Abstract
Patients receiving ifosfamide as part of their cancer treatment are at risk for ifosfamide-related encephalopathy (IRE), a potentially serious adverse event affecting up to 60% of patients. Symptoms range from transient altered mental status to coma and death. Consensus regarding risk factors for the development of IRE has not been reached in the literature. The purpose of this review is to identify risk factors for the development of IRE in adult cancer patients. A literature review was completed by searching PubMed and Scopus databases to identify articles published between 2008 and 2018. A total of 76 search results were reduced to a final sample of seven articles after applying inclusion and exclusion criteria. Published data suggest that Eastern Cooperative Oncology Group (ECOG) performance status of greater than or equal to 2, impaired renal function, hypoalbuminemia, and having multiple risk factors are risk factors for the development of IRE. Knowledge of which patients are at increased risk for the development of IRE could help clinicians to appropriately counsel patients and families regarding personal risk for the development of IRE. Clinicians may also more closely monitor patients with risk factors for IRE.
Ifosfamide is an antineoplastic medication that is used to treat a variety of malignancies but has the potential for inducing serious adverse effects. One such adverse effect is central nervous system toxicity, known as ifosfamide-related encephalopathy (IRE). Ifosfamide is most commonly administered intravenously, as the oral formulation is known to increase the risk of IRE (David & Picus, 2005). Even with intravenous administration, IRE still occurs in 10% to 30% of patients, with some estimates up to 60% (Patel, 2006; Pelgrims et al., 2000). The mechanism of IRE is not fully understood but may be due to the production of neurotoxic metabolites including chloroacetaldehyde, which is capable of crossing the blood-brain barrier (Kim, Isola, & Oh, 2016).
Symptoms of IRE typically occur within 48 hours of drug initiation and are graded on a scale of 1 to 5 (David & Picus, 2005; National Cancer Institute, 2017). While symptoms often resolve spontaneously within 72 hours of stopping ifosfamide, there have been cases of long-term neurologic deficits and fatalities associated with IRE (Brunello et al., 2007; Heim, Fiene, Schick, Wolpert, & Queiber, 1981; Shin et al., 2011; Szabatura et al., 2015). Survival outcomes for patients may be impacted if they are no longer able to receive ifosfamide as part of their treatment plan due to IRE (Sweiss, Beri, & Shord, 2008).
IRE is a clinical diagnosis. It is important for advanced practitioners to exclude other causes of encephalopathy, including infection and metabolic abnormalities. Management of IRE mainly consists of cessation of ifosfamide therapy and intravenous hydration.
There are limited data on the effectiveness of other interventions for the treatment and prevention of IRE. Methylene blue (MB), thiamine, and albumin have been utilized. Data supporting the use of MB for treatment and prophylaxis of IRE are limited to case studies (Gharaibeh, Telfah, Powers, & Salacz, 2018; Patel, 2006; Vakiti, Pilla, Moustafa, Joseph, & Shenoy, 2018) and one retrospective study (Pelgrims et al., 2000). Results from other retrospective studies have not shown any benefit of MB for prevention of IRE (Richards, Marshall, & McQuary, 2010; Sweiss et al., 2008).
Thiamine has also been suggested as an agent for the treatment and prevention of IRE. However, data in support of thiamine are limited to case studies (Gharaibeh et al., 2018; Hamadani & Awan, 2006; Imtiaz & Muzaffar, 2010). In one retrospective analysis examining prophylaxis with MB, thiamine, and albumin, alone or in combination, prophylactic treatment did not reduce the incidence of IRE (Richards et al., 2010).
Research findings evaluating risk factors for the development of IRE have been inconsistent. This review aims to summarize the most recent evidence regarding risk factors for the development of IRE. This information will allow clinicians to counsel patients and identify patients in need of close monitoring for IRE.
METHOD
A review of the literature was performed to evaluate risk factors for IRE in adult cancer patients. Electronic databases searched included PubMed and Scopus. A combination of keywords and MeSH terms, including risk, induced, incidence, neurotoxicity, encephalopathy, and ifosfamide, were used.
The inclusion criteria consisted of peer-reviewed, original research published in English between January 1, 2008, and December 31, 2018, with human subjects who received ifosfamide. Case studies and research regarding pediatric patients were excluded.
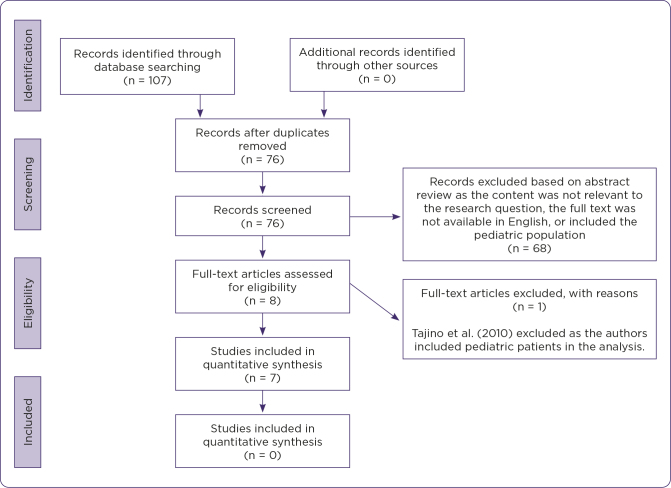
PubMed and Scopus initially yielded 107 articles. References were reviewed to identify additional articles, although none were identified. After removing duplicates, 76 articles were identified for review. Sixty-eight articles were excluded based on abstract review. Full-text review of the remaining eight articles identified seven meeting the inclusion criteria for review (Figure 1).
Figure 1.
Flow diagram of literature search. Adapted from Moher, Liberati, Tetzlaff, and Altman (2009).
RESULTS
Categories used to organize risk factors include patient characteristics, physiologic factors, treatment characteristics, tumor burden, and drug interactions. A summary of the evidence is presented in Tables 1 and 2.
