Abstract
Background
A novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified at the end of 2019. The disease caused by SARS-CoV-2 was named COVID-19. The main purpose of this review was to provide an overview of SARS-CoV-2.
Methods
The authors searched the MEDLINE database for clinical studies related to virus characteristics, pathogenesis, diagnosis, transmission mechanisms, and treatment options.
Results
As of January 27, 2021, the number of infected people and deaths associated with COVID-19 worldwide were approximately 100 million and 2 million, respectively. The manifestations of COVID-19 are variable, and the severity is affected by age and preexisting medical conditions. Children and adolescents are usually asymptomatic or have mild symptoms. Older adults, in comparison, may experience severe illness and have disproportionally elevated mortality. Among those who survive, some may experience enduring deficits. The viral load is particularly elevated in saliva and oropharynx, which constitute potential sources of infection. The diagnosis of the disease may be confounded by factors related to the replicating cycle of the virus, viral load, and sensitivity of the diagnostic method used. As of January 2021, COVID-19 has no cure but can be prevented. Its treatment is based on supportive care along with antiviral medications and monoclonal antibodies. In severe cases with multiorgan involvement, mechanical ventilation, dialysis, and hemodynamic support may be necessary.
Conclusions
COVID-19 is a transmittable disease with a variable course. A substantial number of patients, particularly children, remain asymptomatic. Important advances have been made in the development of new treatments. However, the mortality in vulnerable populations remains elevated.
Practical Implications
The elevated viral load in the oral cavity and pharynx suggests that oral health care professionals could get infected through occupational exposure. Providers should understand the variables that influence the yield of diagnostic studies because false-negative results can occur.
Key Words: COVID-19, SARS-CoV-2, testing, treatment, epidemiology
Abbreviation Key: ACE2, Angiotensin-converting enzyme 2; CP, Convalescent plasma; ECMO, Extracorporeal membrane oxygenation; EUA, Emergency use authorization; FDA, US Food and Drug Administration; MIS-C, Multisystem inflammatory syndrome in children; PCR, Polymerase chain reaction; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
In December 2019, an outbreak of pneumonia of unidentified origin began in Hubei province of China, raising global health concerns owing to the ease of transmission and elevated case-fatality rate reported in vulnerable populations. Researchers discovered that the etiology was a new coronavirus, which they named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The illness caused by the virus was called COVID-19.1
Over the next few months, the viral infection spread rapidly to the rest of the world, causing a pandemic.2 On March 11, 2020, the World Health Organization announced a Public Health Emergency of International Concern. The crude mortality ratio, defined as the number of reported deaths divided by the reported cases, was originally reported as 3% through 4%. However, with the implementation of screening programs, milder and asymptomatic cases were identified, and the mortality ratio was estimated as 2% as of December 2020.3 In comparison, the annual mortality of seasonal influenza is less than 0.1%. In this article, we present an overview of the etiology, epidemiology, pathogenesis, diagnosis, and treatment of SARS-CoV-2 infection. The science of vaccines for SARS-CoV-2 is rapidly evolving and is beyond the scope of this review.
Viral Cycle and Structure
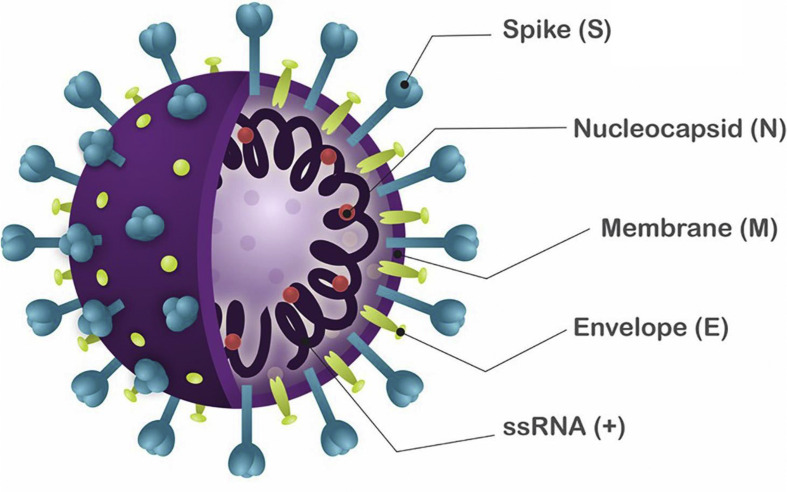
The first human coronavirus was isolated from a boy with a common cold in 1965.4 Coronaviruses are single-stranded RNA viruses with 4 structural proteins called spike glyoprotein, membrane protein, nucleoprotein, and envelope small membrane protein (Figure 1 ). The spike glycoprotein, a spikelike glycoprotein that radiates from the viral surface like a solar corona, has a critical role in the attachment of a virus to the host cell. The spike glycoprotein binds to the angiotensin-converting enzyme 2 (ACE2) receptor located on human epithelial cells, and this is followed by penetration of the cell. The expression of viral spike glycoprotein at the host cell membrane may facilitate cell-to-cell fusion, resulting in the formation of a syncytium, which permits the direct spread of coronaviruses between cells.6 , 7 SARS-CoV-2 has a particular tropism for tissues with elevated expression of ACE2 such as lung, intestine, kidney, and blood vessels.8 The membrane protein is the most abundant structural protein and defines the shape of the viral envelope. The envelope small membrane protein is the smallest structural protein and may activate the inflammasome to drive the hyperinflammatory response observed in COVID-19. The nucleoprotein binds to the viral genome, makes up the nucleocapsid, and has a role in the replication cycle.
