Abstract
In both preclinical animal studies and human clinical trials, adult females tend to develop greater adaptive immune responses than males following receipt of either viral or bacterial vaccines. While there is currently no approved malaria vaccine, several anti-sporozoite vaccines, including RTS,S/AS01 and attenuated sporozoite vaccines, are in development, but the impact of sex and age on their efficacy remains undefined. To examine sex differences in the efficacy of anti-sporozoite stage malaria vaccination, adult (10 weeks of age) or juvenile (11 days of age) male and female C3H mice were twice vaccinated with irradiated transgenic Plasmodium berghei sporozoites expressing the P. falciparum circumsporozoite (CSP) protein and 45 days post boost vaccination, mice were challenged with transgenic P. berghei via mosquito bite or intradermal challenge. Immunization with irradiated sporozoites resulted in greater protection against challenge in adult females, which was associated with greater anti-CSP antibody production and avidity, as well as greater hepatic, but not splenic, CD8+ T cell IFNƴ production in adult females than adult males. No sex differences in adaptive immune responses or protection were observed in mice vaccinated prior to puberty, suggesting a role for sex steroid hormones. Depletion of testosterone in males increased, whereas rescue of testosterone decreased, anti-CSP antibody production, the number of antigen-specific CD8+ T cells isolated from the liver, and protection following parasite challenge. Conversely, depletion of sex steroids in female mice did not alter vaccine-induced responses or protection following challenge. These data suggest that elevated testosterone concentrations in males reduce adaptive immunity and contribute to sex differences in malaria vaccine efficacy.
Keywords: circumsporozoite protein, CSP, estrogen, gender, Plasmodium, testosterone
1. Introduction
Malaria is a significant global burden of disease worldwide, with most cases occurring in sub-Saharan Africa. In 2017, malaria was estimated to result in 216 million clinical cases and 445,000 deaths [1]. Worldwide, malaria causes approximately 5% of all deaths in children under 5 years of age, primarily due to Plasmodium falciparum in sub-Saharan Africa [2]. In response, the World Health Organization (WHO) has a goal to achieve a 90% reduction in malaria cases and mortality by the year 2030 [3]. Current methods to interrupt malaria transmission include the early diagnosis and treatment of cases, vector control (e.g., insecticide treated bed net use and residual indoor insecticide spraying), prophylactic drug treatment, and healthcare capacity improvements, all of which are economically costly and unlikely to achieve this goal on their own [4]. To complement these approaches, the need for a malaria vaccine that is at least 75% protective has been identified as the most cost-effective method for controlling malaria [4, 5].
The RTS,S/AS01 vaccine is currently the leading candidate malaria vaccine undergoing pilot implementation in several regions of sub-Saharan Africa [5]. The RTS,S/AS01 vaccine targets the pre-erythrocytic stage of the malarial life-cycle and consists of the carboxy-terminal region of the P. falciparum circumsporozoite (CSP) surface protein along with the hepatitis B surface antigen. In Phase III clinical trials, the RTS,S/AS01 vaccine was moderately efficacious, with 18–36% protection against clinical disease in young boys and girls (i.e., ages 6–12 weeks and 5–17 months) [6]. The RTS,S/AS01 vaccine, however, was also associated with higher all-cause mortality in girls, but not boys, in both age groups in which the vaccine was tested [7]. Though significant, the mechanisms underlying this sex differential outcome remain unclear, but these observations highlight the potential for sex-specific differences in the outcomes of malaria vaccination [8]. Although no other candidate malaria vaccine has reached Phase III clinical trials, other candidates, including several whole sporozoite vaccines, are currently undergoing preliminary clinical trials [9]. Regardless of the candidate vaccine, to date, clinical trials have been conducted solely in healthy adult volunteers or children without consideration of the influence of sex as a biological variable [10].
Clinical data illustrate that in both children and adults, males and females can differ in vaccine-induced immune responses and protection [11]. Following vaccination, juvenile and to a greater extent adult females often develop higher antibody responses, generate more robust cell-mediated immunity, and are better protected by vaccination, but also experience more frequent and severe adverse reactions than age-matched males [11–13]. The mechanisms mediating these vaccine-associated differences are incompletely understood, but have been attributed to the effects of sex steroid signaling, sex chromosome complement, epigenetic regulation, and the microbiome on immune responses to vaccine antigens [11]. Our current knowledge of the influence of sex on vaccination has generally been informed by vaccines targeting viruses and bacteria [11, 12], with little clinical data pertaining to vaccines against parasite infections, including malaria.
Preclinical animal models have proven useful for studying vaccine efficacy and the immune response to malaria [14]. Little consideration, however, has been given to sex as a biological variable, with most studies either not reporting the sex of the animals or only using female animals [15–18]. In murine studies of malaria blood stage infection, females have reduced mortality, experience faster resolution of infection-associated anemia and weight loss, and mount a more robust immune response to infection (e.g. increased IFNƴ, IL-10, and Plasmodium specific IgG1 antibody production) than males [19]. Consistent with these observations, females are better protected against challenge than males following vaccination with the surface membranes of P. chabaudi-infected red blood cells [20]. To date, no pre-clinical study has been designed to study sex differences in the immunogenicity and efficacy of pre-erythrocytic stage malaria vaccination. In this study, we examined the influence of sex, age, and sex hormones on the efficacy and immune response to pre-erythrocytic malaria vaccination using an irradiated sporozoite vaccine model.
2. Methods
2.1. Mice
All experiments were performed in compliance with the standards outlined in the National Research Council’s Guide to the Care and Use of Laboratory Animals. All animal procedures were reviewed and approved by the Johns Hopkins Animal Care and Use Committee (M016H35). All efforts were made to minimize animal suffering. Adult (8–10 weeks old) male and female C3H/HeNCr MTV mice were purchased from Charles River (Wilmington, MA) and housed at 5 animals per microisolator cage. For studies using juvenile mice, time pregnant (arrived at embryonic day 12) female C3H/HeNCr MTV mice were purchased from Charles River (Wilmington, MA) and housed as individual dams with pups until weaning. Pups were weaned at post-natal day (PND) 21, separated by sex, and housed at 3–5 animals per microisolator cage. All mice were housed under standard BSL-2 housing conditions in a specific pathogen free facility and given food and water ad libitum. Where feasible (e.g., for hormone manipulation), animals were randomly assigned to treatment groups. Details about animal numbers and replicates are contained in the figure legends.
2.2. Irradiated sporozoite vaccination
Previously generated transgenic P. berghei sporozoites expressing the immunodominant P. falciparum circumsporozoite (CSP) protein (P.b.-P.f.) were used for all vaccinations [21]. For studies in adult mice, mice were twice vaccinated at 14-day intervals by tail vein injection with 1 × 105 sporozoites gamma irradiated at a dose of 25gy using Cs-137 as the source (GammaCell 1000). Due to the body size and inaccuracy of accessing the tail vein, juvenile mice were twice vaccinated by intraperitoneal injection at 14-day intervals beginning at PND 11 with 1 × 105 gamma irradiated P.b.-P.f. sporozoites. For all studies, whole irradiated sporozoites were administered in unadjuvanted DMEM (Corning) using a 28g × 12.7mm needle (BD) within one hour of irradiation. All vaccinations were performed in the afternoon. Because irradiated sporozoites are processed directly in the spleen and liver to induce an immune response and are not in solution, doses did not account for adiposity or body mass.
2.3. Anti-circumsporozoite protein enzyme-linked immunosorbent assays (ELISA)
ELISA plates (Greiner Bio-One) were coated with 100μl per well of purified recombinant CSP peptide [21] diluted in 1X Phosphate buffered Saline (PBS, Gibco) at a concentration 1μg/ml. After incubation overnight at room temperature (RT), plates were washed three times with 200μl of PBS, before blocking with 200μl PBS-BSA (1X PBS with 1% BSA, Sigma) for 2 hours at RT. Following three washes with PBS, serially diluted plasma samples were added, and incubated at RT for 1 hour. The plates were washed two times with PBS-Tween (1X PBS with 0.5% Tween20, Sigma) followed by two washes with PBS and 100μl of secondary antibody (IgG; KPL, IgG1; ThermoScientific, or IgG2a; Invitrogen) at a 1:1000 dilution was added for 1 hour at RT. Plates were then washed three times with PBS-Tween followed by three times with PBS and 100μl of horseradish peroxidase substrate (KPL) was added to each well and developed in the dark for 15minutes. The reaction was stopped using 50μl of 1% SDS (Fisher) and the plates were read at 405nm. Antibody titers were calculated as the highest plasma dilution with an optical density (OD) value greater than three times the average OD of the negative controls. Titer curves were plotted after normalization to the negative control and the area under the titer curve (AUC) was calculated and the dilution titer equal to an optical density of 1 (OD1) was determined by non-linear regression. For semiquantitative assessment of antibody concentration, a titer curve was generated using known quantities of the P. falciparum CSP specific monoclonal antibody 2A10 [22], with a concentration of 400μg/ml assigned a value of 1000 ELISA Units (EU). Non-linear regression was then used to generate an equation for the reference antibody curve and the OD values for each experimental sample were interpolated into the reference antibody equation to determine the relative concentration in EUs. For all calculations, only the linear portion of the curve was used, and resulting concentrations were multiplied by the dilution factor to obtain the final relative antibody concentration for each experimental sample.
