Key Points
Question
What are the long-term outcomes experienced by patients with diffuse type 2 dominant or eosinophilic chronic rhinosinusitis following multimodal intervention with neosinus cavity creation and long-term corticosteroid irrigation maintenance?
Findings
In this cohort study including 222 patients, disease control was achieved in more than 85% of patients with eosinophilic chronic rhinosinusitis, and a substantial reduction in symptom burden was achieved across all patients with eosinophilic chronic rhinosinusitis regardless of whether further medical intervention was required. Poor disease control was not associated with inadequate adherence to irrigation use.
Meaning
The findings of this study suggest that long-term multimodal therapy of eosinophilic chronic rhinosinusitis is successful in most patients to control disease and reduce symptoms; maintenance corticosteroid irrigations can be self-tapered to disease activity.
Abstract
Importance
Eosinophilic chronic rhinosinusitis (eCRS), contemporarily classified as diffuse type 2 dominant chronic rhinosinusitis (CRS), is characterized by eosinophil-dominant mucosal inflammation. Contemporary management of eCRS as an inflammatory airway condition is multimodal with corticosteroid irrigations after the surgical creation of a neosinus cavity.
Objectives
To assess long-term treatment outcomes in patients with primary diffuse type 2 CRS or eCRS receiving multimodal treatment.
Design, Setting, and Participants
A prospective cohort study of patients seen in a tertiary rhinology practice recruited from May 2010 to November 2018 was conducted. Follow-up duration was 12 months or more following endoscopic sinus surgery (ESS) with a neosinus cavity formed. Data analysis was performed from August to November 2020. Consecutive adult (≥18 years) patients diagnosed with primary diffuse type 2 dominant CRS or eCRS based on the European Position Paper on Rhinosinusitis and Nasal Polyps 2020 criteria were included. Type 2 inflammation was defined as more than 10 eosinophils per high-power field obtained from sinus mucosal biopsy and managed with neosinus cavity ESS and ongoing corticosteroid irrigations. Exclusion criteria were less than 12 months of follow-up and secondary CRS.
Interventions
Endoscopic sinus surgery with complete removal of intersinus bony partitions to create a neosinus cavity. Nasal irrigation (240 mL) with betamethasone, 1 mg, or budesonide, 1 mg, daily for 3 to 6 months after ESS and tapered to an as-needed basis (minimum, 2-3 per week).
Main Outcomes and Measures
Poor control was defined as polyp recurrence (polyp growth in >1 sinus area on a single side), use of long-term systemic therapy (biologic therapy or ≥3 consecutive months of oral corticosteroids), and revision surgery involving polypectomy. The disease in patients with no poor control criteria was defined as well controlled, and the disease in those with 1 or more criteria was considered poorly controlled. Maintenance medical therapy use and patient-reported outcomes based on the 22-item Sinonasal Outcomes Test for preoperative and last follow-up were collected.
Results
Of the 222 participants recruited with primary diffuse type 2 dominant CRS or eCRS and minimum of year of follow-up, 126 were men (56.8%). Mean (SD) age was 54.8 (13.6) years, and median (SD) follow-up was 2.2 (2.2) years. Of the 222 patients, 195 (87.8%) had well-controlled disease, 16 (7.2%) had polyp recurrence, 7 (3.2%) continued to receive long-term oral corticosteroid therapy, 5 (2.3%) received biologic therapy, and 8 (3.6%) underwent a revision polypectomy. Clinically meaningful change on the 22-item Sinonasal Outcomes Test and the nasal subdomain score was maintained at the last follow-up in 134 patients (67.0%). Poor disease control was not associated with poor adherence to irrigation use.
Conclusions and Relevance
The findings of this cohort study suggest that long-term disease control and reduction in symptom burden in patients with primary diffuse type 2 CRS or eCRS might be achieved when managed as an inflammatory disorder. Maintenance corticosteroid irrigations in the population examined appeared to be successfully self-tapered to disease activity.
This cohort study examines long-term treatment outcomes in patients with eosinophilic rhinosinusitis who have received corticosteroid irrigations after surgical creation of a neosinus cavity.
Introduction
An endotype classification of chronic rhinosinusitis (CRS) has emerged that guides treatment therapies and further research. Eosinophilic CRS (eCRS) is a unique endotype defined by tissue eosinophilia. Histopathologic assessment of sinonasal tissue is needed in the classification of this endotype.1,2 Current literature suggests an inappropriate mucosal inflammatory response to specific environmental stimuli, such as viruses, pollution (particulate matter), and bacterial and fungal antigens.3 Molecular studies suggest that eCRS is influenced by TH2-skewed inflammation that perpetuates persistent eosinophilic activity in the sinonasal mucosa.4,5,6,7 Contemporary classification describes this group as a primary diffuse type 2–dominant CRS, highlighting its inflammatory nature.8,9
Previous studies emphasized that tissue eosinophilia suggests the probability of worse postoperative outcomes in patients with CRS undergoing endoscopic sinus surgery (ESS) compared with those who had noneosinophilic CRS, with higher rates of revision surgery10,11,12,13 and polyp recurrence.14,15,16,17,18,19,20,21 However, most of these studies adopted variable surgical modification of the sinus cavities, and local control was often simple corticosteroid nasal sprays or was not reported. For many surgical studies on eCRS, long-term maintenance therapy is often not detailed or standardized. For a known inflammatory disorder, it is not surprising that single-modality surgical interventions were often associated with failure.22
Contemporary management of eCRS has evolved around its inflammatory source.23 Our current treatment philosophy is multimodal, involving a wide-open ESS technique that creates a single neosinus cavity through the complete removal of intersinus bony partitions.24 This approach to sinus surgery aims to maximize the postoperative delivery of corticosteroids to the sinonasal mucosa rather than focus on optimization of ventilation.25 Topical corticosteroids play an important role in the control of mucosal inflammation in this phenotype.26 The goal of this study was to assess the long-term effectiveness of a contemporary multimodal approach in the management of patients with primary diffuse type 2 CRS or eCRS with consistent surgical and maintenance medical treatment.
