Abstract
Hypertension is associated with body mass index (BMI) and cardiovascular and cerebrovascular diseases (CCDs). Whether hypertension modifies the relationship between BMI and CCDs is still unclear. We examined the association between BMI and CCDs and tested whether effect measure modification was present by hypertension. We identified a population-based sample of 3,942 participants in Shuncheng, Fushun, Liaoning, China. Hypertension was defined as any past use of antihypertensive medication or having a measured systolic/diastolic blood pressure ≥130/80 mm Hg. BMI was calculated from measured body weight and body height. Data on diagnosed CCDs were self-reported and validated in the medical records. We used logistic regression models to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for associations between BMI and CCDs. Higher BMI was associated with increased odds of having CCDs (OR = 1.19, 95% CI: 1.07–1.31). This association was significantly modified by hypertension (P for interaction <0.001), with positive associations observed among hypertensive individuals (OR = 1.28, 95% CI: 1.14–1.42). Age, sex, and diabetic status did not modify the relationship between BMI and CCDs (all P for interaction >0.10). Although higher BMI was associated with increased odds of CCDs, the relationship was mainly limited to hypertensive patients.
Keywords: cardiovascular and cerebrovascular diseases, body mass index, hypertension
1. Introduction
Cardiovascular and cerebrovascular diseases (CCDs) are major public health problems worldwide [1,2,3,4], with 121.5 million individuals suffering from cardiovascular disease and ∼10.3 million new stroke cases during 2016 [5]. The incidence of CCDs is predicted to triple over the next few decades [6]. Stroke and ischemic heart disease were the top two causes for years of life lost globally in 2013 [7]. The total cost for hospitalization of acute myocardial infarction and stroke was 905.3 billion RMB (approximately US $137.4 billion) in 2016 [5]. In 2016, the number of deaths related to CCDs reached 17.9 million, accounting for 31% of all deaths worldwide [8].
It is well documented that higher body mass index (BMI) is associated with higher risk of CCDs [9]. Whether the relationship is modified by hypertensive status is unknown. For individuals with BMI ≥25 kg/m2, each 5 kg/m2 increase in BMI was associated with 40% higher risk of CCDs [9]. In addition, BMI is positively correlated with blood pressure [10]. For every 3 kg/m2 increase in BMI, the risk of hypertension increases by 50% in men and 57% in women [11]. Individuals with a BMI ≥ 24 kg/m2 have 3–4 times the risk of hypertension compared to individuals with a normal BMI in the general population [12]. Meanwhile, a prospective study in a Chinese population reported that for every 10 and 5 mm Hg increase in systolic blood pressure (SBP) and diastolic blood pressure (DBP), respectively, the risk of CCDs increased by 49 and 46% [13].
Hypertension may modify the association between BMI and CCDs, but there are few studies that have examined this [14,15]. Therefore, the present study sought to (1) examine the relationship between BMI and CCDs and (2) determine whether this relationship is modified by hypertensive status.
2. Materials and methods
2.1. Study population
Using a random sampling method, this cross-sectional study enrolled 4,553 population-based residents in Shuncheng, Fushun, Liaoning, China from 2013 to 2016. Shuncheng is a district located in northern Fushun, a northeastern city in China approximately 742 km away from Beijing. The area of Shuncheng is 348 km2, with a population of ∼0.4 million. This study randomly selected eight regions in Shuncheng and distributed 600 questionnaires in each region, totaling 4,800 questionnaires. Written informed consent was obtained from all participants before study enrollment. Among 4,800 returned questionnaires, 247 were excluded from the study because they were incomplete or failed to meet our quality control standards following an assessment. Although the response rate was 100%, we were only able to use 94.9% of the returned questionnaires. All data were fully anonymized before analyses. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Centers for Disease Control (CDC) of Fushun.
For the present study, we included all participants aged 18 years or older. We excluded individuals with missing or invalid data on body weight, height, status of CCDs, age, and sex.
2.2. Study measures
Our primary outcome was CCDs, which included cardiovascular diseases (e.g., coronary heart disease, angina, etc.) and cerebrovascular diseases (e.g., cerebral hemorrhage, cerebral thrombosis, etc.). CCDs were further categorized into cardiovascular diseases and cerebrovascular diseases. Information for CCDs was obtained via self-reported questionnaire inquiring whether they were ever diagnosed by specialists from government-funded hospitals. We also validated all reported diagnoses of CCDs based on their medical records.
