Abstract
Background
There are few studies that directly compare the effects of osimertinib on patients with non‐small cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) T790M mutation between the generation of prior EGFR tyrosine kinase inhibitors (TKIs).
Methods
We retrospectively reviewed clinical data from the medical records of consecutive patients with advanced NSCLC who had developed resistance to first‐ or second‐generation EGFR‐TKIs due to EGFR T790M mutation and were subsequently treated with osimertinib at Juntendo University Hospital. In patients with available tumor samples, target amplicon sequencing analyses were performed to explore the genetic biomarkers.
Results
A total of 38 patients with NSCLC harboring EGFR T790M mutation were treated with osimertinib. Eight patients were classified into group A (afatinib followed by osimertinib) and 30 patients were classified into group B (first‐generation EGFR‐TKI followed by osimertinib). Progression‐free survival (PFS) was significantly longer in group A than in group B (median PFS; not reached vs. 11.0 months, P = 0.018). Fourteen patients had available tissue samples collected before osimertinib treatment for target sequencing. In group A we found no additional mutations, other than EGFR T790M mutation. On the other hand, there were three samples in which other mutations emerged, in addition to EGFR T790M mutation, in group B.
Conclusions
PFS of osimertinib was significantly longer in patients with NSCLC harboring EGFR T790M mutation after treatment with afatinib than in patients after treatment with first generation EGFR‐TKIs. Additional mutations other than EGFR T790M may affect the efficacy of osimertinib treatment.
Key points
Significant findings of the study:
PFS of osimertinib was significantly longer in patients with NSCLC harboring EGFR T790M mutation after treatment with afatinib than in patients after treatment with first generation EGFR‐TKIs.
What this study adds:
Additional mutations other than EGFR T790M may affect the efficacy of osimertinib treatment.
Keywords: EGFR mutation, EGFR‐TKI, non‐small cell lung cancer
There are few studies that directly compare the difference between the generation of prior EGFR tyrosine kinase inhibitors (TKIs) before osimertinib treatment in patients with non‐small cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) T790M mutation. In our retrospective study, progression‐free survival of osimertinib was significantly longer in patients with NSCLC harboring EGFR T790M mutation after treatment with afatinib than in patients after treatment with first generation EGFR‐TKIs.

Introduction
Lung cancer is a neoplasm mostly associated with a dismal prognosis. In particular, advanced non‐small cell lung cancer (NSCLC) is considered as a chemoresistant neoplasm, and the survival of the patients is known to be extremely short, even after systemic chemotherapy. In recent years, the treatments using epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) have improved the prognosis of patients with advanced NSCLC harboring EGFR activating mutations, compared with conventional platinum‐doublet chemotherapy. Five EGFR‐TKIs are currently approved for first‐line treatment of EGFR mutation positive NSCLC in Japan, based on positive phase III trials 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 : first‐generation TKIs, erlotinib and gefitinib; second‐generation TKIs, afatinib and dacomitinib; andthird‐generation TKI, osimertinib. Afatinib and dacomitinib are oral second‐generation EGFR‐TKIs that are irreversible, Erb B1 (EGFR), Erb B2 (HER2), and Erb B4 (HER4) blockers. 5 , 6 , 7 Osimertinib is an oral third‐generation EGFR‐TKI that selectively inhibits both EGFR‐activating and T790M‐resistance mutations. 8 , 9
Regardless of initial tumor shrinkage by EGFR‐TKIs, most cancers progress after 10–18 months. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 Various mechanisms of resistance to first‐ and second‐generation EGFR‐TKIs have been reported. Notably, secondary EGFR T790M mutation occurs in about 50% of the patients with EGFR‐mutated NSCLC that present EGFR‐TKIs resistance. 10 , 11 , 12 In a phase 3 trial (AURA 3), osimertinib improved the progression‐free survival (PFS) (median PFS; 10.1 vs. 4.4 months) versus platinum‐doublet chemotherapy in patients with EGFR‐TKI‐resistant NSCLC harboring EGFR T790M mutation. 9 Based on this result, osimertinib monotherapy has been approved as the standard treatment for these patients. Recently, several mechanisms of resistance to osimertinib have been reported. 13 , 14 However, the standard treatment in patients with osimertinib‐resistance NSCLC has not yet been established.
In the first‐line treatment of patients with EGFR‐mutated NSCLC, osimertinib showed the longest PFS (median 18.9 months) 8 in comparison to first‐ or second‐generation EGFR‐TKIs. 1 , 2 , 3 , 4 , 5 , 6 , 7 Based on these results, osimertinib was selected as a first choice EGFR‐TKI in first‐line setting. However, if patients treated with another EGFR‐TKI could acquire EGFR T790M mutation and receive the subsequent treatment using osimertinib, the total efficacy would be expected to exceed the outcome of osimertinib monotherapy as a first‐line treatment. 15 In the survival data of Asian patients from the FLAURA trial, osimertinib showed a better prognosis than the first‐generation EGFR‐TKIs in the early days of treatment, but the long‐term survival rate was better with the first‐generation EGFR‐TKIs. 16 These results indicate that front‐line osimertinib treatment may not be the best option for some patients.
