Abstract
Background
Acute kidney injury (AKI) occurs among patients with coronavirus disease-19 (COVID-19) and has also been indicated to be associated with in-hospital mortality. Remdesivir has been authorized for the treatment of COVID-19. We conducted a systematic review to evaluate the incidence of AKI in hospitalized COVID-19 patients. The incidence of AKI in different subgroups was also investigated.
Methods
A thorough search was performed to find relevant studies in PubMed, Web of Science, medRxiv and EMBASE from 1 Jan 2020 until 1 June 2020. The systematic review was performed using the meta package in R (4.0.1).
Results
A total of 16,199 COVID-19 patients were included in our systematic review. The pooled estimated incidence of AKI in all hospitalized COVID-19 patients was 10.0% (95% CI: 7.0–12.0%). The pooled estimated proportion of COVID-19 patients who needed continuous renal replacement therapy (CRRT) was 4% (95% CI: 3–6%). According to our subgroup analysis, the incidence of AKI could be associated with age, disease severity and ethnicity. The incidence of AKI in hospitalized COVID-19 patients being treated with remdesivir was 7% (95% CI: 3–13%) in a total of 5 studies.
Conclusion
We found that AKI was not rare in hospitalized COVID-19 patients. The incidence of AKI could be associated with age, disease severity and ethnicity. Remdesivir probably did not induce AKI in COVID-19 patients. Our systematic review provides evidence that AKI might be closely associated with SARS-CoV-2 infection, which should be investigated in future studies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-021-02244-x.
Background
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to more than 60 million infections and over 1 million deaths worldwide [1]. The mortality due to COVID-19 is particularly high among older patients with chronic diseases, including hypertension, diabetes, obesity, chronic kidney disease and cardiac disease [2]. In 2003, the incidence of acute kidney injury (AKI) in patients with SARS was reported to be 6.7, and 91.7% of patients who died were diagnosed with AKI as a complication [3]. Recent studies have suggested that the incidence of AKI during hospitalization in patients with COVID-19 has a wide range and that AKI is associated with a poor prognosis [4–6]. Continuous renal replacement therapy (CRRT) is usually required for critically ill COVID-19 patients, not only for the treatment of AKI but also to effectively eliminate the cytokine storm [7]. The need for CRRT in COVID-19 patients should be evaluated.
Given the current ongoing pandemic of COVID-19, there is a need to identify safe and effective treatment options. Remdesivir, a broad-spectrum antiviral agent, has been shown to have antiviral activity against several RNA viruses, including MERS-CoV and Ebola virus (EV) [8, 9]. As remdesivir was found to effectively inhibit SARS-CoV-2 in vitro and in a mouse model [10, 11], it has been authorized for the treatment of COVID-19 patients in some countries, including the United States [12]. The incidence of AKI in COVID-19 patients being treated with remdesivir is still uncertain. Overall, the exact incidence rate and characteristics of AKI associated with COVID-19 are not well understood. In this study, we performed a systematic review of the incidence of AKI in hospitalized patients with COVID-19.
Methods
Search strategy
A systematic literature search was performed using PubMed, Web of Science, medRxiv and EMBASE from 1 Jan 2020 until 1 June 2020 to summarize the incidence of AKI in patients hospitalized with COVID-19. Two authors independently carried out systematic literature searches employing the terms “kidney” OR “renal” OR “acute kidney injury” OR “acute renal failure” AND “COVID-19” OR “SARS-COV-2” to obtain the AKI incidence in patients hospitalized with COVID-19. No language restrictions were applied.
Inclusion and exclusion criteria
Studies were included if they met the following criteria: 1) observational studies that reported the incidence of AKI in all hospitalized patients with COVID-19 and 2) observational studies or randomized, placebo-controlled trials (RCTs) that reported the incidence of AKI in hospitalized patients with COVID-19 being treated with remdesivir.
Studies that 1) were editorials, review articles or case reports, 2) were preprint articles, 3) had incomplete information about AKI, and 4) did not utilize the 2012 KDIGO criteria to define AKI were excluded.
Quality assessment
The methodological quality of the retrospective cross-sectional studies was assessed independently by two reviewers (Chen and Xu) using the method of the Agency for Healthcare Research and Quality (AHRQ) (http://www.ncbi.nlm.nih.gov/books/NBK.
35,156). An item was scored as 0 if it was answered NO or UNCLEAR; if it was answered YES, then the item was scored as 1. Studies achieving a score of 8 or above were considered high quality. At the same time, the RCTs in our study were analysed using the Cochrane Collaboration tool (http://handbook-5-1.cochrane.org/). Studies were divided into groups A, B and C. Studies that were assigned to the A group were considered high quality.
