Abstract
Oocyte composition can directly influence offspring fitness, particularly in oviparous species such as most insects, where it is the primary form of parental investment. Oocyte production is also energetically costly, dependent on female condition and responsive to external cues. Here, we investigated whether mating influences mature oocyte composition in Drosophila melanogaster using a quantitative proteomic approach. Our analyses robustly identified 4,485 oocyte proteins and revealed that stage-14 oocytes from mated females differed significantly in protein composition relative to oocytes from unmated females. Proteins forming a highly interconnected network enriched for translational machinery and transmembrane proteins were increased in oocytes from mated females, including calcium binding and transport proteins. This mating-induced modulation of oocyte maturation was also significantly associated with proteome changes that are known to be triggered by egg activation. We propose that these compositional changes are likely to have fitness consequences and adaptive implications given the importance of oocyte protein composition, rather than active gene expression, to the maternal-to-zygotic transition and early embryogenesis.
Subject terms: Proteomic analysis, Oogenesis
Introduction
For species with minimal parental care, such as the majority of insects and other invertebrates, parental investment predominantly consists of the material invested in oocytes1,2. Variation in oocyte investment therefore has a direct and substantial impact on early development and hence viability. Oocyte quantity and quality have also been shown to vary in response to female condition3–5. Thus, allocation mechanisms may evolve to maximize female expenditure into oocytes when fertilization opportunities are abundant and environmental conditions are favorable for offspring survival.
Direct transfer of nutrients to the female during mating is one adaptive strategy by which males can influence oocyte attributes. In a variety of insects, males provide nuptial gifts of nutritional resources through ejaculate components, secretions or body parts1,6,7. Intraspecific variation in nuptial gift quality and quantity has experimentally been shown to correspond with numbers of oocytes8–13 and oocyte size9,12,14, as well as offspring maturation time14, size, and lifespan15. Supporting female utilization of male-derived nutrients in oogenesis are observations that ejaculate components become incorporated into the oocytes or ovaries of multiple fruit fly species16,17, butterflies and moths18,19, stink bugs20, cockroaches21, weevils22, lampyrid beetles23, and grasshoppers24. However, we note that in D. melanogaster male-derived chemical elements, but not ejaculate proteins, have been detected in oocytes17,25. Another strategy is male stimulation of increased female investment into oocytes following mating. Seminal fluid proteins of diverse insect species have been found to influence oocyte production through the stimulation of endogenous, female-mediated oogenesis mechanisms26,27. For example, ejaculate receipt by females can stimulate expression of vitellogenins necessary for oocyte production28, increased oocyte size29,30 and increased quantity of oocytes oviposited31.
Here, we investigated whether female mating status influenced the protein composition of D. melanogaster oocytes. We compared the proteome of stage 14 oocytes between those matured in unmated versus mated females. As these oocytes were not ovulated or fertilized, protein differences detected were primarily attributable to the influence of mating on oocyte development. This proteomic variation indicates that mating is likely to alter female-mediated aspects of oocyte maturation and provides insights into maternal investment strategies.
Results
Characterization of the mature oocyte proteome with respect to female mating status
We characterized the proteome of mature oocytes (stage 14) collected from mated and unmated females (Fig. 1A). Overall, we identified 4,485 high confidence oocyte proteins, with proteome composition highly reproducible across replicates (all pairwise protein abundance correlations were greater than 95%; Fig. 1B). These results were consistent with previous studies in terms of proteome coverage, including an 89% overlap with previously described D. melanogaster oocyte proteomes32–34. The three most abundant proteins were yolk proteins (Yp1, Yp2, Yp3), which have previously been shown to be the predominant protein component of oocytes35. Heat shock proteins (Hsp83 and Hsp26), which have also been shown to accumulate in the oocyte during oogenesis and contribute to oocyte stability36, were also among the top 20 most abundant proteins. The top 10% of oocyte proteins by abundance had Gene Ontology (GO) enrichments for: microtubule associated complex (GO:0005875, p < 0.001), lipid particle (GO:0005811, p < 0.001), proteasome complex (GO:0000502, p = 0.004), translation (GO:0006412, p < 0.001), protein folding (GO:0006457, p < 0.001), and centrosome duplication (GO:0051298, p < 0.001). Notable amongst these abundant proteins were those critical to oocyte differentiation or embryo viability (i.e., tudor, vasa, abnormal spindle, and belle)37–40.
Figure 1.
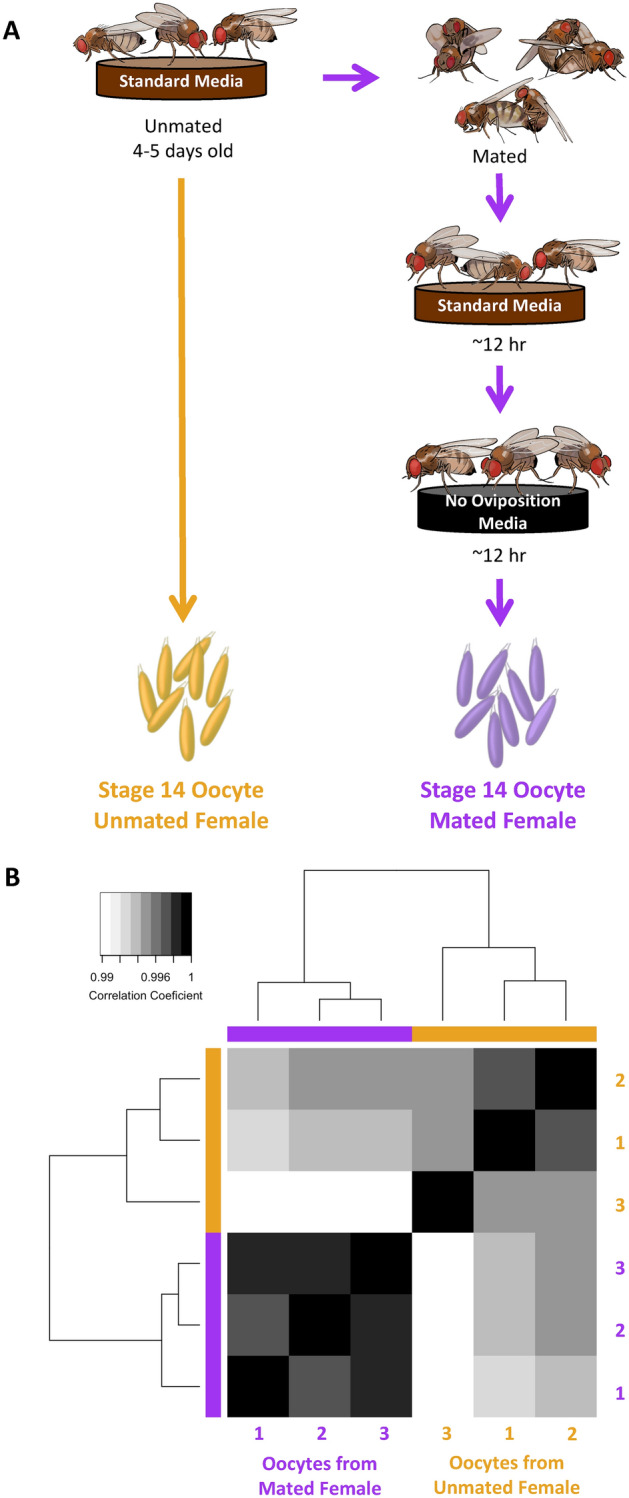
Experimental design and hierarchical clustering of oocyte proteomes. (A) Schematic of experimental design (image permission: Siyuan Cong). Oocytes were collected either from unmated females (left) or mated females (right). After mating, females were allowed to ovulate and clear out oocytes that had matured prior to mating and then transferred to a standard no oviposition media for 12hrs to accumulate oocytes that matured in a mated female environment. (B) Euclidean distance hierarchical clustering of oocytes proteome replicates based on protein abundance.