Recently, several reports have supported the benefit of sequential treatment with afatinib followed by osimertinib. In an observational study (GioTag study), the median period of sequential treatment with afatinib followed by osimertinib was reported to be 27.6 months. 17 Additionally, in a retrospective analysis of patients treated in LUX‐Lung 3, LUX‐Lung 6, and LUX‐Lung 7, the median duration of treatment with osimertinib was 20.2 months in patients with EGFR T790M mutated‐NSCLC who had previously received afatinib. 18 This duration is considerably longer than PFS of osimertinib in the AURA 3 trial. 9 We speculated that the efficacy of osimertinib in patients with EGFR T790M mutated‐NSCLC might depend on the generation of prior EGFR‐TKIs. However, there are few studies to directly compare these differences between the generation of prior EGFR‐TKIs before osimertinib. In addition, the mechanism how upfront EGFR‐TKI treatment affects the efficacy of osimertinib is still unclear.
Based on these backgrounds, we retrospectively assessed the impact of the prior first‐ or second‐ generation EGFR‐TKIs on the efficacy of the subsequent osimertinib treatment. Furthermore, we explored the mechanism of this difference by using tissue samples from patients with EGFR T790M positive NSCLC who were treated with osimertinib.
Methods
Patients
We retrospectively reviewed clinical data from the medical records of consecutive patients with advanced NSCLC harboring EGFR T790M mutations who were treated with osimertinib after acquiring resistance to first‐ or second‐generation EGFR‐TKIs of any lung cancer cell lines at Juntendo University Hospital, between April 2016 and September 2018. The patients with EGFR uncommon mutations were excluded from this study. Osimertinib was administered at 80 mg/day until disease progression or unacceptable toxicity. Treatment change was based on the physician's decision. Additionally, the tissue samples of the patients who gave written informed consent were analyzed using next generation sequencing. (NGS) This study protocol was approved by the Institutional Review Board of Juntendo University Graduate School of Medicine, under number 2017134.
Evaluation of patient characteristics
All pretreatment and treatment parameters were compared between the following two groups: patients pretreated with afatinib (group A) and patients pretreated with first‐generation EGFR‐TKI (group B) before osimertinib treatment. The patients pretreated with both first‐generation EGFR‐TKI and afatinib were included in group A. All patients underwent systemic evaluation and standardized staging procedures before starting the systemic treatment. Clinical stage was assigned based on the results of physical examination, chest radiography, computed tomography scans of the chest and abdomen, computed tomography or magnetic resonance imaging of the brain, and bone scintigraphy or positron emission tomography. Performance status (PS) was evaluated based on the Eastern Cooperative Oncology Group (ECOG) PS scale. EGFR mutations were examined by clinical laboratories (Scorpion‐Amplification Refractory Mutation System methods 19 or cobas EGFR Mutation Test v2). 20
Evaluation of efficacy
The response to osimertinib treatment was evaluated according to the guidelines of the Response Evaluation Criteria in Solid Tumors version 1.1. 21 After the start of osimertinib treatment, chest radiography was performed at monthly intervals. Computed tomography of the chest and abdomen was also performed every 2–3 months. When patients had been treated with osimertinib longer than one year, the frequency of radiological examinations was suitably adjusted according to the physician's judgment. If disease progression was suspected using chest radiography, additional computed tomography was performed as necessary. When clinical signs and symptoms suspicious for brain and bone involvement were present, magnetic resonance imaging of the brain and positron emission tomography were performed based on the physician's decision. PFS was defined as the period between the start of osimertinib treatment and progressive disease or death from any cause. Treatment duration was defined as the period between the start of osimertinib treatment and the discontinuation of osimertinib from any cause.
Next generation sequencing (NGS) analysis
In patients with available tissue samples, genetic analysis was performed on samples prior to initiation of systemic treatment and to administration of osimertinib after resistance to the first‐generation EGFR‐TKIs or afatinib.
Genomic DNA was extracted from formalin fixed paraffin embedded (FFPE) tissues using GeneRead DNA FFPE kit, according to the manufacturer's instructions (Qiagen). Purified genomic DNA obtained from tumor tissues was used to make a library for multiplexed paired‐end sequencing using QIAseq Targeted DNA Panel (Actionable Solid Tumor Panel, Qiagen) and QIAseq 96‐Index A I. Next generation sequencing (NGS) was performed on an Illumina NextSeq 550 platform with a NextSeq 500/550 Mid Output Kit v2 kit (300 cycles).
The genes presented in Table 1 were analyzed in this study. For quality control, samples with a mean read depth of coverage over 90 000 and a base quality score of 20, which accounted for 90% of the total reads, were selected. Data analysis, including adapter trimming, alignment to the reference genome, UMI clustering, and variant calling were performed using smCounter2 of QIAGEN GeneGlobe Data Analysis Center (NGS module). 22 Annotations of called variants were based on dbSNP150, CCDS (NCBI, Release 15), RefSeq (UCSC Genome Browser, Feb 2018), Gencode (UCSC Genome Browser, ver. 19), and 1000Genomes (phase3 release v5). In addition, somatic mutations were selected using the following criteria: (i) the variant allele frequency was higher than 1%, (ii) the mutations were registered as “pathogenic/likely pathogenic variants” in ClinVar, 23 or “pathogenic/likely pathogenic variants” in dbSNP dataset. 24
Table 1.