2.4. Anti-circumsporozoite (CSP) avidity assay
ELISA plates were coated with of 100μl per well of purified CSP peptide [21] diluted in PBS at a concentration 1μg/ml. After incubation overnight at RT, plates were washed three times with 200μl of PBS, before being blocked with 200μl PBS-BSA for 2hrs at RT. Following three washes with 200μl PBS, plasma samples were plated in quadruplicate at a 1:200 dilution in PBS-BSA and incubated for 1 hour at room temperature. Plates were washed two times with 200μl PBS-Tween followed by two washes with PBS. To measure antibody avidity, 2M ammonium thiocyanate (NH4SCN; Sigma) or PBS was added to the plates for exactly 15 minutes. The plates were washed two times with PBS-Tween followed by two times with PBS and peroxidase- labeled goat anti-mouse IgG antibody was added at a concentration of 500ng/ml and incubated for 1 hour at RT. Plates were washed three times with PBS-Tween followed by three times with PBS and 100μl of horseradish peroxidase substrate was added to each well and developed in the dark for 15minutes. The reaction was stopped using 50μl of 1% SDS and the plates were read at 405nm. The antibody avidity index was determined by dividing the NH4SCN treated optical density values by the corresponding PBS (untreated) values for each sample in duplicate.
2.5. Sporozoite challenge
Mice were sedated with Ketamine-Xylazine and challenged by mosquito bite using 10 P.b.-P.f. infected female Anopheles stephensi mosquitoes or challenged by intradermal injection with 3 × 103 chimeric P.b.-P.f. CSP sporozoites in the afternoon, 45 days after the boost vaccination. Hepatic parasite loads were quantified forty-two hours post-challenge by RT-qPCR targeting P. berghei 18s rRNA using forward primer 5’ -TGGGAGATTGGTTTTGACGTTTATGT- 3’ and reverse primer 5’ -AAGCATTAAATAAAGCGAATACATCCTTAC-3’ as described previously [23]. Resulting parasite loads were expressed as P. berghei 18s rRNA copy number as well as the log and percent reduction relative to naïve controls.
2.6. CD8+ T cell responses
Single cells suspensions were generated by homogenizing tissue through a 100μm nylon filter (Falcon) followed by ACK lysis of red blood cells (Quality biologicals) for splenic tissue or Percoll gradient separation (GE) for hepatic tissue. Due to the low frequency of CSP-specific CD8+ T cells in the liver, pools of 3 mice were used for Percoll gradient separation of hepatic samples. The total numbers of live cells for both splenic and hepatic samples were determined using a hemocytometer and trypan blue (Invitrogen) exclusion, and cells were resuspended at 40 × 106 cells/ml in RPMI 1640 (Cellgro) supplemented with 10% fetal bovine serum (Fisher Scientific), 1% L-glutamine (Gibco), and 1% penicillin-streptomycin (Gibco). Isolated cells were plated out at 2 × 106 cells/well and incubated for 5–6hrs at 37°C with 5 × 105 cells/well of either peptide pulsed (DYENDIEKKI – 10 μg/10 × 106 cells) or non-pulsed LM1 target cells [24] in the presence of GolgiPlug (BD) and GolgiStop (BD) as described in [21]. LM1 cells express the H-2K MHC haplotype allowing them to effectively present the protective CD8 epitope of P. falciparum CSP to CD8+ T cells derived from C3H mice. Following incubation, Fc receptors were blocked using anti-CD16/32 (BD Biosciences) and cells were stained with CD8-PerCPCy5.5 (Clone 53–6.7; BD). Cells were then permeabilized and fixed (BD Cytofix/Cytoperm) prior to intracellular staining with IFNƴ -FITC (Clone XMG1.2; BD) and TNFα-PE (Clone MP6-XT22; BD). Data were acquired using a FACSCalibur flow cytometer (BD) running Cell Quest Pro and analyzed using FlowJo (v.10) software (Tree Star, Inc.).
2.7. Gonadectomy and hormone replacement
Adult male and female mice (9–10 weeks) were randomly assigned to be bilaterally gonadectomized (gdx) or receive sham surgeries [25]. For males, two weeks following gonadectomy, mice were implanted subcutaneously with silastic tubing capsules (inner diameter-0.04”, outer diameter- 0.085”; HelixMark) containing crystalline testosterone propionate (gdx + T; 10.0 mm; Sigma) of left empty (gdx) [26]. The capsules were sealed with 2.5 mm of medical adhesive (Factor II, A-100), and incubated at 37°C overnight in sterile saline solution prior to implantation. Capsules were replaced prior to depletion every 28 days for the duration of the study.
2.8. Sex-hormone enzyme immunosorbent assays
Plasma was collected 3 days prior to challenge and total testosterone or estradiol concentrations were quantified by commercial EIA kits according to the manufacturer’s instructions (IBL America – testosterone; Calbiotech – estradiol), with performance characteristics, including analytical sensitivity and coefficients of variation contained in the online protocols. To prevent sample degradation, care was taken to limit light and thermal exposure of plasma samples prior to hormone quantification.
2.9. Statistical analysis
Data were tested for normality (Shapiro-Wilk test) and did not meet the assumptions of a normal distribution. Therefore, data were analyzed by Mann-Whitney U test or Kruskal-Wallis test followed by the Dunn’s test for pairwise multiple comparisons. Statistical analyses were performed using GraphPad Prism 8.1.2 software and mean differences were considered significant at a two-sided P < 0.05. Confidence intervals represent the uncertainty in the mean differences between comparison groups. Following the ARRIVE guidelines, specific details about the statistical methods, numbers of animals analyzed, and estimations for each experiment are contained in either the Results or individual figure legends.
3. Results
3.1. Adult female mice mount greater antibody responses to irradiated sporozoite vaccination
To test if the sexes respond differently to malaria vaccination, we used an established model where adult male and female C3H mice were twice vaccinated with irradiated P.b.-P.f. sporozoites [27]. Forty-two days post boost vaccination, anti-CSP IgG titers were greater in adult females than males (Mann-Whitney: P < 0.0001, 95% CI [6400, 12800]; Fig. 1A). To confirm these findings, anti-CSP IgG data were further analyzed for the area under the curve (AUC), the titer equal to an optical density-1 (OD1), and antibody quantity. Regardless of the method used to quantitatively measure anti-CSP IgG antibody responses, adult females exhibited greater responses following vaccination than adult males (Mann-Whitney; P < 0.0001, 95% CI [3514, 10427] for AUC, P = 0.0002, 95% CI [219.3, 828.2] for OD1, and P = 0.0002, 95% CI [11.76, 44.40] for antibody quantity; Fig. 1B–D).
Fig. 1.
