Abstract
Introduction
Severe COVID-19 cases have a detrimental hyper-inflammatory host response and different cytokine-blocking biologic agents were explored to improve outcomes. Anakinra blocks the activity of both IL-1α and IL‑1β and is approved for different autoinflammatory disorders, but it is used off-label for conditions characterized by an excess of cytokine production. Several studies on anakinra in COVID-19 patients reported positive effects. We performed a meta-analysis of all published evidence on the use of anakinra in COVID19 to investigate its effect on survival and need for mechanical ventilation.
Methods
We searched for any study performed on adult patients with acute hypoxemic failure related to 2019-nCoV infection, receiving anakinra versus any comparator. Primary endpoint was mortality at the longest available follow-up. Adverse effects, need for mechanical ventilation and discharge at home with no limitations were also analysed.
Results
Four observational studies involving 184 patients were included. Overall mortality of patients treated with anakinra was significantly lower than mortality in the control group (95% CI 0.14-0.48, p<0.0001). Moreover, patients treated with anakinra had a significantly lower risk of need for mechanical ventilation than controls (95% CI 0.250.74, p=0.002). No difference in adverse events and discharge at home with no limitations was observed. The Trial Sequential Analysis z-cumulative line reached the monitoring boundary for benefit and the required sample size.
Conclusions
Administration of anakinra in COVID-19 patients was safe and might be associated with reductions in both mortality and need for mechanical ventilation. Randomized clinical trials are warranted to confirm these findings.
Keywords: Anakinra, COVID-19, SARS-CoV-2, Meta-analysis, Mortality
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) affected 104.956.439 people worldwide and caused the death of 2.290.488 as of February 7, 2121[1]. Life-threatening cases of the coronavirus disease 2019 (COVID-19) are typically characterized by a detrimental hyper-inflammatory host response to the virus, which is reminiscent of the cytokine storm which develops in macrophage activation syndrome or after chimeric antigen receptor T-cell treatment, with massive release of pro-inflammatory cytokines [2,3]. Thereby, treatment with cytokine-blocking biologic agents was explored in order to reduce mortality of patients with COVID-19 and hyper-inflammation [2].
Targetable cytokines involved in the pathogenesis of lung inflammation in COVID-19 include IL-1, IL-6, tumour necrosis factor, GM-CSF, and interferon γ [2,4,5]. Already during the early phases of the pandemic, the IL-1 inhibitor anakinra emerged as a candidate treatment to quench hyper-inflammation and reduce mortality in COVID-19 [6]. Anakinra is a recombinant form of the naturally occurring IL-1 receptor antagonist (IL-1Ra), a regulatory molecule blocking the activity of both IL-1α and IL‑1β [7]. It is approved for the treatment of rheumatoid arthritis and autoinflammatory disorders; however, it is mostly used off-label for the treatment of conditions characterized by excess cytokine production [7,8]. Of note, anakinra was previously used to treat cardiopulmonary insufficiency [9], [10], [11], [12], as well as macrophage activation syndrome and septic shock [13,14], both catastrophic conditions sharing clinical and molecular features with COVID-19 hyper-inflammation.
Several studies evaluated treatment with anakinra in COVID-19 and reported encouraging effects on mortality [6,[15], [16], [17], [18], [19]. Nevertheless, use of anakinra in COVID-19 remains empirical. Potential benefits arise from inhibition of a pivotal cytokine found upstream most pro-inflammatory cascades, and include suppression of systemic inflammation, control of fever, reduced infiltration of myeloid cells into the lung, and reduced exudate formation into the alveolar spaces [20,21]. Potential harms may follow inhibition of a beneficial host inflammatory response aimed at controlling viral replication, as well as clinical risks related to immunosuppression in general.
Despite increasing use of anakinra to treat severe COVID-19, it remains to be determined whether IL-1 inhibition confers an advantage over standard management alone, while no evidence from randomized clinical trials is currently available. To address this critical question, we conducted a meta-analysis of all published evidence on the use of anakinra in patients with COVID-19 and hyper-inflammation, to investigate whether IL-1 inhibition had beneficial effects on survival and need for mechanical ventilation.
Materials and methods
Search Strategy
Eligible studies were individually searched in Embase, PubMed, BioMedCentral, medRxiv, bioRxiv and the Cochrane Central Register of Controlled Trials (CENTRAL) by two investigators (LP, GL). The full PubMed search strategy ((Anakinra OR Kineret) AND (COVID OR coronavirus OR SARS-CoV-2) NOT (animal[mh] NOT human[mh]) NOT (comment[pt] OR editorial[pt] OR meta-analysis[pt] OR practice-guideline[pt] OR review[pt]) aimed to include any studies ever performed with anakinra in COVID-19 patients. Backward snowballing was also employed (i.e., looking through of references of collected articles and relevant reviews) and international expert were contacted for additional studies with no language restriction.