The covariates for this study included age, body weight, body height, sex, hypertensive status, smoking, alcohol use, and diabetic status. Body weight, without shoes, was measured (to the nearest 0.1 kg) on an electronic scale. Body height, without shoes and in light clothing, was measured to the nearest 0.1 cm by a wall-mounted stadiometer. BMI was calculated using: body weight (kg) divided by body height squared (m2). The subjects were classified into the following categories according to their BMI: non-obese (<28 kg/m2) and obese (≥28 kg/m2); this classification was based on the 2002 recommendations of the CDC, China [16,17]. Each participant had his/her blood pressure measured after a period of rest of 5 min. SBP and DBP were measured with an automated sphygmomanometer thrice. Investigators asked each participant whether he/she had taken any antihypertensive medications. Hypertension was defined as any past use of antihypertensive medication or a having a measured SBP/DBP ≥130/80 mm Hg based on the guidelines released by the American College of Cardiology/American Heart Association (ACC/AHA) [18]. For our sensitivity analysis, we also defined hypertension as past antihypertensive medication use or SBP/DBP ≥140/90 mm Hg [19]. Fasting blood glucose was measured using a portable blood glucose meter. Diabetic status was defined as having a fasting blood glucose >7.1 mmol/L (http://www.diabetes.org/diabetes-basics/diagnosis/). A structured questionnaire was used to obtain sociodemographics as well as lifestyle and behavioral characteristics. Smoking was defined as current tobacco use. Individuals were classified as alcohol users if he/she reported a history of alcohol intake within the past 12 months.
2.3. Statistical analysis
In the descriptive analysis, continuous variables with a normal distribution are shown as means and standard deviations (SDs) and categorical variables were expressed as frequencies and percentages. The baseline characteristics of participants with and without CCDs were compared using independent t-test and chi-square test.
We used logistic regression models to estimate covariate-adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for BMI (per SD increase) and CCDs. This model adjusted for age (continuous), sex (male/female), hypertensive status (yes/no), smoking (yes/no), alcohol use (yes/no), and diabetic status (yes/no). These covariates were selected a priori based on a literature review of the relationship between BMI and CCDs [5,8,20,21]. We further tested whether effect measure modification was present between BMI (per SD increase) and CCDs by the following variables: (1) age (≤50, >50), (2) sex (male/female), (3) diabetic status (yes/no), and (4) hypertension (yes/no). To do this, we built interaction terms between BMI and each variable in the logistic regression models. Interaction was considered to be present if the P-value was <0.05. All statistical analyses were performed using SPSS software (version: 24.0; SPSS, Chicago, IL, USA).
3. Results
Of the 4,553 individuals recruited, 3,942 individuals met our inclusion and exclusion criteria and were included in the present study (Figure 1). Average age of our study population was 48.9 years (SD = 15.9 years). There were 385 (9.8%) individuals with CCDs (Supplemental Table S1). Individuals with CCDs had significantly higher BMI, were older, more likely to be females, hypertensive (based on both hypertensive cut-points), and diabetic individuals (Table 1). The prevalence of hypertension based on a blood pressure of ≥130/80 and ≥140/90 mm Hg was 64.5 and 23.7%, respectively, in the overall study population. Smoking, alcohol use, and diabetes were observed in 24.0, 26.6, and 4.4% of the 3,942 study participants, respectively.
Figure 1.
Flowchart for subject inclusion and exclusion.
Table 1.
Descriptive characteristics by status of cardiovascular and cerebrovascular diseases (CCDs)
| Variable | With CCDs | Without CCDs | P-value |
|---|---|---|---|
| N (%) | N (%) | ||
| (n = 385) | (n = 3,557) | ||
| Age (years) | 61.5 (11.5) | 47.5 (15.7) | <0.001 |
| BMI (kg/m2) | 24.7 (4.1) | 23.5 (3.5) | <0.001 |
| Male (n, %) | 154 (40.0) | 1,801 (50.6) | <0.001 |
| Hypertension (≥130/80 mm Hg) (n, %) | 310 (80.5) | 2,234 (62.8) | <0.001 |
| Hypertension (≥140/90 mm Hg) (n, %) | 197 (51.2) | 737 (20.7) | <0.001 |
| Smoking (n, %) | 89 (23.1) | 857 (24.1) | 0.670 |
| Alcohol use (n, %) | 81 (21.0) | 968 (24.6) | 0.009 |
| Diabetic status (n, %) | 39 (10.1) | 133 (3.7) | <0.001 |
Abbreviations: CCDs, cardiovascular and cerebrovascular diseases; BMI, body mass index. Values are presented as the mean (standard deviation) or frequency (percentage). Percentages have been rounded.
