Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide, placing an increasing burden on human health. NAFLD is a complex multifactorial disease involving genetic, metabolic, and environmental factors. It is closely associated with metabolic syndrome, obesity, and type 2 diabetes, of which insulin resistance is the main pathophysiological mechanism. Over the past few decades, investigation of the pathogenesis, diagnosis, and treatments has revealed different aspects of NAFLD, challenging the accuracy of definition and therapeutic strategy for the clinical practice. Recently, experts reach a consensus that NAFLD does not reflect the current knowledge, and metabolic (dysfunction) associated fatty liver disease (MAFLD) is suggested as a more appropriate term. The new definition puts increased emphasis on the important role of metabolic dysfunction in it. Herein, the shared features and potential changes in epidemiology, pathophysiology, diagnosis, and pharmacotherapy of the newly defined MAFLD, as compared with the formerly defined NAFLD, are reviewed for updating our understanding.
Keywords: Nonalcoholic fatty liver disease, Metabolic (dysfunction) associated fatty liver disease, Epidemiology, Pathophysiology, Diagnosis, Pharmacotherapy
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease worldwide.[1] NAFLD is defined as the evidence of steatosis in >5% of hepatocytes detected by imaging techniques or histology, in the absence of known causes such as alcohol, viral hepatitis, hereditary liver diseases, or long-term use of steatogenic medication.[2] It is an exclusive diagnosis, and for which liver biopsy is the golden standard. It is a spectrum of progressive liver disease from steatosis to nonalcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and hepatocellular carcinoma (HCC).[3] NAFLD is strongly associated with metabolic syndrome, the components of which include hyperglycemia, hypertension, abdominal obesity, and dyslipidemia.[4] Given the dramatically growing prevalence of NAFLD,[5] the lack of clear nomenclature for nonalcohol-use-disorder fatty liver disease, alongside the absence of a properly defined “positive” diagnosis and lack of approved drugs for this disease, constitute urgent unmet needs in this field.
Recently, a consensus of international experts has proposed the disease name being changed from NAFLD to metabolic (dysfunction) associated fatty liver disease (MAFLD).[6] The criteria are based on the evidence of hepatic steatosis, plus any of the following three conditions: overweight/obesity, presence of type 2 diabetes mellitus (T2DM), or evidence of metabolic dysregulation.[7] In this review, we will discuss the shared features and potential differences between MAFLD and NAFLD in epidemiology, pathophysiology, diagnosis, and pharmacotherapy.
Epidemiology
Over the past two decades, NAFLD has become the most common chronic liver disease globally and it is estimated that 25.24% of the world's populations have NAFLD, with the highest prevalence rates in the Middle East and South America.[8] The previous study has shown that diabetic individuals had an approximately three-fold higher risk of chronic liver disease, mainly associated with a non-virus and non-alcohol-related etiology which is largely attributable to NAFLD.[9] Indeed, the prevalence of NAFLD in patients with T2DM is more than two-fold higher than in the general population, occurring in up to 55.5%.[10] A recent meta-analysis of NAFLD in China has reported an overall NAFLD prevalence is 29.88%, of which the prevalence is higher in participants with T2DM (51.83% in diabetic vs. 30.76% in nondiabetic) and those with obesity (66.21% in obese vs. 11.72% in lean).[11] The prevalence of NAFLD is paralleled with the rising trend of obesity in China (the prevalence from approximately 2% in 2000 to 7% in 2014).[12] Moreover, T2DM and obesity also increase the risk of progression from simple steatosis to NASH, cirrhosis, and HCC.[13,14] Notably, in Asia, China has the highest prevalence, incidence, and annual NAFLD-related mortality rate.[15] The growing epidemic of T2DM and obesity will fuel an increasing prevalence of MAFLD worldwide.
The new definition, MAFLD, puts more emphasis on the role of metabolic dysfunction in it, and the exclusion of significant alcohol intake or other chronic liver disease is not required for the diagnosis anymore.[6] Indeed, the prevalence of obesity and metabolic syndrome in alcoholic liver disease (ALD) patients in the Third National Health and Nutrition Examination Survey (NHANES III) cohort are as high as 44.5% and 32.4%, respectively.[16] Obesity and metabolic syndrome even exacerbate the progression of ALD.[17] In clinical practice, NAFLD is recognized to coexist frequently with other conditions such as viral hepatitis.[18,19] Concomitant MAFLD with other liver diseases is now defined as dual (or more) etiology fatty liver disease. All together will increase the prevalence of MAFLD further.[20]
The presence of fatty liver with at least two metabolic risk abnormalities in lean or normal-weight individuals is also included in the diagnostic criterion of MAFLD. Lean or nonobese NAFLD is previously characterized as a unique phenotype,[21] and its pathogenesis is still not entirely clear. Differences in metabolic adaptation between patients with lean and obese NAFLD, at least in part, explain the pathophysiology of lean NAFLD.[22] A recent meta-analysis encompassing 93 studies from 24 countries or areas shows that in the general population, 5.1% of people have lean NAFLD and 12.1% have nonobese NAFLD.[23] It is worth noting that NAFLD is not uncommon in lean adults even with normal waist circumference (12.9%).[24] Thus, lean or normal-weight individuals but with metabolic abnormalities will constitute a certain proportion of MAFLD.
As mentioned above, the new definition of MAFLD and the new criteria incorporating other fatty liver diseases may result in a higher prevalence.
Pathophysiology of NAFLD/MAFLD
A “two-hit” theory was proposed in 1998 to describe the pathogenesis of NAFLD.[25] It proposed that at the onset of disease, the “first hit” was represented by an increase in liver fat. Subsequently, the “second hit”, including inflammatory cytokines, adipokines, mitochondrial dysfunction, and oxidative stress, was needed for the progression to NASH and advanced fibrosis.[25] However, it was insufficient to include the various molecular and metabolic involvements in NAFLD-NASH-HCC progression since the pathogenic drivers of NAFLD are highly heterogeneous. A “multiple-hit” hypothesis, which incorporates various processes, such as insulin resistance, lipotoxicity, inflammation, imbalance of cytokines, activation of innate immunity, and microbiota, in the context of environmental and genetic factors, offers a more comprehensive delineation of the pathogenesis of NAFLD.[26] In addition, the new terminology MAFLD proposes to define metabolic dysfunction as the corpus of the disease with these variable driving factors, resulting in further disease subtyping.[27]
Nevertheless, fat accumulation in the liver caused by insulin resistance (IR) still represents the first and the core hit. In contrast to the high prevalence of NAFLD in patients with T2DM, the prevalence of NAFLD in patients with type 1 diabetes mellitus (T1DM) is relatively lower (8.8%).[28] This finding further supports the hypothesis that insulin resistance, which is manifested in obesity and T2DM but rarely in T1DM, is the main contributing factor in the pathogenesis of NAFLD. Under conditions of IR, insulin is not capable of switching off hepatic glucose production anymore, but its ability to promote lipogenesis is retained.[29] In this section, multiple factors contributing to the disease pathogenesis of NAFLD/MAFLD are reviewed [Figure 1].
Figure 1.
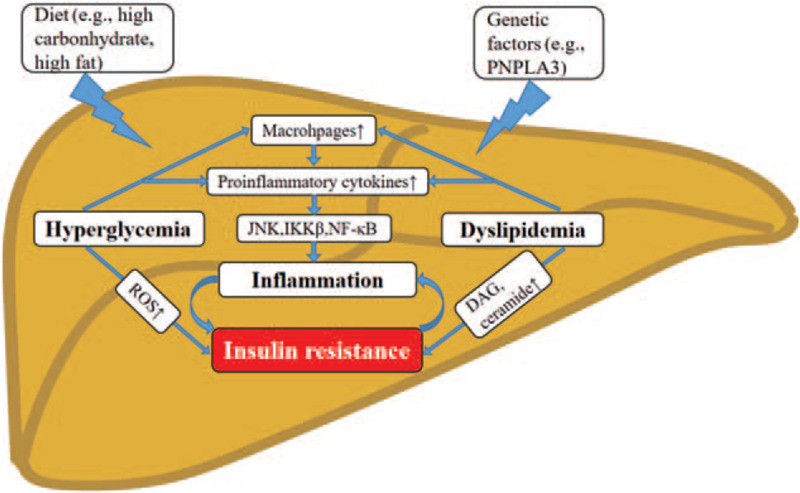
Pathophysiology of MAFLD involving genetic factors, glucotoxicity, and lipotoxicity. High-carbohydrate or high-fat diets contribute to the effect of glucotoxicity and lipotoxicity in the development of hepatic steatosis. Hyperglycemia and dyslipidemia induced hepatic insulin resistance and inflammation through different mechanisms in MAFLD. ROS: reactive oxygen species; IKKβ: kinases IκB kinase-β; JNK: JUN N-terminal kinase; DAG: diacylglycerol; MAFLD: metabolic (dysfunction) associated fatty liver disease.