To test whether oocyte proteome composition was influenced by female mating status, we compared protein abundance in mature oocytes from unmated females to those from females 24 h after mating. Principal components (PC) analysis revealed that the first PC (59.2% of variation explained) distinguished oocyte samples by mating status (Fig S1). PC1 rotation values were significantly correlated with the log fold difference in abundance between oocytes from mated and unmated females (R2 = 0.98, p < 0.001), confirming that PC1 captured variance associated with mating status (Fig. 2A). We next identified 496 proteins that were significantly differentially abundant between oocytes from unmated and mated females (Fig. 2B). Altogether, 11.1% of the proteome exhibited significant abundance differences, including 20 proteins that exceed a two-fold change in abundance. These differentially abundant proteins were significantly biased towards greater abundances in oocytes from mated females (59.7%; binomial probability, p < 0.001) and there was a larger magnitude of protein abundance increases in mated female oocytes relative to unmated (mean log2FC of 0.45 ± 0.02) versus unmated (mean log2FC of 0.27 ± 0.01); Kruskal-Wallace χ2 = 357.34, p < 0.001; Fig. 2C). These differences in protein composition indicate that oocyte content is influenced by female mating status.
Figure 2.
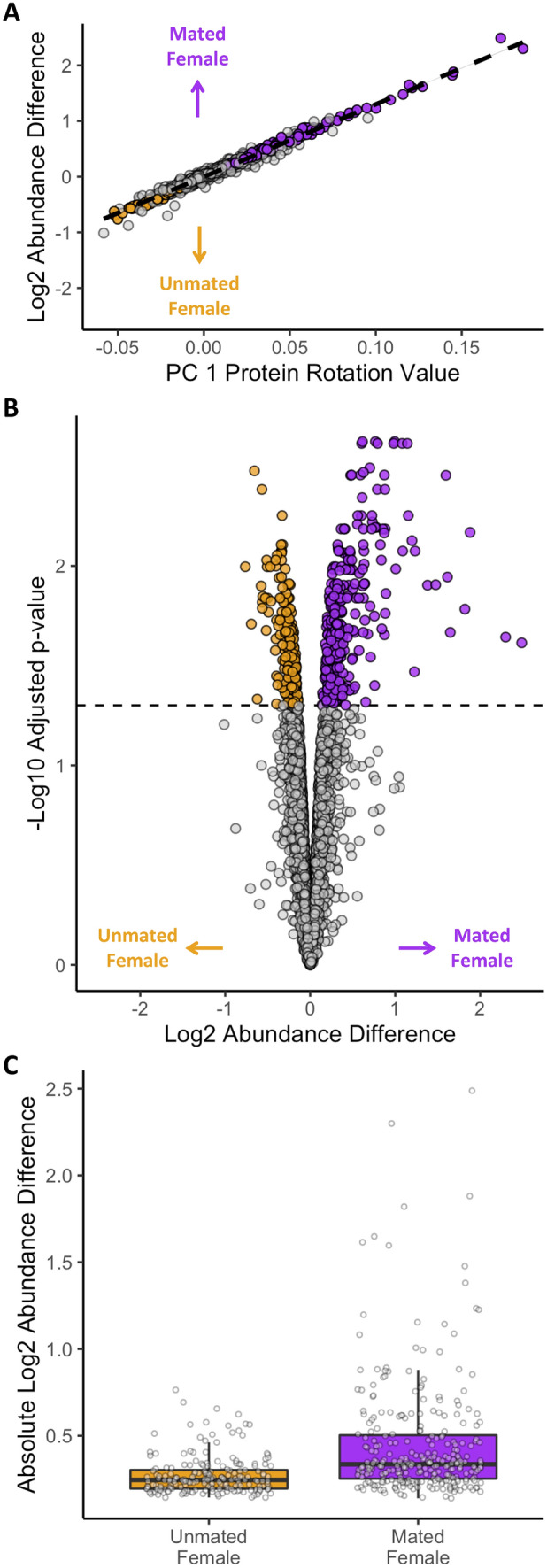
Oocyte proteome composition differences were dependent on female mating status. (A) Linear correlation between principle component 1(PC1) protein loadings with differential abundance between oocytes from unmated and mated females (log2 fold abundance difference) indicates that this axis of variation is associated with mating status. Colored points indicate proteins with significant differential abundance. (B) Volcano plot of log2 fold differential protein abundance between oocytes from unmated and mated females and -log10 adjusted p-values. (C) Boxplot of log2 fold abundance differences of proteins exhibiting significant abundance differences in oocytes from either unmated or mated females. A significantly greater magnitude of abundance change (p < 0.001) was observed in proteins with greater abundance in oocytes from mated females.
Oocyte proteome variation is not consistent with follicle cell contributions or aging
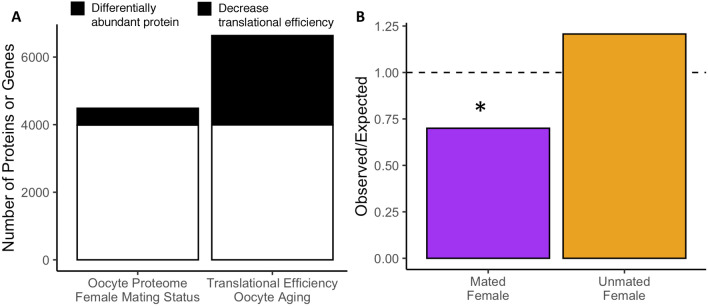
To ensure that the identified oocyte proteomic differences were due to female mating status, we evaluated two alternative hypothesized mechanisms: (1) that proteome differences were due to changes in follicle cells surrounding the oocyte rather than the oocyte itself and (2) that age-dependent oocyte effects contributed to the observed differences (i.e., the duration an oocyte remains in the ovary before ovulation). To address the first alternative hypothesis, we examined proteins commonly identified in follicle cells33,34 and found that they comprise less than 1% of the oocyte proteome (40 out of 4485 proteins) and were not significantly enriched among differentially abundant proteins (lower-tail binomial cumulative probability test p = 0.63; Table S1). To address the second alternative hypothesis, we assessed whether proteomic differences in oocytes from unmated and mated females corresponded to age-dependent changes in translational efficiency that would be expected to influence proteome composition41. Our experimental design necessitated consideration of this possibility because oocytes from unmated females could range from 0 to 96 h old, whereas those from mated females were 24 h old or less. However, we note that oocyte age differences in Greenblatt et al. were not accompanied by changes in oocyte protein content or hating rate. A comparison of our proteomic observations to the global patterns of reduced translational efficiency in aging oocytes revealed three primary differences41. First, in contrast to age-dependent reductions in translational efficiency which were widespread (39.8%; 2644 out of 6637), mating-dependent changes in the oocyte proteome were significantly more specific in nature (11.1%; 496 out of 4,485) (Fig. 3A; Table S1). Second, while age-dependent changes overwhelmingly resulted in reduced translational efficiency (97.7%, 2644 out of 2707), mating-dependent changes were significantly more balanced in their direction of change (59.7% or 296 out of 496 exhibited increased abundance in oocytes of mated females; χ2 = 1065.2, p < 0.001). Third, proteins with lower abundance in potentially older oocytes from unmated females (i.e., those with greater abundance in potentially younger oocytes from mated females) were significantly underrepresented among genes with age-dependent decreases in translational efficiency (lower-tail binomial cumulative probability test p = 0.03, expected 134.0, observed 94 proteins differentially abundant proteins with decreased translational efficiency; Fig. 3B). These results indicated that our proteomic observations were distinct in magnitude, direction and composition from translational efficiency reductions associated with aging. Instead, we conclude that the observed proteomic variation is more likely to be primarily due to differences in oocyte maturation between mated and unmated females. However, we also note that mated females had to be kept on no oviposition media for 12 h and that this nutritional variable may also impact oogenesis.