After adjusting for covariates, a SD increase in BMI was associated with greater odds of having hypertension (≥130/80 mm Hg) (OR = 1.50, 95% CI: 1.38–1.64, Figure 2) and CCDs (OR = 1.19, 95% CI: 1.07–1.31). Hypertension (≥130/80 mm Hg) was associated with increased odds of CCDs (OR = 1.46, 95% CI: 1.10–1.93). Similar associations were noted when we further explored the relationship between BMI, hypertension (≥130/80 mm Hg), and cardiovascular diseases (Supplemental Figure S1) and cerebrovascular diseases (Supplemental Figure S2). Specifically, a SD increase in BMI was associated with increased odds of cardiovascular diseases (OR = 1.20, 95% CI: 1.07–1.34) but not with cerebrovascular diseases (OR = 1.03, 95% CI: 0.79–1.35) (Supplemental Table S2). Among individuals with BMI ≥28 kg/m2 and hypertension (≥130/80 mm Hg), the point estimate suggests a potential stronger relationship with CCDs than among individuals with a BMI <28 kg/m2 (Supplemental Table S3).
Figure 2.
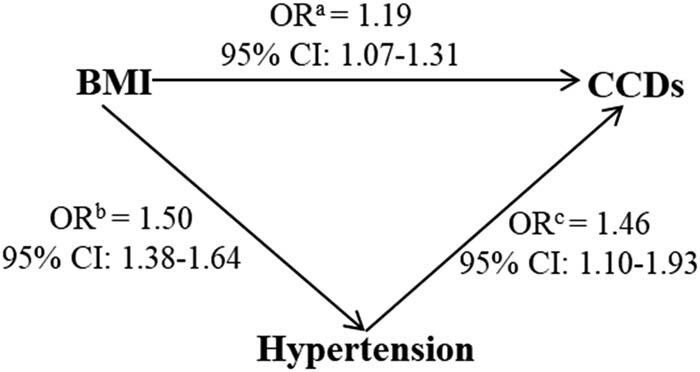
Associations between BMI per 1-SD increase, hypertension (≥130/80 mm Hg), and CCDsa. Abbreviations: CCDs, cardiovascular and cerebrovascular diseases; BMI, body mass index. The values are presented as the odds ratios (ORs) and 95% confidence intervals (95% CIs); aadjusted for age, sex, hypertensive status, alcohol use, smoking, and diabetic status; badjusted for age, sex, alcohol use, smoking, and diabetic status; cadjusted for BMI, age, sex, alcohol use, smoking, and diabetic status. The statistical analysis was performed with multivariate logistic regression analysis. The significance threshold was P <0.05.
Significant effect measure modification by hypertension (≥130/80 mm Hg) was noted in the relationship between BMI and CCDs (P for interaction <0.001) (Table 2). The significant positive association between BMI and CCDs was only observed among individuals with hypertension (≥130/80 mm Hg) (OR = 1.28, 95% CI: 1.14–1.42) but not among non-hypertensive individuals (OR = 0.73, 95% CI: 0.55–0.99). Furthermore, findings indicate a significant inverse relationship between BMI and CCDs among non-hypertensive individuals. However, when hypertensive status was diagnosed as blood pressure ≥140/90 mm Hg, the interaction term between BMI and hypertension failed to reach statistical significance (P for interaction = 0.575). We did not observe interaction by age, sex, or diabetic status in the relationship between BMI and CCDs (all P for interaction >0.10).
Table 2.
Adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs) for associations between BMI (per 1-SD increase) and CCDs by age, sex, diabetic status, and hypertensive status
| Variable | Subgroup | n | OR | 95% CI | P for interaction |
|---|---|---|---|---|---|
| Age (years) | ≤50 | 2,056 | 1.06 | 0.82–1.37 | 0.791 |
| >50 | 1,886 | 1.18 | 1.05–1.33 | ||
| Sex | Male | 1,955 | 1.10 | 0.94–1.29 | 0.200 |
| Female | 1,987 | 1.26 | 1.09–1.46 | ||
| Diabetic status | Yes | 172 | 1.81 | 1.20–2.72 | 0.237 |
| No | 3,770 | 1.10 | 0.99–1.23 | ||
| Hypertension (≥130/80 mm Hg) | Yes | 2,544 | 1.28 | 1.14–1.42 | <0.001 |
| No | 1,398 | 0.73 | 0.55–0.99 | ||
| Hypertension (≥140/90 mm Hg) | Yes | 934 | 1.17 | 1.01–1.34 | 0.575 |
| No | 3,008 | 1.08 | 0.92–1.28 |
Abbreviations: CCDs, cardiovascular and cerebrovascular diseases; BMI, body mass index. The values are presented as the odds ratios (ORs) and 95% confidence intervals (95% CIs); ORs were obtained after adjusting for age, sex, hypertensive status, alcohol use, smoking, and diabetic status.