Statistical analysis
The systematic review was performed using the meta package in R (4.0.1). The incidence of AKI in COVID-19 patients (proportion) was used in our study. The incidences and their 95% CIs are presented as forest plots generated by the Metaprop function. Statistical heterogeneity among studies was assessed using the I2 statistic. The random-effects model was used if there was heterogeneity between studies (I2 < 50%); otherwise, the fixed-effects model was adopted. Rate consolidation was conducted using five methods (untransformed, log transformation, logit transformation, arcsine transformation, and Freeman-Tukey double arcsine transformation), and the logit transformation that yielded the results with the lowest I2 was selected for inclusion in our study. Sensitivity analysis was performed by the leave-one-out method. Peter’s test was performed to assess publication bias, and significance was determined by a P < 0.05.
Results
Literature search and study characteristics
A total of 1852 papers were identified according to our search criteria. After an initial round of exclusion based on titles and abstracts, two authors independently assessed 204 papers. Of those 204 papers, 159 publications were unrelated to AKI and therefore excluded from the study. Forty-five papers received a full-text review, and 23 were excluded based on the exclusion criteria. The flow diagram of the selection process is shown in Fig. 1. Finally, 22 studies including 16,199 COVID-19 patients met the predefined inclusion criteria and were used to determine the incidence of AKI in COVID-19 patients. Five of the 22 studies including 972 patients were used to determine the incidence of AKI in COVID-19 patients being treated with remdesivir.
Fig. 1.
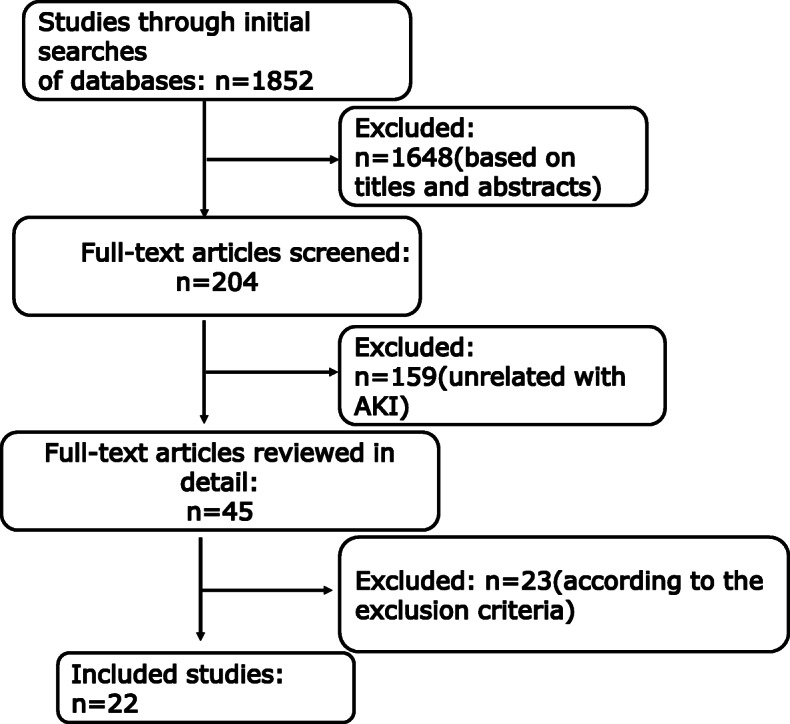
Flow diagram of studies identified, included, and excluded
Table 1 shows the characteristics of the studies in this systematic review. All studies in our systematic review reporting the incidence of AKI were retrospective cross-sectional studies, and most of them were of high quality (12/19). The RCTs included in our study were also of high quality.
Table 1.
