Non-invasive visual cortex stimulation studies have reported varying fellow eye effects. Studies using inhibitory stimulation protocols (1 Hz rTMS and continuous theta burst stimulation; cTBS) have reported reduced fellow eye contrast sensitivity35,36 whereas those using excitatory protocols (anodal tDCS and tRNS)24,42, including the present study, observed improvements. This pattern of results is consistent with the homeostasis hypothesis which predicts relatively impaired fellow eye function following inhibitory stimulation and does not rule out improved fellow eye function following excitatory stimulation. This is because excitatory effects may still occur within neuronal populations dominated by the fellow eye, just to a lesser extent than those dominated by the amblyopic eye.
Successive and cumulative tRNS effects on contrast sensitivity
A day by day analysis of the contrast sensitivity data revealed that tRNS effects were pronounced for both eyes on day 1. However, the within-session tRNS effects waned across sessions, becoming non-significant by day 2 for both eyes. This reduction in within-session tRNS effects was accompanied by stable session to session baseline performance indicating the absence of a cumulative tRNS effect on contrast sensitivity. The waning of within-session effects is consistent with Clavagnier et al.’s40 study of repeated cTBS sessions in amblyopia, however cTBS did induce cumulative effects that improved baseline performance across sessions. One possible explanation for the waning of within-session tRNS effects and the absence of a cumulative effect on contrast sensitivity relates to stimulation intensity. The relationship between tRNS intensity and visual function improvement during stimulation is an “inverted U”, whereby stimulation that is weaker or stronger than an optimum level has limited effects52,53. Lasting changes in cortical excitability induced by prior sessions of tRNS might shift the optimal stimulation intensity towards lower levels, causing a waning of tRNS effects across sessions if stimulation intensity remains constant. If this is the case, tapering stimulation intensity across sessions would be a possible solution.
Another possible explanation for an effect on day 1 and not subsequent days is a placebo effect. Although this cannot be completely ruled out, the use of a single masked, between subjects design combined with automated collection of outcome measures was intended to minimize this source of bias. In addition, participant reported sensations did not differ significantly between the two groups suggesting the adequate masking was preserved throughout the study.
tRNS effects of crowded and uncrowded visual acuity
It is not possible to draw strong conclusions relating to the amblyopic eye datasets for crowded and uncrowded visual acuity because there were large between-group differences in baseline performance that occurred by chance. However, baseline group differences were minimal for the fellow eye datasets and the results followed those for contrast sensitivity very closely; significant differences between groups that were characterized by within-session improvements early in the experiment and a gradual waning of tRNS effects. This suggests that transient tRNS effects occur for a range of visual functions and that long lasting effects may occur for uncrowded visual acuity.
Study limitations
The primary limitation of this study is the relatively small sample size. However, there is no indication in our data that the lack of long-term effects of visual cortex tRNS on amblyopic eye contrast sensitivity is due to insufficient statistical power. We had sufficient power to detect an effect of tRNS on day 1 and this effect waned across subsequent sessions. The small sample size did preclude the use of stratification for amblyopia subtype and baseline clinical characteristics within our randomization procedure and this likely contributed to the between group differences in baseline amblyopic eye visual acuity that are present in our data.
Conclusions
tRNS can induce short-term contrast sensitivity improvements in adult amblyopic eyes, however repeated sessions of tRNS with a fixed set of stimulation parameters do not lead to enhanced or long-lasting effects. In agreement with previous non-invasive brain stimulation studies, these results demonstrate considerable short-term plasticity within the visual cortex of human adults with amblyopia and identify new pathways for future research such as the modification of stimulation parameters across sessions to maximize cumulative stimulation effects and the exploration of specific rather than random stimulation frequency bands.
Acknowledgements
This work was supported by CIHR Grant 390283, CFI Grant 34095 and NSERC Grants RPIN-05394 and RGPAS-477166 to BT.
