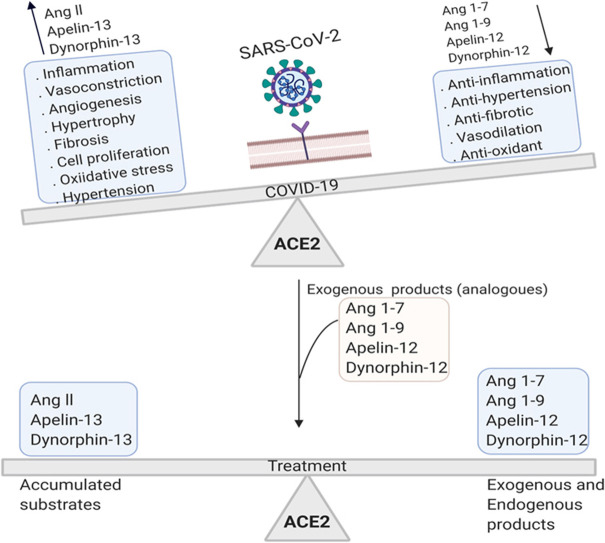
Abstract
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the cause of COVID-19, is reported to increase the rate of mortality worldwide. COVID-19 is associated with acute respiratory symptoms as well as blood coagulation in the vessels (thrombosis), heart attack and stroke. Given the requirement of angiotensin converting enzyme 2 (ACE2) receptor for SARS-CoV-2 entry into host cells, here we discuss how the downregulation of ACE2 in the COVID-19 patients and virus-induced shift in ACE2 catalytic equilibrium, change the concentrations of substrates such as angiotensin II, apelin-13, dynorphin-13, and products such as angiotensin (1–7), angiotensin (1–9), apelin-12, dynorphin-12 in the human body. Substrates accumulation ultimately induces inflammation, angiogenesis, thrombosis, neuronal and tissue damage while diminished products lead to the loss of the anti-inflammatory, anti-thrombotic and anti-angiogenic responses. In this review, we focus on the viral-induced imbalance between ACE2 substrates and products which exacerbates the severity of COVID-19. Considering the roadmap, we propose multiple therapeutic strategies aiming to rebalance the products of ACE2 and to ameliorate the symptoms of the disease.
Keywords: COVID-19, ACE2, Acute Respiratory Syndrome, Angiotensin, Cardiovascular Disease
Graphical Abstract
1. Introduction
SARS-CoV-2 enters the host cells mainly by targeting the Angiotensin Converting Enzyme 2 (ACE2) receptors that is highly expressed in lungs. As ACE2 is expressed in endothelial cells and also throughout the body, COVID-19 can be associated with other symptoms such as hypertension, thrombosis, pulmonary embolism and endothelial dysfunction [1].
ACE2, a key enzyme in renin-angiotensin system, binds to a range of substrates including Angiotensin I (Ang I), Angiotensin II (Ang II), apelin-13, neurotensin- (1–11), dynorphin A-(1–13), β-casomorphin-(1–7), and ghrelin [2]. SARS-CoV-2 interacts with ACE2 receptors via the surface glycoprotein S (homotrimer) [3], [4]. ACE2 has been identified as the main receptor for the binding of the SARS-CoV-2 protein S to the host cells. Other studies have shown that transmembrane serine protease 2 (TMPRSS2) [5], sialic acid receptors and extracellular matrix metalloproteinase may also mediate the entry of the virus to some extent [6]. The N-terminal of the SARS-CoV-2 glycoprotein contains a peptide signal, S1 and S2 subunits. S1 subunit binds to the peptidase domain of ACE2 via receptor binding domain (RBD) and S2 subunit mediates the fusion of viral membrane and host cell membrane, and facilitates the viral genomes entry into the host cells [3], [7].
Host protease-induced cleavage and dissociation of protein S are essential for infection [4]. Protein S is cleaved to a receptor-bound N-terminal S1 subunit and a C-terminal membrane fusion S2 subunit by host proteases such as type II transmembrane serine proteases (TTSPs), HAT, catepsin B and L (pH-dependent endolysosomal protease), elastase, trypsin and furin. A lysosomal protease then cleaves the S2 at S2′ and releases the hydrophobic fusion peptide for integration into the host cell membrane [8], [9].
Sequence homology studies identified several aminoacid replacement in RBD of SARS-CoV-2 protein S in comparison with SARS-CoV which increases the affinity to ACE2 and strengthen the interactions between them. The interaction site is bigger in SARS-CoV-2 than SARS-CoV, as 21 and 17 aminoacides contribute to direct interaction with ACE2, respectively [10], [11], [12]. Shang et al. reported that, unlike SARS-CoV, the cells infected by SARS-CoV-2 induce the proprotein convertase furin-mediated protein S pre-activation which increase the affinity of RBD to ACE2. This leads to efficient the virus entry into the host cells and also facilitates the escape of the virus from immune machinery. The authors hypothesized that this is the reason for high rate of the virus spread [11], [13]. Most of our knowledge about SARS-CoV-2 entery into the cells originates from SARS-CoV. Therefore, it has been suggested that once SARS-CoV-2 binds to ACE-2 receptor, fusion peptide (FP) in S2 subunit interacts with lipid layers in host cell membrane and induces the fusion of the virus and host membranes and formtion of endosomes, in which cysteine proteases cathepsin B and L and serine protease TMPRSS2 cleave protein S and faciliate the release of viral genome into the cytoplasm [13]. However, much remained to be discovered on how spike protein is cleaved by the proteases.
Xia et al. showed that unlike SARS-CoV, an additional RRAR motif exists in SARS-CoV-2 S1/S2 cleavage site. This motif is a furin cleavage site (FCS) that might be cleaved by furin-like enzymes and facilitate virus spread and infection [14], [15]. Huang et al. and Hoffmann showed that serine protease TMPRSS2 on host cell membrane might activate SARS-CoV-2 spike protein to enhance endosome-mediated viral entry into the cells [4], [11].
Based on a previous report virus-bound ACE-2 endocytosis is pH dependent and requires acidic pH (pH 3). Therefore, lysosome and endosome acidic pH activates cathepsin B and L which in turn induce the cleavage of glycoprotein S to S1 and S2 subunits. S1 binds to ACE-2 and S2 is used for membrane fusion [16].
The contribution of a range of proteases to virus-ACE2 interaction can explain many aspects of SARS-CoV-2 pathogenesis. Hoffmann et al. demonstrated that the inhibition of TMPRSS2 serine protease by camostat mesylate blocked SARS-CoV-2 infection of lung cells. They concluded that TMPRSS2 plays a role in priming the viral spike protein to enter the cells. According to the later research, the full inhibition of viral infection is attained when endosomal cathepsin L and B (cysteine proteases) and TMPRSS2 are co-inhibited by E-64d and camostat mesylate respectively. In addition, ammonium choloride strongly inhibited viral entry into the TMPRSS2‾293T cells implied the ammonium choloride-induced inhibition of endosomal cathepsin L and B cysteine proteases [4].
In cells, virus amplification takes place by viral RNA polymerase and the viruses then infect the surrounding cells.This might lead to organ dysfunction and faster spread of the virus. Coronaviruses forms bilayer vesicles and prevents the expression of Receptor Recognition Pattern (PRP) and thus, immune system fails to recognize the viral particles [16], [17], [18].
The entry of the SARS-CoV-2 and SARS-CoV depends ACE-2 [19], [20], [21], SARS-CoV-2 downregulates ACE2 expression similar to SARS-CoV [21], [22]. However, sialic acid of the glycoproteins on the surface of host cell may also act as the receptor for hemagglutinin esterase (HE) on SARS-CoV-2. HE contains a carbohydrate binding domain (lectin) linked to a domain with esterase activity. HE binds to sialic acid moieties and facilitates the virus entry. It seems that HE-mediated fusion of the virus and host cell membrane are necessary for the entry of SARS-CoV-2 [23], [24]. ACE2 is the well-known receptor for the entry of SARS-CoV-2 [1]. ACE2 dysregulation changes the equilibrium of the ACE2-catalyzed reaction. As a result, ACE2 substrates are accumulated while the concentrations of its products are decreased. This might worsen the pathology of the disease and may lead to death. Thereby, ACE2 functions will be reviewed and discussed in detail to elucidate the effects of ACE2 dysregulation upon the entery of the SARS-COV-2.
2. ACE2
ACE2 is a cell-surface non-raft protein with the extracellular N-terminal and intracellular C-terminal domains. Binding to SARS-CoV-2 protein S induces the ACE2 internalization [25]. ACE2 is mainly expressed in cardiac muscle cells, cardiac fibroblasts, the coronary vascular endothelium, kidney, liver, small intestine, testes, brain, lung alveolar epithelial cells, lymphocytes within oral mucosa, enterocytes, arterial and venous endothelial cells as well as arterial smooth muscle cells [26], [27], [28], [29], [30], [31], [32].
ACE2 is a transmembrane protein, however, a low level of soluble form is detectable in the plasma of patients with COVID-19 [33]. Compared with apical and basolateral localization of ACE, ACE2 is mainly on the apical cell surface [34]. Although ACE2 is a similar carboxypeptidase to ACE (EC 3.4.15.1) with 42% sequence similarity, it is a mono-carboxypeptidase (cleaves one amino acid from the substrate) and ACE is a dipeptidyl-carboxypeptidase which cleaves a dipeptide at the C-terminal of substrate [35], [36].
Enzyme structure contains a signal peptide, transmembrane domain and Zn2+-binding active site. Catalytic site is exposed to vasoactive peptides [35]. ACE inhibitory antibodies do not inhibit the ACE-2, in spite of the similar structures [35], [36].
In contrast, ACE2 is overexpressed in patients treated with ACE inhibitory antibodies [37], [38]. ACE2 converts Ang II to Angiotensin (1–7) (Ang 1–7), Ang I to angiotensin 1–9 (Ang 1–9), apelin (1–13) to apelin (1–12), dynorphin A (1–13) to dynorphin (1–12), β-casomorphin (1–7) to β-casomorphin (1-6) [2]. ACE2 is a potential regulator of renin angiotensin system (RAS). SARS-CoV-2 interaction with ACE2, interferes RAS and enhances the severity of acute respiratory symptoms [39]. Since SARS-CoV-2 infection is associated with a downregulation of ACE2 expression [21], this can exacerbates the COVID-19 symptoms. For better understanding of the harmful effects of the accumulation of ACE2 substrates and decrease in the concentrations of the products due to the ACE2 viral- induced dysregulation, we have a closer look at the substrates and products ( Table 1). In this review, we focus on the consequences of the SARS-CoV-2 induced imbalance between ACE2 substrates and products aiming to discover the relation among substrates accumulation, decreased products and disease complications to propose therapeutic strategies.
Table 1.
Properties of the main substrates and products of ACE2.
| Factors | Sequence | kcat/Km | Half-life | Circulation levels | Receptor | Ref |
|---|---|---|---|---|---|---|
| Ang I1 | DRVYIHPFH↓L | – | – | – | [2] | |
| Ang 1–92 | DRVYIHPFH | – | – | AT2R5 | ||
| Ang II3 | DRVYIHP↓F | 15 min in heart, kidney, and adrenal, 0.5 min in circulation (in pigs) | 5–50 fmol/ml (in human, mouse, and rat plasma) | AT1R6 | [227], [228] | |
| Ang 1–74 | DRVYIHP | – | 9 s in rat, 0.5 h in human | 5–80 fmol/ml (in human, mouse, and rat plasma), in human 20 pg/ml | Mas R7 | [227], [229] |
| Apein 1–13 | QRPRLSHKGPMP↓F | Less than 3 min in rat and less than 8 min in human | APJ8 | [2], [163] | ||
| Apelin 1–12 | QRPRLSHKGPMP | – | 10–15 min in human plasma | APJ | [161] | |
| Dynorphin A 1–13 | YGGFLRRIRPKL↓K | Less than 1 min in human blood (ex vivo) | KOP9,NADM10 | [2], [230] | ||
| Dynorphin A 1–12 | YGGFLRRIRPKL | – | – | – | KOP | [2], [231] |
1: Angiotensin I, 2: Angiotensin 1–9, 3: Angiotensin 1–7, 5: angiotensin II type-2 receptor, 6: angiotensin II type-1 receptor, 7: Mas receptor, 8: apelin receptor, 9: κ-opioid receptor, 10: N-Methyl-D-aspartate receptor.
3. Ang II
In RAS, Angiotensin I is produced from angiotensinogen by the action of renin (released from kidney juxtaglomerular cells) [40]. Ang I is converted to Ang II by the catalytic activity of ACE particularly in pulmonary endothelial, plasma, kidney, brain and heart coronaries. In rat model, Ang II, with a half-life of approximately 16 s in plasma [41], is quickly converted to either Ang (1–7) by ACE2 or Ang III by angiotensinases [42]. Ang II is an active octapeptide which triggers its physiological functions by binding to Angiotensin II receptor type 1 (AT-1) ( Fig. 1), AT-2 and AT-4 receptors [43]. The main functions of AT-1 mediated Ang II [44] are vasocontraction [45], inotropy and cardiac regeneration (via myocard receptors), sympathetic nervous system enhancement [46], vasopressin diffusion, regulation of peripheral sympathetic nervous system and pituitary, regulation of aldosterone secretion (via receptors in adrenal glands) [47]. AT-2 receptors are widely expressed in emberyonic tissues, however their expression is limited to adrenal gland, vascular endothelial, brain, kidney and ovary, which acts as vasodilator by releasing bradykinin and nitric oxide [48]. AT-2 mediates anti-inflammatory in vitro and in vivo [49], [50] and anti-proliferating responses in rat pheochromocytoma cell line.
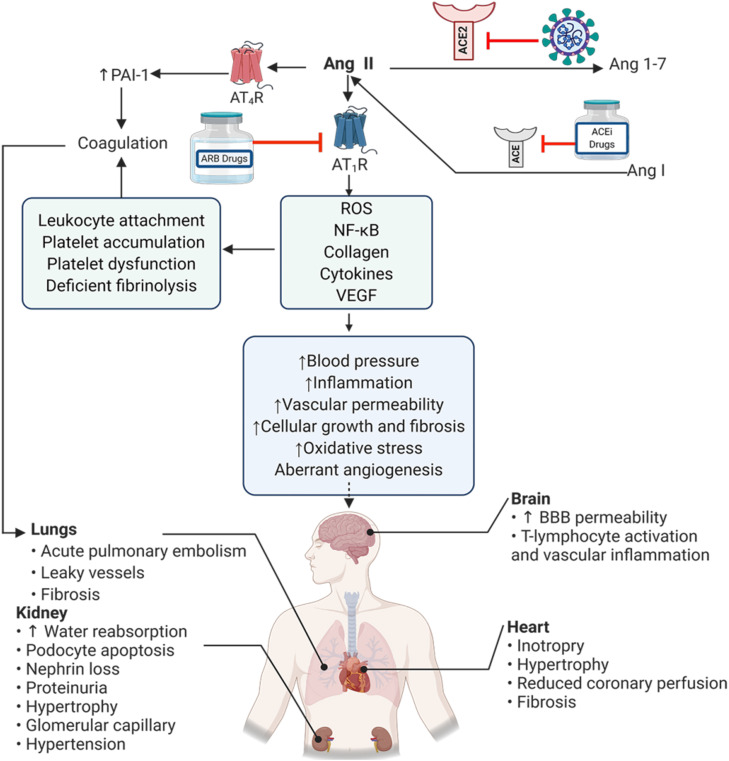
Fig. 1.