Genetics
Findings from the genome-wide association study (GWAS) identified the main risk variants of the NAFLD population.[30] Currently, at least five variants in different genes are robustly associated with the susceptibility to the progression of NAFLD. They are PNPLA3, TM6SF2, GCKR, MBOAT7, and HSD17B13.[31–33] Of these genetic variants, some are associated with an increased risk of T2DM, including TM6SF2,[34] TCFL2,[35] and SREBF-2.[36] Others are associated with the risk of developing obesity, such as ADIPOQ[37] and SH2B1.[38] These genes have been reported to be involved in IR, glucose, and lipid homeostasis.[39,40] The shared genes mentioned above indicate that NAFLD might have shared functional mechanisms that are involved in the pathogenesis of T2DM and obesity. They also support the diagnostic criteria of MAFLD in which the presence of metabolic dysfunction should be included.
In addition, genetic studies have revealed shared inherited determinants of NAFLD and other liver diseases. NAFLD associated variants in PNPLA3, TM6SF2, and MBOAT7 have been identified as risk loci for alcoholic cirrhosis.[41] PNPLA3 and MBOAT7 are also associated with hepatic steatosis in patients with viral hepatitis.[42,43] These findings hint that NAFLD is genetically interrelated with other liver diseases, supporting the inclusion of dual or more etiology to define MAFLD. The list of common variants associated with NAFLD, which also play a role in the development of metabolic disorders and other liver diseases, are presented in Table 1.
Table 1.
Summary of gene variants associated with the presence and/or severity of NAFLD
| Gene name | Variant | In NAFLD | In metabolic disorders | In other liver diseases | Effects of the variant |
| PNPLA3 | rs738409 (I148M) | Increase susceptibility to the whole spectrum of NAFLD.[145] | 1. Increase risk of T2DM.[34]2. Reduce risk of T2DM.[146] | A risk loci for ALD,[41] viral hepatitis.[147,148] | 1. Gain of function: overexpression causes hepatic triacylglycerol accumulation.[149]2. Loss of function: reduce the lipidation of VLDL.[150]3. No function: deficiency in mice showed no hepatic steatosis.[151] |
| TM6SF2 | rs58542926 (E167K) | Associated with risk of NAFLD.[46] | 1. Increase risk of T2DM.[34]2. Protect against CVD.[152,153] | Associated with ALD.[41] | Loss of function: favoring lipid accumulation into the liver.[46] |
| GCKR | rs1260326 (P446L) | Associated with predisposition to NAFLD.[31] | Protect against T2DM.[44] | NA | Loss of function: Elevate hepatic glucose uptake and boost lipogenesis by increasing active cytosolic GCK.[12] |
| MBOAT7 | rs641738 (G17E) | Associated with risk of NAFLD.[43] | NA | A risk factor for ALD[41] and viral hepatitis.[154,155] | Reduced expression.[158] |
| HSD17B13 | rs72613567 (TA) | Reduce risk of NASH, but not steatosis.[33] | NA | Protect against advanced ALD.[156–158] | Loss-of-function[33]: result in an unstable and truncated protein with reduced enzymatic activity[159] |
| TCF7L2 | rs7903146 (CT/TT) | Increase risk of NAFLD.[31] | Increase risk of T2DM.[35] | NA | Loss of function in T2DM: associated with beta-cell dysfunction.[35] |
| SREBF-2 | rs133291 (CT/TT)[36] | Predicts incident NAFLD | Predicts risk of T2DM. | NA | Gain of function |
| ADIPOQ | G45T and G276T | Associated with predisposition to develop NAFLD.[37] | Predict risk of T2DM and obesity.[40] | NA | NA |
| SH2B1 | rs7359397[38] | A higher risk of developing NASH | A higher risk of developing obesity | NA | NA |
ALD: alcoholic liver disease (ALD); CVD: cardiovascular disease; NAFLD: non-alcoholic fatty liver disease; NASH: non-alcoholic steatohepatitis; NA: not applicable; T2DM: type 2 diabetes mellitus; VLDL: very low-density lipoproteins.
However, some of the disease-predisposing variants conferred in NAFLD are associated with a decreased risk of other metabolic disorders. For example, the NAFLD susceptible variants, GCRK P446L variant,[44] are reported to have protective roles in developing T2DM. NAFLD risk allele at PNPLA3 I148M[45] and TM6SF2[46] are inversely associated with the risk of cardiovascular disease (CVD). The opposite effect of these variants on NAFLD and metabolic disorders (ie, T2DM and CVD) might be attributed to their divergent metabolic effects. For example, GCKR P446L variant induces de novo lipogenesis and improves hepatic glucose metabolism, resulting in elevated triglycerides (TG) in the liver but decreased glucose level.[31] Thus, the effect of risk genes on liver fat content and metabolic variables are complex. Carriers with PNPLA3 I148M variant, the first and the most common variant of NAFLD, are associated with a small increase risk of T2DM,[34] but not accompanied by IR.[45]
It is still unclear why patients with “genetic NAFLD” accumulate hepatic fat, which might be influenced by gain or loss of function of the risk variant. The differences in disease risk indicated by these variants may help to explain why individuals without diabetes or obesity could suffer from liver steatosis. Investigation of the genetic architecture of MAFLD should better characterize the role of risk variants in the disease heritability and pathogenesis in the future.
Glucotoxicity
Epidemiological studies indicate a correlation between high-carbohydrate diets and NAFLD.[47] Diet with high sugars, such as fructose or sucrose, increases the risk of NAFLD.[48] The abundant carbohydrate consumption and the resultant increased levels of blood glucose exert deleterious effects on cells, a phenomenon termed glucotoxicity. This concept is intrinsically linked to IR in the liver manifested with increased gluconeogenesis and decreased glycogenesis, leading to hyperglycemia.[49]
T2DM is a chronic condition of glucotoxicity with characteristics of impaired glucose metabolism and hyperglycemia.[50] The relation between NAFLD and T2DM is bidirectional and mutually causal. Studies in T2DM population have demonstrated that plasma glucose level is positively correlated with the histological severity of NAFLD.[51] Glycemic variability is an independent predictive factor for the progression of NAFLD.[52] Baseline IR is a good predictor of NAFLD incidence in the general population.[53] Studies in rodents indicate that exposure to high glucose induces hepatic IR,[54] which could be a consequence of suppressed insulin receptor signaling.[55,56] IR is also an independent risk factor associated with liver fibrosis in patients with T2DM.[57] Given the strong correlation between T2DM and fatty liver, it will be of great interest to investigate the cause and effect interaction among glucotoxicity, IR, and MAFLD.
The chronic low-degree inflammation, which could be provoked by glucotoxicity and leads to IR,[58] is also a shared pathological feature of NAFLD and T2DM.[59] The process of NAFLD inflammation is induced by two classical pathways: kinases IκB kinase-β (IKKβ) pathway and JUN N-terminal kinase (JNK) pathway.[60] In serum of patients with T2DM, levels of interleukin-1β (IL-1β), IL-6, tumor necrosis factor (TNF)-alpha, and C-reactive protein (CRP) are higher than those without T2DM.[61] These inflammatory regulators activate IKKβ and JNK pathway and thus promote IR in the liver.[60] The down-stream transcription factor nuclear factor-κB (NF-κB) of these two inflammatory pathways amplifies the expression of the aforementioned pro-inflammatory cytokines.[62] Besides, the increasing oxidative stress in hepatocytes also accounts for the glucotoxicity-related inflammation.[63] In mice exposed to glucose fluctuation, liver inflammation is enhanced by the mitochondrial permeability transition and dysfunction.[64]
To sum up, glucotoxicity-related systematic IR and chronic inflammation act as shared mechanisms in the progression of hepatic steatosis and T2DM. It supports the new definition of MAFLD with the involvement of glucotoxicity in the pathogenesis. Future study is required to investigate the mechanism by which glucotoxicity induces hepatic IR and the therapeutic options that could block this process.
Lipotoxicity
Hepatic steatosis develops within days of a high-fat diet (HFD) feeding in both human beings and rodents.[65,66] A recent study found that a diet enriched in saturated fat was more harmful for elevating intrahepatic TG content than a diet enriched in free sugars in overweight males.[67] These findings support the predominant role of lipotoxicity in NAFLD. Tracer studies in individuals with obesity have demonstrated that ∼60% of liver TG content is derived from free fatty acids (FFAs) from adipose tissue.[68] Obese individuals have increased visceral adipose tissue that could lead to IR and hyperinsulinemia, which will enhance adipose tissue lipolysis.[3] Rodent models have shown that decreasing hepatic TG content could improve insulin sensitivity,[69,70] suggesting a strong link between lipotoxicity and hepatic IR.[71]
The potential lipids in inducing hepatic IR consist of two major classes of lipid intermediates: diacylglycerol (DAG) and ceramide.[72] In obese, nondiabetic patients, hepatic DAG content is the best predictor of NAFLD-related IR.[73] Later research verifies that the development of hepatic IR is ascribed to hepatic DAG-induced PKCε activation in NAFLD.[74] In mice, hepatocyte deletion of DAG acyltransferase 2 successfully reduces diet-induced hepatic steatosis.[75] Intracellular DAG near the membrane results in PKC activation, which in turn inhibits insulin signaling.[76] Besides, studies in rats show that the hepatic ceramide level is increased in HFD induced hepatic steatosis and IR,[77] and inhibiting ceramide synthesis could attenuate hepatic steatosis and IR.[78] Thus, lipid metabolites act as inter-mediators in the causal link between lipotoxicity and IR, which both contribute to the development of NAFLD/MAFLD.