Figure 3.
Oocyte proteome composition differences are distinct from age-dependent changes. (A) Stacked bar chart displaying the proportions of oocyte proteins exhibiting mating-dependent changes in abundance and genes exhibiting age-dependent decreases in translational efficiency. (B) Observed versus expected overlap of proteins exhibiting age-dependent reductions in translational efficiency and proteins that change in abundance between oocytes from mated and unmated females (*p < 0.001).
Highly connected protein networks contribute to oocyte proteome variation
We investigated the functional coherence of mating-dependent changes in oocyte proteome composition using GO enrichment analyses (Table S2). Proteins more abundant in oocytes from mated females exhibited enrichments for integral membrane component (GO: 0016021, adj. p < 0.001), nucleolus (GO:0005730, adj. p < 0.001) and endomembrane system (GO:0012505, adj. p < 0.001). Proteins more abundant in oocytes from unmated females exhibited enrichments for components of the nucleus (GO:0005634, adj. p = 0.006). We next investigated patterns of functional enrichment among differentially abundant oocyte proteins using protein network interaction information. Proteins with greater abundance in oocytes from mated females (296 proteins) comprised a significantly interconnected protein network (PPI enrichment p < 0.001) with 1.9 times more interactions per node than expected (408 edges observed and 210 edges expected; Fig. 4) This protein network was enriched for ribosome biogenesis (GO: 0042254, adj. p = 0.03) and proteins with a transmembrane helix (KW-1133, adj. p < 0.001; Table S2). The potential importance of ribosomal protein accumulation is supported by studies showing high rates of ribosome synthesis in the ovary, the reduced size of oocytes when ribosomal proteins are mutated, and longer oocyte development times when rRNA levels are disrupted42,43. It is also plausible that elevated amounts of translational machinery could result in oocytes better primed for egg activation, which would influence embryonic development prior to zygotic genome activation44. The enrichment of proteins containing transmembrane helixes included membrane proteins involved in calcium-related processes. Calcium influx is the critical trigger for egg activation45 and several of these membrane proteins were involved in calcium channel activity (i.e., painless, Tmem63), calcium ion binding (i.e., alpha-Man-la, AnxB11, CG17271, CG17272, Edem2, LPCAT, mgl, Ndg, qua) and calcium homeostasis (i.e., CG6230).
Figure 4.
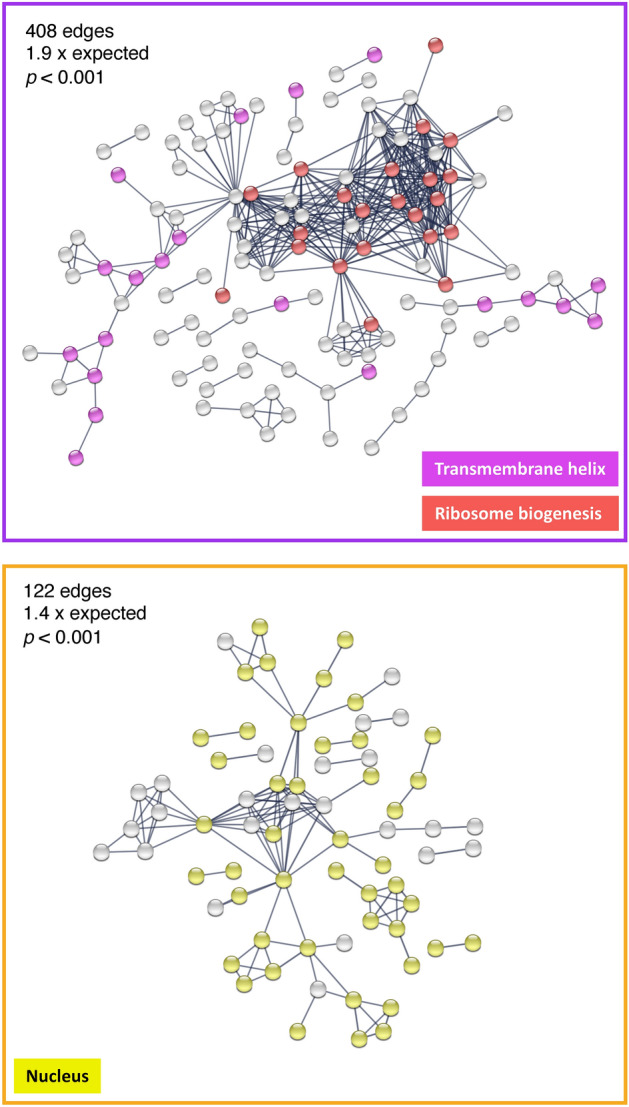
Network enrichment of differentially abundant oocyte proteins. Differentially abundant proteins were analyzed for connectivity and functional enrichment using high confidence protein interactions (confidence > 0.9; line thickness indicates strength of support for protein interactions). Proteins with increased abundance in oocytes from mated (top) and unmated (bottom) females had significantly more interactions than expected. Proteins with greater abundance in oocytes from mated females were significantly enriched for ribosome biogenesis (red) and transmembrane proteins (purple) whereas proteins with greater abundance in oocytes from unmated females were significantly enriched for associations with the nucleus (yellow).
For proteins with greater abundance in oocytes from unmated females (200 proteins), we also observed protein networks with significant interconnectivity, including 1.4 times the expected interactions among proteins (PPI enrichment adj. p < 0.001; 122 edges observed 85 expected). However, the average protein connectedness was less than half that of proteins found to be more abundant in oocytes from mated females (1.22 average for proteins greater in oocytes from unmated females vs. 2.77 average for proteins greater in mated females; Fig. 4). The network of proteins with greater abundance in oocytes from unmated females was significantly enriched for association with the nucleus (GO: 0005634, adj. p < 0.001) and chromosomes (GO:0098687, adj. p = 0.005). This network further included proteins involved in chromosome segregation, such as microtubules, spindle, kinetochore, and centriole, that could contribute to the resumption and completion of meiosis or mitosis following egg activation32,33.