Genetic variants that could be analyzed in the QIAseq targeted DNA panel
| Exons | BRAF, PDGFRA, EGFR (ERBB1), KRAS, NRAS, KIT(CD117) |
| Hotspots | AKT1, ALK, CTNNB1, ERBB3, ESR1(Era), FOXL2, GNA11, GNAQ, IDH1, IDH2, MET, RAF1, RET |
| Whole coding region | ERBB2(HER‐2, NEU), PIK3CA(p110‐alpha), TP53(p53) |
Statistical analysis
The chi‐squared and Mann‐Whitney U tests were used to evaluate differences in categorical and continuous variables between the two groups, respectively. PFS was evaluated using the Kaplan‐Meier method and compared using the log‐rank test. Cox proportional hazards models were used to adjust for potential confounding factors. A P‐value <0.05 was considered statistically significant. All analyses were performed using JMP 10 for Windows statistical software (SAS Institute Japan Inc., Tokyo, Japan).
Results
Patient characteristics
A total of 38 patients with NSCLC harboring EGFR T790M mutation were included in this retrospective study. Eight patients were classified into group A (afatinib followed by osimertinib) and 30 patients were classified into group B (first‐generation EGFR‐TKI, followed by osimertinib). The characteristics of patients at the start of osimertinib treatment were stratified by the groups are summarized in Table 2. The distribution of age, sex, PS, smoking status, histology, presence of brain metastases, status of EGFR activating mutation, total treatment duration with EGFR‐TKIs before osimertinib administration, and the period between the end of pretreatment EGFR‐TKI and osimertinib administration were similar between the two groups. Almost all patients in group A were treated with osimertinib directly after acquiring resistance with EGFR T790M to afatinib. However, one patient in this group received gefitinib treatment between afatinib and osimertinib. There was a significant difference in the number of prior treatments between the two groups. Six patients (75%) in group A were treated with osimertinib as a late‐line treatment.
Table 2.
Patient characteristics at the start of osimertinib treatment
| Group A (n = 8) | Group B (n = 30) | ||
|---|---|---|---|
| n (%) | n (%) | P‐value | |
| Sex | 0.767 | ||
| Male | 3 (38) | 13 (43) | |
| Female | 5 (62) | 17 (57) | |
| Age | |||
| Median (range) | 66.5 (47–78) | 69.5 (39–83) | 0.622 |
| Performance status | 0.275 | ||
| 0–1 | 8 (100) | 26 (87) | |
| 2 | 0 | 4 (13) | |
| Smoking status | 0.767 | ||
| Never | 5 (62) | 17 (57) | |
| Previous/current | 3 (38) | 13 (43) | |
| Histology | |||
| Adenocarcinoma | 8 (100) | 30 (100) | |
| Brain metastasis | 0.611 | ||
| Present | 4 (50) | 12 (40) | |
| Absent | 4 (50) | 18 (60) | |
| Status of EGFR activating mutation | 0.898 | ||
| Exon 19 deletion | 5 (62) | 18 (60) | |
| L858R | 3 (38) | 12 (40) | |
| Specimen to be measured T790M | 0.129 | ||
| Tissue | 6 (75) | 17 (57) | |
| Cytology | 0 | 10 (33) | |
| Plasma | 2 (25) | 3 (10) | |
| Last EGFR‐TKI before osimertinib treatment | < 0.001 | ||
| Afatinib | 7 (88) | 0 | |
| Gefitinib | 1 (12) | 12 (40) | |
| Erlotinib | 0 | 18 (60) | |
| Number of prior regimens | 0.034 | ||
| 1–2 | 2 (25) | 20 (67) | |
| 3– | 6 (75) | 10 (33) | |
| Total treatment duration with EGFR‐TKIs before osimertinib administration | 0.737 | ||
| Median, months (95% confidence interval) | 24.9 (13.2–40.6) | 19.3 (15.7–34.1) | |
| Period between end of pretreatment EGFR‐TKI and osimertinib administration | 0.351 | ||
| Median, days (95% confidence interval) | 1 (1–20) | 1 (1–8) | |
Group A; pretreated with afatinib before osimertinib treatment, Group B; pretreated with first‐generation EGFR‐TKI before osimertinib treatment.
Efficacy of osimertinib
The median follow‐up period from the start of osimertinib treatment was 16.1 months. The response rate of osimertinib treatment in group A was relatively higher than that in group B (88 vs. 50%, P = 0.056) (Table 3), though there was no significant difference. PFS was significantly longer in group A than in group B (median PFS; not reached [95% confidence interval, 7.5 ‐ not reached] vs. 11.0 [95% confidence interval, 5.8–14.9] months, P = 0.018) (Fig 1). There was no significant difference in treatment duration of osimertinib between group A and B (median duration; not reached [95% confidence interval, 7.4 ‐ not reached] vs. 15.5 [95% confidence interval, 11.4 ‐ not reached] months, P = 0.073) (Fig 2). The result of univariate analyses for PFS of osimertinib treatment is shown in Table 4. In this analysis, age, smoking history, status of EGFR activating mutation, number of prior treatments, and brain metastases were not significantly associated with osimertinib treatment PFS. Previous treatments with afatinib (hazard ratio, 0.203; 95% confidence interval, 0.032–0.702; P = 0.009) and PS 0–1 (hazard ratio, 0.247; 95% confidence interval, 0.082–0.906; P = 0.037) were significant favorable prognostic factors in the univariate analysis of PFS.