Characteristics of the studies included in the analysis of the incidence of AKI in hospitalized COVID-19 patients
| Study | Year | Country | Design | Sample size | Age (median/ mean) |
Male (%) | The diagnosis criteria of AKI | Department | Quality score |
|---|---|---|---|---|---|---|---|---|---|
| Yichun Cheng [6] | 2020 |
China, Wuhan |
Retrospective Cross-sectional study |
701 | 63 | 52.4% |
2012 KDIGO criteria Stage 1(n = 13) Stage 2 (n = 9) Stage 3(n = 14) |
Hospitalized Patients |
AHRQ 8 |
| Weijie Guan [13] | 2020 |
China, Wuhan |
Retrospective Cross-sectional study |
1099 | 47 | 58.1% | 2012 KDIGO criteria | Hospitalized Patients |
AHRQ 9 |
| Chaolin Huang [14] | 2020 |
China, Wuhan |
Retrospective Cross-sectional study |
41 | 49 | 73.0% |
2012 KDIGO criteria CRRT 3(7%) |
Hospitalized Patients |
AHRQ 8 |
| Shaobo Shi [15] | 2020 |
China, Wuhan |
Retrospective Cross-sectional study |
416 | 64 | 49.7% |
2012 KDIGO criteria CRRT 2(0.5%) |
Hospitalized Patients |
AHRQ 9 |
| Luwen Wang [16] | 2020 |
China, Wuhan |
Retrospective Cross-sectional study |
116 | 54 | 57.8% | 2012 KDIGO criteria | Hospitalized Patients |
AHRQ 6 |
| Dawei Wang [17] | 2020 |
China, Wuhan |
Retrospective Cross-sectional study |
138 | 56 | 54.3% |
2012 KDIGO criteria CRRT 2(1.45%) |
Hospitalized Patients |
AHRQ 8 |
| Fei Zhou [18] | 2020 |
China, Wuhan |
Retrospective Cross-sectional study |
191 | 56 | 62.0% |
2012 KDIGO criteria CRRT 10(5%) |
Hospitalized Patients |
AHRQ 8 |
| Dawei Wang [19] | 2020 |
China, Wuhan and Huanggang |
Retrospective Cross-sectional study |
107 | 51 | 53.3% | 2012 KDIGO criteria | Hospitalized Patients |
AHRQ 7 |
| Tao Chen [20] | 2020 |
China, Wuhan |
Retrospective Cross-sectional study |
274 | 62.0 | 62.4% |
2012 KDIGO criteria CRRT 3(1%) |
Hospitalized Patients |
AHRQ 8 |
| Xiaochen Li [21] | 2020 |
China, Wuhan |
Retrospective Cross-sectional study |
548 | 60 | 50.9% |
2012 KDIGO criteria CRRT 2(0.4%) |
Hospitalized Patients |
AHRQ 8 |
| Xiaobo Yang [22] | 2020 |
China, Wuhan |
Retrospective Cross-sectional study |
52 | 51.9 | 70% |
2012 KDIGO criteria CRRT 9(17%) |
ICU Patients |
AHRQ 7 |
| Yuan Yu [23] | 2020 |
China, Wuhan |
Retrospective Cross-sectional study |
226 | 64 | 61.5% |
2012 KDIGO criteria Stage 1 (n = 23); Stage2 (n = 12); Stage 3 (n = 22) |
ICU Patients |
AHRQ 7 |
| KyungSoo Hong [24] | 2020 |
Korea, Daegu |
Retrospective Cross-sectional study |
98 | 55.4 | 38.8% |
2012 KDIGO criteria CRRT 3(3.1%) |
Hospitalized Patients |
AHRQ 6 |
| Safiya Richardson [25] | 2020 |
USA, New York |
Retrospective Cross-sectional study |
5700 | 63 | 60.3% |
2012 KDIGO criteria CRRT 81(3.2%) |
Hospitalized Patients |
AHRQ 8 |
| Jamie S. Hirsch [5] | 2020 |
USA, New York |
Retrospective Cross-sectional study |
5449 | 64.0 | 60.9% |
2012 KDIGO criteria CRRT 285(5.2%) |
Hospitalized Patients |
AHRQ 8 |
| Jessica Ferguson [26] | 2020 |
USA, California |
Retrospective Cross-sectional study |
72 | 60.4 | 52.8% | 2012 KDIGO criteria | Hospitalized Patients |
AHRQ 6 |
| Matt Arentz [27] | 2020 |
USA, Washington |
Retrospective Cross-sectional study |
21 | 79 | 52% | 2012 KDIGO criteria | ICU Patients |
AHRQ 8 |
| J.H. Beigel [28] | 2020 |