Figure 1.
Schematic representation of the severe acute respiratory syndrome coronavirus 2. Reproduced from Santos and colleagues.5
It has been hypothesized that SARS-CoV-2 originated in horseshoe bats in China and jumped species into humans.9 COVID-19 constitutes the third coronavirus of zoonotic origin that infects humans. The first was the severe acute respiratory syndrome coronavirus that emerged in China in 2002, originating in civets. The second was the Middle Eastern respiratory syndrome coronavirus that appeared in Saudi Arabia in 2012, originating from dromedary camels.10
Epidemiology
People of all ages may be infected by SARS-CoV-2; however, almost 80% of cases of COVID-19 occur in adults aged 30 through 69 years. In addition, there is emerging evidence of maternal-fetal transmission in pregnant women infected with the virus.11 , 12
Although the number of cases is similar in males and females, the risk of death due to COVID-19 is higher in men. There are different hypotheses for this, including sex-specific differences in immunity, expression of ACE2, lifestyles, and the prevalence of comorbid conditions.11 , 12
Hispanic-Latino and Black people are 3 times more likely to become infected with COVID-19 and are nearly twice as likely to die as White people. The high burden of the disease in these diverse groups probably is related to the higher prevalence of preexisting medical conditions and lifestyles that prevent social distancing. As an example, the proportion of workers employed in service and manufacturing sectors is 25% for White people and 43% for non-White people. In addition, Hispanic-Latino and Black people are also twice as likely to live in crowded dwellings White people.13 The epidemiology of COVID-19 also may be influenced by circumstances that compromise social distancing, including civil unrest and social gatherings during holidays. However, the effect of these and other phenomena on the incidence of the disease are only partially understood.14 , 15
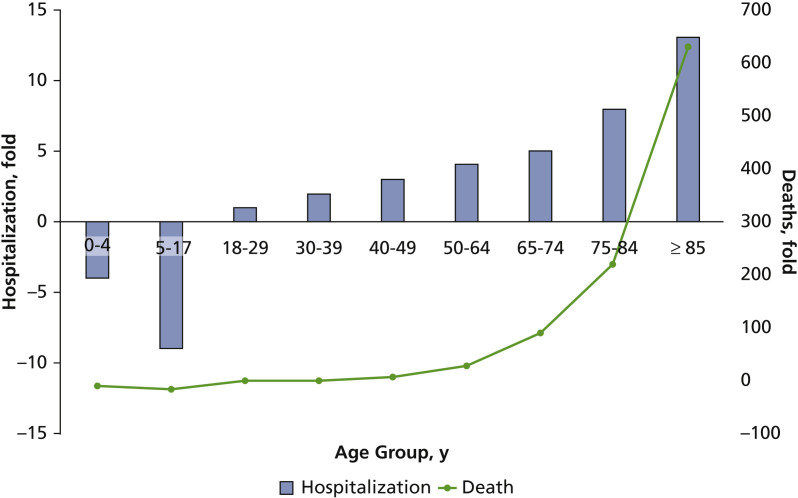
Risk factors for COVID-19 include older age, obesity, and chronic medical conditions, such as heart or lung disease and diabetes. According to the Centers for Disease Control and Prevention, patients with COVID-19 with underlying medical conditions are 6 times more likely to be hospitalized and 12 times more likely to die than those without.16 The need for hospitalization, as well as mortality, increase exponentially with age (Figure 2 ).17 , 18
Figure 2.
COVID-19 hospitalization and deaths by age. Unadjusted ratios expressed as fold relative to the 18- to 29-year-old age group. Source: Centers for Disease Control and Prevention.18
Transmission
The routes of human-to-human transmission of SARS-CoV-2 include direct inhalation of contaminated droplets released into the environment via sneezing or coughing and contact transmission via oral, nasal, and ocular mucous membranes.19 Microbes in aerosols (droplets greater than 5 micrometers in diameter) can be suspended in the air for a long period and transmitted to other people over distances of more than 3 feet, or 1 meter.20 Transmission may also occur through direct contact with infected objects.21
The number of people 1 person could infect is the reproductive number (also known as R0 or R-naught). SARS-CoV-2 has a reproductive number of 2 to 3. If each COVID-19–positive person infects 2 people, the size of the outbreak doubles quickly. Resistance to the spread of the virus can be improved by means of barrier measures (that is, use of masks and face shields), social distancing, and the development of population immunity.22 , 23 Herd immunity occurs when the infection is no longer growing exponentially; depending on multiple factors, it is estimated that this occurs when 60% to 80% of the population has developed immunity.24 Herd immunity provides indirect protection via minimizing the probability of contact between a susceptible person and an infected host. There are 2 possible approaches to build population immunity: natural immunity through exposure of a significant proportion of the population to the wild-type virus or artificial immunity through mass vaccination campaigns.
Establishing herd immunity through natural exposure is not a viable or safe strategy. In response to this dilemma, new vaccines against SARS-CoV-2 have been developed and are being implemented at the time of publication of this article. Long-term outcomes, including effectiveness in preventing viral transmission, preventing manifestations of COVID-19, safety, and duration of protection, are still unknown. Therefore, policies that raise awareness about protective measures such as mask wearing and strategies to prevent spread such as distancing, in combination with increased detection of asymptomatic carriers, contact tracing, and isolation, are likely to continue for some time despite the availability of new vaccines.