Study Selection
Firstly, references were individually explored at a title/abstract level by two investigators (LP; GL) and controversies were settled by agreement or by mediation of a third author (GC), if necessary. If potentially eligible, references were retrieved as full articles.
Inclusion criteria suitable studies were: adult COVID-19 patients treated with anakinra with no restrictions on dose or time of administration; patients admitted either in ordinary ward or intensive care unit (ICU). Exclusion criteria were overlapping publications (in this in that event we r cited the first article published but we collected data from the paper with the longest available follow-up), non-adult patients, case-report and missing data on all of the following: mortality, need for mechanical ventilation, occurrence of bacterial infection, markedly elevated serum transaminases, rate of thromboembolism. Two investigators individually estimated compliance to inclusion criteria and choose studies for the final analysis, with controversies settled by agreement.
Data Abstraction
Data on baseline, procedural, and outcome were individually retrieved by two investigators. In case of missing data, two or more attempts at contacting corresponding authors were made. The primary endpoint of our study was mortality rate at the longest available follow-up. Adverse effects, need for mechanical ventilation, occurrence of bacterial infection, markedly elevated serum transaminases and rate of thromboembolism, discharge at home with no limitations were also analysed.
Assessment of risk of bias in included studies
The risk of bias of non-randomized studies was appraised according to the Risk Of Bias In Non-Randomized Studies Of Interventions (ROBINS-I) tool [22,23], with controversies settled by agreement or by mediation of a third author.
Data Analysis and Synthesis
Analyses were performed with Review Manager version 5.4. Statistical heterogeneity was measured with Cochran Q test, setting the level of statistical significance at 0.10 (for a two-tailed test). Statistical consistency was calculated with I2, according to the formula: I2 = 100% X (Cochran's Q, heterogeneity statistic – degrees of freedom).
Binary outcomes from single studies were analysed to calculate individual and pooled risk ratio (RR) with pertinent 95% confidence interval (CI), by means of inverse variance method. In case of low statistical inconsistency (I2 <25%) or high statistical inconsistency (I2 >25%) a fixed effect model or a random-effect model (which better accommodates clinical and statistical variations) were respectively applied. For continuous variables, mean differences (MD) and 95% CI were calculated for using the same models. Sensitivity analyses were done by sequentially removing each study and reanalysing the residual dataset (producing a new analysis for each study removed). The level of statistical significance was set at 0.05 (for a two-tailed test). Unadjusted p values were reported throughout.
A pre-specified Trial Sequential Analysis (TSA) was performed on primary outcome. We estimated the required information size on the calculated minimal intervention effect, considering a type I error of 5% and a power of 80%. The accumulating number of included patients were reported on the x-axis. The cumulative Z-Scores were reported on the y-axis and represented the statistical summary of the gathered data. The aligned brown lines indicated the conventional levels to discriminate statistically significant results (a constant Z value of 1.96, representing a p value=0.05). The blue line was the incremental Z curve and represented the growing volume of information added by published trials, each square indicating a single study. Trial sequential boundaries (the curved lines at the upper and lower left‐hand corners) represented the sequential analysis thresholds for statistical significance. The red diagonal lines between the aligned brown lines represented the futility boundaries.
This study was registered on PROSPERO (CRD42020196808) and performed according to The Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [23,24].
Results
Study characteristics
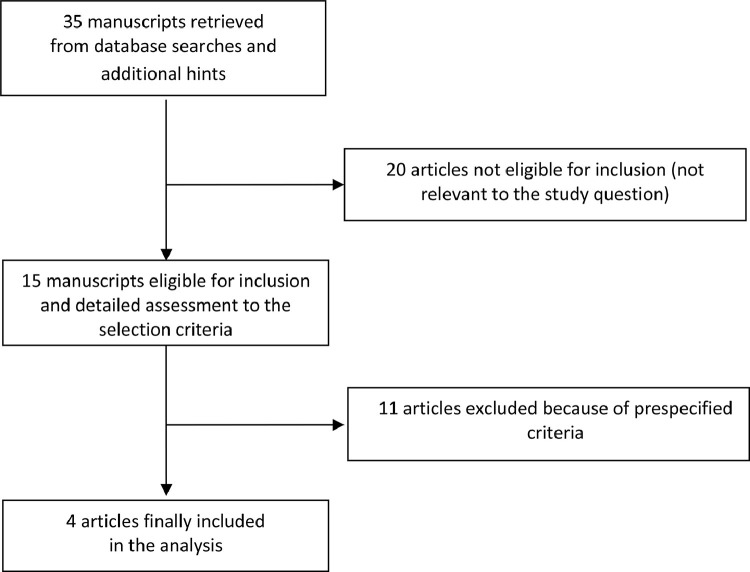
Database searches, snowballing, and contacts with experts yielded a total of 35 articles. Excluding 20 non-pertinent titles or abstracts, we retrieved in complete form and assessed 15 studies according to the selection criteria (Fig. 1 ). Eleven studies were further excluded because of our prespecified exclusion criteria: five case reports [25], [26], [27], [28], [29]; four case-series with no comparators [15], [16], [17],30]; two because including patients treated with anakinra before COVID-19 infection [26,31].