Effects of Ang II on organs. ACE degrades Ang I and produces Ang II which binds to its receptors (ATRs). Then, ACE2 converts Ang II into Ang 1–7. SARS-CoV-2 downregulates the expression of ACE2 and causes the pathological symptoms in several human organs due to accumulation of Ang II (Created in BioRender.com). Ang I: angiotensin I, Ang II angiotensin II, Ang 1–7. angiotensin 1–7, ACE: angiotensin-converting enzyme, ACE2: angiotensin-converting enzyme2, AT1R: Angiotensin II receptor type 1, AT4R: Angiotensin II receptor type 4, PAI-1: Plasminogen activator inhibitor-1 ROS: Reactive Oxygen Species, VEGF: Vascular endothelial growth factor, ARB drugs: angiotensin II receptor blocker drugs, ACEi drugs: Angiotensin-converting enzyme inhibitor drugs, BBB: blood–brain barrier.
[51], [52] which attenuates AT-1 signaling in turn. Moreover, it seems that AT-4 regulates extracellular matrix in central nervous system and modulates oxytocin diffusion [53], [54].
Ang II stimulates inflammatory responses, via AT-1, in leukocytes, endothelial cells and smooth muscle cells by activating NF-κB which altogether enhance the transcription of TNF-α, IL-1 and Interleukin 6 (IL-6) [55], adhesive molecules such as E-selectin and P-selectin, vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), different chemokines and other proinflammatory factors [56]. These functions of Ang II are consistent with the findings from COVID-19 patients where SARS-CoV-2 entry to pulmonary cells was associated with high secretion of inflammatory cytokines like IL-1β, IL-6 and TNFα [1], [57]. Among all, IL-6 is known to be one of the strongest predictors of death during hospitalization [58]. Downregulation of ACE-2 in COVID-19 patients can lead to Ang II accumulation [57], [59]. Studies on patient samples and animal models have shown that Ang II binding to AT-1 can further internalize ACE2 through extracellular signal-regulated kinases (ERK1/2) and P38 mitogen-activated protein kinase (MAPK) signaling and hence downregulates ACE2 [60], [61]. Hypertension in COVID-19 patients [62], [63], [64] might be severed by Ang II accumulation which activates proinflammatory signaling [65]. Clinical studies have shown that arterial pressure is correlated to increased plasma inflammatory mediators and vascular inflammation. In hypertensive patients circulating monocytes, lymphocytes and pro-inflammatory cytokines such as TNF-α, IL-6 and C-reactive protein (CRP) are increased [66], [67], [68].
Accumulated Ang II can be metabolized to Ang IV by aminopeptidase A [69]. Ang IV binds to AT-4 receptor [54]. Accumulation of Ang II (in mice model) and Ang IV (in rat model) can increase the likeliness of thrombosis by increasing the plasminogen activator inhibitor-1 (PAI-1) [70], [71], [72]. Thrombotic role of Ang II can be explained by overexpressing and activating endothelin-1 as PAI-1 activator [65], [73]. Ang II-stimulated inflammation influences the vascular permeability in rat endothelial cells [74], alters vascular morphogenic responses, leukocyte attachment to endothelium (in rat model) [75] and platelet accumulation in human platelet rich plasma (in vitro study) [76], [77]. Hypertension is prevalent among the COVID-19 patients and might be induced by Ang II accumulation associated with accumulation of platelets, platelet dysfunction, deficient fibrinolysis and coagulation [71], [78]. Therefore, thrombosis in COVID-19 patients can be attributed to harmful effects of Ang II accumulation due to dysregulation of ACE2 after the entry of the SARS-CoV-2 into host cells [8], [79]. COVID-19 patients have more or less impaired coagulation system which increases the rate of ischemic mortality [80], [81]. Acute pulmonary embolism in COVID-19 patients is one of the important predictor of clinical deterioration during viral pneumonia [82], [83], [84]. Therefore, a constant checking of the coagulation markers such a Prothrombin Time/Partial Thromboplastin Time (PT/PTT), fibrinogen, D-dimer and prescribing the anticoagulation agents like heparin has been recommended for patients with D-dimer value above normal range (4 times higher in COVID-19 patients) [1].
Ang II also acts as a pathologic mechanism in angiogenesis during hypoxia. Ang II upregulates angiopoietin-2, the factor that binds to TEK receptor tyrosine kinase (Tie2) and induces angiogenesis. At the early stages of angiogenesis, angiopoietin-2 antagonizes Tie2 and inhibits angiopoietin-1/Tie2 signaling axis. Cornea micropocket assay has showed that the angiopoietin induces neovascularization in mice mediated by vascular endothelial growth factor (VEGF) [85], [86]. This allows VEGF and other growth factors to enhance the migration of endothelial cells, and form new vessels [85], [86]. Consequently, angiopoietin-1 is increased while angiopoietin-2 is decreased [87], [88]. However, angiopoietin-1 activates Tie2 and induces endothelial survival as well as vessel maturation [87], [88]. Therefore, the ratio of angiopoietin-2 to angiopoietin-1 is well controlled during angiogenesis and shifts toward either of the proteins associated with pathological conditions [87], [88]. Angiopoietin-2 is increased during acute respiratory distress syndrome (ARDS) in COVID-19 patients [89]. This is consistent with the increased (angiopoietin-2)/(angiopoietin-1) ratio in ALI/ARDS patients. Increased Angiopoetin-2 can be a prognosis marker for lethality [90]. Ang II upregulates angiopoietin-2 and increases angioprotein-2 as well as VEGF expression and thus play a key role in increasing vascular permeability in patients with acute pulmonary injury [91]. Vascular permeability is increased in patients with acute respiratory syndrome as a result of hypoxia-induced VEGF expression [92]. and focal adhesion tyrosine kinase (FAK) induced inflammatory responses [93]. Angioprotein-2 has been shown to induce inflammation and cell death during hypoxia in vivo as well as epithelial necrosis in mouse lung epithelial cell and mice in vitro (hyperoxia causes angiopoietin 2–mediated acute lung injury and necrotic cell death).
These raise the possibility that Ang II-induced angiogenesis and high vascular permeability worsen the pathology of the COVID-19 [8]. Regarding the role of Ang II in the induction of oxidative stress, Ang II phosphorylates c-Src, [the regulator of NOX (a family of NAD(P)H oxidases) that is the main source of reactive oxygen species (ROS)] [94], [95]. In addition, Ang II facilitates ROS generation by the mitochondrial potential depolarization [96]. There is evidence that Ang II downregulates the ROS scavengers and hence increases ROS [97]. ROS activates redox-sensitive transcription factors like activator protein 1 (AP-1) and NF-κB [98], leading to vascular permeability, leukocyte infiltration, and etc [98]. As oxidative stress is generated during coronavirus induced ADRS, COVID-19 patients show the aforementioned symptoms due to Ang II accumulation and impaired ACE2 signaling axis, less access of endothelial cells to nitric oxide (NO) and oxidative stress [99]. Moreover, the increased ROS during oxidative stress can upregulate VEGF in macrophages and endothelial cells [100], [101]. ROS induces Hypoxia-Inducible Factor 1-alpha (HIF1-α) and Ets-1 and resulting in VEGF upregulation [102]. By-products of ROS such as lipid peroxide can interact with VEGFR2 and induce angiogenesis in vivo [103]. Multiple in vitro studies on normal and tumor cell types have shown that the lipid oxidation products increase VEGF which induces angiogenesis in association with IL-8 and COX-2 products [104]. Animal studies have shown that lipid oxidation products can induce angiogenesis through VEGF-independent Toll-like receptor (TLR) 2/MyD88 dependent signaling (Fig. 1) [105].
As discussed above, Ang II can affect the severity of COVID-19. A recent study in human (non-hypertensive patients) showed that there is a correlation between the plasma concentration of Ang II and severity of COVID-19 [59]. Since it negatively regulates the activity of ACE2 and also plays a role in hypertension, infalamation, oxidative stress and angiogenesis whose accumulation may lead to severity of COVID-19.
4. Ang (1–7)
Ang II is mainly degraded by ACE2 and to some extent by prolylcarboxypeptidase (PRCP) and prolyloligopeptidase (POP/PEP/ PREP/PE), where these carboxypeptidases remove the C-terminal phenylalanine from Ang II [33]. In contrast to Ang II, Ang (1–7) is a biologically active peptide and acts by binding to MAS receptor and inhibiting the vasocontraction, angiogenesis and inflammation ( Fig. 2) [106], [107].
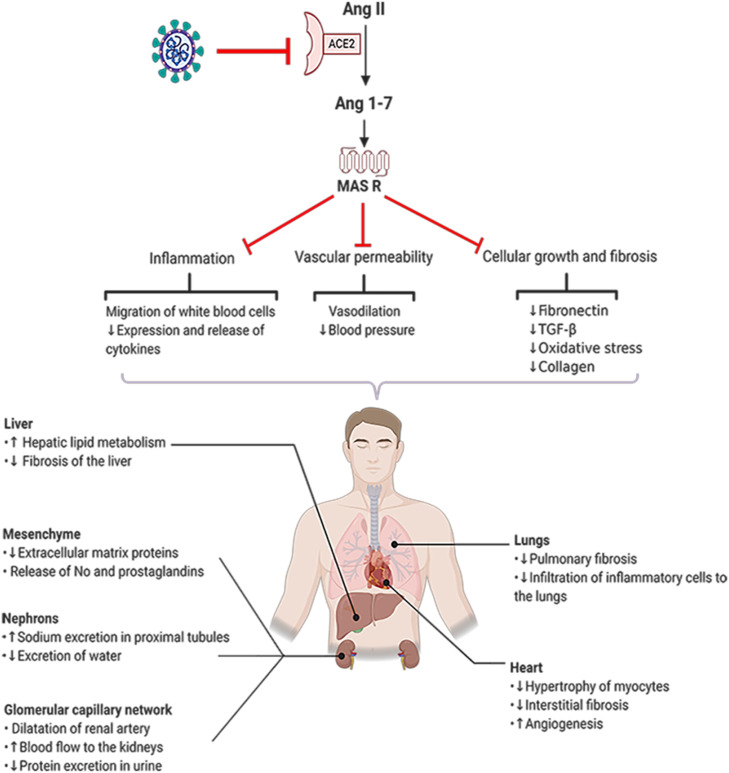
Fig. 2.
Ang 1–7 functions in several organs. ACE2 converts Ang II into Ang 1–7 which acts through its receptors (MasR). SARS-CoV-2 downregulates the expression of ACE2 and reduces the concentration of Ang 1–7 leading to progressive deterioration of physiological functions in patients with COVID-19 (Created in BioRender.com). ACE2: angiotensin converting enzyme2, Ang 1–9: Angiotensin 1–9, Ang 1–7: Angiotensin 1–7, MasR: mitochondrial assembly receptor, TGF-β: Transforming growth factor beta.
In addition, Ang (1–7) has showed beneficial effects on hypertensive patients. It also alters renal water retention, and enhances vasodilation [108]. Ang (1–7) suppresses angiogenesis, suggesting that Ang (1–7) can be an endogenous regulator of cell growth. It should be noted that Ang (1–7) reduces VEGF and consequently induces the angiogenesis in xenograft mice model of lung cancer. Altogether, Ang (1–7) reduces the expression of VEGF, placental growth factor (PlGF), HIF-1α and VEGF receptors in human nasopharyngeal carcinoma xenografts in nude mice model. These processes have been further confirmed by in vitro studies [109], [110]. In vivo study on prostate cancer model has showed that Ang (1–7) decreases angiogenesis by reducing the expression of PLGF and VEGF and increasing the expression of fms-like tyrosine kinase-1 (sFlt-1) [111]. Anti-inflammatory functions of Ang (1–7) are induced by binding to MAS receptors on leukocytes [106] Anti-inflammatory and antifibrotic activities of Ang (1–7)/Mas are regulated by reducing the leukocyte migration, downregulation of pro-inflammatory cytokines, and reduction of tissue fibrosis factors [106]. In patients with kidney disease, Ang (1–7)/Mas attenuates inflammatory responses by reducing neutrophil influx, downregulating CXC chemokine ligand (CXCL), IL-6, TNF-α, IL-1b, Endothelin-1 (a vasocontractor) and monocyte chemoattractant protein-1 (MCP-1, a key chemokine responsible for white blood cells migration) [112]. Considering the renal failure in COVID-19 patients, administration of Ang (1–7) might help the recovery of the patients by its anti-inflammatory and anti-fibrotic effects [113]. Ang (1–7) decreases oxidative stress and suppress NF-κB and thus improves permanent middle cerebral artery occlusion [114]. In addition, intracerebroventricular administered Ang (1–7) in brain ischemic mice inhibited NF-κB activity which in turn is associated with downregulation of TNF-α, IL-1β and cyclooxygenase-2 (COX-2) and hence could rescue the brain [115]. COVID-19 damages patients’ nervous system as virus is able to attack nervous system suggesting the use of Ang (1–7) to reduce the clinical complications [115]. Study on heart tissue (Sprague–Dawley rats) has shown the beneficial role of Ang (1–7) in maintaining the cardiovascular hemostasis and protection against heart diseases. In heart (Sprague–Dawley rats), Ang (1–7) reduces the expression of inflammatory cytokines such as TNF-α and IL-6 and increases IL-10 [116]. As ARDS is caused by SARS-CoV-2 infections, Ang (1–7) can be a useful drug candidate for improving lung function. Reduction in the concentration of Ang (1–7) in COVID-19 patients is due to dysregulation, internalization and shedding of ACE2. Ang (1–7) has anti-thrombotic effect by releasing an antithrombotic factor NO via MAS receptor-induced signaling. When concentration of Ang II is low, Ang (1–7) binds to AT2R and increases the concentration of NO and prostacyclin in plasma and these factors facilitate clot formation by activating the platelets [115], [117], [118], [119], [120].
Considering the anti-inflammatory and anti-hypertensive roles and stimulatory effect on clot formation, Ang (1–7) might be beneficial for COVID-19 patients to protect them against thrombosis (Fig. 2).
5. Ang (1–9)
Ang (1–9) is produced from Ang II in a reaction catalyzed by ACE2 or catepsin A [121]. Ang (1–9) binds to AT2R, inhibits the Ang II-AT1R signaling axis [122] and balances the vasoconstrictive/proliferative to vasodilatory/antiproliferative axis and consequently, improves cardiovascular conditions [123]. AT2R-derived signaling functions in cell differentiation, vasodilation and reduces the cell proliferation, inflammation and fibrosis [124].