Methods
Study Design and Participants
A prospective cohort study was conducted including patients with eCRS who underwent neosinus ESS and had a postsurgical corticosteroid irrigation regimen. Ethical approval for this project was obtained from the St Vincent’s Hospital Human Research Ethics Committee, Sydney, Australia, and patients provided verbal informed consent for research data collection. Participants did not receive financial compensation. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Consecutive adult (age ≥18 years) patients diagnosed with eCRS and seen at a single tertiary center across an 8-year period (May 2010 to November 2018) were included in this study. All participants underwent neosinus cavity ESS, were managed with ongoing postsurgical corticosteroid irrigation therapy, and had follow-up of 12 months or more. Patients were excluded if they had less than 12 months’ follow-up or secondary CRS, such as odontogenic sinusitis, cystic fibrosis, or Churg-Strauss syndrome (eosinophilic granulomatosis with polyangiitis).
Definition of eCRS
Eosinophilic CRS was defined as primary CRS with tissue eosinophilia of greater than 10 eosinophils per high-power field assessed perioperatively, according to The European Position Paper on Rhinosinusitis and Nasal Polyps 2020 criteria.8 Past patients were retrospectively classified based on European Position Paper on Rhinosinusitis and Nasal Polyps 2020 criteria. The tissue eosinophil count was analyzed at the time of primary ESS by standard hematoxylin and eosin staining of sinus mucosal samples and was assessed on 2 separate high-power fields (×400 magnification). All patients discontinued use of systemic corticosteroid medications at least 4 weeks before surgery.
Neosinus ESS and Corticosteroid Irrigation Maintenance Therapy
All patients had a neosinus cavity created as part of treatment. This cavity included a Draf2a frontal recess clearance if the maximum dimension of the frontal recess was 10 mm or larger; otherwise, a Draf3 frontal opening was created. All surgeries were performed by the same surgeon (R.J.H.). In the first 3 weeks after surgery, all patients received betamethasone, 1 mg, or budesonide, 1 mg, delivered once daily by a 240-mL nasal irrigation device (Sinus Rinse; Neilmed) and continued daily until 3 to 6 months postoperatively. Once the sinonasal mucosa had normalized or a stable state was achieved as noted on follow-up, patients could self-adjust their irrigation use but were advised to maintain at least a minimum of 2 to 3 times per week. This corticosteroid irrigation regimen was not routinely used for disease management before the decision for surgery. Postoperative corticosteroid therapy was used in all patients for 3 weeks (25 mg/day for 1 week, 12.5 mg/day for 1 week and 5 mg/day for 1 week).
Defining the Success of Local Therapy
The multimodal therapy was defined as successful if the inflammatory process was controlled locally. Any of the following criteria indicated a failure of local therapy: (1) symptomatically significant polyp recurrence within a sinus cavity (see definition in next paragraph), (2) use of long-term systemic therapy (corticosteroid or biologic therapy), or (3) further surgery to remove recurrent polyps (revision polypectomy). Patients without failure outcomes were identified as well controlled. Patients who received further management in addition to neosinus ESS and a corticosteroid irrigation regimen, as indicated by fulfilling 1 or more of the failure criteria, were considered poorly controlled.
Symptomatically Significant Polyp Recurrence Within a Sinus Cavity
Polyp recurrence was determined by the assessment of video recordings of sinus endoscopies performed during the patient’s latest follow-up clinical consultation. The recordings were scored based on the Modified Lund Mackay Postoperative Endoscopy Score.27 Mucosal edema (scored from 0 [normal mucosa] to 6 [polyp extending beyond cavity) was assessed in the 5 post-ESS cavities on left and right sides. Nasal polyp recurrence was defined by a mucosal score of 5 (polyp within cavity) or 6 (polyp extending outside of cavity) in more than 1 postoperative cavity on a single side. Patients with polyp recurrence in both sides were included in this definition. This cutoff was defined as patients with polyp growth in more than 1 sinus cavity on any one side had significantly worse symptoms at the last follow-up within the nasal subdomain score (NSS) of the 22-item Sinonasal Outcomes Test (SNOT-22) compared with those with no polyp growth.
The need for long-term systemic therapy was defined as a need for continuous oral corticosteroid therapy for 3 or more consecutive months or biologic therapy despite postsurgical corticosteroid nasal irrigations. Revision polypectomy was defined as whether a revision ESS that involved the removal of inflammatory polyps was performed during the follow-up period. The indications for revision polypectomy included nasal obstruction, mucus trapping, and recurrent exacerbations. Minor revision procedures without polypectomy, such as for division of adhesions, were not considered an indicator of poor mucosal disease control, but rather optimization of the sinus cavity anatomy.