Table 1. Studies Examining Risk Factors for Ifosfamide-Related Encephalopathy.
| Author (year) | Evidence type, level of evidence, sample | Study findings | Limitations |
|---|---|---|---|
| Howell (2008) | Case-control study Evidence level IV N = 45 Adult sarcoma patients receiving chemotherapy regimens with MAI (mesna, doxorubicin, and ifosfamide) between 01/01/2004 and 12/31/2006 | Patient characteristics: Eight patients (18%) developed IRE. There was no significant difference in mean age or baseline MMSE between the two groups. Patients with IRE did have a higher baseline delirium score on admission (p = .03) and on day 1 of chemotherapy (p < .05). | Retrospective study design. Patient population was limited to sarcoma patients. No cases of patients with brain metastasis were included, as brain metastasis is not common in sarcoma. All patients received antiemetic medications, including steroids, benzodiazepines, and phenothiazines, which may have side effects similar to IRE. Dexamethasone use was standard, and the other medications were typically given on an as-needed basis, making their impact on the development of IRE difficult to define. |
| Physiologic factors: Mean SCr was lower for patients with IRE than without, 0.61 vs. 0.79 (p < .05), but both groups were WNL. Mean albumin was lower for patients with IRE, 2.88 vs. 3.85 (p < 0.05) and total bilirubin was higher, 0.86 vs. 0.42 (p < .05) at baseline. | |||
| Treatment characteristics: Total dose of ifosfamide was lower in patients with IRE. Five patients (62%) developed IRE in cycle 1 and discontinued further treatment. The other three patients developed IRE in later cycles. | |||
| Tumor burden: Not examined | |||
| Drug interactions: Six of the eight patients had received aprepitant as an antiemetic before treatment. A RR of 2.6 (95% CI = 0.47–26.6) was associated with the use of aprepitant. A trend of an increased occurrence of IRE with aprepitant use was noted, although this was not statistically significant | |||
| Kettle (2010) | Case-control study Evidence level IV N = 41 patients, 93 cycles of ifosfamide All adults treated with ifosfamide at an academic medical center over 13 months | Patient characteristics: Six cases of IRE occurred in four patients (6.5% of all treatments, 9.8% of all patients). Mean age was not significantly different between the two groups. Patients with IRE had a higher mean total number of risk factors, 2.67 vs. 1.43 (p = 0.02). All cases of IRE occurred among patients identified as high risk according to the risk-stratification model developed by the authors (p = 0.01). | Retrospective study design. Size of the patient population was small, and the risk of IRE occurrences was also low. Further research is needed to validate the accuracy of the risk stratification tool. |
| Physiologic factors: Patients with IRE had higher mean SCr, 1.05 vs. 0.85 (p = 0.04), although mean creatinine clearance was higher in IRE patients, 107.7 vs. 103.0 (not statistically significant). Albumin prophylaxis (typically given to patients with serum albumin < 3.0 before ifosfamide infusion with repeat dosing if needed) was given in 32 (34%) of cycles. Five events of IRE occurred in patients receiving albumin prophylaxis. Albumin prophylaxis was not felt to reduce risk of IRE. Patients with IRE had lower baseline albumin, 2.88 vs. 3.42 (p = 0.01). Patients with IRE had higher baseline LFTs, AST 69.5 vs. 30.1, ALT 141.3 vs. 34 (p < 0.01) | |||
| Treatment characteristics: Mean ifosfamide dose was not significantly different between the two groups, 2.2 g/m2/day vs. 2.8 g/m2/day. | |||
| Tumor burden: Not examined | |||
| Drug interactions: Not examined | |||
| Kim (2016) | Case-control study Evidence level IV N = 28 patients, 47 cycles of ifosfamide chemotherapy All hospitalized patients who received ifosfamidebased chemotherapy during the 1-year study period (age range: 17–90) | Patient characteristics: Seven cases of IRE occurred in six patients (14.9% of all treatments, 21.4% of all patients). Patients with IRE had a greater number of risk factors for IRE, 3 vs. 2 (p = 0.012). There was no significant difference in the history of IRE between the two groups. | Retrospective study design. The size of the sample was small and not adequate to conduct a statistical analysis of all risk factors between the two groups. While the authors stated that drug-drug interactions between the two groups were not significant, the authors did not describe any details regarding the drug interactions examined. The authors captured patients to include in the analysis by identifying orders for methylene blue and by reviewing adverse drug events data, a voluntary reporting system. Accordingly, cases of IRE eligible for analysis may have been missed if not reported, and if the patient did not receive methylene blue. |
| Physiologic factors: There was no significant difference in renal function between patients who had IRE and those that did not. There was no significant difference in median albumin between patients who had IRE and those that did not. Patients with IRE had a lower median nadir WBC count, 0.4 vs. 3.9 (p = 0.003) and higher reduction in median WBC count from baseline, –6.2 vs. –4.1 (p < .001). There was no significant difference in pretreatment WBC count and number of days until WBC nadir. | |||
| Treatment characteristics: Not examined. | |||
| Tumor burden: Patients with pelvic disease were more likely to have IRE (p = .009) | |||
| Drug interactions: Drug-drug interactions between the two groups were not significant. | |||
| Lo (2016) | Case-control study Evidence level IV N = 337 patients Adult patients who had completed at least one cycle of ifosfamide between January 2008 and December 2010 at National Taiwan University Hospital | Patient characteristics: 38 patients (11%) developed IRE. Age and gender were not significantly different between the two groups. Patients with IRE were more likely to have ECOG PS 2–4 (p < .001) on both multivariate and univariate analysis. | Retrospective study design. |
| Physiologic factors: Mean SCr was significantly higher in patients with IRE (p = .004) on both multivariate and univariate analysis. Mean baseline albumin was significantly lower in patients with IRE (p = .002) on both multivariate and univariate analysis. Mean baseline AST was significantly higher in patients with IRE (p = 0.039) on univariate analysis only. Mean baseline ALT and total bilirubin were not significantly different between the groups, although patients with total bilirubin > 3 were at significantly higher risk for IRE (p = .007) on univariate analysis only. Mean baseline WBC count was significantly higher in patients with IRE (p = .026) on univariate analysis only. Platelet count was not significantly different between the two groups. | |||