During post-myocardial infarction, Ang (1–9) improves cardiovascular conditions and left ventricular systolic performance [124], [125], [126]. In cardiomyocytes, similar to Ang (1–7), Ang (1–9) exerts positive inotropic effects by increasing calcium transient amplitude and contraction force, and anti-ventricular hypertrophy effect, in vivo and in vitro (in animal models) [124]. Ang (1–9) reduces blood pressure by affecting endothelial cells [127]. The vascular enhancement is attributed to the release of NO and arachidonic acid in endothelial cells, which improves bradykinin-induced endothelial repair (in vitro study on human right atrial and left ventricular tissues) [128]. Ang (1–9) also reduces tissue fibrosis especially in the heart and the lungs [122], [129]. Pulmonary fibrosis has been demonstrated in COVOD-19 patients [130], suggesting that Ang (1–9) might be a potential treatment for lung fibrosis. SA Cha et al. have shown that Ang (1–9) downregulates the expression of proinflammatory cytokines such as IL-6, IL-1β, TNF-α, MCP-1 in pulmonary arterial in monocrotaline (MCT)-induced pulmonary hypertensive rats [131]. Furthermore, Ang (1–9) downregulates proinflammatory cytokines such as IL-6, IL-1β, TNF-α, MCP-1 in pulmonary arterial hypertension in rat model [131]. In hypertensive rats, Ang (1–9) affects heart, aortic wall, and kidney independent of AR2R [132] Therefore, it is anticipated that Ang (1–9) synergizes Ang (1–7) and suppresses the inflammation in COVID-19 patients. Low level of Ang (1–9) and the consequent complications can be attributed to downregulation of ACE2 ( Fig. 3).
Fig. 3.
Ang 1–9 functions in human organs. ACE2 converts Ang1 to the Ang 1–9 and the latter acts through its receptor (AT2R). SARS-CoV-2 downregulates the expression of ACE2 which ultimately reduces the concentration of Ang 1–9. The decrease in the concentration of Ang 1–9 results in the loss of its beneficial functions in several organs in patients with COVID-19 (Created in BioRender.com). ACE2: angiotensin converting enzyme2, Ang I: Angiotensin I, Ang 1–9: Angiotensin 1–9, AT2R; Angiotensin II receptor type 2, MI: myocardial infarction, vWF: von Willebrand factor, α-SMA: α-smooth muscle actin.
6. Apelin (1–13)
Apelin (1–13) is a member of apelin family, named after first extraction from bovine stomach extract. This peptide acts as a ligand for previously-identified GPCR AR (APJ) receptors. Apelin in mammalian has 77 amino acids and is engaged to the production of several peptides with various lengths (12, 13, 17 and 36 residues in length) [133], [134].
It is the second catalytic substrate for ACE2 with the powerful positive inotropic actions [133]. The isoforms can be generated by the processing enzymes. Apelin-13 is converted to Pyr-apelin-13 by spontaneous rotation of N-terminal glutamine which produces a more stable isoform with higher half-life [134], [135], [136], [137], [138].
Apelin-13 is the most abundant isoform in plasma, heart and brain [134]. Apelin and its receptor APJ are expressed in CNS, cardiovascular, circulation, digestive and reproductive systems, fat tissue and skeletal muscles [139], [140].
It seems that the main physiological role of apelin is the vascular tone and cardiac contraction modulation [139], [140]. In rat model, apelins modulate cardiocontraction and vascular tone likely via activation of protein kinase Cε (PKCε) and ERK1/2 signaling pathway [141]. In addition, it has been suggested that apelin upregulates VEGFA, VEGFR2 (kdr Kinase Insert Domain Receptor), angiopoetin-1, Tie2 and endothelial nitric oxide synthase (eNOS) as angiogenic and antifibrotic factors to target the heart (in rat model of post-MI) [142]. Apelin (1–13) also plays anti-apoptotic roles via phosphatidylinositol-3-kinase (PI3K)/Akt, ERK1/2, caspase signaling and autophagy [143]. Hypoxia induces the expression of apelin via HIF-1α-mediated mechanism in smooth muscle cells in the lungs [144].
Moreover, expression of apelin is induced both directly and indirectly by hypoxia and VGEF in endothelial cells respectively [144], [145]. Owing to ACE2 downregulation [21], [22], apelin-13 might be increased in the plasma of patients with COVID-19. Upregulations of HIF-1α, VEGF and VEGFR2 and activation of eNOS-generating signaling pathway (AMPK and PI3K / Akt) and angiogenesis are induced by apelin (1–13). VEGF, one of the most important growth factors required for angiogenesis, binds to kdr receptor, induces migration and replication of endothelial cells and inhibits the apoptosis. NO produced during downstream signaling contributes to migration, proliferation, and anti-apoptotic function of endothelial cells, stimulates the proliferation of endothelial cells and prevents vascular degeneration. Furthermore, apelin (1–13) has been reported to induce angiogenesis in heart, retina and lungs [146], [147], [148], [149], [150].
Similar to Ang II accumulation, increased angiogenesis in COVID-19 patients [8] is probably correlated to the level of apelin. Based on the previous research, during oxidative stress, apelin (1–13) increases antioxidant scavengers and reduces mitochondrial ROS [151], [152], [153], [154].
As COVID-19 is a severe respiratory disease, it is hypothesized that apelin-13 is increased in the plasma of the patients with COVID-19. Some studies have shown that administration of apelin (1–13) inhibits NF-κB pathway which reduces proinflammatory cytokines IL-6 E, TNF-α and IL-1β [154], [155].
Moreover, LPS-treated mice showed that apelin (1–13) prevents the nuclear migration of NF-κB 65P and subsequently inhibits the NF-κB signaling and the macrophage infiltration [154].
Interestingly, apelin (1–13) prevents the activation of LPS-induced NLR family pyrin domain containing 3 (NLRP3) inflammation in mice lungs and hence reduces the inflammatory cytokines [152], [154]. It can be imagined that due to the low half-life, anti-inflammatory and anti-oxidant properties, endogenous apelin (1–13) is not quite stable.
One of the most remarkable roles of apelin (1–13) is to regulate blood pressure. It has been found that APJ receptor interacts with AT1R to decrease the affinity of Ang II binding to AT1R. Therefore, it acts as a negative allosteric regulator for AT1 and negatively regulates Ang II function during hypertension [156]. Moreover, apelin upregulates the expression of ACE2 and reduces the concentration of Ang II (in vitro and in vivo studies: in mice) [157], [158].
Studies on the administration of apelin, to compare the effects of apelin (1–12), apelin (1–13) and apelin (1–36) on arterial pressure, showed that apelin (1–12) is most potent for arterial pressure reduction in anesthetized mice [159]. Therefore, it is hypothesized that downregulation of ACE2 by SARS-CoV-2 in COVID-19 patients can reduce Apelin (1–12), the product of ACE2. Considering the low half-life of apelin (1–13), it might be accumulated in body upon the entery of SARS-CoV-2 into the cells ( Table 2).
Table 2.
Some disease-related functions of apelin-13 and APJ.
| Organ | Diseases | Experiment model | Effects | Outcomes | Ref |
|---|---|---|---|---|---|
| Lung | Pulmonary hypertension (PAH) | Mice, human lung tissues and pulmonary artery endothelial cells | miR1424/503 overexpression, FGF2 and FGFR13 downregulation | pulmonary and vascular homeostasis | [232], [233] |
| Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) | Mice | Suppression of nuclear translocation and activation NF-κB4, ↓NLRP35, ↓Inflammasome-induced inflammatory responses, ↓ROS6 production, Mitochondrial apoptosis. | Cytoprotective effects | [154] | |
| Bronchopulmonary dysplasia | Rat | ↓ Fibrin deposition and inflammation. | Reduction of pulmonary fibrosis. | [234] | |
| Heart | Cardiac fibrosis | Mouse and neonatal cardiac fibroblasts | ↓ Collagen production, ↓CTGF7 and ↓TGF-β8. | Increased cardiac fibrosis. | [235] |
| Chronic Heart failure | Human | ↑ Peripheral and coronary vasodilatation. | Increased cardiac output. | [236] | |
| Kidney | Diabetic nephropathy | Mice and mesangial cells | ↓glomerular filtration rate. | Suppression of the development of nephropathy via regulation of histone acetylation. | [237] |
| ↓ Proteinuria, ↓ glomerular hypertrophy, ↓mesangial expansion and renal inflammation and inhibition of histone hyperacetylation. | |||||
| Chronic kidney disease | Mice and HK-2 cells | Antagonist of the TGF-β1/Smad9 pathway. ↓matrix collagen production and improved tubulointerstitial lesions. | Retardation of CKD progression. | [238] | |
| Brain | Cerebral I/R injury | Rat and neurons cells | Activation of Gαi/Gαq-CK210 signaling attenuates eIF211-ATF412-CHOP13-mediated neuronal apoptosis. | Protection of neurons against I/R injury-induced apoptosis, facilitation of post-stroke recovery | [239] |
| Parkinson’s disease | Human neuroblastoma SH-SY5Y cell | ↓ ROS and the inhibition of caspase-3 and release of cytochrome c | Protective effects against dopaminergic neural toxicity by antioxidant and antiapoptotic properties. | [240] |
1: microRNA, 2: Fibroblast growth factors, 3: Fibroblast growth factor receptors, 4: Nuclear factor kappa B, 5: NLR family pyrin domain containing 3, 6: Reactive oxygen species, 7: Connective tissue growth factor, 8: Transforming growth factor beta, 9: Mothers against decapentaplegic homolog 4, 10: Casein kinase II, 11: Eukaryotic initiation factor 2, 12: Activating transcription factor 4, 13: C/EBP homologous protein.
7. Apelin (1–12)
Hydrolysis of phenylalanine residue at the C-terminal decreases the half-life of apelin (1–13) in the circulation resulting in the production of apelin (1–12) [157]. Removal of phenylalanine from apelin (1–13) reduces the affinity of the produced apelin (1–12) to APJ receptor three times without significantly changing the biological activity [160].
Tatemoto et al. have shown that in rat model the biological activity of apelin is inversely related to the length of the peptide and therefore, apelin-12 is a highly active isoform [159]. However, the length of at least 12 residues seems to be essential for the biological activity of the apelin derivatives because apleins with 9, 10 and 11 residues are biologically inactive. The half-life of apelin (1–12) in human plasma is 10–15 min [161] while it is about 3 and 8 min for apelin (1–13) in rats [162] and humans plasma [163], respectively. Apelin knockout mice showed hypertension and age-related impaired ventricular contraction similar to the cardiac phenotype of ACE2 knockout mice [164]. Apparently, stable responses following apelin (1–13) injection is due to ACE2-induced apelin (1–13) cleavage and the production of apelin (1–12). Studies on rats with coronary artery occlusion have shown the apelin (1–12) in heart which reduced the mean arterial pressure [165]. Apelin (1–12) injection following local ischemia ameliorates myocard infarction and damage to heart membrane in rats [166].
In humans, apelin (1–12) increases blood pressure in a dose-dependent manner. Apelin (1–12) induces the overexpression of ACE2 [158]. On the other hand, SARS-CoV-2 infection is associated with a downregulation of ACE2 expression [21]. Therefore, it has been suggested that induction of the expression of ACE2 particularly apelin (1–12), would lead to increase in the concentrations of Ang (1–7) and Ang (1–9) which in turn might promote the recovery from COVID-19. Since acute cardiac injury is among the most common complications in COVID-19 [167], apelin (1–12) reduction may induce cardiac injury as it normally plays protective roles in heart tissue ( Table 3). This reduction also causes cardiovascular failure, as it reduces systemic oxygenation during pneumonia and activates T cells and immune signaling [168]. Downregulation of ACE2 in COVID-19 might attenuate the immune response to SARS-CoV-2 due to decrease in the concentration of apelin (1–12) which can explain cardiac injury by taking into account the protective role of apelin (1–12) for heart tissue. It is crucial to investigate the effects of Apelin (1–12) and Apelin (1–13) on the repair of endothelial cells as immunoreactivity of [Pyr1] apelin-13 (1–12)-like has been discovered in neighboring endothelial cells [161].
Table 3.
Disease-related effects and outcomes of the administration of apelin-12.
| Organ | Diseases | Experiment model | Effects | Outcomes | Ref |
|---|---|---|---|---|---|
| Heart | Conscious rat | Rat | ↓Mean arterial pressure and ↑ heart rate | Arterial and venous dilator | [241] |
| Hypertension | Rat | ↓Systolic and diastolic blood pressure | Hypotension | [242] | |
| Failing rat cardiac muscle | Rat | ↑ Transients [Ca2+]I and ↑ force development | Selective positive inotropic effect | [243] | |
| Cardiac I/R5 injury | Rat | Mediating PLC1 and survival kinases, PKC2, PI3K3, and MEK41/2 signaling pathways with activation of downstream targets, NO synthase and mito K ATP channels, and sarcolemmal Na+/H+ and Na+/Ca2+ exchangers. | Reduction of irreversible cardiomyocyte damage, improvement of cardiac dysfunction, enhancement of metabolic restoration and membrane integrity. | [244] | |
| Brain | Cerebral infarction | Mice | Improvement of the neurobehavioral score and brain edema. | Inhibition of the JNK and P38/MAPK10 signaling pathway. protection to neurons | [245] |
| Inhibition of the morphological changes and apoptosis of neuronal cells, Activation of caspase-3. ↑ Bcl6-2. ↓Bax7, ↓caspase-3, ↓p-JNK8 and p-p389 |
1: Phospholipase C, 2: Protein kinase C, 3: Phosphatidylinositol 3-kinase, 4: Mitogen-activated protein kinase, 5: Ischemia/reperfusion, 6: B-cell lymphoma 2, 7: B-cell lymphoma protein 2 -associated X, 8: C-Jun N-terminal kinase, 9: Phosphorylated p38, 10: Mitogen-activated protein kinase.
8. Dynorphins A (1–13) and (1–12)
Dynorphins (endogenous opioid neuropeptides) are the ACE2 substrates with pain relieving effects [169], [170].
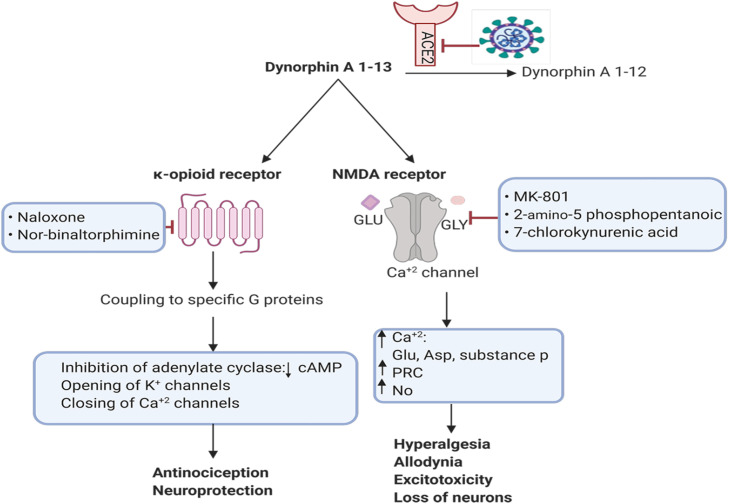
Dynorphins binds to κ-opioid receptors and act as inhibitory neurotransmitters and induce pain desensitization [171]. Dynorphin A (1–13) is a breakdown product of dynorphin A which is synthesized as preprodynorphin [172]. Apart from pain relieving roles, high concentration of dynorphins can induce hyperalgesia and allodynia or even the production of neurodegeneration metabolites [173].
and dynorphin A3–13 reduce rat spinal cord blood flow by non-opioid mechanisms. Brain research. 1987;436(2)Intrathecal injection of dynorphins into rat subarachnoid causes persistent flaccid paralysis of hindlimbs and loss of pain response [173]. Dynorphin A (1–13) acts via NMDA-glutamatergic and κ-opioid receptors [174]. It also affects neuron survival so that the toxic effects of dynorphin on the neurons are mediated by glutamatergic receptors, because high concentrations of dynorphin A (1–13) (µM) can activate NMDA receptors which in turn increase intracellular concentration of Ca2+ and kill many neuron cells ( Fig. 4) [175]. Neurological complications were common in patients with Covid-19 who were hospitalized for treatment in Wuhan [176], [177], [178]. Considering the destructive effects of dynorphins accumulation [21], the plasma concentration of dynorphin A (1–13) in COVID-19 patients might be increased due to ACE2 downregulation which can induce brain neurotoxic effect. Thus, dysregulation of ACE2 after SARS-CoV-2 entry and consequent accumulation of dynorphin A (1–13) versus decreased concentration of dynorphin A (1–12) (1–13) [179] might be of the causes of the loss of taste and smell in COVID-19 patients due to the neurotoxic effects of dynorphin A. Furthermore, a study has shown that immune cells secrete dynorphin A and also IL-1 can induce the production of dynorphin A from these cells (in the rat model of localized hindpaw inflammation) [180]. Since IL-1 is increased in COVID-19 patients [181], it is thought that production of dynorphin A might be increased more in COVID-19.
