Abstract
Purpose
The number of newly approved cancer medications continues to grow; many of these newly approved medications are oral agents. Oral oncolytic agents have advantages including patient convenience, prolonged drug exposure, and noninvasive administration. However, these advantages come at a cost premium that many patients cannot afford, which can lead to change in therapy or abandonment. This study evaluates the perceptions of health-care workers regarding the cost and safety of oral oncolytic agents.
Methods
This is a descriptive, nonexperimental, cross-sectional study of health-care professionals in hematology/oncology patient care settings across the United States. Data were collected using a 35-item online questionnaire to measure quality improvement areas when using oral oncolytic agents.
Results
Results are based on 503 survey respondents comprising mainly pharmacists (54%), pharmacy administrators (15%), and nurses (10%). Adherence to oral oncolytics was not included in outcome measurements at 31.5% of respondents’ facilities. Treatment abandonment due to cost was reported by 46.6% of respondents. The most common agents abandoned due to cost included capecitabine, abiraterone, and palbociclib. To reduce cost, some respondents (6.1%) have utilized drug interactions to increase drug half-life. Prior authorization delays were perceived to occur in 1 to 2 patients weekly, creating a 4- to 6-day wait to initiate therapy; 24.0% of respondents spend more than 30 hours weekly resolving these issues.
Conclusions
Health-care workers underscore their concerns about the prevalence of issues related to oral oncolytic therapy, by reporting on the incidence of abandonment of therapy, delay in therapy initiation, resources needed to assure patient access to oral oncolytic agents, and impact on patient care. Patients diagnosed with cancer require prompt access to appropriate treatments to produce favorable outcomes. In many instances, patients are unable to understand the extensive process involved in determining an appropriate course of treatment. Many factors aid in deciding on a particular course of therapy, including efficacy, safety, access to medication, adherence, and out-of-pocket cost of medication. Until recently, cancer therapy consisted primarily of IV infusion therapy, but oral oncolytic agents have been added to the therapy options over the past several years.
Oral oncolytic agents are being approved by the US Food and Drug Administration (FDA) at a rapid pace. There are currently more than 800 new oncology therapies in the pipeline, 25% of which are oral agents (American Cancer Society [ACS], 2016). Oral oncolytic agents have several advantages over the parenteral route, including patient convenience, prolonged drug exposure, and noninvasive administration. However, many of these oral agents can be a cost burden to patients (Abbott, Edwards, Edwards, Dranitsaris, & McCarthy, 2011). In the United States, the reported direct medical costs associated with cancer were estimated at $87.3 billion in 2014, and are estimated to reach almost $174 billion by 2020 (ACS, 2016). Streeter and colleagues (2011) studied the out-of-pocket costs associated with oral oncolytic agents compared to the abandonment rate. High out-of-pocket costs were associated with 1 in every 10 patients who abandon oral oncolytic therapy. A quarter of the patients who abandoned their oral oncolytic treatment exhibited reverse medical claims on their oral oncolytic therapy to follow up with another oncolytic prescription (Streeter et al., 2011). As oral oncolytic therapy becomes more prevalent, adherence to therapy is critical to achieve beneficial outcomes; thus, cost of care needs to be further evaluated.
The purpose of this study was to evaluate the perceptions of health-care workers regarding the cost and safety of oral oncolytic agents, and to formally highlight perceptions of impact on patient care.
METHODS
Design
This study was based on a descriptive, nonexperimental, cross-sectional survey of health-care professionals. Research was granted an exemption from the Institutional Review Board of the University of Arizona. Subjects included pharmacists, physicians, advanced practitioners (nurse practitioners or PAs), nurses, pharmacy administrators (supervisors, managers, directors), and others with experience in hematology/oncology patient care across the United States.
Measures
Data were collected using a 35-item online questionnaire to measure what financial improvement areas require further development when using oral oncolytic agents (May & Figgins, 2016; O’Bryant & Crandell, 2008). The questionnaire was completed using Qualtrics through the University of Arizona. Sixteen items obtained respondents’ demographics. Six items focused on the prevalence of cost issues, while nine items addressed the impact on patient care and safety. Four items assessed alternative methods to obtain medications. A copy of the questionnaire can be found in Appendix A.
Data Collection
The questionnaire was distributed with the assistance of the American Society of Health-System Pharmacists (ASHP), the Association of Community Cancer Centers (ACCC), the American Society of Clinical Oncology (ASCO), the Community Oncology Pharmacy Association (COPA), the National Community Oncology Dispensing Association (NCODA), the Oncology Nursing Society (ONS), and the Advanced Practitioner Society for Hematology and Oncology (APSHO). Emails embedded with a QR code linked to the open-response online survey were sent to potential respondents, using physical or digital membership lists provided by the above-mentioned partner societies and associations. There was no incentive, and participation was voluntary. The survey was available from March 23, 2017, to January 6, 2018.
Data Analysis
Results were presented in the form of descriptive statistics. Continuous variables were summarized using measures of central tendency (mean and standard deviation) and categorized variables as percentages. Responses to open questions were numbered consecutively and grouped into common themes independently. The themes were then ranked from the most to the least commonly reported.