| Treatment characteristics: Daily dose, dose per cycle, cumulative dose of ifosfamide were not significantly different between the two groups. | |||
| Tumor burden: There was no significant difference between the two groups for the presence of brain metastasis. | |||
| Drug interactions: There was no difference between the two groups for concurrent cisplatin use. Non-cytotoxic medications, which could have neurologic effects, were not examined. | |||
| Richards (2010) | Case-control study Evidence level IV N = 63 patients, 166 cycles of ifosfamide chemotherapy Adult patients hospitalized receiving any high-dose ifosfamide-containing regimen to treat sarcoma from 9/30/2006–2008. | Patient characteristics: Age > 50, and history of IRE are risk factors for IRE. | Retrospective study design. Only sarcoma patients were included. Risk factors were a secondary objective. Statistical analyses with a level of significance were not reported for risk factors for IRE. |
| Physiologic factors: Sodium < 135 and creatinine > 1 are risk factors for IRE. Albumin < 3.5, AST > 40, ALT > 65, total bilirubin > 1.3 are risk factors. | |||
| Treatment characteristics: 1–2 infusion time of ifosfamide, total dose per cycle of ifosfamide greater than or equal to 8 g/m2, every 12-hour frequency of ifosfamide administration are risk factors for IRE. | |||
| Tumor burden: History of brain irradiation and the presence of pelvic disease are risk factors for IRE. | |||
| Drug interactions: Cisplatin use and use of more than three concomitant neurotoxic medications are risk factors for IRE. Aprepitant is not a risk factor. | |||
| Sweiss (2008) | Case-control study Evidence level IV N = 19 patients All adult patients who received high-dose ifosfamide for soft tissue sarcomas from January 2000 to December 2004. | Patient characteristics: 8 patients (42%) experienced IRE. 5 experienced more than one episode of IRE after receiving one or more subsequent cycles of ifosfamide. Female gender and bodyweight were risk factors for IRE. | Retrospective study design. The sample size was very small; therefore, statistical analysis for the level of significance was not conducted. Adverse drug event reports were reviewed to identify patients for study analysis. |
| Physiologic factors: No difference was reported between the two groups. Serum albumin was substantially lower in the IRE group. Liver enzymes were similar between the two groups. Differences in the bilirubin level were not felt to be clinically relevant. Anemia and decreased WBC count from baseline to completion of treatment were risk factors for IRE. | |||
| Treatment characteristics: Too few patients received a continuous infusion of ifosfamide rather than fractionated doses to assess if this is a risk factor. | |||
| Tumor burden: No difference was reported between the two groups for the presence of pelvic disease. | |||
| Drug interactions: Not examined. | |||
| Szabatura (2015) | Case-control study Evidence level IV N = 200, 100 lymphoma and 100 sarcoma patients. Adult patients receiving ifosfamide for either lymphoma or sarcoma at Dana Farber/Brigham and Women’s Cancer Center | Patient characteristics: 29 patients (14.5%) experienced IRE. Age and gender were not statistically significant between the two groups. | Retrospective study design. Only patients with lymphoma and sarcoma were included in the study. |
| Physiologic factors: The odds of having IRE were nine times higher for a one-unit increase in SCr (p = .02). The odds of having IRE were 0.15 times lower for a one-unit increase in albumin (p = .001). The odds of having IRE were 1.4 times higher for a one-unit increase in hemoglobin (p = .031) | |||
| Treatment characteristics: Patients with IRE received a significantly higher ifosfamide daily dose (p = .009), although ifosfamide total dose and infusion duration were not significantly different between the two groups. | |||
| Tumor burden: 24 (83%) patients with IRE had a sarcoma diagnosis, and 5 (17%) had lymphoma (p < .0001). | |||
| Drug interactions: Concomitant CYP2B6 inhibitor use, opioid use, and previous cisplatin exposure were significant for the development of IRE. |
Note. IRE= ifosfamide-related encephalopathy; MMSE = Mini-Mental State Exam; SCr = serum creatinine; WNL = within normal limits; RR = relative risk; LFT = liver function test; AST = aspartate aminotransferase; ALT = alanine aminotransferase; WBC = white blood cell.
Table 2. Summary of Evidence for Risk Factors for Ifosfamide-Related Encephalopathy.
| Risk factor | Risk factor status based on summary of the evidence | |
|---|---|---|
| Patient characteristics | Age | Not a risk factor |
| Gender | Insufficient data | |
| History of IRE | Insufficient data | |
| Multiple risk factors | Risk factor | |
| Performance status | Risk factor | |
| Bodyweight | Insufficient data | |
| Baseline cognitive impairment and delirium | Insufficient data | |
| Physiologic | Renal insufficiency | Risk factor |
| Hypoalbuminemia | Risk factor | |
| Hyperbilirubinemia | Insufficient data | |
| CBC abnormalities (platelet count, hemoglobin level, baseline and nadir WBC) | Insufficient data | |
| Transaminitis | Insufficient data | |
| Electrolyte abnormalities | Insufficient data | |
| Treatment characteristics | Ifosfamide dose | Insufficient data |
| Rate of infusion | Insufficient data | |
| Frequency of infusion | Insufficient data | |
| Tumor burden | Pelvic disease | Insufficient data |
| Brain metastasis | Insufficient data | |
| Drug-drug interactions | Cisplatin exposure | Insufficient data |
| Neurotoxic medications | Insufficient data | |
| Aprepitant | Not a risk factor |
Note. IRE = ifosfamide-related encephalopathy; CBC = complete blood count; WBC = white blood cell.
Patient Characteristics
Patient characteristics evaluated as risk factors for IRE include age, gender, a history of IRE during previous chemotherapy cycles, total number of risk factors for IRE, Eastern Cooperative Oncology Group (ECOG) performance status (PS), weight, and baseline cognitive impairment and delirium.