Fig. 4.
The binding of dynorphin-13 to κ-opioid and NMDA receptors. Dynorphin A (1–13) binds to κ-opioid receptor and NMDA-glutamatergic. ACE2 degrades dynorphin A 1–13 and subsequently produces dynorphin A 1–12. SARS-CoV-2 downregulates the expression of ACE2 and thus the harmful effects of dynorphin A 1–13 accumulation causes the loss of neurons via NMDA receptor (Created in BioRender.com). PKC: Protein kinase C, NO: nitric oxide, cAMP: Cyclic Adenosine Monophosphate, NMDA: N-Methyl-D-aspartic acid.
Finally, dynorphin A (1–12) is produced by the cleavage of dynorphin A (1–13) by ACE2 [170], [182]. Dynorphin A (1–12) is one of the main metabolites of dynorphin A (1–13) in human CSF and plasma which binds to κ-opioid receptor with the less affinity than dynorphin A (1–13) [183], [184]. In human blood half-life of dynorphin A (1–12) is 1.9 min (more than dynorphin A1–13, half life: 0.9 min) [185]. It is anticipated that its accumulation shows only mild side effects, ACE2 dysregulation induced by the entry of SARS-CoV-2 into the cells decreases the concentration of dynorphin A (1–12) and might lead to loss of its counterbalancing effects against accumulated dynorphin A (1–13).
9. Link between immune responses and renin-angiotensin system (RAS)
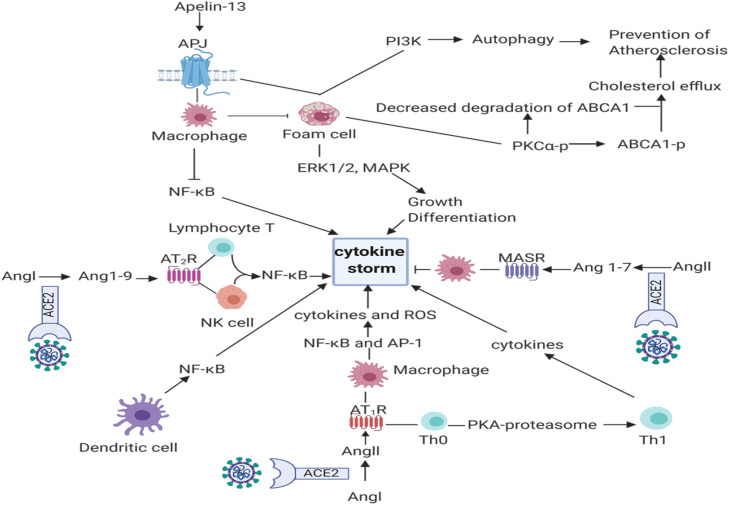
Adoptive and innate immunities play a key role against SARS-CoV-2, however, the virus can induce hyperinflamation or cytokine storm [57]. Although different factors induce cytokine storm, studying the dysregulation of ACE2 and correlation with immune responses seems interesting ( Fig. 5). A previously-published study has reported that downregulation of ACE2 in macrophages in COVID-19 patients increases the inflammatory cytokine and production of NO. On the other hand, Ang II induces cell proliferation and cytokine production implying the role of Ang II in regulating the cell signaling in inflammation and immune-related diseases [186]. IL-2 is an immune cytokine that stimulates the proliferation of T cells [187]. Studies on Jurkat T-cells showed that Ang II-ATR1-ERK axis activates lymphocyte T and leads to production of IL-2 [186]. For instance, AT1R found on the surfaces of various immune cell and T cells contains endogenous RAS and thus express renin, renin receptor, angiotensinogen and ACE2 [188]. Ang II-AT1R axis in T cells regulates activation of T lymphocytes and secretion of IL-2, IFN-γ and reduces IL-4 [189], [190]. The axis also induces differentiation of Th0 to Th1 cell through activation of AT1R-PKA-proteasome pathway [191]. Th1 is responsible for the production of IL-1B, IFNγ, CXCL10, and CCL2 [192]. In human Jurkat T cells (in vitro study), Apelin-APJ system upregulates the expression of CD69 and CD25 on the surface of T lymphocytes and stimulates the cells proliferation and activity [193]. Apelin (1–13) suppresses IFN-γ, IL-2 and IL-4 in activated T lymphocytes in mice [194]. AT1R is also expressed on macrophages when monocytes are differentiated to macrophages [195] and upregulates inflammatory cytokines such as TNF-α, IL-1b, IL-6, and IL-10 and produces ROS through NF-κB and AP-1 pathway [196]. Apelin (1–13) promotes growth and differentiation of macrophages by binding to APJ through ERK1/2 and mitogen-activated protein (MAP) kinase pathways and by inhibition of apoptosis [197], [198]. Apelin (1–13) also reduces the production of cytokine and ROS in macrophages and prevents the conversion of macrophage to foam cells [199]. Moreover, dendritic cells express AT1R and hence activate NF-κB for the production of proinflammatory cytokines [200], [201].
Fig. 5.
Schematic presentation of the interactions among the immune system and ACE2 substrates and products (Created in BioRender.com). ACE2: Angiotensin converting enzyme2, Ang I: Angiotensin I, Ang II: Angiotensin II, Ang 1–9: Angiotensin 1–9, Ang 1–7. Angiotensin 1–7, AT1R; Angiotensin II receptor type 1, AT2R; Angiotensin II receptor type 2, MAS R: Mas receptor, APJ: Apelin receptor, ROS: Reactive Oxygen Species, ERK1/2: Extracellular signal-regulated kinases, MAPK: Mitogen-activated protein kinase, PI3K: Phosphatidylinositol-3-kinase, PKC: Protein kinase C, Th1: T helper cell 1, Th0: Naive T cell, NF-κB: Nuclear factor-κB, AP-1: Activator protein 1, ABCA1-p: ATP binding cassette transporter A1- phosphorylated.
Studies on DC2.4 cells, a murine bone marrow-derived DC line, showed that Ang II activates dendritic cells in part through p65 / NF-κB ،ERK2/1 and STAT1 [202].
Furthermore, dynorphin A induces the secretion of IL-1, TNFα, macrophage-mediated phagocytosis, chemotaxis in mononuclear cells and neutrophils, and reduces the release of NO [203]. The anti-inflammatory functions of Ang (1–9) and Ang (1–7) have been already discussed, however, there is not much information about their functions on immune cells. Ang (1–7) has been shown to induce macrophage-mediated anti-inflammatory responses via MAS axis (in murine models of autoimmune neuroinflammation and atherosclerosis) [204]. AT2R, a receptor for Ang (1–9), was found on T cells and NK cells and anti-inflammatory function of AT2R ligands or agonists is dependent on the formation of epoxyeicosatrienoic acid and direct inhibition of NF-κB inflammatory signaling. In addition, lymphopenia is one of the most informative diagnostic markers of COVID-19 which is produced as a consequence of cytokine storm [205]. The cytokine storm and increased inflammatory cytokines like TNF-α and IL-6 induce apoptosis and necrosis in T cells [206]. In patients with COVID-19, the increased cytokines were correlated to decreased T lymphocytes and according to the function of Ang II, it is involved in the production of these inflammatory cytokines and acts to reduce lymphocytes [207]. On the other hand, Ang 1–7, Ang 1–9 can be effective as anti-inflammatory substance on preventing the reduction of lymphocytes.
Moreover, pyroptosis, a novel inflammatory form of programmed cell death following infection, is the other cause of lymphopenia induced by SARS-CoV [208].
Pyroptosis is a much faster process than apoptosis and is associated with diffusion of proinflammatory factors [209]. Pathogen-associated molecular patterns (PAMPs) binding to pattern recognition receptors (PRR) such as nitric oxide synthase and NLRP3 on host cells, activate caspase-1, a key component of innate immunity which in turn leads to cell perforation, inflammation, cell lysis and secretion of IL-1β and IL-18 [96]. Similarity between SARS-COV and SARS-COV-2 on one hand and increased IL 1β as a marker for pyroptosis on the other hand suggests that pyroptosis may explain the lymphopenia in COVID-19 patients [92], [118]. It could be initiation of inflammatory signaling induced by increased Ang II as well as pyroptosis-mediated release of cytokines (Fig. 5) [201]. In sum, these conditions can exacerbate the inflammation induced by the imbalance between ACE2 substrates and products in the COVID-19 patients.
10. Conclusion
Considering the key role of ACE2 as a functional receptor for SARS-CoV-2, targeting the receptor to block the virus entry is studying [210]. We suggest that downregulation of ACE2 can be one of the main causes of SARS-CoV-2 symptoms. COVID-19 severity can be changed by reduction in ACE2 products such as Ang (1–7), Ang (1–9), apelin (1–12) and accumulation of substrates such as apelin (1–13) and Ang II [211]. Given the downregulation of ACE2, accumulated apelin (1–13) can stimulate the pulmonary embolism. On the other hand, when the ACE2 is downregulated by SARS-CoV-2, the concentration of apelin (1–12) can be decreased. It is also possible to hypothesize that decrease in the concentration of apelin 1–12 in the plasma of COVID-19 patients may increase the damages of endothelial cells (apelin-12 repairs endothelial cells). However, further studies are required to test these hypotheses. Moreover, higher concentration of apelin (1–13) during severe pulmonary embolism [212], a common complication in COVID-19 and the major cause of death in the patients [213], [214], might be suggested as a possible biomarker for diagnosis of the stages of the COVID-19 severity. Losses of taste and smell have also been listed as COVID-19 symptoms which might be due to accumulation of the dynorphin A (1–13). Possible neuronal damage for the losses of taste and smell induced by coronaviruses might be attributed to virus entry into the nervous system and subsequent hypoxia-induced damages following the pulmonary disorders and the production of inflammatory cytokines (cytokine storm) [215], [216], [217]. It has been suggested that targeting ACE2 expression can be used as a treatment for COVID-19 and thus, ARB and ACE inhibitors (ACEi) have been used to increase the level of ACE2 but failed to treat [211], [218]. It should be noted that ACEi have been already used in asian brain ischemic patients [219] as well as Parkinson disease which questions the negative effects of ACEi [220], [221], [222], [223].
Considering the severe thrombotic complications in the COVID-19 patients [224], it has been suggested that the use of anti-coagulants may reduce the symptoms at the expense of serious side effects. Inflammatory responses can play a key role against SARS-CoV-2 by removing the infection, however, hyperinflamation or cytokine storm can lead to death, and thus the use of non-steroidal anti-inflammatory drugs is not a safe and promising therapeutic approach to treat COVID-19. Considering the anti-thrombotic, anti-hypertensive and anti-oxidative stress properties of ACE2 products such as Ang (1–7) and cardiovascular protective function of Ang (1–9), we suggest the use of the more stable analogs of these compounds to reverse the harmful effects of Ang II accumulation induced by dysregulation of ACE2. As inflammation, vascular failure, blood pressure and von Willebrand factor play roles in vascular thrombosis [225], [226], Ang (1–9) might have protective role against vascular thrombosis in COVID-19 patients. In addition, Ang (1–7) may exert more efficient protection against COVID-19 than recombinant soluble ACE2 or AT1 blockers, however this needs to be investigated and clinically evaluated. We also suggest that the administration of apelin (1–12) can be tested for the treatment of the COVID-19 due to prolonged half-life, higher affinity to AT1 and higher expression in endothelial cells. According to the results presented previously, the lack of apelin (1–12) causes the failure of vascular repair and pulmonary embolism and thereby administration of apelin (1–12) may ameliorate the symptoms of COVID-19. Finally, antagonists of dynorphin A (1–12) may reduce the harmful effects of dynorphin A (1–13) accumulation triggered by dysregulation of ACE2 upon virus entry. Nevertheless, these hypotheses should be evaluated and tested as potential therapeutic strategies for COVID-19 treatment.
Source of funding
This research was done without any financial support in the form of grant or otherwise.
Conflict of interest statement
The authors declare that they have no conflict of interest.
References
- 1.Sardu C., Gambardella J., Morelli M.B., Wang X., Marfella R., Santulli G. Is COVID-19 an endothelial disease? Clin. Basic Evid. 2020 doi: 10.3390/jcm9051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vickers C., Hales P., Kaushik V., Dick L., Gavin J., Tang J., Godbout K., Parsons T., Baronas E., Hsieh F. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002;277(17):14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 3.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;183:1735. doi: 10.1016/j.cell.2020.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuyama S., Nao N., Shirato K., Kawase M., Saito S., Takayama I., Nagata N., Sekizuka T., Katoh H., Kato F. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. 2020;117(13):7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tortorici M.A., Walls A.C., Lang Y., Wang C., Li Z., Koerhuis D., Boons G.-J., Bosch B.-J., Rey F.A., de Groot R.J. Structural basis for human coronavirus attachment to sialic acid receptors. Nat. Struct. Mol. Biol. 2019;26(6):481–489. doi: 10.1038/s41594-019-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kam Y.-W., Okumura Y., Kido H., Ng L.F., Bruzzone R., Altmeyer R. Cleavage of the SARS coronavirus spike glycoprotein by airway proteases enhances virus entry into human bronchial epithelial cells in vitro. PloS One. 2009;4(11) doi: 10.1371/journal.pone.0007870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y., Yang C., Xu X.-f, Xu W., Liu S.-w. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020;41(9):1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., Lu G., Qiao C., Hu Y., Yuen K.-Y. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181(4):894–904.e9. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia S., Lan Q., Su S., Wang X., Xu W., Liu Z., Zhu Y., Wang Q., Lu L., Jiang S. The role of furin cleavage site in SARS-CoV-2 spike protein-mediated membrane fusion in the presence or absence of trypsin. Signal Transduct. Target. Ther. 2020;5(1):1–3. doi: 10.1038/s41392-020-0184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020;176 doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.J. Kearney, Chloroquine as a potential treatment and prevention measure for the 2019 novel coronavirus: a review, (2020).