Timeline of the Disease
The incubation period is the time from infection to emergence of symptoms. For COVID-19, the incubation period ranges from 2 through 14 days, and 50% of those infected by the virus will become ill by day 5. The infectious period, in comparison, is the time during which an infected person may transmit the virus to others. In the case of COVID-19, the infectious period begins 2 days before the start of signs and symptoms and ends when at least 10 days have passed, symptoms have disappeared, and there has been no fever for the past 72 hours. In comparison, the incubation period for the flu is 1 through 4 days, and the infectious period 3 through 7 days. The extended incubation and infectious periods of COVID-19 enhance its spreading potential.25
Pathogenesis
Human ACE2, the major cell-surface receptor for the viral spike glycoprotein, provides the entry point for SARS-CoV-2 into the organism.26 In normal circumstances, ACE2 regulates blood pressure and inflammation. In pathologic conditions, ACE2 causes inflammation and tissue injury. The manifestation of COVID-19 is variable and may resemble seasonal flu (Box ).27 A considerable proportion of those infected remains asymptomatic. Most people experience mild to moderate disease and do not require hospitalization. Vulnerable populations may develop difficulty breathing, hemoptysis, dyspnea, chest pain, or hemodynamic instability, which requires urgent medical attention.
Box.
Signs and symptoms of COVID-19.∗
| ▪ Fever or chills ▪ Cough ▪ Shortness of breath or labored breathing ▪ Fatigue or malaise ▪ Myalgia ▪ Headache ▪ Ageusia or dysgeusia ▪ Anosmia ▪ Sore throat ▪ Rhinorrhea ▪ Nausea or vomiting ▪ Diarrhea ▪ Chest pain ▪ Conjunctivitis ▪ Confusion or lethargy |
Source: Centers for Disease Control and Prevention.27
The lungs are the primary site of the infection. The computed tomographic scans of infected patients' chests usually shows bilateral ground-glass opacities consistent with viral pneumonia.11 Thrombotic microangiopathy, hypercoagulability, hemorrhage, and severe systemic inflammation (called a cytokine storm) are important causes of multiorgan failure and death.28 Multifocal hepatic necrosis, lymphocytic infiltration, and steatosis have been described in the liver of patients with COVID-19 who have moderate to severe illness. In addition, mild myocardial hypertrophy and focal fibrosis have been reported in heart biopsies of patients with COVID-19.
When adequate oxygen saturation cannot be maintained spontaneously, mechanical ventilation support may be necessary. If this is insufficient, extracorporeal membrane oxygenation (ECMO) is used. With ECMO, the mortality rate of critically ill patients is 45%, in contrast to 60% through 70% with mechanical ventilation.29
Knowledge about the long-term sequelae of COVID-19 is just emerging. Some people, called long-haulers, have a protracted recovery and experience cough, fatigue, and low-grade fever that can linger for months after the original infection. Other symptoms include headache, neurocognitive decline, muscle pain, gastrointestinal dysfunction, shortness of breath, and chest pain.30 , 31 The pathogenesis of this phenomenon is poorly defined, and different mechanisms have been proposed, including chronic inflammatory or immune reaction, persistent viremia due to incomplete immune response to the infection, deconditioning, and psychogenic factors such as posttraumatic stress disorder.30
COVID-19 in Children and Adolescents
Approximately 2% through 5% of people with laboratory-confirmed COVID-19 are younger than 18 years. Screening efforts largely have targeted adults who are considered vulnerable.32 Thus, the epidemiology of SARS-CoV-2 infection in children is incomplete and rapidly evolving. The reasons for the low prevalence of COVID-19 observed in children relative to adults are not fully understood. ACE2 expression may be lower in pediatric populations. In addition, children may have a qualitatively different immune response to SARS-CoV-2 than adults. Furthermore, other viruses in the mucosa of the lungs and airways, common in young children, may compete with SARS-CoV-2 and limit its infectivity.33
Most children with a diagnosis of COVID-19 have mild symptoms and require only supportive care. However, despite being asymptomatic, children may have elevated viral loads and contribute to the spread of the infection.34 Similar to adults, the main signs and symptoms of the disease in children are cough and fever (Table 1 ).35 Other symptoms may include shortness of breath, pharyngeal erythema, abdominal pain, conjunctivitis, poor feeding, and generalized rash.36 Dysgeusia, with or without anosmia, is commonly reported in adults and may be an early or lone manifestation of COVID-19 infection; however, this is uncommon in children.36, 37, 38
Table 1.