Lipotoxicity related chronic low-grade inflammation is involved in the development of NAFLD.[79] In obese individuals, the degree of inflammation indicated by IL-6 and TNF-α were in a dose-dependent manner correlated with the severity of NAFLD.[80] The production of pro-inflammatory mediators further activates the key transcriptional factors such as JNK and NF-κB,[81] leading to steatohepatitis. At the same time, the impaired release of anti-inflammatory adipokines (eg, adiponectin) also diminishes insulin sensitivity.[82] Apart from inflammatory molecules, the recruitment of macrophages in the liver is associated with IR and steatohepatitis.[83]
From the above, lipotoxicity-related IR and inflammation contribute to the pathogenesis of fatty liver. As obesity and dyslipidemia are implicated in the new definition of MAFLD, further research is needed to clarify the mechanisms of lipotoxicity induced MAFLD.
Diagnosis
The proposed criteria for the diagnosis of MAFLD rely on the evidence of hepatic steatosis, which could be detected either by imaging techniques, blood biomarkers, or liver histology. Several noninvasive screening and diagnostic assessments have been developed in recent years for liver steatosis evaluation.[84] Here, we summarize the current status of noninvasive (imaging, biomarkers) and invasive (liver biopsy) methods available for the diagnosis of NAFLD/MAFLD.
Imaging techniques
Owing to the asymptomatic features of NAFLD, hepatic steatosis is often incidentally diagnosed on imaging checks such as abdominal ultrasound, CT scan, or magnetic resonance imaging (MRI). The most common imaging method for diagnosis is an abdominal ultrasound which is easily accessible and can sonographically demonstrate the fat infiltration of the liver.[85] However, when steatosis is less than 30%, the sensitivity reduces significantly.[86,87] Although the diagnostic accuracy of CT is much more precise than ultrasound for grading moderate to severe steatosis, its capability is also limited for mild steatosis. Moreover, the radiation exposure limits its use as a screening or early diagnostic tool.[88] An alternative diagnostic method is MRI, which is highly sensitive for small amounts of isolated steatosis. But this modality is not readily available or cost-effective.[89]
More recently, transient elastography (TE, FibroScan) performed with ultrasound has gained attraction due to the allowance of rapid measurements of liver stiffness, an indicator that is strongly related to the stage of liver fibrosis.[90] Controlled attenuation parameter (CAP) has been developed to assess the quantification of hepatic steatosis and fibrosis simultaneously, based on the properties of ultrasonic signals through the TE in patients with T2DM.[91] Screening for NAFLD in T2DM individuals using FibroScan/CAP is recommended in the latest Asia-Pacific Working Party on Non-Alcoholic Liver Disease guidelines.[92] Studies using FibroScan/CAP has found a high prevalence of NAFLD (ranging from 60.7% to 70.4%) in patients with T2DM.[93,94] This range is in agreement with the prevalence of NAFLD (47.26%–63.67%) in T2DM.[10] It will be plausible to optimize its use in screening fatty liver in patients manifested with metabolic dysfunction.
Blood biomarkers
Intensive efforts have been exerted to develop non-invasive biomarkers for the detection of fibrosis in patients with NAFLD.[95] In clinical practice, NAFLD fibrosis score (NFS) and fibrosis-4 index (FIB-4) scoring systems work well to exclude advanced fibrosis-cirrhosis (with negative predictive values >90%); therefore, they could be used as a first-line classification to identify patients with low risk of advanced fibrosis.[96] However, it may be late to take action in reversing the fibrosis stage. The ability to detect simple steatosis and steatohepatitis early and noninvasively in patients with fatty liver is crucial for preventing disease progression. Previous studies demonstrated that the biochemical parameters including alanine aminotransferase (ALT), aspartate aminotransferase (AST), hemoglobin A1c, and homeostatic model assessment (HOMA)-IR could provide good prediction indices of NAFLD in patients with metabolic syndrome[97,98] or even in the general population.[99] The advances in novel serum markers, including cytokeratin 18 fragment (CK18-F) and insulin-like growth factor-1 (IGF-1), have shown desirable performance in routine screening for NAFLD.[100] Besides, the serum miRNAs test exhibits robust diagnostic efficacy for NASH in obese subjects.[101]
Nevertheless, a recent study shows that the well-validated biomarker panels for the diagnosis of different stages of NAFLD such as simple steatosis, steatohepatitis, and advanced fibrosis may underperform in patients with T2DM.[97] It suggests that patients with T2DM may require predictive models that have been specifically developed for them.
Histological biopsy
Liver biopsy remains the golden standard for the diagnosis of NAFLD, especially for the diagnosis of NASH.[102] However, limitations of liver biopsy should be noted. For instance, a biopsy procedure is an invasive test with interobserver variability that could produce complications, and the mortality percentage of biopsy is about 0.05%.[103] In addition to technical problems, liver biopsy is a costly procedure that requires operators and pathologists trained to obtain adequate and representative results, which limits its use for mass screening.[96] The accuracy of liver biopsy to evaluate fibrosis has been questioned, mainly due to the sampling errors and intra- and inter-observer variability, which can lead to an over or underestimation of the hepatic fibrosis stage.[104] For these reasons, noninvasive methods might be preferred as the first choice to detect NAFLD/MAFLD. Liver biopsy is reserved for the patients in whom the etiology of the liver disease needs to be clarified, or when NASH and the degree of liver fibrosis cannot be ascertained with noninvasive measures.[105]
Pharmacotherapy
No specific pharmaceuticals are currently FDA approved for NASH. Drugs that target inflammation and fibrogenesis are under investigation,[106] but with limited clinical success. The likely cause of failures of phase 2 and phase 3 studies involving the anti-apoptosis and anti-fibrosis treatment[107] may be that the drug targets a later stage in the development of NASH. In addition, the American Association for the Study of Liver Diseases (AASLD) guidance suggests that drugs should be limited to patients with NASH and fibrosis,[2] leaving a gap in pharmacotherapy for earlier stage of NAFLD. The new criteria for the diagnosis of MAFLD will promisingly encourage the initiation of drugs in the early stage.
Since T2DM and obesity shared the common pathophysiological factors with NAFLD/MAFLD, anti-obesity and anti-hyperglycemic drugs may exert efficacy in improving liver histology and clinical outcomes in NAFLD/MAFLD. Particularly, CVD is the leading cause of death in patients with NAFLD and NASH.[108] Drugs that have beneficial CVD outcomes in T2DM, such as glucagon-like peptide 1 receptor agonists (GLP-1 RAs) and sodium-glucose cotransporter 2 (SGLT2) inhibitors, might also reduce the risk of cardiovascular mortality in patients with NAFLD/MAFLD. A composite of current glucose and lipid-lowering drugs with trials undergoing in NAFLD is listed in Table 2.
Table 2.
Summary of pharmacotherapy with currently undergoing clinical trials in NAFLD.
| Class | Agents | Mechanism of action | Outcomes in treating NAFLD/NASH | Recommendation in AASLD 2018 |
| Anti-oxidant agents | Vitamin E | Antioxidant effect | 1. Improve steatosis, inflammation, and resolution of NASH.[110]2. Significantly reduce serum hepatobiliary enzymes, hepatic steatosis, inflammation, and hepatocellular ballooning.[160] | Nondiabetic adults with biopsy-proven NASH |
| Insulin sensitizers | Pioglitazone | PPAR-γ agonism | 1. Significantly improve hepatic steatosis and lobular inflammation in NASH with[110] and without T2DM with additional weight loss.[111]2. Improved liver histology in combination with vitamin E.[112] | Patients with biopsy-proven NASH |
| Metformin | IR alleviation | No effect of metformin on liver histology.[116] | Not recommended | |
| GLP1 analogues | Liraglutide | GLP1 receptor agonism | Meet the primary endpoint of histological resolution of NASH with no worsening in fibrosis.[123] | Premature as a specific treatment for NASH |
| Semaglutide | 1. Reduce ALT and hypersensitive CRP.[126]2. Resulted in a significantly higher percentage of patients with NASH resolution than placebo.[127] | |||
| SGLT2 inhibitors | Empagliflozin | SGLT2 inhibition | Reduces liver fat, improves ALT levels in patients with T2DM and NAFLD.[131] | NA |
| Statins | Simvastatin | HMG-CoA reductase inhibitor | Reduce the risk of steatosis by 71% after 4 years of treatment in combination with vitamins C and E in patients with NAFLD.[161] | Can be used to treat dyslipidemia and should be avoided in decompensated cirrhosis. |
| FXR agonist | Obeticholic acid | FXR agonism | 1. Reduce NAFLD activity[141] and improving fibrosis.[142]2. Combination with statin mitigate OCA-induced increases in LDLc.[145] | Should not be used off-label to treat NASH. |
AASLD: American Association for the Study of Liver Diseases; ALT: alanine aminotransferase; CRP: C-reactive protein; FXR: farnesol X receptor; GLP1: glucagon-like peptide 1; HMG-CoA: hydroxymethylglutaryl-CoA; IR: insulin resistance; LDLc: low-density lipoprotein cholesterol; NA: not applicable; NAFLD: non-alcoholic fatty liver disease; NASH: non-alcoholic steatohepatitis; OCA: obeticholic acid; PPAR: peroxisome proliferator-activated receptor; RCT: randomized controlled trial; SGLT2: sodium-glucose cotransporter 2; T2DM: type 2 diabetes mellitus.