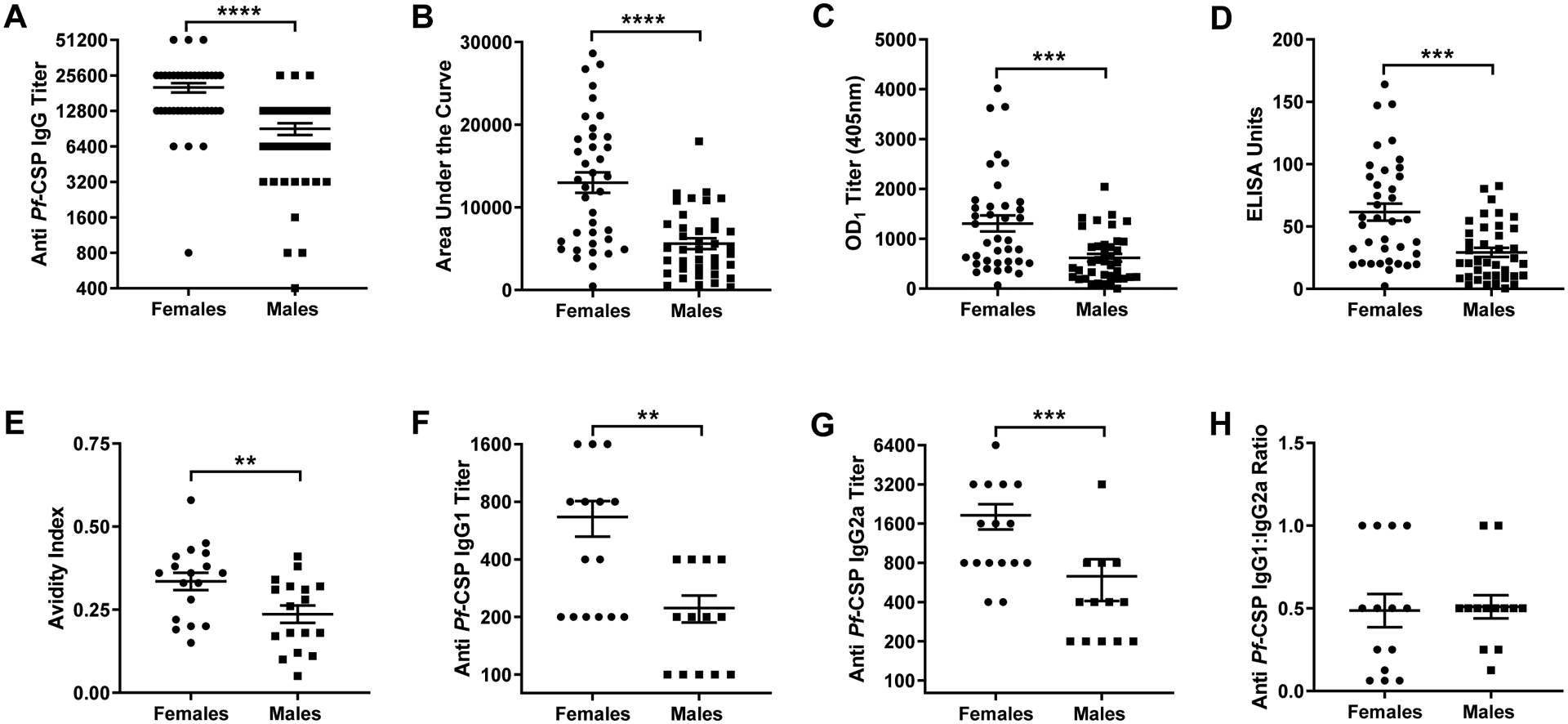
Effects of sex on antibody responses to irradiated sporozoite vaccination. Adult (8–10 weeks) male and female mice were twice vaccinated with irradiated sporozoites at 14-day intervals. Forty-two days post boost vaccination, plasma was collected and anti-CSP IgG titers (A), areas under the curve (AUC) (B), anti-CSP titers equal to an optical density-1 (C), anti-CSP antibody concentrations (D), anti-CSP antibody avidity (E), anti-CSP IgG1 titers (F), anti-CSP IgG2a titers (G), and the ratio of IgG1/IgG2a (H) were measured. Data represent means ± the SEM from two independent replications (n = 34–39/sex) and significant differences are denoted with asterisks (**P < 0.01, *** < 0.001, ****P < 0.0001).
In addition to antibody quantity, qualitative differences in humoral responses can influence vaccine efficacy. To assess the impact of sex on qualitative antibody traits following irradiated sporozoite vaccination, antibody avidity, which is associated with protection from sporozoite challenge [16], was measured. Anti-CSP antibody avidity was greater in adult female than adult male mice (Mann-Whitney; P = 0.007, 95% CI [0.02, 0.18]; Fig. 1E). Because avidity can be influenced by antibody isotype and IgG1, but not IgG2a/c, titers are associated with sterile immunity against P. berghei [16], we evaluated sex differences in IgG isotypes. Overall, females had greater anti-CSP IgG1 (Mann-Whitney; P = 0.005, 95% CI [0.0, 700]; Fig. 1F) and IgG2a antibody titers than males (Mann-Whitney; P = 0.0007, 95% CI [400, 2400] ;Fig. 1G). The IgG1:IgG2a ratio, however, was similar between the sexes (Mann-Whitney, P = 0.61; Fig. 1H), suggesting no sex-specific shift in Th2:Th1 skewing. These data suggest that the quality and quantity of antibody following receipt of an irradiated sporozoite vaccine is greater in adult female than male mice.
3.2. Adult female mice have increased numbers of antigen-specific hepatic CD8+ T cells and are better protected following parasite challenge
To evaluate sex differences in irradiated sporozoite vaccine efficacy, 45 days post boost vaccination adult male and female mice were challenged by mosquito bite using 10 P.b.-P.f. infected female mosquitoes. Forty-two hours after challenge, hepatic and splenic CD8+ T cells were isolated and the number of cells producing IFNƴ in response to CSP-specific peptide stimulation was quantified. Following CSP peptide stimulation, the number of hepatic CD8+ T cells producing IFNƴ was greater in adult females than males (Mann-Whitney; P = 0.004, 95% CI [2275, 6459]; Fig. 2A). In contrast, while peptide stimulation increased production of IFNƴ by splenic CD8+ T cells in vaccinated mice, no sex differences were observed (Mann-Whitney, P = 0.99 for peptide stimulation; Fig. 2B).
Fig. 2.
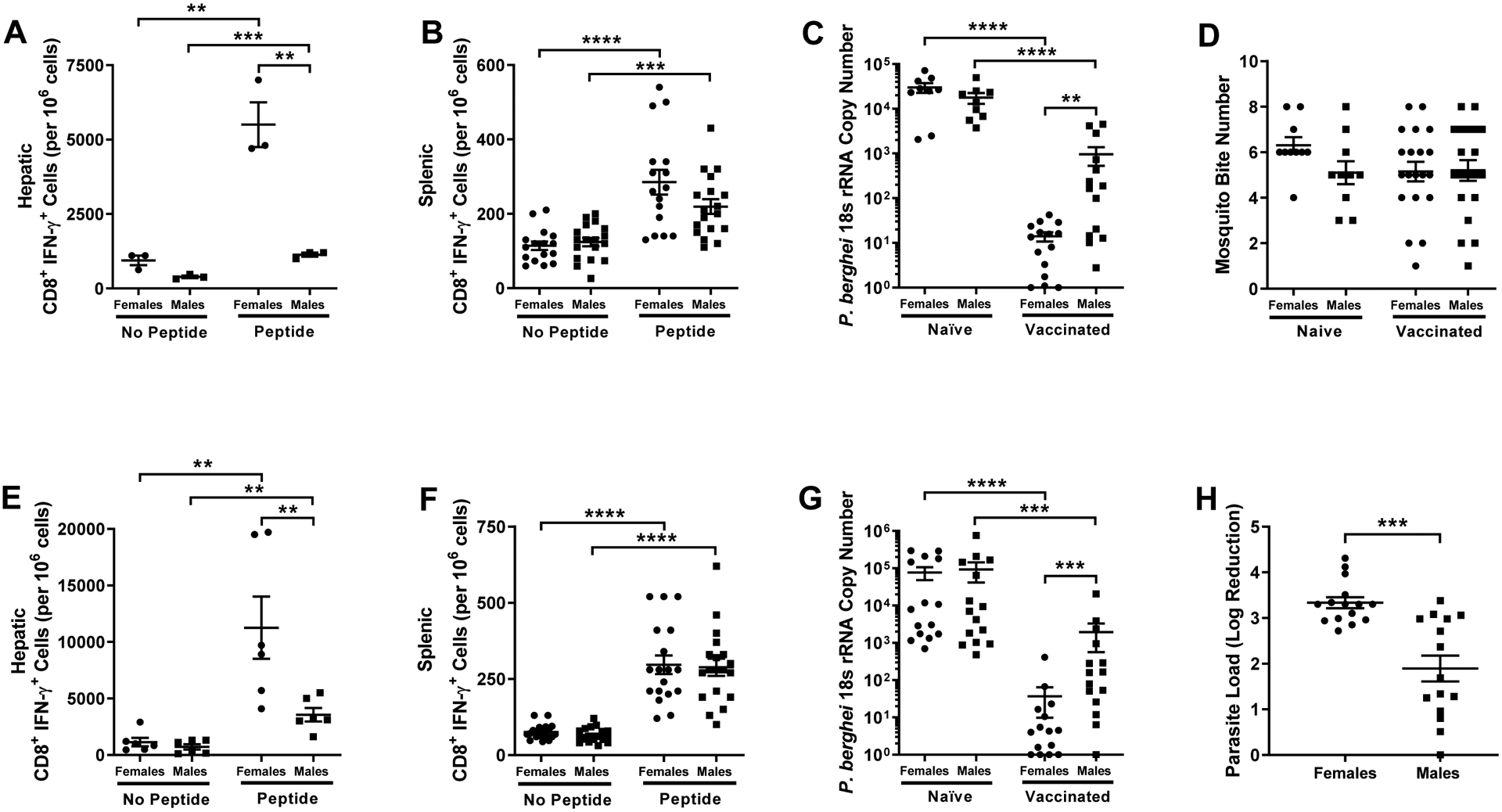
Effects of sex on CD8+ T cell responses and protection from sporozoite challenge. Naïve and irradiated sporozoite vaccinated adult (8–10 weeks) male and female mice were challenged by mosquito bite 45 days post boost vaccination. The numbers of hepatic (n = 3 pools of 3/sex/treatment) (A) and splenic (n = 16–18/sex/treatment) (B) CD8+ T cells producing IFNƴ in response to CSP-specific peptide stimulation were measured 42 hours post challenge. Hepatic P. berghei 18s rRNA copy number (C) was measured (n = 9–15/sex/treatment) in naïve and vaccinated male and female mice. The number of blood fed mosquitos was recorded for each challenge (n = 18–20 mice/sex/treatment) (D). Challenge experiments were repeated with intradermal inoculation, and the numbers of hepatic (n = 6 pools of 3/sex/treatment) (E) and splenic (n = 16–18/sex/treatment) (F) CD8+ T cells producing IFNƴ in response to CSP peptide stimulation were measured 42 hours post challenge. Hepatic P. berghei 18s rRNA copy number was measured (n = 15/sex/treatment) (G) and the log reduction (H) in parasite load relative to naïve controls was determined (n = 15/sex). Data represent means ± the SEM from two independent replications and significant differences are denoted with asterisks (**P < 0.01, *** < 0.001, ****P < 0.0001).