Table 3.
Osimertinib objective response rate
| Group A (n = 8) | Group B (n = 30) | P‐value | |
|---|---|---|---|
| CR | 2 | 1 | |
| PR | 5 | 14 | |
| SD | 1 | 13 | |
| PD | 0 | 2 | |
| Response rate (CR + PR) | 88% | 50% | 0.056 |
| Disease control rate (CR + PR + SD) | 100% | 93% | 0.453 |
Group A; pretreated with afatinib before osimertinib treatment, Group B; pretreated with first‐generation EGFR‐TKI before osimertinib treatment.
CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 1.
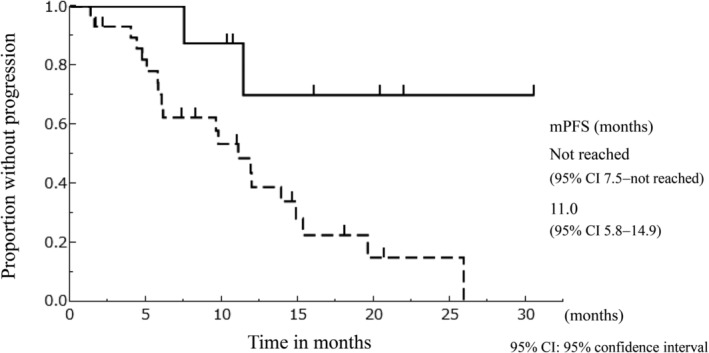
The Kaplan‐Meier curve of progression‐free survival of osimertinib stratified according to the type of EGFR‐TKIs used for the treatment before osimertinib. ( ) Group A; (
) Group A; ( ) Group B
) Group B
Figure 2.
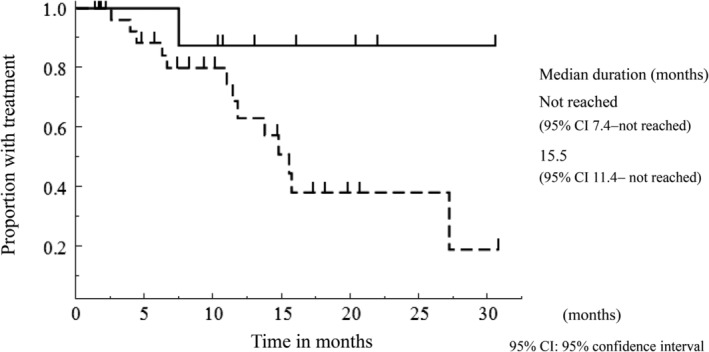
The Kaplan‐Meier curve of treatment duration of osimertinib stratified according to the type of EGFR‐TKIs used for the treatment before osimertinib. ( ) Group A; (
) Group A; ( ) Group B
) Group B
Table 4.
Univariate analysis for progression‐free survival (PFS) following osimertinib treatment
| n | mPFS, months (95% CI) | Hazard rate (95% CI) | P‐value | |
|---|---|---|---|---|
| Age | 0.24 | |||
| ≥75 | 10 | 11.9 (1.6–15.3) | 1 | |
| <75 | 28 | 11.9 (6.1–25.9) | 0.562 (0.226–1.512) | |
| Smoking history | 0.354 | |||
| Never | 22 | 11.4 (5.8–19.6) | 1 | |
| Previous/current | 16 | 15.3 (9.6–NR) | 0.655 (0.247–1.585) | |
| Performance status | 0.037 | |||
| 2 | 4 | 7.7 (1.6–11.0) | 1 | |
| 0–1 | 34 | 13.9 (9.7–25.9) | 0.247 (0.082–0.906) | |
| EGFR activating mutation | 0.055 | |||
| L858R | 15 | 9.6 (5.8–11.9) | 1 | |
| exon 19 del | 23 | 19.6 (9.7–NR) | 0.418 (0.170–1.018) | |
| Treatment line of osimertinib | 0.297 | |||
| Second or third | 22 | 11.4 (5.7–25.9) | 1 | |
| Fourth or later | 16 | 14.9 (9.6–NR) | 0.636 (0.261–1.485) | |
| Brain metastasis | 0.742 | |||
| Present | 16 | 14.9 (5.7–19.6) | 1 | |
| Absent | 22 | 11.9 (9.6–25.9) | 0.867 (0.371–2.070) | |
| Previous treatment with afatinib | 0.009 | |||
| No | 30 | 11.0 (5.8–14.9) | 1 | |
| Yes | 8 | NR (7.5–NR) | 0.203 (0.032–0.702) | |
CI, confidence interval; NR, not reached.