United States, Denmark, the United Kingdom,Greece, Germany, Korea, Mexico, Spain, Japan, and Singapore |
RCT (Remdesivir) |
1062 | 58.9 | 64.3% | 2012 KDIGO criteria | Hospitalized Patients |
Cochrane A |
| Spinello Antinori [29] | 2020 |
Italy, Milan |
Prospective, Cross-sectional study (Remdesivir) | 35 | 63.0 | 74.3% | 2012 KDIGO criteria | Hospitalized Patients |
AHRQ 6 |
| J. Grein [30] | 2020 | United States, Japan, Italy, Austria, France, Germany, Netherlands, Spain, and Canada | Prospective, Cross-sectional study (Remdesivir) | 53 | 64 | 75% | 2012 KDIGO criteria | Hospitalized Patients |
AHRQ 8 |
| Yeming Wang [31] | 2020 | China, Wuhan |
RCT (Remdesivir) |
236 | 66.0 | 56% | 2012 KDIGO criteria | Hospitalized Patients |
Cochrane A |
| Jason D. Goldman [32] | 2020 |
United States, Italy, Spain, Germany, Hong Kong, Singapore, South Korea, and Taiwan |
RCT (Remdesivir) |
397 | 62 | 64% | 2012 KDIGO criteria | Hospitalized Patients |
Cochrane A |
Incidence of AKI in COVID-19 patients
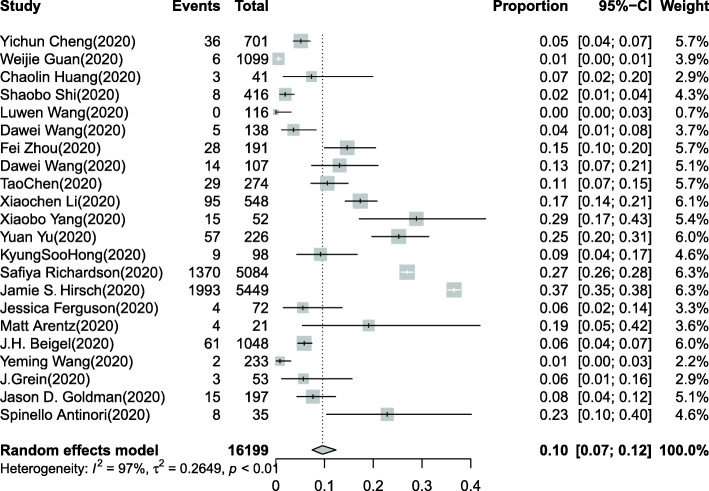
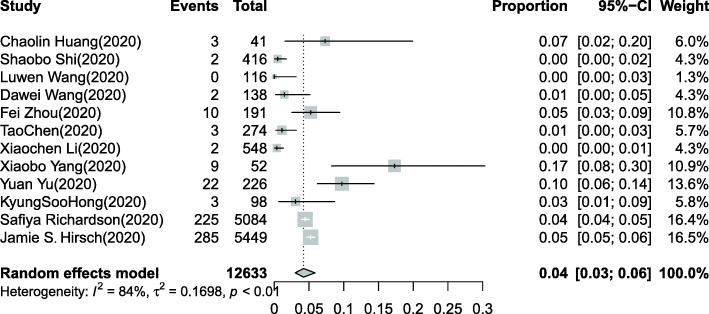
Overall, 16,199 COVID-19 patients were included in our systematic review [5, 6, 13–32]. The pooled estimated incidence of AKI in all hospitalized COVID-19 patients was 10% (95% CI: 7–12%, Fig. 2), and significant heterogeneity (I2 = 97%, chi-square = 0.26, P < 0.0001) was observed. Meanwhile, a total of 12,633 COVID-19 patients in 12 studies were included to investigate the need for CRRT [5, 14–18, 20–25]. A total of 566 patients (15.6%) needed CRRT among 3612 COVID-19 patients with AKI. The pooled estimated proportion of COVID-19 patients who needed CRRT was 4% (95% CI: 3–6%, Fig. 3).
Fig. 2.
Forest plot of the incidence of AKI in COVID-19 patients. Overall, 16,199 COVID-19 patients in 22 studies were included. I2 > 50% indicated that heterogeneity existed among the studies. The random-effects model was used to pool the data. The pooled estimated incidence of AKI in all hospitalized COVID-19 patients was 10% (95% CI: 7–12%)
Fig. 3.
Forest plot of the proportion of COVID-19 patients who needed CRRT. A total of 12,633 COVID-19 patients in 12 studies were included. I2 > 50% indicated that heterogeneity existed among the studies. The random-effects model was used to pool the data. The pooled estimated proportion of hospitalized COVID-19 patients who needed CRRT was 4% (95% CI: 3–6%)
Incidence of AKI in different subgroups of COVID-19 patients
Subgroup analyses were performed according to ethnicity, age and disease severity (Supplementary Fig. 1, 2, 3). The pooled estimated AKI incidences in the Asian subgroup and non-Asian subgroup were 7% (95% CI: 4–11%) and 15% (95% CI: 11–20%), respectively (Supplementary Fig. 1). At the same time, the incidences of AKI in the subgroup with a median/mean age greater than 60 years and the subgroup with a median/mean age less than 60 years were 12% (95% CI: 9–16%) and 6% (95% CI: 3–12%), respectively (Supplementary Fig. 2). In the subgroup of hospitalized patients, the incidence of AKI was 8% (95% CI: 6–11%), but it was 26% (95% CI: 21–31%) in ICU patients (Supplementary Fig. 3). There was still significant heterogeneity in most of the subgroups in our subgroup analysis.