To assess whether sex differences in immune responses to the vaccine resulted in differential vaccine efficacy, we measured hepatic P. berghei 18s rRNA copy number as a surrogate for parasite load 42 hours post challenge. Parasite load was lower in vaccinated mice relative to naïve mice of both sexes; mean parasite load among vaccinated adult females, however, was lower relative to adult males (Kruskal-Wallis; P = 0.003, 95% CI [−688.7, −11.1]; Fig 2C). To determine whether sex differences in parasite load were reflective of differential mosquito bite numbers, and hence exposure, the number of blood fed mosquitoes (i.e. those with visible midgut blood content) was recorded for each mouse. Though the number of challenge mosquito bites varied among individual mice, no sex differences in mosquito bite numbers were observed in either vaccinated or naïve mice (Kruskal-Wallis, P = 0.30; Fig 2D).
Because of the variation in mosquito bite numbers, and presumably challenge dose, challenge experiments were repeated using intradermal inoculation with 3 × 103 P.b.-P.f. sporozoites at 45 days post boost vaccination. Consistent with mosquito bite challenge, vaccinated mice had greater numbers of IFNƴ + CD8+ T cells in the liver and spleen following ex vivo peptide simulation (Mann-Whitney, P < 0.05 in each case; Fig. 2E and Fig. 2F). Following CSP-specific peptide stimulation, numbers of CD8+ T cells producing IFNƴ in the liver, but not the spleen, were greater in vaccinated female than male mice following intradermal challenge (Mann-Whitney; P = 0.009 and 95% CI [1000, 16500] for hepatic CD8+ T cells and P = 0.99 for splenic CD8+ T cells; Fig. 2E and Fig. 2F). Also, consistent with mosquito bite challenge, hepatic P. berghei 18s rRNA copy number was reduced in both sexes following vaccination, with the magnitude of the reduction being greater in adult females than males (Kruskal-Wallis; P = 0.0006, 95% CI [−288.9, −24.1]; Fig 2G). As another way to analyze the change in parasite load following vaccination, we calculated the log reduction in parasite load relative to the average parasite load in naïve mice for each sex and experimental replication. Consistent with the relative parasite load (Fig 2G), as compared with naïve mice, the log reduction in parasite load was greater for vaccinated females than males (Mann-Whitney; P = 0.0002, 95% CI [−0.45, −2.17]; Fig. 2H). These data demonstrate that irradiated sporozoite vaccine efficacy is greater in adult females than in male mice.
3.3. Prior to puberty, vaccine responses and efficacy do not differ between the sex
Sex differences in the immune responses to vaccines are often not evaluated in studies of childhood vaccines [11], but there are some vaccines, including the RTS,S vaccine, that are primarily or exclusively administered prior to puberty [5, 28]. To characterize the impact of sex on irradiated sporozoite vaccine response and efficacy prior to puberty, juvenile mice were twice vaccinated by intraperitoneal injection beginning at PND 11. Forty-two days post boost vaccination, antibody responses were analyzed by measuring anti-CSP IgG titers, AUC, the OD1 titer, and antibody quantity. Although vaccination resulted in detectable antibody responses against CSP, no sex differences in any measure of the antibody response to CSP were detected among mice vaccinated as juveniles (Mann-Whitney, P = 0.37; Fig. 3A and Suppl. Fig. 1). The avidity of anti-CSP IgG also did not differ between males and female vaccinated as juveniles (Mann-Whitney, P = 0.27; Fig. 3B). Finally, the titers of anti-CSP IgG1 and IgG2a were quantified and neither titers of IgG isotypes nor the ratio of IgG1:IgG2a differed between sexes among mice vaccinated as juveniles (Mann-Whitney; P = 0.85, P = 0.57, and P = 0.32 respectively; Fig. 3C–E).
Fig. 3.