Next generation sequencing (NGS) analysis
Fourteen patients (five patients in group A, nine in group B) had available tissue samples collected before osimertinib treatment for NGS analysis. Thirteen patients had both samples at diagnosis and at the time of acquired resistance with EGFR T790M mutation by treatment using afatinib or first‐generation EGFR‐TKI. However, one patient in group A had a tissue sample at acquired EGFR T790M mutation by using afatinib only. The results of NGS analysis using these tissue samples are summarized in Table 5. In group A, all four samples before afatinib treatment showed coexistence of gene mutations, other than EGFR. In group B, four of nine samples before first‐generation EGFR‐TKIs administration showed co‐mutations, other than EGFR mutation, and one sample showed an EGFR compound mutation. After treatment with afatinib or first‐generation EGFR‐TKIs, EGFR T790M mutation was found in all samples tested. In group A, the only newly emerged gene mutation after treatment was EGFR T790M mutation. On the other hand, three samples acquired other mutations, in addition to EGFR T790M mutation, after treatment with first‐generation EGFR‐TKIs in group B. Patient 8 had simultaneously acquired CTNNB1 D32N and PIK3CA E542K mutations, in addition to EGFR T790M mutation at the time of resistance to first‐generation EGFR‐TKI. Patient 10 had acquired PIK3CA Q546K mutation along with EGFR T790M mutation. Intriguingly, EGFR compound mutation (A289V) emerged along with EGFR T790M mutation in the sample of patient 11.
Table 5.
Next generation sequencing (NGS) analysis using tissue samples before and after afatinib or first‐generation EGFR‐TKI
| Before EGFR‐TKI | After EGFR‐TKI | Efficacy of osimertinib | |||||
|---|---|---|---|---|---|---|---|
| EGFR mutations | Other mutations | EGFR mutations | Other mutations | PFS (months) | DOT (months) | ||
| Afatinib (Group A) | Patient 1 | Exon 21 L858R (26.3) |
CTNNB1 exon 2 S37Y (12.6) PIK3CA exon 20 G1049R (22.0) |
Exon 21 L858R (18.1) Exon 20 T790M (28.4) |
CTNNB1 exon 2 S37Y (9.1) PIK3CA exon 20 G1049R (13.9) |
11.4 | 13.0+ |
| Patient 2 | Exon 19 deletion (20.8) |
CTNNB1 Exon 2 S37F (1.1) TP53 exon 6 R248Q (1.0) Exon 5 Q192* (44.4) |
Exon 19 deletion (1.6) Exon 20 T790M (1.0) |
TP53 Exon 5 Q192* (1.6) |
30.1+ | 30.1+ | |
| Patient 3 |
Exon 21 L858R (2.0) Exon 20 T790M (1.9) |
RET Exon 11 R635C (2.3) TP53 Exon 7 E298K (1.8) |
Exon 21 L858R (32.7) Exon 20 T790M (22.3) |
22.0+ | 22.0+ | ||
| Patient 4 | Exon 19 deletion (45.2) |
TP53 Exon 6 N239S (83.7) |
Exon 19 deletion (9.8) Exon 20 T790M (8.7) |
TP53 Exon 6 N239S (7.1) |
16.1+ | 16.1+ | |
| Patient 5 | No available sample | No available sample |
Exon 19 deletion (46.1) Exon 20 T790M (33.9) |
20.4+ | 20.4+ | ||
| First‐generation EGFR‐TKI (Group B) | Patient 6 | Exon 19 deletion (28.3) |
CTNNB1 exon 2 S45P (27.1) |
Exon 19 deletion (25.5) Exon 20 T790M (17.3) |
CTNNB1 exon 2 S45P (18.4) |
4.4 | 4.4 |
| Patient 7 | Exon 19 deletion (37.4) |
TP53 exon 6 N239D (46.8) |
Exon 19 deletion (88.8) Exon 20 T790M (8.2) |
TP53 exon 6 N239D (90.6) |
20.7+ | 20.7+ | |
| Patient 8 | Exon 19 deletion (18.7) |
Exon 19 deletion (26.8) Exon 20 T790M (21.2) |
CTNNB1 exon 2 D32N (27.7) PIK3CA exon 9 E542K (28.5) |
19.6 | 19.8+ | ||
| Patient 9 |
Exon 19 deletion (8.3) Exon 20 T790M (1.5) Exon 20 R776H (2.0) |
ALK exon 20 D1091N (1.8) TP53 exon 4 P152L (2.5) |
Exon 20 T790M (28.5) |
18.1+ | 18.1+ | ||
| Patient 10 | Exon 19 deletion (11.0) |
Exon 19 deletion (33.4) Exon 20 T790M (39.6) |
PIK3CA exon 9 Q546K (7.0) |
25.9 | 27.1 | ||
| Patient 11 | Exon 21 L858R (2.1) |
Exon 21 L858R (6.1) Exon 20 T790M (1.9) Exon 7 A289V (1.1) |
11.9 | 30.8+ | |||
| Patient 12 | Exon 19 deletion (23.1) |
Exon 19 deletion (7.4) Exon 20 T790M (2.2) |
15.3 | 15.7 | |||
| Patient 13 | Exon 19 deletion (56.2) |
Exon 19 deletion (62.3) exon20 T790M (52.9) |
2.2+ | 2.2+ | |||
| Patient 14 | Exon 19 deletion (33.6) |
TP53 exon 7 R282W (35.2) |
Exon 19 deletion (37.1) Exon 20 T790M (31.1) |
TP53 exon 7 R282W (11.2) |
1.7+ | 1.7+ | |
Parentheses indicate allele frequency, %.
