Key Points
Question
Is magnetic resonance imaging (MRI) with targeted biopsy only noninferior to systematic biopsy for the diagnosis of clinically significant prostate cancer (PCa)?
Findings
In this prospective phase 3 randomized clinical trial of 453 men, clinically significant cancer was found in 35% vs 30% in the MRI and systematic biopsy arms, respectively, which demonstrated noninferiority. A total of 79 participants in the MRI arm (37%) avoided a biopsy, and diagnosis of grade group 1 PCa was reduced by more than 50%.
Meaning
Magnetic resonance imaging with targeted biopsy alone resulted in similar detection rates of clinically significant PCa while avoiding biopsy in more than one-third of men and reducing the diagnosis of clinically insignificant cancer.
Abstract
Importance
Magnetic resonance imaging (MRI) with targeted biopsy is an appealing alternative to systematic 12-core transrectal ultrasonography (TRUS) biopsy for prostate cancer diagnosis, but has yet to be widely adopted.
Objective
To determine whether MRI with only targeted biopsy was noninferior to systematic TRUS biopsies in the detection of International Society of Urological Pathology grade group (GG) 2 or greater prostate cancer.
Design, Setting, and Participants
This multicenter, prospective randomized clinical trial was conducted in 5 Canadian academic health sciences centers between January 2017 and November 2019, and data were analyzed between January and March 2020. Participants included biopsy-naive men with a clinical suspicion of prostate cancer who were advised to undergo a prostate biopsy. Clinical suspicion was defined as a 5% or greater chance of GG2 or greater prostate cancer using the Prostate Cancer Prevention Trial Risk Calculator, version 2. Additional criteria were serum prostate-specific antigen levels of 20 ng/mL or less (to convert to micrograms per liter, multiply by 1) and no contraindication to MRI.
Interventions
Magnetic resonance imaging–targeted biopsy (MRI-TB) only if a lesion with a Prostate Imaging Reporting and Data System (PI-RADS), v 2.0, score of 3 or greater was identified vs 12-core systematic TRUS biopsy.
Main Outcome and Measures
The proportion of men with a diagnosis of GG2 or greater cancer. Secondary outcomes included the proportion who received a diagnosis of GG1 prostate cancer; GG3 or greater cancer; no significant cancer but subsequent positive MRI results and/or GG2 or greater cancer detected on a repeated biopsy by 2 years; and adverse events.
Results
The intention-to-treat population comprised 453 patients (367 [81.0%] White, 19 [4.2%] African Canadian, 32 [7.1%] Asian, and 10 [2.2%] Hispanic) who were randomized to undergo TRUS biopsy (226 [49.9%]) or MRI-TB (227 [51.1%]), of which 421 (93.0%) were evaluable per protocol. A lesion with a PI-RADS score of 3 or greater was detected in 138 of 221 men (62.4%) who underwent MRI, with 26 (12.1%), 82 (38.1%), and 30 (14.0%) having maximum PI-RADS scores of 3, 4, and 5, respectively. Eighty-three of 221 men who underwent MRI-TB (37%) had a negative MRI result and avoided biopsy. Cancers GG2 and greater were identified in 67 of 225 men (30%) who underwent TRUS biopsy vs 79 of 227 (35%) allocated to MRI-TB (absolute difference, 5%, 97.5% 1-sided CI, −3.4% to ∞; noninferiority margin, −5%). Adverse events were less common in the MRI-TB arm. Grade group 1 cancer detection was reduced by more than half in the MRI arm (from 22% to 10%; risk difference, −11.6%; 95% CI, −18.2% to −4.9%).
Conclusions and Relevance
Magnetic resonance imaging followed by selected targeted biopsy is noninferior to initial systematic biopsy in men at risk for prostate cancer in detecting GG2 or greater cancers.
Trial Registration
ClinicalTrials.gov Identifier: NCT02936258
This randomized clinical trial compares magnetic resonance imaging with targeted biopsy with systematic biopsy for the diagnosis of clinically significant prostate cancer.
Introduction
For 35 years, the standard pathway for prostate cancer diagnosis has been systematic transrectal ultrasonography biopsy (TRUSBx) of the prostate in patients with elevated prostate-specific antigen (PSA) levels. Transrectal ultrasonography is used primarily for anatomic guidance, as traditional ultrasonography discriminates poorly between cancerous and noncancerous tissue.1
With multiparametric magnetic resonance imaging (MRI), the additional conspicuity of cancer offered by diffusion-weighted imaging and dynamic contrast-enhancing imagine has improved the diagnostic accuracy of MRI for cancer detection.2 In studies examining the correlation between MRI and radical prostatectomy specimens, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value were 45% to 90%, 40% to 88%, 77% to 81%, and 67% to 95%, respectively, for the identification of prostate tumors greater than 0.5 mL3,4,5 or clinically significant disease.6,7,8,9,10 These metrics are also a function of grade, insofar as MRI is more sensitive for higher-grade cancers.3,11 The evidence is less clear in active surveillance.12,13,14
There is an unmet need for a test that identifies clinically significant prostate cancer without overdiagnosing insignificant cancer. The objective of this study was to determine if MRI with only targeted biopsy is noninferior to 12-core systematic biopsies for the diagnosis of clinically significant prostate cancer.
This study was designed independently but in coordination with the PRECISION study, a recent European-based, prospective randomized multicenter study that compared MRI-targeted biopsy (MRI-TB) alone with systematic biopsy.15,16 Achieving funding, implementation, and accrual was a more prolonged process for PRECISE. Despite the encouraging results of MRI-TB reported by multiple high-quality studies, including PRECISION,15 PROMIS,17 MRI-FIRST,18 4M,19 and a Cochrane meta-analysis,20 in many jurisdictions, MRI before biopsy is not yet part of routine clinical practice.21
In contrast to PRECISION, the PRECISE trial included risk-based eligibility, systematic follow-up of all patients for 2 years, including a repeated MRI in all untreated patients, the investigation of fluid- and tissue-based biomarkers in the cohort, and an economic analysis.
