The coronavirus disease 2019 (COVID-19) pandemic has affected over twenty million people worldwide since its outbreak,[1] in which approximately 19% are expected to progress to severe or critical disease, constituting the high-risk group for death.[2] The reported case-fatality rates among the severe COVID-19 varied a lot across different regions from zero to 61.5%[1,3] and the reason behind remained unclear. Very limited data concerning management of severe cases were reported from low case-fatality areas. In this study, we described the clinical features, multi-strategy management, and respiratory support resources usage for the severe COVID-19 in Sichuan province, where the 28-day case-fatality rate was 0.6% in all patients and 3.7% in severe cases, which was much lower compared with that reported in most of the studies worldwide.
Using data from Sichuan Provincial Department of Health, the multicentre cohort study (StUdy of 2019 Novel coRonavirus pneumonia Infected critically ill patients in Sichuan provincE, SUNRISE, ChiCTR2000029758) was performed.[4] All 21 hospitals designated for patients with severe COVID-19 in the province were included. Data were prospectively collected for patients who were still in the hospital after study enrolment, and otherwise retrospectively collected, between January 16 and March 15 [Supplementary Figure 1]. All microbiologically confirmed COVID-19 patients who met any of the five following criteria[2] were included as severe cases: (1) dyspnoea or respiratory frequency ≥30 breaths/min; (2) pulse oxygen saturation (SpO2) ≤93% without oxygen therapy in resting state; (3) partial pressure of oxygen/fraction of inspired oxygen (P/F) ratio ≤300 mmHg; (4) lung infiltrates >50% within 24 to 48 h; (5) respiratory failure, septic shock, and/or multiple organ dysfunction. The day of enrolment of each patient with severe COVID-19 was considered day 1 (D1). Each patient was followed up from D1 until discharge, death, or the end of the study. Clinical outcomes by D28, including rapid recovery (RR), prolonged recovery (PR), and no recovery (NR), were defined as follows: (1) RR: patient fully meeting the discharge criteria before D28, with normal body temperature ≥3 days, obvious improvement in respiratory symptoms and pulmonary imaging, and twice-negative nucleic acid tests (sampling interval being at least 24 h) on respiratory samples; (2) PR: patient partially meeting the discharge criteria on D28 and still requiring hospitalization but without advanced respiratory support; (3) NR: death or the patient still in need of advanced respiratory support on D28. The study protocol was approved by the Ethics Committee of the West China Hospital and the participating hospitals (No. 2020-131). Informed consent was obtained from the patient or the patient's legally authorized representative.
Eighty-one out of 539 patients were identified as severe cases. The median (interquartile range [IQR]) durations from the onset of symptoms to the first hospitalization and the diagnosis of severe condition were 3 (1–6) and 9 (6–11) days, respectively. Among the five severe diagnostic criteria,[2] P/F ≤300 mmHg, SpO2 ≤93% without oxygen therapy in resting state, and dyspnoea were the most commonly reported, accounting for 87.7% (71/81), 66.7% (54/81), and 27.2% (22/81), respectively. The median age (IQR) of the patients was 50 (39–65) years, 37.0% (30/81) were female, and 50.6% (41/81) were with a body mass index ≥24 kg/m2. The elderly (age ≥65 years) accounted for 28.4% (23/81) of the patients and chronic comorbidities were observed in 43 (53.1%) patients. All patients were followed up to the end of the study. Fifty-three (65.4%) were discharged before D28 and regarded as RR. Eighteen (22.2%) patients were regarded as PR, including 13 still in need of conventional oxygen therapy (COT) and five awaiting negative results of reverse transcription-polymerase chain reaction on D28. Ten patients (12.3%) were in the NR group, including three deaths and seven still in need of advanced respiratory support including high flow nasal cannula (HFNC), non-invasive (NIV) or invasive mechanical ventilation (IV) [Supplementary Table 1].
Among the 18 designated hospitals receiving severe cases, only two had standard intensive care unit (ICU) wards, while 16 had provisional ICUs which were transformed from general wards for infectious disease and equipped with ICU team and equipment needed. All 81 severe cases were centralized to the 18 ICUs, among whom 51 (63.0%) were treated in 16 provisional ICUs. Seventy (86.4%) patients were transferred from non-designated hospitals or designated hospitals for non-severe cases, and only 11 (13.6%) were admitted directly. In total, 77 patients (95.1%) were admitted to ICUs by D1 [Supplementary Figure 2].
Respiratory support was the most commonly used organ support method for patients with severe COVID-19. On D1, 76 patients (93.8%) received respiratory support, including 55 (67.9%) with COT, 13 (16.0%) with NIV, and 8 (9.9%) with HFNC. No patient was intubated or given extracorporeal membrane oxygenation (ECMO) [Supplementary Figure 3]. During the study period, 79 (97.5%) patients used COT, 31 (38.3%) used HFNC, 22 (27.2%) used NIV, 10 (12.3%) used IV, and 1 (1.2%) used ECMO. Thirty-four patients (42.0%) used only COT among which 79.4% (27/34) were discharged before D28. In the 25 patients who started with COT and needed escalation to advanced respiratory support methods, 12 (48.0%) were discharged by D28. In total, all forms of respiratory support were used 1579 person-day, of which COT took up 62.7% (990 person-day), HFNC 19.3% (305 person-day), NIV 9.4% (149 person-day), IV 8.5% (134 person-day), and ECMO 0.1% (one person-day). The peak needs of respiratory support, which developed on February 5, had a significant lag of 9 days behind the peak of newly diagnosed patients in Sichuan, lasted for 20 days and paralleled with hospitalization needs for severely ill patients [Figure 1].