Insulin sensitizers and vitamin E
As mentioned previously, IR and oxidative stress contribute to the process of liver lipid accumulation. Pioglitazone, a peroxisome proliferator-activated receptor (PPAR)-γ agonist that belongs to thiazolidinediones (TZDs), is approved for the treatment of T2DM by alleviating IR.[109] Vitamin E is a potent antioxidant that may reduce oxidative stress in NAFLD. In the PIVENS (Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis) trial, both pioglitazone and vitamin E were associated with ameliorated hepatic steatosis in NASH patients without diabetes,[110] with no benefit in fibrosis improvement. In patients with T2DM, pioglitazone has shown effects in alleviating IR with improvement in liver steatosis and inflammation compared to placebo.[111] A recent proof-of-concept study has revealed that pioglitazone in combination with vitamin E is better than a placebo in improving liver histology in patients with NASH and T2DM.[112] Based on these studies, pioglitazone and vitamin E are now recommended by guidelines as treatment options for biopsy-proven NASH patients with and without diabetes, respectively.[2] However, the use of pioglitazone has been restricted by the risk of congestive heart failure and postmenopausal bone loss.[113] Long-term use of high-dose vitamin E has been reported to be associated with an increased incidence of hemorrhagic stroke.[114] Thus, the benefits of these drugs must be balanced against the potential risks when the decision is made in clinical practice.
Apart from TZDs, metformin, another kind of insulin sensitizer, has been positioned as the first-line agent for T2DM for many years.[115] Although there is a lack of evidence for metformin as an adequate treatment to improve liver histology of NAFLD,[116] the weight loss-promoting and insulin-sensitizing properties of metformin are desirable.[117] A recent study in T2DM patients with biopsy-proven NASH and fibrosis showed that long-term metformin use is associated with a lower risk of overall mortality, liver transplant, and HCC.[118] These benefits together with its low cost and safe profile[119] could make metformin an ideal candidate for the treatment of T2DM and NAFLD/MAFLD.
New antidiabetic agents
Over the past few decades, there have been efforts to study the effect of new antidiabetic agents, such as GLP-1 RAs, dipeptidyl-peptidase 4 (DPP-4) inhibitors, and SGLT2 inhibitors with the goal of reversing hepatic steatosis and preventing the progression to NASH with advanced fibrosis.[120] GLP-1 RAs along with SGLT2 inhibitors become the first-line therapy for patients at high risk of major adverse cardiovascular events in T2DM.[121] According to a recent meta-analysis of seven trials, GLP-1 RAs have beneficial effects on cardiovascular, mortality, and kidney outcomes in patients with T2DM.[122] The efficacy of liraglutide was reported in NASH patients in the Liraglutide Efficacy and Action in NASH (LEAN) study[123] and Japanese studies (LEAN-J study).[124] The phase 2 of the LEAN study showed that liraglutide met the primary endpoint of histological resolution of NASH with no worsening in fibrosis. Semaglutide, a novel GLP-1 RA, has been proved for glucose control and weight loss in patients with T2DM.[125] In sub-analyses of the SUSTAIN-6 study, semaglutide has been shown to reduce ALT and hypersensitive CRP.[126] A recent phase 2 trial has shown that semaglutide treatment resulted in a significantly higher percentage of patients with NASH resolution and with no worsening of fibrosis than placebo (59% in the 0.4-mg group and 17% in the placebo group ).[127] Although DPP-4 inhibitors prolong the effect of endogenous GLP-1, it is not effective for patients with hepatic steatosis or steatohepatitis as shown by a recent systematic review.[128] Therefore, GLP-1 RAs will be a promising incretin-based drug for the treatment of MAFLD, especially in patients with T2DM.
SGLT2 inhibitors decrease blood glucose levels in patients with T2DM via the promotion of renal excretion of glucose.[129] A meta-analysis of randomized controlled trials (RCTs) with the reported outcome up to October 1, 2019, has shown that SGLT2 inhibitors could significantly decrease ALT level, reduce liver fat content and body weight in patients with T2DM, which indicates a positive effect of SGLT2 inhibitor on improving fatty liver.[130] In an open-label pilot study, empagliflozin (an SGLT2 inhibitor) has reduced liver steatosis, ballooning, and fibrosis in T2DM patients with liver-biopsy proven NASH.[131] Robust clinical data has demonstrated that apart from renal protection, SGLT2 inhibitors improve cardiovascular outcomes in patients with T2DM.[132] Given CVD being the most common cause of death in NAFLD, the effect of SGLT2 inhibitors on the reduction of cardiovascular death will be beneficial to the prognosis of NAFLD. Studies have shown that the combination of GLP-1 RAs and SGLT2 inhibitors results in significant improvements in glycemic control with acceptable tolerability in patients with T2DM.[133,134] Based on the above evidence, GLP-1 RAs, SGLT2 inhibitors, the combination of them, or an add-on to each other might be useful in the treatment of MAFLD in the future. However, more clinical trials and evidence are urgently needed.
Statins
Statins, a class of lipid-lowering drugs, reduce the risk of cardiovascular morbidity and mortality in patients with NASH and dyslipidemia.[135] Though no evidence proves the benefits of statins on liver histology in NASH patients,[136] statins may reduce the risk of HCC in diabetic patients.[137,138] The current guideline has pointed out that statins can be safely used to treat dyslipidemia and prevent CVD in patients with NAFLD/NASH.[2] As lipid accumulation is closely associated with the progression and cardiovascular outcomes of NAFLD, statins are promising drugs for the treatment of MAFLD.
Farnesoid X receptor (FXR) agonists
FXR is a member of the nuclear receptor superfamily controlling a variety of genes involved in bile acid synthesis and transport, also in glucose and lipid metabolism.[139] Obeticholic acid (OCA), an FXR agonist, is now waiting for the FDA decision to approve its marketing authorization application by June 2020. In phase 2 RCT, OCA improved IR and liver function tests better than placebo in patients with NAFLD and T2DM.[140] Following the encouraging results of the “FLINT” trial which has shown the superiority of OCA at reducing NAFLD activity score (NAS) by two points without fibrosis worsening in adults with NASH,[141] a phase 3 trial called “REGENERATE” is currently underway. Its interim analysis has shown that OCA significantly improved fibrosis and NASH activity.[142] Nevertheless, pruritus was reported to be the most common side effect of OCA treatment in this interim analysis,[142] leading to discontinuation of treatment in some patients. In post hoc analyses of the phase 2b FLINT trial, OCA was associated with an increase in serum alkaline phosphatase, LDL cholesterol (LDLc), and hemoglobin A1c levels.[143,144] Combination treatment of OCA with atorvastatin has subsequently been proven efficacious in preventing the LDLc increase present in OCA monotherapy.[145] Novel candidate compounds for selective FXR modulation is in urgent demand to improve the tolerability in the treatment of NAFLD.
Since MAFLD is associated with disturbance in lipid and glucose metabolism, a combination of drugs targeting different pathogenic mechanisms will be the highly anticipated treatment.
Conclusion
A growing epidemic worldwide of T2DM and obesity, and the new definition of MAFLD may lead to a higher prevalence of MAFLD in the future. The pathophysiology of MAFLD is closely associated with metabolic syndrome, T2DM, and obesity. The complex interactions between genetics, glucotoxicity, and lipotoxicity make it difficult to distinguish the precise mechanisms underlying the increased risk of MAFLD in T2DM or obesity. There is thus an imperative need to clarify the epidemiological features and mechanisms that drive the development and progression of MAFLD. Rising the awareness of early screening for MAFLD is important for timely diagnosis and effective interventions. As the patient population is heterogeneous and the pathophysiology is complex, the area of drug development is particularly challenging. Drugs that target the advanced stage of NASH, including liver fibrosis and cirrhosis, are currently under investigation, but with limited clinical success. It suggests that interventions should be initiated at the early stage of NAFLD/MAFLD to obtain cardiovascular benefits and prevent the progression to the advanced stages. Pharmacological strategies aiming at improving metabolic dysfunctions will be promising for improving the clinical outcomes of NAFLD/MAFLD.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Nos. 81670782 and 81970741), the Local Innovative and Research Teams Projects of Guangdong Pearl River Talents Program (No. 2017BT01S131), the Guangdong High-Level Talents Special Support Program (No. 2016TQ03R590), and the Pearl River S&T Nova Program of Guangzhou (No. 201610010175).
Conflicts of interest
None.