Age. The association of age with IRE was addressed in five articles (Howell, Szabatura, Hatfield Seung, & Nesbit, 2008; Kettle et al., 2010; Lo, Shen, Chen, Dong, & Wu, 2016; Richards et al., 2010; Szabatura et al., 2015). There was no significant age difference between patients with and without IRE in four of these studies (Howell et al., 2008; Kettle et al., 2010; Lo et al., 2016; Szabatura et al., 2015). Howell and colleagues (2008) completed a retrospective case-control study of 45 sarcoma patients who received ifosfamide, with and without the use of aprepitant. Eight patients developed IRE. Kettle and colleagues (2010) conducted a retrospective case-control study that included 41 patients who received 93 cycles of ifosfamide. Patients who received prophylaxis for IRE with albumin were compared with those who did not receive prophylaxis. Six cases of IRE were identified in four patients. Lo and colleagues (2016) carried out a retrospective case-control study that included 337 patients who had received ifosfamide. Thirty-eight patients developed IRE. Szabatura and colleagues (2015) performed a retrospective case-control study that included 100 lymphoma and 100 sarcoma patients who received ifosfamide. Twenty-nine patients developed IRE.
In contrast, Richards and colleagues (2010) identified age as a potential risk factor. This retrospective case-control study included 63 sarcoma patients who received 166 cycles of ifosfamide. Patients who received prophylaxis for IRE with methylene blue, thiamine, and/or albumin were compared with those who did not receive prophylaxis. The study authors reported that age greater than 50 years was a risk factor for IRE. The authors did not report any statistical analysis for the identification of risk factors included in the study.
Gender. The authors of three studies examined gender as a risk factor for IRE (Lo et al., 2016; Sweiss et al., 2008; Szabatura et al., 2015). Gender was reported to be nonsignificant in two of the studies (Lo et al., 2016; Szabatura et al., 2015). Sweiss and colleagues (2008) conducted a retrospective case-control study that included 19 sarcoma patients who received ifosfamide. Of the eight patients who developed encephalopathy, seven were female. Only three of the non-IRE patients were female. Therefore, the authors reported female gender as a possible risk factor for IRE, although the sample size was too small for any statistical analysis.
History of IRE. Kim and colleagues (2016) and Richards and colleagues (2010) examined a history of IRE as a risk factor for the development of IRE in subsequent chemotherapy cycles. Kim and colleagues (2016) completed a retrospective case-control study that included 28 patients who received 47 cycles of ifosfamide. Six patients developed seven cases of IRE. Only one patient with a history of IRE during previous ifosfamide therapy developed IRE while two patients with a history of IRE did not develop IRE. Therefore, history of IRE was not significantly associated with the development of IRE. In contrast, Richards and colleagues (2010) reported that a history of IRE was a risk factor for subsequent development of IRE.
Number of risk factors. Kettle and colleagues (2010) and Kim and colleagues (2016) examined whether having multiple risk factors predicted the development of IRE. Kettle and colleagues (2010) reported that patients with IRE had a mean of 2.67 risk factors (age greater than 65, history of IRE, hypoalbuminemia, renal insufficiency, hepatic insufficiency, electrolyte imbalances, high dose ifosfamide), compared with 1.43 risk factors in patients without IRE (p = .02). The authors developed a risk stratification model for IRE, and all cases of IRE occurred among patients identified as high risk (p = .001). Kim and colleagues (2016) reported that patients with IRE had a higher median number of risk factors for IRE (renal dysfunction, hypoalbuminemia, pelvic disease, history of IRE, and potential drug-drug interactions), with three compared with two (p = .012).
Performance status. Lo and colleagues (2016) addressed PS as a risk factor for IRE. They reported that the rate of encephalopathy was significantly higher in patients with a PS of 2 to 4, compared with patients with a PS of 0 to 1 (p < .001). Performance status was significant on both univariate and multivariate analyses.
Weight. In the Sweiss and colleagues (2008) study, investigators examined weight as a risk factor for IRE. They reported the ratio of actual bodyweight to mean ideal bodyweight was substantially higher in patients who developed IRE.
Cognitive impairment. Howell and colleagues (2008) examined the association of baseline cognitive impairment with the development of IRE. Upon admission for chemotherapy, patients were evaluated with a Mini-Mental State Exam (MMSE) and the Delirium Rating Scale (Trzepacz, Baker, & Greenhouse, 1988). There was no significant difference in baseline MMSE scores between the patients who developed IRE and those who did not. However, patients who developed IRE had a higher mean delirium score on admission (0.2 vs. 0) and on day one of chemotherapy administration (3 vs. 0.23) compared with those without IRE (p = .03, p < .05, respectively).
Physiologic Factors
Renal insufficiency. The authors of all seven studies examined renal insufficiency as a risk factor for IRE. Renal insufficiency, measured by serum creatinine (SCr) in mg/dL and creatinine clearance (CrCl), was reported as a risk factor in four studies (Kettle et al., 2010; Lo et al., 2016; Richards et al., 2010; Szabatura et al., 2015). Kettle and colleagues (2010) reported that patients with IRE had higher mean SCr, 1.05 vs. 0.85, than patients without IRE (p = .04), although CrCl was not significantly different. Lo and colleagues (2016) reported that patients with IRE had higher mean SCr, 1.18 vs. 0.92, than patients without IRE (p = .004). Richards and colleagues (2010) identified creatinine greater than 1 as a risk factor for IRE. Szabatura and colleagues (2015) reported the odds of having IRE was nine times higher for a one-unit (0.10) increase in SCr. Conversely, Howell and colleagues (2008) reported that mean SCr was lower for patients with IRE than those without (p < 0.05), 0.61 vs. 0.79. Two studies reported no significant difference in SCr between patients with and without IRE (Kim et al., 2016; Sweiss et al., 2008).
Hypoalbuminemia. The authors of all seven studies examined hypoalbuminemia as a risk factor for IRE, and all except Kim and colleagues (2016) identified hypoalbuminemia as a risk factor. Howell and colleagues (2008) reported that mean albumin (g/dL) was lower for patients with IRE compared with patients without IRE, 2.88 vs. 3.85 (p < 0.05). Kettle and colleagues (2010) reported that patients with IRE had lower baseline albumin than patients without IRE, 2.88 vs. 3.42 (p = .01). Lo and colleagues’ (2016) multivariate and univariate analysis demonstrated a significantly lower (p = 0.002) mean baseline albumin in patients with IRE. In the Richards and colleagues (2010) study, an albumin level of less than 3.5 was a risk factor for IRE. Sweiss and colleagues (2008) reported that albumin was substantially lower in the IRE group. Szabatura and colleagues (2015) calculated the odds of having IRE were 0.15 times lower for a one-unit increase in albumin (p = 0.001). Only Kim and colleagues (2016) reported no significant difference in albumin levels between patients with and without IRE.