Frequency of reported signs and symptoms of COVID-19 in pediatric and adult populations.∗
| SIGNS AND SYMPTOMS | PEDIATRIC, % | ADULT, % |
|---|---|---|
| Fever | 56 | 71 |
| Cough | 54 | 80 |
| Shortness of Breath | 13 | 43 |
| Fever, Cough, or Shortness of Breath | 73 | 93 |
| Headache | 28 | 58 |
| Sore Throat | 24 | 35 |
| Myalgia | 23 | 61 |
| Diarrhea | 13 | 31 |
| Nausea or Vomiting | 11 | 16 |
| Rhinorrhea | 7.2 | 6.9 |
| Abdominal Pain | 5.8 | 12 |
Source: Bialek and colleagues.35
The severe manifestation of COVID-19 in children is called multisystem inflammatory syndrome in children (MIS-C). It was first described in the United Kingdom by the Royal College of Pediatrics.39 It resembles Kawasaki disease, an acute febrile illness characterized by systemic vasculitis. If the heart vasculature is affected, there is a potential for the development of coronary artery aneurysms and sudden death. Five criteria are used for the diagnosis of MIS-C:
-
▪
younger than 21 years;
-
▪
high fever for more than 1 day;
-
▪
laboratory evidence of inflammation;
-
▪
more than 2 organs involved (that is, disseminated erythema or skin rash, swollen extremities, red eyes, and cracked lips);
-
▪
laboratory evidence of SARS-CoV-2 or exposure to someone known to have COVID-19.
Statistically, most cases occur in children aged 1 through 14 years. In addition, almost two-thirds of affected patients are boys, and 70% of the cases reported in the United States have occurred in Hispanic-Latino or Black children. Furthermore, 96% of the cases tested positive for SARS-CoV-2, and the remaining 4% were in contact with someone COVID-19–positive. Most children developed MIS-C 3 through 4 weeks after infection with SARS-CoV-2, slightly later than the severe infection seen in adults.40
Diagnosis
The diagnosis of COVID-19 is based on clinical signs and symptoms, laboratory testing, and imaging studies, including chest radiographs or computed tomographic scans. Infected patients may develop flulike symptoms that manifest at days 2 through 14 (Box).27 , 41 The most common laboratory abnormalities are lymphopenia, leukopenia, hypoalbuminemia, hypertroponinemia, and elevated levels of inflammatory markers such as C-reactive protein, erythrocyte sedimentation rate, lactic dehydrogenase, interleukin-6, and ferritin. Patients with severe cases may also have laboratory evidence of renal and liver dysfunction as well as coagulopathy. This is characterized by elevated levels of D-dimer and fibrin degradation products, thrombocytopenia, and prolonged prothrombin time and activated partial prothrombin time.42
Testing is crucial for diagnosing SARS-CoV-2 and understanding the disease prevalence, spread, and severity. Two main types of tests are available: 1 to detect the virus and 1 to detect presence of antibodies. Real-time reverse transcriptase polymerase chain reaction (PCR) is a molecular diagnostic technique that permits the identification of the viral genetic material in a specimen obtained via swab sampling of the nose, throat, or mouth or saliva. This is typically used in people with signs and symptoms of the disease or those with confirmed exposure. Different PCR platforms are available. The positivity rate of this methodology depends on the viral load, the quality and type of specimen tested, and the sensitivity and specificity of the platform used.8 , 10 A negative test does not exclude the infection conclusively, particularly if the viral load is low, as may occur in people who are presymptomatic, asymptomatic, and paucisymptomatic.
The serologic test identifies human antibodies that recognize the virus. Three isotypes of antibodies—immunoglobulin G, immunoglobulin M, and immunoglobulin A—may be detected in blood.43 A positive test means that the person was exposed to SARS-CoV-2 and developed antibodies against it. However, the degree of protection of these antibodies and the duration of immunity are unclear.
Another important factor that affects the sensitivity of both types of tests is the window period. This is defined as the time from exposure to the pathogen until the laboratory test can reliably confirm the infection. The window period depends on the viral replication time (for real-time PCR test) and the time required for the body to mount a measurable immune response (for serologic test). Extended window periods increase the rate of false-negative results and subsequent asymptomatic spread.44 Studies using serologic tests have reported highly variable immune responses, including a broad range of antibodies among people with mild symptoms of the virus, fewer antibodies among younger people, and no trace of antibodies in some people.45
Immunity
The production of antibodies against the spike glycoprotein and its receptor-binding domain appears to be protective against SARS-CoV-2.46 Immunoglobulin M titers increase within the first week of the SARS-CoV-2 infection, peak after 2 weeks, and then decrease progressively. Immunoglobulin G levels increase after the first week, peak 3 weeks after, and remain elevated for at least 3 months.47 , 48 Antibody titers decrease over time, particularly in mild cases.47 , 49 Cases of reinfection have been reported, some of which may be explained by the emergence of new viral strains.50 Nonetheless, the duration of immunity against SARS-CoV-2 remains to be determined. However, as the immune response matures, memory T cells and B cells are produced.51 These cells, as opposed to circulating antibodies, ensure long-term protection. Thus, the decrease in the titers of circulating antibodies does not imply lack of immunity as the presence of memory immune cells allows a rapid and effective response against possible reinfections.52
COVID-19 in Dentistry
A previous study reported a higher seroprevalence of antibodies against respiratory viruses in oral surgeons than in age-matched controls.53 The authors of this study concluded that “... dentists are at occupational risk of infection with respiratory tract viruses ... .” However, there are limited reports documenting the development of clusters of contagion among dental care providers in other infectious outbreaks, owing to routine infection-control practices.