RESULTS
Demographics
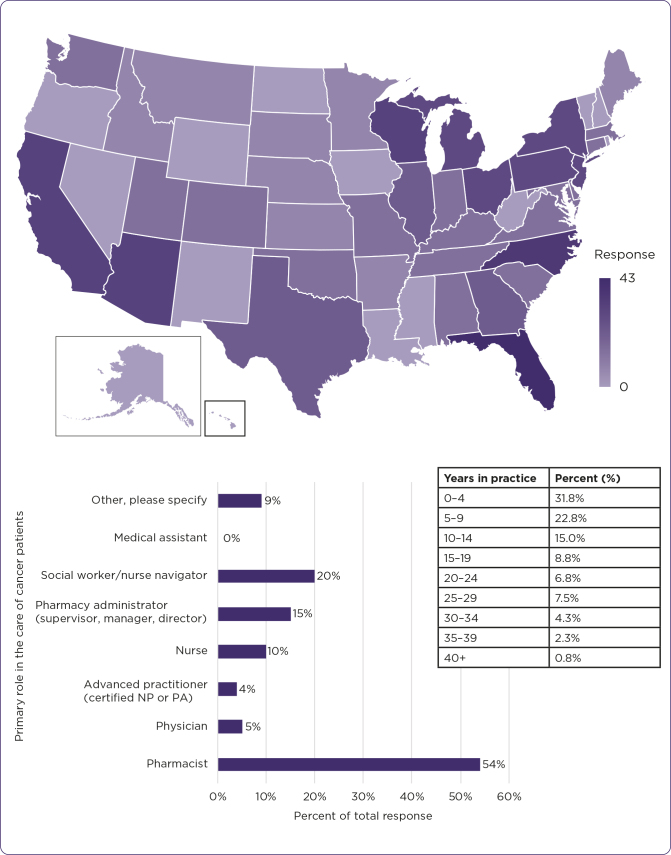
Several organizations and individuals participated in both completion and distribution of the questionnaire, yielding 503 responses. The majority of respondents reported learning of this questionnaire through an email from a colleague or friend. Respondents identified their primary role in the care of cancer patients as “other” listed roles such as financial counselor, pharmacy technician, nursing director, patient advocate, nurse/practice manager, practice/office administrator, director of cancer services, or resident. Regarding their experience in the cancer care setting, respondents reported a range from 0 to 40 years. The majority of respondents had at least 2 years’ experience, and the average collective experience was 11.2 years (standard deviation, 9.8 years) in the cancer care setting (Figure 1). The map displayed in Figure 1 shows the distribution of questionnaire respondents based on the state in which their practice site is located. Representation was obtained from 46 out of 50 states. Alaska, New Mexico, North Dakota, and Rhode Island are not represented. The bar graph shows the distribution of respondents based on their primary role in the care of cancer patients. The table shows the distribution of respondents based on years of experience in the hematology/oncology patient care profession.
Figure 1.
Respondent demographics, including location, profession, and experience.
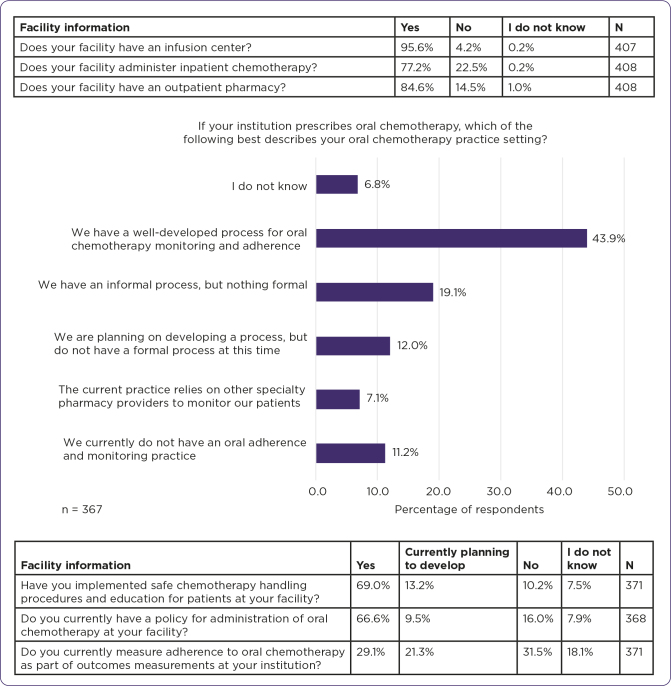
Facility type included academic medical center (42.5%), community hospital (24.7%), community retail/specialty pharmacy (8.9%), standardized patient program health system (2.7%), standardized patient program independent (2.5%), other (17.3%), and unknown (1.5%). Facilities with outpatient pharmacies included retail pharmacies (32.5%), specialty pharmacies (33.1%), in-office dispensing facilities (15.5%), and other (18.9%). Other types of outpatient pharmacies reported included some combination of the three, or government agency. Number of oncolytic doses (parenterally) administered at each facility per month was reported as < 100 doses (5.7%), 100–499 doses (17.1%), 500–999 doses (16.8%), 1000–4999 doses (29.8%), ≥ 5000 doses (8.1%), and unknown number of doses (22.5%). Respondents reported oral oncolytic dispensing per month at a frequency of zero oral oncolytic prescriptions filled at this facility (14.0%), 1–9 prescriptions (5.1%), 10–99 prescriptions (18.1%), 100–499 prescriptions (21.3%), 500–999 prescriptions (7.5%), 1000–4999 prescriptions (3.5%), ≥ 5000 prescriptions (1.3%), and did not know (29.1%). Patients primarily fill their prescriptions for oral oncolytic medications at community pharmacies (14.1%), standardized patient program health systems (2.5%), physicians’ offices (8.0%), specialty mail order pharmacies (30.8%), specialty pharmacies (34.8%), pharmaceutical manufacturers (6.4%), and did not know (3.5%), according to health-care respondents (Figure 2).