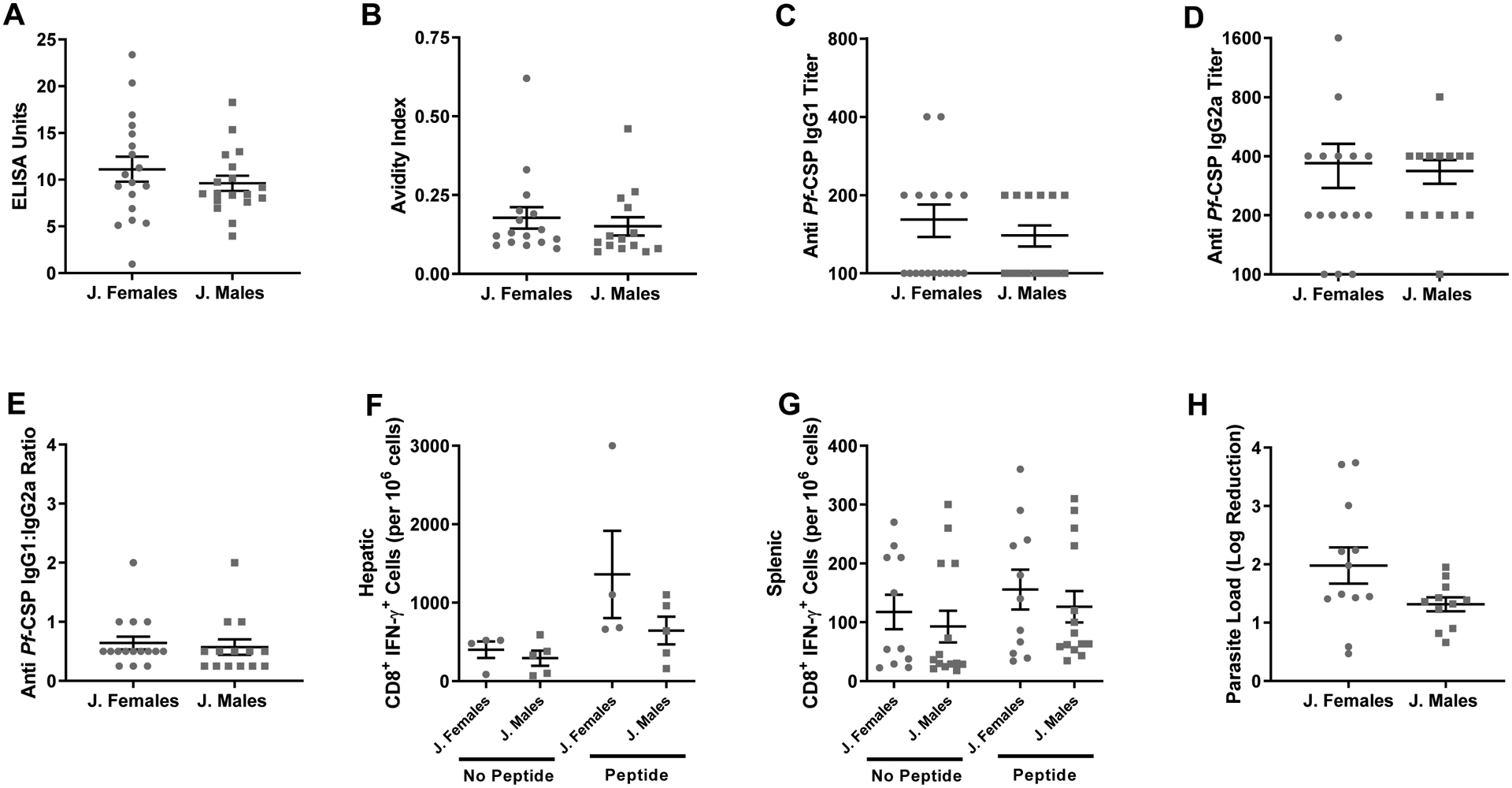
Effects of sex on vaccine-induced immunity and protection in juvenile mice. Pre-pubertal (2 weeks of age) male and female mice were twice vaccinated with irradiated sporozoites. Forty-two days post boost vaccination, plasma was collected and anti-CSP IgG concentrations (A), anti-CSP antibody avidity (B), anti-CSP IgG1 titers (C), anti-CSP IgG2a titers (D), and the ratio of IgG1/IgG2a (E) were measured (n = 15–18/sex). Naïve and vaccinated juvenile mice were challenged as adults by intradermal inoculation 45 days post boost vaccination and the numbers of hepatic (n = 4–5 pools of 3/sex) (F) and splenic (n = 11–14/sex) (G) CD8+ T cells producing IFNƴ in response to CSP peptide stimulation were measured 42 hours post challenge. The log reduction (H) in parasite load relative to naïve controls was determined (n = 9–12/sex). Data represents means ± SEM from two independent replications. No differences by sex were observed in mice vaccinated as juveniles.
As adults, mice that were vaccinated with irradiated sporozoites as juveniles received an intradermal challenge with transgenic P.b.-P.f. parasites. Unlike mice that were both vaccinated and challenged as adults, among mice vaccinated as juveniles and challenged as adults there was no significant increase in the number of hepatic or splenic CD8+ T cells producing IFNƴ in response to CSP specific peptide stimulation, which also did not differ between the sexes (Kruskal-Wallis, P = 0.99 for both; Fig. 3F and Fig. 3G). Although juvenile vaccination was efficacious, there was no sex difference in the log reduction in parasite load (Mann-Whitney; P = 0.06, 95% CI [−1.40, 0.07]; Fig. 3H). These data suggest vaccination prior to puberty does not result in sex differences in vaccine efficacy, suggesting that sex steroid hormones may be involved.
3.4. Removal of ovaries has no effect on vaccine-induced immune responses or protection among adult females
To begin to test whether sex hormones in females contribute to greater vaccine-induced immune responses and protection, a subset of female had their ovaries removed thereby reducing concentrations of sex steroids, including 17β-estradiol (Mann-Whitney; P = 0.0002, 95% CI [−3.6, −14.7]; Fig. 4A). Removal of the ovaries in adult female mice, however, did not s alter either the quality or quantity of anti-CSP IgG at 42 days post boost vaccination as compared with gonad-intact (i.e., sham) female mice (Mann-Whitney; P = 0.61 for antibody quantity, P = 0.61 for avidity, P = 0.54 for IgG1 titers, and P = 0.22 for IgG2a titers; Fig. 4B–E and Suppl. Fig. 2). Following sporozoite challenge, while peptide stimulation increased production of IFNƴ by both splenic and hepatic CD8+ T cells from vaccinated mice, ovariectomy increased CSP-specific splenic, but not hepatic, CD8+ T cell production of IFNƴ following peptide stimulation (Kruskal-Wallis; P = 0.04, 95% CI [8.5, 348.4] for splenic CD8+ T cells and P = 0.99 for hepatic CD8+ T cells; Fig. 4F and Fig. 4G). Finally, the log reduction in hepatic parasite load following intradermal challenge did not differ between intact or gonadectomized female mice (Mann-Whitney, P = 0.82; Fig. 4H). These data suggest that immunity and protection from parasite challenge in female mice may be independent of female gonadal sex hormones.
Fig. 4.
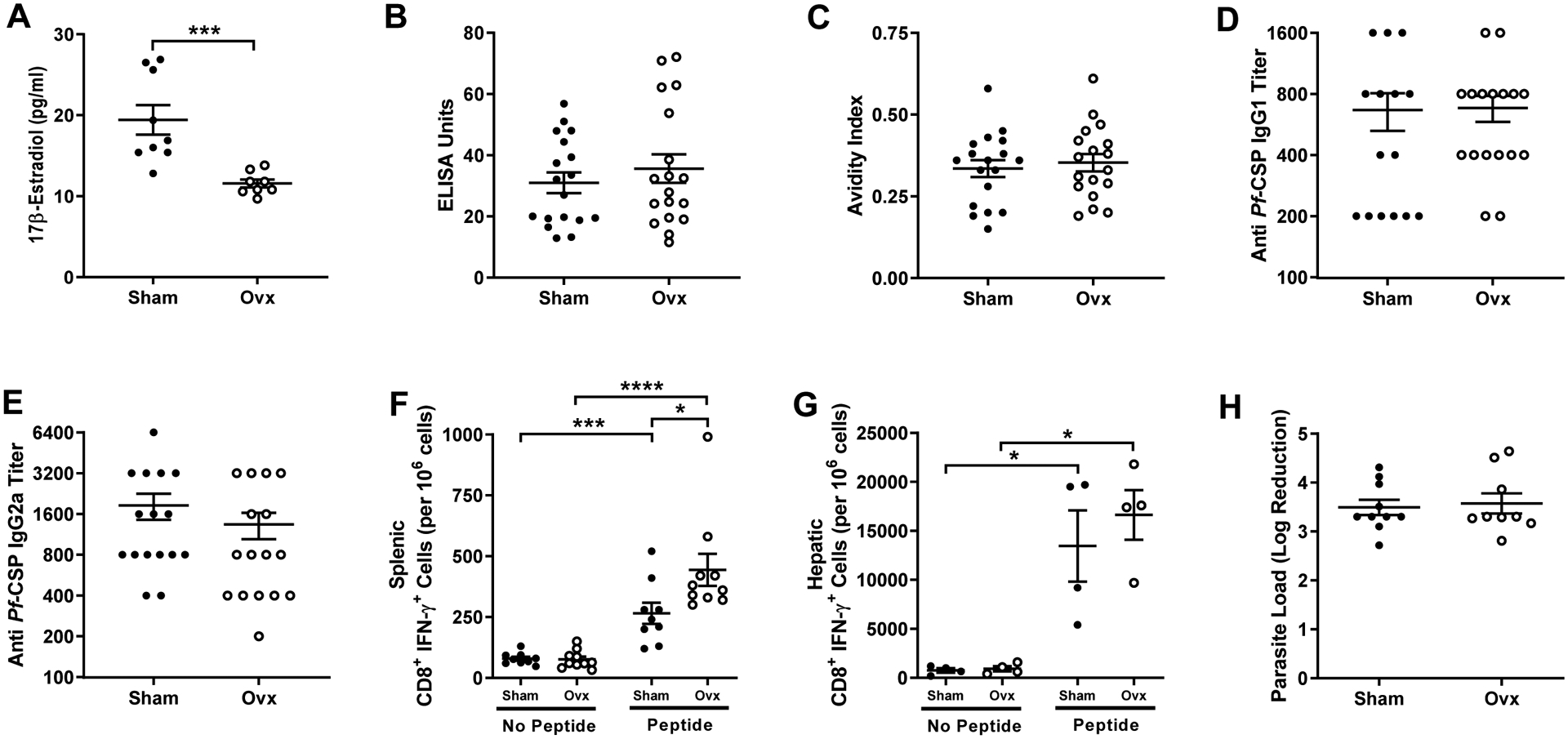
Effects of ovariectomy on irradiated sporozoite vaccination in adult female mice. Adult (8–10 weeks) female mice were assigned to remain intact (i.e., receive a sham surgery) or be ovariectomized (ovx) two weeks prior to being twice vaccinated with irradiated sporozoites. Plasma was collected 42 days post boost vaccination and estrogen (A) (n = 8–9/treatment), anti-CSP IgG antibody concentrations (B), anti-CSP antibody avidity (C), anti-CSP IgG1 titers (D), and anti-CSP IgG2a titers (E) were measured (n = 15–18/ treatment). Mice were challenged by intradermal inoculation and the numbers of hepatic (n = 4 pools of 3/treatment) (F) and splenic (n = 9–10/treatment) (G) CD8+ T cells producing IFNƴ in response to CSP-specific peptide stimulation were quantified. The log reduction in parasite load was measured (n = 9–10/treatment) (H) relative to naïve female mice. Data represent means ± SEM from two independent replications and significant differences between treatment groups are denoted by asterisks (*P < 0.05, *** < 0.001, ****P < 0.0001).