Figure 1.
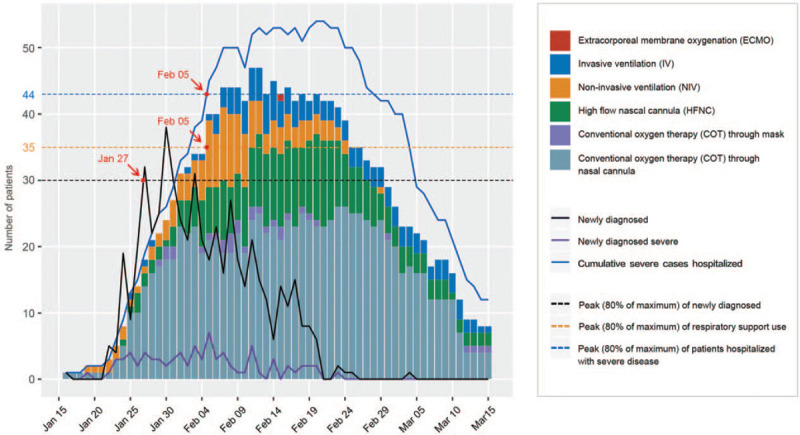
Daily respiratory support needs for patients with severe COVID-19. The bar plot shows, for each calendar day, counts of the respiratory support used for patients with severe COVID-19. Daily number of newly diagnosed patients with COVID-19 disease in Sichuan, newly diagnosed severe cases, and cumulative severe cases hospitalized are shown in lines. COVID-19: Coronavirus disease 2019.
Early identification of severe COVID-19 is a prerequisite for timely interventions for severe COVID-19. For the diagnosis of severe cases, different criteria were used in the previous studies while some did not report clear criteria. In this study, using the criteria for severe cases proposed by the National Health Commission of the People's Republic of China, none of the severe cases needed mechanical ventilation or ECMO on the day of diagnosis. In particular, our data showed that, on the day of diagnosis of severe COVID-19, up to 72.8% did not present symptoms of dyspnoea and only 33.3% had an SpO2 no less than 93%. Using only dyspnoea or SpO2 as the diagnostic criteria would fail to identify a large number of severe cases of COVID-19 in the early stage and miss the opportunity of intervening in time. P/F ratio was more likely a sensitive indicator for early identification of severe COVID-19, as 87.7% severe cases were identified by using this criterion. With early identification of the severe illness, 93.8% patients in this study received various respiratory support by D1, with a median P/F ratio of 204 mmHg. In the previous studies, the reported median P/F ratio on ICU admission was much lower (103.8–169.0 mmHg).[3,5] Similar to the patients in Sichuan, the patients outside Wuhan were less severe than those in Wuhan in China. The use of sensitive diagnostic criteria might be crucial for improving prognosis of patients with old age or comorbidity, for whom symptoms may be more atypical and the progression of the disease may be faster than others.
The shortage of advanced support equipment such as ventilators is a challenging issue. As there's no definite effective drug to treat COVID-19, appropriate respiratory therapy is essential for severe cases. According to data reported in studies from Wuhan and a study from the US, IV was administered in 38.9% to 71% of severely ill patients.[5,6] However, in our study, IV was used in only 12.3% of the patients and the case-fatality rate was much lower than that in these studies. This difference may be explained, at least in part, by the timing of intervention. It is reasonable to hypothesize that hypoxemia may participate in multiple organ injury if the hypoxic compensatory period was missed or the patients were not treated timely. Given that the COVID-19 pandemic is spreading rapidly, this finding is of particular interest for treating newly diagnosed severe cases and for regions facing a shortage of ventilators.
Acknowledgements
The authors would like to thank Ms. Hai-Xin Miao and Mr. De-Song Qiu from Sichuan Zhikang Technology CO., Chengdu, for their help of establishing the electronic data capture and analysis system; the authors thank Ms. Yu Ma and Mr. Bi-Wei Zhan from Chengdu Urban Planning Information Technology Centre for the localization and mapping for the designated hospital. The authors also thank Yi Liu, Ph D. from Shanghai, for his assistance in data visualization.
Funding
This project was supported by the Project of Novel Coronavirus Pneumonia in West China Hospital (No. HX2019nCoV027).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Liao XL, Chen H, Li Z, Wang B, Zhang ZW, Li WM, Liang ZA, Tang J, Wang J, Shi R, Jin XD, Kang Y, SUNRISE Group. Critical care for severe coronavirus disease 2019: a population-based study from a province with low case-fatality rate in China. Chin Med J 2021;134:98–100. doi: 10.1097/CM9.0000000000001187
Xue-Lian Liao, Hong Chen, and Zhen Li contributed equally to this work.
Supplemental digital content is available for this article.
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard. Geneva: World Health Organization, 2020. Available from: https://covid19.who.int. [Accessed August 22, 2020]. [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao X, Wang B, Kang Y. Novel coronavirus infection during the 2019-2020 epidemic: preparing intensive care units-the experience in Sichuan Province, China. Intensive Care Med 2020; 46:357–360. doi: 10.1007/s00134-020-05954-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