Data on age, sex, smoking status, aspirin sensitivity, previous sinus surgery, asthma status, and atopic status were collected. Patients were considered smokers if they currently smoked any number of cigarettes at least weekly or had previously smoked but ceased in the past 12 months. Aspirin sensitivity was defined as a well-described history of bronchospasm after aspirin or nonsteroidal anti-inflammatory use or a positive oral or nasal lysine aspirin challenge test with a greater than 15% change in forced expiratory volume in the first second of expiration or greater than 40% total nasal airway resistance on rhinomanometry. Prior sinus surgery was self-reported by the patient or via clinical and radiologic evidence of prior surgery. Asthma status was indicated either through current use of bronchodilator or inhaled corticosteroid therapy or a greater than 15% change in forced expiratory volume in the first second of expiration after bronchodilator use on spirometry. Atopic status was determined by automated immunoassay (ImmunoCap) to detect serum-specific immunoglobulin E (IgE) antibodies to 4 aeroallergen mixes: (1) grass, (2) dust mite, (3) mold, and (4) animal epithelium. A serum-specific IgE level greater than 0.35 kU/L (to convert to milligrams per liter, multiply by 0.0024) for any of these mixes was considered a positive result. Patients were identified as atopic if they tested positive to any mix.
Patients were also given a retrospective classification as either CRS with nasal polyps or CRS without nasal polyps phenotypes based on intraoperative endoscopic findings. Evidence of systemic inflammatory response was sought by preoperative blood eosinophil count, total serum IgE level, C-reactive protein level, and erythrocyte sedimentation rate.
Long-term Symptom Changes
Preoperative patient-reported outcome measures were quantified using the 22-item SNOT-22 completed by the patient before surgery. The SNOT-22 consists of 22 questions that require the patient to rate the severity of a sinonasal disease based on a 5-point ordinal scale: 0 indicates no problem; 1, very mild problem; 2, mild or slight problem; 3, moderate problem; 4, severe problem; and 5, problem as bad as it can be. The SNOT-22 total score was calculated by the addition of the individual scores for all 22 items with a possible range from 0 to 110. The NSS was calculated by the addition of the following SNOT-22 items: need to blow nose, nasal obstruction, loss of smell/taste, thick nasal discharge, and facial pain/pressure.28 The possible score of the NSS ranges from 0 to 25. Patients completed an electronic format of the SNOT-22 questionnaire at their last follow-up. The change in SNOT-22 score and change in the NSS was the difference between the presurgical and follow-up scores. The minimal clinically important difference in the SNOT-22 score was defined as greater than or equal to 9.0.28
Maintenance Therapy
The extent of reliance on postoperative topical corticosteroid therapy was defined as the weekly frequency of nasal corticosteroid irrigation use at the last follow-up. This information was self-reported by the patient at the last follow-up. Data were collected based on a 4-point ordinal scale: 1 indicates requires irrigations 4 or more days/week; 2, requires irrigations 2 to 3 days/week; 3, uses irrigations only when unwell or as needed; and 4, does not use any irrigations. For statistical analysis, this outcome was dichotomized as greater than or equal to 4 or less than 4 corticosteroid irrigations per week to distinguish between high- and low-frequency use.
Short courses of systemic corticosteroid and antibiotic therapy in the period ranging after the initial perioperative period 3 months following ESS to the last follow-up were investigated to assess the risk of inflammatory and/or infective disease in the postoperative period. The cumulative number of courses of systemic antibiotics and corticosteroids used was divided by the duration of follow-up (years) to normalize the data. A short-term prednisone therapy course was standardized to 25 mg/day for 7 days, 12.5 mg/day for 7 days, and then 5 mg/day for 7 days.
Analysis was performed to examine whether the number of reported corticosteroid irrigations per week correlated with symptom improvement based on the SNOT-22 score and NSS.
Statistical Analysis
SPSS Statistics, version 25 (IBM Corp) was used for statistical analysis. Changes in continuous data were summarized in mean (SD), 95% CI, and Cohen d effect sizes. A Cohen d effect size of 0.2 was considered a small but important effect, 0.5 indicated a medium effect, and greater than or equal to 0.8 represented large effect.29 Odds ratios (ORs) and 95% CIs were used to compare ordinal data. Findings were considered significant if 95% CI values did not cross null values (OR, 1; mean difference, 0). Data analysis was conducted from August to November 2020.
Results
Participants
A total of 260 potential participants were assessed; of these, 222 participants were recruited (126 [56.8%] men, 96 [43.2%] women, mean [SD] age, 54.8 [13.6] years) and 38 were excluded owing to less than 12 months' follow-up. Characteristics of the group included in the analysis comprised smoking (25 [12.3%]), asthma (114 [51.4%]), atopy (118 [53.2%]), aspirin sensitivity (17 [7.7%]), CRS with nasal polyps (162 [73.0%]), and previous sinus surgery (110 [49.5%]). The mean (SD) laboratory values were blood eosinophil level, 400/μL (300/μL) (to convert to ×109 per liter, multiply by 0.001); serum IgE level, 182.1 U/L (263.0 U/L); C-reactive protein level, 0.26 mg/dL (0.48 mg/dL) (to convert to milligrams per liter, multiply by 10); and erythrocyte sedimentation rate, 8.7 (8.7) mm/h. Median (SD) follow-up duration was 2.2 (2.2) years.