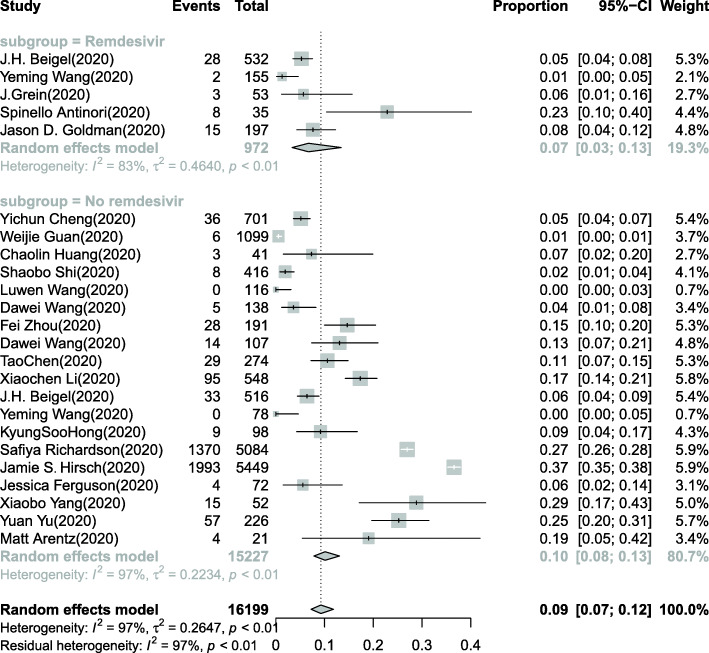
Incidence of AKI in the subgroup of COVID-19 patients being treated with remdesivir
A total of 5 studies with 972 COVID-19 patients investigated the incidence of AKI in hospitalized COVID-19 patients being treated with remdesivir [28–32]. The pooled estimated AKI incidence in hospitalized COVID-19 patients being treated with remdesivir was 7% (95% CI: 3–13%) (Fig. 4). In the subgroup of COVID-19 patients not treated with remdesivir, the incidence of AKI was 10% (95% CI: 8–13%).
Fig. 4.
Forest plot of the incidence of AKI in the remdesivir and no remdesivir subgroups of COVID-19 patients. A total of 972 COVID-19 patients in 5 studies were included in the remdesivir subgroup, and 15,227 patients were included in the no remdesivir subgroup. The pooled estimated incidence of AKI in COVID-19 patients being treated with remdesivir was 7% (95% CI: 3–13%). In the no remdesivir subgroup of COVID-19 patients, the incidence of AKI was 10% (95% CI: 8–13%)
Sensitivity analysis and publication bias
In the sensitivity analysis, we used the leave-one-out method (Supplementary Figs. 4 and 5) and found similar results to those in our main study. Peter’s test was performed to evaluate publication bias (Table 2), and no significant difference was detected in the incidence of AKI in COVID-19 patients.
Table 2.
Results of the systematic review of the incidence of AKI and the proportion of patients who needed CRRT among all COVID-19 patients
| Study No. |
COVID-19 patients No. | Proportion/OR (95%CI) |
Study heterogeneity | ||||
|---|---|---|---|---|---|---|---|
| Chi-square test | df | I2 | Peter’s test (P value) |
||||
| The incidence of AKI in COVID-19 patients | 22 | 16,199 | 0.10(0.07–0.12) | 0.26 | 21 | 97% | 0.18 |
| The incidence of CRRT in COVID-19 patients | 12 | 12,633 | 0.04(0.03–0.06) | 0.17 | 11 | 84% | 0.24 |
Discussion
In this systematic review, the results from 22 retrospective cross-sectional studies including 16,199 patients hospitalized with COVID-19 from 1 January 2020 to 1 June 2020 demonstrated that AKI was not rare in COVID-19 patients. The incidence of AKI might be associated with age, disease severity and ethnicity, according to our subgroup analyses.
COVID-19 is primarily a respiratory disease, but other organs, including the kidneys, are often involved. SARS-CoV-2 enters cells via the angiotensin-converting enzyme 2 (ACE2) receptor and is highly homologous to SARS-CoV [33]. High ACE2 expression in proximal tubular epithelial cells may make the kidneys a potential target, leading to kidney injury [34]. Renal abnormalities, such as proteinuria, haematuria, and AKI, occur in patients with COVID-19 [35]. AKI is characterized by a rapid increase in serum creatinine, a decrease in urine output, or both [36]. The current widely used AKI definition was developed by the Kidney Disease Improving Global Outcomes (KDIGO) group in 2012 [37]. The most common causes of AKI are septic shock, major surgery, cardiogenic shock, drug toxicity and hypovolemia [38]. The cause of AKI in COVID-19 patients is likely to be multifactorial, including a direct attack by SARS-CoV-2 (COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup) or haemodynamic instability, microcirculatory dysfunction, tubular cell injury, renal congestion, microvascular thrombi and endothelial dysfunction [39], which are commonly found in critically ill patients. Pathological reports from autopsies of patients with COVID-19 with renal failure revealed that the kidneys contained viral particles within both the tubular epithelium and the podocytes that were visible with electron microscopy [40], varying degrees of acute tubular necrosis (ATN), diffuse proximal tubule injury with the loss of the brush border, nonisometric vacuolar degeneration, haemosiderin granules and pigmented casts [40, 41].