Author contributions
Conceptualization R.D. and B.T.; experimental design R.D., B.T., C.T. and M.W.D.; data collection R.D. and C.T.; data analysis R.D., A.S., A.P.J. and B.T.; data interpretation R.D., A.S., A.P.J. and B.T.; manuscript writing R.D. and B.T., revision and review of manuscript all authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Attebo K, Mitchell P, Cumming R, Smith W, Jolly N, Sparkes R. Prevalence and cause of amblyopia in an adult population. Ophthalmology. 1998 doi: 10.1016/S0161-6420(98)91862-0. [DOI] [PubMed] [Google Scholar]
- 2.Webber AL, Wood J. Amblyopia: Prevalence, natural history, functional effects and treatment. Clin. Exp. Optom. 2005 doi: 10.1111/j.1444-0938.2005.tb05102.x. [DOI] [PubMed] [Google Scholar]
- 3.Pai ASI, Rose KA, Leone JF, Sharbini S, Burlutsky G, Varma R, et al. Amblyopia prevalence and risk factors in Australian preschool children. Ophthalmology. 2012 doi: 10.1016/j.ophtha.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Asper L, Crewther D, Crewther SG. Strabismic amblyopia: Part 1: Psychophysics. Clin. Exp. Optom. 2000 doi: 10.1111/j.1444-0938.2000.tb04892.x. [DOI] [PubMed] [Google Scholar]
- 5.Hamm LM, Black J, Dai S, Thompson B. Global processing in amblyopia: A review. Front. Psychol. 2014;5:583. doi: 10.3389/fpsyg.2014.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKee SP, Levi DM, Movshon JA. The pattern of visual deficits in amblyopia. J. Vis. 2003;3:5. doi: 10.1167/3.5.5. [DOI] [PubMed] [Google Scholar]
- 7.Levi DM, Klein S. Hyperacuity and amblyopia. Nature. 1982 doi: 10.1038/298268a0. [DOI] [PubMed] [Google Scholar]
- 8.Levi DM, Klein SA. Vernier acuity, crowding and amblyopia. Vis. Res. 1985 doi: 10.1016/0042-6989(85)90208-1. [DOI] [PubMed] [Google Scholar]
- 9.Stager DR, Everett ME, Birch EE. Comparison of crowding bar and linear optotype acuity in amblyopia. Am. Orthopt. J. 1990 doi: 10.1080/0065955X.1990.11981816. [DOI] [Google Scholar]
- 10.Bradley A, Freeman RD. Contrast sensitivity in anisometropic amblyopia. Investig. Ophthalmol Vis. Sci. 1981;21:467. [PubMed] [Google Scholar]
- 11.Kiorpes L, Kiper DC, Movshon JA. Contrast sensitivity and vernier acuity in amblyopic monkeys. Vis. Res. 1993 doi: 10.1016/0042-6989(93)90107-8. [DOI] [PubMed] [Google Scholar]
- 12.Hess RF, Howell ER. The threshold contrast sensitivity function in strabismic amblyopia: Evidence for a two type classification. Vis. Res. 1977 doi: 10.1016/0042-6989(77)90009-8. [DOI] [PubMed] [Google Scholar]
- 13.Birch EE. Amblyopia and binocular vision. Prog. Retin. Eye Res. 2013 doi: 10.1016/j.preteyeres.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levi DM, Knill DC, Bavelier D. Stereopsis and amblyopia: A mini-review. Vis. Res. 2015 doi: 10.1016/j.visres.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black JM, Hess RF, Cooperstock JR, To L, Thompson B. The measurement and treatment of suppression in amblyopia. J. Vis. Exp. 2012 doi: 10.3791/3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Thompson B, Lam CSY, Deng D, Chan LYL, Maehara G, et al. The role of suppression in amblyopia. Investig. Ophthalmol. Vis. Sci. 2011;52:4169–4176. doi: 10.1167/iovs.11-7233. [DOI] [PubMed] [Google Scholar]
- 17.Hess RF, Thompson B. Amblyopia and the binocular approach to its therapy. Vis. Res. 2015;114:4–16. doi: 10.1016/j.visres.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Kiorpes L, Daw N. Cortical correlates of amblyopia. Vis. Neurosci. 2018;35:E016. doi: 10.1017/S0952523817000232. [DOI] [PubMed] [Google Scholar]
- 19.Loudon SE, Polling JR, Simonsz HJ. Electronically measured compliance with occlusion therapy for amblyopia is related to visual acuity increase. Graefe’s Arch. Clin. Exp. Ophthalmol. 2003 doi: 10.1007/s00417-002-0570-z. [DOI] [PubMed] [Google Scholar]
- 20.Holmes JM, Lazar EL, Melia BM, Astle WF, Dagi LR, Donahue SP, et al. Effect of age on response to amblyopia treatment in children. Arch. Ophthalmol. 2011 doi: 10.1001/archophthalmol.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inukai Y, Saito K, Sasaki R, Tsuiki S, Miyaguchi S, Kojima S, et al. Comparison of three non-invasive transcranial electrical stimulation methods for increasing cortical excitability. Front. Hum. Neurosci. 2016;10:668. doi: 10.3389/fnhum.2016.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terney D, Chaieb L, Moliadze V, Antal A, Paulus W. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J. Neurosci. 2008;28:14147. doi: 10.1523/JNEUROSCI.4248-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paulus W. Transcranial electrical stimulation (tES - tDCS; tRNS, tACS) methods. Neuropsychol. Rehabil. 2011;21:602–617. doi: 10.1080/09602011.2011.557292. [DOI] [PubMed] [Google Scholar]