Footnotes
How to cite this article: Xian YX, Weng JP, Xu F. MAFLD vs. NAFLD: shared features and potential changes in epidemiology, pathophysiology, diagnosis, and pharmacotherapy. Chin Med J 2021;134:8–19. doi: 10.1097/CM9.0000000000001263
References
- 1.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018; 15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018; 67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 3.Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2014; 2:901–910. doi: 10.1016/S2213-8587(14)70032-4. [DOI] [PubMed] [Google Scholar]
- 4.Huang TD, Behary J, Zekry A. Non-alcoholic fatty liver disease (NAFLD): A review of epidemiology, risk factors, diagnosis and management. Intern Med J 2020; 50:1038–1047. doi: 10.1111/imj.14709. [DOI] [PubMed] [Google Scholar]
- 5.Younossi Z, Tacke F, Arrese M, Chander SB, Mostafa I, Bugianesi E, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2019; 69:2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- 6.Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020; 158:1999–2014. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 7.Eslam M, Newsome PN, Anstee QM, Targher G, Gomez MR, Zelber-Sagi S, et al. A new definition for metabolic associated fatty liver disease: an international expert consensus statement. J Hepatol 2020; 73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 8.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 9.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol 2015; 62:S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol 2019; 71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y, Zheng Q, Zou B, Yeo YH, Li X, Li J, et al. The epidemiology of NAFLD in Mainland China with analysis by adjusted gross regional domestic product: a meta-analysis. Hepatol Int 2020; 12:259–269. doi: 10.1007/s12072-020-10023-3. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Zhou F, Wang W, Zhang XJ, Ji YX, Zhang P, et al. Epidemiological features of NAFLD from 1999 to 2018 in China. Hepatology 2020; 71:1851–1864. doi: 10.1002/hep.31150. [DOI] [PubMed] [Google Scholar]
- 13.Rhee EJ. Nonalcoholic fatty liver disease and diabetes: An epidemiological perspective. Endocrinol Metab (Seoul) 2019; 34:226–233. doi: 10.3803/EnM.2019.34.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism 2019; 92:82–97. doi: 10.1016/j.metabol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2019; 4:389–398. doi: 10.1016/S2468-1253(19)30039-1. [DOI] [PubMed] [Google Scholar]
- 16.Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut 2010; 59:1410–1415. doi: 10.1136/gut.2010.213553. [DOI] [PubMed] [Google Scholar]
- 17.Chiang DJ, McCullough AJ. The impact of obesity and metabolic syndrome on alcoholic liver disease. Clin Liver Dis 2014; 18:157–163. doi: 10.1016/j.cld.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunt EM, Ramrakhiani S, Cordes BG, Neuschwander-Tetri BA, Janney CG, Bacon BR, et al. Concurrence of histologic features of steatohepatitis with other forms of chronic liver disease. Mod Pathol 2003; 16:49–56. doi: 10.1097/01.MP.0000042420.21088.C7. [DOI] [PubMed] [Google Scholar]
- 19.Choi H, Brouwer WP, Zanjir W, de Man RA, Feld JJ, Hansen BE, et al. Nonalcoholic steatohepatitis is associated with liver-related outcomes and all-cause mortality in chronic hepatitis B. Hepatology 2020; 71:539–548. doi: 10.1002/hep.30857. [DOI] [PubMed] [Google Scholar]
- 20.Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int 2020; 40:2082–2089. doi: 10.1111/liv.14548. [DOI] [PubMed] [Google Scholar]
- 21.Feldman A, Eder SK, Felder TK, Kedenko L, Paulweber B, Stadlmayr A, et al. Clinical and metabolic characterization of lean Caucasian subjects with non-alcoholic fatty liver. Am J Gastroenterol 2017; 112:102–110. doi: 10.1038/ajg.2016.318. [DOI] [PubMed] [Google Scholar]
- 22.Chen F, Esmaili S, Rogers GB, Bugianesi E, Petta S, Marchesini G, et al. Lean NAFLD: A distinct entity shaped by differential metabolic adaptation. Hepatology 2020; 71:1213–1227. doi: 10.1002/hep.30908. [DOI] [PubMed] [Google Scholar]
- 23.Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020; 5:739–752. doi: 10.1016/S2468-1253(20)30077-7. [DOI] [PubMed] [Google Scholar]
- 24.Zeng J, Yang RX, Sun C, Pan Q, Zhang RN, Chen GY, et al. Prevalence, clinical characteristics, risk factors, and indicators for lean Chinese adults with nonalcoholic fatty liver disease. World J Gastroenterol 2020; 26:1792–1804. doi: 10.3748/wjg.v26.i15.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology 1998; 114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 26.Fang YL, Chen H, Wang CL, Liang L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: from “two hit theory” to “multiple hit model”. World J Gastroenterol 2018; 24:2974–2983. doi: 10.3748/wjg.v24.i27.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng KI, Fan JG, Shi JP, Wong VW, Eslam M, George J, et al. From NAFLD to MAFLD: a “redefining” moment for fatty liver disease. Chin Med J 2020; 133:2271–2273. doi: 10.1097/CM9.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cusi K, Sanyal AJ, Zhang S, Hartman ML, Bue-Valleskey JM, Hoogwerf BJ, et al. Non-alcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes Obes Metab 2017; 19:1630–1634. doi: 10.1111/dom.12973. [DOI] [PubMed] [Google Scholar]
- 29.Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci USA 2010; 107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: clinical impact. J Hepatol 2018; 68:268–279. doi: 10.1016/j.jhep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Speliotes EK, Yerges-Armstrong LM, Wu J, Hernaez R, Kim LJ, Palmer CD, et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. Plos Genet 2011; 7:e1001324.doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjærg-Hansen A, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2014; 46:352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abul-Husn NS, Cheng X, Li AH, Xin Y, Schurmann C, Stevis P, et al. A protein-truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med 2018; 378:1096–1106. doi: 10.1056/NEJMoa1712191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahajan A, Wessel J, Willems SM, Zhao W, Robertson NR, Chu AY, et al. Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat Genet 2018; 50:559–571. doi: 10.1038/s41588-018-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyssenko V, Lupi R, Marchetti P, Del GS, Orho-Melander M, Almgren P, et al. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest 2007; 117:2155–2163. doi: 10.1172/JCI30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musso G, Cassader M, Bo S, De Michieli F, Gambino R. Sterol regulatory element-binding factor 2 (SREBF-2) predicts 7-year NAFLD incidence and severity of liver disease and lipoprotein and glucose dysmetabolism. Diabetes 2013; 62:1109–1120. doi: 10.2337/db12-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musso G, Gambino R, De Michieli F, Durazzo M, Pagano G, Cassader M. Adiponectin gene polymorphisms modulate acute adiponectin response to dietary fat: Possible pathogenetic role in NASH. Hepatology 2008; 47:1167–1177. doi: 10.1002/hep.22142. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Diaz-Del-Campo N, Abete I, Cantero I, Marin-Alejandre BA, Monreal JI, Elorz M, et al. Association of the SH2B1 rs7359397 gene polymorphism with steatosis severity in subjects with obesity and non-alcoholic fatty liver disease. Nutrients 2020; 12.doi: 10.3390/nu12051260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musso G, Gambino R, Pacini G, Pagano G, Durazzo M, Cassader M. Transcription factor 7-like 2 polymorphism modulates glucose and lipid homeostasis, adipokine profile, and hepatocyte apoptosis in NASH. Hepatology 2009; 49:426–435. doi: 10.1002/hep.22659. [DOI] [PubMed] [Google Scholar]
- 40.Ramya K, Ayyappa KA, Ghosh S, Mohan V, Radha V. Genetic association of ADIPOQ gene variants with type 2 diabetes, obesity and serum adiponectin levels in south Indian population. Gene 2013; 532:253–262. doi: 10.1016/j.gene.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Buch S, Stickel F, Trépo E, Way M, Herrmann A, Nischalke HD, et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet 2015; 47:1443–1448. doi: 10.1038/ng.3417. [DOI] [PubMed] [Google Scholar]
- 42.Eslam M, Mangia A, Berg T, Chan HL, Irving WL, Dore GJ, et al. Diverse impacts of the rs58542926 E167K variant in TM6SF2 on viral and metabolic liver disease phenotypes. Hepatology 2016; 64:34–46. doi: 10.1002/hep.28475. [DOI] [PubMed] [Google Scholar]