Methods
This was a multicenter, randomized, noninferiority trial at 5 centers in Canada. Following acquisition of written informed consent, men were randomized in a 1:1 ratio to undergo either systematic 12-core TRUSBx or MRI, with targeted biopsy of lesions with a Prostate Imaging Reporting and Data System (PI-RADS), v 2.0, score of 3 or greater. In the absence of lesions with a PI-RADS score of 3 or greater (a negative MRI result), no biopsy was performed. The protocol was approved by the research ethics board at each participating institution and monitored by the trial steering committee and an independent data safety monitoring committee (Supplement 1). Participants provided written informed consent.
The primary outcome was the proportion of men with clinically significant cancer (grade group [GG] ≥2) diagnosed in each arm. Secondary outcomes included the proportion of men in the 2 arms who were found to have clinically insignificant cancer (GG1), GG3 or greater cancer, no significant cancer but subsequent positive MRI results and/or GG2 or greater cancer detected on a repeated biopsy by 2 years, postbiopsy adverse events, and definitive local treatment (eg, radical prostatectomy, external beam radiotherapy, or brachytherapy) or systemic treatment (eg, hormone therapy and chemotherapy). In the MRI arm, we evaluated the proportion of men who avoided biopsy and the number of men with lesions with a PI-RADS score of 3, 4, or 5 but no detection of clinically significant cancer. Health-related quality of life scores were also assessed using the EuroQol-5D validated questionnaire.
Eligible patients were men recruited from the outpatient clinics with clinical suspicion of prostate cancer and were advised to undergo a prostate biopsy. Additional eligibility criteria included a 5% or greater chance of GG2 or greater prostate cancer as calculated individually using the Prostate Cancer Prevention Trial Risk Calculator (PCPTRAC), v 2 (http://deb.uthscsa.edu/URORiskCalc/Pages/calcs.jsp), serum PSA levels of 20 ng/mL or less (to convert to micrograms per liter, multiply by 1), no prior biopsy or treatment for prostate cancer, suitable candidates for a biopsy, and no contraindication for an MRI. A dynamic allocation process was used for randomization, including stratification factors of (1) individualized risk of GG2 or greater prostate cancer (5%-25%; >25% as measured by the PCPTRC 2.0 calculator) and (2) treatment center. A web-based interactive system was used. Allocation of the first 20 patients was performed so that patients were allocated to each arm with a probability of 0.5. From patient 21 onwards, if an imbalance was observed based on the 2 stratification factors, patients were allocated to the treatment arm, which minimized the imbalance with a probability of 0.8. The dynamic allocation process was programmed by individuals who had no patient contact or involvement in patient enrollment or selection.
The men in the MRI-TB arm who had a negative MRI result and those in the TRUSBx arm whose biopsy results were negative were scheduled to have a repeated MRI at 2 years. The MRI was performed according to PI-RADS, v 2.0, guidelines.22,23 All centers used a 3-T scanner without an endorectal coil. The MRI was interpreted according to PI-RADS, v 2.0, guidelines by the site radiologists, all of whom were experienced in the interpretation of at least 500 prostate MRIs. Men whose MRI showed a score of 3, 4, or 5 underwent MRI- TB using TRUS guidance with 4 cores per lesion. Coregistration was performed using TRUS MRI fusion software at all centers (Artemis, UroNav, or Koelis). The fusion biopsies were performed by experienced radiologists or urologists who had performed at least 50 prior MRI-informed fusion-targeted biopsies.
Statistical Analysis
The primary analysis was based on an intention-to-treat (ITT) noninferiority outcome that was defined as the proportion of men in each arm who received a diagnosis of clinically significant prostate cancer (≥GG2). The rate of detection of GG2 or greater cancer by targeted-alone biopsy in a population with no prior biopsy was estimated at between 42%24 and 50%,25 vs 27% with 12-core TRUSBx.26 Therefore, it was predicted that MRI-TB would identify more than 15% more GG2 or greater cancer than systematic biopsy. For sample size calculations, it was conservatively hypothesized that systematic biopsy would detect clinically significant cancer in 30% of men, and MRI-TB would detect clinically significant cancer in 10% more men (ie, 40% total). For the noninferiority hypothesis, using 90% power and a 2.5% 1-sided α, assuming an MRI-TB detection rate of clinically significant cancer of 40% and a detection rate for TRUSBx of 30%, and using a margin of clinical unimportance of 5%, 211 men per arm would be required (422 total). The choice of 5% as the margin of noninferiority represented a clinically important difference based on expert consensus.22 If noninferiority was met, a superiority analysis was to be performed, with superiority met if the bound of the 1-sided 97.5% CI exceeded 0%. To account for potential withdrawal/loss to follow-up and the effect of stratification, the sample size was inflated by 5%, and a target of 450 men was established.
Descriptive statistics were used to summarize the patient characteristics and outcomes of interest (Supplement 2). Absolute risk differences and 95% CIs were constructed using the Wald method. A multivariable logistic regression analysis was performed that investigated the effect of treatment arm, adjusted for stratification factors (study center and baseline risk). Logistic regression was also used to explore the effects prognostic of detecting GG2 or greater cancer within each treatment arm separately. A multivariable model was constructed within each treatment arm based on forward stepwise selection using the P < .05 criterion. Linearity assumptions were examined using a visual inspection of residuals. Logarithmic transformations of nonlinear continuous variables were used when appropriate. No interpolation for missing data occurred, and patients with missing data were categorized in a separate group for regression analyses. All the statistical analysis were conducted using SAS, version 9 for Microsoft Window (SAS Institute) or R, v 3.2.2 (R Foundation; http://www.r-project.org). The plan for the statistical analysis was prespecified and approved by the data safety monitoring committee.