- 17.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busse L.W., Chow J.H., McCurdy M.T., Khanna A.K. COVID-19 and the RAAS—a potential role for angiotensin II? Crit. Care. 2020;24:136. doi: 10.1186/s13054-020-02862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo J., Huang Z., Lin L., Lv J. Coronavirus disease 2019 (covid‐19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J. Am. Heart Assoc. 2020;9(7) doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Triana S., Zumaran C.M., Ramirez C., Kee C., Doldan P., Shahraz M., Schraivogel D., Gschwind A.R., Steinmetz L.M., Herrmann C. Single-cell analyses reveal SARS-CoV-2 interference with intrinsic immune response in the human gut. bioRxiv. 2020 doi: 10.15252/msb.202110232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seltzer S. Linking ACE2 and angiotensin II to pulmonary immunovascular dysregulation in SARS-CoV-2 infection. Int. J. Infect. Dis. 2020;101:42–45. doi: 10.1016/j.ijid.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofmann H., Geier M., Marzi A., Krumbiegel M., Peipp M., Fey G.H., Gramberg T., Pöhlmann S. Susceptibility to SARS coronavirus S protein-driven infection correlates with expression of angiotensin converting enzyme 2 and infection can be blocked by soluble receptor. Biochem. Biophys. Res. Commun. 2004;319(4):1216–1221. doi: 10.1016/j.bbrc.2004.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashour H.M., Elkhatib W.F., Rahman M., Elshabrawy H.A. Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens. 2020;9(3):186. doi: 10.3390/pathogens9030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren X., Glende J., Al-Falah M., de Vries V., Schwegmann-Wessels C., Qu X., Tan L., Tschernig T., Deng H., Naim H.Y. Analysis of ACE2 in polarized epithelial cells: surface expression and function as receptor for severe acute respiratory syndrome-associated coronavirus. J. Gen. Virol. 2006;87(6):1691–1695. doi: 10.1099/vir.0.81749-0. [DOI] [PubMed] [Google Scholar]
- 26.Doobay M.F., Talman L.S., Obr T.D., Tian X., Davisson R.L., Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292(1):R373–R381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gembardt F., Sterner-Kock A., Imboden H., Spalteholz M., Reibitz F., Schultheiss H.-P., Siems W.-E., Walther T. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides. 2005;26(7):1270–1277. doi: 10.1016/j.peptides.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamming I., Timens W., Bulthuis M., Lely A., Navis Gv, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. A J. Pathol. Soc. G. B. Irel. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532(1–2):107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 30.Patel V.B., Clarke N., Wang Z., Fan D., Parajuli N., Basu R., Putko B., Kassiri Z., Turner A.J., Oudit G.Y. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J. Mol. Cell. Cardiol. 2014;66:167–176. doi: 10.1016/j.yjmcc.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 31.Patel V.B., Zhong J.-C., Fan D., Basu R., Morton J.S., Parajuli N., McMurtry M.S., Davidge S.T., Kassiri Z., Oudit G.Y. Angiotensin-converting enzyme 2 is a critical determinant of angiotensin II–induced loss of vascular smooth muscle cells and adverse vascular remodeling. Hypertension. 2014;64(1):157–164. doi: 10.1161/HYPERTENSIONAHA.114.03388. [DOI] [PubMed] [Google Scholar]
- 32.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serfozo P., Wysocki J., Gulua G., Schulze A., Ye M., Liu P., Jin J., Bader M., Myöhänen T., García-Horsman J.A. Ang II (angiotensin II) conversion to angiotensin-(1–7) in the circulation is POP (prolyloligopeptidase)-dependent and ACE2 (angiotensin-converting enzyme 2)-independent. Hypertension. 2020;75(1):173–182. doi: 10.1161/HYPERTENSIONAHA.119.14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warner F.J., Lew R.A., Smith A.I., Lambert D.W., Hooper N.M., Turner A.J. Angiotensin-converting enzyme 2 (ACE2), but not ACE, is preferentially localized to the apical surface of polarized kidney cells. J. Biol. Chem. 2005;280(47):39353–39362. doi: 10.1074/jbc.M508914200. [DOI] [PubMed] [Google Scholar]
- 35.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R. A novel angiotensin-converting enzyme–related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 2000;87(5):e1–e9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 36.Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme: cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000;275(43):33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 37.Ishiyama Y., Gallagher P.E., Averill D.B., Tallant E.A., Brosnihan K.B., Ferrario C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43(5):970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 38.Rice G.I., Thomas D.A., Grant P.J., Turner A.J., Hooper N.M. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem. J. 2004;383(1):45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.-C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fountain J.H., Lappin S.L. Physiol. Renin Angiotensin Syst. 2017 [Google Scholar]
- 41.Al-Merani S., Brooks D., Chapman B., Munday K. The half‐lives of angiotensin II, angiotensin II‐amide, angiotensin III, Sar1–Ala8–angiotensin II and renin in the circulatory system of the rat. J. Physiol. 1978;278(1):471–490. doi: 10.1113/jphysiol.1978.sp012318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kopf P.G., Campbell W.B. Endothelial metabolism of angiotensin II to angiotensin III, not angiotensin (1–7), augments the vasorelaxation response in adrenal cortical arteries. Endocrinology. 2013;154(12):4768–4776. doi: 10.1210/en.2013-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhuo J., Moeller I., Jenkins T., Chai S.Y., Allen A.M., Ohishi M., Mendelsohn F.A. Mapping tissue angiotensin-converting enzyme and angiotensin AT1, AT2 and AT4 receptors. J. Hypertens. 1998;16(12):2027–2037. doi: 10.1097/00004872-199816121-00026. [DOI] [PubMed] [Google Scholar]
- 44.Dasgupta C., Zhang L. Angiotensin II receptors and drug discovery in cardiovascular disease. Drug Discov. Today. 2011;16(1–2):22–34. doi: 10.1016/j.drudis.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gohla A., Schultz Gn, Offermanns S. Role for G12/G13 in agonist-induced vascular smooth muscle cell contraction. Circ. Res. 2000;87(3):221–227. doi: 10.1161/01.res.87.3.221. [DOI] [PubMed] [Google Scholar]
- 46.Castrén E., Kurihara M., Gutkind J.S., Saavedra J.M. Specific angiotensin II binding sites in the rat stellate and superior cervical ganglia. Brain Res. 1987;422(2):347–351. doi: 10.1016/0006-8993(87)90942-5. [DOI] [PubMed] [Google Scholar]
- 47.Foster R.H., MacFarlane C.H., Bustamante M.O. Recent progress in understanding aldosterone secretion. Gen. Pharmacol. Vasc. Syst. 1997;28(5):647–651. doi: 10.1016/s0306-3623(96)00290-x. [DOI] [PubMed] [Google Scholar]
- 48.Katada J., Majima M. AT2 receptor‐dependent vasodilation is mediated by activation of vascular kinin generation under flow conditions. Br. J. Pharmacol. 2002;136(4):484–491. doi: 10.1038/sj.bjp.0704731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rompe F., Artuc M., Hallberg A., Alterman M., Ströder K., Thöne-Reineke C., Reichenbach A., Schacherl J., Dahlöf Br, Bader M. Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor κB. Hypertension. 2010;55(4):924–931. doi: 10.1161/HYPERTENSIONAHA.109.147843. [DOI] [PubMed] [Google Scholar]
- 50.Dhande I., Ma W., Hussain T. Angiotensin AT 2 receptor stimulation is anti-inflammatory in lipopolysaccharide-activated THP-1 macrophages via increased interleukin-10 production. Hypertens. Res. 2015;38(1):21–29. doi: 10.1038/hr.2014.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stoll M., Steckelings U.M., Paul M., Bottari S.P., Metzger R., Unger T. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J. Clin. Investig. 1995;95(2):651–657. doi: 10.1172/JCI117710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolf G., Harendza S., Schroeder R., Wenzel U., Zahner G., Butzmann U., Freeman R.S., Stahl R.A. Angiotensin II’s antiproliferative effects mediated through AT2-receptors depend on down-regulation of SM-20. Lab. Investig. 2002;82(10):1305–1317. doi: 10.1097/01.lab.0000029207.92039.2f. [DOI] [PubMed] [Google Scholar]
- 53.Hall A., Busse L.W., Ostermann M. Angiotensin in critical care. Crit. Care. 2018;22(1):69. doi: 10.1186/s13054-018-1995-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hall A., Busse L.W., Ostermann M. Angiotensin in critical care. Crit. Care. 2018;22(1):1–6. doi: 10.1186/s13054-018-1995-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim J.M., Heo H.-S., Ha Y.M., Ye B.H., Lee E.K., Choi Y.J., Yu B.P., Chung H.Y. Mechanism of Ang II involvement in activation of NF-κB through phosphorylation of p65 during aging. Age. 2012;34(1):11–25. doi: 10.1007/s11357-011-9207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alvarez A., Cerdá-Nicolás M., Naim Abu Nabah Y., Mata M., Issekutz A.C., Panés J., Lobb R.R., Sanz M.-J. Direct evidence of leukocyte adhesion in arterioles by angiotensin II. Blood. 2004;104(2):402–408. doi: 10.1182/blood-2003-08-2974. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., Aaron J.G., Claassen J., Rabbani L.E., Hastie J. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu Z., Hu R., Zhang C., Ren W., Yu A., Zhou X. Elevation of plasma angiotensin II level is a potential pathogenesis for the critically ill COVID-19 patients. Crit. Care. 2020;24(1):1–3. doi: 10.1186/s13054-020-03015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernandes T., Hashimoto N.Y., Magalhaes F.C., Fernandes F.B., Casarini D.E., Carmona A.K., Krieger J.E., Phillips M.I., Oliveira E.M. Aerobic exercise training–induced left ventricular hypertrophy involves regulatory MicroRNAs, decreased angiotensin-converting enzyme-angiotensin II, and synergistic regulation of angiotensin-converting enzyme 2-angiotensin (1–7) Hypertension. 2011;58(2):182–189. doi: 10.1161/HYPERTENSIONAHA.110.168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koka V., Huang X.R., Chung A.C., Wang W., Truong L.D., Lan H.Y. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am. J. Pathol. 2008;172(5):1174–1183. doi: 10.2353/ajpath.2008.070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schiffrin E.L., Flack J.M., Ito S., Muntner P., Webb R.C. Oxford University Press; US: 2020. Hypertension and COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;1–8:2020. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hong H.-J., Chan P., Liu J.-C., Juan S.-H., Huang M.-T., Lin J.-G., Cheng T.-H. Angiotensin II induces endothelin-1 gene expression via extracellular signal-regulated kinase pathway in rat aortic smooth muscle cells. Cardiovasc. Res. 2004;61(1):159–168. doi: 10.1016/j.cardiores.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 66.McMaster W.G., Kirabo A., Madhur M.S., Harrison D.G. Inflammation, immunity, and hypertensive end-organ damage. Circ. Res. 2015;116(6):1022–1033. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fliser D., Buchholz K., Haller H. Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation. 2004;110(9):1103–1107. doi: 10.1161/01.CIR.0000140265.21608.8E. [DOI] [PubMed] [Google Scholar]
- 68.Blake G.J., Ridker P.M. Novel clinical markers of vascular wall inflammation. Circ. Res. 2001;89(9):763–771. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- 69.Wolf G., Wenzel U., Assmann K.J., Stahl R.A. Renal expression of aminopeptidase A in rats with two‐kidney, one‐clip hypertension. Nephrol. Dial. Transplant. 2000;15(12):1935–1942. doi: 10.1093/ndt/15.12.1935. [DOI] [PubMed] [Google Scholar]
- 70.Senchenkova E.Y., Russell J., Esmon C.T., Granger D.N. Roles of coagulation and fibrinolysis in angiotensin II‐enhanced microvascular thrombosis. Microcirculation. 2014;21(5):401–407. doi: 10.1111/micc.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Senchenkova E.Y., Russell J., Almeida-Paula L.D., Harding J.W., Granger D.N. Angiotensin II–mediated microvascular thrombosis. Hypertension. 2010;56(6):1089–1095. doi: 10.1161/HYPERTENSIONAHA.110.158220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mogielnicki A., Chabielska E., Pawlak R., Szemraj J., Buczko W. Angiotensin II enhances thrombosis development in renovascular hypertensive rats. Thromb. Haemost. 2005;93(06):1069–1076. doi: 10.1160/TH04-10-0701. [DOI] [PubMed] [Google Scholar]
- 73.Lin Y.-J., Kwok C.-F., Juan C.-C., Hsu Y.-P., Shih K.-C., Chen C.-C., Ho L.-T. Angiotensin II enhances endothelin-1-induced vasoconstriction through upregulating endothelin type A receptor. Biochem. Biophys. Res. Commun. 2014;451(2):263–269. doi: 10.1016/j.bbrc.2014.07.119. [DOI] [PubMed] [Google Scholar]
- 74.Liu L., Meng L., Zhang P., Lin H., Chi J., Peng F., Guo H. Angiotensin II inhibits the protein expression of ZO‑1 in vascular endothelial cells by downregulating VE‑cadherin. Mol. Med. Rep. 2018;18(1):429–434. doi: 10.3892/mmr.2018.8991. [DOI] [PubMed] [Google Scholar]
- 75.Kiarash A., Pagano P.J., Tayeh M., Rhaleb N.-E., Carretero O.A. Upregulated expression of rat heart intercellular adhesion molecule-1 in angiotensin II–but not phenylephrine-induced hypertension. Hypertension. 2001;37(1):58–65. doi: 10.1161/01.hyp.37.1.58. [DOI] [PubMed] [Google Scholar]
- 76.Akwii R., Sajib M., Zahra F., Mikelis C. Role of Angiopoietin-2 in vascular physiology and pathophysiology. Cells. 2019;8:471. doi: 10.3390/cells8050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jagroop I., Mikhailidis D. Angiotensin II can induce and potentiate shape change in human platelets: effect of losartan. J. Hum. Hypertens. 2000;14(9):581–585. doi: 10.1038/sj.jhh.1001102. [DOI] [PubMed] [Google Scholar]
