To the Editor: Psoriasis is a chronic inflammatory proliferative disease mediated by T lymphocytes under a multi-gene genetic background.[1] Moreover, its pathogenesis is not fully understood. Recent research shows that T cell-mediated immune inflammation plays a key role in the pathophysiology of psoriasis, and its most common downstream mediator, interleukin (IL)-17, is the primary effector cytokine in this condition. Secukinumab is a high-affinitive, fully humanized, G1κ monoclonal antibody that can simultaneously block IL-17A from multiple sources.[2] In a global, core, phase III trial, secukinumab was confirmed to rapidly improve psoriasis symptoms; however, the efficacy of its use in Chinese population remains unknown. This study was performed in a Chinese population for a period of 16 weeks to explore the efficacy and safety of secukinumab after its introduction in clinical settings in May 2019.
A total of 54 patients with moderate-to-severe psoriasis, who were treated at the Guangzhou Institute of Dermatology and Guangdong Second Provincial General Hospital from May 23, 2019, to March 2020, were enrolled. These comprised 41 males and 13 females with the mean age of 36.5 ± 3.6 years (18–58 years); the course of the disease was 2 to 32 years (9.6 ± 5.5 years). Exclusion criteria included the following: systematic use of methotrexate, acitretin capsules, immunosuppressive agents, and biological agents in the past 6 months; patients under additional conditions such as infection, pregnancy, childbirth, and trauma; patients with malignant tumors or low immunity; patients with active tuberculosis, hepatitis B, hepatitis C, or abnormal liver and kidney function; and patients with human immunodeficiency virus infection.
Fifty-four patients received secukinumab (COSENTYX; Novartis, Basel, Switzerland) monotherapy comprising initial subcutaneous injections at 0, 1, 2, 3, and 4 weeks; the dose was then maintained once every 4 weeks until 16 weeks. Drug was injected subcutaneously into both the arms (150 mg each). Psoriasis area and severity index (PASI) and dermatology life quality index (DLQI) were recorded for patients at different periods. Physician's Global Assessment (PGA) improvement, observed clinical efficacy, and adverse drug reactions were considered statistically significant at P < 0.05.
All patients were treated and observed for 16 weeks. At 2 weeks, a PASI of 75 was noted in three patients for the first time; at 3 weeks, a PASI of 90 was noted in three patients (5.6%) for the first time; at 4 weeks, a PASI of 75 was noted in 34 patients (63.0%) and PASI of 100 in one patient (1.7%) for the first time; at 8 weeks, a PASI of 50 and 90 was noted in 54 (100.0%) and 29 patients (53.7%), respectively. At 12 weeks, 52 patients (96.3%) attained a PASI of 75; at 16 weeks, 52 patients (96.3%) attained a PASI of 75, 46 (85.2%) attained a PASI of 90, and 17 (31.5%) attained a PASI of 100. Overall the PASI decline continued to 16 weeks, and the average PASI decreased by more than 50% in the third week [Figure 1A and 1B]. DLQI of 54 patients at 2, 4, 8, and 12 weeks after treatment was significantly lower than that before treatment. Average DLQI at 16 weeks was only 1.25 ± 0.96 points, which was significantly lower than that before treatment (t = 10.88, P < 0.0001) [Figure 1C]. The PGA score of 54 patients at 16 weeks was significantly lower than that before treatment (t = 14.83, P < 0.0001) [Figure 1D]. Efficacy of treatment in one patient is shown below [Figure 1E–1G].
Figure 1.
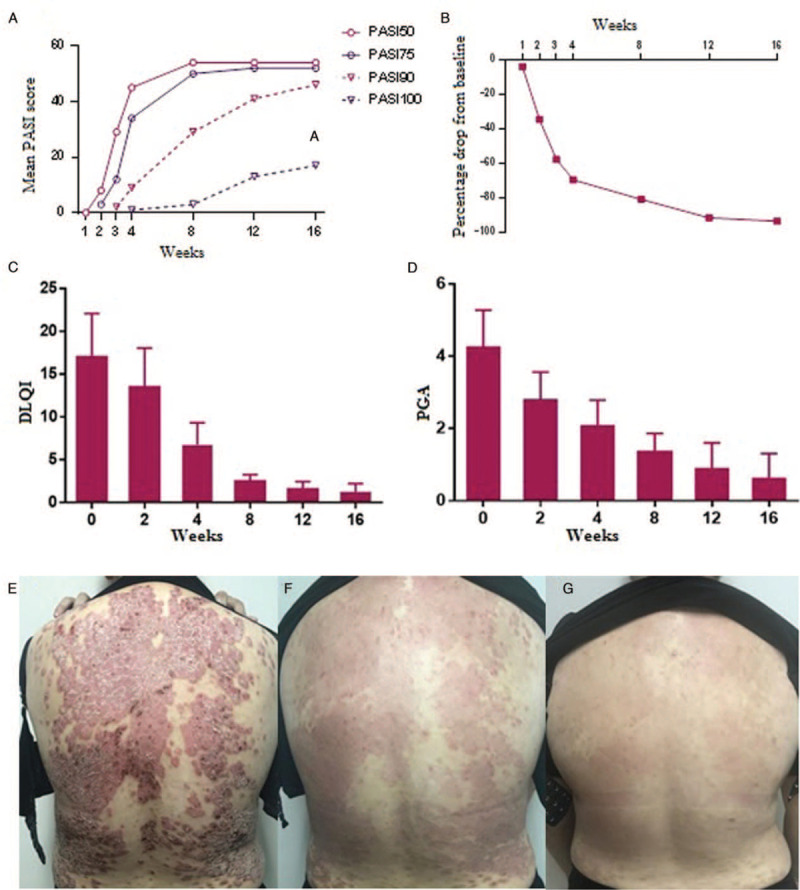
(A) Summary of a 16-week response to secukinumab. (B) Percentage change between values at baseline and those until 16 weeks. (C) Comparison of DLQI before and after treatment. (D) Comparison of PGA before and after treatment. (E–G) Conditions of one patient with psoriasis after treatment (0–8 weeks). DLQI: Dermatology life quality index; PASI: Psoriasis area and severity index; PGA: Physician's global assessment.