SARS-CoV-2, in particular, is present in high concentrations in the mouth and oropharynx.54 Dental procedures generate aerosols that could carry the virus and increase the risk of spreading.55 Thus, it is possible that dental care providers could get infected if exposed to a patient with SARS-CoV-2. Despite this potential risk, a 2020 survey found that the rate of COVID-19 among dentists is less than 1%.56 More than 95% of dental practices either closed or worked at reduced capacity during the initial peak of the pandemic.57 Thus, the results of the 2020 study may underestimate the real risk for dental care providers at this time. It is also possible that routine practices could reduce the risk of the disease spreading in the dental office. For example, it has been proposed that water or air spray could dilute the viral load. In addition, high-volume suction could reduce aerosol at source, and this process could be increased further if a dental rubber dam is used to isolate the affected tooth from the oropharynx.58 The use of appropriate personal protective equipment, including respirators, can prevent spread, as outlined in the Centers for Disease Control and Prevention guidelines for dental settings, summarized in Table 2 .59 Most dental practices have developed screening questionnaires to identify patients with signs or symptoms suggestive of COVID-19. PCR testing is used commonly before medical procedures but has not been adopted universally in dentistry. In our office, we performed PCR testing on more than 1,000 consecutive patients undergoing dental treatment who were asymptomatic and denied exposure to confirmed cases. The positivity rate in our cohort reached approximately 2% through 3%.60 Our experience indicates that, although they are easy to implement, the use of screening questionnaires does not identify asymptomatic patients who are a source of contagion.34
Table 2.
Centers for Disease Control and Prevention recommendations for dental practices.∗
| AREA | RECOMMENDATION |
|---|---|
| General |
|
| Work Practice |
|
| Physical Plant |
|
| Personnel |
|
Source: Centers for Disease Control and Prevention.59
Treatment
An effective therapy against COVID-19 should target the virus or address complications such as thrombotic microangiopathy, hemostatic disorders, and severe systemic inflammatory response.44
The most commonly used medications include
-
▪
immune-based therapy, such as corticosteroids, interferons, antimalarial drugs, and monoclonal antibodies;
-
▪
agents against viral proteins, including proteinases, helicases, and polymerases;
-
▪
adjunctive therapy, such as zinc, vitamin D, azithromycin, ascorbic acid, and nitric oxide.
In cases of procoagulability, anticoagulation with heparin, low molecular weight heparin, or direct thrombin inhibitors may be indicated.61 The treatment of the disease may require, in severe cases, mechanical ventilation, dialysis, or pharmacologic hemodynamic support.
The US Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for the use of bamlanivimab in nonhospitalized patients with mild to moderate COVID-19 at risk of deterioration.
Bamlanivimab (also known as LY-CoV555) is a neutralizing monoclonal antibody against the spike protein. In a phase 2, randomized, placebo-controlled trial, the infusion of bamlanivimab decreased the viral load by more than 99%, accelerated the resolution of symptoms, and reduced the number of COVID-19–related hospitalizations or visits to the emergency department from 6.3% in the placebo group to 1.6%.62
The use of single-antibody therapy against respiratory viruses has been found to result in the emergence of treatment-resistant sequence variations. To circumvent this complication, cocktails of antibodies have been investigated. REGN-COV2 is an intravenous combination of noncompeting neutralizing monoclonal antibodies (casirivimab and imdevimab) against the spike glycoprotein. In the interim analysis of a placebo-controlled double-blind study that included 275 nonhospitalized patients with COVID-19, REGN-COV2 enhanced the clearance of the virus and reduced COVID-19–related clinical visits from 6% to 3% relative to placebo.63 The beneficial effects of REGN-COV2 were observed particularly in people who were SARS-CoV-2 antibody negative. This suggests that REGN-COV2 is effective in patients with slow immune response to the virus. The additional results of the 799 patients included in the original trial confirmed the beneficial effect of REGN-COV2. On the basis of these data, on November 21, 2020, the FDA issued an EUA for the use of casirivimab and imdevimab to be administered in conjunction as an infusion for the treatment of mild to moderate COVID-19 in patients older than 12 years with positive SARS-CoV-2 viral infection.64
The antiviral agents investigated for the treatment of SARS-CoV-2 infection include remdesivir, umifenovir, lopinavir, oseltamivir, and favipiravir.65 Remdesivir is an inhibitor of the viral RNA-dependent RNA polymerase that has in vitro activity against severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus. In a randomized clinical trial including 1,062 adults with COVID-19, the use of remdesivir was associated with a significantly shorter time to recovery than the use of a placebo (10 days versus 15 days; P < .001) with no effect on mortality.66 On the basis of the results of this and 2 other smaller studies, on October 22, 2020, the FDA approved remdesivir for use in adults and children older than 12 years with COVID-19 requiring hospitalization.67, 68, 69