- 78.Vital S.A., Terao S., Nagai M., Granger D.N. Mechanisms underlying the cerebral microvascular responses to angiotensin II‐induced hypertension. Microcirculation. 2010;17(8):641–649. doi: 10.1111/j.1549-8719.2010.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., Der Nigoghossian C., Ageno W., Madjid M., Guo Y. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., Chen H., Ding X., Zhao H., Zhang H. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020;382(17) doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xie Y., Wang X., Yang P., Zhang S. COVID-19 complicated by acute pulmonary embolism. Radiol. Cardiothorac. Imaging. 2020;2(2) doi: 10.1148/ryct.2020200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur. Heart J. 2020;41(19):1858. doi: 10.1093/eurheartj/ehaa254. 1858-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jolobe O.M. Similarities between community-acquired pneumonia and pulmonary embolism. Am. J. Med. 2019;132(12) doi: 10.1016/j.amjmed.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 85.Otani A., Takagi H., Oh H., Koyama S., Honda Y. Angiotensin II induces expression of the Tie2 receptor ligand, angiopoietin-2, in bovine retinal endothelial cells. Diabetes. 2001;50(4):867–875. doi: 10.2337/diabetes.50.4.867. [DOI] [PubMed] [Google Scholar]
- 86.Asahara T., Chen D., Takahashi T., Fujikawa K., Kearney M., Magner M., Yancopoulos G.D., Isner J.M. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ. Res. 1998;83(3):233–240. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- 87.Hashimoto T., Pittet J.-F. Angiopoietin-2: modulator of vascular permeability in acute lung injury? PLoS Med. 2006;3(3) doi: 10.1371/journal.pmed.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ong T., McClintock D.E., Kallet R.H., Ware L.B., Matthay M.A., Liu K.D. Ratio of angiopoietin-2 to angiopoietin-1 as a predictor of mortality in acute lung injury patients: Crit. Care Med. 2010;38(9):1845–1851. doi: 10.1097/CCM.0b013e3181eaa5bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Calfee C.S., Delucchi K.L., Sinha P., Matthay M.A., Hackett J., Shankar-Hari M., McDowell C., Laffey J.G., O’Kane C.M., McAuley D.F. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir. Med. 2018;6(9):691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wada T., Jesmin S., Gando S., Yanagida Y., Mizugaki A., Sultana S.N., Zaedi S., Yokota H. The role of angiogenic factors and their soluble receptors in acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) associated with critical illness. J. Inflamm. 2013;10(1):1–8. doi: 10.1186/1476-9255-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Akwii R.G., Sajib M.S., Zahra F.T., Mikelis C.M. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells. 2019;8(5):471. doi: 10.3390/cells8050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu Y., Cox S.R., Morita T., Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells: identification of a 5′ enhancer. Circ. Res. 1995;77(3):638–643. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- 93.Chen X.L., Nam J.-O., Jean C., Lawson C., Walsh C.T., Goka E., Lim S.-T., Tomar A., Tancioni I., Uryu S. VEGF-induced vascular permeability is mediated by FAK. Dev. Cell. 2012;22(1):146–157. doi: 10.1016/j.devcel.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Callera G.E., Yogi A., Briones A.M., Montezano A.C., He Y., Tostes R.C., Schiffrin E.L., Touyz R.M. Vascular proinflammatory responses by aldosterone are mediated via c-Src trafficking to cholesterol-rich microdomains: role of PDGFR. Cardiovasc. Res. 2011;91(4):720–731. doi: 10.1093/cvr/cvr131. [DOI] [PubMed] [Google Scholar]
- 95.Montezano A.C., Touyz R.M. Reactive oxygen species and endothelial function–role of nitric oxide synthase uncoupling and Nox family nicotinamide adenine dinucleotide phosphate oxidases. Basic Clin. Pharmacol. Toxicol. 2012;110(1):87–94. doi: 10.1111/j.1742-7843.2011.00785.x. [DOI] [PubMed] [Google Scholar]
- 96.Dai D.-F., Johnson S.C., Villarin J.J., Chin M.T., Nieves-Cintrón M., Chen T., Marcinek D.J., Dorn G.W., Kang Y.J., Prolla T.A. Mitochondrial oxidative stress mediates angiotensin II–induced cardiac hypertrophy and Gαq overexpression–induced heart failure. Circ. Res. 2011;108(7):837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lijnen P.J., Van Pelt J.F., Fagard R.H. Downregulation of manganese superoxide dismutase by angiotensin II in cardiac fibroblasts of rats: Association with oxidative stress in myocardium. Am. J. Hypertens. 2010;23(10):1128–1135. doi: 10.1038/ajh.2010.128. [DOI] [PubMed] [Google Scholar]
- 98.Dandona P., Dhindsa S., Ghanim H., Chaudhuri A. Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J. Hum. Hypertens. 2007;21(1):20–27. doi: 10.1038/sj.jhh.1002101. [DOI] [PubMed] [Google Scholar]
- 99.Delgado-Roche L., Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch. Med. Res. 2020;51:384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Knighton D.R., Hunt T.K., Scheuenstuhl H., Halliday B.J., Werb Z., Banda M.J. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science. 1983;221(4617):1283–1285. doi: 10.1126/science.6612342. [DOI] [PubMed] [Google Scholar]
- 101.Wang Y., Zang Q.S., Liu Z., Wu Q., Maass D., Dulan G., Shaul P.W., Melito L., Frantz D.E., Kilgore J.A. Regulation of VEGF-induced endothelial cell migration by mitochondrial reactive oxygen species. Am. J. Physiol. Cell Physiol. 2011;301(3):C695–C704. doi: 10.1152/ajpcell.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bir S.C., Kolluru G.K., Fang K., Kevil C.G. Seminars in Cell & Developmental Biology. Elsevier; 2012. Redox balance dynamically regulates vascular growth and remodeling; pp. 745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Birukova A.A., Lee S., Starosta V., Wu T., Ho T., Kim J., Berliner J.A., Birukov K.G. A role for VEGFR2 activation in endothelial responses caused by barrier disruptive OxPAPC concentrations. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bochkov V.N., Philippova M., Oskolkova O., Kadl A., Furnkranz A., Karabeg E., Afonyushkin T., Gruber F., Breuss J., Minchenko A. Oxidized phospholipids stimulate angiogenesis via autocrine mechanisms, implicating a novel role for lipid oxidation in the evolution of atherosclerotic lesions. Circ. Res. 2006;99(8):900–908. doi: 10.1161/01.RES.0000245485.04489.ee. [DOI] [PubMed] [Google Scholar]
- 105.West X.Z., Malinin N.L., Merkulova A.A., Tischenko M., Kerr B.A., Borden E.C., Podrez E.A., Salomon R.G., Byzova T.V. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010;467(7318):972–976. doi: 10.1038/nature09421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Simões e Silva A., Silveira K., Ferreira A., Teixeira M. ACE2, angiotensin‐(1–7) and M as receptor axis in inflammation and fibrosis. Br. J. Pharmacol. 2013;169(3):477–492. doi: 10.1111/bph.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Magalhaes G.S., da Gloria Rodrigues-Machado M., Motta-Santos D., Campagnole-Santos M.J., Santos R.A.S. Activation of Ang-(1–7)/mas receptor is a possible strategy to treat coronavirus (SARS-CoV-2) infection. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schindler C., Bramlage P., Kirch W., Ferrario C.M. Role of the vasodilator peptide angiotensin-(1–7) in cardiovascular drug therapy. Vasc. Health Risk Manag. 2007;3(1):125. [PMC free article] [PubMed] [Google Scholar]
- 109.Pei N., Wan R., Chen X., Li A., Zhang Y., Li J., Du H., Chen B., Wei W., Qi Y. Angiotensin-(1–7) decreases cell growth and angiogenesis of human nasopharyngeal carcinoma xenografts. Mol. Cancer Ther. 2016;15(1):37–47. doi: 10.1158/1535-7163.MCT-14-0981. [DOI] [PubMed] [Google Scholar]
- 110.Soto-Pantoja D.R., Menon J., Gallagher P.E., Tallant E.A. Angiotensin-(1–7) inhibits tumor angiogenesis in human lung cancer xenografts with a reduction in vascular endothelial growth factor. Mol. Cancer Ther. 2009;8(6):1676–1683. doi: 10.1158/1535-7163.MCT-09-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Krishnan B., Torti F.M., Gallagher P.E., Tallant E.A. Angiotensin‐(1–7) reduces proliferation and angiogenesis of human prostate cancer xenografts with a decrease in angiogenic factors and an increase in sFlt‐1. Prostate. 2013;73(1):60–70. doi: 10.1002/pros.22540. [DOI] [PubMed] [Google Scholar]
- 112.Giani J.F., Burghi V., Veiras L.C., Tomat A., Muñoz M.C., Cao G., Turyn D., Toblli J.E., Dominici F.P. Angiotensin-(1–7) attenuates diabetic nephropathy in Zucker diabetic fatty rats. Am. J. Physiol. Ren. Physiol. 2012;302(12):F1606–F1615. doi: 10.1152/ajprenal.00063.2012. [DOI] [PubMed] [Google Scholar]
- 113.Y. Cheng, R. Luo, K. Wang, M. Zhang, Z. Wang, L. Dong, Kidney impairment is associated with in-hospital death of COVID-19 patients, (2020) [e-pub ahead of print] (medRxiv 2020.02. 18.20023242, Accepted Article).
- 114.Sriramula S., Cardinale J.P., Lazartigues E., Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc. Res. 2011;92(3):401–408. doi: 10.1093/cvr/cvr242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jiang T., Gao L., Guo J., Lu J., Wang Y., Zhang Y. Suppressing inflammation by inhibiting the NF‐κB pathway contributes to the neuroprotective effect of angiotensin‐(1–7) in rats with permanent cerebral ischaemia. Br. J. Pharmacol. 2012;167(7):1520–1532. doi: 10.1111/j.1476-5381.2012.02105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qi Y., Shenoy V., Wong F., Li H., Afzal A., Mocco J., Sumners C., Raizada M.K., Katovich M.J. Lentivirus‐mediated overexpression of angiotensin‐(1–7) attenuated ischaemia‐induced cardiac pathophysiology. Exp. Physiol. 2011;96(9):863–874. doi: 10.1113/expphysiol.2011.056994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fraga-Silva R.A., Pinheiro S.V.B., Gonçalves A.C.C., Alenina N., Bader M., Santos R.A.S. The antithrombotic effect of angiotensin-(1–7) involves mas-mediated NO release from platelets. Mol. Med. 2008;14(1–2):28–35. doi: 10.2119/2007-00073.Fraga-Silva. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fang C., Stavrou E., Schmaier A.A., Grobe N., Morris M., Chen A., Nieman M.T., Adams G.N., LaRusch G., Zhou Y. Angiotensin 1–7 and Mas decrease thrombosis in Bdkrb2–/– mice by increasing NO and prostacyclin to reduce platelet spreading and glycoprotein VI activation, Blood. J. Am. Soc. Hematol. 2013;121(15):3023–3032. doi: 10.1182/blood-2012-09-459156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kucharewicz I., Pawlak R., Matys T., Pawlak D., Buczko W. Antithrombotic effect of captopril and losartan is mediated by angiotensin-(1–7) Hypertension. 2002;40(5):774–779. doi: 10.1161/01.hyp.0000035396.27909.40. [DOI] [PubMed] [Google Scholar]
- 120.Fraga-Silva R.A., Pinheiro S.V.B., Gonçalves A.C.C., Alenina N., Bader M., Santos R.A.S. The antithrombotic effect of angiotensin-(1–7) involves mas-mediated NO release from platelets. Mol. Med. 2008;14(1):28–35. doi: 10.2119/2007-00073.Fraga-Silva. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Erdös E.G., Jackman H.L., Brovkovych V., Tan F., Deddish P.A. Products of angiotensin I hydrolysis by human cardiac enzymes potentiate bradykinin. J. Mol. Cell. Cardiol. 2002;34(12):1569–1576. doi: 10.1006/jmcc.2002.2080. [DOI] [PubMed] [Google Scholar]
- 122.Bruce E., Shenoy V., Rathinasabapathy A., Espejo A., Horowitz A., Oswalt A., Francis J., Nair A., Unger T., Raizada M. Selective activation of angiotensin AT 2 receptors attenuates progression of pulmonary hypertension and inhibits cardiopulmonary fibrosis. Br. J. Pharmacol. 2015;172(9):2219–2231. doi: 10.1111/bph.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ocaranza M.P., Jalil J.E. Protective role of the ACE2/Ang-(1–9) axis in cardiovascular remodeling. Int. J. Hypertens. 2012;2012:1–12. doi: 10.1155/2012/594361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ocaranza M.P., Lavandero S., Jalil J.E., Moya J., Pinto M., Novoa U., Apablaza F., Gonzalez L., Hernandez C., Varas M. Angiotensin-(1–9) regulates cardiac hypertrophy in vivo and in vitro. J. Hypertens. 2010;28(5):1054–1064. doi: 10.1097/hjh.0b013e328335d291. [DOI] [PubMed] [Google Scholar]
- 125.Fattah C., Nather K., McCarroll C.S., Hortigon-Vinagre M.P., Zamora V., Flores-Munoz M., McArthur L., Zentilin L., Giacca M., Touyz R.M. Gene therapy with angiotensin-(1–9) preserves left ventricular systolic function after myocardial infarction. J. Am. Coll. Cardiol. 2016;68(24):2652–2666. doi: 10.1016/j.jacc.2016.09.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Flores‐Muñoz M., Smith N., Haggerty C., Milligan G., Nicklin S. Angiotensin1–9 antagonises pro‐hypertrophic signalling in cardiomyocytes via the angiotensin type 2 receptor. J. Physiol. 2011;589(4):939–951. doi: 10.1113/jphysiol.2010.203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ocaranza M.P., Moya J., Barrientos V., Alzamora R., Hevia D., Morales C., Pinto M., Escudero N., García L., Novoa U. Angiotensin-(1–9) reverses experimental hypertension and cardiovascular damage by inhibition of the angiotensin converting enzyme/Ang II axis. J. Hypertens. 2014;32(4):771–783. doi: 10.1097/HJH.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 128.Jackman H.L., Massad M.G., Sekosan M., Tan F., Brovkovych V., Marcic B.M., Erdös E.G. Angiotensin 1–9 and 1–7 release in human heart: role of cathepsin A. Hypertension. 2002;39(5):976–981. doi: 10.1161/01.hyp.0000017283.67962.02. [DOI] [PubMed] [Google Scholar]
- 129.Flores-Munoz M., Work L.M., Douglas K., Denby L., Dominiczak A.F., Graham D., Nicklin S.A. Angiotensin-(1–9) attenuates cardiac fibrosis in the stroke-prone spontaneously hypertensive rat via the angiotensin type 2 receptor. Hypertension. 2012;59(2):300–307. doi: 10.1161/HYPERTENSIONAHA.111.177485. [DOI] [PubMed] [Google Scholar]
- 130.George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir. Med. 2020;8:807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cha S.A., Park B.M., Kim S.H. Angiotensin-(1–9) ameliorates pulmonary arterial hypertension via angiotensin type II receptor. Korean J. Physiol. Pharmacol. 2018;22(4):447. doi: 10.4196/kjpp.2018.22.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gonzalez L., Novoa U., Moya J., Gabrielli L., Jalil J.E., García L., Chiong M., Lavandero S., Ocaranza M.P. Angiotensin-(1–9) reduces cardiovascular and renal inflammation in experimental renin-independent hypertension. Biochem. Pharmacol. 2018;156:357–370. doi: 10.1016/j.bcp.2018.08.045. [DOI] [PubMed] [Google Scholar]