Hyperbilirubinemia. The authors of four studies examined hyperbilirubinemia as a risk factor for IRE (Howell et al., 2008; Lo et al., 2016; Richards et al., 2010; Sweiss et al., 2008). Hyperbilirubinemia was a significant risk factor in three of the studies (Howell et al., 2008; Lo et al., 2016; Richards et al., 2010). Howell and colleagues (2008) reported that total bilirubin (mg/dL) at baseline was higher in patients with IRE, 0.86 vs. 0.42 (p < 0.05). Lo and colleagues (2016) reported no significant difference in mean baseline total bilirubin levels between the groups, although patients with total bilirubin greater than 3 were at significantly higher risk for IRE (p = 0.007) on univariate analysis. Richards and colleagues (2010) identified total bilirubin greater than 1.3 as a risk factor for IRE. Sweiss and colleagues (2008) reported that mean total bilirubin levels were lower in patients with IRE, 0.5 vs. 0.8. Also, the mean change in bilirubin from baseline to the final dose of ifosfamide was higher in patients without IRE, 0.05 vs. –0.45.
Complete blood count. Abnormal CBC indices as risk factors for IRE were investigated in four studies (Kim et al., 2016; Lo et al., 2016; Sweiss et al., 2008; Szabatura et al., 2015). Kim and colleagues (2016) reported that patients with IRE had a lower median nadir white blood cell (WBC) count (× 103/∝L), 0.4 vs. 3.9 (p = 0.003) and a higher reduction in median WBC count from baseline than patients without IRE, –6.2 vs. –4.1 (p < 0.001). There was no significant difference in pretreatment WBC count and number of days until WBC nadir. Lo and colleagues (2016) reported that mean baseline WBC count was significantly higher (p = 0.026) in patients with IRE than those without, on univariate analysis. Platelet count was not significantly different between the two groups. Sweiss and colleagues (2008) reported that anemia, measured by hemoglobin and hematocrit, and decreased WBC count from baseline to completion of treatment were risk factors for IRE. Mean baseline hemoglobin (g/dL) and hematocrit for patients with IRE were 10.5 and 31.3%, respectively, compared with 12.4 and 37% for patients without IRE. Fluctuation in mean WBC count was –7.1 for patients with IRE compared with –3.0 in patients without IRE. Szabatura and colleagues (2015) calculated that the odds of having IRE were 1.4 times higher for a one-unit increase in hemoglobin.
Transaminitis. The authors of four studies examined transaminitis as a risk factor for IRE (Kettle et al., 2010; Lo et al., 2016; Richards et al., 2010; Sweiss et al., 2008). Kettle and colleagues (2010) reported that mean aspartate aminotransferase (AST) and alanine aminotransferase (ALT; units/L) were significantly higher in patients with IRE (p < 0.01). Lo and colleagues (2016) reported that mean baseline AST was significantly higher in patients with IRE (p = .039) on univariate analysis. Mean baseline ALT was not significantly different between the groups. Richards and colleagues (2010) reported that ALT greater than 65 and AST greater than 40 were risk factors for IRE. Sweiss and colleagues (2008) found no difference in liver enzymes between patients with and without IRE.
Electrolyte abnormalities. Kettle and colleagues (2010) and Richards and colleagues (2010) examined electrolyte abnormalities as risk factors for IRE. In the risk stratification model for IRE developed by Kettle and colleagues (2010), electrolyte abnormalities including sodium (mEq/L) greater than 150 or less than 135 and potassium (mEq/L) greater than 5.0 or less than 3.5 were included as minor risk factors for IRE. However, the investigators did not include electrolytes in the risk factor analysis for statistical significance. In Richards and colleagues (2010), for patients who developed IRE, more patients who received prophylaxis for IRE had hyponatremia (less than 135) compared with patients who did not receive prophylaxis.
Treatment Characteristics
The authors of five studies examined various treatment characteristics as risk factors for IRE (Howell et al., 2008; Kettle et al., 2010; Lo et al., 2016; Richards et al., 2010; Szabatura et al., 2015). Howell and colleagues (2008) reported that the total dose of ifosfamide was lower in patients with IRE, although no statistical analysis was reported. In the Kettle and colleagues (2010) study, the mean dose of ifosfamide was not significantly different between patients with and without IRE. Lo and colleagues (2016) reported that daily ifosfamide dose, dose per cycle, and cumulative ifosfamide dose were not significantly different between patients with and without IRE. Richards and colleagues (2010) established a 1- to 2-hour infusion time of ifosfamide, total ifosfamide dose per cycle greater than or equal to 8 g/m2, and every 12-hour frequency of ifosfamide administration as risk factors for IRE. Szabatura and colleagues (2015) reported that patients with IRE had received a significantly higher ifosfamide daily dose (p = 0.009), although ifosfamide total dose and infusion duration were not significantly different between patients with and without IRE.
Tumor Burden
The presence of pelvic disease as a risk factor for IRE was examined in four studies (Kim et al., 2016; Richards et al., 2010; Sweiss et al., 2008; Szabatura et al., 2015). Kim and colleagues (2016) reported that patients with pelvic disease were significantly more likely to develop IRE (p = 0.009). Richards and colleagues (2010) also identified pelvic disease as a risk factor for IRE. However, Sweiss and colleagues (2008) reported no difference in the presence of pelvic disease between patients with and without IRE. In the Szabatura and colleagues (2015) study, IRE was more common in patients with sarcoma than lymphoma (p < 0.0001) and occurred most often in sarcoma patients with disease in the pelvis and retroperitoneum.