Fig. 1.
Flow diagram for selection of articles.
The four manuscripts included in the present meta-analysis involved 184 patients (111 received anakinra and 73 received standard treatment)[6], [18], [19], [32]. (Table 1 ) No randomized clinical trials were identified and all studies were retrospective. Characteristics of included studies are presented in Table 1. (Table 1) Clinical heterogeneity was mostly due to inclusion criteria, duration of anakinra administration and standard treatment. (Table 1) Overall risk of bias of included studies was high. (Supplemental material)
Table 1.
Description of the studies included in the meta-analysis.
| First author | Year | Setting | Inclusion criteria | Anakinra patients | Control patients | Anakinra dosage | Duration on study treatment | Comparator | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Cauchois R | 2020 | Ordinary ward | Adult patients with a positive PCR for SARS-CoV-2 in nasopharyngeal samples, respiratory symptoms, and a concordant pneumonia on low-dose computed tomography (CT) scan, scored from 0 to 5 according to the severity. Between the 5th and 13th days of the diagnosis, the patients presented severe hypoxia requiring oxygen therapy and were classified as stage 2b or 3, according to the 2020 clinical staging proposal. Anakinra was started with a rapidly deteriorating condition consisting of increased oxygen requirement of >4 L/min within the previous 12 h, and CRP above 110 mg/L with or without fever higher than 38.5°C. | 12 | 10 | nfused intravenously (i.v.) over 2 h as a single daily dose of 300 mg for 5 days, then tapered to 200 mg•d−1 for 2 days, and then 100 mg for 1 day | 8 days | Standard treatment: antibiotics and hydroxychloroquine. Two patients received ritonavir/lopinovir. | 20 days |
| Cavalli G | 2020 | Ordinary ward | Adults patients with COVID-19 ARDS, and hyperinflammation: increase in serum C-reactive protein (≥100 mg/L) or ferritin (≥900 ng/mL), or both. COVID-19 was diagnosed by quantitative RT-PCR and either chest radiography or CT. ARDS (acute-onset respiratory failure with bilateral infiltrates on chest radiography or CT, hypoxaemia ([PaO2:FiO2] ≤200 mm Hg with a positive end-expiratory pressure [PEEP] of at least 5 cm H2O), and no evidence of left atrial hypertension. | 36 | 16 | High-dose anakinra: intravenously at a dose of 10 mg/kg per day (5 mg/kg twice daily, infused over 1 h), in addition to standard treatment. | Until sustained clinical benefit: 75% reduction in serum C-reactive protein and sustainedrespiratory improvement (PaO2:FiO2 >200 mm Hg) for at least 2 days, or until death, bacteraemia or side-effects arousal. | Standard treatment and continuous positive airway pressure (CPAP) outside of the ICU; no anti-inflammatory agents or glucocorticoids. | 21 days |
| Huet T | 2020 | Ordinary ward | Adult patients with severe COVID-19-related bilateral pneumonia; SARS-CoV-2 infection confirmed by either a positive result from an RT-PCR assay or a typical aspect on CT scan of the lungs; bilateral lung infiltrates on a lung CT scan or chest x-ray; and had critical pulmonary function defined by oxygen saturation ≤93% under ≥6 L/min of oxygen or oxygen saturation <93% on 3 L/min with a saturation on ambient air decreasing by 3% in the previous 24 h. | 52 | 44 | Subcutaneous anakinra at a dose of 100 mg twice daily for 72 h, followed by 100 mg daily for 7 days, in addition to standard treatment. | 10 days | Standard treatment included oral hydroxychloroquine 600 mg/day for 10 days, oral azithromycin 250 mg/day for 5 days, and parenteral β-lactam antibiotics for 7 days. All patients received thromboembolic prophylaxis. No oral corticosteroids or vasopressors were used, but some patients received an intravenous bolus of methylprednisolone (500 mg). Supportive care included low-flow or high-flow oxygen therapy (>6 L/min with high-flow nasal cannula or face mask). None of the patients had invasive or non-invasive mechanical ventilation at baseline. | Hospital stay |