- 133.Tatemoto K., Hosoya M., Habata Y., Fujii R., Kakegawa T., Zou M.-X., Kawamata Y., Fukusumi S., Hinuma S., Kitada C. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 1998;251(2):471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 134.Zhen E.Y., Higgs R.E., Gutierrez J.A. Pyroglutamyl apelin-13 identified as the major apelin isoform in human plasma. Anal. Biochem. 2013;442(1):1–9. doi: 10.1016/j.ab.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 135.Shin K., Kenward C., Rainey J.K. Apelinergic system structure and function. Compr. Physiol. 2011;8(1):407–450. doi: 10.1002/cphy.c170028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.De Mota N., Reaux-Le Goazigo A., El Messari S., Chartrel N., Roesch D., Dujardin C., Kordon C., Vaudry H., Moos F., Llorens-Cortes C. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc. Natl. Acad. Sci. 2004;101(28):10464–10469. doi: 10.1073/pnas.0403518101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shin K., Pandey A., Liu X.-Q., Anini Y., Rainey J.K. Preferential apelin-13 production by the proprotein convertase PCSK3 is implicated in obesity. FEBS Open Bio. 2013;3:328–333. doi: 10.1016/j.fob.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Valle A., Hoggard N., Adams A., Roca P., Speakman J. Chronic central administration of apelin‐13 over 10 days increases food intake, body weight, locomotor activity and body temperature in C57BL/6 mice. J. Neuroendocrinol. 2008;20(1):79–84. doi: 10.1111/j.1365-2826.2007.01617.x. [DOI] [PubMed] [Google Scholar]
- 139.Yu X.-H., Tang Z.-B., Liu L.-J., Qian H., Tang S.-L., Zhang D.-W., Tian G.-P., Tang C.-K. Apelin and its receptor APJ in cardiovascular diseases. Clin. Chim. Acta. 2014;428:1–8. doi: 10.1016/j.cca.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 140.Ouyang Q., You T., Guo J., Xu R., Guo Q., Lin J., Zhao H. Effects of apelin on left ventricular-arterial coupling and mechanical efficiency in rats with ischemic heart failure. Dis. Mark. 2019;2019:1–9. doi: 10.1155/2019/4823156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Perjés Á., Skoumal R., Tenhunen O., Kónyi A., Simon M., Horváth I.G., Kerkelä R., Ruskoaho H., Szokodi I. Apelin increases cardiac contractility via protein kinase Cε-and extracellular signal-regulated kinase-dependent mechanisms. PloS One. 2014;9(4) doi: 10.1371/journal.pone.0093473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Azizi Y., Faghihi M., Imani A., Roghani M., Zekri A., Mobasheri M.B., Rastgar T., Moghimian M. Post-infarct treatment with [Pyr1] apelin-13 improves myocardial function by increasing neovascularization and overexpression of angiogenic growth factors in rats. Eur. J. Pharmacol. 2015;761:101–108. doi: 10.1016/j.ejphar.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 143.Liu J., Liu M., Chen L. Novel pathogenesis: regulation of apoptosis by Apelin/APJ system. Acta Biochim. Biophys. Sin. 2017;49(6):471–478. doi: 10.1093/abbs/gmx035. [DOI] [PubMed] [Google Scholar]
- 144.Eyries M., Siegfried G., Ciumas M., Montagne K., Agrapart M., Lebrin F., Soubrier F. Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis. Circ. Res. 2008;103(4):432–440. doi: 10.1161/CIRCRESAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 145.Pi J., Cheng Y., Sun H., Chen X., Zhuang T., Liu J., Li Y., Chang H., Zhang L., Zhang Y. Apln-CreERT: mT/mG reporter mice as a tool for sprouting angiogenesis study. BMC Ophthalmol. 2017;17(1):163. doi: 10.1186/s12886-017-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lakkisto P., Kytö V., Forsten H., Siren J.-M., Segersvärd H., Voipio-Pulkki L.-M., Laine M., Pulkki K., Tikkanen I. Heme oxygenase-1 and carbon monoxide promote neovascularization after myocardial infarction by modulating the expression of HIF-1α, SDF-1α and VEGF-B. Eur. J. Pharmacol. 2010;635(1–3):156–164. doi: 10.1016/j.ejphar.2010.02.050. [DOI] [PubMed] [Google Scholar]
- 147.Zhang J., Liu Q., Fang Z., Hu X., Huang F., Tang L., Zhou S. Hypoxia induces the proliferation of endothelial progenitor cells via upregulation of Apelin/APLNR/MAPK signaling. Mol. Med. Rep. 2016;13(2):1801–1806. doi: 10.3892/mmr.2015.4691. [DOI] [PubMed] [Google Scholar]
- 148.Mitsos S., Katsanos K., Koletsis E., Kagadis G.C., Anastasiou N., Diamantopoulos A., Karnabatidis D., Dougenis D. Therapeutic angiogenesis for myocardial ischemia revisited: basic biological concepts and focus on latest clinical trials. Angiogenesis. 2012;15(1):1–22. doi: 10.1007/s10456-011-9240-2. [DOI] [PubMed] [Google Scholar]
- 149.Zhang J., Liu Q., Hu X., Fang Z., Huang F., Tang L., Zhou S. Apelin/APJ signaling promotes hypoxia‑induced proliferation of endothelial progenitor cells via phosphoinositide‑3 kinase/Akt signaling. Mol. Med. Rep. 2015;12(3):3829–3834. doi: 10.3892/mmr.2015.3866. [DOI] [PubMed] [Google Scholar]
- 150.Zeng X., He H., Yang J., Yang X., Wu L., Yu J., Li L. Temporal effect of Guanxin No. 2 on cardiac function, blood viscosity and angiogenesis in rats after long-term occlusion of the left anterior descending coronary artery. J. Ethnopharmacol. 2008;118(3):485–494. doi: 10.1016/j.jep.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 151.Than A., Zhang X., Leow M.K.-S., Poh C.L., Chong S.K., Chen P. Apelin attenuates oxidative stress in human adipocytes. J. Biol. Chem. 2014;289(6):3763–3774. doi: 10.1074/jbc.M113.526210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pisarenko O., Shulzhenko V., Studneva I., Pelogeykina Y., Timoshin A., Anesia R., Valet P., Parini A., Kunduzova O. Structural apelin analogues: mitochondrial ROS inhibition and cardiometabolic protection in myocardial ischaemia reperfusion injury. Br. J. Pharmacol. 2015;172(12):2933–2945. doi: 10.1111/bph.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bircan B., Çakır M., Kırbağ S., Gül H.F. Effect of apelin hormone on renal ischemia/reperfusion induced oxidative damage in rats. Ren. Fail. 2016;38(7):1122–1128. doi: 10.1080/0886022X.2016.1184957. [DOI] [PubMed] [Google Scholar]
- 154.Zhang H., Chen S., Zeng M., Lin D., Wang Y., Wen X., Xu C., Yang L., Fan X., Gong Y. Apelin-13 administration protects against LPS-induced acute lung injury by inhibiting NF-κB pathway and NLRP3 inflammasome activation. Cell. Physiol. Biochem. 2018;49(5):1918–1932. doi: 10.1159/000493653. [DOI] [PubMed] [Google Scholar]
- 155.Fan X.-F., Xue F., Zhang Y.-Q., Xing X.-P., Liu H., Mao S.-Z., Kong X.-X., Gao Y.-Q., Liu S.F., Gong Y.-S. The Apelin-APJ axis is an endogenous counterinjury mechanism in experimental acute lung injury. Chest. 2015;147(4):969–978. doi: 10.1378/chest.14-1426. [DOI] [PubMed] [Google Scholar]
- 156.Siddiquee K., Hampton J., McAnally D., May L.T., Smith L.H. The apelin receptor inhibits the angiotensin II type 1 receptor via allosteric trans‐inhibition. Br. J. Pharmacol. 2013;168(5):1104–1117. doi: 10.1111/j.1476-5381.2012.02192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang W., McKinnie S.M., Farhan M., Paul M., McDonald T., McLean B., Llorens-Cortes C., Hazra S., Murray A.G., Vederas J.C. Angiotensin-converting enzyme 2 metabolizes and partially inactivates pyr-apelin-13 and apelin-17: physiological effects in the cardiovascular system. Hypertension. 2016;68(2):365–377. doi: 10.1161/HYPERTENSIONAHA.115.06892. [DOI] [PubMed] [Google Scholar]
- 158.Sato T., Suzuki T., Watanabe H., Kadowaki A., Fukamizu A., Liu P.P., Kimura A., Ito H., Penninger J.M., Imai Y. Apelin is a positive regulator of ACE2 in failing hearts. J. Clin. Investig. 2013;123(12):5203–5211. doi: 10.1172/JCI69608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tatemoto K., Takayama K., Zou M.-X., Kumaki I., Zhang W., Kumano K., Fujimiya M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul. Pept. 2001;99(2–3):87–92. doi: 10.1016/s0167-0115(01)00236-1. [DOI] [PubMed] [Google Scholar]
- 160.Medhurst A.D., Jennings C.A., Robbins M.J., Davis R.P., Ellis C., Winborn K.Y., Lawrie K.W., Hervieu G., Riley G., Bolaky J.E. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J. Neurochem. 2003;84(5):1162–1172. doi: 10.1046/j.1471-4159.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 161.Yang P., Kuc R.E., Brame A.L., Dyson A., Singer M., Glen R.C., Cheriyan J., Wilkinson I.B., Davenport A.P., Maguire J.J. Pyr1]Apelin-13(1–12) Is a biologically Active ACE2 metabolite of the endogenous cardiovascular peptide [Pyr1]Apelin-13. Front. Neurosci. 2017;11:92. doi: 10.3389/fnins.2017.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Brame A.L., Maguire J.J., Yang P., Dyson A., Torella R., Cheriyan J., Singer M., Glen R.C., Wilkinson I.B., Davenport A.P. Design, characterization, and first-in-human study of the vascular actions of a novel biased apelin receptor agonist. Hypertension. 2015;65(4):834–840. doi: 10.1161/HYPERTENSIONAHA.114.05099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Japp A.G., Cruden N.L., Amer D.A., Li V.K., Goudie E.B., Johnston N.R., Sharma S., Neilson I., Webb D.J., Megson I.L. Vascular effects of apelin in vivo in man. J. Am. Coll. Cardiol. 2008;52(11):908–913. doi: 10.1016/j.jacc.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 164.Kuba K., Zhang L., Imai Y., Arab S., Chen M., Maekawa Y., Leschnik M., Leibbrandt A., Markovic M., Schwaighofer J. Impaired heart contractility in Apelin gene–deficient mice associated with aging and pressure overload. Circ. Res. 2007;101(4):e32–e42. doi: 10.1161/CIRCRESAHA.107.158659. [DOI] [PubMed] [Google Scholar]
- 165.Pisarenko O.I., Serebryakova L.I., Studneva I.M., Pelogeykina Y.A., Tskitishvili O.V., Bespalova Z.D., Sidorova M.V., Az’muko A.A., Khatri D.N., Pal’keeva M.E. Effects of structural analogues of apelin-12 in acute myocardial infarction in rats. J. Pharmacol. Pharmacother. 2013;4(3):198. doi: 10.4103/0976-500X.114600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pisarenko O., Serebryakova L., Pelogeykina Y.A., Studneva I., Khatri D., Tskitishvili O., Bespalova Z.D., Az’muko A., Sidorova M., Pal’keeva M. In vivo reduction of reperfusion injury to the heart with apelin-12 peptide in rats. Bull. Exp. Biol. Med. 2011;152(1):79–82. doi: 10.1007/s10517-011-1459-9. [DOI] [PubMed] [Google Scholar]
- 167.Santoso A., Pranata R., Wibowo A., Al-Farabi M.J., Huang I., Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Babapoor-Farrokhran S., Gill D., Walker J., Rasekhi R.T., Bozorgnia B., Amanullah A. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vanderah T.W., Gardell L.R., Burgess S.E., Ibrahim M., Dogrul A., Zhong C.-M., Zhang E.-T., Malan T.P., Ossipov M.H., Lai J. Dynorphin promotes abnormal pain and spinal opioid antinociceptive tolerance. J. Neurosci. 2000;20(18):7074–7079. doi: 10.1523/JNEUROSCI.20-18-07074.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Warner F., Smith A., Hooper N., Turner A. Angiotensin-converting enzyme-2: a molecular and cellular perspective. Cell. Mol. Life Sci.: CMLS. 2004;61(21):2704–2713. doi: 10.1007/s00018-004-4240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Beyer A., Schäfer M., Stein C. Antinociceptive effects of dynorphin peptides in a model of inflammatory pain. Pain. 1997;70:141–147. doi: 10.1016/s0304-3959(97)03327-7. [DOI] [PubMed] [Google Scholar]
- 172.Caudle R.M., Mannes A.J. Dynorphin: friend or foe? Pain. 2000;87(3):235–239. doi: 10.1016/S0304-3959(00)00360-2. [DOI] [PubMed] [Google Scholar]
- 173.Long J.B., Kinney R.C., Malcolm D.S., Graeber G.M., Holaday J.W. Intrathecal dynorphin A1–13 and dynorphin A3–13 reduce rat spinal cord blood flow by non-opioid mechanisms. Brain Res. 1987;436(2):374–379. doi: 10.1016/0006-8993(87)91683-0. [DOI] [PubMed] [Google Scholar]
- 174.Shukla V.K., Lemaire S. Non-opioid effects of dynorphins: possible role of the NMDA receptor. Trends Pharmacol. Sci. 1994;15(11):420–424. doi: 10.1016/0165-6147(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 175.Hauser K.F., Foldes J.K., Turbek C.S. Dynorphin A (1–13) neurotoxicity in vitro: opioid and non-opioid mechanisms in mouse spinal cord neurons. Exp. Neurol. 1999;160(2):361–375. doi: 10.1006/exnr.1999.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.L. Mao, M. Wang, S. Chen, Q. He, J. Chang, C. Hong, Y. Zhou, D. Wang, X. Miao, Y. Hu, Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study, (2020). [DOI] [PMC free article] [PubMed]
- 177.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á., Layos-Romero A., García-García J., González E., Redondo-Peñas I., Perona-Moratalla A.B., Del Valle-Pérez J.A. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95(8):e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gautier J.F., Ravussin Y., New A. A new symptom of COVID‐19: loss of taste and smell. Obesity. 2020;28(5):848. doi: 10.1002/oby.22809. (848-848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cabot P.J., Carter L., Schäfer M., Stein C. Methionine-enkephalin-and Dynorphin A-release from immune cells and control of inflammatory pain. Pain. 2001;93(3):207–212. doi: 10.1016/S0304-3959(01)00322-0. [DOI] [PubMed] [Google Scholar]
- 181.van de Veerdonk F.L., Netea M.G. Blocking IL-1 to prevent respiratory failure in COVID-19. Crit. Care. 2020;24(1):1–6. doi: 10.1186/s13054-020-03166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kaushik V.C.H.P., Tang V.D.L.G.J., Godbout J., Parsons K., Baronas T., Hsieh F E. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 183.Müller S., Ho B., Gambus P., Millard W., Hochhaus G. An HPLC/RIA method for dynorphin A1–13 and its main metabolites in human blood. J. Pharm. Biomed. Anal. 1997;16(1):101–109. doi: 10.1016/s0731-7085(97)00010-1. [DOI] [PubMed] [Google Scholar]
- 184.Müller S., Grundy B.L., Hochhaus G. Metabolism of dynorphin A1–13 in human CSF. Neurochem. Res. 1996;21(10):1213–1219. doi: 10.1007/BF02532398. [DOI] [PubMed] [Google Scholar]
- 185.Müller S., Hochhaus G. Metabolism of dynorphin A 1–13 in human blood and plasma. Pharm. Res. 1995;12(8):1165–1170. doi: 10.1023/a:1016211910107. [DOI] [PubMed] [Google Scholar]
- 186.Tawinwung S., Petpiroon N., Chanvorachote P. Blocking of type 1 angiotensin II receptor inhibits T-lymphocyte activation and IL-2 production. In Vivo. 2018;32(6):1353–1359. doi: 10.21873/invivo.11386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ross S.H., Cantrell D.A. Signaling and function of interleukin-2 in T lymphocytes. Annu. Rev. Immunol. 2018;36:411–433. doi: 10.1146/annurev-immunol-042617-053352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jurewicz M., McDermott D.H., Sechler J.M., Tinckam K., Takakura A., Carpenter C.B., Milford E., Abdi R. Human T and natural killer cells possess a functional renin-angiotensin system: further mechanisms of angiotensin ii–induced inflammation. J. Am. Soc. Nephrol. 2007;18(4):1093–1102. doi: 10.1681/ASN.2006070707. [DOI] [PubMed] [Google Scholar]