4. Discussion
In this cross-sectional study, we found a significant positive relationship between BMI and CCDs. Interestingly, the positive relationship between BMI and CCDs was only found among hypertensive individuals (blood pressure ≥130/80 mm Hg). When hypertension was defined using ≥140/90 mm Hg, no interaction was noted for hypertensive status in the relationship between BMI and CCDs. These results suggested that early intervention and management of hypertensive individuals (blood pressure ≥130/80 mm Hg) with higher BMIs may be important to prevent CCD outcomes.
We found a significant association between BMI with cardiovascular disease but not with cerebrovascular disease. This is also in line with previous studies, in which BMI was more strongly associated with cardiovascular diseases than cerebrovascular diseases [22,23,24]. Huxley et al. and Barry et al. found that obesity was a risk factor for cardiovascular disease [22,24], whereas Sun et al. reported that the risk of cerebrovascular disease was not significantly different between overweight, obese, and normal-weight individuals [23].
In our study, we found that hypertension was more strongly associated with CCDs among obese individuals compared to non-obese people. These findings are consistent with previous studies [25,26,27]. As the baseline BMI level increases, individuals with hypertension have 2–3 times higher risk of CCDs than those with normal blood pressure [25]. It is likely that hypertension is an earlier predictor for CCDs among obese people.
We did not find significant interaction by age, sex, or diabetic in the association between BMI and CCDs. This is consistent with many previous studies, in which age, sex, and diabetes status were independent of BMI for assessing risk of CCDs [28,29,30].
We only found significant interaction by hypertension in the relationship between BMI and CCDs when hypertension was defined as having a blood pressure ≥130/80 mm Hg, but not ≥140/90 mm Hg. The underlying reasons for this finding are unclear. However, there is in vitro evidence suggesting that there are interrelated mechanisms between high blood pressure, BMI, and CCDs [14,15,31,32]. As this is the first epidemiological study suggesting these interrelationships, our findings warrant further confirmation.
The interrelationships between hypertension, BMI, and CCDs are likely to be attributed to numerous mechanisms. First, adipokines are highly deregulated under obesity and may control cardiovascular homeostasis [33,34]. Adipose tissue can release free fatty acids (FFA) in the proximity and around the coronary arteries, modulating vascular responsiveness to vasoactive agents [35] and turning into an adverse lipotoxic, pro-thrombotic, and pro-inflammatory factor (IFNγ) to overexpress chemotactic cytokines (i.e., MCP-1, IL-6) [33,36,37]. In addition, adipose tissue can discharge FFA into the bloodstream, disturbing vascular homeostasis and endothelial dysfunction, which leads to increased risk of CCDs [33,38]. Second, elevated blood pressure leads to systemic arteriole spasm by increasing the permeability of the vascular endothelium, prolonging the contact time of lipoproteins with the vascular wall, and reducing the endothelium-dependent vasodilation. The systemic arteriole spasm was suggested to increase risk of CCDs [39]. Third, blood pressure regulation is centered on endothelial function, which is regulated by the interaction of the renin–angiotensin–aldosterone system, adrenergic receptors, and metabolic reactions; these endothelial function-related mechanisms are also closely related to adipose tissue [40,41]. Furthermore, obesity-related FFA inhibits the sodium/potassium exchange pump and sodium-ATP pump, which increases smooth muscle tone, peripheral resistance, and blood pressure [42,43].
The present study’s findings have clinical implications at the population level. According to the latest ACC/AHA hypertension guidelines [18], we were able to screen more hypertensive patients compared to the previous standard (blood pressure ≥140/90 mm Hg). The advantage of using the new hypertension definition is that it will allow us to prevent adverse hypertension-related outcomes (i.e., CCDs) at an early stage.
There are several strengths for our study. First, we used a community-based population with random sampling methods. The possibility for generalizing our findings to the population is high. Second, this study had a high response and validity rate, further increasing the representativeness of the findings. Lastly, blood pressure for each participant was directly measured thrice at a stable and consistent condition. Antihypertension medications were also considered. All these factors ensured validity and reliability of hypertensive status.
Several limitations must be considered in the interpretation of our results. First, because of the cross-sectional study design, we cannot conclude a temporal relationship between hypertension and BMI with CCDs. Second, this study is conducted in a Shuncheng, Fushun, Liaoning, which is relatively a small area. Thus, the generalizability for our findings may be limited. Third, only some major CCD risk factors were considered in this study. Data for dietary intake, physical activity, and genetics were not included in this study, because they are not available. Potential residual confounding cannot be fully excluded.
In summary, there are interrelated relationships between BMI, CCDs, and hypertension. Furthermore, hypertension modifies the relationship between BMI and CCDs. Although BMI was independently associated with CCDs, this association was primarily limited to individuals with hypertension (≥130/80 mm Hg). These results suggested that prevention efforts for CCDs among obese individuals may need to focus on individuals with a blood pressure ≥130/80 mm Hg. Further studies are warranted to confirm our results.