Control of Disease
Of the 222 patients with eCRS, 195 (87.8%) had well-controlled disease, 16 (7.2%) had polyp recurrence, 7 (3.2%) received continuous oral corticosteroid therapy, 5 (2.3%) received biologic therapy, and 8 (3.6%) underwent a revision polypectomy by the time of the last follow-up. In total, 27 patients with eCRS (12.2%) had poorly controlled disease, having fulfilled at least 1 failure outcome.
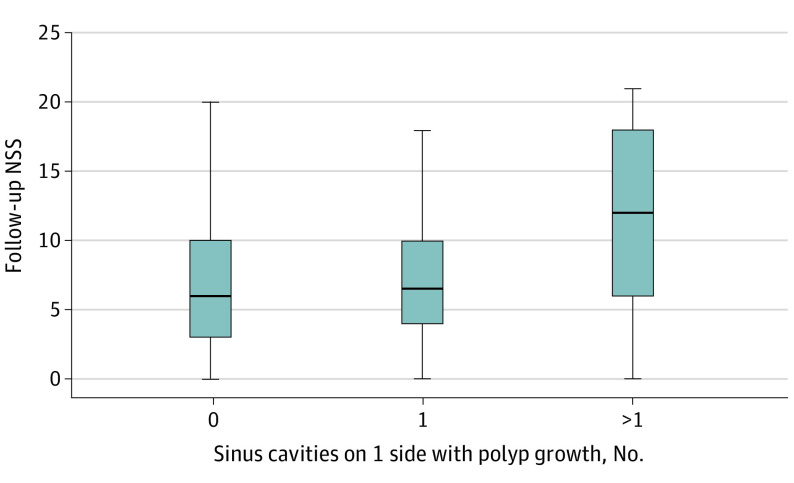
Endoscopy assessment was performed on a total of 342 sinus cavities across 171 patients. The criteria for polyp recurrence were determined through statistical analysis. Patients with more than 1 sinus cavity with polyps in any of the 5 sinus cavities on 1 side had significantly worse symptoms at last follow-up within the NSS of the SNOT-22 compared with those with no polyp growth (mean difference, 6.0; 95% CI, 2.00-10.04; Cohen d, 1.014). In contrast, patients with only 1 sinus cavity involvement on 1 side had a similar symptom burden to those with no polyp growth (mean difference, 0.9; 95% CI, −3.52 to 1.81; Cohen d, 0.175) (Figure).
Figure. Comparison of Follow-up Nasal Subdomain Score (NSS) Based on Number of Sinus Cavities With Polyp Growth.
For 0 vs more than 1 sinus cavity with polyp growth on 1 side, the mean difference was 6.0 (95% CI, 2.00-10.04; Cohen d, 1.014). For 0 vs 1 sinus cavity with polyp growth, the mean difference was 0.9 (95% CI, −3.52 to 1.81; Cohen d, 0.175).
Using the criteria of more than 1 polyp in any of the 5 sinus cavities on 1 side, 16 (7.2%) patients with eCRS had polyp recurrence. Fifteen patients (6.3%) had polyp growth in only 1 of the 5 sinus cavities and 141 (63.5%) had no polyp recurrence in any cavity. An additional 15 patients (6.8%) had revision surgery for surgical cavity modification without polypectomy for procedures such as adhesions, mucus recirculation, sumping/mucostasis, and scarring.
Comparison of Disease Baseline Characteristics Between Groups
Table 1 presents a comparison of baseline characteristics between patients with well-controlled and poorly controlled disease. Patients with poorly controlled CRS were more likely to have asthma (OR, 3.1; 95% CI, 1.24-7.59) and aspirin sensitivity (OR, 4.78; 95% CI, 1.60-14.25) and to have undergone previous sinus surgery (OR, 4.2; 95% CI, 1.61-10.78). Patients with well-controlled disease had lower blood eosinophil levels compared with those who had poorly controlled disease (400/μL [300/μL] vs 600/μL [400/μL]; mean difference, 0.19; 95% CI, −0.35 to 0.02; and Cohen d effect size, 0.48; 95% CI, 0.06-0.90). Receiver operating characteristic curve analysis showed that blood eosinophil levels estimated the probability of poorly controlled disease at a cutoff level of 430/μL (sensitivity, 59.3%; specificity, 69.6%; area under the curve, 0.664; Youden index, 0.288; positive likelihood ratio, 1.95; and diagnostic OR, 3.33).
Table 1. Comparison of Baseline Characteristics Between Patients With Well-Controlled and Poorly Controlled eCRS.