Relationship between oocyte maturation and mating-induced changes in oocyte composition
The observed proteomic variation was likely to have occurred during oocyte maturation, the last stage of oogenesis (release from prophase I arrest to metaphase I arrest) when substantial changes to oocyte composition occur46. Previous characterization of maturation-associated proteome changes (i.e. between stage 11 vs. stage 14 of oocyte development) found that approximately 30% of the proteome changed in abundance33. Proteins that increased during maturation were enriched for functions related to meiotic progression, whereas proteins that decreased were enriched for translational machinery that may degrade concomitantly with nurse cells. To evaluate whether female mating status influenced oocyte maturation, we next investigated how our results correspond to the established global proteomic changes associated with maturation (Table S1).
We identified a fairly weak, albeit significant, negative correlation between protein abundance differences during maturation and protein abundance differences associated with mating status (r = − 0.25, df = 4131, p < 0.0001; Fig S2). Next, we focused specifically on the overlap between proteins exhibiting mating-dependent abundance differences to those observed to change in abundance during oocyte maturation (Fig. 5A). Among proteins that increase in abundance during maturation (i.e., greater abundance in stage 14 oocytes), we observed a larger than expected overlap with proteins that had greater abundance in oocytes from unmated females (upper-tail cumulative probability test p = 0.002). In contrast, proteins that decrease in abundance during maturation (i.e., greater abundance in stage 11 oocytes) exhibited a significantly larger than expected overlap with proteins that had greater abundance in oocytes from mated females (upper-tail cumulative probability test p < 0.001). Notably, 25% of the overlapping proteins that decreased in abundance during maturation and were more abundant in oocytes from mated females had a transmembrane domain. Thus, mating status does appear to influence oocyte maturation dynamics, particularly in relation to transmembrane proteins.
Figure 5.
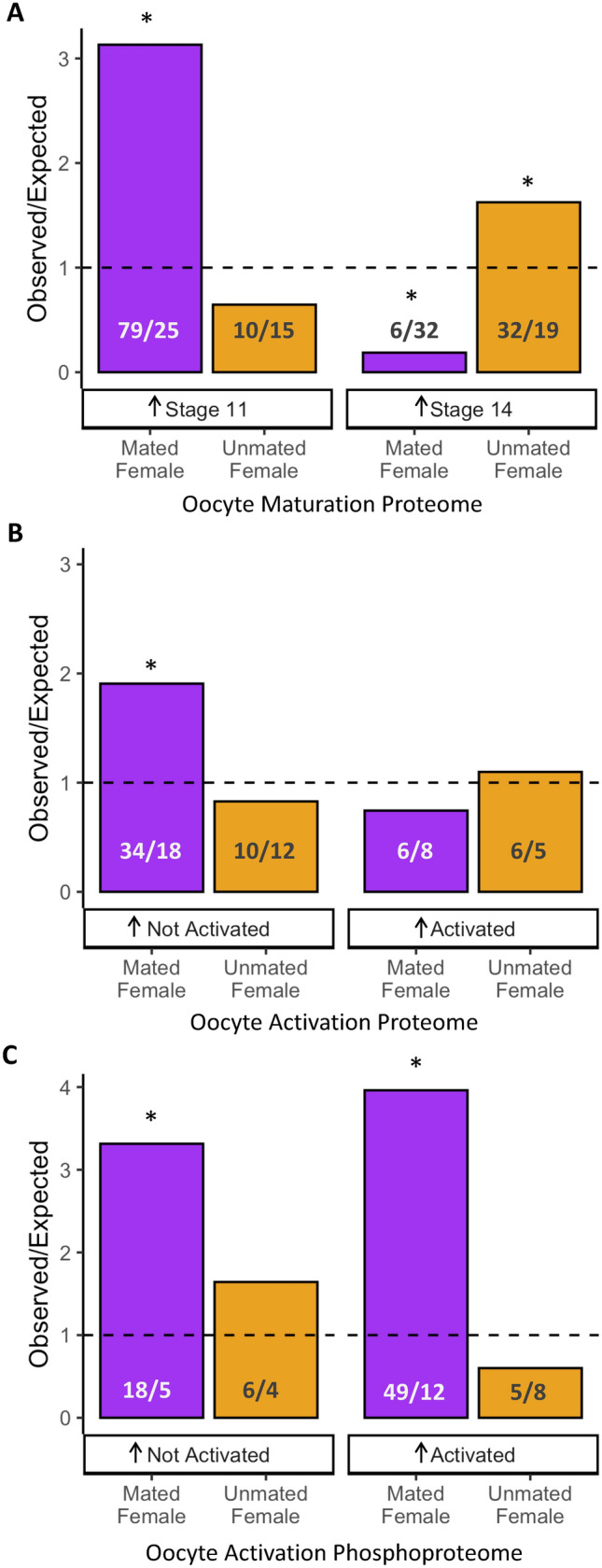
Comparison to protein changes that occur during oocyte maturation and activation. Bar plots of the observed versus expected protein overlap between protein abundances differences in oocytes from mated (purple) and unmated females (orange) with (A) proteomic changes during oocyte maturation, (B) proteomic changes during oocyte activation and (C) proteomic changes in phosphoproteins during oocyte activation. The number of observed versus expected (rounded to the nearest whole number) proteins is indicated. Significance is indicated by asterisks (*p < 0.01).
Relationship between egg activation and mating-induced changes in oocyte composition
Following maturation, the next transition for oocytes is activation, which involves the resumption of meiosis as the ovulated oocyte passes through the oviduct47. Egg activation is a transcriptionally silent process and characterized by dynamic changes in protein abundance and phosphorylation state32,34. Kronja et al. found that many proteins that increased in abundance during egg activation were important for embryonic development, such as proteins involved in chromosome organization and replication. We compared our data to proteomic changes during activation to evaluate the extent to which oocyte composition differences associated with mating status could influence post-activation dynamics (Table S1). We observed an enrichment for proteins that decreased in abundance during activation and had greater abundance in oocytes from mated females (upper-tail cumulative binomial probability test p < 0.001; Fig. 5B). Notably, the majority of these proteins (61.8%; 21 out of 34) also decreased in abundance during maturation (see above)33. Thus, proteins that normally decrease in abundance during maturation and activation appear to do so to a lesser extent in already mated females.
Finally, we compared our data to an analysis of changes in the phosphoproteome (i.e., proteins that are phosphorylated) during egg activation (Table S1)34. We found that phosphorylated proteins that changed in abundance (both increased and decreased) were more likely than expected to be of greater abundance in oocytes from mated females (increased at activation upper-tail cumulative binomial probability test p < 0.001; decreased at activation upper-tail cumulative binomial probability test p < 0.001; Fig. 5c). Thus, mating-associated changes in oocyte composition could influence downstream proteome dynamics following egg activation.