| Navarro-Millan I | 2020 | Ordinary ward | Adult COVID-19 patients with molecular documentation of SARS-CoV-2, fever (documented or historical), ferritin >1,000 ng/mL with one additional laboratory marker of hyperinflammation, and acute hypoxic respiratory failure (requiring either 15 liters (L) of supplemental oxygen (O2) via non-rebreather mask combined with 6L nasal cannula or ≥95% oxygen by high flow nasal cannula. | 11 | 3 | Subcutaneous anakinra 100 mg every 6 hours; however, while a uniform treatment plan and secure supply of medication was being established, the first two patients were treated initially with doses below the proposed 100 mg every 6 hours. The dosing frequency of anakinra was gradually decreased to every 8, 12, and 24 hours. | Maximum 20 days | Standard treatment: methylprednisolone, empiric antibiotics and hydroxychloroquine. | Hospital stay |
Quantitative Data Synthesis
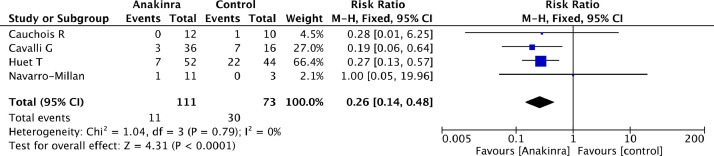
Overall mortality of patients treated with anakinra was significantly lower than mortality of patients in the control group (11/111 [10%] in the anakinra group vs 30/73 [41%] in the control group, RR=0.26 [95% CI 0.14 to 0.48], p for effect <0.0001, p for heterogeneity 0.79, I2 = 0%). (Table 2 ; Fig. 2 ) Results were confirmed at sensitivity analysis performed by sequentially removing each study and reanalysing the remaining dataset. (Supplemental material). Switching from fixed to random effects model made no difference to the estimates.
Table 2.
Outcomes.
| Outcome | Number of included studies | Anakinra patients | Control patients | RR | 95% CI | P for effect | I2 (%) |
|---|---|---|---|---|---|---|---|
| Overall studies | 4 | 111 | 73 | ||||
| Mortality* | 4 | 11/111 [10%] | 30/73 [41%] | 0.26 | 0.14 to 0.48 | <0.0001 | 0 |
| Need for invasive MV* | 4 | 18/111 [16%] | 26/73 [36%] | 0.45 | 0.25 to 0.82 | 0.008 | 19 |
| Bacterial infection | 3 | 9/99 [9%] | 2/63 [3%] | 1.59 | 0.35 to 7.16 | 0.55 | 7 |
| Thromboembolic events | 3 | 13/99 [13%] | 7/63[11%] | 1.35 | 0.58 to 3.12 | 0.35 | 0 |
| Elevated serum transaminases | 3 | 13/99 [13%] | 9/63 [14%] | 0.81 | 0.21 to 3.13 | 0.11 | 55 |
| Discharged from hospital with no limitations | 2 | 20/47 [43%] | 6/19[32%] | 1.29 | 0.61 to 2.74 | 0.50 | 0 |
RR: relative risk; CI: confidence interval; P: p-value; MV: mechanical ventilation
Additional data provided by corresponding author (Navarro-Millán)
Fig. 2.
Forest plot for mortality.
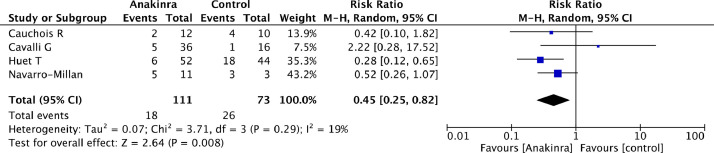
Moreover, patients treated with anakinra had a significantly lower risk of need for mechanical ventilation than controls (18/111[16%] in the anakinra group vs 26/73 [36%] in the control group, RR=0.45 [95% CI 0.25 to 0.82], p for effect=0.008, p for heterogeneity 0.29, I2 = 19%). (Fig. 3 ) Results were not confirmed at sensitivity analysis. (Supplemental material)
Fig. 3.
Forest plot for need for invasive mechanical ventilation.
The number of patients discharged from hospital with no limitations was available in only two studies and similar between groups. (Table 2) No differences in rate of bacterial infection, thromboembolism or elevation of serum transaminases were found. (Table 2).
Switching from fixed to random effects model made no difference to the estimates. Visual inspection of funnel plot did not identify a skewed or asymmetrical shape, excluding the presence of small publication bias. (Supplemental material).
The TSA z-cumulative line reached the monitoring boundary for benefit and the required sample size. In fact, the z-cumulative line lied outside the horizontal brown lines. Therefore, conventional meta-analysis demonstrated statistical significance. Moreover, TSA showed that we achieved the required information size to determine the anticipated effect on mortality with certainty. (Supplemental material)
Discussion
This meta-analysis of non-randomized cohort studies is the first to investigate the effect of IL-1 inhibition with anakinra on overall mortality and need for mechanical ventilation in patients with COVID-19. Across comparative, non-randomized studies, administration of anakinra was safe and associated with significant reductions in both mortality and need for mechanical ventilation. These findings are of great interest because safe and effective treatments to inhibit inflammation and reduce mortality are needed in COVID-19, and because the safety and efficacy of anakinra in quenching hyper-inflammation are documented extensively in multiple diseases other than COVID-19 [9].