List of abbreviations
- ACC/AHA
The American College of Cardiology/American Heart Association
- BMI
body mass index
- CCDs
cardiovascular and cerebrovascular diseases
- CDC
Centers for Disease Control
- CHD
coronary heart disease
- 95% CIs
95% confidence intervals
- DBP
diastolic blood pressure
- FFA
free fatty acids
- MI
myocardial infarction
- ORs
odds ratios
- RAAS
renin–angiotensin–aldosterone system
- SBP
systolic blood pressure
- SD
standard deviation
Acknowledgments
We acknowledge the institutional review board at Fushun Center for Disease Prevention and Control (CDC) for approving this research.
Footnotes
Data availability: The data used in this study are owned and managed by the Fushun CDC. Because of ethical restrictions regarding individual privacy, data are available upon request. Data are available upon request from Fushun CDC. The authors did not have special access privileges and interested researchers can access the data in the same manner as the authors.
Funding: This work has no funding body.
Author contributions: The authors made the following contributions: W. Q. and S. Y. analyzed the data. X. Z., S. X., Y. Z., B. K., B. L., Q. Z., and D. G. collected data. W. Q. drafted the article. S. Y., X. S., and A. M. V. reviewed the manuscript. All authors contributed to the study design. S. Y. managed the overall project. All the authors critically revised the article for important intellectual content and approved the final version of the manuscript.
Conflict of interest: The authors declare no potential conflict of interest.
References
- [1].Joseph P, Leong D, McKee M, Anand SS, Schwalm JD, Teo K, et al. Reducing the global burden of cardiovascular disease, part 1: the epidemiology and risk factors. Circ Res. 2017;121(6):677–94. [DOI] [PubMed]; Joseph P, Leong D, McKee M, Anand SS, Schwalm JD, Teo K. et al. Reducing the global burden of cardiovascular disease, part 1: the epidemiology and risk factors. Circ Res. 2017;121(6):677–94. doi: 10.1161/CIRCRESAHA.117.308903. [DOI] [PubMed] [Google Scholar]
- [2].Kotseva K, Wood D, De Backer G, De Bacquer D, Pyorala K, Keil U, et al. Cardiovascular prevention guidelines in daily practice: a comparison of EUROASPIRE I, II, and III surveys in eight European countries. Lancet. 2009;373(9667):929–40. [DOI] [PubMed]; Kotseva K, Wood D, De Backer G, De Bacquer D, Pyorala K, Keil U. et al. Cardiovascular prevention guidelines in daily practice: a comparison of EUROASPIRE I, II, and III surveys in eight European countries. Lancet. 2009;373(9667):929–40. doi: 10.1016/S0140-6736(09)60330-5. [DOI] [PubMed] [Google Scholar]
- [3].Volpe M, Tocci G. Global cardiovascular risk management in primary prevention. Curr Vasc Pharmacol. 2012;10(6):709–11. [DOI] [PubMed]; Volpe M, Tocci G. Global cardiovascular risk management in primary prevention. Curr Vasc Pharmacol. 2012;10(6):709–11. doi: 10.2174/157016112803520729. [DOI] [PubMed] [Google Scholar]
- [4].Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(8):631–9. [DOI] [PubMed]; Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(8):631–9. doi: 10.1016/S2213-2600(15)00241-6. [DOI] [PubMed] [Google Scholar]
- [5].Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. 2019;139(10):e56–66. [DOI] [PubMed]; Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP. et al. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. 2019;139(10):e56–66. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- [6].Strazzullo P, D’Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke. 2010;41(5):e418–26. [DOI] [PubMed]; Strazzullo P, D’Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke. 2010;41(5):e418–26. doi: 10.1161/STROKEAHA.109.576967. [DOI] [PubMed] [Google Scholar]
- [7].Toyoda K, Ninomiya T. Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol. 2014;13(8):823–33. [DOI] [PubMed]; Toyoda K, Ninomiya T. Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol. 2014;13(8):823–33. doi: 10.1016/S1474-4422(14)70026-2. [DOI] [PubMed] [Google Scholar]