- 43.Mancina RM, Dongiovanni P, Petta S, Pingitore P, Meroni M, Rametta R, et al. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology 2016; 150:1219–1230. doi: 10.1053/j.gastro.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaxillaire M, Cavalcanti-Proença C, Dechaume A, Tichet J, Marre M, Balkau B, et al. The common P446L polymorphism in GCKR inversely modulates fasting glucose and triglyceride levels and reduces type 2 diabetes risk in the DESIR prospective general French population. Diabetes 2008; 57:2253–2257. doi: 10.2337/db07-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franko A, Merkel D, Kovarova M, Hoene M, Jaghutriz BA, Heni M, et al. Dissociation of fatty liver and insulin resistance in I148M PNPLA3 carriers: Differences in diacylglycerol (DAG) FA18:1 lipid species as a possible explanation. Nutrients 2018; 10.doi: 10.3390/nu10091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luukkonen PK, Zhou Y, Nidhina HP, Dwivedi OP, Hyötyläinen T, Ali A, et al. Impaired hepatic lipid synthesis from polyunsaturated fatty acids in TM6SF2 E167K variant carriers with NAFLD. J Hepatol 2017; 67:128–136. doi: 10.1016/j.jhep.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 47.Basaranoglu M, Basaranoglu G, Bugianesi E. Carbohydrate intake and nonalcoholic fatty liver disease: Fructose as a weapon of mass destruction. Hepatobiliary Surg Nutr 2015; 4:109–116. doi: 10.3978/j.issn.2304-3881.2014.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, et al. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol 2018; 68:1063–1075. doi: 10.1016/j.jhep.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology 2014; 59:713–723. doi: 10.1002/hep.26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gastaldelli A, Miyazaki Y, Pettiti M, Buzzigoli E, Mahankali S, Ferrannini E, et al. Separate contribution of diabetes, total fat mass, and fat topography to glucose production, gluconeogenesis, and glycogenolysis. J Clin Endocrinol Metab 2004; 89:3914–3921. doi: 10.1210/jc.2003-031941. [DOI] [PubMed] [Google Scholar]
- 51.Pang Y, Kartsonaki C, Turnbull I, Guo Y, Clarke R, Chen Y, et al. Diabetes, plasma glucose, and incidence of fatty liver, cirrhosis, and liver cancer: a prospective study of 0.5 million people. Hepatology 2018; 68:1308–1318. doi: 10.1002/hep.30083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hashiba M, Ono M, Hyogo H, Ikeda Y, Masuda K, Yoshioka R, et al. Glycemic variability is an independent predictive factor for development of hepatic fibrosis in nonalcoholic fatty liver disease. PloS One 2013; 8:e76161.doi: 10.1371/journal.pone.0076161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zelber-Sagi S, Lotan R, Shlomai A, Webb M, Harrari G, Buch A, et al. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. J Hepatol 2012; 56:1145–1151. doi: 10.1016/j.jhep.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Burgeiro A, Cerqueira MG, Varela-Rodríguez BM, Nunes S, Neto P, Pereira FC, et al. Glucose and lipid dysmetabolism in a rat model of prediabetes induced by a high-sucrose diet. Nutrients 2017; 9.doi: 10.3390/nu9060638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsuda S, Kobayashi M, Kitagishi Y. Roles for PI3K/AKT/PTEN pathway in cell signaling of nonalcoholic fatty liver disease. ISRN Endocrinol 2013; 2013:472432.doi: 10.1155/2013/472432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rebollo A, Roglans N, Baena M, Padrosa A, Sánchez RM, Merlos M, et al. Liquid fructose down-regulates liver insulin receptor substrate 2 and gluconeogenic enzymes by modifying nutrient sensing factors in rats. J Nutr Biochem 2014; 25:250–258. doi: 10.1016/j.jnutbio.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 57.Aller R, Sigüenza R, Pina M, Laserna C, Antolín B, Burgueño B, et al. Insulin resistance is related with liver fibrosis in type 2 diabetic patients with non-alcoholic fatty liver disease proven biopsy and Mediterranean diet pattern as a protective factor. Endocrine 2020; 68:557–563. doi: 10.1007/s12020-020-02268-7. [DOI] [PubMed] [Google Scholar]
- 58.Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ Res 2020; 126:1549–1564. doi: 10.1161/CIRCRESAHA.119.315896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weng JP, Bi Y, Zhu DL, Guan YF. Non-alcoholic fatty liver disease: a starting point of the onset of type 2 diabetes mellitus? (in Chinese). J Univ Sci Tech China 2018; 48:777–780. doi: CNKI:SUN:ZKJD.0.2018-10-001. [Google Scholar]
- 60.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006; 116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011; 11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 62.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 2005; 11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salvadó L, Palomer X, Barroso E, Vázquez-Carrera M. Targeting endoplasmic reticulum stress in insulin resistance. Trends Endocrinol Metab 2015; 26:438–448. doi: 10.1016/j.tem.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Yin X, Zheng F, Pan Q, Zhang S, Yu D, Xu Z, et al. Glucose fluctuation increased hepatocyte apoptosis under lipotoxicity and the involvement of mitochondrial permeability transition opening. J Mol Endocrinol 2015; 55:169–181. doi: 10.1530/JME-15-0101. [DOI] [PubMed] [Google Scholar]
- 65.Lindeboom L, Nabuurs CI, Hesselink MK, Wildberger JE, Schrauwen P, Schrauwen-Hinderling VB. Proton magnetic resonance spectroscopy reveals increased hepatic lipid content after a single high-fat meal with no additional modulation by added protein. Am J Clin Nutr 2015; 101:65–71. doi: 10.3945/ajcn.114.094730. [DOI] [PubMed] [Google Scholar]
- 66.Turner N, Kowalski GM, Leslie SJ, Risis S, Yang C, Lee-Young RS, et al. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 2013; 56:1638–1648. doi: 10.1007/s00125-013-2913-1. [DOI] [PubMed] [Google Scholar]
- 67.Parry SA, Rosqvist F, Mozes FE, Cornfield T, Hutchinson M, Piche ME, et al. Intrahepatic fat and postprandial glycemia increase after consumption of a diet enriched in saturated fat compared with free sugars. Diabetes Care 2020; 43:1134–1141. doi: 10.2337/dc19-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005; 115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang D, Wang A, et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest 2006; 116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dentin R, Benhamed F, Hainault I, Fauveau V, Foufelle F, Dyck JR, et al. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes 2006; 55:2159–2170. doi: 10.2337/db06-0200. [DOI] [PubMed] [Google Scholar]
- 71.Item F, Konrad D. Visceral fat and metabolic inflammation: the portal theory revisited. Obes Rev 2012; 13: Suppl 2: 30–39. doi: 10.1111/j.1467-789X.2012.01035.x. [DOI] [PubMed] [Google Scholar]
- 72.Petersen MC, Shulman GI. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends Pharmacol Sci 2017; 38:649–665. doi: 10.1016/j.tips.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumashiro N, Erion DM, Zhang D, Kahn M, Beddow SA, Chu X, et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci USA 2011; 108:16381–16385. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ter Horst KW, Gilijamse PW, Versteeg RI, Ackermans MT, Nederveen AJ, la Fleur SE, et al. Hepatic diacylglycerol-associated protein kinase C ε translocation links hepatic steatosis to hepatic insulin resistance in humans. Cell Rep 2017; 19:1997–2004. doi: 10.1016/j.celrep.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gluchowski NL, Gabriel KR, Chitraju C, Bronson RT, Mejhert N, Boland S, et al. Hepatocyte deletion of triglyceride-synthesis enzyme Acyl CoA: Diacylglycerol acyltransferase 2 reduces steatosis without increasing inflammation or fibrosis in mice. Hepatology 2019; 70:1972–1985. doi: 10.1002/hep.30765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jornayvaz FR, Birkenfeld AL, Jurczak MJ, Kanda S, Guigni BA, Jiang DC, et al. Hepatic insulin resistance in mice with hepatic overexpression of diacylglycerol acyltransferase 2. Proc Natl Acad Sci U S A 2011; 108:5748–5752. doi: 10.1073/pnas.1103451108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Longato L, Tong M, Wands JR, de la Monte SM. High fat diet induced hepatic steatosis and insulin resistance: role of dysregulated ceramide metabolism. Hepatol Res 2012; 42:412–427. doi: 10.1111/j.1872-034X.2011.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang M, Li C, Liu Q, Wang A, Lei M. Inhibiting ceramide synthesis attenuates hepatic steatosis and fibrosis in rats with non-alcoholic fatty liver disease. Front Endocrinol (Lausanne) 2019; 10:665.doi: 10.3389/fendo.2019.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zatterale F, Longo M, Naderi J, Raciti GA, Desiderio A, Miele C, et al. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front Physiol 2019; 10:1607.doi: 10.3389/fphys.2019.01607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jorge A, Andrade J, Paraíso AF, Jorge G, Silveira CM, de Souza LR, et al. Body mass index and the visceral adipose tissue expression of IL-6 and TNF-alpha are associated with the morphological severity of non-alcoholic fatty liver disease in individuals with class III obesity. Obes Res Clin Pract 2018; 12:1–8. doi: 10.1016/j.orcp.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 81.Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol 2018; 68:280–295. doi: 10.1016/j.jhep.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 82.Di Maira G, Pastore M, Marra F. Liver fibrosis in the context of nonalcoholic steatohepatitis: the role of adipokines. Minerva Gastroenterol Dietol 2018; 64:39–50. doi: 10.23736/S1121-421X.17.02427-8. [DOI] [PubMed] [Google Scholar]