The pathogenesis of COVID-19 involves an exuberant, maladaptive inflammatory response, which is mirrored systemically by elevations in serum C-reactive protein. [2,3,5] Inflammatory responses to lung damage are centrally mediated by IL-1α and IL‑1β: these two cytokines have different, non-redundant pro-inflammatory functions, as IL-1α is released by damaged epithelial and endothelial tissues and triggers inflammation, whereas IL‑1β is produced by infiltrating myeloid cells and propagates inflammation [33]. The main physiologic mechanism preventing excessive inflammation driven by either cytokine is the IL-1 receptor antagonist (IL-1Ra), which blocks the receptor transducing the pro-inflammatory effects of both IL-1α and IL‑1β. [20,21] In patients with acute respiratory distress syndrome (ARDS), evaluation of serial bronchoalveolar lavage fluids for cytokine concentrations reveals that increases in IL‑1β herald disease onset; however, an increase in IL-1Ra eventually follows, which provides an endogenous regulatory mechanism for dampening excessive inflammation in the lung [34].
Anakinra is a recombinant form of IL-1Ra and the first-in-class IL-1 inhibitor drug. It is approved for the treatment of rheumatoid arthritis and autoinflammatory disorders [9,35], but it is used off-label for the treatment of multiple conditions characterized by excess cytokine production, including critical disease states [7,11,12,36,37]. Notable therapeutic applications include macrophage activation syndrome, a condition sharing similarities with COVID-19 and hyper-inflammation [13,38,39], as well as cytokine release syndromes following infection in predisposed patients (i.e. patients with autoimmune and rheumatic conditions).[40], [41], [42] In addition, re-analysis of data from a phase 3 randomized controlled trial of anakinra in sepsis confirmed survival benefits in patients with features of hyper-inflammation [14]. A good safety profile and a short half-life of 3 hours, which ensures rapid clearance from the circulation, contribute to making anakinra a suitable treatment for critically ill patients [9]. Based on these features, anakinra was among the first cytokine-blocking agents evaluated for the treatment of COVID-19, as documented by multiple reports [6,[15], [16], [17], [18], [19].
Clinical trials of anakinra are ongoing. Other strategies to quench IL-1-mediated inflammation in COVID-19 undergoing controlled testing include oral inhibitors of the NLRP3 inflammasome, which is responsible for the processing and activation of IL‑1β prior to secretion by myeloid cells [43]. Of note, a monoclonal antibody selectively blocking IL‑1β (but not IL-1α), canakinumab [44], was also evaluated in a RCT of COVID-19 (NCT04362813), which did not meet the primary efficacy endpoint of a greater chance of survival without the need for invasive mechanical ventilation, or its key secondary endpoint of reduced COVID-19 mortality, compared with the standard of care [45]. Potential reasons for the lack of efficacy of canakinumab in this trial include the potential role of IL-1α in the pathogenesis of COVID-19. Also, although inclusion criteria included serum CRP and ferritin levels, the thresholds set for enrollment were relatively low (C-reactive protein ≥20 mg/L or ferritin level ≥600 µg/L) and thereby not necessarily indicative of hyper-inflammation in all patients. Alternative cytokine-blocking agents used to treat COVID-19 include tocilizumab and sarilumab, monoclonal antibodies blocking the IL-6 receptor, whose use in uncontrolled as well as controlled settings yielded less convincing results [46], [47], [48], [49]. The anti-GM-CSF agent mavrilimumab also emerged as a promising option in preliminary observational studies, and is presently in controlled trials (NCT04397497) [4]. Corticosteroids, which non-selectively inhibit cytokine production, were evaluated in the massive RECOVERY trial and found to reduce mortality compared to usual care alone, and became the standard of care for moderate-to-sever COVID-19 [50,51].