Discussion
Mating induces a myriad of postmating physiological responses in Drosophila females, including the stimulation of oocyte maturation and increased rates of oogenesis28,31,48. Our analyses suggest that, concomitant with these responses, there are functionally coherent changes in oocyte proteome composition. Although the mechanisms responsible for these differences remain to be elucidated, the stimulation of oocyte maturation may accelerate the temporal progression of oogenesis and this, in turn, may alter the dynamics of protein translation and degradation, as well as the duration of interactions with nurse and follicle cells49. Our analysis suggests that mating-induced changes influence proteome dynamics associated with maturation, including transmembrane proteins. Intriguingly, we also found that mating-induced changes also bear a significant relationship to molecular hallmarks of egg activation32,33 and may therefore have downstream effects on fertilization success and early embryogenesis. Syncytial embryonic development in Drosophila is a highly coordinated process that relies heavily on maternal contributions50. Although zygotic gene expression occurs far earlier than previously recognized51, many aspects of pre-cellular development are likely to be programmed during oogenesis and thus may be influenced by variation in oocyte protein composition. At this time we cannot predict the potential functional ramifications of this variation but we note that protein abundance variation was relatively widespread (11.1% of proteins), although only a relatively small set (20 proteins) exceeded two-fold changes in abundance.
Two hypotheses address the possible adaptive value of mating-dependent modulation of oocyte composition. First, the acceleration of oogenesis in response to mating may be energetically costly. Investment into oocytes may therefore trade off with the quantitative rate of oocyte production. Under this scenario, it may be adaptive for females to maximize their investment in oocyte number and only transition to investing in the final stages of oogenesis after mating when oocytes can be fertilized. Note that this hypothesis assumes a fixed total energy available for oogenesis. An alternative hypothesis is that mating triggers a net increase of female investment in oogenesis, thus increasing both oocyte quantity and quality. Testing these "adaptive maternal investment" hypotheses will prove challenging. Nevertheless, fitness consequences of the mating-induced changes could be examined by comparing differences in offspring development and fitness indexes (e.g., lifetime fecundity of daughters and competitive mating/fertilization success of sons) between offspring from oocytes matured in an unmated female (i.e., the first cohort of eggs laid immediately after mating) with eggs laid at later timepoints when mating-induced aspects of oogenesis are manifest. We also predict that variation in oocyte investment will be dependent on interactions with male genotype or phenotype. Female × male interactions have been shown to extensively influence postmating reproductive events in D. melanogaster52, including egg volume53. In addition, male quality may also influence oocyte investment. For example, the stimulation of egg laying by older males is reduced relative to younger males, which may correspond with age-related changes in ejaculate composition54,55.
The phenomenon we demonstrate here is also likely to differ among species, with variation arising through selection associated with oocyte production/oviposition strategies and mating system evolution. For example, there is dramatic variation in the relative and absolute size of oocytes and in the pattern of oviposition (i.e., clutching versus continuous production and oviposition) among Drosophila species56,57 and insects in general58. Further, female remating behavior can vary dramatically, ranging from multiple matings each day, to a remating latency of multiple days, to a single mating per lifetime59. Species that remate rarely might exhibit more pronounced modulation of oocyte investment to optimize reproductive potential when they have had the opportunity to mate. Alternatively, those with greater competition may have greater differential postmating responses based on male quality. A comparative evolutionary approach may be valuable to understand the potential for adaptive maternal investment in species with frequent remating where mechanisms of cryptic female choice are prominent or selection favors a balance in investment across current and future mating opportunities.
Methods
Fly maintenance and sample preparation
Wildtype D. melanogaster LHM strain was maintained in standard laboratory conditions at room temperature (~ 23 °C) with a natural light cycle. Flies were reared in glass bottles on a yeast, cornmeal, agar, and molasses media. Unmated females were collected and matured in vials of 10–15 flies with 1.5 cm3 of media supplemented with live yeast for 4 to 5 days. Males were reared separately to isotopically label all proteins60. In brief, embryos were collected and reared on an agar and sucrose media supplemented with heavy labeled (13C6 15N2 arginine and 13C6 15N4 lysine) yeast. Males were 8 to 14 days old and had mated at least once prior to this experiment. For mated samples females were mated en masse to an excess of heavy-labeled males. Mated females were kept on standard media for approximately 12 h to allow oviposition of accumulated, matured oocytes. Females were then transferred to media not conducive to oviposition (molasses and agar without yeast) for approximately 12 h to ensure the accumulation of oocytes that had matured in a postmating female.
Females were frozen at − 80o C with a drop of water and stored until dissection. After thawing, ovaries were dissected away from all remaining tissues in approximately 20 females per sample in 1 × phosphate buffered saline (PBS). Ovarioles were then opened from the base to specifically isolate unfertilized, mature (stage 14) oocytes, which were identified by long and differentiated dorsal appendages46. Oocytes, including surrounding follicle cells of the egg chamber, were rinsed through a fresh drop of PBS and collected in a 1.5 ml Eppendorf tube with PBS. Approximately 100 oocytes were collected per sample, and three replicates were collected from both unmated and mated females. Samples were washed 3 × with PBS and solubilized in ~ 50 μL detergent (1 M HEPES with 2% SDS and 5% β-mercaptoethanol) with alternating cycles of heating (95 °C) and homogenization until completely solubilized. Prepared protein samples were stored at − 80 °C.
Mass spectrometry
Protein isolation and tandem mass spectrometry (MS/MS) was conducted by Cambridge Proteomics following standard protocols, as previously described61. In brief, 30 μg of each sample was reduced (TCEP), alkylated (iodacetamide), trypsin digested, and labeled with 6-plex tandem mass tags (TMT, Thermo Scientific). Replicate oocyte samples from unmated females were labeled with 127N, 128N, 129C and those from mated females were labeled 129N, 130N, 130C. Resulting peptides were combined in equal volumes, cleaned and desalted on a Sep-Pak C18 Cartridge (Waters), and reconstituted in 0.1 mL 20 mM ammonium formate with 4% acetonitrile. Peptides were separated with high pH reverse-phase chromatography (Acquity UPLC bridged ethyl hybrid C18 column; 1.7 um particle, 2.1 mm; Waters) over 60 min (linear gradient 5–60% acetonitrile with 20 mM ammonium nitrate; flow rate 0.25 mL/min). Fractions were collected in 1 min increments dried and then resuspended in 0.1% formic acid and combined into 15 fractions.
Liquid chromatography with MS/MS was performed on a Dionex Ultimate 3000 rapid separation liquid chromatography nanoUPLC system (Thermo Scientific) coupled with a Lumos Orbitrap mass spectrometer. Peptides of each fraction were first loaded onto an Acclaim PepMap 100 C18 pre-column (5 µm particle size, 100 Å pore size, 300 µm inner diameter × 5 mm length, Thermo Scientific) with 0.1% formic acid for 3 min at 10ul/min. Eluted peptides were then separated on a reverse-phase nano EASY-spray column (PepMap C18; 2 µm particle size, 100 Å pore size, 75 µm inner diameter × 500 mm length, Thermo Scientific) for 90 min at 300nL/min in a gradient of 1.6% to 32% acetonitrile in 0.1% formic acid. Peptides from fractions were eluted from the column and sprayed (Easy-Spray Source; Thermo Fisher Scientific) into the mass spectrometer.