- 189.Hoch N.E., Guzik T.J., Chen W., Deans T., Maalouf S.A., Gratze P., Weyand C., Harrison D.G. Regulation of T-cell function by endogenously produced angiotensin II. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R208–R216. doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shao J., Nangaku M., Miyata T., Inagi R., Yamada K., Kurokawa K., Fujita T. Imbalance of T-cell subsets in angiotensin II–infused hypertensive rats with kidney injury. Hypertension. 2003;42(1):31–38. doi: 10.1161/01.HYP.0000075082.06183.4E. [DOI] [PubMed] [Google Scholar]
- 191.Qin X.-Y., Zhang Y.-L., Chi Y.-F., Yan B., Zeng X.-J., Li H.-H., Liu Y. Angiotensin II regulates Th1 T cell differentiation through angiotensin II type 1 receptor-PKA-mediated activation of proteasome. Cell. Physiol. Biochem. 2018;45(4):1366–1376. doi: 10.1159/000487562. [DOI] [PubMed] [Google Scholar]
- 192.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wei M., Gan L., Hou J., Chen L., Liu Y., Ren L. Effects of (Pyr1) apelin-13 on the proliferation and activation of Jarkat T lymphocyte. Chin. J. Cell. Mol. Immunol. 2016;32(12):1641–1645. (Xi bao yu fen zi mian yi xue za zhi) [PubMed] [Google Scholar]
- 194.Habata Y., Fujii R., Hosoya M., Fukusumi S., Kawamata Y., Hinuma S., Kitada C., Nishizawa N., Murosaki S., Kurokawa T. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1999;1452(1):25–35. doi: 10.1016/s0167-4889(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 195.Okamura A., Rakugi H., Ohishi M., Yanagitani Y., Takiuchi S., Moriguchi K., Fennessy P.A., Higaki J., Ogihara T. Upregulation of renin-angiotensin system during differentiation of monocytes to macrophages. J. Hypertens. 1999;17(4):537–545. doi: 10.1097/00004872-199917040-00012. [DOI] [PubMed] [Google Scholar]
- 196.Guo F., Chen X.-L., Wang F., Liang X., Sun Y.-X., Wang Y.-J. Role of angiotensin II type 1 receptor in angiotensin II-induced cytokine production in macrophages. J. Interferon Cytokine Res. 2011;31(4):351–361. doi: 10.1089/jir.2010.0073. [DOI] [PubMed] [Google Scholar]
- 197.Zhu N., Cui J., Qiao C., Li Y., Ma Y., Zhang J., Shen B. cAMP modulates macrophage development by suppressing M-CSF-induced MAPKs activation. Cell. Mol. Immunol. 2008;5(2):153–157. doi: 10.1038/cmi.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hartmann S., Nusbaum D.J., Kim K., Alameh S., Ho C.-L.C., Cruz R.L., Levitin A., Bradley K.A., Martchenko M. Role of a small molecule in the modulation of cell death signal transduction pathways. ACS Infect. Dis. 2018;4(12):1746–1754. doi: 10.1021/acsinfecdis.8b00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yao F., Lv Y.-C., Zhang M., Xie W., Tan Y.-L., Gong D., Cheng H.-P., Liu D., Li L., Liu X.-Y. Apelin-13 impedes foam cell formation by activating Class III PI3K/Beclin-1-mediated autophagic pathway. Biochem. Biophys. Res. Commun. 2015;466(4):637–643. doi: 10.1016/j.bbrc.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 200.Lapteva N., Ide K., Nieda M., Ando Y., Hatta-Ohashi Y., Minami M., Dymshits G., Egawa K., Juji T., Tokunaga K. Activation and suppression of renin–angiotensin system in human dendritic cells. Biochem. Biophys. Res. Commun. 2002;296(1):194–200. doi: 10.1016/s0006-291x(02)00855-0. [DOI] [PubMed] [Google Scholar]
- 201.Li N., Karin M. Is NF‐κB the sensor of oxidative stress? FASEB J. 1999;13(10):1137–1143. [PubMed] [Google Scholar]
- 202.Meng Y., Chen C., Liu Y., Tian C., Li H.-H. Angiotensin II regulates dendritic cells through activation of NF-κB/p65, ERK1/2 and STAT1 pathways. Cell. Physiol. Biochem. 2017;42(4):1550–1558. doi: 10.1159/000479272. [DOI] [PubMed] [Google Scholar]
- 203.Podolnikova N.P., Brothwell J.A., Ugarova T.P. The opioid peptide dynorphin A induces leukocyte responses via integrin Mac-1 (αMβ2, CD11b/CD18) Mol. Pain. 2015;11 doi: 10.1186/s12990-015-0027-0. s12990-015-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hammer A., Yang G., Friedrich J., Kovacs A., Lee D.-H., Grave K., Jörg S., Alenina N., Grosch J., Winkler J. Role of the receptor Mas in macrophage-mediated inflammation in vivo. Proc. Natl. Acad. Sci. 2016;113(49):14109–14114. doi: 10.1073/pnas.1612668113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhao Q., Meng M., Kumar R., Wu Y., Huang J., Deng Y., Weng Z., Yang L. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020;99:47–56. doi: 10.1016/j.ijid.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Aggarwal S., Gollapudi S., Gupta S. Increased TNF-α-induced apoptosis in lymphocytes from aged humans: changes in TNF-α receptor expression and activation of caspases. J. Immunol. 1999;162(4):2154–2161. [PubMed] [Google Scholar]
- 207.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.M. Yang, Cell pyroptosis, a potential pathogenic mechanism of 2019-nCoV infection, Available at SSRN 3527420, (2020).
- 209.Fink S.L., Cookson B.T. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005;73(4):1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Teralı K., Baddal B., Gülcan H.O. Prioritizing potential ACE2 inhibitors in the COVID-19 pandemic: Insights from a molecular mechanics-assisted structure-based virtual screening experiment. J. Mol. Graph. Model. 2020;100 doi: 10.1016/j.jmgm.2020.107697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Watkins J. Preventing a covid-19 pandemic. BMJ. 2020:m810. doi: 10.1136/bmj.m810. [DOI] [PubMed] [Google Scholar]
- 212.Selimoglu Şen H., Kaplan I., Abakay Ö., Sezgi C., Yilmaz S., Taylan M., Abakay A., Tanrikulu A.Ç. Serum apelin 13 levels in patients with pulmonary embolism. Clin. Appl. Thromb. Hemost. 2016;22(6):543–547. doi: 10.1177/1076029615572467. [DOI] [PubMed] [Google Scholar]
- 213.Koller G.M., Schafer C., Kemp S.S., Aguera K.N., Lin P.K., Forgy J.C., Griffin C.T., Davis G.E. Proinflammatory mediators, IL (Interleukin)-1β, TNF (Tumor Necrosis Factor) α, and thrombin directly induce capillary tube regression. Arterioscler. Thromb. Vasc. Biol. 2020;40(2):365–377. doi: 10.1161/ATVBAHA.119.313536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology. 2020;296:E186–E188. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.N. Ahmadirad, Z. Ghasemi, COVID-19 and central nervous system: entry routes and, Basic and clinical neuroscience 11(2) (2020) 217. [DOI] [PMC free article] [PubMed]
- 216.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chen C., Zhang X., Ju Z., He W. Advances in the research of cytokine storm mechanism induced by Corona Virus Disease 2019 and the corresponding immunotherapies. Chin. J. Burns. 2020;36 doi: 10.3760/cma.j.cn501120-20200224-00088. (Zhonghua shao shang za zhi= Zhonghua shaoshang zazhi) (E005-E005) [DOI] [PubMed] [Google Scholar]
- 218.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8(4) doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shinohara Y., Origasa H. Post-stroke pneumonia prevention by angiotensin-converting enzyme inhibitors: results of a meta-analysis of five studies in Asians. Adv. Ther. 2012;29(10):900–912. doi: 10.1007/s12325-012-0049-1. [DOI] [PubMed] [Google Scholar]
- 220.Wang H.C., Lin C.C., Lau C.I., Chang A., Kao C.H. Angiotensin‐converting enzyme inhibitors and bacterial pneumonia in patients with Parkinson disease. Mov. Disord. 2015;30(4):593–596. doi: 10.1002/mds.26136. [DOI] [PubMed] [Google Scholar]
- 221.Rossi G.P., Sanga V., Barton M. Potential harmful effects of discontinuing ACE-inhibitors and ARBs in COVID-19 patients. Elife. 2020;9 doi: 10.7554/eLife.57278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Caldeira D., Alarcão J., Vaz-Carneiro A., Costa J. Risk of pneumonia associated with use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers: systematic review and meta-analysis. BMJ. 2012;345 doi: 10.1136/bmj.e4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liu C.-L., Shau W.-Y., Wu C.-S., Lai M.-S. Angiotensin-converting enzyme inhibitor/angiotensin II receptor blockers and pneumonia risk among stroke patients. J. Hypertens. 2012;30(11):2223–2229. doi: 10.1097/HJH.0b013e328357a87a. [DOI] [PubMed] [Google Scholar]
- 224.Harzallah I., Debliquis A., Drénou B. Lupus anticoagulant is frequent in patients with Covid‐19: Response to Reply. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chauhan A.K., Kisucka J., Lamb C.B., Bergmeier W., Wagner D.D. von Willebrand factor and factor VIII are independently required to form stable occlusive thrombi in injured veins. Blood. 2007;109(6):2424–2429. doi: 10.1182/blood-2006-06-028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Brill A., Fuchs T.A., Chauhan A.K., Yang J.J., De Meyer S.F., Köllnberger M., Wakefield T.W., Lämmle B., Massberg S., Wagner D.D. von Willebrand factor–mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood J. Am. Soc. Hematol. 2011;117(4):1400–1407. doi: 10.1182/blood-2010-05-287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chappell M.C. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am. J. Physiol. Heart Circ. Physiol. 2016;310(2):H137–H152. doi: 10.1152/ajpheart.00618.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.van Kats J.P., de Lannoy L.M., Danser A.J., van Meegen J.R., Verdouw P.D., Schalekamp M.A. Angiotensin II Type 1 (AT1) receptor–mediated accumulation of angiotensin II in tissues and its intracellular half-life in vivo. Hypertension. 1997;30(1):42–49. doi: 10.1161/01.hyp.30.1.42. [DOI] [PubMed] [Google Scholar]
- 229.Petty W.J., Miller A.A., McCoy T.P., Gallagher P.E., Tallant E.A., Torti F.M. Phase I and pharmacokinetic study of angiotensin-(1–7), an endogenous antiangiogenic hormone. Clin. Cancer Res. 2009;15(23):7398–7404. doi: 10.1158/1078-0432.CCR-09-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chou J.Z., Chait B.T., Wang R., Kreek M.J. Differential biotransformation of dynorphin A (1–17) and dynorphin A (1–13) peptides in human blood, ex vivo. Peptides. 1996;17(6):983–990. doi: 10.1016/0196-9781(96)00154-4. [DOI] [PubMed] [Google Scholar]
- 231.Chavkin C., Goldstein A. Specific receptor for the opioid peptide dynorphin: structure--activity relationships. Proc. Natl. Acad. Sci. 1981;78(10):6543–6547. doi: 10.1073/pnas.78.10.6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kim J., Kang Y., Kojima Y., Lighthouse J.K., Hu X., Aldred M.A., McLean D.L., Park H., Comhair S.A., Greif D.M. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat. Med. 2013;19(1):74–82. doi: 10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Young A., Wu W., Sun W., Larman H.B., Wang N., Li Y.-S., Shyy J.Y., Chien S., García-Cardeña G. Flow activation of AMP-activated protein kinase in vascular endothelium leads to Kruppel-like factor 2 expression. Arterioscler. Thromb. Vasc. Biol. 2009;29(11):1902–1908. doi: 10.1161/ATVBAHA.109.193540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Visser Y.Pd, Walther F.J., Laghmani E.H., v.d. Laarse A., Wagenaar G.T. Apelin attenuates hyperoxic lung and heart injury in neonatal rats. Am. J. Respir. Crit. Care Med. 2010;182(10):1239–1250. doi: 10.1164/rccm.200909-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhou Y., Deng L., Zhao D., Chen L., Yao Z., Guo X., Liu X., Lv L., Leng B., Xu W. Micro RNA‐503 promotes angiotensin II‐induced cardiac fibrosis by targeting Apelin‐13. J. Cell. Mol. Med. 2016;20(3):495–505. doi: 10.1111/jcmm.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Japp A., Cruden N., Barnes G., Van Gemeren N., Mathews J., Adamson J., Johnston N., Denvir M., Megson I., Flapan A. Acute cardiovascular effects of apelin in humans. Circulation. 2010;121(16):1818–1827. doi: 10.1161/CIRCULATIONAHA.109.911339. [DOI] [PubMed] [Google Scholar]
- 237.Chen H., Li J., Jiao L., Petersen R.B., Li J., Peng A., Zheng L., Huang K. Apelin inhibits the development of diabetic nephropathy by regulating histone acetylation in Akita mouse. J. Physiol. 2014;592(3):505–521. doi: 10.1113/jphysiol.2013.266411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wang L.-Y., Diao Z.-L., Zhang D.-L., Zheng J.-F., Zhang Q.-D., Ding J.-X., Liu W.-H. The regulatory peptide apelin: a novel inhibitor of renal interstitial fibrosis. Amino Acids. 2014;46(12):2693–2704. doi: 10.1007/s00726-014-1826-8. [DOI] [PubMed] [Google Scholar]
- 239.Wu F., Qiu J., Fan Y., Zhang Q., Cheng B., Wu Y., Bai B. Apelin-13 attenuates ER stress-mediated neuronal apoptosis by activating Gαi/Gαq-CK2 signaling in ischemic stroke. Exp. Neurol. 2018;302:136–144. doi: 10.1016/j.expneurol.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 240.Pouresmaeili-Babaki E., Esmaeili-Mahani S., Abbasnejad M., Ravan H. Protective effect of neuropeptide apelin-13 on 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y dopaminergic cells: involvement of its antioxidant and antiapoptotic properties. Rejuvenation Res. 2018;21(2):162–167. doi: 10.1089/rej.2017.1951. [DOI] [PubMed] [Google Scholar]
- 241.Cheng X., Cheng X.S., Pang C.C. Venous dilator effect of apelin, an endogenous peptide ligand for the orphan APJ receptor, in conscious rats. Eur. J. Pharmacol. 2003;470(3):171–175. doi: 10.1016/s0014-2999(03)01821-1. [DOI] [PubMed] [Google Scholar]
- 242.Lee D.K., Saldivia V.R., Nguyen T., Cheng R., George S.R., O’Dowd B.F. Modification of the terminal residue of apelin-13 antagonizes its hypotensive action. Endocrinology. 2005;146(1):231–236. doi: 10.1210/en.2004-0359. [DOI] [PubMed] [Google Scholar]
- 243.Dai T., Ramirez-Correa G., Gao W.D. Apelin increases contractility in failing cardiac muscle. Eur. J. Pharmacol. 2006;553:222–228. doi: 10.1016/j.ejphar.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Pisarenko O.I., Shulzhenko V.S., Studneva I.M., Serebryakova L.I., Pelogeykina Y.A., Veselova O.M. Signaling pathways of a structural analogue of apelin-12 involved in myocardial protection against ischemia/reperfusion injury. Peptides. 2015;73:67–76. doi: 10.1016/j.peptides.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 245.Liu D., Hu W., Chen G. Apelin-12 exerts neuroprotective effect against ischemia-reperfusion injury by inhibiting JNK and P38MAPK signaling pathway in mouse. Eur. Rev. Med. Pharm. Sci. 2018;22(12):3888–3895. doi: 10.26355/eurrev_201806_15273. [DOI] [PubMed] [Google Scholar]