The positive findings of this meta-analysis are to be interpreted with caution in view of the high risk of bias (i.e. single-center study bias, small study bias), as well as the limited number and uncontrolled nature of the included studies. Moreover, we acknowledge that another important bias is the fact that the severity of illness seems to have gone down during the pandemic. Therefore, just treating patients later in the course inserted a bias favoring lower mortality in the later cohort.[52] Of note, when compared to mortality rate of COVID-19 patients included in high quality randomized clinical trials, we found that mortality rate in the control arms of the included studies was notably high and may well represent biases in the selection of patients related to many factors (i.e inclusion criteria, difference in diagnostic yields, epidemiology of the infection and availability of other treatments).[50] In particular, it should be underlined that these cohort studies were completed before the widespread use of remdesivir and dexamethasone [50,[53], [54], [55]. Furthermore, the dosage regimens for anakinra varied across studies, ranging from high-dose intravenous administration in the study by Cavalli et al., to relatively low dose subcutaneous administration in the study by Huet et al. [6,19]. The timing of administration also differed between studies due to practical reasons, although all investigators shared a conceptual attitude towards the earliest possible administration. For these limitations, no indication on which anakinra regimen is most suitable for COVID-19 can be extrapolated from these studies. Also, a limitation of the present study is the lack of a standardized corticosteroid regimen among the usual care. Since corticosteroids became the standard of care for COVID-19, future studies of IL-1 inhibitors will have to prove incremental benefit over corticosteroid treatment. Of note, previous evidence of incremental efficacy of anakinra over corticosteroids comes from macrophage activation syndrome, a hyper-inflammatory condition sharing similarities with COVID-19 [56], and myocarditis [57,58]. Another limitation of our analysis is that, due to the lack of adjustment in the primary studies we were not able to adjust for potential confounders and, therefore, the effect of anakinra on mortality could be either over or underestimated.
Strengths of this study include the timely and systematic review of an emerging therapeutic strategy for a new disease; comparison of anakinra to standard of care throughout all studies; and evaluation of clear-cut, clinically relevant outcomes such as mortality and need for mechanical ventilation. Moreover, despite the limited number of included studies, TSA showed that our data were convincing enough to prove the effect.
Clinical trials of anakinra in COVID-19 are ongoing (i.e. NCT04443881). If ever available, controlled evidence from these investigations will supersede currently available observational evidence. However, it should be noted that ongoing clinical trials of anakinra lack a shared core set of inclusion criteria as well as clear-cut outcomes, which is likely to yield inconclusive or conflicting results.[59] Also of note, while obviously superior to observational studies at generating evidence, randomized clinical trials are not nearly as pragmatic under emergency circumstances: controlled evidence may only become available after the cusp of the pandemic, with limited impact on patient management.[60] In this meta-analysis of low-quality available evidence, anakinra seemed to be associated with reduced mortality and need for mechanical ventilation, without safety concerns. These findings are in line with, and add robustness to, the increasing number of real-life reports of the clinical usefulness of anakinra for the treatment of patients with COVID-19. High-quality randomized clinical trials are urgently warranted to confirm these positive findings.
Declaration of Competing Interest
None.
Acknowledgments
Fundings
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
We thank Prof. Ines Navarro-Millian for sending us additional data.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ejim.2021.01.016.
Appendix. Supplementary materials
References
- 1.WHO Coronavirus Disease . WHO Coronavirus Disease (COVID-19) Dashboard; 2021. COVID-19) Dashboard. https://covid19.who.int/, accessed February 7, 2021. [Google Scholar]
- 2.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Luca G, Cavalli G, Campochiaro C, Della-Torre E, Angelillo P, Tomelleri A. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 6.Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavalli G, Franchini S, Aiello P, Guglielmi B, Berti A, Campochiaro C. Efficacy and safety of biological agents in adult-onset Still's disease. Scand J Rheumatol. 2015;44:309–314. doi: 10.3109/03009742.2014.992949. [DOI] [PubMed] [Google Scholar]
- 8.Cavalli G, Dinarello CA. Anakinra therapy for non-cancer inflammatory diseases. Front Pharmacol. 2018;9:1157. doi: 10.3389/fphar.2018.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavalli G, Dinarello CA. Treating rheumatological diseases and co-morbidities with interleukin-1 blocking therapies. Rheumatol (United Kingdom) 2015;54:2134–2144. doi: 10.1093/rheumatology/kev269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavalli G, Pappalardo F, Mangieri A, Dinarello CA, Dagna L, Tresoldi M. Treating life-threatening myocarditis by blocking interleukin-1. Crit Care Med. 2016;44:e751–e754. doi: 10.1097/CCM.0000000000001654. [DOI] [PubMed] [Google Scholar]