Populations
All participants who underwent randomization were included in the ITT analysis. Analyses based on the per protocol and biopsy population were performed as supportive efficacy analyses (eTable 1 in Supplement 3). Safety analyses were performed on the men who underwent a biopsy.
Results
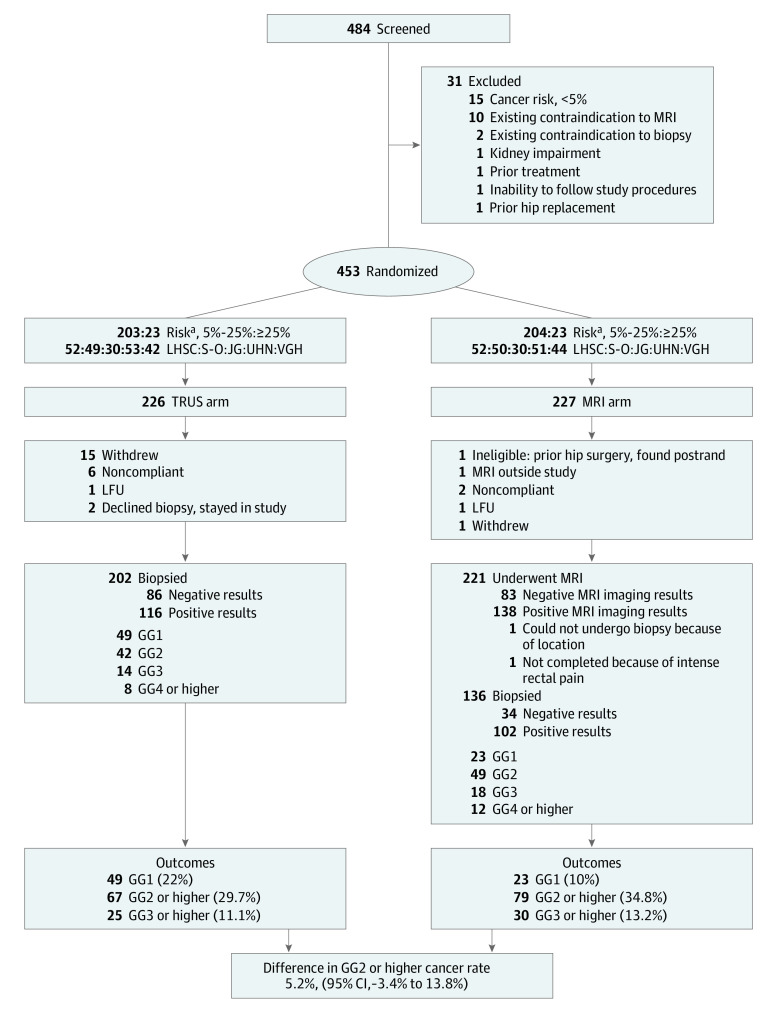
Between April 2017 and November 2019, 453 patients were enrolled, with 226 and 227 men allocated to the TRUSBx arm and MRI arm, respectively. Twenty-four men in the TRUSBx arm (10.6%) left the study before the first study intervention, including 15 who withdrew. Six men in the MRI arm (2.6%) left the study before the MRI (Figure).
Figure. CONSORT Diagram.
GG indicates International Society of Urological Pathology grade group; LFU, lost to follow-up; MRI, magnetic resonance imaging; postrand, postrandomization; TRUS, transrectal ultrasonography.
aAs measured using the Prostate Cancer Prevention Trial Risk Calculator, v 2.0 calculator, found at http://deb.uthscsa.edu/URORiskCalc/Pages/calcs.jsp.
Patient characteristics are summarized in Table 1. Patient characteristics were well balanced.
Table 1. Baseline Characteristics.
| Baseline characteristics | No. (%) | |
|---|---|---|
| Control (TRUS-guided biopsy) | Experimental (MRI-guided biopsy) | |
| Participants, No. | 226 | 227 |
| Eligibility and stratum | ||
| Riska | ||
| 5%-25% | 232 (90) | 204 (90) |
| ≥25% | 23 (10) | 23 (10) |
| Study center | ||
| London | 52 (23) | 52 (23) |
| Sunnybrook | 49 (22) | 50 (22) |
| Jewish General | 30 (13) | 30 (13) |
| UHN | 53 (23) | 51 (22) |
| Vancouver General | 42 (10) | 44 (19) |
| Demographic characteristics | ||
| Age at registration, mean (SD), y | 64.5 (8.8) | 65.3 (7.6) |
| Race | ||
| White | 184 (81) | 183 (81) |
| African Canadian | 8 (3.5) | 11 (4.9) |
| Asian | 17 (7.5) | 15 (6.6) |
| Hispanic | 4 (1.8) | 6 (2.6) |
| Other | 12 (5.3) | 12 (5.3) |
| Unknown | 1 (0.4) | 0 |
| Family history | ||
| No | 133 (59) | 152 (67) |
| Yes | 74 (33) | 63 (28) |
| Do not know | 19 (8.4) | 12 (5.3) |
| ECOG performance status score, ≥1 | 1 (0.4) | 2 (0.9) |
| Height, mean (SD), cm | 174.8 (7.1) | 175.6 (7.0) |
| Weight, mean (SD), kg | 84.5 (13) | 83.6 (14) |
| BMI, mean (SD) | 27.7 (4.1) | 27.1 (4.0) |
| Body surface area, mean (SD) | 2.0 (0.2) | 2.0 (0.2) |
| Prostate characteristics | ||
| PSA levels, ng/dL | ||
| Mean (SD) | 6.8 (3.0) | 7.5 (3.6) |
| Median (range) | 6.2 (1.0-19) | 6.7 (1.1-24) |
| Prostate volume, mean (SD), cca | 48 (25) | 60 (45) |
| NA | 17 | 11 |
| ≤20 | 4 (1.9) | 5 (2.3) |
| 21-34 | 67 (32) | 58 (27) |
| 35-49 | 101 (48) | 96 (44) |
| ≥50 | 37 (18) | 57 (26) |
| PSA density, mean (SD) | 0.17 (0.11) | 0.16 (0.11) |
| Palpable tumor | ||
| NA | 2 | 6 |
| Normal | 165 (74) | 161 (73) |
| Nodule, cm | ||
| ≤1.5 | 48 (21) | 48 (22) |
| >1.5 | 8 (3.6) | 12 (5.4) |
| Both lobes | 3 (1.3) | 0 (0.0) |
| Kidney impairment, yes, No./No. (%) | 23/76 (30) | 26/79 (33) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ECOG, Eastern Cooperative Oncology Group; MRI, multiparametric magnetic resonance imaging; NA, not applicable; PSA, prostate-specific antigen; TRUS, transrectal ultrasonography; UHN, University Health Network.