Figure 2.
Composition of respondent facility pharmacy, policies, and practices.
The table above the graph in Figure 2 characterizes which respondents come from facilities with infusion capabilities, inpatient, or outpatient pharmacies. The graph identifies the respondents’ institutional policies on monitoring adherence to oral oncolytic therapy. The table below the graph shows implementation status of handling practices, administration practices, and adherence measurement as part of outcomes measurements.
Reported Prevalence of Cost Issues
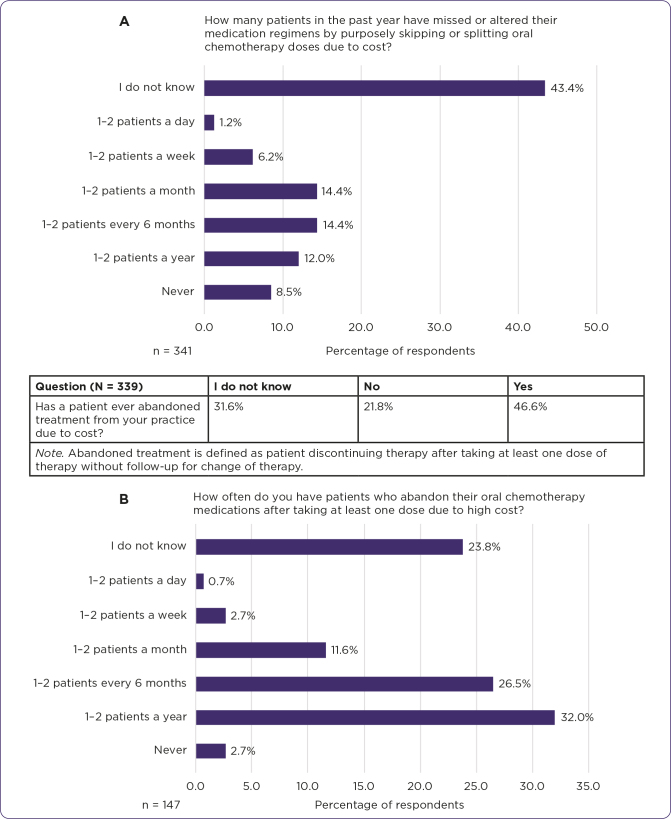
Respondents (n = 340) encounter patients unable to initiate therapy due to the cost of their oral oncolytic medications at a frequency of never (5.0%), 1–2 patients a year (4.1%), 1–2 patients every 6 months (13.5%), 1–2 patients a month (28.5%), 1–2 patients a week (25.6%), 1–2 patients a day (9.1%), and did not know (14.1%). Respondents reported that changing an oral oncolytic regimen to an IV regimen due to the cost of an oral oncolytic agent has happened at a frequency of never (10.6%), 1–2 patients a year (16.2%), 1–2 patients every 6 months (23.3%), 1–2 patients a month (19.5%), 1–2 patients a week (3.5%), 1–2 patients a day (0.9%), and did not know (26.0%; n = 339). In the respondents’ experience, the most common oral oncolytic agents reported (156 respondents who named 356 medications) to have been abandoned by patients due to cost include capecitabine (15.5%), palbociclib (Ibrance, 9.3%), and abiraterone (Zytiga, 9.1%). Respondents’ answers included 61 different common oral oncolytic agents seen abandoned by patients due to cost (Figure 3).
Figure 3.
(A) Respondents reported prevalence of cost issues in the past year based on their experience. The table shows abandonment of treatment due to cost, based on the respondents’ experience. (B) Frequency of abandonment of oral oncolytic medications due to high cost after taking at least one dose was reported based on respondents’ experience.