3.5. Testosterone suppresses vaccine-induced immune responses and protection in male mice
To determine whether testosterone in males affected irradiated sporozoite vaccine responses, adult male mice underwent sham surgeries or gonadectomy, with half the gonadectomized males receiving endogenous testosterone, which increased circulating testosterone to within the physiological range of gonad-intact males (Kruskal-Wallis; P = 0.022, 95% CI [−8.44, −0.23] for sham vs gdx and P= 0.0004, 95% CI [4.82, 11.18] for gdx vs gdx + T; Fig. 5A). Following vaccination, depletion of testosterone significantly increased, whereas rescue of testosterone significantly decreased, anti-CSP IgG antibody titers (P = 0.0003, 95% CI [5.77, 22.61] for sham vs gdx and P < 0.0001, 95% CI [−29.31, −10.13] for gdx vs gdx + T) and avidity (P = 0.025, 95% CI [0.00, 0.20] for sham vs gdx and P < 0.0001, 95% CI [−0.30, −0.08] for gdx vs gdx + T) as well as anti-CSP IgG1 antibody titers (Kruskal-Wallis; P = 0.001, 95% CI [300 to 1500] for sham vs gdx and P < 0.0001, 95% CI [−1500, −300] for gdx vs gdx + T; Fig. 5B–D and Suppl. Fig. 3). Rescue of testosterone in gonadectomized males significantly reduced IgG2a antibody titers (Kruskal-Wallis; P = 0.0013, 95% CI [−1005.0, 11.7]; Fig. 5E) and the ratio of IgG1:IgG2a relative to testosterone-depleted males (Kruskal-Wallis; P = 0.0014, 95% CI [−3.0, 0.0]; Fig. 5F).
Fig. 5.
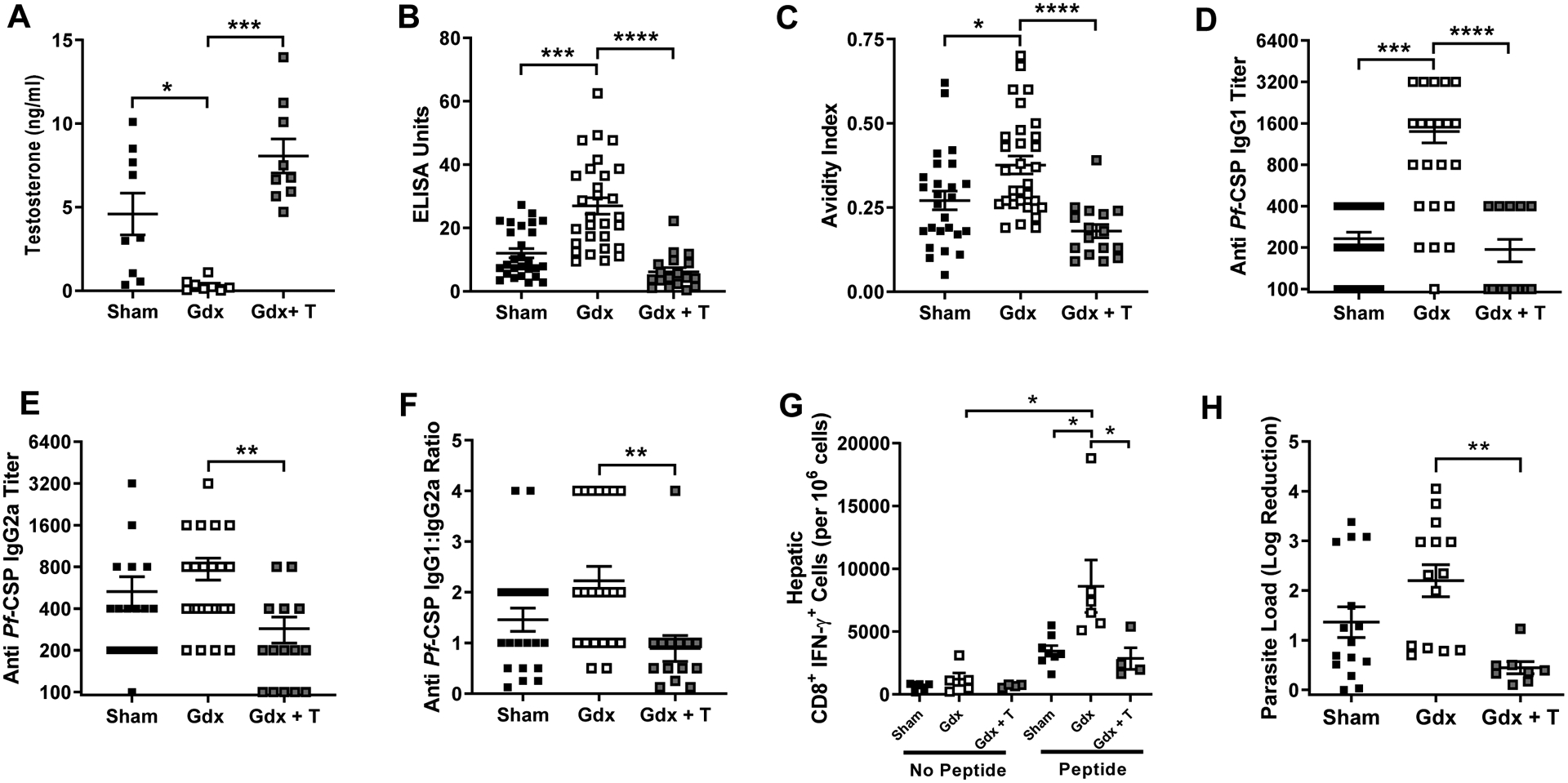
Effects of testosterone on irradiated sporozoite vaccination in adult males. Adult (8–10 weeks) male mice were gonadectomized and implanted with placebo (gdx) or testosterone (gdx + T) capsules or received sham surgeries (sham). Mice were twice vaccinated with irradiated sporozoites and 42 days post boost vaccination, plasma was collected and testosterone concentrations (A) were quantified (n = 8–9/treatment). Anti-CSP IgG antibody concentrations (B), anti-CSP antibody avidity (C), anti-CSP IgG1 titers (D), anti-CSP IgG2a titers (E), and the ratio of IgG1/IgG2a titers were measured (n = 18–29/ treatment group). Mice were challenged by intradermal inoculation and the numbers of hepatic (n = 4–8 pools of 3/treatment) (G) CD8+ T cells producing IFNƴ in response to CSP-specific peptide stimulation were quantified. The log reduction in parasite load (n = 8–15/treatment) (H) relative to naïve male mice was measured 42 hours post challenge. Data represents means ± SEM from two independent replications and significant differences across treatment groups are denoted by asterisks (*P < 0.05, **P < 0.01, *** < 0.001, ****P < 0.0001).