SI conversion factor: To convert PSA to micrograms per liter, multiply by 1.
As measured using the Prostate Cancer Prevention Trial Risk Calculator, v 2.0 calculator, found at http://deb.uthscsa.edu/URORiskCalc/Pages/calcs.jsp.
Primary Outcome
Study outcomes in the ITT population are summarized in Table 2. Of the patients who underwent MRI, 138 of 221 (62.4%) had positive results (PI-RADS score, ≥3), with lesions with a PI-RADS score of 3, 4, or 5 in 26 (12.1%), 82 (38.1%), and 30 (14.0%) men, respectively. Eighty-three of 221 men (36.6%; 95% CI, 30.3%- 43.2%) had a negative MRI result (PI-RADS score, ≤2) and therefore avoided a biopsy. After undergoing biopsy, 67 men (29.7%) in the TRUSBx arm and 79 men (34.8%) in the MRI arm had GG2 or greater cancer detected, resulting in an absolute risk difference of 5.2% (97.5% 1-sided CI, −3.4% to ∞). The lower bound of this confidence interval exceeded the prespecified noninferiority boundary of −5%, thus demonstrating the noninferiority of the MRI-TB approach. A test for superiority was then conducted and observed to be not statistically significant (P = .27).
Table 2. Outcomes.
| Characteristic | No. (%) | |
|---|---|---|
| TRUS-guided biopsy | MRI-guided biopsy | |
| Participants, No. | 226 | 227 |
| MRI result | ||
| PI-RADS score | NA | |
| 1-2/negative | 83 (38) | |
| 3-5/positive | 138 (61) | |
| 3 | 26 (12) | |
| 4 | 82 (38) | |
| 5 | 30 (14) | |
| Biopsy | ||
| Did not undergo | 24 (11) | 91 (40) |
| Underwent | 202 (89) | 136 (60) |
| No cancer on biopsy | 86 (38) | 34 (15) |
| Cancer | ||
| GG1 or none (including no biopsy) | 159 (70) | 148 (65) |
| GG1 | 49 (22) | 23 (10) |
| GG2 | 42 (19) | 49 (22) |
| GG2 or highera | 67 (30) | 79 (35) |
| GG3 | 17 (8) | 18 (8) |
| GG3 or higher | 25 (11) | 30 (13) |
| GG4 | 3 (1) | 5 (2) |
| GG5 | 5 (2) | 7 (3) |
Abbreviations: GG, International Society of Urological Pathology grade group; MRI, multiparametric magnetic resonance imaging; NA, not applicable; PI-RADS, Prostate Imaging Reporting and Data System; TRUS, transrectal ultrasonography.
Primary outcome.
Results were similar in the per protocol population. However, the lower bound of the 95% 1-sided CI was slightly lower than the predetermined 5% threshold. Of 202 men in the TRUSBx arm, there were 67 (33.2%) with GG2 or greater cancers detected compared with 79 of 219 (36.1%) in the MRI-TB arm. The risk difference was 2.9% (95% CI, −6.2% to 12.0%), with the superiority test deemed not statistically significant (P = .54).
Secondary Outcomes
A total of 25 men (11.1%) in the TRUSBx arm and 30 men (13.2%) in the MRI arm had GG3 or greater cancer detected, resulting in an absolute risk difference of 2.2% (97.5% CI, −3.9% to ∞). The test for superiority was not statistically significant (P = .57). There were fewer diagnoses of GG1 cancer in the MRI-TB arm (23 [10.1%] vs 49 [21.7%]; absolute difference, 11.6%; 95% CI, −18.2% to −4.9%; P < .001). The PI-RADS score and biopsy grade correlated closely (Table 3). The rate of diagnosis of GG2 or greater cancer for PI-RADS scores of 3, 4, and 5 was 4 of 24 (16.7%), 49 of 82 (59.8%), and 26 of 30 (86.7%), respectively, while for GG3 or greater cancer it was 2 of 24 (8.3%), 16 of 82 (19.5%), and 12 of 30 (40.0%). The core number was higher in the TRUSBx arm (mean [SD] of 11.4 [1.3] vs 6.3 [2.6] per patient) despite having the same number (mean = 4.4 in each arm) of positive cores per patient. Across all patients, 36.8% of TRUSBx cores were positive compared with 55.2%of the MRI-TB cores. No substantial differences were observed in the type of treatment received between intervention arms.