- [8].Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American heart association. Circulation. 2017;135(10):e146–603. [DOI] [PMC free article] [PubMed]; Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R. et al. Heart disease and stroke statistics-2017 update: a report from the American heart association. Circulation. 2017;135(10):e146–603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–96. [DOI] [PMC free article] [PubMed]; Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J. et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Heianza Y, Kodama S, Arase Y, Hsieh SD, Yoshizawa S, Tsuji H, et al. Role of body mass index history in predicting risk of the development of hypertension in Japanese individuals: Toranomon hospital health management center study 18 (TOPICS 18). Hypertension. 2014;64(2):247–52. [DOI] [PubMed]; Heianza Y, Kodama S, Arase Y, Hsieh SD, Yoshizawa S, Tsuji H. et al. Role of body mass index history in predicting risk of the development of hypertension in Japanese individuals: Toranomon hospital health management center study 18 (TOPICS 18) Hypertension. 2014;64(2):247–52. doi: 10.1161/HYPERTENSIONAHA.113.02918. [DOI] [PubMed] [Google Scholar]
- [11].Zou ZY, Yang YD, Wang S, Dong B, Li XH, Ma J. The importance of blood lipids in the association between BMI and blood pressure among Chinese overweight and obese children. Br J Nutr. 2016;116(1):45–51. [DOI] [PubMed]; Zou ZY, Yang YD, Wang S, Dong B, Li XH, Ma J. The importance of blood lipids in the association between BMI and blood pressure among Chinese overweight and obese children. Br J Nutr. 2016;116(1):45–51. doi: 10.1017/S0007114516001744. [DOI] [PubMed] [Google Scholar]
- [12].Chen Z, Smith M, Du H, Guo Y, Clarke R, Bian Z, et al. Blood pressure in relation to general and central adiposity among 5,00,000 adult Chinese men and women. Int J Epidemiol. 2015;44(4):1305–19. [DOI] [PMC free article] [PubMed]; Chen Z, Smith M, Du H, Guo Y, Clarke R, Bian Z. et al. Blood pressure in relation to general and central adiposity among 5,00,000 adult Chinese men and women. Int J Epidemiol. 2015;44(4):1305–19. doi: 10.1093/ije/dyv012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bundy JD, He J. Hypertension and related cardiovascular disease burden in China. Ann Glob Health. 2016;82(2):227–33. [DOI] [PubMed]; Bundy JD, He J. Hypertension and related cardiovascular disease burden in China. Ann Glob Health. 2016;82(2):227–33. doi: 10.1016/j.aogh.2016.02.002. [DOI] [PubMed] [Google Scholar]
- [14].Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1·8 million participants. Lancet. 2014;383(9921):970–83. [DOI] [PMC free article] [PubMed]; Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1·8 million participants. Lancet. 2014;383(9921):970–83. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bogers RP, Bemelmans WJ, Hoogenveen RT, Boshuizen HC, Woodward M, Knekt P, et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 3,00,000 persons. Arch Intern Med. 2007;167(16):1720–8. [DOI] [PubMed]; Bogers RP, Bemelmans WJ, Hoogenveen RT, Boshuizen HC, Woodward M, Knekt P. et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 3,00,000 persons. Arch Intern Med. 2007;167(16):1720–8. doi: 10.1001/archinte.167.16.1720. [DOI] [PubMed] [Google Scholar]
- [16].He W, Li Q, Yang M, Jiao J, Ma X, Zhou Y, et al. Lower BMI cutoffs to define overweight and obesity in China. Obesity (Silver Spring, Md). 2015;23(3):684–91. [DOI] [PubMed]; He W, Li Q, Yang M, Jiao J, Ma X, Zhou Y. et al. Lower BMI cutoffs to define overweight and obesity in China. Obesity (Silver Spring, Md) 2015;23(3):684–91. doi: 10.1002/oby.20995. [DOI] [PubMed] [Google Scholar]
- [17].Oakkar EE, Stevens J, Truesdale KP, Cai J. BMI and all-cause mortality among Chinese and Caucasians: the People’s Republic of China and the atherosclerosis risk in communities studies. Asia Pac J Clin Nutr. 2015;24(3):472–9. [DOI] [PMC free article] [PubMed]; Oakkar EE, Stevens J, Truesdale KP, Cai J. BMI and all-cause mortality among Chinese and Caucasians: the People’s Republic of China and the atherosclerosis risk in communities studies. Asia Pac J Clin Nutr. 2015;24(3):472–9. doi: 10.6133/apjcn.2015.24.3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Whelton PK, Carey RM, Aronow WS, Casey DE, Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71(19):e127–248. [DOI] [PubMed]; Whelton PK, Carey RM, Aronow WS, Casey DE, Jr., Collins KJ, Dennison Himmelfarb C. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71(19):e127–248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- [19].Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., et al. The Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72. [DOI] [PubMed]; Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr.. et al. The Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- [20].Yatsuya H, Matsunaga M, Li Y, Ota A. Risk factor of cardiovascular disease among older individuals. J Atheroscler Thromb. 2017;24(3):258–61. [DOI] [PMC free article] [PubMed]; Yatsuya H, Matsunaga M, Li Y, Ota A. Risk factor of cardiovascular disease among older individuals. J Atheroscler Thromb. 