Lo and colleagues (2016) and Richards and colleagues (2010) reported on brain metastasis as a risk factor for IRE. Lo and colleagues (2016) found no significant difference between patients with and without IRE for the presence of brain metastasis. However, Richards and colleagues (2010) noted that concurrent or previous brain irradiation was a risk factor for IRE.
Drug Interactions
The authors of three studies examined the relationship between cisplatin exposure and IRE (Lo et al., 2016; Richards et al., 2010; Szabatura et al., 2015). Lo and colleagues (2016) reported no difference for concurrent administration of cisplatin and ifosfamide between patients with and without IRE. In contrast, Richards and colleagues (2010) identified cisplatin use as a risk factor for IRE. Szabatura and colleagues (2015) reported that previous cisplatin exposure was a significant risk factor for the development of IRE (p = 0.007).
The authors of two studies reported on the relationship between ifosfamide and aprepitant. Howell and colleagues (2008) reported a trend of an increased occurrence of IRE with aprepitant use, although this was not statistically significant. Richards and colleagues (2010) noted that the use of aprepitant was not a risk factor for IRE.
Kim and colleagues (2016) reported that the presence of possible drug-drug interactions at baseline was not significantly different between patients with and without IRE. Richards and colleagues (2010) reported that the concomitant use of more than three neurotoxic medications, including benzodiazepines, opioids, dexamethasone, and sedating antiemetics were risk factors for IRE. Szabatura and colleagues (2015) reported that concomitant CYP2B6 inhibitor use and opioid use was significant for the development of IRE (p = 0.003, p = 0.048, respectively).
DISCUSSION
Patient Characteristics
Advanced age may be a risk factor for IRE, possibly because elderly patients are at risk for delirium. An age of greater than 65 years has been proposed as a cut-off point (Kettle et al., 2010). However, only Richards and colleagues (2010) reported age as a risk factor for IRE, and this study did not include statistical analysis. The four other studies that examined this risk factor reported that age was not significant. Thus, it appears that age is not a risk factor for the development of IRE.
Only Sweiss and colleagues (2008) reported that female gender and bodyweight were risk factors for IRE, and with a sample of 19 patients, no statistical analysis was conducted. The authors noted that gender and weight as risk factors might not be independent of each other as more women than men developed IRE, and women tend to have a higher fat to muscle ratio than men. Therefore, there are insufficient data to determine if gender and bodyweight are risk factors for IRE.
While ifosfamide is often discontinued once a patient has experienced IRE, it may be continued in subsequent chemotherapy cycles based on symptom severity and provider discretion. IRE is most common in the first cycle of treatment, although it can occur in later cycles (Sweiss et al., 2008). Due to conflicting results between the studies that examined a history of IRE as a risk factor for IRE (Kim et al., 2016; Richards et al. 2010), there is insufficient evidence to state whether or not a history of IRE is a risk factor for the subsequent development of IRE.
Kettle and colleagues (2010) reported that the development of IRE is likely the result of a multifaceted metabolic process and not one individual risk factor. Therefore, these authors and Kim and colleagues (2016) examined whether having multiple risk factors is a risk factor for the development of IRE. Both reported that patients with IRE had more risk factors than patients without IRE. Accordingly, having multiple risk factors does appear to be a risk factor for IRE, although further research is needed to develop risk stratification models. The risk factor model developed by Kettle and colleagues (2010) could be updated to reflect additional risk factors identified in more recent studies.
Although only Lo and colleagues (2016) evaluated PS as a risk factor for IRE, findings from this study demonstrated robust evidence that a PS of 2 to 4 is a risk factor. The difference in PS between the groups with and without IRE was highly significant on both univariate and multivariate analysis. After controlling for other covariates, a patient with a PS of 2 to 4 had 5.15 times higher risk of developing IRE than a patient with a PS of 0 to 1. Performance status is a subjective measure not always documented consistently in the medical record, which limited the ability of other authors to evaluate PS as a risk factor (Sweiss et al., 2008). Based on available evidence, a PS of 2 to 4 does appear to be a risk factor for IRE.
Cognitive status was evaluated in only one study (Howell et al., 2008). Patients received antiemetic medications, including steroids, benzodiazepines, and phenothiazines, which may have side effects similar to IRE, confounding mental status measurement. With these limited data, there is insufficient evidence to determine if baseline cognitive impairment or delirium are risk factors for IRE.
Physiologic Factors
Renal insufficiency may not allow for adequate clearance of the neurotoxic metabolites of ifosfamide, thus contributing to the development of encephalopathy. Authors of four studies (Kettle et al., 2010; Lo et al., 2016; Richards et al., 2010; Szabatura et al., 2015) identified higher SCr as a risk factor for IRE, while authors of two others (Kim et al., 2016; Sweiss et al., 2008) reported no significant difference. Howell and colleagues (2008) reported a lower mean SCr for patients with IRE. However, the mean SCr for both groups was within normal limits, which limits the ability to draw conclusions about renal insufficiency from this study. The authors who reported no significant difference had small samples sizes (28 and 19 patients, respectively), thus limiting their ability to detect differences. The study with the most robust data reported impaired renal function was a risk factor on both multivariate and univariate analysis (Lo et al., 2016). These results provide evidence to support renal insufficiency as a risk factor for the development of IRE.
Impaired liver function is a potential risk factor for IRE, as ifosfamide is metabolized in the liver (Richards et al., 2010). Evaluation of this risk factor is complicated by the fact that there are multiple ways to measure liver function. All study authors included in this review, except Kim and colleagues (2016), reported hypoalbuminemia as a risk factor for IRE. However, Kim and colleagues (2016) defined hypoalbuminemia at a higher level than the other studies, which may have influenced results. Thus, the data suggest that hypoalbuminemia is a risk factor for the development of IRE.