Impact of Cost Issues on Patient Care/Safety
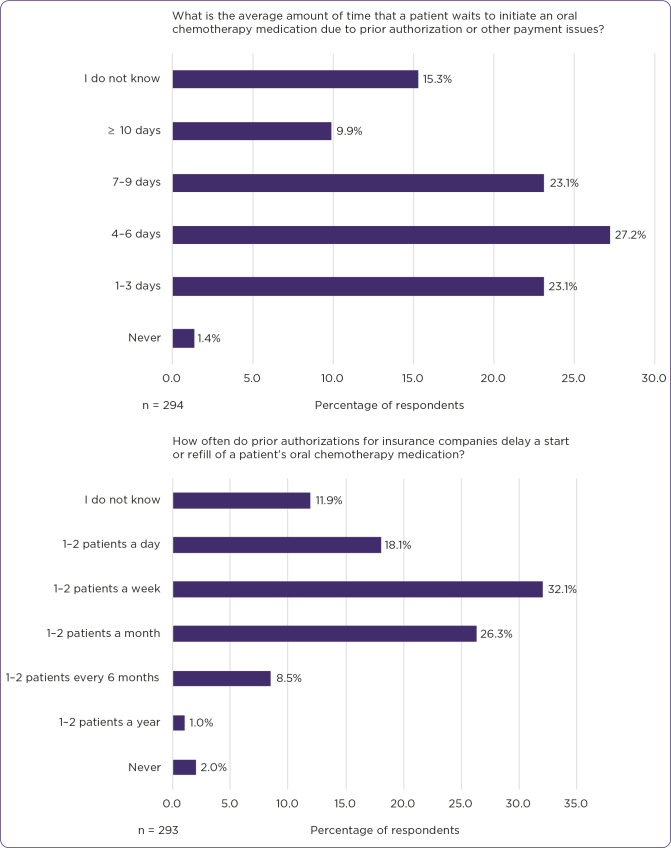
Changing a patient’s treatment course due to the patient not being able to procure the oral drug therapy was reported by 67.8% of respondents, 10.8% of respondents have never had to change a patient’s treatment course due to inability to procure oral therapy, 10.5% reported providing samples to continue a patient’s course of therapy, and 10.8% of respondents did not know (n = 295). Therapy being changed due to insurance denial for oral oncolytic medications was reported at a frequency of never (4.4%), 1–2 patients a year (16.9%), 1–2 patients every 6 months (29.2%), 1–2 patients a month (20.7%), 1–2 patients a week (5.8%), 1–2 patients a day (0.3%), and did not know (22.7%) by respondents (n = 295). On average, 1.4% respondents reported zero days waiting, 23.1% reported 1–3 days waiting, 27.2% reported 4–6 days waiting, 23.1% reported 7–9 days waiting, 9.9% reported ≥ 10 days waiting, and 15.3% did not know the amount of time a patient waits to initiate an oral oncolytic medication due to prior authorization or other payment issues (n = 294; Figure 4).
Figure 4.
Prevalence of delays due to prior authorizations and average patient waiting time due to cost issues, per respondents’ experience.
A number of respondents reported having utilized drug interactions to provide patients with equivalent oral oncolytic benefit while taking less actual drug product, to reduce the overall cost of medications. Of the respondents who reported having utilized drug interactions to increase half-life of the drug and reduce overall cost, the most common drug interactions used included dasatinib (Sprycel) with proton pump inhibitors, voriconazole with CYP3A4 inhibitors, voriconazole with tyrosine kinase inhibitors (TKIs), cimetidine with lapatinib (Tykerb), trametinib (Mekinist) with everolimus, azole antifungals with busulfan, fluconazole with venetoclax (Venclexta), and proton pump inhibitors with BCR-ABL TKIs (Table 1).
Table 1. Survey Question on Use of Drug Interactions to Reduce Overall Cost of Medications (N = 295).
| Question (N = 295) | I do not know | No | Yes |
|---|---|---|---|
| Have you used a drug interaction in order to increase half-life of the drug and reduce overall cost for a patient’s oral chemotherapy medication? | 19.7% | 74.2% | 6.1% |
Alternative Methods to Obtain Medications
The top three types of medication assistance programs respondents reported using at their facilities to help patients obtain oral oncolytic agents at a reduced cost were manufacturer patient assistance programs, disease-based assistance co-pay assistance grants, and manufacturer co-pay cards. Other medication assistance programs used by respondents included grants, local cancer services programs, charity funds, HUB International, foundations, PACE (Program of All-Inclusive Care for the Elderly), hospital pricing, and 340B pricing. Pharmaceutical manufacturer programs were reported as being used never (1.4%), for 1–2 patients a year (1.4%), for 1–2 patients every 6 months (5.1%), for 1–2 patients a month (25.0%), for 1–2 patients a week (34.8%), for 1–2 patients a day (13.8%), and did not know (18.5%), according to respondents (n = 276). Disease-based assistance grants were reported by respondents (n = 279) to be used never (3.9%), for 1–2 patients a year (3.2%), for 1–2 patients every 6 months (7.5%), for 1–2 patients a month (17.9%), for 1–2 patients a week (22.6%), for 1–2 patients a day (16.8%), and did not know (28.0%). Pharmaceutical manufacturer co-pay reduction coupons were reported by respondents (n = 277) to be used never (2.9%), for 1–2 patients a year (2.9%), for 1–2 patients every 6 months (10.5%), for 1–2 patients a month (17.7%), for 1–2 patients a week (28.2%), for 1–2 patients a day (14.1%), and did not know (23.8%).
Adjudication of oral oncolytic agents under the Oral Oncology Parity Law was reported by 12.4% of respondents, no adjudication was reported by 27.7% of respondents, and 59.9% did not know their adjudication status (n = 314). When running billing under the Oral Oncology Parity Law, 26.8% reported issues, 26.3% reported no issues, and 47.4% did not know if they had issues running billing under the Oral Oncology Parity Law (n = 38). Some of the concerns the respondents expanded upon included issues with self-funded insurance companies being excluded from the Oral Oncology Law in some states, no impact on the patient cost of the medication, a very small subset qualifying under state law, and the fact that oral targeted therapies without IV counterparts were not typically covered.