- 24.Moret B, Camilleri R, Pavan A, Lo Giudice G, Veronese A, Rizzo R, et al. Differential effects of high-frequency transcranial random noise stimulation (hf-tRNS) on contrast sensitivity and visual acuity when combined with a short perceptual training in adults with amblyopia. Neuropsychologia. 2018;114:125–133. doi: 10.1016/j.neuropsychologia.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Camilleri R, Pavan A, Campana G. The application of online transcranial random noise stimulation and perceptual learning in the improvement of visual functions in mild myopia. Neuropsychologia. 2016;89:225–231. doi: 10.1016/j.neuropsychologia.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Spiegel DP, Li J, Hess RF, Byblow WD, Deng D, Yu M, et al. Transcranial direct current stimulation enhances recovery of stereopsis in adults with amblyopia. Neurotherapeutics. 2013;10:831–839. doi: 10.1007/s13311-013-0200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Brien AT, Bertolucci F, Torrealba-Acosta G, Huerta R, Fregni F, Thibaut A. Non-invasive brain stimulation for fine motor improvement after stroke: A meta-analysis. Eur. J. Neurol. 2018 doi: 10.1111/ene.13643. [DOI] [PubMed] [Google Scholar]
- 28.Norise C, Hamilton RH. Non-invasive brain stimulation in the treatment of post-stroke and neurodegenerative aphasia: Parallels, differences, and lessons learned. Front. Hum. Neurosci. 2017;10:1–16. doi: 10.3389/fnhum.2016.00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubis N. Non-invasive brain stimulation to enhance post-stroke recovery. Front. Neural Circuits. 2016;10:56. doi: 10.3389/fncir.2016.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlaug G, Renga V, Nair D. Transcranial direct current stimulation in stroke recovery. Arch. Neurol. 2008;65:1571–1576. doi: 10.1001/archneur.65.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu X-S, Fisher CA, Munz SM, Toback RL, Nascimento TD, Bellile EL, et al. Feasibility of non-invasive brain modulation for management of pain related to chemoradiotherapy in patients with advanced head and neck cancer. Front. Hum. Neurosci. 2016;10:466. doi: 10.3389/fnhum.2016.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palm U, Chalah MA, Padberg F, Al-Ani T, Abdellaoui M, Sorel M, et al. Effects of transcranial random noise stimulation (tRNS) on affect, pain and attention in multiple sclerosis. Restor. Neurol. Neurosci. 2016;34:189–199. doi: 10.3233/RNN-150557. [DOI] [PubMed] [Google Scholar]
- 33.Vanneste S, Fregni F, De Ridder D. Head-to-head comparison of transcranial random noise stimulation, transcranial AC stimulation, and transcranial DC stimulation for tinnitus. Front. Psychiatry. 2013 doi: 10.3389/fpsyt.2013.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pal N, Maire R, Stephan MA, Herrmann FR, Benninger DH. Transcranial direct current stimulation for the treatment of chronic tinnitus: A randomized controlled study. Brain Stimul. 2015;8:1101–1107. doi: 10.1016/j.brs.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Perin C, Viganò B, Piscitelli D, Matteo BM, Meroni R, Cerri CG. Non-invasive current stimulation in vision recovery: A review of the literature. Restor. Neurol. Neurosci. 2019 doi: 10.3233/rnn-190948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabel BA, Thut G, Haueisen J, Henrich-Noack P, Herrmann CS, Hunold A, et al. Vision modulation, plasticity and restoration using non-invasive brain stimulation—An IFCN-sponsored review. Clin. Neurophysiol. 2020 doi: 10.1016/j.clinph.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Sabel BA, Fedorov AB, Naue N, Borrmann A, Herrmann C, Gall C. Non-invasive alternating current stimulation improves vision in optic neuropathy. Restor. Neurol. Neurosci. 2011;29:493–505. doi: 10.3233/RNN-2011-0624. [DOI] [PubMed] [Google Scholar]