2017;24(3):258–61. doi: 10.5551/jat.Ed064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham heart study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383(9921):999–1008. [DOI] [PMC free article] [PubMed]; Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham heart study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383(9921):999–1008. doi: 10.1016/S0140-6736(13)61752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk – a review of the literature. Eur J Clin Nutr. 2010;64(1):16–22. [DOI] [PubMed]; Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk – a review of the literature. Eur J Clin Nutr. 2010;64(1):16–22. doi: 10.1038/ejcn.2009.68. [DOI] [PubMed] [Google Scholar]
- [23].Sun W, Huang Y, Xian Y, Zhu S, Jia Z, Liu R, et al. Association of body mass index with mortality and functional outcome after acute ischemic stroke. Sci Rep. 2017;7(1):2507. [DOI] [PMC free article] [PubMed]; Sun W, Huang Y, Xian Y, Zhu S, Jia Z, Liu R. et al. Association of body mass index with mortality and functional outcome after acute ischemic stroke. Sci Rep. 2017;7(1):2507. doi: 10.1038/s41598-017-02551-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Barry VW, Caputo JL, Kang M. The joint association of fitness and fatness on cardiovascular disease mortality: a meta-analysis. Prog Cardiovasc Dis. 2018;61(2):136–41. [DOI] [PubMed]; Barry VW, Caputo JL, Kang M. The joint association of fitness and fatness on cardiovascular disease mortality: a meta-analysis. Prog Cardiovasc Dis. 2018;61(2):136–41. doi: 10.1016/j.pcad.2018.07.004. [DOI] [PubMed] [Google Scholar]
- [25].Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of the obesity society and the American society of hypertension. J Clin Hypertens (Greenwich). 2013;15(1):14–33. [DOI] [PMC free article] [PubMed]; Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D. et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of the obesity society and the American society of hypertension. J Clin Hypertens (Greenwich) 2013;15(1):14–33. doi: 10.1111/jch.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhao D, Wang W, Liu J, Cheng J, Liu J, Qin LP. Association between body mass index and ten-year-accumulative-risk of hypertension. Zhonghua Liu Xing Bing Xue Za Zhi. 2009;30(5):435–8. [PubMed]; Zhao D, Wang W, Liu J, Cheng J, Liu J, Qin LP. Association between body mass index and ten-year-accumulative-risk of hypertension. Zhonghua Liu Xing Bing Xue Za Zhi. 2009;30(5):435–8. [PubMed] [Google Scholar]
- [27].Schütten MT, Houben AJ, de Leeuw PW, Stehouwer CD. The link between adipose tissue renin-angiotensin-aldosterone system signaling and obesity-associated hypertension. Physiology (Bethesda, Md). 2017;32(3):197–209. [DOI] [PubMed]; Schütten MT, Houben AJ, de Leeuw PW, Stehouwer CD. The link between adipose tissue renin-angiotensin-aldosterone system signaling and obesity-associated hypertension. Physiology (Bethesda, Md) 2017;32(3):197–209. doi: 10.1152/physiol.00037.2016. [DOI] [PubMed] [Google Scholar]
- [28].Urtamo A, Jyväkorpi SK, Kautiainen H, Pitkälä KH, Strandberg TE. Major cardiovascular disease (CVD) risk factors in midlife and extreme longevity. Aging Clin Exp Res. 2020;32(2):299–304. [DOI] [PMC free article] [PubMed]; Urtamo A, Jyväkorpi SK, Kautiainen H, Pitkälä KH, Strandberg TE. Major cardiovascular disease (CVD) risk factors in midlife and extreme longevity. Aging Clin Exp Res. 2020;32(2):299–304. doi: 10.1007/s40520-019-01364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kane AE, Howlett SE. Differences in cardiovascular aging in men and women. Adv Exp Med Biol. 2018;1065:389–411. [DOI] [PubMed]; Kane AE, Howlett SE. Differences in cardiovascular aging in men and women. Adv Exp Med Biol. 2018;1065:389–411. doi: 10.1007/978-3-319-77932-4_25. [DOI] [PubMed] [Google Scholar]
- [30].Ortega-Loubon C, Fernández-Molina M, Singh G, Correa R. Obesity and its cardiovascular effects. Diabet Metab Res Rev. 2019;35(4):e3135. [DOI] [PubMed]; Ortega-Loubon C, Fernández-Molina M, Singh G, Correa R. Obesity and its cardiovascular effects. Diabet Metab Res Rev. 2019;35(4):e3135. doi: 10.1002/dmrr.3135. [DOI] [PubMed] [Google Scholar]
- [31].Corden B, Keenan NG, de Marvao AS, Dawes TJ, Decesare A, Diamond T, et al. Body fat is associated with reduced aortic stiffness until middle age. Hypertension. 2013;61(6):1322–7. [DOI] [PubMed]; Corden B, Keenan NG, de Marvao AS, Dawes TJ, Decesare A, Diamond T. et al. Body fat is associated with reduced aortic stiffness until middle age. Hypertension. 2013;61(6):1322–7. doi: 10.1161/HYPERTENSIONAHA.113.01177. [DOI] [PubMed] [Google Scholar]