DOT, duration of treatment; PFS, progression‐free survival.
Discussion
Our study showed significantly longer PFS in patients harboring EGFR T790M mutation treated with osimertinib after afatinib than in patients treated with osimertinib after first generation EGFR‐TKIs. There have been several reports in the literature that support our results. Park et al. reported a retrospective study to evaluate the efficacy of osimertinib in patients with NSCLC harboring EGFR T790M mutation, after treatment with afatinib in prospective trials (LUX‐Lung 3, LUX‐Lung 6, and LUX‐Lung 7). This study showed a promising result, with median treatment duration with osimertinib of 20.2 months. 18 Tamiya et al. conducted a retrospective study to compare the effects of osimertinib in patients with NSCLC harboring EGFR T790M mutation after afatinib treatment with those after first generation EGFR‐TKIs treatment. PFS tended to be longer in patients after treatment with afatinib (median PFS; 17.0 vs. 9.7 months, P = 0.164). 25 These data, including our results, suggest that the efficacy of osimertinib in patients with NSCLC harboring EGFR T790M mutation might depend on the generation of EGFR‐TKI previously administered.
The most promising hypothesis to explain these results is based on genetic heterogeneity of NSCLC with EGFR mutations. 26 Blakely et al. reported co‐occurring genetic alterations, other than EGFR mutations, present in most advanced‐stage EGFR‐mutant lung cancer, some of which have been shown to affect the efficacy of EGFR‐TKIs. In addition, they reported that tumor genomic complexity increased over the course of EGFR‐TKI treatment, and co‐occurring mutations were very commonly observed in EGFR T790M mutation positive tumors. 27 In our study, PIK3CA and CTNNB1 mutations were observed simultaneously with EGFR T790M positive tumors after acquisition of resistance to first‐generation EGFR‐TKIs. On the other hand, no additional mutations were found in tumors after resistance to afatinib. In a preclinical study, co‐occurring mutations in PIK3CA and CTNNB1 exhibited nonredundant functions that cooperatively promoted tumor metastasis or limited EGFR‐inhibitor response. 27 The presence of these mutations, which were observed after first‐generation EGFR‐TKI treatment, could affect the efficacy of osimertinib. However, the clinical impact of EGFR‐independent mechanism, together with EGFR activating and T790M mutations on the effect of osimertinib remains unclear. It is also unclear whether there is a definitive difference in the status of non‐EGFR gene mutations during resistance between first‐generation EGFR‐TKIs or afatinib. First‐generation EGFR‐TKIs inhibit only EGFR, whereas afatinib has been reported to also have inhibitory activity against HER2 and HER4. 28 In fact, some clinical benefit has been reported for HER2 mutation‐positive lung cancer. 29 In addition, afatinib may have antitumor activity in lung cancer without EGFR mutations. 30 It is possible that these characteristics of afatinib may affect the status of non‐EGFR gene mutations during resistance. 26 Larger scale studies are warranted.
Furthermore, EGFR mutations have been reported to have heterogeneity. 31 , 32 , 33 Kohsaka et al. performed detailed genetic analysis of 390 NSCLC samples with EGFR mutations and showed that more than one type of EGFR mutation (EGFR compound mutation) was present in 15.9% of these NSCLCs. They also created cell line models with EGFR compound mutations and demonstrated that different combinations of coexisting EGFR mutations affected in the sensitivity to EGFR‐TKIs. In this preclinical study, afatinib had been reported to be more inhibitory to tumor cells with EGFR compound mutation than first‐generation EGFR‐TKIs and osimertinib. 31 In addition, it has been reported that EGFR compound mutations confer shorter osimertinib PFS in advanced NSCLC with a secondary T790M mutation. 34 These data suggest that there is a difference in the status of EGFR compound mutation, other than T790M, during resistance between afatinib and first‐generation EGFR‐TKIs, and this difference may have influenced the effect of osimertinib. In our study, EGFR A289V mutation was observed in a tissue sample after acquisition of resistance to first‐generation EGFR‐TKI. On the other hand, there were no EGFR compound mutations in the samples after afatinib treatment. EGFR A289V mutation has been reported to be relatively frequent in glioblastoma, and it is considered a poor prognostic factor. 35 This mutation is a very rare mutation in lung cancer, and there is only one case report. 36 The patient with NSCLC harboring EGFR A289V single mutation in this report was treated with first‐generation EGFR‐TKI and obtained the efficacy of partial response. The impact of A289V compounding with L858R and T790M on the effect of osimertinib is not clear. Further studies are awaited on the clinical impact of EGFR compound mutations on the effect of osimertinib treatment. In addition, the association between the generation of EGFR‐TKIs administered and the status of EGFR compound mutations at the time of acquired resistance should be examined.