- 83.Remmerie A, Martens L, Scott CL. Macrophage subsets in obesity, aligning the liver and adipose tissue. Front Endocrinol (Lausanne) 2020; 11:259.doi: 10.3389/fendo.2020.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang JZ, Cai JJ, Yu Y, She ZG, Li H. Nonalcoholic fatty liver disease: an update on the diagnosis. Gene Expr 2019; 19:187–198. doi: 10.3727/105221619X15553433838609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 2011; 54:1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maurice J, Manousou P. Non-alcoholic fatty liver disease. Clin Med (Lond) 2018; 18:245–250. doi: 10.7861/clinmedicine.18-3-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Esterson YB, Grimaldi GM. Radiologic imaging in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Clin Liver Dis 2018; 22:93–108. doi: 10.1016/j.cld.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 88.Lee DH. Imaging evaluation of non-alcoholic fatty liver disease: Focused on quantification. Clin Mol Hepatol 2017; 23:290–301. doi: 10.3350/cmh.2017.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology 2016; 150:626–637. doi: 10.1053/j.gastro.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 90.Mikolasevic I, Milic S, Orlic L, Stimac D, Franjic N, Targher G. Factors associated with significant liver steatosis and fibrosis as assessed by transient elastography in patients with one or more components of the metabolic syndrome. J Diabetes Complications 2016; 30:1347–1353. doi: 10.1016/j.jdiacomp.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 91.Roulot D, Roudot-Thoraval F, NKontchou G, Kouacou N, Costes JL, Elourimi G, et al. Concomitant screening for liver fibrosis and steatosis in French type 2 diabetic patients using Fibroscan. Liver Int 2017; 37:1897–1906. doi: 10.1111/liv.13481. [DOI] [PubMed] [Google Scholar]
- 92.Wong VW, Chan WK, Chitturi S, Chawla Y, Dan YY, Duseja A, et al. Asia-pacific working party on non-alcoholic fatty liver disease guidelines 2017-part 1: definition, risk factors and assessment. J Gastroenterol Hepatol 2018; 33:70–85. doi: 10.1111/jgh.13857. [DOI] [PubMed] [Google Scholar]
- 93.Sobhonslidsuk A, Pulsombat A, Kaewdoung P, Petraksa S. Non-alcoholic fatty liver disease (NAFLD) and significant hepatic fibrosis defined by non-invasive assessment in patients with type 2 diabetes. Asian Pac J Cancer Prev 2015; 16:1789–1794. doi: 10.7314/apjcp.2015.16.5.1789. [DOI] [PubMed] [Google Scholar]
- 94.Tuong T, Tran DK, Phu P, Hong T, Dinh TC, Chu DT. Non-alcoholic fatty liver disease in patients with type 2 diabetes: evaluation of hepatic fibrosis and steatosis using Fibroscan. Diagnostics (Basel) 2020; 10:159.doi: 10.3390/diagnostics10030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee DH. Noninvasive evaluation of nonalcoholic fatty liver disease. Endocrinol Metab (Seoul) 2020; 35:243–259. doi: 10.3803/EnM.2020.35.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.European Association for Study of Liver. Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015; 63:237–264. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 97.Bril F, McPhaul MJ, Caulfield MP, Castille JM, Poynard T, Soldevila-Pico C, et al. Performance of the SteatoTest, ActiTest, NashTest and FibroTest in a multiethnic cohort of patients with type 2 diabetes mellitus. J Investig Med 2019; 67:303–311. doi: 10.1136/jim-2018-000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen LW, Huang PR, Chien CH, Lin CL, Chien RN. A community-based study on the application of fatty liver index in screening subjects with nonalcoholic fatty liver disease. J Formos Med Assoc 2020; 119:173–181. doi: 10.1016/j.jfma.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 99.Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. The visceral adiposity index is a predictor of incident nonalcoholic fatty liver disease: a population-based longitudinal study. Clin Res Hepatol Gastroenterol 2020; 44:375–383. doi: 10.1016/j.clinre.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 100.Lin B, Ma Y, Wu S, Liu Y, Liu L, Wu L. Novel serum biomarkers for noninvasive diagnosis and screening of nonalcoholic fatty liver disease-related hepatic fibrosis. Omics 2019; 23:181–189. doi: 10.1089/omi.2019.0035. [DOI] [PubMed] [Google Scholar]
- 101.Xin S, Zhan Q, Chen X, Xu J, Yu Y. Efficacy of serum miRNA test as a non-invasive method to diagnose nonalcoholic steatohepatitis: a systematic review and meta-analysis. BMC Gastroenterol 2020; 20:186.doi: 10.1186/s12876-020-01334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gunn NT, Shiffman ML. The use of liver biopsy in nonalcoholic fatty liver disease: when to biopsy and in whom. Clin Liver Dis 2018; 22:109–119. doi: 10.1016/j.cld.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 103.Zhang JZ, Cai JJ, Yu Y, She ZG, Li HL. Nonalcoholic fatty liver disease: An update on the diagnosis. Gene Expr 2019; 19:187–198. doi: 10.3727/105221619X15553433838609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Castera L. Diagnosis of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: non-invasive tests are enough. Liver Int 2018; 38: Suppl 1: 67–70. doi: 10.1111/liv.13658. [DOI] [PubMed] [Google Scholar]
- 105.Boyd A, Cain O, Chauhan A, Webb GJ. Medical liver biopsy: background, indications, procedure and histopathology. Frontline Gastroenterol 2020; 11:40–47. doi: 10.1136/flgastro-2018-101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Polyzos SA, Kang ES, Boutari C, Rhee EJ, Mantzoros CS. Current and emerging pharmacological options for the treatment of nonalcoholic steatohepatitis. Metabolism 2020; 154203.doi: 10.1016/j.metabol.2020.154203. [DOI] [PubMed] [Google Scholar]
- 107.Francque S, Vonghia L. Pharmacological treatment for non-alcoholic fatty liver disease. Adv Ther 2019; 36:1052–1074. doi: 10.1007/s12325-019-00898-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lonardo A, Ballestri S, Targher G, Loria P. Diagnosis and management of cardiovascular risk in nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol 2015; 9:629–650. doi: 10.1586/17474124.2015.965143. [DOI] [PubMed] [Google Scholar]
- 109.Yki-Järvinen H. Thiazolidinediones. N Engl J Med 2004; 351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 110.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010; 362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med 2016; 165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 112.Bril F, Biernacki DM, Kalavalapalli S, Lomonaco R, Subbarayan SK, Lai J, et al. Role of vitamin E for nonalcoholic steatohepatitis in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care 2019; 42:1481–1488. doi: 10.2337/dc19-0167. [DOI] [PubMed] [Google Scholar]
- 113.Yau H, Rivera K, Lomonaco R, Cusi K. The future of thiazolidinedione therapy in the management of type 2 diabetes mellitus. Curr Diab Rep 2013; 13:329–341. doi: 10.1007/s11892-013-0378-8. [DOI] [PubMed] [Google Scholar]
- 114.Schürks M, Glynn RJ, Rist PM, Tzourio C, Kurth T. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ 2010; 341:c5702.doi: 10.1136/bmj.c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.American Diabetes Association. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care 2018; 41:S73–S85. doi: 10.2337/dc18-S008. [DOI] [PubMed] [Google Scholar]
- 116.Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Rep 2013; 1:57–64. doi: 10.3892/br.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia 2017; 60:1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vilar-Gomez E, Vuppalanchi R, Desai AP, Gawrieh S, Ghabril M, Saxena R, et al. Long-term metformin use may improve clinical outcomes in diabetic patients with non-alcoholic steatohepatitis and bridging fibrosis or compensated cirrhosis. Aliment Pharmacol Ther 2019; 50:317–328. doi: 10.1111/apt.15331. [DOI] [PubMed] [Google Scholar]
- 119.Iogna PL, Tsochatzis EA. The effect of antidiabetic medications on non-alcoholic fatty liver disease (NAFLD). Hormones (Athens) 2018; 17:219–229. doi: 10.1007/s42000-018-0021-9. [DOI] [PubMed] [Google Scholar]
- 120.Ranjbar G, Mikhailidis DP, Sahebkar A. Effects of newer antidiabetic drugs on nonalcoholic fatty liver and steatohepatitis: think out of the box!. Metabolism 2019; 101:154001.doi: 10.1016/j.metabol.2019.154001. [DOI] [PubMed] [Google Scholar]
- 121.Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, et al. 2019 Update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020; 43:487–493. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 2019; 7:776–785. doi: 10.1016/S2213-8587 (19)30249-9. [DOI] [PubMed] [Google Scholar]
- 123.Cegla J. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomized, placebo-controlled phase 2 study. Ann Clin Biochem 2016; 53:518.doi: 10.1177/0004563216648250. [DOI] [PubMed] [Google Scholar]
- 124.Eguchi Y, Kitajima Y, Hyogo H, Takahashi H, Kojima M, Ono M, et al. Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J). Hepatol Res 2015; 45:269–278. doi: 10.1111/hepr.12351. [DOI] [PubMed] [Google Scholar]
- 125.Pratley RE, Aroda VR, Lingvay I, Lüdemann J, Andreassen C, Navarria A, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): A randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol 2018; 6:275–286. doi: 10.1016/S2213-8587 (18)30024-X. [DOI] [PubMed] [Google Scholar]