DISCUSSION
The continued development and approval of oral oncolytic agents has brought about a considerable shift in cancer treatment. The shift to oral oncology-based treatment regimens requires clinicians to empower patients and caregivers with strategies to manage and adhere to medication regimens (Vachon, 2017). Oral oncolytic agents can be expensive, making it difficult for patients to afford and access these medications. While there has been significant informal discussion about these issues, they have not been formally evaluated. The purpose of this survey was to quantify these concerns formally and to provide a reference for future practice-related, formally evaluated discussions, as well as a foundation for future research and policy development.
With any treatment, a certain level of medication adherence is required to maintain efficacy (Ruddy, Mayer, & Partridge, 2009). Although it is not the only hurdle during cancer treatment, cost is a substantial obstacle to adherence. Cost-related medication nonadherence is associated with negative health outcomes including poorer physical and mental functioning, myocardial infarction, stroke, increased use of emergency and hospital services, and death (Lee, Khan, & Salloum, 2018). During the period from 1999 to 2012, 1,788 cancer survivors reported cost-related nonadherence of 8.7% (Lee et al., 2018). The trends in cost-related nonadherence among younger cancer survivors increased from 9.9% in 1999 to 16.9% in 2012 (p < .001; Lee et al., 2018). The overwhelming majority of respondents (67.8%) have had to change a patient’s treatment course due to the patient not being able to procure the oral drug therapy, while treatment abandonment due to cost was seen by 46.6% of respondents. Although the largest group of respondents denied using a drug interaction to increase half-life of the drug and reduce overall cost for a patient’s oral oncolytic medication, 6.1% did report utilizing this technique for cost-saving purposes. Administration of medications in ways that are unresearched or under-researched can lead to unpredictable outcomes with the potential for discouraging results due to common narrow therapeutic windows. Several responses reported their patients missing or altering their medication regimens by purposely skipping or splitting oral oncolytic doses due to cost. Appropriate adherence is essential for safe use of these oral oncolytic agents to improve therapy efficacy.
Treatment abandonment is not uncommon; it is seen in 1 in 10 newly initiated oral oncolytic patients (Streeter et al., 2011). The ADAGIO study assessed the impact of nonadherence on patient clinical response with imatinib. According to ADAGIO, patients with suboptimal response had significantly higher mean percentages of imatinib not taken (23.2% + 23.8%) than those with optimal response (7.3% + 19.3%, p = .005; Noens et al., 2009). Results regarding the inclusion of adherence as part of an outcome measurement for oral oncolytic monitoring was split similarly between yes, no, currently planning to develop a protocol, and I do not know. The majority (31.5%) reported not utilizing adherence as part of the oral oncolytic outcome. Due to suboptimal adherence and discontinuation of therapy both adversely impacting the efficacy and toxicity of oral oncolytic agents, it is recommended to have a formal and structured pharmacist-driven adherence program to proactively screen and identify patients who may have barriers to adherence, including cost-related challenges, incorporated as part of outcome measurement policies (Dusetzina, Muluneh, Khan, Richards, & Keating, 2014). This is recommended as an ongoing assessment due to patients’ constantly evolving financial status (Association of Community Cancer Centers, 2016).
Therapy delays have been associated with worse overall survival in patients being treated with oncolytic agents (Chavez-Mac-Gregor, Clarke, Lichtensztajn, & Giordano, 2016; Gallagher et al., 2016) and are often due to payment barriers. The American Medical Association (AMA) published results from a survey that evaluated the impact of prior authorizations on patients and physician practices, finding that almost two-thirds (64%) of physicians reported waiting at least 1 business day for prior authorization decisions, and approximately one-third (30%) reported waiting 3 business days or longer (AMA, 2017). These findings are comparable with the respondents in our survey reporting that prior authorizations or other payment issues can delay oral oncolytic initiation by 4 to 6 days. The AMA physicians reported this issue becoming increasingly common over the past 5 years, with 92% reporting that prior authorizations resulted in care delays for patients, and 78% reporting that prior authorizations resulted in treatment abandonment by patients (AMA, 2017). The AMA survey found that physicians receive an average of 29.1 prior authorization requests per week, and processing those requests takes an average of 14.6 hours per week (AMA, 2017).
Our survey results were analogous, but respondents reported the average time used to process prior authorizations as ranging from zero to more than 30 hours per week. The length of time used to process prior authorizations should be addressed, along with further studies regarding prior authorizations that are not completed. The AMA is working with the American Hospital Association, the America’s Health Insurance Plans, the American Pharmacists Association, the Blue Cross Blue Shield Association, and the Medical Group Management Association to improve prior authorization processes and lower administrative burdens (AMA, 2018a; Butt & Ream, 2016; AMA, 2018b).