There are some limitations in this study. First, we retrospectively collected the data from a single institution, and our sample size was small. This small sample size results from the difficulty of rebiopsy in clinical practice. As this is a retrospective study, the intervals between CT scans are not constant, especially in patients who have been treated with osimertinib for more than one year. In addition, there are still many censored cases in the PFS of Group A. These should be kept in mind when evaluating the PFS results of this study. Second, the mutational panel used in this study has a small number of genes that can be analyzed to assess the number of gene variants, such as mutational diversity. Using a genetic panel capable of detecting more genetic variants may clarify differences in intratumor heterogeneity, according to the generation of EGFR‐TKIs that was previously administered. There can be other co‐occurring mutations that affect the efficacy of EGFR‐TKIs, including osimertinib, than the mutations evaluated in this study. More extensive and detailed studies are needed in order to maximize the efficacy of osimertinib treatment.
In conclusion, PFS of osimertinib was significantly longer in patients with NSCLC harboring EGFR T790M mutation after treatment with afatinib than in patients after treatment with first generation EGFR‐TKIs. Although the mechanism could not be clarified in this study, it may be related the coexisting gene mutations with EGFR activating and T790M mutations in the tumor. Larger scale studies are warranted.
Disclosure
Ryo Ko reports grants and personal fees from Boehringer Ingelheim and AstraZeneca, personal fees from Taiho Pharmaceutical, Chugai Pharmaceutical, Ono Pharmaceutical, and Pfizer, outside the submitted work. Takehito Shukuya reports grants and personal fee from MSD and Boehringer Ingelheim, personal fees from Nichi‐Iko Pharmaceutical Co., Daiichi Sankyo, Ono Pharmaceutical, Bristol‐Myers Squibb, Eli Lilly, Novartis, and Taiho Pharmaceutical, outside the submitted work. Kazuhisa Takahashi reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, and Chugai Pharmaceutical, grants from MSD, and Bristol‐Myers Squibb, personal fees from Eli Lilly, Astellas, Ono Pharmaceutical, Taiho Pharmaceutical, Shionogi, Novartis, Pfizer, Glaxo Smith Kline, and Actelion, outside the submitted work. The other authors have no conflicts of interest to declare.
Acknowledgments
This study was funded by the Department of Respiratory Medicine, Juntendo University Graduate School of Medicine, and Boehringer Ingelheim.
References
- 1. Maemondo M, Inoue A, Kobayashi K et al Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–8. [DOI] [PubMed] [Google Scholar]
- 2. Mitsudomi T, Morita S, Yatabe Y et al Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010; 11 (2): 121–8. [DOI] [PubMed] [Google Scholar]
- 3. Rosell R, Carcereny E, Gervais R et al Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): a multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012; 13 (3): 239–46. [DOI] [PubMed] [Google Scholar]
- 4. Zhou C, Wu YL, Chen G et al Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): A multicentre, open‐label, randomised, phase 3 study. Lancet Oncol 2011; 12 (8): 735–42. [DOI] [PubMed] [Google Scholar]
- 5. Sequist LV, Yang JC, Yamamoto N et al Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31 (27): 3327–34. [DOI] [PubMed] [Google Scholar]
- 6. Wu YL, Zhou C, Hu CP et al Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐Lung 6): An open‐label, randomised phase 3 trial. Lancet Oncol 2014; 15 (2): 213–22. [DOI] [PubMed] [Google Scholar]
- 7. Wu YL, Cheng Y, Zhou X et al Dacomitinib versus gefitinib as first‐line treatment for patients with EGFR‐mutation‐positive non‐small‐cell lung cancer (ARCHER 1050): A randomised, open‐label, phase 3 trial. Lancet Oncol 2017; 18 (11): 1454–66. [DOI] [PubMed] [Google Scholar]
- 8. Soria JC, Ohe Y, Vansteenkiste J et al Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med 2018; 378 (2): 113–25. [DOI] [PubMed] [Google Scholar]
- 9. Mok TS, Wu YL, Ahn MJ et al Osimertinib or platinum‐pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med 2017; 376 (7): 629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sequist LV, Waltman BA, Dias‐Santagata D et al Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011; 3: 75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu HA, Arcila ME, Rekhtman N et al Analysis of tumor specimens at the time of acquired resistance to EGFR‐TKI therapy in 155 patients with EGFR‐mutant lung cancers. Clin Cancer Res 2013; 19 (8): 2240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ko R, Kenmotsu H, Serizawa M et al Frequency of EGFR T790M mutation and multimutational profiles of rebiopsy samples from non‐small cell lung cancer developing acquired resistance to EGFR tyrosine kinase inhibitors in Japanese patients. BMC Cancer 2016; 16 (1): 864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oxnard GR, Hu Y, Mileham KF et al Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M‐positive lung cancer and acquired resistance to osimertinib. JAMA Oncol 2018; 4 (11): 1527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramalingam SS, Cheng Y, Zhou C et al Mechanisms of acquired resistance to first‐line osimertinib: Preliminary data from the phase III FLAURA study. Ann Oncol 2018; 29 (Suppl. 8): viii740, LBA50. [Google Scholar]