Table 3. Secondary Outcomes.
| Characteristic | Participants, No. (%) | |
|---|---|---|
| TRUS-guided biopsy | MRI-guided biopsy | |
| Randomization to MRI, median (IQR), d | NA | 19 (11-36) |
| MRI to biopsy, median (IQR), d | NA | 23 (14-39) |
| Randomization to biopsy, median (IQR), d | 30 (16-46) | 53 (34-82) |
| Further treatments | ||
| Further diagnostic testing | ||
| No. (%) | 92 (41) | 92 (40) |
| PSA | 66 (29) | 74 (33) |
| MRI | 43 (19) | 25 (11) |
| Biopsy | 4 (1.8) | 3 (1.3) |
| Active surveillance | 75 (33) | 69 (30) |
| Radical treatment | ||
| No. (%) | 37 (16) | 43 (19) |
| Prostatectomy | 21 (9.3) | 30 (13) |
| Radiotherapy | 13 (5.8) | 13 (5.7) |
| Unknown | 3 (1.3) | 0 |
| Minimally invasive therapy (HIFU) | 3 (1.3) | 3 (1.3) |
| Hormone therapy | 6 (2.7) | 3 (1.3) |
| Other treatment | 18 (8.0) | 22 (9.7) |
| Deaths during follow-up | 0 | 2 (0.9) |
| AEs | ||
| No. of patients with at least 1 AE | 145 (64) | 89 (40) |
| Most frequent AE experienced | ||
| Erectile dysfunction | 8 (4) | 10 (4) |
| Hematochezia | 36 (16) | 22 (10) |
| Hematospermia | 95 (42) | 51 (22) |
| Hematuria | 109 (48) | 44 (19) |
| Pain | 72 (32) | 34 (15) |
| Prostatitis | 9 (4) | 1 (<1) |
| Urinary incontinence | 12 (5) | 5 (2) |
| No. of patients with at least 1 grade ≥3 AE | 10 (4.4) | 8 (3.5) |
| Appendicitis | 0 | 1 (0.4) |
| Arthritis | 0 | 1 (0.4) |
| Chills | 1 (0.4) | 0 |
| Erectile dysfunction | 0 | 1 (0.4) |
| Fever | 1 (0.4) | 0 |
| Hematuria | 2 (0.9) | 0 |
| Hematochezia | 0 | 1 (0.4) |
| Pain | 1 (0.4) | 0 |
| Prostatitis | 1 (0.4) | 0 |
| Sepsis | 4 (1.8) | 1 (0.4) |
| Urinary retention | 1 (0.4) | 0 |
| Urinary tract infection | 3 (1.3) | 1 (0.4) |
Abbreviations: AE, adverse event; HIFU, high intensity focal ultrasonography; IQR, interquartile range; MRI, multiparametric magnetic resonance imaging; PSA, prostate-specific antigen; TRUS, transrectal ultrasonography.
A logistic regression analysis demonstrated no significant difference in the detection of GG2 or greater cancer (odds ratio, 1.28 for MRI-TB vs TRUSBx; 95% CI, 0.86-1.93; P = .23) or GG3 or greater cancer (odds ratio, 1.23 for MRI-TB vs TRUSBx; 95% CI, 0.69- 2.20; P = .49). In those men who underwent a biopsy, GG2 or greater cancer was detected in 79 (58.1%) by MRI-TB and 67 (33.2%) by TRUSBx (P < .001). This represents an absolute difference of 24.9% (95% CI, 14.4%-35.5%). In the same biopsy population, GG1 was detected in 23 men (16.9%) in the MRI-TB arm compared with 49 (24.3%) in the TRUSBx arm (P = .14; absolute risk difference, 7.4%; 95% CI, −1.3% to 16.0.
Adverse events are summarized in Table 3. The MRI arm experienced 25% fewer adverse events. Prostatitis, hematuria, hematospermia, and incontinence occurred less frequently in the MRI arm. Among only those men who underwent biopsy, the rate of adverse events remained in favor of the MRI-TB arm, although with a reduced risk difference. The proportion of patients who indicated that another biopsy would be a major or moderate problem was 35 (15.4%) vs 14 (6.2%) (absolute risk difference, 9.2%; 95% CI, 3.5%-14.9%; P = .002). Further, TRUSBx patients were more likely to consider undergoing another biopsy a problem in 91 of 170 patients (53%) compared with 45 of 120 MRI-TB patients (37%). No significant differences were observed in the self-reported quality of life as measured by the EuroQol-5D at baseline or at the follow-up visit (eTable 2 in Supplement 3).
Prognostic Factors
The results of logistic regression analyses that evaluated potential prognostic factors of GG2 or greater cancer are presented in Table 4. In the multivariable model baseline risk, PSA levels, PSA density, and palpable tumor were prognostic for the diagnosis of GG2 or greater cancer among patients who underwent TRUSBx. In the MRI arm, age, PSA density, body mass index (calculated as weight in kilograms divided by height in meters squared), and palpable tumor were prognostic.