Different strategies to mitigate the hyperinflammatory state triggered by COVID-19 have been investigated. In an open-label study, the 28-day mortality was 25.7% in patients receiving usual care and 22.9% in those treated with dexamethasone (P < .001), and this finding was more pronounced in patients requiring respiratory support.70 Baricitinib is an oral selective inhibitor of Janus kinase 1 and 2. In a randomized, double-blind, placebo-controlled study including 1,033 adults with COVID-19, the combination of baricitinib plus remdesivir reduced the median time to recovery relative to remdesivir plus placebo (7 days versus 8 days; P = .03), with no significant effect on mortality. In addition, in the subgroups of patients receiving high-flow oxygen or noninvasive ventilation at enrollment, the time to recovery was 10 days in the baricitinib plus remdesivir group and 18 days in the remdesivir group (rate ratio for recovery, 1.51; 95% confidence interval, 1.10 to 2.08).71 On November 19, 2020, the FDA issued an EUA for the use of baricitinib in combination with remdesivir in hospitalized adults and children 2 years or older with COVID-19 who require supplemental oxygen, invasive mechanical ventilation, or ECMO. The treatment potential of different interleukin-6 receptor inhibitors, including tocilizumab, sarilumab, and siltuximab, has been investigated in a randomized clinical trial. This study has yielded conflicting results and, as of March 11, 2021, the FDA has not approved their use except in clinical trials.72
Chloroquine and hydroxychloroquine have been used as antimalarial medications for more than 70 years and against HIV, and small clinical studies suggested potential as therapy for COVID-19. However, the administration of chloroquine and hydroxychloroquine in COVID-19 patients has been associated with an increased risk of experiencing cardiac arrhythmia and cardiac arrest, so they are not recommended.61 The beneficial effects of vitamin D73 and zinc74 have yet to be reported in properly powered randomized trials.61
Convalescent plasma (CP) refers to the acellular blood fraction from people who recovered from COVID-19 that contains antibodies that recognize SARS-CoV-2. On August 23, 2020, the FDA authorized the use of CP for the treatment of patients hospitalized with COVID-19. However, the panel also recognized the lack of adequately powered controlled, randomized studies addressing the benefits and risks of this treatment. Therefore, the FDA considers that there is insufficient evidence to support or reject the use of CP or other blood products, such as SARS-CoV-2 immunoglobulins, for the treatment of COVID-19.75
Conclusions
COVID-19 has affected our lives at multiple levels owing to its rapid spread, elevated mortality rate, social impact, and economic damage worldwide. Critical thinking is necessary to interpret the results of diagnostic studies, given that false-negative results can occur. Older adults and people with chronic medical conditions are vulnerable populations who are at high risk of experiencing disease and complications. In contrast, children are frequently asymptomatic but may be a source of infection. Different pharmacologic approaches were identified for the treatment of SARS-CoV-2 infection, and several others are in the pipeline. In addition, the development of new vaccines against SARS-CoV-2 is likely to change the landscape of the pandemic. Our understanding of the disease is rapidly evolving, and future findings may challenge some of the information included in our review.
Biographies
Dr. Lamberghini is a clinical assistant professor, Department of Pediatric Dentistry, College of Dentistry, University of Illinois at Chicago, Chicago, IL, and the director, Apple Dental Care, Chicago, IL.
Dr. Testai is a professor, Department of Neurology and Rehabilitation, College of Medicine, University of Illinois at Chicago, Chicago, IL.
Footnotes
Disclosures. Drs. Lamberghini and Testai did not report any disclosures.
References
- 1.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 2.WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int Available at:
- 3.Ritchie H., Ortiz-Ospina E., Beltekian D. Mortality risk of COVID-19. https://ourworldindata.org/mortality-risk-covid Available at:
- 4.Stawicki S.P., Jeanmonod R., Miller A.C. The 2019-2020 novel coronavirus (severe acute respiratory syndrome coronavirus 2) pandemic: a joint American College of Academic International Medicine-World Academic Council of Emergency Medicine Multidisciplinary COVID-19 Working Group consensus paper. J Glob Infect Dis. 2020;12(2):47–93. doi: 10.4103/jgid.jgid_86_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos I.A., Grosche V.R., Bergamini F.R.G., Sabino-Silva R., Jardim A.C.G. Antivirals against coronaviruses: candidate drugs for SARS-CoV-2 treatment? Front. Microbiol. 2020;11:1818. doi: 10.3389/fmicb.2020.01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1):1–22. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoeman D., Fielding B.C. Is there a link between the pathogenic human coronavirus envelope protein and immunopathology? A review of the literature. Front Microbiol. 2020;11:2086. doi: 10.3389/fmicb.2020.02086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7):e00127-20. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhama K., Patel S.K., Sharun K. SARS-CoV-2 jumping the species barrier: zoonotic lessons from SARS, MERS and recent advances to combat this pandemic virus. Travel Med Infect Dis. 2020;37:101830. doi: 10.1016/j.tmaid.2020.101830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hui D.S.C., Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect Dis Clin North Am. 2019;33(4):869–889. doi: 10.1016/j.idc.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song F., Shi N., Shan F. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295(1):210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baghizadeh Fini M. What dentists need to know about COVID-19. Oral Oncol. 2020;105:104741. doi: 10.1016/j.oraloncology.2020.104741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Randolph H.E., Barreiro L.B. Herd immunity: understanding COVID-19. Immunity. 2020;52(5):737–741. doi: 10.1016/j.immuni.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horton J. Covid: Thanksgiving the cause of a spike in US infections? BBC News. https://www.bbc.com/news/55363256 Available at: Accessed December 20, 2020.