Following intradermal parasite challenge, the depletion of testosterone increased the number of hepatic CD8+ T cells producing IFNƴ in response to CSP-specific peptide stimulation, while the rescue of testosterone levels reduced hepatic CD8+ T cell production of IFNƴ to levels comparable with gonad-intact male mice (Kruskal-Wallis; P =0.021, 95% CI [1600, 15510] for sham vs gdx and P = 0.012, 95% CI [−17160, 300] for gdx vs gdx + T; Fig. 5G). Depletion of testosterone also increased the log reduction in parasite load, whereas the rescue of testosterone reduced the log reduction in parasite load as compared with naïve mice from the same hormone treatment group (Kruskal-Wallis; P = 0.0011, 95% CI [−2.98, −0.40] for gdx vs gdx + T; Fig. 5H). These data suggest that the decreased efficacy of irradiated sporozoite vaccination in adult males may be mediated, in part, by testosterone.
4. Discussion
Sex differences in vaccine-induced immune responses are documented primarily for vaccines that protect against viruses and bacteria [11, 29], with less attention paid to parasite vaccines, including those against malaria. In the present study, following irradiated sporozoite vaccination, adult female mice were better protected against sporozoite challenge than age-matched males, with these differences in protection being associated with greater malaria-specific antibody and CD8+ T cell responses in females compared with males. These findings are consistent with both human and murine studies showing greater immunogenicity and efficacy of either erythrocytic stage malaria vaccination [20, 30] or Plasmodium infection [19] in adult females relative to males. Sex differences in sporozoite vaccine-induced immunity and protection were not observed among mice that were vaccinated prior to puberty. The surge of testosterone during puberty in males as opposed to hormonal changes following puberty in females appeared to be associated with sex differences in vaccine-induced immunity and protection in adults. Manipulation of sex steroid hormones (i.e., estradiol and progesterone) via ovariectomy in females had no impact on vaccine-induced immunity and protection, whereas manipulation of testosterone in males dramatically affected the outcome of vaccination.
The observation that sex influences the immunogenicity and efficacy of irradiated sporozoite vaccination in adults, but not in mice vaccinated as juveniles, led us to hypothesize that changes in sex hormone concentrations associated with puberty may be involved. At puberty, the ovaries of females begin synthesizing and releasing elevated concentrations of estradiol and progesterone, whereas the testes of males begin synthesizing greater concentrations of testosterone. These sex steroid hormones affect adaptive immune responses both directly by binding to sex steroid receptors on B and T cells as well as indirectly by altering the activity of innate immune cells [32, 33].
In females, estradiol, in particular, can enhance antibody responses to vaccines and infections [11, 19, 34–36]. In murine models of malaria blood stage infection, treatment of female mice with estradiol alone or in combination with progesterone increased Plasmodium-specific IgG1 antibody production as well as IFNƴ production by splenocytes relative to sex-hormone depleted females [35]. In the current study, depletion of sex steroids in females did not alter either the adaptive immune response to vaccination or protection following parasite challenge. Whether these discordant effects of female sex hormone depletion represent differences in the context of vaccination versus infection, the route of inoculation, or differences in the pathways of immune activation warrant future study. Moreover, there may be other sex-specific factors (e.g., Y chromosomal factors in males or increased X-linked gene expression in females) that could be contributing to the more robust immune response in adult females relative to adult males.
The immunosuppressive properties of testosterone are well characterized, but few studies have evaluated the impacts of testosterone on the immune response to vaccination. In the current study, depletion of testosterone in vaccinated males increased, whereas the testosterone treatment of castrated, vaccinated males decreased, parasite load following sporozoite challenge. These data are consistent with previous murine studies demonstrating that testosterone suppresses resistance to blood stage malaria infection and reduces protection against challenge following erythrocytic stage vaccination [20, 40]. Depletion of testosterone, however, still did not elevate antibody titers to the level seen in gonad-intact females, suggesting that other sex specific factors in addition to sex hormones are involved.
Anti-CSP IgG1 antibodies, which are associated with a Th2 cellular responses and antibody avidity, play an important role in sterile immunity against sporozoite challenge [16, 43]. In the current study, depletion of testosterone increased IgG1, but not Th1 associated IgG2a, titers relative to testosterone-replete males, resulting in a skewing of the anti-sporozoite IgG1/IgG2a ratio. Whether testosterone induced changes in CD4+ T helper cell polarization was not evaluated but should be considered in future studies as these are a likely cell population impacting both antibody and CD8+ T cell activity following vaccination and challenge.
Most pre-clinical malaria vaccine studies use exclusively female mice or do not consider the sex of the animals used [15]. In this study, we demonstrate that testosterone in males is a significant factor mediating sex differences in the immunogenicity and efficacy of a preclinical, pre-erythrocytic malaria vaccine design. Greater consideration of biological sex and sex-specific factors are needed in the experimental design and analysis of preclinical animal studies as well as clinical trials of malaria vaccines.
Supplementary Material
Supplemental Fig. 1. Effects of sex on vaccine-induced antibody responses in juvenile mice. Juvenile mice were twice vaccinated with irradiated sporozoites beginning at postnatal day 11. Forty-two days post boost vaccination, plasma was collected and anti-CSP IgG titers (A), areas under the curve (AUC) (B), and the anti-CSP IgG titers equal to an optical density-1 were measured (n = 16–18/sex). Data represents means ± SEM from two independent replications. No differences by sex were observed in juvenile mice.
Supplemental Fig. 2. Effects of ovariectomy on vaccine-induced antibody responses in adult female mice. Adult (8–10 weeks) female mice were ovariectomized (ovx) or received sham surgeries (sham) and mice were twice vaccinated with irradiated sporozoites. Plasma was collected 42 days post boost vaccination and anti-CSP IgG titers (A), areas under the curve (AUC) (B), and the anti-CSP IgG titers equal to an optical density-1 were measured (n = 16–19/treatment). Data represents means ± SEM from two independent replications. No differences by treatment group were observed.
Supplemental Fig. 3. Effects of testosterone on vaccine-induced antibody responses in adult males. Adult (8–10 weeks) male mice were gonadectomized and implanted with placebo (gdx) or testosterone (gdx + T) capsules or received sham surgeries (sham). Mice were twice vaccinated with irradiated sporozoites and 42 days post boost vaccination, plasma was collected and anti-CSP IgG titers (A), areas under the curve (AUC) (B), and the anti-CSP IgG titers equal to an optical density-1 were measured (n = 17–28/treatment). Data represents means ± SEM from two independent replications and significant differences across treatment groups are denoted by asterisks (*P < 0.05, *** < 0.001, ****P < 0.0001).
Acknowledgements:
The authors would like to thank the Pekosz, Zavala, and Davis laboratories for discussions about these data, Megan Vermillion for technical assistance with surgeries, and the Johns Hopkins Malaria Research Institute Parasite and Mosquito Cores as well as the Johns Hopkins BD Immune Function Core.
Funding: This work was supported by a pilot award from the Johns Hopkins Malaria Research Institute (SK).
Footnotes
Data availability statement: All available data are contained in the manuscript.