Table 4. Logistic Regression of Prognostic Factors of GG2 or Higher Cancer.
| Factor | TRUS Bx arm (n = 202) | MRI arm (n = 221) | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age, y | 1.06 (1.01-1.10) | .01 | 1.07 (1.03-1.11) | .001 |
| Risk (high vs low risk) | 11.13 (3.57-34.71) | <.001 | 1.93 (0.80-4.67) | .15 |
| PSA (log-transform) | 5.40 (2.41-12.11) | <.001 | 2.76 (1.43-5.33) | .003 |
| BMI, U | 0.98 (0.91-1.05) | .53 | 1.05 (0.98-1.13) | .17 |
| Volume, U | 0.97 (0.95-0.99) | <.001 | 1.00 (0.99-1.00) | .41 |
| PSA density/0.1 U | 2.78 (1.88-4.10) | <.001 | 1.68 (1.25-2.27) | <.001 |
| Center | ||||
| LHSC | 1.77 (0.73-4.26) | .01 | 0.58 (0.24-1.41) | .35 |
| Sunnybrook | 0.50 (0.18-1.39) | 1.41 (0.60-3.30) | ||
| Jewish General | 2.86 (1.01-8.12) | 0.91 (0.34-2.47) | ||
| UHN | 0.75 (0.29-1.97) | 1.06 (0.45-2.50) | ||
| Vancouver | 1 [Reference] | 1 [Reference] | ||
| Family history | ||||
| Yes | 1 [Reference] | .59 | 1 [Reference] | .94 |
| No | 1.06 (0.57-2.00) | 1.12 (0.60-2.09) | ||
| Do not know | 1.87 (0.56-6.22) | 1.12 (0.29-4.25) | ||
| Palpable tumor | ||||
| Normal | 1 [Reference] | .01 | 1 [Reference] | .01 |
| Abnormal, cm | ||||
| ≤1.5 | 2.55 (1.27-5.12) | 3.04 (1.54-6.01) | ||
| >1.5 | 6.99 (1.30-37.49) | 1.74 (0.53-5.76) | ||
| Both lobes | 5.59 (0.49-63.38) | - | ||
| Prostate volume | ||||
| ≤20 | 7.67 (0.70-83.74) | .40 | Undefined | .32 |
| 21-35 | 1.38 (0.54-3.51) | 1.44 (0.64-3.24) | ||
| 36-50 | 1.20 (0.49-2.91) | 1.97 (0.96-4.06) | ||
| ≥51 | 1 [Reference] | 1 [Reference] | ||
| Multivariable analysis | ||||
| Risk (high vs low risk) | 4.98 (1.38-17.97) | .01 | NA | NA |
| Palpable tumor | ||||
| Normal | 1 [Reference] | .07 | 1 [Reference] | .001 |
| Abnormal, cm | ||||
| ≤1.5 | 2.51 (1.10-5.72) | 4.40 (1.96-9.89) | ||
| >1.5 | 6.05 (0.85-42.97) | 1.94 (0.51-7.45) | ||
| Both lobes | 2.36 (0.07-79.04) | |||
| PSA density/0.1 U | 2.24 (1.59-3.69) | <.001 | 2.16 (1.52-3.09) | <.001 |
| Age/y | NA | NA | 1.08 (1.03-1.13) | .001 |
| BMI | 1.15 (1.05-1.26) | .003 | ||
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GG, International Society of Urological Pathology grade group; LHSC, London Health Sciences Centre; MRI, multiparametric magnetic resonance imaging; NA, not applicable; OR, odds ratio; PSA, prostate-specific antigen; TRUS Bx, systematic transrectal ultrasonography–guided biopsy; UHN, University Health Network.
Intersite Analyses
eTable 3 in Supplement 3 shows differences in outcomes of interest among participating sites to explore potential effects because of differences in familiarity with the MRI fusion software as well as intersite variability in terms of population/operator/treatment differences. The frequency of detecting cancers (ie, having a cancer with a PI-RADS score ≥3) using MRI was significantly different, ranging from 42% to 83% (P = .01), as was the number of GG2 or greater cancer (from 50% to 82%; P = .02) among men who underwent a biopsy; however, the number of men with GG3 or greater cancers (from 42% to 76%; P = .14) detected was not different among patients who underwent a biopsy in the MRI arm. The center with the highest GG2 or greater MRI biopsy cancer detection rate had the lowest TRUSBx rate, and vice versa.
Discussion
This study met its primary end point, demonstrating the noninferiority of MRI-TB to conventional systematic biopsy based on the ITT population. Secondary outcomes demonstrated that there was a reduction in the rate of men undergoing biopsy of almost 40%, a substantially reduced rate of GG1 cancers or no cancer found in men who underwent biopsy, and a decreased adverse event profile. In those who underwent biopsy, targeted biopsy was superior to systematic biopsy for the detection of GG2 or greater cancer. Therefore, the strategy of MRI before biopsy appears to be superior to systematic TRUSBx.
The performance of the MRI-TB varied between centers, with differences in positive MRI rates and target biopsy yields. This difference occurred despite the fact that all MRIs were reviewed, and biopsies performed, by experienced radiologists or urologists. This underscores the need for quality control measures to enable the broad application of MRI. A central review of the MRI images in this study is planned for a subsequent analysis.
This study was designed along similar lines to the PRECISION study.15,20 The results are similar, albeit with some differences. In this study, 38% of participants had a negative MRI result and avoided biopsy; in PRECISION, it was 28%. The absolute difference in the finding of clinically significant cancer was 5.2% in PRECISE vs 12% in PRECISION. The diagnosis rate for GG1 in the MRI vs systematic biopsy arms was 10.4% vs 24.3% in PRECISE and 9% vs 22% in PRECISION, which was remarkably similar. Among men who underwent a biopsy, the likelihood of GG2 or greater cancer in the MRI arm vs TRUSBx arm was 58% vs 33% in PRECISE and 44$ vs 18% in PRECISION (a 25% absolute difference in both studies). There are many potential reasons for these differences, including variation in expertise in MRI interpretation and targeted biopsy, differences between the cohorts, and variations in the systematic biopsy approach. We hope to gain greater insight by central review of the MRI evaluations as well as follow-up MRI at 2 years.