- 15.Neyman G, Dalsey W. Black Lives Matter protests and COVID-19 cases: relationship in two databases [published online ahead of print November 20, 2020]. J Public Health (Oxf). 10.1093/pubmed/fdaa212. [DOI] [PMC free article] [PubMed]
- 16.Killerby M.E., Link-Gelles R., Haight S.C., CDC COVID-19 Response Clinical Team Characteristics associated with hospitalization among patients with COVID-19: metropolitan Atlanta, Georgia—March-April 2020. Morb Mortal Wkly Rep. 2020 doi: 10.15585/mmwr.mm6925e1. 26;69(25):790-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omori R., Matsuyama R., Nakata Y. The age distribution of mortality from novel coronavirus disease (COVID-19) suggests no large difference of susceptibility by age. Sci Rep. 2020;10(1):16642–16648. doi: 10.1038/s41598-020-73777-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. COVID-19 Hospitalization and death by age https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html Available at:
- 19.Riou J., Althaus C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Euro Surveill. 2020;25(4):2000058. doi: 10.2807/1560-7917.ES.2020.25.4.2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dancer S.J., Tang J.W., Marr L.C., Miller S., Morawska L., Jimenez J.L. Putting a balance on the aerosolization debate around SARS-CoV-2. J Hosp Infect. 2020;105(3):569–570. doi: 10.1016/j.jhin.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lotfi M., Hamblin M.R., Rezaei N. COVID-19: Transmission, prevention, and potential therapeutic opportunities. Clin Chim Acta. 2020;508:254–266. doi: 10.1016/j.cca.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Guidance for wearing masks: help slow the spread of COVID-19 https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html Available at: Accessed March 11, 2021.
- 23.Lindsley W.G., Noti J.D., Blachere F.M., Szalajda J.V., Beezhold D.H. Efficacy of face shields against cough aerosol droplets from a cough simulator. J Occup Environ Hyg. 2014;11(8):509–518. doi: 10.1080/15459624.2013.877591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones D., Helmreich S. A history of herd immunity. Lancet. 2020 doi: 10.1016/S0140-6736(20)31924-3. 19;396(10254):810-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Similarities and differences between flu and COVID-19 https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm#:∼:text=Because%20some%20of%20the%20symptoms,differences%20between%20the%20two Available at:
- 26.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus: a first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Symptoms of coronavirus https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html Available at:
- 28.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt M., Hajage D., Lebreton G., for the Groupe de Recherche Clinique en REanimation et Soins intensifs du Patient en Insuffisance Respiratoire aiguE (GRC-RESPIRE) Sorbonne Université and the Paris-Sorbonne ECMO-COVID investigators; for the EOLIA Trial Group, REVA and ECMONet Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med. 2020;8(11):1121–1131. doi: 10.1016/S2213-2600(20)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenhalgh T., Knight M., A’Court C., Buxton M., Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 31.Rubin R. As their numbers grow, COVID-19 “long haulers” stump experts. JAMA. 2020;324:1381–1393. doi: 10.1001/jama.2020.17709. [DOI] [PubMed] [Google Scholar]
- 32.Milani G.P., Bottino I., Marchisio P., Elli S., Agostoni C., Costantino G. Frequency of children vs adults carrying severe acute respiratory syndrome coronavirus 2 asymptomatically. JAMA Pediatr. 2020;175(2):193–194. doi: 10.1001/jamapediatrics.2020.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215:108427. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yonker L.M., Neilan A.M., Bartsch Y. Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, and immune responses. J Pediatr. 2020;227:45–52. doi: 10.1016/j.jpeds.2020.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bialek S., Gierke R., Hughes M., McNamara L.A., Pilishvili T., Skoff T. Coronavirus disease 2019 in children: United States, February 12-April 2, 2020. Morb Mortal Wkly Rep. 2020;69(14):422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasmussen S.A., Thompson L.A. Coronavirus disease 2019 and children: what pediatric health care clinicians need to know. JAMA Pediatr. 2020;174(8):743–744. doi: 10.1001/jamapediatrics.2020.1224. [DOI] [PubMed] [Google Scholar]
- 37.Erdede O., Sari E., Uygur Kulcu N., Uyur Yalcin E., Sezer Yamanel R.G. An overview of smell and taste problems in paediatric COVID-19 patients. Acta Paediatr. 2020;109(11):2184–2186. doi: 10.1111/apa.15515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lozada-Nur F., Chainani-Wu N., Fortuna G., Sroussi H. Dysgeusia in COVID-19: possible mechanisms and implications. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130(3):344–346. doi: 10.1016/j.oooo.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong Y., Mo X., Hu Y. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6) doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 40.Alcendor D.J. Racial disparities-associated COVID-19 mortality among minority populations in the US. J Clin Med. 2020;9(8):2442. doi: 10.3390/jcm9082442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lovato A., de Fiis C., Marioni G. Upper airway symptoms in coronavirus disease 2019 (COVID-19) Am J Otolaryngol. 2020;41(3):102474. doi: 10.1016/j.amjoto.2020.102474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu J., Zhong Z., Ji P. Clinicopathological characteristics of 8697 patients with COVID-19 in China: a meta-analysis. Fam Med Community Health. 2020;8(2) doi: 10.1136/fmch-2020-000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peeling R.W., Wedderburn C.J., Garcia P.J. Serology testing in the COVID-19 pandemic response. Lancet Infect Dis. 2020;20(9):e245–e249. doi: 10.1016/S1473-3099(20)30517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Damme W., Dahake R., Delamou A. The COVID-19 pandemic: diverse contexts; different epidemics-how and why? BMJ Glob Health. 2020;5(7) doi: 10.1136/bmjgh-2020-003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirkcaldy R.D., King B.A., Brooks J.T. COVID-19 and postinfection immunity: limited evidence, many remaining questions. JAMA. 2020;323(22):2245–2246. doi: 10.1001/jama.2020.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lumley SF, O’Donnell D, Stoesser NE, et al; Oxford University Hospitals Staff Testing Group. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384(6):533-540. [DOI] [PMC free article] [PubMed]