Several studies have confirmed that medications with higher prescription co-payments or cost sharing are associated with greater nonadherence (Neugut et al., 2011; Streeter et al., 2011). One difficulty in making oral oncolytic agents more affordable is generic competition. Manufacturers need to recoup their investment before their patent expires, which may cause elevated prices for therapies new to the market (Dusetzina et al., 2014). In our study, the most frequently reported oral oncolytic agent that respondents have seen abandoned due to cost was capecitabine (15.5%). Despite rapid uptake of generic capecitabine, with four generic entrants, in 2014, the list price for generic capecitabine was 17% lower than the projected branded price (mean generic price, $2598; 95% CI, $2570–$2625) and dropped to 36% from the projected branded price in 2016 (mean generic price, $2328; 95% CI = $2289–$2367), leading to the conclusion that few novel oncology drugs have sufficient competition to decrease prices, possibly owing to smaller patient populations and limited potential profit (Cole, Sanoff, & Duetzina, 2017). This study found that many patients are unable to initiate therapy and must change regimens due to the cost of their oral oncolytic medications. Regardless of brand or generic status, cost-associated nonadherence remains a problem for oncology patients.
Alternative methods to assist patients with oral oncolytic costs include new laws along with cost assistance programs. Oral oncology parity laws improved financial protection for many patients without increasing total health-care spending (Dusetzina, Huskamp, Winn, Basch, & Keating, 2018). These laws require health insurance plans to cover oral oncolytic medications at the same out-of-pocket rate as parenteral oncolytic medications. Study results have shown that those that utilize the Oral Oncology Parity Laws have found issues with ineffective lowering of costs and affordability. Momentum to better understand the value of high-cost drugs and to assess whether a value-based insurance design could improve affordability and access is needed (Dusetzina et al., 2014; Greenapple, 2012).
Many patients requiring oral oncology agents rely on assistance provided by manufacturer patient assistance programs, disease-based assistance co-pay assistance grants, manufacturer co-pay cards, and many other resources to obtain therapies. Current data from a Rhode Island specialty pharmacy between 2014 and 2017 showed that up-front charity assistance was associated with a longer time to filling the first prescription (median = 9 vs. 7 days; p = .011) and longer duration of therapy (median = 261 vs. 134 days, p = .014; Olszewski, Zullo, Nering, & Huynh, 2018). Opportunities to reduce the cost burden of oral oncolytic agents are plentiful, and laws are currently being integrated with the intent to aid those that struggle financially. However, more change is required to address the growth in spending for cancer therapies and to ensure that patients and payers receive maximum value for their health-care dollars spent (Dusetzina et al., 2014; Raborn, Pelletier, Smith, & Reyes, 2012).
Study limitations include response bias, recruitment techniques, and questionnaire respondents’ recall. Not all questions were answered by each respondent, as demonstrated by a fluctuating response rate for each question. This creates missing data and a reduced sample size for select questions. It is the assumption of this study that the questionnaire respondents answered accurately and truthfully. This may explain the missing data, as respondents were able to skip questions they did not choose to or feel they could accurately complete, favoring more accurate answers over a consistent response rate. The survey response rate is undetermined due to the open response format of distribution. The vast majority of respondents were pharmacists (54%), but input was included from several other fields of practice within the oncology setting with varying levels of oncology knowledge, experience, training, and education. There was not an equal distribution of respondents from each career field, size of the patient population, or experience within the field of oncology. The survey design was limited in its ability to assess any benefit between in-office dispensing vs. external mail order pharmacies, as each practice may have unique barriers to care. This is a descriptive analysis based on health-care workers’ report/recall, limiting the data to what the health-care worker can remember.
CONCLUSIONS
Health-care professional responses indicate a high incidence of abandonment of therapy, delay in therapy initiation, and significant resources allocated to resolving issues in assuring patient access to oral oncolytic therapy. In conclusion, there is a need to further evaluate cost issues in therapy and develop approaches to minimize impact on patient care, to ensure that patients treated with oral oncolytic agents are receiving care commensurate with those treated through the parenteral route. Future objectives should include increasing adherence and improving overall survival for oral oncology patients.
Key Points
• The purpose of this study was to evaluate the perceptions of health-care workers regarding the cost and safety of oral oncolytic agents, and to formally highlight perceptions of impact on patient care.
• Responses indicated a high incidence of abandonment of therapy, delay in initiation, and significant resources allocated to resolving issues with cost of oral oncolytic agents.
• Further evaluation of cost issues in therapy and how to minimize the impact on patient care is needed to ensure that patients treated with oral anticancer agents are receiving care commensurate with that of their counterparts receiving parenteral treatment.
Acknowledgment
We gratefully thank all the individuals for completing and participating in this survey. A very special thank you to the American Society of Health-System Pharmacists (ASHP), the Association of Community Cancer Centers (ACCC), the American Society of Clinical Oncology (ASCO), the Community Oncology Pharmacy Association (COPA), the National Community Oncology Dispensing Association (NCODA), the Oncology Nursing Society (ONS), the Advanced Practitioner Society for Hematology and Oncology (APSHO), and many others in their assistance in distributing this survey.