For each peptide ion, m/z values (MS1 scans) were measured at a resolution of 120,00 and range between 380 and 1500 Da. Data dependent MS/MS (MS2) scans (Top Speed) of the most abundant precursor ions (excluding those that were singly charged, had unassigned charge states, or were outside of the 70 s dynamic exclusion window) were isolated and fragmented by collision-induced disassociation (35% Normalized Collision Energy). From each MS2 scan the top 10 most abundant fragment ions were selected by Synchronous Precursor Selection for MS3 fragmentation by high energy collisional disassociation (65% normalized collision energy). For each fragment ion (mass range 100–500 Da) m/z values and relative abundances of reporter ions were measured in the Orbitrap analyzer (60,000 resolution).
Protein identification and differential abundance analyses
Raw data files were processed using Proteome Discoverer v 2.3 (Thermo Fisher Scientific) and Mascot v 2.6 (Matrix Science), allowing for a MS tolerance of ± 10 ppm, MS/MS tolerance of ± 0.8 Da, and up to two missed tryptic cleavages. Peptides and proteins were identified in reference to a database of the longest isoform of the D. melanogaster genome (r6.21)62 accounting for common contaminant proteins (cRAP v 1.0; thegpm.org). Standard protein modifications of carbamidomethylation (cysteine, fixed), oxidation (methionine, variable) and deamidation (glutamine and arginine, variable) were included. The isotopic labelling of male proteins ensured that they were not identified in this analysis. Protein abundance estimates for each sample was calculated as the sum of centroid TMT receptor ions (± 2 millimass unit window) corrected for isotopic label purity. Labeling efficiency of TMT reporter ions was 99.8%.
In total, 761,774 MS/MS spectra were analyzed resulting in 87,683 peptide spectral matches and 5407 proteins. Heavy labelling of males prevented the detection of male-derived proteins and all proteomic differences can therefore be attributed to females. Proteins were filtered to include only Drosophila proteins that were high confidence in all samples (FDR ≤ 0.01) and identified by at least two unique peptides, for a total of 4,485 proteins. Protein intensities were log transformed and median difference normalized in MSnbase63. Differential abundance was calculated with empirical Bayes moderated t-tests using LIMMA64 and p-values were corrected for multiple comparisons using the Benjamini–Hochberg method. All analyses, following protein identification, were conducted in R.
Statistical analysis, functional annotation, and data visualization
Pearson’s correlations between samples were visualized with complete-linkage hierarchical clustering heatmap in gplots. Sample relationships were also analyzed with a principal component analysis (PCA) using prcomp. Differential abundance plots were visualized with ggplot2. Departures from parity in the direction and magnitude of abundance changes were calculated with a weighted binomial test and Kruskal–Wallis test, respectively. The likelihood of observed overlaps between protein datasets was calculated using a cumulative weighted binomial distribution. Relationships between data sets were assessed using a Spearman’s correlation. Whether data sets had similar proportional changes was calculated with a chi-square test.
Functional enrichments were conducted with the Database for Annotation, Visualization and Integrated Discovery (DAVID) v 6.865 with the D. melanogaster genome as the background and considered significant with an adjusted Benjamini–Hochberg of p < 0.05. Protein–protein interaction (PPI) networks and functional enrichment amongst differentially abundant proteins were analyzed and visualized using highest confidence (> 0.9 interaction score) interconnected proteins as implemented by the STRING database (v11) with all oocyte proteins designated as the background66.
Supplementary Information
Acknowledgements
The authors thank Yagnesh Umrania, Renata Feret, and Mike Deery at Cambridge Centre for Proteomics for their contributions to data acquisition and analysis, and Siyuan Cong for her contribution of fruit fly drawings used in Fig. 1. We also thank Sharleen Buel, Emma Whittington and Scott Erdman for their assistance with SILAC labelling. This work was enhanced by feedback from all members of the Center for Reproductive Evolution and, in particular, analytical support and editorial comments from Yasir Ahmed-Braimah, Sharleen Buel, Martin Garlovsky, Erin McCullough and Emma Whittington. This work was supported by a National Science Foundation Graduate Research Fellowship (to C.E.M-G.), by grants from the National Science Foundation (DEB-1655840 to S.D. and S.P.) and the Eunice Shriver National Institute for Child Health and Human Development (R21-HD088910 to S.D. and S.P.) and by a generous gift by Mike and Jane Weeden to Syracuse University.
Abbreviations
- FC
Fold change
- GO
Gene ontology
- PCA
Principal components analysis
- PPI
Protein–protein interaction
Author contributions
Conceptualization/Methodology (C.E.M-G., S.P., S.D.), Investigation/Formal Analysis (C.E.M-G., S.P, S.D.), Writing (C.E.M-G., S.P., S.D.).
Data availability
Raw spectral files are available from the ProteomeXchange Consortium (PXD022142). Pre-computed protein intensities, differential abundance, and comparisons to existing data sets are available in Table S1. GO functional enrichments and network analyses are available in Table S2. Analysis code is available at github.com/CEMcDonoughGoldstein/OocyteProteome_FemaleMatingStatus.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Caitlin E. McDonough-Goldstein, Email: mcdonouce@gmail.com
Steve Dorus, Email: sdorus@syr.edu.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-82801-4.