- 126.Newsome P, Francque S, Harrison S, Ratziu V, Van Gaal L, Calanna S, et al. Effect of semaglutide on liver enzymes and markers of inflammation in subjects with type 2 diabetes and/or obesity. Aliment Pharmacol Ther 2019; 50:193–203. doi: 10.1111/apt.15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dougherty JA, Guirguis E, Thornby KA. A systematic review of newer antidiabetic agents in the treatment of nonalcoholic fatty liver disease. Ann Pharmacother 2020; published ahead of print. doi: 10.1177/1060028020935105. [DOI] [PubMed] [Google Scholar]
- 128.Dougherty JA, Guirguis E, Thornby KA. A systematic review of newer antidiabetic agents in the treatment of nonalcoholic fatty liver disease. Ann Pharmacother 2020; 27511233.doi: 10.1177/1060028020935105. [DOI] [PubMed] [Google Scholar]
- 129.Vivian EM. Sodium-glucose co-transporter 2 (SGLT2) inhibitors: a growing class of antidiabetic agents. Drugs Context 2014; 3:212264.doi: 10.7573/dic.212264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xing B, Zhao Y, Dong B, Zhou Y, Lv W, Zhao W. Effects of SGLT2 inhibitors on nonalcoholic fatty liver disease in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. J Diabetes Investig 2020; 11:1238–1247. doi: 10.1111/jdi.13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lai LL, Vethakkan SR, Nik MN, Mahadeva S, Chan WK. Empagliflozin for the treatment of nonalcoholic steatohepatitis in patients with type 2 diabetes mellitus. Dig Dis Sci 2020; 65:623–631. doi: 10.1007/s10620-019-5477-1. [DOI] [PubMed] [Google Scholar]
- 132.Ray EC. Evolving understanding of cardiovascular protection by SGLT2 inhibitors: Focus on renal protection, myocardial effects, uric acid, and magnesium balance. Curr Opin Pharmacol 2020; 54:11–17. doi: 10.1016/j.coph.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ludvik B, Frías JP, Tinahones FJ, Wainstein J, Jiang H, Robertson KE, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2018; 6:370–381. doi: 10.1016/S2213-8587 (18)30023-8. [DOI] [PubMed] [Google Scholar]
- 134.Ishihara H, Yamaguchi S, Nakao I, Sakatani T. Ipragliflozin add-on therapy to a GLP-1 receptor agonist in Japanese patients with type 2 diabetes (AGATE): a 52-week open-label study. Diabetes Ther 2018; 9:1549–1567. doi: 10.1007/s13300-018-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kothari S, Dhami-Shah H, Shah SR. Antidiabetic drugs and statins in nonalcoholic fatty liver disease. J Clin Exp Hepatol 2019; 9:723–730. doi: 10.1016/j.jceh.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dongiovanni P, Petta S, Mannisto V, Mancina RM, Pipitone R, Karja V, et al. Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol 2015; 63:705–712. doi: 10.1016/j.jhep.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 137.El-Serag HB, Johnson ML, Hachem C, Morgana RO. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology 2009; 136:1601–1608. doi: 10.1053/j.gastro.2009.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim G, Jang SY, Nam CM, Kang ES. Statin use and the risk of hepatocellular carcinoma in patients at high risk: A nationwide nested case-control study. J Hepatol 2018; 68:476–484. doi: 10.1016/j.jhep.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 139.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 2009; 89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 140.Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013; 145:574–582. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 141.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015; 385:956–965. doi: 10.1016/S0140-6736 (14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: Interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019; 394:2184–2196. doi: 10.1016/S0140-6736 (19)33041-7. [DOI] [PubMed] [Google Scholar]
- 143.Hameed B, Terrault NA, Gill RM, Loomba R, Chalasani N, Hoofnagle JH, et al. Clinical and metabolic effects associated with weight changes and obeticholic acid in non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2018; 47:645–656. doi: 10.1111/apt.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Siddiqui MS, Van Natta ML, Connelly MA, Vuppalanchi R, Neuschwander-Tetri BA, Tonascia J, et al. Impact of obeticholic acid on the lipoprotein profile in patients with non-alcoholic steatohepatitis. J Hepatol 2020; 72:25–33. doi: 10.1016/j.jhep.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pockros PJ, Fuchs M, Freilich B, Schiff E, Kohli A, Lawitz EJ, et al. CONTROL: a randomized phase 2 study of obeticholic acid and atorvastatin on lipoproteins in nonalcoholic steatohepatitis patients. Liver Int 2019; 39:2082–2093. doi: 10.1111/liv.14209. [DOI] [PubMed] [Google Scholar]
- 146.Xia MF, Lin HD, Chen LY, Wu L, Ma H, Li Q, et al. The PNPLA3 rs738409 C>G variant interacts with changes in body weight over time to aggravate liver steatosis, but reduces the risk of incident type 2 diabetes. Diabetologia 2019; 62:644–654. doi: 10.1007/s00125-018-4805-x. [DOI] [PubMed] [Google Scholar]
- 147.Valenti L, Rumi M, Galmozzi E, Aghemo A, Del MB, De Nicola S, et al. Patatin-like phospholipase domain-containing 3 I148M polymorphism, steatosis, and liver damage in chronic hepatitis C. Hepatology 2011; 53:791–799. doi: 10.1002/hep.24123. [DOI] [PubMed] [Google Scholar]
- 148.Viganò M, Valenti L, Lampertico P, Facchetti F, Motta BM, D’Ambrosio R, et al. Patatin-like phospholipase domain-containing 3 I148M affects liver steatosis in patients with chronic hepatitis B. Hepatology 2013; 58:1245–1252. doi: 10.1002/hep.26445. [DOI] [PubMed] [Google Scholar]
- 149.Li JZ, Huang Y, Karaman R, Ivanova PT, Brown HA, Roddy T, et al. Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis. J Clin Invest 2012; 122:4130–4144. doi: 10.1172/JCI65179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pirazzi C, Adiels M, Burza MA, Mancina RM, Levin M, Ståhlman M, et al. Patatin-like phospholipase domain-containing 3 (PNPLA3) I148M (rs738409) affects hepatic VLDL secretion in humans and in vitro. J Hepatol 2012; 57:1276–1282. doi: 10.1016/j.jhep.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 151.Basantani MK, Sitnick MT, Cai L, Brenner DS, Gardner NP, Li JZ, et al. Pnpla3/Adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. J Lipid Res 2011; 52:318–329. doi: 10.1194/jlr.M011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dongiovanni P, Petta S, Maglio C, Fracanzani AL, Pipitone R, Mozzi E, et al. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology 2015; 61:506–514. doi: 10.1002/hep.27490. [DOI] [PubMed] [Google Scholar]
- 153.Rees MG, Wincovitch S, Schultz J, Waterstradt R, Beer NL, Baltrusch S, et al. Cellular characterisation of the GCKR P446L variant associated with type 2 diabetes risk. Diabetologia 2012; 55:114–122. doi: 10.1007/s00125-011-2348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Thabet K, Asimakopoulos A, Shojaei M, Romero-Gomez M, Mangia A, Irving WL, et al. MBOAT7 rs641738 increases risk of liver inflammation and transition to fibrosis in chronic hepatitis C. Nat Commun 2016; 7:12757.doi: 10.1038/ncomms12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Thabet K, Chan H, Petta S, Mangia A, Berg T, Boonstra A, et al. The membrane-bound O-acyltransferase domain-containing 7 variant rs641738 increases inflammation and fibrosis in chronic hepatitis B. Hepatology 2017; 65:1840–1850. doi: 10.1002/hep.29064. [DOI] [PubMed] [Google Scholar]
- 156.Thangapandi VR, Knittelfelder O, Brosch M, Patsenker E, Vvedenskaya O, Buch S, et al. Loss of hepatic Mboat7 leads to liver fibrosis. Gut 2020; published ahead of print. doi: 10.1136/gutjnl-2020-320853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stickel F, Lutz P, Buch S, Nischalke HD, Silva I, Rausch V, et al. Genetic variation in HSD17B13 reduces the risk of developing cirrhosis and hepatocellular carcinoma in alcohol misusers. Hepatology 2020; 72:88–102. doi: 10.1002/hep.30996. [DOI] [PubMed] [Google Scholar]
- 158.Yang J, Trépo E, Nahon P, Cao Q, Moreno C, Letouzé E, et al. A 17-beta-hydroxysteroid dehydrogenase 13 variant protects from hepatocellular carcinoma development in alcoholic liver disease. Hepatology 2019; 70:231–240. doi: 10.1002/hep.30623. [DOI] [PubMed] [Google Scholar]
- 159.Ma Y, Belyaeva OV, Brown PM, Fujita K, Valles K, Karki S, et al. 17-beta hydroxysteroid dehydrogenase 13 is a hepatic retinol dehydrogenase associated with histological features of nonalcoholic fatty liver disease. Hepatology 2019; 69:1504–1519. doi: 10.1002/hep.30350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sato K, Gosho M, Yamamoto T, Kobayashi Y, Ishii N, Ohashi T, et al. Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: A meta-analysis of randomized controlled trials. Nutrition 2015; 31:923–930. doi: 10.1016/j.nut.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 161.Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis Heart Study randomized clinical trial. Am J Gastroenterol 2011; 106:71–77. doi: 10.1038/ajg.2010.299. [DOI] [PubMed] [Google Scholar]