| Characteristic | Well-controlled eCRS (n = 195) | Poorly controlled eCRS (n = 27) | Mean difference (95% CI) | Odds ratio (95% CI) |
|---|---|---|---|---|
| Population characteristics | ||||
| Age, mean (SD), y | 55.3 (13.5) | 51.2 (14.3) | 4.09 (−1.41 to 9.60) | NA |
| Sex, No. (%) | ||||
| Women | 82 (42.1) | 14 (51.9) | NA | 1.5 (0.7 to 3.3) |
| Men | 113 (57.9) | 13 (48.1) | NA | 0.80 (0.36 to 1.79) |
| Smoking | 24 (13.6) | 1 (3.7) | NA | 0.24 (0.03 to 1.88) |
| Asthma | 94 (48.2) | 20 (74.1) | NA | 3.07 (1.24 to 7.59) |
| Atopy | 99 (51.3) | 18 (66.7) | NA | 1.90 (0.81 to 4.44) |
| Aspirin sensitivity | 11 (5.6) | 6 (22.2) | NA | 4.78 (1.60 to 14.25) |
| Prior sinus surgery | 89 (45.6) | 21 (77.8) | NA | 4.17 (1.61 to 10.78) |
| Disease characteristics | ||||
| CRS with nasal polyps | 139 (71.3) | 23 (85.2) | NA | 2.32 (0.77 to 7.0) |
| Blood eosinophils, mean (SD), /μL | 0.4 (0.3) | 0.6 (0.4) | −0.19 (−0.35 to −0.02) | NA |
| Serum IgE, mean (SD), U/L | 187.8 (277.6) | 140.8 (107.6) | 47.0 (−10.19 to 104.22) | NA |
| CRP, mean (SD), mg/L | 2.3 (4.1) | 4.9 (8.2) | −2.5 (5.82 to 0.75) | NA |
| ESR, mean (SD), mm/h | 8.5 (7.8) | 10.2 (13.3) | −1.7 (−5.2 to 1.84) | NA |
| Patient-reported characteristics | ||||
| Preoperative SNOT-22 score (range, 0-110), mean (SD)a | 42.8 (22.8) | 58.9 (29.6) | −16.2 (−28.26 to −4.06) | NA |
| Preoperative NSS (range, 0-25), mean (SD)b | 13.0 (6.2) | 16.7 (6.4) | −3.7 (−6.26 to −1.20) | NA |
Abbreviations: CRP, C-reactive protein; eCRS, eosinophilic chronic rhinosinusitis; ESR, erythrocyte sedimentation rate; IgE, immunoglobulin E; NA, not applicable; NSS, nasal symptom score; SNOT-22, 22-item Sinonasal Outcome Test.
SI conversion factors: To convert CRP to milligrams per liter, multiply by 10; eosinophils to ×109 per liter, multiply by 0.001; and IgE to kilounits per liter, multiply by 1.
Possible score range, 0 to 110, with 0 indicating no problem; 1, very mild problem; 2, mild or slight problem; 3, moderate problem; 4, severe problem; and 5, problem as bad as it can be.
Possible score range, 0 to 25, calculated by the addition of the following SNOT-22 items: need to blow nose, nasal obstruction, loss of smell/taste, thick nasal discharge, and facial pain/pressure.
There were no significant differences in age, sex, smoking status, atopic status, CRS with nasal polyp phenotype, serum IgE level, C-reactive protein level, and erythrocyte sedimentation rate.
For preoperative symptoms, patients with well-controlled vs poorly controlled disease had lower mean preoperative SNOT-22 scores of 42.8 (22.8) vs 58.9 (29.6) (mean difference, 16.2; 95% CI, 4.06-28.26; and Cohen d effect size, 0.56; 95% CI, 0.16-0.98) and mean preoperative NSS of 13.0 (6.2) vs 16.7 (6.4) (mean difference, 3.7; 95% CI, 1.20-6.26 and Cohen d effect size, 0.60; 95% CI, 0.22-1.00).
Long-term Symptom Changes Between Groups
This cohort of postsurgical patients with eCRS experienced a decrease in their SNOT-22 scores of −20.2 (23.4) points and NSS −6.3 (6.5) points. The mean change in SNOT-22 and NSS score based on treatment outcome group is summarized in Table 2. A total of 134 patients (67.0%) reached minimal clinically significant improvements in their SNOT-22 score. When comparing well-controlled and poorly controlled groups, there were no significant differences in the degree of improvement in SNOT-22 scores (mean [SD], −20.1 [23.1] vs −20.7 [26.0]; mean difference, 0.6; 95% CI, −9.0 to 10.2; and Cohen d effect size, 0.02; 95% CI, −0.38 to 0.43) and NSS (mean [SD], −6.5 [6.7] vs −5.2 [5.6]; mean difference, 1.3; 95% CI, −4.0 to 1.4; and Cohen d effect size, 0.20; 95% CI, 0.0-0.60). There were also similar odds of having minimal clinically significant improvement in SNOT-22 scores: 65.9% vs 74.1% (OR, 1.48; 95% CI, 0.59-3.70).
Table 2. Changes in SNOT-22 Score Within Treatment Outcome Groups.
| Outcome | Mean (SD) | ||||
|---|---|---|---|---|---|
| Well controlled | Poorly controlled | ||||
| Polyp recurrence | Use of systemic therapy | Revision polypectomy | |||
| Continuous oral corticosteroids | Biologic therapy | ||||
| SNOT-22 score change | −20.1 (23.1) | −14.7 (30.6) | −17.5 (4.8) | −35.7 (31.5) | −25.0 (28.8) |
| NSS change | −6.5 (6.7) | −3.1 (7.2) | −5.5 (3.2) | −6.7 (2.3) | −6.9 (5.4) |
Abbreviations: NSS, nasal subdomain score; SNOT-22, 22-item Sinonasal Outcome Test.
Maintenance Therapy
A comparison of weekly corticosteroid irrigation frequency at last follow-up between the well-controlled and poorly controlled groups showed that more patients with poorly controlled disease used corticosteroid irrigation more frequently at 4 or more times per week: 40.5% vs 56.0%; OR, 1.84; 95% CI, 0.82-4.13) (Table 3).