Appendix
Appendix A.
| 1. Introduction |
| The purpose of this study is to examine healthcare workers’ perceptions of the cost and safety of oral oncolytic agents to determine their impact on patient care. As oral chemotherapy agents become more widely used due to their convenience for patients, there is a greater need to evaluate the cost of therapy and impact on patient care to ensure that patients treated with oral anti-cancer agents are getting comparable care to those patients receiving IV anti-cancer agents. |
| In many instances, patients are unable to understand the extensive process to determine an appropriate course of treatment. Many factors aid in deciding on a particular course of therapy, including out-of-pocket cost of medication, adherence, access to medication, effectiveness, and efficacy. This survey consists of questions for healthcare practitioners working in the chemotherapy setting to determine oral oncolytic therapy cost and impact on patient care from a healthcare worker perspective. |
| This survey contains 35 questions and should take roughly 15 to 20 minutes to complete. |
| 2. Privacy Policy |
| It is the goal of this survey to keep all answers submitted anonymous. Please answer all questions honestly and to the best of your ability. |
| • agree to answer the following questions honestly and to the best of my ability. Authorizing all information obtained from this survey to be used for research purposes. |
| ○ I agree |
| ○ I disagree |
| 3. Demographics |
| Oral chemotherapy has deviating impact in different care settings. The following questions will focus on practice site and clinical practice setting. Please answer the following questions accordingly. |
| • How did you find out about this survey? |
| ○ AZPA - Arizona Pharmacy Association |
| ○ ASCO – American Society of Clinical Oncology |
| ○ APhA – American Pharmacists Association |
| ○ HOPA – Hematology/Oncology Pharmacy Association |
| ○ ASHP – American Society of Health-System Pharmacist |
| ○ NCPA – National Community Pharmacists Association |
| ○ ACCC – Association of Community Cancer Centers |
| ○ Other, please specify: |
| • Identify your primary role in the care of cancer patients. |
| ○ Pharmacist |
| ○ Physician |
| ○ Advanced Practitioner (certified nurse practitioner or physician assistant) |
| ○ Nurse |
| ○ Pharmacy administrator (supervisor, manager, director) |
| ○ Social Worker/Oncology Navigator |
| ○ Medical Assistant |
| ○ Other, please specify: |
| • How many years have you practiced in the cancer care setting? |
| ○ Enter years of practice in the cancer care setting: |
| • Location of Practice |
| ○ Enter zip code of practice site: |
| • How would you define your facility type? |
| ○ Academic Medical Center |
| ○ Community Hospital |
| ○ Community Retail/ Specialty Pharmacy |
| ○ Standardized Patient Program Health System |
| ○ Standardized Patient Program Independent |
| ○ Standardized Patient Program Pharmacy Benefit Manager |
| ○ I do not know |
| ○ Other, please specify: |
| • Does your facility have an infusion center? |
| ○ Yes |
| ○ No |
| ○ I do not know |
| • Does your facility administer inpatient chemotherapy? |
| ○ Yes |
| ○ No |
| ○ I do not know |
| • Does your facility have an outpatient pharmacy? |
| ○ Yes |
| ○ No |
| ○ I do not know |
| • How many chemotherapy doses (parenteral) are administered per month at your facility? |
| ○ <100 |
| ○ 100-499 |
| ○ 500-999 |
| ○ 1000-4999 |
| ○ ≥5000 |
| ○ I do not know |
| • How many oral chemotherapy prescriptions are filled per month at your facility? |
| ○ 0, No oral chemotherapy prescriptions are filled at this facility |
| ○ 1-9 |
| ○ 10-99 |
| ○ 100-499 |
| ○ 500-999 |
| ○ 1000-4999 |
| ○ ≥5000 |
| ○ I do not know |
| • Where do your patients primarily fill their prescriptions for oral chemotherapy medications? |
| ○ Community Pharmacy |
| ○ Standardized Patient Program Health System |
| ○ Physician’s Office |
| ○ Specialty Mail Order Pharmacy |
| ○ Specialty Pharmacy |
| ○ Pharmaceutical Manufacturer |
| ○ I do not know |
| • If your institution prescribes oral chemotherapy, which of the following best describes your oral chemotherapy practice setting? |
| ○ We currently do not have an oral adherence and monitoring practice |
| ○ The current practice relies on other specialty pharmacy providers to monitor our patients |
| ○ We are planning on developing a process, but do not have a formal process at this time |
| ○ We have an informal process, but nothing formal |
| ○ We have a well-developed process for oral chemotherapy monitoring and adherence |
| ○ I do not know |
| • Have you implemented safe chemotherapy handing procedures and education for patients at your facility? |
| ○ Yes |
| ○ Currently planning to develop an education procedure |
| ○ No |
| ○ I do not know |
| • Have you implemented safe chemotherapy handling procedures and education for patients at your facility? |
| ○ Yes |
| ○ Currently planning to develop an education procedure |
| ○ No |
| ○ I do not know |
| • Do you currently have a policy for administration of oral chemotherapy at your facility? |
| ○ Yes |
| ○ Currently planning to develop a protocol |
| ○ No |
| ○ I do not know |
| • Do you currently measure adherence to oral chemotherapy as part of outcomes measurements at your institution? |
| ○ Yes |
| ○ Currently planning to develop a protocol |
| ○ No |
| ○ I do not know |
| 4. Prevalence of Cost Issues |