We found that the incidence of AKI in COVID-19 patients was 10%. A similar AKI incidence in COVID-19 patients (10.8%) was also reported in another study [34]. The diversity of patients included in our systematic review resulted in heterogeneity. According to the subgroup analysis, the estimated AKI incidence in patients with an average age greater than 60 years old was 12%, while that in patients with an average age less than 60 years old was 6%. Many reports on COVID-19 have highlighted age-related differences in health outcomes, and the mortality due to COVID-19 is particularly high among older patients [42, 43]. Age is also an important risk factor for AKI [44]. The pooled estimated AKI incidence in the Asian subgroup was 7%. However, in the non-Asian subgroup, it was 15%. African ancestry is also a risk factor for AKI [45]. In a large cohort study of hospitalized COVID-19 patients, 76.9% of the patients who were hospitalized with COVID-19 and 70.6% of those who died were Black, whereas the Black population only accounted for 31% of the total population [46]. There might be a difference between the criteria for hospital admission in Asian and non-Asian COVID-19 patients. A European study showed that 190/1457 (13%) COVID-19 patients were diagnosed with AKI on arrival [47]. The incidence of AKI in ICU patients with COVID-19 is particularly high, ranging from 8 to 62% [14, 17, 22–24, 26, 27]. In our subgroup analysis, we found that the incidence of AKI was 26% in ICU patients. Critically ill patients hospitalized with COVID-19 who stayed in the ICU were more likely to develop AKI [5]. Lin L proved that disease severity was associated with the incidence of AKI in COVID-19 patients [34].
The proportion of COVID-19 patients who needed CRRT was 4%, according to our investigation. CRRT has been administered to many sepsis patients complicated with AKI [48]. Growing evidence suggests that patients with severe COVID-19 may develop cytokine storm syndrome [49, 50]. CRRT can remove inflammatory factors, thus blocking cytokine storm syndrome and ultimately reducing the damage inflicted on multiple organs [51]. However, the timing of the initiation of CRRT in patients with severe COVID-19 remains controversial [49]. Additional research is needed to determine whether the early initiation of CRRT could improve the prognosis of COVID-19 patients with AKI.
The initiation of treatment with antiviral drugs is a common cause of drug-induced AKI [52, 53]. As shown in Fig. 4, the incidence of AKI in hospitalized COVID-19 patients being treated with remdesivir was 7%. In clinical studies of remdesivir, AKI was the most frequent adverse event leading to drug discontinuation [29, 31]. Antiviral drugs cause AKI through many mechanisms, including direct renal tubular toxicity, allergic interstitial nephritis (AIN), and crystal nephropathy [54, 55]. However, in animal models, remdesivir was effective against MERS-CoV and did not cause any side effects, such as AKI [56]. According to a recently published multicentre matched cohort study of remdesivir, remdesivir was not significantly associated with an increased incidence of AKI in COVID-19 patients, even in patients who had a baseline eCrCl< 30 mL/min [57]. In our study, we also did not observe remdesivir-associated AKI in COVID-19 patients. More RCTs should be performed on this topic in the future.
Limitations
Our systematic review had some limitations. First, most of the studies included were retrospective cross-sectional studies, although the majority of them (65%) were of high quality. Second, the systematic review was performed using studies with single groups, leading to greater heterogeneity. There was statistically significant heterogeneity in the systematic review of the incidence of AKI in COVID-19 patients. The diversity of the included studies, which involved different disease stages or activities, ages, ethnicities and sexes, might also be associated with the heterogeneity. Although we performed subgroup analyses, the results still had significant heterogeneity. As COVID-19 is a new and unknown infectious disease, our review could only summarize the studies that have already been published on this topic. The potential bias in the reported COVID-19 patients means that they may not represent all of the patients hospitalized with COVID-19 worldwide. Third, there were few original studies (n < 10) that could be included in the systematic review of the incidence of AKI in hospitalized COVID-19 patients being treated with remdesivir. Finally, since investigations of COVID-19 are ongoing, additional clinical data are expected to be published.
Conclusion
According to our study, AKI is common in hospitalized COVID-19 patients. The incidence of AKI could be associated with age, disease severity and ethnicity. Remdesivir probably does not induce AKI in COVID-19 patients. Our systematic review demonstrated the clinical characteristics of AKI in COVID-19 patients, providing evidence that AKI might be closely associated with SARS-CoV-2 infection, which should be assessed in future studies.
Supplementary Information
Additional file 1: Figure S1. Forest plot of the incidence of AKI in the Asian and non-Asian subgroups of COVID-19 patients.
Additional file 2: Figure S2. Forest plot of the incidence of AKI in the median/mean age more than 60 years and less than 60 years subgroups of COVID-19 patients.
Additional file 3: Figure S3. Forest plot of the incidence of AKI in the ICU and hospitalized subgroups of COVID-19 patients.
Additional file 4: Figure S4. Sensitivity analysis for the incidence of AKI in COVID-19 patients.
Additional file 5: Figure S5. Sensitivity analysis for the proportion of COVID-19 patients who needed CRRT.