- 11.Tassell B, Canada J, Carbone S, Trankle C, Buckley L, Oddi EC. 2017. Interleukin-1 blockade in recently decompensated systolic heart failure: the recently decompensated heart failure anakinra response trial (REDHART). Eur J Hear Fail Conf Hear Fail 2017 4th World Congr Acute Hear Fail Fr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbate A, Kontos MC, Abouzaki NA, Melchior RD, Thomas C, Van Tassell BW. Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies) Am J Cardiol. 2015;115:288–292. doi: 10.1016/j.amjcard.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Grom AA, Horne A, De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat Rev Rheumatol. 2016;12:259–268. doi: 10.1038/nrrheum.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA. Interleukin-1 Receptor Blockade Is Associated with Reduced Mortality in Sepsis Patients with Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial∗. Crit Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pontali E, Volpi S, Antonucci G, Castellaneta M, Buzzi D, Tricerri F. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J Allergy Clin Immunol. 2020;146:213–215. doi: 10.1016/j.jaci.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aouba A, Baldolli A, Geffray L, Verdon R, Bergot E, Martin-Silva N. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217706. annrheumdis-2020-217706https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 17.Dimopoulos G, de Mast Q, Markou N, Theodorakopoulou M, Komnos A, Mouktaroudi M. Favorable Anakinra Responses in Severe Covid-19 Patients with Secondary Hemophagocytic Lymphohistiocytosis. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navarro-Millán I, Sattui S, Lakhanpal A, Zisa D, Siegel C, Crow M. Use of Anakinra to Prevent Mechanical Ventilation in Severe COVID-19: A Case Series. Arthritis Rheumatol. 2020 doi: 10.1002/art.41422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huet T, Beaussier H, Voisin O, Jouveshomme S, Dauriat G, Lazareth I. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arena WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: Role in biology. Annu Rev Immunol. 1998 doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 21.Gabay C, Smith MF, Eidlen D, Arend WP. Interleukin 1 receptor antagonist (IL-1Ra) is an acute-phase protein. J Clin Invest. 1997 doi: 10.1172/JCI119488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016 doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.2019. Cochrane Handbook for Systematic Reviews of Interventions. [DOI] [Google Scholar]
- 24.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009 doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González-García A, García-Sánchez I, Lopes V, Moreno-Arrones OM, Tortosa-Cabañas M, Elías-Sáenz I. Successful treatment of severe COVID-19 with subcutaneous anakinra as a sole treatment. Rheumatology (Oxford) 2020;59:2171–2173. doi: 10.1093/rheumatology/keaa318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez-Piedra C, Diaz-Torne C, Manero J, Pego-Reigosa JM, Rúa-Figueroa Í, Gonzalez-Gay MA. Clinical features and outcomes of COVID-19 in patients with rheumatic diseases treated with biological and synthetic targeted therapies. Ann Rheum Dis. 2020;79:988–990. doi: 10.1136/annrheumdis-2020-217948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filocamo G, Mangioni D, Tagliabue P, Aliberti S, Costantino G, Minoia F. The COVID-19 resource centre is hosted on Elsevier Connect. the company ’ s public news and information; 2020. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19. [Google Scholar]
- 28.Karadeniz H, Yamak BA, Özger HS, Sezenöz B, Tufan A, Emmi G. 2020. Anakinra for the Treatment of COVID-19-Associated Pericarditis: A Case Report. Cardiovasc Drugs Ther. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franzetti M, Pozzetti U, Carugati M, Pandolfo A, Molteni C, Faccioli P. Interleukin-1 receptor antagonist anakinra in association with remdesivir in severe coronavirus disease 2019: A case report. Int J Infect Dis. 2020;97:215–218. doi: 10.1016/j.ijid.2020.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Day JW, Fox TA, Halsey R, Carpenter B, Kottaridis PD. Interleukin-1 blockade with anakinra in acute leukaemia patients with severe COVID-19 pneumonia appears safe and may result in clinical improvement. Br J Haematol. 2020;190:e80–e83. doi: 10.1111/bjh.16873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fredi M, Cavazzana I, Moschetti L, Andreoli L, Franceschini F, Airò P. COVID-19 in patients with rheumatic diseases in northern Italy: a single-centre observational and case–control study. Lancet Rheumatol. 2020;9913:1–8. doi: 10.1016/s2665-9913(20)30169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cauchois R, Koubi M, Delarbre D, Manet C, Carvelli J, Blasco VB. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc Natl Acad Sci. 2020;117 doi: 10.1073/pnas.2009017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011 doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Radella F, Park DR. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001 doi: 10.1164/ajrccm.164.10.2104013. [DOI] [PubMed] [Google Scholar]