References
- 1.Thornhill R. Sexual selection and paternal investment in insects. Am. Nat. 1976;110:153–163. doi: 10.1086/283055. [DOI] [Google Scholar]
- 2.Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the Decent of Man, 1871–1971. Venice: Aldine; 1972. pp. 136–179. [Google Scholar]
- 3.Armstrong AR. Drosophila melanogaster as a model for nutrient regulation of ovarian function. Reproduction. 2020;159:R69–R82. doi: 10.1530/REP-18-0593. [DOI] [PubMed] [Google Scholar]
- 4.Chapman T, Partridge L. Female fitness in Drosophila melanogaster: An interaction between the effect of nutrition and of encounter rate with males. Proc. R. Soc. Lond. B. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- 5.Wheeler D. The role of nourishment in oogenesis. Annu. Rev. Entomol. 1996;41:407–431. doi: 10.1146/annurev.en.41.010196.002203. [DOI] [PubMed] [Google Scholar]
- 6.Boggs CL. Male nuptial fifts: Phenotypic consequences and evolutionary implications. In: Leather SR, Hardie J, editors. Insect Reproduction. Boca Raton: CRC Press; 2018. pp. 215–242. [Google Scholar]
- 7.Lewis S, South A. The evolution of animal nuptial gifts. Adv. Study Behav. 2012;44:53–97. doi: 10.1016/B978-0-12-394288-3.00002-2. [DOI] [Google Scholar]
- 8.Engqvist L. Nuptial gift consumption influences female remating in a scorpionfly: Male or female control of mating rate? Evol. Ecol. 2007;21:49–61. doi: 10.1007/s10682-006-9123-y. [DOI] [Google Scholar]
- 9.Gwynne DT. Courtship feeding increases female reproductive success in bushcrickets. Nature. 1984;307:361–363. doi: 10.1038/307361a0. [DOI] [Google Scholar]
- 10.Karlsson B. Nuptial gifts, resource budgets, and reproductive output in a polyandrous butterfly. Ecology. 1998;79:2931–2940. doi: 10.1890/0012-9658(1998)079[2931:NGRBAR]2.0.CO;2. [DOI] [Google Scholar]
- 11.Rönn JL, Katvala M, Arnqvist G. Interspecific variation in ejaculate allocation and associated effects on female fitness in seed beetles. J. Evol. Biol. 2008;21:461–470. doi: 10.1111/j.1420-9101.2007.01493.x. [DOI] [PubMed] [Google Scholar]
- 12.Simmons LW. Nuptial feeding in tettigoniids male costs and the rates of fecundity increase. Behav. Ecol. Sociobiol. 1990;27:43–47. doi: 10.1007/BF00183312. [DOI] [Google Scholar]
- 13.Steele RH. Courtship feeding in Drosophila subobscura: The nutritional significance of courtship feeding. Anim. Behav. 1986;34:1087–1098. doi: 10.1016/S0003-3472(86)80168-3. [DOI] [Google Scholar]
- 14.Gwynne DT. Courtship feeding and the Fitness of female katydids (Orthoptera: Tettigoniidae) Evolution. 1988;42:545–555. doi: 10.1111/j.1558-5646.1988.tb04159.x. [DOI] [PubMed] [Google Scholar]
- 15.Reinhold K. Paternal investment in Poecilimon veluchianus bushcrickets: Beneficial effects of nuptial feeding on offspring viability. Behav. Ecol. Sociobiol. 1999;45:293–299. doi: 10.1007/s002650050564. [DOI] [Google Scholar]
- 16.Markow TA, Ankney PF. Insemination reaction in Drosophila: Found in species whose males contribute material to oocytes before fertilization. Evolution. 1988;42:1097–1101. doi: 10.1111/j.1558-5646.1988.tb02529.x. [DOI] [PubMed] [Google Scholar]
- 17.Pitnick S, Spicer GS, Markow T. Phylogenetic examination of female incorporation of ejaculates in Drosophila. Evolution. 1997;51:833–845. doi: 10.1111/j.1558-5646.1997.tb03665.x. [DOI] [PubMed] [Google Scholar]
- 18.Boggs CL, Gilbert LE. Male Contribution to egg production in butterflies: Evidence for transfer of nutrients at mating. Science. 1979;206:83–84. doi: 10.1126/science.206.4414.83. [DOI] [PubMed] [Google Scholar]
- 19.Greenfield MD. The question of paternal investment in Lepidoptera: male-contributed proteins in Plodia interpunctella. Int. J. Invert. Reprod. 1982;5:323–330. doi: 10.1080/01651269.1982.10553485. [DOI] [Google Scholar]
- 20.Koshiyama Y, Tsumuki H, Fujisaki K, Nakasuji F. Nutritional contribution to females of 14C-labeled male secretions transferred during mating in Menida scotti (Heteroptera: Pentatomidae) Res. Popul. Ecol. 1996;38:51–56. doi: 10.1007/BF02514970. [DOI] [Google Scholar]
- 21.Mullins DE, Keil CB. Paternal investment of urates in cockroaches. Nature. 1980;283:567–569. doi: 10.1038/283567a0. [DOI] [Google Scholar]
- 22.Sirot LK, Lapointe SL, Shatters R, Bausher M. Transfer and fate of seminal fluid molecules in the beetle, Diaprepes abbreviatus: Implications for the reproductive biology of a pest species. J. Insect Physiol. 2006;52:300–308. doi: 10.1016/j.jinsphys.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Rooney J, Lewis SM. Differential allocation of male-derived nutrients in two lampyrid beetles with contrasting life-history characteristics. Behav. Ecol. 1999;10:97–104. doi: 10.1093/beheco/10.1.97. [DOI] [Google Scholar]
- 24.Friedel T, Gillott C. Contribution of male-produced proteins to vitellogenesis in Melanoplus sanguinipes. J. Insect Physiol. 1977;23:145–151. doi: 10.1016/0022-1910(77)90120-2. [DOI] [PubMed] [Google Scholar]
- 25.Markow TA, Coppola A, Watts TD. How Drosophila males make eggs: it is elemental. Proc. R. Soc. Lond. B. 2001;268:1527–1532. doi: 10.1098/rspb.2001.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: Identification and function. Annu. Rev. Entomol. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillott C. Chemoecology of Insect Eggs and Egg Deposition. New York: Springer; 2003. Insect accessory reproductive glands: Key players in production and protection of eggs; pp. 37–59. [Google Scholar]
- 28.Soller M, Bownes M, Kubli E. Mating and sex peptide stimulate the accumulation of yolk in oocytes of Drosophila Melanogaster. Eur. J. Biochem. 1997;243:732–738. doi: 10.1111/j.1432-1033.1997.00732.x. [DOI] [PubMed] [Google Scholar]
- 29.Markow TA, Ankney PF. Drosophila males contribute to oogenesis in a multiple mating species. Science. 1984;224:302–303. doi: 10.1126/science.224.4646.302. [DOI] [PubMed] [Google Scholar]
- 30.Pitnick S, Miller GT, Schneider K, Markow TA. Ejaculate-female coevolution in Drosophila mojavensis. Proc. R. Soc. Lond. B. 2003;270:1507–1512. doi: 10.1098/rspb.2003.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soller M, Bownes M, Kubli E. Control of oocyte maturation in sexually mature Drosophila females. Dev. Biol. 1999;208:337–351. doi: 10.1006/dbio.1999.9210. [DOI] [PubMed] [Google Scholar]
- 32.Kronja I, et al. Widespread changes in the posttranscriptional landscape at the Drosophila oocyte-to-embryo transition. Cell Rep. 2014;7:1495–1508. doi: 10.1016/j.celrep.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kronja I, et al. Quantitative proteomics reveals the dynamics of protein changes during Drosophila oocyte maturation and the oocyte-to-embryo transition. Proc. Natl. Acad. Sci. USA. 2014;111:16023–16028. doi: 10.1073/pnas.1418657111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Ahmed-Braimah YH, Goldberg ML, Wolfner MF. Calcineurin-dependent protein phosphorylation changes during Eeg activation in Drosophila melanogaster. Mol. Cell Proteom. 2019;18:S145–S158. doi: 10.1074/mcp.RA118.001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bownes M, Hames BD. Accumulation and degradation of three major yolk proteins in Drosophila melanogaster. J. Exp. Zool. 1977;200:149–156. doi: 10.1002/jez.1402000118. [DOI] [PubMed] [Google Scholar]