Acknowledgements
The authors appreciate the assistance of all participants.
Consent to publication
Not applicable.
Abbreviations
- AKI
Acute kidney injury
- COVID-19
Coronavirus disease 2019
- CRRT
Continuous renal replacement therapy
- ICU
Intensive care unit
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- EV
Ebola virus
- RCTs
Randomized controlled trials
Authors’ contributions
Concept and design: AX and JC. Acquisition, analysis, or interpretation of data: ZX and JC. Drafting of the manuscript: ZX and YT. Critical revision of the manuscript: AX and JC. Statistical analysis: QH, SF, XL and BL. The authors read and approved the final manuscript.
Funding
This work was in part supported by grants from the National Natural Science Foundation of China (General Program: 81870481), the Sun Yat-Sen Clinical Research Cultivating Program (SYS-C-201905) and the Medical Scientific Research Foundation of Guangdong Province of China (A2020431).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
This study was approved by the institutional review board of Sun Yat-sen University.
Competing interests
All of the authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhenjian Xu and Ying Tang contributed equally to this work.
Contributor Information
Anping Xu, Email: xuanping@mail.sysu.edu.cn.
Junzhe Chen, Email: chenjzh23@mail.sysu.edu.cn.
References
- 1.Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/
- 2.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu KH, Tsang WK, Tang CS, Lam MF, Lai FM, To KF, et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67(2):698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31(7):1380–1383. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forest SJ, Michler RE, Skendelas JP, DeRose JJ, Friedmann P, Parides MK, et al. De novo renal failure and clinical outcomes of patients with critical coronavirus disease 2019. Crit Care Med. 2021;49(2):161–9. [DOI] [PubMed]
- 8.Sheahan TP, Sims AC, Leist SR, Schafer A, Won J, Brown AJ, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(1):222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulangu S, Dodd LE, Davey RT, Jr, Tshiani Mbaya O, Proschan M, Mukadi D, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheahan TP, Sims AC, Zhou S, Graham RL, Pruijssers AJ, Agostini ML, et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020;12(541):eabb5883. [DOI] [PMC free article] [PubMed]
- 12.Lim S, DeBruin DA, Leider JP, Sederstrom N, Lynfield R, Baker JV, et al. Developing an ethics framework for allocating Remdesivir in the COVID-19 pandemic. Mayo Clin Proc. 2020;95(9):1946–1954. doi: 10.1016/j.mayocp.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of Cardiac Injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Li X, Chen H, Yan S, Li D, Li Y, et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;51(5):343–348. doi: 10.1159/000507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D, Yin Y, Hu C, Liu X, Zhang X, Zhou S, et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care. 2020;24(1):188. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Y, Xu D, Fu S, Zhang J, Yang X, Xu L, et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. 2020;24(1):219. doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong KS, Lee KH, Chung JH, Shin KC, Choi EY, Jin HJ, et al. Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in Daegu, South Korea: a brief descriptive study. Yonsei Med J. 2020;61(5):431–437. doi: 10.3349/ymj.2020.61.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson J, Rosser JI, Quintero O, Scott J, Subramanian A, Gumma M, et al. Characteristics and outcomes of coronavirus disease patients under nonsurge conditions, northern California, USA, March-April 2020. Emerg Infect Dis. 2020;26(8):1679–1685. doi: 10.3201/eid2608.201776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19- final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antinori S, Cossu MV, Ridolfo AL, Rech R, Bonazzetti C, Pagani G, et al. Compassionate remdesivir treatment of severe Covid-19 pneumonia in intensive care unit (ICU) and non-ICU patients: clinical outcome and differences in post-treatment hospitalisation status. Pharmacol Res. 2020;158:104899. doi: 10.1016/j.phrs.2020.104899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate use of Remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Zhou F, Zhang D, Zhao J, Du R, Hu Y, et al. Evaluation of the efficacy and safety of intravenous remdesivir in adult patients with severe COVID-19: study protocol for a phase 3 randomized, double-blind, placebo-controlled, multicentre trial. Trials. 2020;21(1):422. doi: 10.1186/s13063-020-04352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020;383(19):1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raza A, Estepa A, Chan V, Jafar MS. Acute renal failure in critically ill COVID-19 patients with a focus on the role of renal replacement therapy: a review of what we know so far. Cureus. 2020;12(6):e8429. doi: 10.7759/cureus.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin L, Wang X, Ren J, Sun Y, Yu R, Li K, et al. Risk factors and prognosis for COVID-19-induced acute kidney injury: a meta-analysis. BMJ Open. 2020;10(11):e042573. doi: 10.1136/bmjopen-2020-042573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31(6):1157–1165. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394(10212):1949–1964. doi: 10.1016/S0140-6736(19)32563-2. [DOI] [PubMed] [Google Scholar]