- 35.Campochiaro C, Farina N, Tomelleri A, De Luca G, Baldissera E, Cavalli G. Drug retention rates of biological agents in adult onset Still's disease. Semin Arthritis Rheum. 2021;51:1–6. doi: 10.1016/j.semarthrit.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Schultz MJ, Hemmes SNT, Neto AS, Binnekade JM, Canet J, Hedenstierna G. Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: LAS VEGAS - An observational study in 29 countries. Eur J Anaesthesiol. 2017;34 doi: 10.1097/EJA.0000000000000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cavalli G, Foppoli M, Cabrini L, Dinarello CA, Tresoldi M, Dagna L. Interleukin-1 receptor blockade rescues myocarditis-associated end-stage heart failure. Front Immunol. 2017 doi: 10.3389/fimmu.2017.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eloseily EM, Weiser P, Crayne CB, Haines H, Mannion ML, Stoll ML. Benefit of Anakinra in Treating Pediatric Secondary Hemophagocytic Lymphohistiocytosis. Arthritis Rheumatol. 2020 doi: 10.1002/art.41103. [DOI] [PubMed] [Google Scholar]
- 39.Ravelli A, Minoia F, Davì S, Horne A, Bovis F, Pistorio A. 2016 Classification Criteria for Macrophage Activation Syndrome Complicating Systemic Juvenile Idiopathic Arthritis: A European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborat. Arthritis Rheumatol. 2016 doi: 10.1002/art.39332. [DOI] [Google Scholar]
- 40.Minoia F, Davì S, Horne A, Demirkaya E, Bovis F, Li C. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: A multinational, multicenter study of 362 patients. Arthritis Rheumatol. 2014;66:3160–3169. doi: 10.1002/art.38802. [DOI] [PubMed] [Google Scholar]
- 41.Fukaya S, Yasuda S, Hashimoto T, Oku K, Kataoka H, Horita T. Clinical features of haemophagocytic syndrome in patients with systemic autoimmune diseases: Analysis of 30 cases. Rheumatology. 2008;47:1686–1691. doi: 10.1093/rheumatology/ken342. [DOI] [PubMed] [Google Scholar]
- 42.Mehta P, Cron RQ, Hartwell J, Manson JJ, Tattersall RS. Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol. 2020;2:e358–e367. doi: 10.1016/S2665-9913(20)30096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cavalli G, Cenci S. Autophagy and Protein Secretion. J Mol Biol. 2020;432:2525–2545. doi: 10.1016/j.jmb.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Cavalli G, Tomelleri A, De Luca G, Campochiaro C, Dinarello CA, Baldissera E. Efficacy of canakinumab as first-line biologic agent in adult-onset Still's disease. Arthritis Res Ther. 2019;21 doi: 10.1186/s13075-019-1843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novartis provides update on CAN-COVID trial in hospitalized patients with COVID-19 pneumonia and cytokine release syndrome (CRS) | Novartis n.d. https://www.novartis.com/news/media-releases/novartis-provides-update-can-covid-trial-hospitalized-patients-covid-19-pneumonia-and-cytokine-release-syndrome-crs (accessed December 22, 2020).
- 46.Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.July N. NASDAQ: SNY) Sanofi and Regeneron provide update on Kevzara® (sarilumab) Phase 3 U.S. trial in COVID-19 patients. Press Release Source: Sanofi (EURONEXT: SAN) 2020 [Google Scholar]
- 48.Campochiaro C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020 doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Della-Torre E, Campochiaro C, Cavalli G, De Luca G, Napolitano A, La Marca S. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: An open-label cohort study. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collaborative Group RECOVERY, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- 51.Pasin L, Navalesi P, Zangrillo A, Kuzovlev A, Likhvantsev V, Hajjar LA. Corticosteroids for COVID-19 patients with different disease severity: a meta-analysis of randomized clinical trials. J Cardiothorac Vasc Anesth. 2020;35 doi: 10.1053/j.jvca.2020.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ciceri F, Ruggeri A, Lembo R, Puglisi R, Landoni G, Zangrillo A. Decreased in-hospital mortality in patients with COVID-19 pneumonia. Pathog Glob Health. 2020;114:281–282. doi: 10.1080/20477724.2020.1785782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19: A Randomized Clinical Trial. JAMA - J Am Med Assoc. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC. Remdesivir for the Treatment of Covid-19 — Final Report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/nejmoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sönmez HE, Demir S, Bilginer Y, Özen S. Anakinra treatment in macrophage activation syndrome: a single center experience and systemic review of literature. Clin Rheumatol. 2018;37:3329–3335. doi: 10.1007/s10067-018-4095-1. [DOI] [PubMed] [Google Scholar]
- 57.De Luca G, Campochiaro C, Dinarello CA, Dagna L, Cavalli G. Treatment of dilated cardiomyopathy with interleukin-1 inhibition. Ann Intern Med. 2018;169:819–820. doi: 10.7326/L18-0315. [DOI] [PubMed] [Google Scholar]
- 58.De Luca G, Cavalli G, Campochiaro C, Tresoldi M, Dagna L. Myocarditis: An interleukin-1-mediated disease? Front Immunol. 2018;9:1335. doi: 10.3389/fimmu.2018.01335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.King A, Vail A, Hannan C, Brough D, Patel H, Galea J, et al. Anakinra in COVID-19: important considerations for clinical trials 2020. https://doi.org/ 10.1016/S2665-9913(20)30160-0. [DOI] [PMC free article] [PubMed]
- 60.Cavalli G, Dagna L. Authors’ reply; 2020. Effect of anakinra in COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.