A multivariable logistic regression analysis identified PSA density and palpable tumor as prognostic factors in both groups. Baseline risk and PSA levels were prognostic in the TRUS arm only, and age and body mass index in the MRI arm only. These parameters are well established prognostic features in men at risk for prostate cancer.
There was a trend toward lower complications from biopsy in the MRI arm. These patients had fewer cases of prostatitis, sepsis events, visits to the emergency room, and hospital admissions. In contrast, PRECISION showed no difference in serious adverse events between the 2 arms (2% in each group). Moderate or major resistance to the prospect of another biopsy was 43% less common (absolute difference, 8.9%) among MRI arm patients. There was greater lack of compliance in the TRUSBx arm, with 15 patients withdrawing because they refused TRUSBx.
The PPV of a PI-RADS score of 3, 4, or 5 for GG2 or greater cancer was 17%, 60%, and 87%, respectively. These figures are markedly superior to the predictive value reported by our group in the ASIST trial recently,12 in which the analogous PPV was 13%, 24%, and 33%. The reported PPV of MRI varies widely.27,28 A recent overview of 26 centers estimated that the PPV for a PI-RADS score of 4 or greater was 49% (95% CI, 40%-58%).28 The PPV results in PRECISE are comparable with those reported in these studies. There are several possible explanations for this variability, including different populations (biopsy-naive patients in PRECISE vs low-risk active surveillance patients in ASIST). The most compelling explanation is that ASIST results reflected the learning curve of fusion-targeted biopsy. Furthermore, fewer samples per lesion were mandated in ASIST (1-3 per lesion vs 4).
Several parameters, particularly PSA density, have been identified as predictors for the risk of significant cancer in men with a negative MRI.23 Long-term follow-up on the PRECISE cohort should provide important information on this question.
Limitations
Among men who underwent MRI, 40% did not have a biopsy, and a few of these individuals may have significant cancer. All of the patients without a diagnosis or with a GG1 diagnosis in this study will be followed up for 2 years and will have an MRI at the end of that period. Patients who had a concerning change in a clinical parameter that suggested undiagnosed significant prostate cancer were able to undergo a biopsy off protocol. The outcome of this strategy, including the proportion of patients in each group who received a diagnosis during the period of follow-up, will be evaluated once all patients have reached the 2-year end point. The incremental value of systematic biopsies in men undergoing targeted biopsies was not addressed by this study design. The MRI interpretation and biopsies were performed by experienced radiologists, thus limiting the generalizability of the results with respect to less experienced clinicians or residents. It is possible that the disease phenotype of targeted biopsy–detected cancers varies from that of systematic biopsy–detected cancers29; this question may be addressed by long-term follow-up studies.
Conclusions
The intervention of MRI followed by MRI-guided biopsy in men at risk of prostate cancer results in similar detection rates of clinically significant prostate cancer in the ITT population compared with systematic biopsy in all men while avoiding biopsy in more than one-third of men and reducing the diagnosis of clinically insignificant cancer. This strategy offers substantial advantages over an initial systematic biopsy.
Trial protocol
Log file
eTable 1. Per protocol analysis
eTable 2. Quality of life data
eTable 3. Between site comparison
Data sharing statement
References
- 1.Das CJ, Razik A, Sharma S, Verma S. Prostate biopsy: when and how to perform. Clin Radiol. 2019;74(11):853-864. doi: 10.1016/j.crad.2019.03.016 [DOI] [PubMed] [Google Scholar]
- 2.Moore CM, Robertson NL, Arsanious N, et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol. 2013;63(1):125-140. doi: 10.1016/j.eururo.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 3.Villers A, Puech P, Mouton D, Leroy X, Ballereau C, Lemaitre L. Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. J Urol. 2006;176(6 pt 1):2432-2437. doi: 10.1016/j.juro.2006.08.007 [DOI] [PubMed] [Google Scholar]
- 4.Johnson DC, Raman SS, Mirak SA, et al. Detection of individual prostate cancer foci via multiparametric magnetic resonance imaging. Eur Urol. 2019;75(5):712-720. doi: 10.1016/j.eururo.2018.11.031 [DOI] [PubMed] [Google Scholar]
- 5.Moldovan PC, Van den Broeck T, Sylvester R, et al. What is the negative predictive value of multiparametric magnetic resonance imaging in excluding prostate cancer at biopsy? a systematic review and meta-analysis from the European Association of Urology Prostate Cancer guidelines panel. Eur Urol. 2017;72(2):250-266. doi: 10.1016/j.eururo.2017.02.026 [DOI] [PubMed] [Google Scholar]
- 6.Kirkham AP, Emberton M, Allen C. How good is MRI at detecting and characterising cancer within the prostate? Eur Urol. 2006;50(6):1163-1174. doi: 10.1016/j.eururo.2006.06.025 [DOI] [PubMed] [Google Scholar]
- 7.Wu LM, Xu JR, Ye YQ, Lu Q, Hu JN. The clinical value of diffusion-weighted imaging in combination with T2-weighted imaging in diagnosing prostate carcinoma: a systematic review and meta-analysis. AJR Am J Roentgenol. 2012;199(1):103-110. doi: 10.2214/AJR.11.7634 [DOI] [PubMed] [Google Scholar]
- 8.Wu LM, Xu JR, Gu HY, et al. Usefulness of diffusion-weighted magnetic resonance imaging in the diagnosis of prostate cancer. Acad Radiol. 2012;19(10):1215-1224. doi: 10.1016/j.acra.2012.05.016 [DOI] [PubMed] [Google Scholar]