Competing interests: none declared
References
- [1].World malaria report 2017. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- [2].Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. The Lancet. 2016;388:3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].WHO: Global Technical Strategy for Malaria 2016–2030. 2015. [DOI] [PMC free article] [PubMed]
- [4].Plebanski M, Flanagan KL. The Economics of Malaria Vaccine Development. Trends Parasitol. 2017;33:154–6. [DOI] [PubMed] [Google Scholar]
- [5].Malaria vaccine: WHO position paper-January 2016. Wkly Epidemiol Rec. 2016;91:33–51. [PubMed] [Google Scholar]
- [6].Rts SCTP. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].GSK. Efficacy of GSK Biologicals’ candidate malaria vaccine (257049) against malaria disease caused by P. falciparum infection in infants and chlidren in Africa 17 February 2016. ed2016. [Google Scholar]
- [8].Klein SL, Shann F, Moss WJ, Benn CS, Aaby P. RTS,S Malaria Vaccine and Increased Mortality in Girls. mBio. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hoffman SL, Vekemans J, Richie TL, Duffy PE. The march toward malaria vaccines. Vaccine. 2015;33 Suppl 4:D13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sugimoto CR, Ahn Y-Y, Smith E, Macaluso B, Larivière V. Factors affecting sex-related reporting in medical research: a cross-disciplinary bibliometric analysis. The Lancet. 2019;393:550–9. [DOI] [PubMed] [Google Scholar]
- [11].Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and Gender Differences in the Outcomes of Vaccination over the Life Course. Annu Rev Cell Dev Biol. 2017;33:577–99. [DOI] [PubMed] [Google Scholar]
- [12].Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. The Lancet Infectious diseases. 2010;10:338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Voysey M, Barker CI, Snape MD, Kelly DF, Truck J, Pollard AJ. Sex-dependent immune responses to infant vaccination: an individual participant data meta-analysis of antibody and memory B cells. Vaccine. 2016;34:1657–64. [DOI] [PubMed] [Google Scholar]
- [14].Wykes MN, Good MF. What have we learnt from mouse models for the study of malaria? Eur J Immunol. 2009;39:2004–7. [DOI] [PubMed] [Google Scholar]
- [15].Potluri T, Engle K, Fink AL, Vom Steeg LG, Klein SL. Sex Reporting in Preclinical Microbiological and Immunological Research. MBio. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Reed RC, Louis-Wileman V, Wells RL, Verheul AF, Hunter RL, Lal AA. Re-investigation of the circumsporozoite protein-based induction of sterile immunity against Plasmodium berghei infection. Vaccine. 1996;14:828–36. [DOI] [PubMed] [Google Scholar]
- [17].Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330:664–6. [DOI] [PubMed] [Google Scholar]
- [18].Purcell LA, Wong KA, Yanow SK, Lee M, Spithill TW, Rodriguez A. Chemically attenuated Plasmodium sporozoites induce specific immune responses, sterile immunity and cross-protection against heterologous challenge. Vaccine. 2008;26:4880–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cernetich A, Garver LS, Jedlicka AE, Klein PW, Kumar N, Scott AL, et al. Involvement of gonadal steroids and gamma interferon in sex differences in response to blood-stage malaria infection. Infect Immun. 2006;74:3190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wunderlich F, Maurin W, Benten WP, Schmitt-Wrede HP. Testosterone impairs efficacy of protective vaccination against P. chabaudi malaria. Vaccine. 1993;11:1097–9. [DOI] [PubMed] [Google Scholar]
- [21].Espinosa DA, Christensen D, Munoz C, Singh S, Locke E, Andersen P, et al. Robust antibody and CD8(+) T-cell responses induced by P. falciparum CSP adsorbed to cationic liposomal adjuvant CAF09 confer sterilizing immunity against experimental rodent malaria infection. NPJ Vaccines. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wirtz RA, Zavala F, Charoenvit Y, Campbell GH, Schneider I, Esser KM, et al. Comparitive testing of monoclonal antibodies against plasmodium falciparum sporozoites for ELISA development. Bulletin of the World Health Organization. 1987;65:39–45. [PMC free article] [PubMed] [Google Scholar]
- [23].Bruna-Romero O, Hafalla JC, Gonzalez-Aseguinolaza G, Sano G, Tsuji M, Zavala F. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int J Parasitol. 2001;31:1499–502. [DOI] [PubMed] [Google Scholar]
- [24].Jaulin C, Romero P, Luescher IF, Casanova JL, Prochnickachalufour A, Langladedemoyen P, et al. Most Residues on the Floor of the Antigen-Binding Site of the Class-I Mhc Molecule H-2kd Influence Peptide Presentation. International immunology. 1992;4:945–53. [DOI] [PubMed] [Google Scholar]
- [25].Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17beta-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog. 2011;7:e1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vom Steeg LG, Vermillion MS, Hall OJ, Alam O, McFarland R, Chen H, et al. Age and testosterone mediate influenza pathogenesis in male mice. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2016;311:L1234–L44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective Immunity produced by the Injection of X-irradiated Sporozoites of Plasmodium berghei. Nature. 1967;216:160. [DOI] [PubMed] [Google Scholar]
- [28].Klein SL, Flanagan KL. Sex differences in immune responses. Nature Reviews Immunology. 2016;16:626. [DOI] [PubMed] [Google Scholar]
- [29].Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. 2015;109:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Migasena S, Heppner DG, Kyle DE, Chongsuphajaisiddhi T, Gordon DM, Suntharasamai P, et al. SPf66 malaria vaccine is safe and immunogenic in malaria naive adults in Thailand. Acta tropica. 1997;67:215–27. [DOI] [PubMed] [Google Scholar]
- [31].Parmar R, Patel H, Yadav N, Patidar M, Tyagi RK, Dalai SK. Route of administration of attenuated sporozoites is instrumental in rendering immunity against Plasmodia infection. Vaccine. 2016;34:3229–34. [DOI] [PubMed] [Google Scholar]
- [32].Wunderlich F, Benten WP, Lieberherr M, Guo Z, Stamm O, Wrehlke C, et al. Testosterone signaling in T cells and macrophages. Steroids. 2002;67:535–8. [DOI] [PubMed] [Google Scholar]
- [33].Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clinical reviews in allergy & immunology. 2011;40:66–73. [DOI] [PubMed] [Google Scholar]
- [34].Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–38. [DOI] [PubMed] [Google Scholar]
- [35].Klein PW, Easterbrook JD, Lalime EN, Klein SL. Estrogen and progesterone affect responses to malaria infection in female C57BL/6 mice. Gend Med. 2008;5:423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nguyen DC, Masseoud F, Lu X, Scinicariello F, Sambhara S, Attanasio R. 17beta-Estradiol restores antibody responses to an influenza vaccine in a postmenopausal mouse model. Vaccine. 2011;29:2515–8. [DOI] [PubMed] [Google Scholar]
- [37].Hall OJ, Nachbagauer R, Vermillion MS, Fink AL, Phuong V, Krammer F, et al. Progesterone-Based Contraceptives Reduce Adaptive Immune Responses and Protection against Sequential Influenza A Virus Infections. J Virol. 2017;91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].LÜ FX, Abel K, Ma Z, Rourke T, Lu D, Torten J, et al. The strength of B cell immunity in female rhesus macaques is controlled by CD8+T cells under the influence of ovarian steroid hormones. Clinical & Experimental Immunology. 2002;128:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiebaut R, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A. 2014;111:869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lopes LN, Folha Santos FA, Marques Oliveira LC, Ferreira Araujo MT, Sequeira CG, Libonati RM, et al. An analysis of the influence of sex hormones on Balb/c mice infected with Plasmodium berghei. Microb Pathog. 2016;90:7–12. [DOI] [PubMed] [Google Scholar]
- [41].Scholfield L y interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature.330. [DOI] [PubMed] [Google Scholar]
- [42].Romero P, Maryanski JL, Corradin G, Nussenzweig RS, Nussenzweig V, Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989;341:323–6. [DOI] [PubMed] [Google Scholar]
- [43].Keitany GJ, Sack B, Smithers H, Chen L, Jang IK, Sebastian L, et al. Immunization of mice with live-attenuated late liver stage-arresting Plasmodium yoelii parasites generates protective antibody responses to preerythrocytic stages of malaria. Infect Immun. 2014;82:5143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Effects of sex on vaccine-induced antibody responses in juvenile mice. Juvenile mice were twice vaccinated with irradiated sporozoites beginning at postnatal day 11. Forty-two days post boost vaccination, plasma was collected and anti-CSP IgG titers (A), areas under the curve (AUC) (B), and the anti-CSP IgG titers equal to an optical density-1 were measured (n = 16–18/sex). Data represents means ± SEM from two independent replications. No differences by sex were observed in juvenile mice.
Supplemental Fig. 2. Effects of ovariectomy on vaccine-induced antibody responses in adult female mice. Adult (8–10 weeks) female mice were ovariectomized (ovx) or received sham surgeries (sham) and mice were twice vaccinated with irradiated sporozoites. Plasma was collected 42 days post boost vaccination and anti-CSP IgG titers (A), areas under the curve (AUC) (B), and the anti-CSP IgG titers equal to an optical density-1 were measured (n = 16–19/treatment). Data represents means ± SEM from two independent replications. No differences by treatment group were observed.
Supplemental Fig. 3. Effects of testosterone on vaccine-induced antibody responses in adult males. Adult (8–10 weeks) male mice were gonadectomized and implanted with placebo (gdx) or testosterone (gdx + T) capsules or received sham surgeries (sham). Mice were twice vaccinated with irradiated sporozoites and 42 days post boost vaccination, plasma was collected and anti-CSP IgG titers (A), areas under the curve (AUC) (B), and the anti-CSP IgG titers equal to an optical density-1 were measured (n = 17–28/treatment). Data represents means ± SEM from two independent replications and significant differences across treatment groups are denoted by asterisks (*P < 0.05, *** < 0.001, ****P < 0.0001).