Table 3. Comparison of Long-term Outcomes Between Patients With Well-Controlled and Poorly Controlled eCRS.
| Outcome | Well-controlled eCRS (n = 195) | Poorly controlled eCRS (n = 27) | Mean difference (95% CI) | Odds ratio (95% CI) |
|---|---|---|---|---|
| Topical corticosteroid use (≥4 per week), No. (%) | 79 (40.5) | 15 (56.0) | NA | 1.84 (0.82 to 4.13) |
| Systemic corticosteroid therapies/y | 0.1 (0.4) | 0.4 (0.3) | −0.26 (−0.41 to −0.10) | NA |
| Systemic antibiotic therapies/y | 0.1 (0.4) | 0.1 (0.2) | −0.01 (−0.15 to 0.14) | NA |
Abbreviations: eCRS, eosinophilic chronic rhinosinusitis; NA, not applicable.
Patients with well-controlled vs poorly controlled disease received fewer courses of systemic corticosteroid medication per year: mean (SD), 0.1 (0.4) vs 0.4 (0.3); mean difference, 0.26; 95% CI, −0.41 to 0.10; and Cohen d effect size, 0.68; 95% CI, 0.29-1.08). There were no significant differences in systemic antibiotic use.
Corticosteroid Irrigation Frequency and Its Association With Topical Therapy Control
When comparing low (<4 per week) and high (≥4 per week) frequency of irrigation, there were no significant differences in symptom improvement based on changes in the SNOT-22 score (mean [SD], −19.4 [22.5] vs −21.2 [24.6]; mean difference, 1.75; 95% CI, −4.82 to 8.33) and NSS (mean [SD], −5.9 [6.1] vs −6.7 [7.0]; mean difference, 0.71; 95% CI, −1.12 to 2.5). There were similar odds of clinically important SNOT-22 score improvement with low vs high frequency of irrigation: 64.8% vs 69.6% (OR, 1.24; 95% CI, 0.69-2.25).
Discussion
A multimodal approach to eCRS includes neosinus cavity ESS that allows for restoration of nasal patency, removal of trapped mucin, and facilitated delivery of topical medications.24 There is evidence that delivery of corticosteroids for this purpose is more effective with high-volume irrigation compared with nasal sprays26,30,31 and corticosteroid nasal drops.32
Tissue eosinophilia typically results in higher rates of postsurgical disease recurrence16,20,21,33 and poorer patient-reported outcomes.33,34,35 Vlaminck et al20 demonstrated a recurrence rate of 32% in eCRS defined as 5 or more eosinophils per high-power field at 3 years or more following surgery; Grgić et al21 found a 41% recurrence rate at 2 years or more in eCRS defined as CRS with nasal polyps with more than 20 eosinophils per high-power field tissue eosinophils. Maintenance therapy is not often reported in these studies.
In contrast, our study demonstrated a 12.2% rate of disease recurrence in a large cohort of patients with eCRS, using current European Position Paper on Rhinosinusitis and Nasal Polyps 2020 criteria for eCRS and a wider definition of treatment failure. The primary intent of the surgery for many of these patients was to establish a local route of administration for anti-inflammatory therapy. Most patients were able to manage their disease with local topical care by using corticosteroid irrigations into the open sinus cavity without recurrence during the long-term postoperative period. Findings of this study suggest that eCRS is a manageable disease when approached as an inflammatory disorder, moving away from the poor prognosis in the surgery-only focused literature.
Previous studies have assessed SNOT-22 scores in an attempt to define eCRS based on poorer baseline and postoperative scores.36,37 This study describes the evolution of symptoms of patients with eCRS treated with multimodal therapy. A substantial reduction in their symptom burden was reflected in their large decreases in SNOT-22 scores and NSS. Overall, primary intervention of eCRS with neosinus surgery and maintenance corticosteroid irrigations appeared to reduce the long-term symptom burden even in patients recommended to receive further intervention.
During the period of this study, mepolizumab and benralizumab were listed on the Australian Pharmaceutical Benefits Scheme for the management of severe asthma, and several patients transitioned to receive one of these biologic therapies from continuous oral corticosteroids because their lower airway disease met the criteria for use of these biologic agents. The patients with eCRS treated with biologics experienced large symptomatic improvement. However, the sample size was small (n = 5). Biologics may potentially play an important role in salvage therapy for patients with disease refractory to topical care.
Poor adherence to maintenance therapy is often cited as a reason for unsuccessful treatment.24 A study by Grayson et al38 assessed the same group of participants as we did in this study and reported that weekly corticosteroid irrigation use was not associated with polyp recurrence. Our study aimed to further elaborate on the outcomes of eCRS, focusing on long-term symptom control. This study found that patients with poorly controlled eCRS were nearly twice as likely to use weekly corticosteroid irrigations compared with patients with well-controlled disease. Overall, patients can self-taper their maintenance therapy to a desired frequency based on day-to-day symptoms and can still achieve good symptom outcomes without risk of failure.
Limitations
This study has limitations. Use of various treatment modalities was not randomized, and the possible roles of confounding factors and treatment selection bias were not accounted for in this study. Patients with less than 12 months of follow-up were excluded to preserve the long-term nature of this study. This study’s results may reflect the outcomes of a cohort of patients with eCRS who have particularly severe disease. For this study, symptom scores were collected only at the final follow-up; thus, the longitudinal clinical trajectory of symptoms across the postoperative period was not illustrated.