- 47.Hou H., Wang T., Zhang B. Detection of IgM and IgG antibodies in patients with coronavirus disease 2019. Clin Transl Immunology. 2020;9(5) doi: 10.1002/cti2.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li G., Chen X., Xu A. Profile of specific antibodies to the SARS-associated coronavirus. New Engl J Med. 2003;349(5):508–509. doi: 10.1056/NEJM200307313490520. [DOI] [PubMed] [Google Scholar]
- 49.Self W.H. Decline in SARS-CoV-2 Antibodies After Mild Infection Among Frontline Health Care Personnel in a Multistate Hospital Network: 12 states, April-August 2020. Morb Mortal Wkly Rep. 2020;69(47):1762–1766. doi: 10.15585/mmwr.mm6947a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tillett R., Sevinsky J. Genomic evidence for a case of reinfection with SARS-CoV-2. Lancet Infect Dis. 2020;21(1):52–58. doi: 10.1016/S1473-3099(20)30764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hartley G.E., Edwards E.S.J., Aui P.M. Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. Sci Immunol. 2020;5(54):1–15. doi: 10.1126/sciimmunol.abf8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cox R.J., Brokstad K.A. Not just antibodies: B cells and T cells mediate immunity to COVID-19. Nat Rev Immunol. 2020;20(10):581–582. doi: 10.1038/s41577-020-00436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davies K.J., Herbert A.M., Westmoreland D., Bagg J. Seroepidemiological study of respiratory virus infections among dental surgeons. Br Dent J. 1994;176(7):262–265. doi: 10.1038/sj.bdj.4808430. [DOI] [PubMed] [Google Scholar]
- 54.Xu R., Cui B., Duan X., Zhang P., Zhou X., Yuan Q. Saliva: potential diagnostic value and transmission of 2019-nCoV. Int J Oral Sci. 2020;12(1):1–6. doi: 10.1038/s41368-020-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ge Z., Yang L., Xia J., Fu X., Zhang Y. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J Zhejiang Univ Sci B. 2020;21(5):361–368. doi: 10.1631/jzus.B2010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Estrich C.G., Mikkelsen M., Morrissey R. Estimating COVID-19 prevalence and infection control practices among US dentists. JADA. 2020;151(11):815–824. doi: 10.1016/j.adaj.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.American Dental Association Health Policy Institute COVID-19 Economic Impact: state dashboard. https://www.ada.org/en/science-research/health-policy-institute/covid-19-dentists-economic-impact/survey-results Available at:
- 58.Epstein JB, Chow K, Mathias R. Dental procedure aerosols and COVID-19 [published online ahead of print August 10, 2020]. Lancet Infect Dis. 10.1016/S1473-3099(20)30636-8. [DOI] [PMC free article] [PubMed]
- 59.Centers for Disease Control and Prevention COVID-19: healthcare workers—guidance for dental settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html Available at:
- 60.Lamberghini F, Trifan G, Testai FD. Severe acute respiratory syndrome coronavirus 2 infection in asymptomatic pediatric dental patients. JADA. 2021;152(4):277-283. [DOI] [PMC free article] [PubMed]
- 61.National Institutes of Health. COVID-19 treatment guidelines https://www.covid19treatmentguidelines.nih.gov/whats-new/. Accessed 25 December 2020. [PubMed]
- 62.Chen P., Nirula A., Heller B., for the BLAZE-1 Investigators SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weinreich DM, Sivapalasingam S, Norton T, et al; for the Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med 2021;384(3):238-251. [DOI] [PMC free article] [PubMed]
- 64.US Food and Drug Administration, Coronavirus (COVID-19) update: FDA authorizes monoclonal antibodies for treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19 Available at:
- 65.National Institutes of Health, COVID-19 treatment guidelines: antiviral drugs that are under evaluation for the treatment of COVID-19 https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/ Available at:
- 66.Beigel J.H., Tomashek K.M., Dodd L.E., for the ACTT-1 Study Group Members Remdesivir for the treatment of Covid-19: final report. New Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.US Food and Drug Administration. FDA approves first treatment for COVID-19 [news release] https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 Available at:
- 68.Goldman J.D., Lye D.C.B., Hui D.S., for the GS-US-540-5773 Investigators Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020;383(19):1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spinner C.D., Gottlieb R.L., Criner G.J. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.The RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704. [DOI] [PMC free article] [PubMed]
- 71.Kalil AC, Patterson TF, Mehta AK, et al; for the ACTT-2 Study Group Members. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795-807 [DOI] [PMC free article] [PubMed]
- 72.National Institutes of Health. Covid-19 treatment guidelines: interleukin-6 inhibitors. Available at: https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/. Accessed February 27, 2021. [PubMed]
- 73.Meltzer D.O., Best T.J., Zhang H., Vokes T., Arora V., Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.National Institutes of Health. COVID treatment guidelines: zinc supplementation and COVID-19 https://www.covid19treatmentguidelines.nih.gov/adjunctive-therapy/zinc/ Available at:
- 75.National Institutes of Health. COVID-19 treatment guidelines: convalescent plasma and immune globulins https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/blood-derived-products/convalescent-plasma/ Available at: [PubMed]