- 38.Gall C, Schmidt S, Schittkowski MP, Antal A, Ambrus GG, Paulus W, et al. Alternating current stimulation for vision restoration after optic nerve damage: A randomized clinical trial. PLoS ONE. 2016 doi: 10.1371/journal.pone.0156134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson B, Mansouri B, Koski L, Hess RF. Brain plasticity in the adult: Modulation of function in amblyopia with rTMS. Curr. Biol. 2008;18:1067–1071. doi: 10.1016/j.cub.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 40.Clavagnier S, Thompson B, Hess RF. Long lasting effects of daily theta burst rTMS sessions in the human amblyopic cortex. Brain Stimul. 2013;6:860–867. doi: 10.1016/j.brs.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Spiegel DP, Byblow WD, Hess RF, Thompson B. Anodal transcranial direct current stimulation transiently improves contrast sensitivity and normalizes visual cortex activation in individuals with amblyopia. Neurorehabil. Neural Repair. 2013;27:760–769. doi: 10.1177/1545968313491006. [DOI] [PubMed] [Google Scholar]
- 42.Ding Z, Li J, Spiegel DP, Chen Z, Chan L, Luo G, et al. The effect of transcranial direct current stimulation on contrast sensitivity and visual evoked potential amplitude in adults with amblyopia. Sci. Rep. 2016;6:19280. doi: 10.1038/srep19280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castaño-Castaño S, Garcia-Moll A, Morales-Navas M, Fernandez E, Sanchez-Santed F, Nieto-Escamez F. Transcranial direct current stimulation improves visual acuity in amblyopic Long-Evans rats. Brain Res. 2017;1657:340–346. doi: 10.1016/j.brainres.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Castaño-Castaño S, Martinez-Navarrete G, Morales-Navas M, Fernández-Jover E, Sanchez-Santed F, Nieto-Escámez F. Transcranial direct-current stimulation (tDCS) improves detection of simple bright stimuli by amblyopic Long Evans rats in the SLAG task and produces an increase of parvoalbumin labelled cells in visual cortices. Brain Res. 2019 doi: 10.1016/j.brainres.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 45.Castaño-Castaño S, Feijoo-Cuaresma M, Paredes-Pacheco J, Morales-Navas M, Ruiz-Guijarro JA, Sanchez-Santed F, et al. tDCS recovers depth perception in adult amblyopic rats and reorganizes visual cortex activity. Behav. Brain Res. 2019 doi: 10.1016/j.bbr.2019.111941. [DOI] [PubMed] [Google Scholar]
- 46.Bachtiar V, Near J, Johansen-Berg H, Stagg CJ. Modulation of GABA and resting state functional connectivity by transcranial direct current stimulation. Elife. 2015;4:1–9. doi: 10.7554/eLife.08789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sengpiel F, Blakemore C, Kind PC, Harrad R. Interocular suppression in the visual cortex of strabismic cats. J. Neurosci. 1994;14:6855–6871. doi: 10.1523/JNEUROSCI.14-11-06855.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hensch TK, Li RW, Bavelier D, Levi DM, Dan Y. Removing brakes on adult brain plasticity: From molecular to behavioral interventions. J. Neurosci. 2010 doi: 10.1523/jneurosci.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho K-A, Taylor J, Loo CP. Transcranial random noise stimulation: A new approach to stimulating the brain. Clin. Neurophysiol. 2013 doi: 10.1016/j.clinph.2013.04.286. [DOI] [Google Scholar]
- 50.Fertonani A, Pirulli C, Miniussi C. Random noise stimulation improves neuroplasticity in perceptual learning. J. Neurosci. 2011 doi: 10.1523/jneurosci.2002-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herpich F, Melnick MD, Agosta S, Huxlin KR, Tadin D, Battelli L. Boosting learning efficacy with noninvasive brain stimulation in intact and brain-damaged humans. J. Neurosci. 2019 doi: 10.1523/JNEUROSCI.3248-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Groen O, Wenderoth N. Transcranial random noise stimulation of visual cortex: Stochastic resonance enhances central mechanisms of perception. J. Neurosci. 2016;36:5289. doi: 10.1523/JNEUROSCI.4519-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pavan A, Ghin F, Contillo A, Milesi C, Campana G, Mather G. Modulatory mechanisms underlying high-frequency transcranial random noise stimulation (hf-tRNS): A combined stochastic resonance and equivalent noise approach. Brain Stimul. 2019 doi: 10.1016/J.BRS.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 54.McDonnell MD, Ward LM. The benefits of noise in neural systems: Bridging theory and experiment. Nat. Rev. Neurosci. 2011 doi: 10.1038/nrn3061. [DOI] [PubMed] [Google Scholar]