- 15. Takeda M, Nakagawa K. First‐ and second‐ generation EGFR‐TKIs are all replaced to osimertinib in chemo‐naïve EGFR mutation‐positive non‐small cell lung cancer? Int J Mol Sci 2019; 20 (1): 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ramalingam SS, Vansteenkiste J, Planchard D et al Overall survival with osimertinib in untreated, EGFR‐mutated advanced NSCLC. N Engl J Med 2020; 382 (1): 41–50. [DOI] [PubMed] [Google Scholar]
- 17. Hochmair MJ, Morabito A, Hao D et al Sequential afatinib and osimertinib in patients with EGFR mutation‐positive non‐small‐cell lung cancer: An observational study. Future Oncol 2018; 14 (27): 2861–74. [DOI] [PubMed] [Google Scholar]
- 18. Park K, Bennouna J, Boyer M et al Sequencing of therapy following first‐line afatinib in patients with EGFR mutation‐positive non‐small cell lung cancer. Lung Cancer 2019; 132: 126–31. [DOI] [PubMed] [Google Scholar]
- 19. Ho HL, Chang FP, Ma HH et al Molecular diagnostic algorithm for epidermal growth factor receptor mutation detection in Asian lung adenocarcinomas: Comprehensive analyses of 445 Taiwanese patients with immunohistochemistry, PCR‐direct sequencing and Scorpion/ARMS methods. Respirology 2013; 18 (8): 1261–70. [DOI] [PubMed] [Google Scholar]
- 20. Brown P. The cobasR EGFR mutation test v2 assay. Future Oncol 2016; 12 (4): 451–2. [DOI] [PubMed] [Google Scholar]
- 21. Eisenhauer EA, Therasse P, Bogaertis J et al New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45 (2): 228–47. [DOI] [PubMed] [Google Scholar]
- 22. Xu C, Gu X, Padmanabhan R et al smCounter2: An accurate low‐frequnecy variant caller for targeted sequencing data with unique molecular identifiers. Bioinhormatics 2019; 35 (8): 1299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Landrum MJ, Lee JM, Benson M et al ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res 2018; 46 (D1): D1062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sherry ST, Ward MH, Kholodov M et al dbSNP: The NCBI database of genetic variation. Nucleic Acids Res 2001; 29 (1): 308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tamiya M, Tamiya A, Suzuki H et al Which is better EGFR‐TKI followed by osimertinib: Afatinib or gefitinib/erlotinib? Anticancer Res 2019; 39 (7): 3923–9. [DOI] [PubMed] [Google Scholar]
- 26. Kohsaka S, Petronczki M, Solca F, Maemondo M. Tumor clonality and resistance mechanisms in EGFR mutation‐positive non‐small‐cell lung cancer: Implications for therapeutic sequencing. Future Oncol 2019; 15 (6): 637–52. [DOI] [PubMed] [Google Scholar]
- 27. Blakely C, Watkins TBK, Wu W et al Evolution and clinical impact of co‐occurring genetic alterations in advanced‐stage EGFR‐mutant lung cancers. Nat Genet 2017; 49 (12): 1693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Solca F, Dahl G, Zoephel A et al Target binding properties and cellular activity of afatinib (BIBW2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther 2012; 343 (2): 342–50. [DOI] [PubMed] [Google Scholar]
- 29. Greve JD, Moran T, Graas MP et al Phase II study of afatinib, an irreversible ErbB family blocker, in demographically and genotypically defined lung adenocarcinoma. Lung Cancer 2015; 88 (1): 63–9. [DOI] [PubMed] [Google Scholar]
- 30. Soria JC, Felip E, Cobo M et al Afatinib versus erlotinib as second‐line treatment of patients with advanced squamous cell carcinoma of the lung (LUX‐Lung 8): An open‐label randomised controlled phase 3 trial. Lancet Oncol 2015; 16 (8): 897–907. [DOI] [PubMed] [Google Scholar]
- 31. Kohsaka S, Nagano M, Ueno T et al A method of high‐throughput functional evaluation of EGFR gene variants of unknown significance in cancer. Sci Transl Med 2017; 9 (416): eaan6566. [DOI] [PubMed] [Google Scholar]
- 32. Kim EY, Cho EN, Park HS et al Compound EGFR mutation in frequency detected with co‐mutations of actionable genes and associated with poor clinical outcome in lung adenocarcinoma. Cancer Biol Ther 2016; 17 (3): 237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu X, Zhang X, Zhang Z et al First‐generation EGFR tyrosine kinase inhibitor therapy in 106 patients with compound EGFR‐mutated lung cancer: A single institution's clinical practice experience. Cancer Commun 2018; 38 (1): 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin YT, Tsai TH, Wu SG, Liu YN, Yu CJ, Shih JY. Complex EGFR mutations with secondary T790M mutation confer shorter osimertinib progression‐free survival and overall survival in advanced non‐small cell lung cancer. Lung Cancer 2020; 145: 1–9. [DOI] [PubMed] [Google Scholar]
- 35. Binder ZA, Thorne AH, Bakas S et al Epidermal growth factor receptor extracellular domain mutations in glioblastoma present opportunities for clinical imaging and therapeutic development. Cancer Cell 2018; 34 (1): 163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dai L, Su X, Lu L, Lv D. Nonsmall cell lung cancer with rare exon 7 p.A289V mutation in the EGFR gene responds to icotinib treatment: A case report. Medicine 2018; 97 (51): e13809. [DOI] [PMC free article] [PubMed] [Google Scholar]