Conclusions
For primary diffuse type 2–dominant CRS or eCRS, multimodal therapy appeared to be associated with long-term disease control for more than 85% of patients in this study and associated with substantial symptom reduction regardless of further medical intervention. The results suggest that maintenance corticosteroid irrigations may be self-tapered to achieve good outcomes.
References
- 1.Ferguson BJ. Categorization of eosinophilic chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2004;12(3):237-242. doi: 10.1097/01.moo.0000124938.46948.c7 [DOI] [PubMed] [Google Scholar]
- 2.Sakuma Y, Ishitoya J, Komatsu M, et al. New clinical diagnostic criteria for eosinophilic chronic rhinosinusitis. Auris Nasus Larynx. 2011;38(5):583-588. doi: 10.1016/j.anl.2011.01.007 [DOI] [PubMed] [Google Scholar]
- 3.Barham HP, Osborn JL, Snidvongs K, Mrad N, Sacks R, Harvey RJ. Remodeling changes of the upper airway with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015;5(7):565-572. doi: 10.1002/alr.21546 [DOI] [PubMed] [Google Scholar]
- 4.Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61(11):1280-1289. doi: 10.1111/j.1398-9995.2006.01225.x [DOI] [PubMed] [Google Scholar]
- 5.Bachert C, Zhang N, Holtappels G, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010;126(5):962-968, 968.e1-968.e6. doi: 10.1016/j.jaci.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 6.Zhang N, Van Zele T, Perez-Novo C, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122(5):961-968. doi: 10.1016/j.jaci.2008.07.008 [DOI] [PubMed] [Google Scholar]
- 7.Jiang XD, Li GY, Li L, Dong Z, Zhu DD. The characterization of IL-17A expression in patients with chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2011;25(5):e171-e175. doi: 10.2500/ajra.2011.25.3645 [DOI] [PubMed] [Google Scholar]
- 8.Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(suppl S29):1-464. doi: 10.4193/Rhin20.401 [DOI] [PubMed] [Google Scholar]
- 9.Grayson JW, Hopkins C, Mori E, Senior B, Harvey RJ. Contemporary classification of chronic rhinosinusitis beyond polyps vs no polyps: a review. JAMA Otolaryngol Head Neck Surg. 2020;146(9):831-838. doi: 10.1001/jamaoto.2020.1453 [DOI] [PubMed] [Google Scholar]
- 10.Younis RT, Ahmed J. Predicting revision sinus surgery in allergic fungal and eosinophilic mucin chronic rhinosinusitis. Laryngoscope. 2017;127(1):59-63. doi: 10.1002/lary.26248 [DOI] [PubMed] [Google Scholar]
- 11.Zadeh MH, Banthia V, Anand VK, Huang C. Significance of eosinophilia in chronic rhinosinusitis. Am J Rhinol. 2002;16(6):313-317. doi: 10.1177/194589240201600606 [DOI] [PubMed] [Google Scholar]
- 12.Matsuwaki Y, Ookushi T, Asaka D, et al. Chronic rhinosinusitis: risk factors for the recurrence of chronic rhinosinusitis based on 5-year follow-up after endoscopic sinus surgery. Int Arch Allergy Immunol. 2008;146(suppl 1):77-81. doi: 10.1159/000126066 [DOI] [PubMed] [Google Scholar]
- 13.Bassiouni A, Ou J, Rajiv S, Cantero D, Vreugde S, Wormald PJ. Subepithelial inflammatory load and basement membrane thickening in refractory chronic rhinosinusitis with nasal polyposis: a histopathological study. Int Forum Allergy Rhinol. 2016;6(3):248-255. doi: 10.1002/alr.21661 [DOI] [PubMed] [Google Scholar]
- 14.Brescia G, Marioni G, Franchella S, et al. Can a panel of clinical, laboratory, and pathological variables pinpoint patients with sinonasal polyposis at higher risk of recurrence after surgery? Am J Otolaryngol. 2015;36(4):554-558. doi: 10.1016/j.amjoto.2015.01.019 [DOI] [PubMed] [Google Scholar]
- 15.Lou H, Meng Y, Piao Y, et al. Cellular phenotyping of chronic rhinosinusitis with nasal polyps. Rhinology. 2016;54(2):150-159. doi: 10.4193/Rhin15.271 [DOI] [PubMed] [Google Scholar]
- 16.Lou H, Meng Y, Piao Y, Wang C, Zhang L, Bachert C. Predictive significance of tissue eosinophilia for nasal polyp recurrence in the Chinese population. Am J Rhinol Allergy. 2015;29(5):350-356. doi: 10.2500/ajra.2015.29.4231 [DOI] [PubMed] [Google Scholar]
- 17.Tosun F, Arslan HH, Karslioglu Y, Deveci MS, Durmaz A. Relationship between postoperative recurrence rate and eosinophil density of nasal polyps. Ann Otol Rhinol Laryngol. 2010;119(7):455-459. doi: 10.1177/000348941011900705 [DOI] [PubMed] [Google Scholar]
- 18.De Corso E, Lucidi D, Battista M, et al. Prognostic value of nasal cytology and clinical factors in nasal polyps development in patients at risk: can the beginning predict the end? Int Forum Allergy Rhinol. 2017;7(9):861-867. doi: 10.1002/alr.21979 [DOI] [PubMed] [Google Scholar]