- 55.Antal A, Herrmann CS. Transcranial alternating current and random noise stimulation: Possible mechanisms. Neural Plast. 2016;2016:1–12. doi: 10.1155/2016/3616807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao TY, Guo CX, Babu RJ, Black JM, Bobier WR, Chakraborty A, et al. Effectiveness of a binocular video game vs placebo video game for improving visual functions in older children, teenagers, and adults with amblyopia: A randomized clinical trial. JAMA Ophthalmol. 2018;136:172–181. doi: 10.1001/jamaophthalmol.2017.6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res. Bull. 2007;72:208–214. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial direct current stimulation: Evidence based update 2016. Brain Stimul. 2016;9:641–661. doi: 10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008 doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Bikson M, Datta A, Elwassif M. Establishing safety limits for transcranial direct current stimulation. Clin. Neurophysiol. 2009;120:1033–1034. doi: 10.1016/j.clinph.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bindman LJ, Lippold OCJ, Redfearn JWT. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J. Physiol. 1964 doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000;3:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Antal A, Nitsche MA, Paulus W. External modulation of visual perception in humans. NeuroReport. 2001;12:3553–3555. doi: 10.1097/00001756-200111160-00036. [DOI] [PubMed] [Google Scholar]
- 64.Paneri B, Adair D, Thomas C, Khadka N, Patel V, Tyler WJ, et al. Tolerability of repeated application of transcranial electrical stimulation with limited outputs to healthy subjects. Brain Stimul. 2016;9:740–754. doi: 10.1016/j.brs.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W, et al. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin. Neurophysiol. 2003;114:2220–2222. doi: 10.1016/S1388-2457(03)00235-9. [DOI] [PubMed] [Google Scholar]
- 66.Bach M. The “Freiburg visual acuity test”—Automatic measurement of visual acuity. Optom. Vis. Sci. 1996;73:49. doi: 10.1097/00006324-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 67.Bach M. The Freiburg visual acuity test-variability unchanged by post-hoc re-analysis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007 doi: 10.1007/s00417-006-0474-4. [DOI] [PubMed] [Google Scholar]
- 68.R Development Core Team. R Development Core Team, R: A Language and Environment for Statistical Computing (2020).
- 69.Ho J, Tumkaya T, Aryal S, Choi H, Claridge-Chang A. Moving beyond P values: Data analysis with estimation graphics. Nat. Methods. 2019 doi: 10.1038/s41592-019-0470-3. [DOI] [PubMed] [Google Scholar]
- 70.Thompson B, Mansouri B, Koski L, Hess RF. From motor cortex to visual cortex: The application of noninvasive brain stimulation to amblyopia. Dev. Psychobiol. 2012;54:263–273. doi: 10.1002/dev.20509. [DOI] [PubMed] [Google Scholar]
- 71.Bocci T, Nasini F, Caleo M, Restani L, Barloscio D, Ardolino G, et al. Unilateral application of cathodal tDCS reduces transcallosal inhibition and improves visual acuity in amblyopic patients. Front. Behav. Neurosci. 2018;12:1–9. doi: 10.3389/fnbeh.2018.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tuna AR, Pinto N, Brardo FM, Fernandes A, Nunes AF, Pato MV. Transcranial Magnetic Stimulation in Adults With Amblyopia. J. Neuroophthalmol. 2020;40(2):185–192. doi: 10.1097/WNO.0000000000000828. [DOI] [PubMed] [Google Scholar]
- 73.van Koningsbruggen MG, Ficarella SC, Battelli L, Hickey C. Transcranial random-noise stimulation of visual cortex potentiates value-driven attentional capture. Soc. Cogn. Affect. Neurosci. 2016;11:1481–1488. doi: 10.1093/scan/nsw056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Groen O, Mattingley JB, Wenderoth N. Altering brain dynamics with transcranial random noise stimulation. Sci. Rep. 2019 doi: 10.1038/s41598-019-40335-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herpich F, Melnick M, Huxlin K, Tadin D, Agosta S, Battelli L. Transcranial random noise stimulation enhances visual learning in healthy adults. J. Vis. 2015 doi: 10.1167/15.12.40. [DOI] [Google Scholar]
- 76.Terney D, Chaieb L, Moliadze V, Antal A, Paulus W. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J. Neurosci. 2008 doi: 10.1523/JNEUROSCI.4248-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chaieb L, Antal A, Paulus W. Transcranial random noise stimulation-induced plasticity is NMDA-receptor independent but sodium-channel blocker and benzodiazepines sensitive. Front. Neurosci. 2015 doi: 10.3389/fnins.2015.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bola M, Gall C, Moewes C, Fedorov A, Hinrichs H, Sabel BA. Brain functional connectivity network breakdown and restoration in blindness. Neurology. 2014 doi: 10.1212/WNL.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 79.Stagg CJ, Bachtiar V, Johansen-Berg H. The role of GABA in human motor learning. Curr. Biol. 2011 doi: 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]