- [32].Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377(9771):1085–95. [DOI] [PMC free article] [PubMed]; Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A. et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377(9771):1085–95. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].González N, Moreno-Villegas Z, González-Bris A, Egido J, Lorenzo Ó. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc Diabetol. 2017;16(1):44. [DOI] [PMC free article] [PubMed]; González N, Moreno-Villegas Z, González-Bris A, Egido J, Lorenzo Ó. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc Diabetol. 2017;16(1):44. doi: 10.1186/s12933-017-0528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Boulet MM, Chevrier G, Grenier-Larouche T, Pelletier M, Nadeau M, Scarpa J, et al. Alterations of plasma metabolite profiles related to adipose tissue distribution and cardiometabolic risk. Am J Physiol Endocrinol Metab. 2015;309(8):E736–46. [DOI] [PubMed]; Boulet MM, Chevrier G, Grenier-Larouche T, Pelletier M, Nadeau M, Scarpa J. et al. Alterations of plasma metabolite profiles related to adipose tissue distribution and cardiometabolic risk. Am J Physiol Endocrinol Metab. 2015;309(8):E736–46. doi: 10.1152/ajpendo.00231.2015. [DOI] [PubMed] [Google Scholar]
- [35].Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25(12):2594–9. [DOI] [PubMed]; Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM. et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25(12):2594–9. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- [36].Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front Endocrinol. 2013;4:71. [DOI] [PMC free article] [PubMed]; Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front Endocrinol. 2013;4:71. doi: 10.3389/fendo.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Aslanabadi N, Salehi R, Javadrashid A, Tarzamni M, Khodadad B, Enamzadeh E, et al. Epicardial and pericardial fat volume correlate with the severity of coronary artery stenosis. J cardiovasc Thorac Res. 2014;6(4):235–9. [DOI] [PMC free article] [PubMed]; Aslanabadi N, Salehi R, Javadrashid A, Tarzamni M, Khodadad B, Enamzadeh E. et al. Epicardial and pericardial fat volume correlate with the severity of coronary artery stenosis. J cardiovasc Thorac Res. 2014;6(4):235–9. doi: 10.15171/jcvtr.2014.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, et al. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes. 2001;50(1):159–65. [DOI] [PubMed]; Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F. et al. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes. 2001;50(1):159–65. doi: 10.2337/diabetes.50.1.159. [DOI] [PubMed] [Google Scholar]
- [39].Yuan X, Braun T. Multimodal regulation of cardiac myocyte proliferation. Circ Res. 2017;121(3):293–309. [DOI] [PubMed]; Yuan X, Braun T. Multimodal regulation of cardiac myocyte proliferation. Circ Res. 2017;121(3):293–309. doi: 10.1161/CIRCRESAHA.117.308428. [DOI] [PubMed] [Google Scholar]
- [40].Mu F, Harris HR, Rich-Edwards JW, Hankinson SE, Rimm EB, Spiegelman D, et al. A prospective study of inflammatory markers and risk of endometriosis. Am J Epidemiol. 2018;187(3):515–22. [DOI] [PMC free article] [PubMed]; Mu F, Harris HR, Rich-Edwards JW, Hankinson SE, Rimm EB, Spiegelman D. et al. A prospective study of inflammatory markers and risk of endometriosis. Am J Epidemiol. 2018;187(3):515–22. doi: 10.1093/aje/kwx272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni-D’Ambrosia G, Arbique D, Vongpatanasin W, et al. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magnetic Reson Med. 2003;49(3):417–23. [DOI] [PubMed]; Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni-D’Ambrosia G, Arbique D, Vongpatanasin W. et al. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magnetic Reson Med. 2003;49(3):417–23. doi: 10.1002/mrm.10372. [DOI] [PubMed] [Google Scholar]
- [42].Bell BB, Rahmouni K. Leptin as a mediator of obesity-induced hypertension. Curr Obes Rep. 2016;5(4):397–404. [DOI] [PMC free article] [PubMed]; Bell BB, Rahmouni K. Leptin as a mediator of obesity-induced hypertension. Curr Obes Rep. 2016;5(4):397–404. doi: 10.1007/s13679-016-0231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Alpert MA, Omran J, Mehra A, Ardhanari S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog Cardiovasc Dis. 2014;56(4):391–400. [DOI] [PubMed]; Alpert MA, Omran J, Mehra A, Ardhanari S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog Cardiovasc Dis. 2014;56(4):391–400. doi: 10.1016/j.pcad.2013.09.003. [DOI] [PubMed] [Google Scholar]