- 36.Lynn Zimmerman J, Petri W, Meselson M. Accumulation of a specific subset of D. melanogaster heat shock mRNAs in normal development without heat shock. Cell. 1983;32:1161–1170. doi: 10.1016/0092-8674(83)90299-4. [DOI] [PubMed] [Google Scholar]
- 37.Golumbeski GS, Bardsley A, Tax F, Boswell RE. Tudor, a posterior-group gene of Drosophila melanogaster, encodes a novel protein and an mRNA localized during mid-oogenesis. Genes Dev. 1991;5:2060–2070. doi: 10.1101/gad.5.11.2060. [DOI] [PubMed] [Google Scholar]
- 38.Johnstone O, et al. Belle is a Drosophila DEAD-box protein required for viability and in the germ line. Dev. Biol. 2005;277:92–101. doi: 10.1016/j.ydbio.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 39.Riparbelli MG, Massarelli C, Robbins LG, Callaini G. The abnormal spindle protein is required for germ cell mitosis and oocyte differentiation during Drosophila oogenesis. Exp. Cell Res. 2004;298:96–106. doi: 10.1016/j.yexcr.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 40.Styhler S, Nakamura A, Swan A, Suter B, Lasko P. Vasa is required for GURKEN accumulation in the oocyte, and is involved in oocyte differentiation and germline cyst development. Development. 1998;125:1569–1578. doi: 10.1242/dev.125.9.1569. [DOI] [PubMed] [Google Scholar]
- 41.Greenblatt EJ, Obniski R, Mical C, Spradling AC. Prolonged ovarian storage of mature Drosophila oocytes dramatically increases meiotic spindle instability. eLife. 2019;8:e49455. doi: 10.7554/eLife.49455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mermod J-J, Jacobs-Lorena M, Crippa M. Changes in rate of RNA synthesis and ribosomal gene number during oogenesis of Drosophila melanogaster. Dev. Biol. 1977;57:393–402. doi: 10.1016/0012-1606(77)90224-X. [DOI] [PubMed] [Google Scholar]
- 43.Qian S, Hongo S, Jacobs-Lorena M. Antisense ribosomal protein gene expression specifically disrupts oogenesis in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 1988;85:9601–9605. doi: 10.1073/pnas.85.24.9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Avilés-Pagán EE, Orr-Weaver TL. Activating embryonic development in Drosophila. Semin. Cell Dev. Biol. 2018;84:100–110. doi: 10.1016/j.semcdb.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaneuchi T, et al. Calcium waves occur as Drosophila oocytes activate. Proc. Natl. Acad. Sci. USA. 2015;112:791–796. doi: 10.1073/pnas.1420589112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cummings MR, King RC. The cytology of the vitellogenic stages of oogenesis in Drosophila melanogaster. I. General staging characteristics. J. Morphol. 1969;128:427–441. doi: 10.1002/jmor.1051280404. [DOI] [Google Scholar]
- 47.Heifetz Y, Yu J, Wolfner MF. Ovulation triggers activation of Drosophila oocytes. Dev. Biol. 2001;234:416–424. doi: 10.1006/dbio.2001.0246. [DOI] [PubMed] [Google Scholar]
- 48.Heifetz Y, Tram U, Wolfner MF. Male contributions to egg production: the role of accessory gland products and sperm in Drosophila melanogaster. Proc. R. Soc. Lond. B. 2001;268:175–180. doi: 10.1098/rspb.2000.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antel M, Inaba M. Modulation of cell–cell interactions in Drosophila oocyte development. Cells. 2020;9:274. doi: 10.3390/cells9020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schüpbach T, Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster I. Maternal effect mutations. Genetics. 1989;121:101–117. doi: 10.1093/genetics/121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ali-Murthy Z, Lott SE, Eisen MB, Kornberg TB. An essential role for zygotic expression in the pre-cellular Drosophila embryo. PLoS Genet. 2013;9:e1003428. doi: 10.1371/journal.pgen.1003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lupold, S. et al. How female x male and male x male interactions influence competitive fertilization in Drosophila melanogaster. Evol. Lett. (In press). [DOI] [PMC free article] [PubMed]
- 53.Pischedda A, Stewart AD, Little MK, Rice WR. Male genotype influences female reproductive investment in Drosophila melanogaster. Proc. R. Soc. B. 2011;278:2165–2172. doi: 10.1098/rspb.2010.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruhmann H, Koppik M, Wolfner MF, Fricke C. The impact of ageing on male reproductive success in Drosophila melanogaster. Exp. Gerontol. 2018;103:1–10. doi: 10.1016/j.exger.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sepil I, et al. Male reproductive aging arises via multifaceted mating-dependent sperm and seminal proteome declines, but is postponable in Drosophila. Proc. Natl. Acad. Sci. USA. 2020;117:17094–17103. doi: 10.1073/pnas.2009053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Markow TA, Beall S, Matzkin LM. Egg size, embryonic development time and ovoviviparity in Drosophila species: Ovoviviparity in Drosophila species. J. Evol. Biol. 2009;22:430–434. doi: 10.1111/j.1420-9101.2008.01649.x. [DOI] [PubMed] [Google Scholar]
- 57.Starmer WT, et al. Phylogenetic, geographic, and temporal analysis of female reproductive trade-offs in Drosophila. Evol. Biol. 2003;33:138–171. [Google Scholar]
- 58.Church SH, Donoughe S, de Medeiros BAS, Extavour CG. Insect egg size and shape evolve with ecology, not developmental rate. bioRxiv. 2018;2018:471946. doi: 10.1038/s41586-019-1302-4. [DOI] [PubMed] [Google Scholar]
- 59.Markow TA, O’Grady PM. Evolutionary genetics of reproductive behavior in Drosophila: Connecting the dots. Annu. Rev. Genet. 2005;39:263–291. doi: 10.1146/annurev.genet.39.073003.112454. [DOI] [PubMed] [Google Scholar]
- 60.Krijgsveld J, et al. Metabolic labeling of C. elegans and D. melanogaster for quantitative proteomics. Nat Biotechnol. 2003;21:927–931. doi: 10.1038/nbt848. [DOI] [PubMed] [Google Scholar]
- 61.McCullough EL, McDonough CE, Pitnick S, Dorus S. Quantitative proteomics reveals rapid divergence in the postmating response of female reproductive tracts among sibling species. Proc. R Soc. B. 2020;287:1030. doi: 10.1098/rspb.2020.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thurmond J, et al. FlyBase 2.0: the next generation. Nucleic Acids Res. 2019;47:D759–D765. doi: 10.1093/nar/gky1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gatto L, Lilley KS. MSnbase-an R/Bioconductor package for isobaric tagged mass spectrometry data visualization, processing and quantitation. Bioinformatics. 2012;28:288–289. doi: 10.1093/bioinformatics/btr645. [DOI] [PubMed] [Google Scholar]
- 64.Smyth GK. limma: Linear models for microarray data. In: Gentleman R, Carey VJ, Huber W, Irizarry RA, Dudoit S, editors. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Berlin: Springer-Verlag; 2005. pp. 397–420. [Google Scholar]
- 65.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szklarczyk D, et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw spectral files are available from the ProteomeXchange Consortium (PXD022142). Pre-computed protein intensities, differential abundance, and comparisons to existing data sets are available in Table S1. GO functional enrichments and network analyses are available in Table S2. Analysis code is available at github.com/CEMcDonoughGoldstein/OocyteProteome_FemaleMatingStatus.