The authors of three of the four studies examining hyperbilirubinemia reported hyperbilirubinemia as a significant risk factor for IRE (Howell et al., 2008; Sweiss et al., 2008; Richards et al., 2010). However, in Lo and colleagues’ research (2016), bilirubin was only a significant risk factor with a total bilirubin of greater than 3 on univariate analysis. Providers may exercise caution in giving ifosfamide to patients with bilirubin over 3, as dose reduction is recommended at this level (Floyd, Mirza, Sachs, & Perry, 2006). Differences in bilirubin between patients with and without IRE in Sweiss and colleagues (2008) were not felt to be clinically significant. Data regarding elevated liver enzymes were also conflicting, as outlined in the results section. Based on available data, there is insufficient evidence to suggest that hyperbilirubinemia or transaminitis are risk factors for IRE.
The authors of four studies investigated CBC indices as risk factors for IRE. However, each study did not examine the same indices, making it difficult to compare results. There were also conflicting results between studies, including Sweiss and colleagues (2008) reporting anemia as a risk factor for IRE, while Szabatura and colleagues (2015) reported an increase in hemoglobin as a risk factor. Therefore, further research is needed to establish CBC indices, including platelet count, hemoglobin, and baseline and nadir WBC count as risk factors for IRE.
The authors of two studies (Kettle et al., 2010; Richards et al., 2010) examined electrolyte abnormalities as risk factors for IRE. However, Kettle and colleagues (2010) did not include electrolytes in risk factor analysis. Richards and colleagues (2010) noted that more patients who received prophylaxis for IRE had hyponatremia. This trend could be because hyponatremia may have similar symptoms to IRE. There is insufficient evidence to suggest that electrolyte abnormalities are risk factors for IRE.
Treatment Characteristics
Howell and colleagues (2008) reported that the total dose of ifosfamide was lower in patients with IRE. However, this may be because 62% of patients in the study with IRE developed IRE in cycle one of chemotherapy and discontinued further treatment with ifosfamide. Sweiss and colleagues (2008) noted that there were too few patients who received a continuous infusion of ifosfamide rather than fractioned doses to assess if different dosing schedules influenced the development of IRE. Kettle and colleagues (2010) and Lo and colleagues (2016) reported no difference between the groups. Richards and colleagues (2010) reported that infusion time, total dose per cycle, and frequency were risk factors for IRE, but no statistics were reported. Szabatura and colleagues (2015) reported that higher daily ifosfamide dose was a risk factor, but total dose and infusion duration were not different. Based on these results, there are insufficient data to suggest that these treatment characteristics are risk factors for IRE.
Tumor Burden
Pelvic disease is a possible risk factor for IRE, as it may lead to urinary obstruction and altered drug elimination (Kim et al., 2016). Symptoms of brain metastases and IRE may overlap (Howell et al., 2008). Results regarding pelvic disease and brain metastasis were mixed among the studies (Kim et al., 2016; Lo et al., 2016; Richards et al., 2010; Sweiss et al., 2008; Szabatura et al., 2015). Only Kim and colleagues (2016) provided statistical analysis for the level of significance for the presence of pelvic disease. Many of the patients included in these studies were sarcoma patients in whom brain metastasis is uncommon (Howell et al., 2008). There is insufficient evidence from the data to suggest that pelvic disease, a history of brain metastasis, or brain radiation are risk factors for IRE.
Drug Interactions
Cisplatin exposure is a possible risk factor for IRE due to cumulative renal tubular injury with the use of both agents (Goren et al., 1987). Aprepitant is an inhibitor of the cytochrome P4503A4 enzyme, which metabolizes ifosfamide, and may increase levels of neurotoxic metabolites (Howell et al., 2008). While Lo and colleagues (2016) did not report concurrent cisplatin as a risk factor for IRE, the authors noted that only a few patients received concurrent cisplatin and did not assess the influence of prior cisplatin exposure. While Kim and colleagues (2016) reported that concomitant use of drugs with potential interactions were not significantly different between patients with and without IRE, the authors did not describe any details regarding the drug interactions examined. The results suggest that aprepitant use is not a risk factor for IRE. There is insufficient evidence regarding whether or not cisplatin exposure and concomitant use of other neurotoxic medications are risk factors for IRE.
CONCLUSION
A PS of 2 to 4, having multiple risk factors, renal insufficiency, and hypoalbuminemia appear to be risk factors for the development of IRE. Other potential risk factors in need of further research are a history of IRE, baseline cognitive impairment or delirium, gender, weight, hyperbilirubinemia, transaminitis, ifosfamide dose, infusion rate and frequency, pelvic disease, brain metastasis, cisplatin exposure, use of other neurotoxic medications, electrolyte abnormalities, and CBC abnormalities (platelet count, hemoglobin level, baseline and nadir WBC). Previously suggested risk factors that do not appear to be risk factors for IRE based on the data available are age and use of aprepitant (see Table 2 for a risk factor summary).
There are limitations to the interpretation of the data in this review. All studies had a retrospective case-control design, which does not allow for control of confounding factors such as other risk factors that may have been present but were not measured. Some patients with IRE may not be included in study analysis as it can be challenging to capture patients with IRE due to differing medical terminology used in medical records. Sample sizes of some studies were small, limiting the generalizability of the results.
Advanced practitioners should be aware of potential risk factors for the development of IRE. Patients should be counseled regarding their own risk for IRE, and at-risk patients should be monitored closely. Although evidence is currently lacking regarding treatment and prevention of IRE, hopefully further research can be done in this area.