- 9.Umbehr M, Bachmann LM, Held U, et al. Combined magnetic resonance imaging and magnetic resonance spectroscopy imaging in the diagnosis of prostate cancer: a systematic review and meta-analysis. Eur Urol. 2009;55(3):575-590. doi: 10.1016/j.eururo.2008.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drost FH, Osses D, Nieboer D, et al. Prostate magnetic resonance imaging, with or without magnetic resonance imaging–targeted biopsy, and systematic biopsy for detecting prostate cancer: a Cochrane systematic review and meta-analysis. Eur Urol. 2020;77(1):78-94. doi: 10.1016/j.eururo.2019.06.023 [DOI] [PubMed] [Google Scholar]
- 11.Borofsky S, George AK, Gaur S, et al. What are we missing? false-negative cancers at multiparametric MR imaging of the prostate. Radiology. 2018;286(1):186-195. doi: 10.1148/radiol.2017152877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klotz L, Loblaw A, Sugar L, et al. Active Surveillance Magnetic Resonance Imaging Study (ASIST): results of a randomized multicenter prospective trial. Eur Urol. 2019;75(2):300-309. doi: 10.1016/j.eururo.2018.06.025 [DOI] [PubMed] [Google Scholar]
- 13.Klotz L, Pond G, Loblaw A, et al. Randomized study of systematic biopsy versus magnetic resonance imaging and targeted and systematic biopsy in men on active surveillance (ASIST): 2-year postbiopsy follow-up. Eur Urol. 2020;77(3):311-317. doi: 10.1016/j.eururo.2019.10.007 [DOI] [PubMed] [Google Scholar]
- 14.Chen RC, Rumble RB, Loblaw DA, et al. Active surveillance for the management of localized prostate cancer (Cancer Care Ontario guideline): American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2016;34(18):2182-2190. doi: 10.1200/JCO.2015.65.7759 [DOI] [PubMed] [Google Scholar]
- 15.Kasivisvanathan V, Rannikko AS, Borghi M, et al. ; PRECISION Study Group Collaborators . MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378(19):1767-1777. doi: 10.1056/NEJMoa1801993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasivisvanathan V, Jichi F, Klotz L, et al. A multicentre randomised controlled trial assessing whether MRI-targeted biopsy is non-inferior to standard transrectal ultrasound guided biopsy for the diagnosis of clinically significant prostate cancer in men without prior biopsy: a study protocol. BMJ Open. 2017;7(10):e017863. doi: 10.1136/bmjopen-2017-017863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. ; PROMIS study group . Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389(10071):815-822. doi: 10.1016/S0140-6736(16)32401-1 [DOI] [PubMed] [Google Scholar]
- 18.Rouvière O, Puech P, Renard-Penna R, et al. ; MRI-FIRST Investigators . Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019;20(1):100-109. doi: 10.1016/S1470-2045(18)30569-2 [DOI] [PubMed] [Google Scholar]
- 19.van der Leest M, Cornel E, Israël B, et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naïve men with elevated prostate-specific antigen: a large prospective multicenter clinical study. Eur Urol. 2019;75(4):570-578. doi: 10.1016/j.eururo.2018.11.023 [DOI] [PubMed] [Google Scholar]
- 20.Drost FH, Osses D, Nieboer D, et al. Prostate magnetic resonance imaging, with or without magnetic resonance imaging-targeted biopsy, and systematic biopsy for detecting prostate cancer: a Cochrane systematic review and meta-analysis. Eur Urol. 2020;77(1):78-94. doi: 10.1016/j.eururo.2019.06.023 [DOI] [PubMed] [Google Scholar]
- 21.Cheung DC, Finelli A. Magnetic resonance imaging diagnosis of prostate cancer: promise and caution. CMAJ. 2019;191(43):E1177-E1178. doi: 10.1503/cmaj.190568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American College of Radiology . PI-RADS: prostate imaging–reporting and data system, 2015, version 2. Accessed September 15, 2020. https://www.acr.org/-/media/ACR/Files/RADS/Pi-RADS/PIRADS-V2.pdf
- 23.Barentsz JO, Weinreb JC, Verma S, et al. Synopsis of the PI-RADS v2 guidelines for multiparametric prostate magnetic resonance imaging and recommendations for use. Eur Urol. 2016;69(1):41-49. doi: 10.1016/j.eururo.2015.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haffner J, Lemaitre L, Puech P, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011;108(8 Pt 2):E171-E178. doi: 10.1111/j.1464-410X.2011.10112.x [DOI] [PubMed] [Google Scholar]
- 25.Kasivisvanathan V, Dufour R, Moore CM, et al. Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol. 2013;189(3):860-866. doi: 10.1016/j.juro.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 26.Presti JC Jr. Prostate biopsy: how many cores are enough? Urol Oncol. 2003;21(2):135-140. doi: 10.1016/S1078-1439(03)00006-1 [DOI] [PubMed] [Google Scholar]
- 27.Pagniez MA, Kasivisvanathan V, Puech P, et al. Predictive factors of missed clinically significant prostate cancers in men with negative magnetic resonance imaging: a systematic review and meta-analysis. J Urol. 2020;204(1):24-32. doi: 10.1097/JU.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 28.Westphalen AC, McCulloch CE, Anaokar JM, et al. Variability of the positive predictive value of PI-RADS for prostate MRI across 26 centers: experience of the Society of Abdominal Radiology Prostate Cancer Disease-focused panel. Radiology. 2020;296(1):76-84. doi: 10.1148/radiol.2020190646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vickers A, Carlsson SV, Cooperberg M, et al. Routine use of magnetic resonance imaging for early detection of prostate cancer is not justified by the clinical trial evidence. Eur Urol. 2020;78(3):304-306. doi: 10.1016/j.eururo.2020.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Log file
eTable 1. Per protocol analysis
eTable 2. Quality of life data
eTable 3. Between site comparison
Data sharing statement