- 37.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. NephronClin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 38.Gameiro J, Fonseca JA, Outerelo C, Lopes JA. Acute kidney injury: from diagnosis to prevention and treatment strategies. J Clin Med. 2020;9(6):1704. [DOI] [PMC free article] [PubMed]
- 39.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farkash EA, Wilson AM, Jentzen JM. Ultrastructural evidence for direct renal infection with SARS-CoV-2. J Am Soc Nephrol. 2020;31(8):1683–1687. doi: 10.1681/ASN.2020040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jing QL, Liu MJ, Zhang ZB, Fang LQ, Yuan J, Zhang AR, et al. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(10):1141–1150. doi: 10.1016/S1473-3099(20)30471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collaborative CO. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396(10243):27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thongprayoon C, Hansrivijit P, Kovvuru K, Kanduri SR, Torres-Ortiz A, Acharya P, et al. Diagnostics, risk factors, treatment and outcomes of acute kidney injury in a new paradigm. J Clin Med. 2020;9(4):1104. [DOI] [PMC free article] [PubMed]
- 45.Demirjian S. Race, class, and AKI. J Am Soc Nephrol. 2014;25(8):1615–1617. doi: 10.1681/ASN.2014030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Portoles J, Marques M, Lopez-Sanchez P, de Valdenebro M, Munez E, Serrano ML, et al. Chronic kidney disease and acute kidney injury in the COVID-19 Spanish outbreak. Nephrol Dial Transplant. 2020;35(8):1353–1361. doi: 10.1093/ndt/gfaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai C, Qiu G, Hong W, Shen Y, Gong X. Clinical effect and safety of continuous renal replacement therapy in the treatment of neonatal sepsis-related acute kidney injury. BMC Nephrol. 2020;21(1):286. doi: 10.1186/s12882-020-01945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen G, Zhou Y, Ma J, Xia P, Qin Y, Li X. Is there a role for blood purification therapies targeting cytokine storm syndrome in critically severe COVID-19 patients? Ren Fail. 2020;42(1):483–488. doi: 10.1080/0886022X.2020.1764369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Antinori S, Bonazzetti C, Gubertini G, Capetti A, Pagani C, Morena V, et al. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: an increased risk for candidemia? Autoimmun Rev. 2020;19(7):102564. doi: 10.1016/j.autrev.2020.102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neveu H, Kleinknecht D, Brivet F, Loirat P, Landais P. Prognostic factors in acute renal failure due to sepsis. Results of a prospective multicentre study. The French study group on acute renal failure. Nephrol Dial Transplant. 1996;11(2):293–299. doi: 10.1093/oxfordjournals.ndt.a027256. [DOI] [PubMed] [Google Scholar]
- 52.Zavras P, Su Y, Fang J, Stern A, Gupta N, Tang Y, et al. Impact of preemptive therapy for cytomegalovirus on toxicities after allogeneic hematopoietic cell transplantation in clinical practice: a retrospective single-center cohort study. Biol Blood Marrow Transplant. 2020;26(8):1482–1491. doi: 10.1016/j.bbmt.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maan R, Al Marzooqi SH, Klair JS, Karkada J, Cerocchi O, Kowgier M, et al. The frequency of acute kidney injury in patients with chronic hepatitis C virus infection treated with sofosbuvir-based regimens. Aliment Pharmacol Ther. 2017;46(1):46–55. doi: 10.1111/apt.14117. [DOI] [PubMed] [Google Scholar]
- 54.Xing W, Gu L, Zhang X, Xu J, Lu H. A metabolic profiling analysis of the nephrotoxicity of acyclovir in rats using ultra performance liquid chromatography/mass spectrometry. Environ Toxicol Pharmacol. 2016;46:234–240. doi: 10.1016/j.etap.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 55.Izzedine H, Launay-Vacher V, Deray G. Antiviral drug-induced nephrotoxicity. Am J Kidney Dis. 2005;45(5):804–817. doi: 10.1053/j.ajkd.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 56.Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 2020;585(7824):273–276. doi: 10.1038/s41586-020-2423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ackley TW, Mcmanus D, Topal JE, Cicali B, Shah S. A valid warning or clinical Lore: an evaluation of safety outcomes of Remdesivir in patients with impaired renal function from a multicenter matched cohort. Antimicrob Agents Chemother. 2020;65(2):e02290–20. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Forest plot of the incidence of AKI in the Asian and non-Asian subgroups of COVID-19 patients.
Additional file 2: Figure S2. Forest plot of the incidence of AKI in the median/mean age more than 60 years and less than 60 years subgroups of COVID-19 patients.
Additional file 3: Figure S3. Forest plot of the incidence of AKI in the ICU and hospitalized subgroups of COVID-19 patients.
Additional file 4: Figure S4. Sensitivity analysis for the incidence of AKI in COVID-19 patients.
Additional file 5: Figure S5. Sensitivity analysis for the proportion of COVID-19 patients who needed CRRT.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.