References
- Brunello, A., Basso, U., Rossi, E., Stefani, M., Ghiotto, C., Marino, D., Crivellari, G., & Monfardini, S. (2007). Ifosfamide-related encephalopathy in elderly patients: Report of five cases and review of the literature. Drugs & Aging, 24(11), 967–973. 10.2165/00002512-200724110-00008 [DOI] [PubMed] [Google Scholar]
- David, K. A., & Picus, J. (2005). Evaluating risk factors for the development of ifosfamide encephalopathy. American Journal of Clinical Oncology, 28(3), 277–280. 10.1097/01.coc.0000158439.02724.5a [DOI] [PubMed] [Google Scholar]
- Floyd, J., Mirza, I., Sachs, B., & Perry, M. C. (2006). Hepatotoxicity of chemotherapy. Seminars in Oncology, 33(1), 50–67. 10.1053/j.seminoncol.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Gharaibeh, E. Z., Telfah, M., Powers, B. C., & Salacz, M. E. (2018). Hydration, methylene blue, and thiamine as a prevention regimen for ifosfamide-induced encephalopathy. Journal of Oncology Pharmacy Practice, 25(7), 1784–1786. 10.1177/1078155218808361 [DOI] [PubMed] [Google Scholar]
- Goren, M. P., Wright, R. K., Pratt, C. B., Horowitz, M. E., Dodge, R. K., Viar, M. J., & Kovnar, E. H. (1987). Potentiation of ifosfamide neurotoxicity, hematotoxicity, and tubular nephrotoxicity by prior cis-diamminedichloroplatinum (II) therapy. Cancer Research, 47(5), 1457–1460. [PubMed] [Google Scholar]
- Hamadani, M., & Awan, F. (2006). Role of thiamine in managing ifosfamide-induced encephalopathy. Journal of Oncology Pharmacy Practice, 12(4), 237–239. 10.1177/1078155206073553 [DOI] [PubMed] [Google Scholar]
- Heim, M. E., Fiene, R., Schick, E., Wolpert, E., & Queiber, W. (1981). Central nervous side effects following ifosfamide monotherapy of advanced renal carcinoma. Journal of Cancer Research and Clinical Oncology, 100, 113–116. 10.1007/BF00405909 [DOI] [PubMed] [Google Scholar]
- Howell, J. E., Szabatura, A. H., Hatfield Seung, A., & Nesbit, S. A. (2008). Characterization of the occurrence of ifosfamide-induced neurotoxicity with concomitant aprepitant. Journal of Oncology Pharmacy Practice, 14(3), 157–162. 10.1177/1078155208093930 [DOI] [PubMed] [Google Scholar]
- Imtiaz, S., & Muzaffar, N. (2010). Ifosfamide neurotoxicty in a young female with a remarkable response to thiamine. Journal of the Pakistan Medical Association, 60(10), 867. [PubMed] [Google Scholar]
- Kettle, J. K., Grauer, D., Folker, T. L., O’Neal, N., Henry, D. W., & Williams, C. B. (2010). Effectiveness of exogenous albumin administration for the prevention of ifosfamide-induced encephalopathy. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 30(8), 812–817. 10.1592/phco.30.8.812 [DOI] [PubMed] [Google Scholar]
- Kim, S. S., Isola, L. M., & Oh, W. K. (2016). Severe post-treatment leukopenia associated with the development of encephalopathy following ifosfamide infusion. Anti-Cancer Drugs, 27(3), 235–238. 10.1097/CAD.0000000000000321 [DOI] [PubMed] [Google Scholar]
- Lo, Y., Shen, L. J., Chen, W. H., Dong, Y. H., & Wu, F. L. L. (2016). Risk factors of ifosfamide-related encephalopathy in adult patients with cancer: a retrospective analysis. Journal of the Formosan Medical Association, 115(9), 744–751. 10.1016/j.jfma.2015.07.016 [DOI] [PubMed] [Google Scholar]
- Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ, 339, b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute (2017). Common terminology criteria for adverse events (CTCAE): version 5.0. Retrieved from https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf [Google Scholar]
- Patel, P. N. (2006). Methylene blue for management of ifosfamide-induced encephalopathy. The Annals of Pharmacotherapy, 40(2), 299–303. 10.1345/aph.1G114 [DOI] [PubMed] [Google Scholar]
- Pelgrims, J., De Vos, F., Van den Brande, J., Schrijvers, D., Prové, A., & Vermorken, J. B. (2000). Methylene blue in the treatment and prevention of ifosfamide-induced encephalopathy: Report of 12 cases and a review of the literature. British Journal of Cancer, 82(2), 291–294. 10.1054/bjoc.1999.0917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, A., Marshall, H., & McQuary, A. (2010). Evaluation of methylene blue, thiamine, and/or albumin in the prevention of ifosfamide-related neurotoxicity. Journal of Oncology Pharmacy Practice, 17(4), 372–380. 10.1177/1078155210385159 [DOI] [PubMed] [Google Scholar]
- Shin, Y. J., Kim, J. Y., Moon, J. W., You, R. M., Park, J. Y., & Nam, J. H. (2011). Fatal ifosfamide-induced metabolic encephalopathy in patients with recurrent epithelial ovarian cancer: Report of two cases. Cancer Research and Treatment, 43(4), 260–263. 10.4143/crt.2011.43.4.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweiss, K. I., Beri, R., & Shord, S. S. (2008). Encephalopathy after high-dose ifosfamide. Drug Safety, 31(11), 989–996. 10.2165/00002018-200831110-00003 [DOI] [PubMed] [Google Scholar]
- Szabatura, A. H., Cirrone, F., Harris, C., McDonnell, A. M., Feng, Y., Voit, D.,…Fisher, D. C. (2015). An assessment of risk factors associated with ifosfamide-induced encephalopathy in a large academic cancer center. Journal of Oncology Pharmacy Practice, 21(3), 188–193. 10.1177/1078155214527143 [DOI] [PubMed] [Google Scholar]
- Tajino, T., Kikuchi, S. I., Yamada, H., Takeda, A., & Konno, S. I. (2010). Ifosfamide encephalopathy associated with chemotherapy for musculoskeletal sarcomas: Incidence, severity, and risk factors. Journal of Orthopaedic Science, 15(1), 104–111. 10.1007/s00776-009-1431-y [DOI] [PubMed] [Google Scholar]
- Trzepacz, P. T., Baker, R. W., & Greenhouse, J. (1988). A symptom rating scale for delirium. Psychiatry Research, 23(1), 89–97. 10.1016/0165-1781(88)90037-6 [DOI] [PubMed] [Google Scholar]
- Vakiti, A., Pilla, R., Moustafa, M. A., Joseph, J. J., & Shenoy, A. G. (2018). Ifosfamide-induced metabolic encephalopathy in 2 patients with cutaneous T-cell lymphoma successfully treated with methylene blue. Journal of Investigative Medicine High Impact Case Reports, 6, 1–4. 10.1177/2324709618786769 [DOI] [PMC free article] [PubMed] [Google Scholar]