No serious adverse drug reactions and deaths occurred in any of the 54 patients during treatment and follow-up, and patients with adverse reactions did not discontinue the drug. Among those with adverse drug reactions, seven patients with latent tuberculosis did not exhibit reactivation of tuberculosis during treatment owing to our strict monitoring protocol and the preventive treatment administered to few patients. While using the biological agent, two patients with hepatitis B virus (HBV) infection were administered antiviral therapy and underwent liver function monitoring and detailed follow-up for HBV DNA. However, no abnormalities were recorded for any indicator within 16 weeks. Eight patients received the injections at home during the coronavirus disease (COVID-19) pandemic, and they reported no infection at the injection site.
Since long, the systemic treatment of psoriasis has relied on traditional medicines, including retinoic acid drugs, immunosuppressants, and light therapy. Traditional treatment methods have limited efficacy and many side effects. Secukinumab, which targets IL-17A and inhibits its binding to IL-17 receptors, can prevent the release of pro-inflammatory cytokines, chemokines, and tissue damage mediators, thus reducing IL-17A-mediated autoimmunity and inflammation.[3]
In the 54 Chinese patients we observed, secukinumab demonstrated significant clinical efficacy. In the 4th week of treatment, the number of patients with a PASI of 75 significantly increased, indicating that more than half of the patients (63.0%) experienced rapid remission; moreover, for the first time, one patient had a PASI of 100. In addition, secukinumab acts rapidly with high efficacy in psoriasis. After comparing clinical studies in Europe and the United States,[4,5] we found that at 16 weeks, the response rate among Chinese patients with a PASI of 75 (96.3%) and 90 (85.2%) was higher than that among European and American patients with a PASI of 75 (93.1%) and 90 (79%), suggesting that IL-17 inhibitors are more suitable for Asians. After treatment, DLQI and PGA were significantly lower than those before treatment, which significantly improved the adherence of patients with psoriasis to maintenance treatment.
During the treatment and follow-up of the Chinese patients in this study, we noted no serious adverse reactions leading to drug discontinuation as well as no malignant tumors or serious infections. During the treatment and follow-up of patients with latent tuberculosis and HBV infection, no incidences of reactivation were observed. Owing to the COVID-19 pandemic, in February 2020, eight patients completed drug regimen at home according to doctor's guidance and operating standards; moreover, no injection site reaction or infections were noted during the follow-up, reflecting good patient compliance with the drug and administration convenience during the critical period. However, biological inhibitors may lead to immunosuppression, which may increase the risk of infection. Therefore, patients should improve immunity during secukinumab administration.
Above all, the assessment of 54 patients with psoriasis treated with secukinumab showed that subcutaneously administered secukinumab has a high response rate in Chinese population and can quickly alleviate the disease, with few adverse reactions. Secukinumab can be used as a novel alternative for the treatment of patients with moderate and severe psoriasis who cannot be effectively treated with traditional therapeutics. However, owing to the limited observation time in this study, the maintenance effect and safety of the drug after skin lesion removal could not be observed, and these require further observation and follow-up.
Funding
This study was supported by a grant from the Guangzhou Medical and Health Technology General Guidance Project (20171A011285).
Conflicts of interest
None.
Footnotes
How to cite this article: Zeng JX, Luo Q, Wen J, Tian X, Zhou X, Li W, Tang YP, Zhang SQ, Liu WY, Zhu HL, Zhang XB. Real-world investigation of the efficacy and safety of secukinumab for psoriasis treatment in a Chinese population. Chin Med J 2021;134:117–119. doi: 10.1097/CM9.0000000000001179
Jing-Xin Zeng and Quan Luo contributed equally to this work.
References
- 1.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009; 361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 2.Krstic A, Mojsilovic S, Jovcic G, Bugarski A. The potential of interleukin-17 to mediate hematopoietic response. Immunol Res 2012; 52:34–41. doi: 10.1007/s12026-012-8276-8. [DOI] [PubMed] [Google Scholar]
- 3.McInnes BI, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin TC, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015; 386:1137–1146. doi: 10.1016/S0140-6736(15)61134-5. [DOI] [PubMed] [Google Scholar]
- 4.Fraser KA. American Academy of Dermatology annual meeting: Washington, DC, USA, 1-5 March 2019. J Am Clin Dermatol 2019; 20:307–310. doi: 10.1007/s40257-019-00433-x. [DOI] [PubMed] [Google Scholar]
- 5.Blauvelt A, Reich K, Tsai TF, Tyring S, Vanaclocha F, Kingo K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol 2017; 76:60–69. doi: 10.1016/j.jaad.2016.08.008. [DOI] [PubMed] [Google Scholar]