- 19.Weibman AR, Huang JH, Stevens WW, et al. A prospective analysis evaluating tissue biopsy location and its clinical relevance in chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2017;7(11):1058-1064. doi: 10.1002/alr.22005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vlaminck S, Vauterin T, Hellings PW, et al. The importance of local eosinophilia in the surgical outcome of chronic rhinosinusitis: a 3-year prospective observational study. Am J Rhinol Allergy. 2014;28(3):260-264. doi: 10.2500/ajra.2014.28.4024 [DOI] [PubMed] [Google Scholar]
- 21.Grgić MV, Ćupić H, Kalogjera L, Baudoin T. Surgical treatment for nasal polyposis: predictors of outcome. Eur Arch Otorhinolaryngol. 2015;272(12):3735-3743. doi: 10.1007/s00405-015-3519-7 [DOI] [PubMed] [Google Scholar]
- 22.Steinke JW, Borish L. Chronic rhinosinusitis phenotypes. Ann Allergy Asthma Immunol. 2016;117(3):234-240. doi: 10.1016/j.anai.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snidvongs K, Chin D, Sacks R, Earls P, Harvey RJ. Eosinophilic rhinosinusitis is not a disease of ostiomeatal occlusion. Laryngoscope. 2013;123(5):1070-1074. doi: 10.1002/lary.23721 [DOI] [PubMed] [Google Scholar]
- 24.Snidvongs K, Pratt E, Chin D, Sacks R, Earls P, Harvey RJ. Corticosteroid nasal irrigations after endoscopic sinus surgery in the management of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2012;2(5):415-421. doi: 10.1002/alr.21047 [DOI] [PubMed] [Google Scholar]
- 25.Harvey RJ, Goddard JC, Wise SK, Schlosser RJ. Effects of endoscopic sinus surgery and delivery device on cadaver sinus irrigation. Otolaryngol Head Neck Surg. 2008;139(1):137-142. doi: 10.1016/j.otohns.2008.04.020 [DOI] [PubMed] [Google Scholar]
- 26.Kalish L, Snidvongs K, Sivasubramaniam R, Cope D, Harvey RJ. Topical steroids for nasal polyps. Cochrane Database Syst Rev. 2012;12(12):CD006549. [DOI] [PubMed] [Google Scholar]
- 27.Snidvongs K, Dalgorf D, Kalish L, Sacks R, Pratt E, Harvey RJ. Modified Lund Mackay Postoperative Endoscopy Score for defining inflammatory burden in chronic rhinosinusitis. Rhinology. 2014;52(1):53-59. doi: 10.4193/Rhin13.056 [DOI] [PubMed] [Google Scholar]
- 28.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34(5):447-454. doi: 10.1111/j.1749-4486.2009.01995.x [DOI] [PubMed] [Google Scholar]
- 29.Faul F, Erdfelder E, Lang A, Buchner A, Cohen J. Statistical Power Analysis for the Behavioral Sciences. Routledge Academic; 1988. [Google Scholar]
- 30.Harvey RJ, Snidvongs K, Kalish LH, Oakley GM, Sacks R. Corticosteroid nasal irrigations are more effective than simple sprays in a randomized double-blinded placebo-controlled trial for chronic rhinosinusitis after sinus surgery. Int Forum Allergy Rhinol. 2018;8(4):461-470. doi: 10.1002/alr.22093 [DOI] [PubMed] [Google Scholar]
- 31.Harvey RJ, Schlosser RJ. Local drug delivery. Otolaryngol Clin North Am. 2009;42(5):829-845, ix. doi: 10.1016/j.otc.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 32.Harvey RJ, Debnath N, Srubiski A, Bleier B, Schlosser RJ. Fluid residuals and drug exposure in nasal irrigation. Otolaryngol Head Neck Surg. 2009;141(6):757-761. doi: 10.1016/j.otohns.2009.09.006 [DOI] [PubMed] [Google Scholar]
- 33.Tokunaga T, Sakashita M, Haruna T, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy. 2015;70(8):995-1003. doi: 10.1111/all.12644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al Abdulla A, Darwish A, Bella MH. Chronic eosinophilic rhinosinusitis clinical implications. Bahrain Med Bull. 2017;39(2):92-95. doi: 10.12816/0047528 [DOI] [Google Scholar]
- 35.Adnane C, Adouly T, Khallouk A, et al. Using preoperative unsupervised cluster analysis of chronic rhinosinusitis to inform patient decision and endoscopic sinus surgery outcome. Eur Arch Otorhinolaryngol. 2017;274(2):879-885. doi: 10.1007/s00405-016-4315-8 [DOI] [PubMed] [Google Scholar]
- 36.Soler ZM, Sauer DA, Mace J, Smith TL. Relationship between clinical measures and histopathologic findings in chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2009;141(4):454-461. doi: 10.1016/j.otohns.2009.06.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soler ZM, Sauer D, Mace J, Smith TL. Impact of mucosal eosinophilia and nasal polyposis on quality-of-life outcomes after sinus surgery. Otolaryngol Head Neck Surg. 2010;142(1):64-71. doi: 10.1016/j.otohns.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grayson JW, Li W, Ho J, et al. Topography of polyp recurrence in eosinophilic chronic rhinosinusitis. Int Forum Allergy Rhinol. 2020;10(5):604-609. doi: 10.1002/alr.22529 [DOI] [PubMed] [Google Scholar]