| Oral chemotherapy often has high out-of-pocket patient costs or cost issues that prevent a patient from initiating or continuing the prescribed oral chemotherapy. The following questions will focus on the incidence of cost issues with patient’s oral chemotherapy. Please answer the questions accordingly. |
| • How often do you encounter patients unable to initiate therapy due to cost of their oral chemotherapy medications? |
| ○ 0, Never |
| ○ 1-2 patients a year |
| ○ 1-2 patients every 6 months |
| ○ 1-2 patients a month |
| ○ 1-2 patients a week |
| ○ 1-2 patients a day |
| ○ I do not know |
| • How many patients in the past year have missed or altered their medication regimens by purposely skipping or splitting oral chemotherapy doses due to cost? |
| ○ 0, Never |
| ○ 1-2 patients a year |
| ○ 1-2 patients every 6 months |
| ○ 1-2 patients a month |
| ○ 1-2 patients a week |
| ○ 1-2 patients a day |
| ○ I do not know |
| • How often have you had to change an oral chemotherapy regimen to an IV regimen due to cost of oral chemotherapy? |
| ○ 0, Never |
| ○ 1-2 patients a year |
| ○ 1-2 patients every 6 months |
| ○ 1-2 patients a month |
| ○ 1-2 patients a week |
| ○ 1-2 patients a day |
| ○ I do not know |
| • Has a patient ever abandoned treatment from your practice due to cost? (Abandoned treatment is defined as: patient discontinuing therapy after taking at least one dose of therapy without follow-up for change of therapy) |
| ○ Yes |
| ○ No |
| ○ I do not know |
| • How often do you have patients who abandon their oral chemotherapy medications after taking at least one dose due to high cost? |
| ○ 0, Never |
| ○ 1-2 patients a year |
| ○ 1-2 patients every 6 months |
| ○ 1-2 patients a month |
| ○ 1-2 patients a week |
| ○ 1-2 patients a day |
| ○ I do not know |
| • In your experience, what is the most common oral chemotherapy agent you have seen abandoned by patients due to cost? (Please fill in the text box): |
| 5. Impact on Patient Care/Safety |
| Cost issues can dangerously impact patient care and safety. Patients risk having a less than optimal treatment course due to abandonment of therapy and delays in initiation of therapy caused by processing/approving for specific oral chemotherapy medications. It is essential that these oral anti-cancer agents allow for equivalent patient care to those patients using IV anti-cancer agents. The following questions focus on evaluating the clinical impact of oral oncolytic agents. Please answer the questions accordingly. |
| • Have you ever had to change a patient’s treatment course due to a patient not being able to procure the oral drug therapy? |
| ○ Yes |
| ○ No |
| ○ Samples were provided to continue a patient’s course of therapy |
| ○ I do not know |
| • How often has your facility had to change therapy due to an insurance denial for oral chemotherapy medications? |
| ○ 0, Never |
| ○ 1-2 patients a year |
| ○ 1-2 patients every 6 months |
| ○ 1-2 patients a month |
| ○ 1-2 patients a week |
| ○ 1-2 patients a day |
| ○ I do not know |
| • How often does prior authorization for insurance companies delay a start or refill of a patient’s oral chemotherapy medication? |
| ○ 0, Never |
| ○ 1-2 patients a year |
| ○ 1-2 patients every 6 months |
| ○ 1-2 patients a month |
| ○ 1-2 patients a week |
| ○ 1-2 patients a day |
| ○ I do not know |
| • On average, how much time is used to process a prior authorization in your facility? |
| ○ 0 hours per week |
| ○ 1-10 hours per week |
| ○ 11-20 hours per week |
| ○ 21-30 hours per week |
| ○ >30 hours per week |
| ○ I do not know |
| • What is the average amount of time that a patient waits to initiate an oral chemotherapy medication due to prior authorization or other payment issues? |
| ○ 0 days |
| ○ 1-3 days |
| ○ 4-6 days |
| ○ 7-9 days |
| ○ ≥10 days |
| ○ I do not know |
| • Have you used a drug interaction in order to increase half-life of the drug and reduce overall cost for a patient’s oral chemotherapy medication? |
| ○ Yes |
| - Name the most common drug interaction used: |
| ○ No |
| ○ I do not know |
| • Which of the following drug therapies is contraindicated with the chronic myelogenous leukemia therapy dasatinib? |
| ○ Pravastatin |
| ○ Omeprazole |
| ○ Amoxicillin |
| ○ Metoprolol |
| ○ I do not know |
| • Which of the following therapies is contraindicated with food, specifically high-fat food and concomitant oral chemotherapy administration? |
| ○ Imatinib |
| ○ Methotrexate |
| ○ Abiraterone |
| ○ Olaparib |
| ○ I do not know |
| • Which of the following therapies has a matched correlated drug interaction? |
| ○ Valproic acid and temozolomide |
| ○ Ciprofloxacin and vemurafenib |
| ○ Esomeprazole and erlotinib |
| ○ Sertraline and crizotinib |
| ○ All of the above |
| ○ I do not know |
| 6. Alternative Methods to Obtain Medications |
| Due to expensive out-of-pocket costs for oral oncolytic medications, many assistance programs are available to financially assist patients in continuing their therapy. The following questions focus on current use of patient assistance programs for patients struggling with the high cost of oral chemotherapy. Please answer questions accordingly. |
| • Please select all of the medication assistance programs utilized at your facility to help patients obtain oral chemotherapy agents at a reduced cost. (Select all that apply) |
| ○ Manufacturer patient assistance programs |
| ○ Disease-based assistance co-pay assistance grants |
| ○ Manufacturer co-pay cards |
| ○ HUB International |
| ○ No patient assistance programs utilized |
| ○ Other, please specify: |
| • How often are pharmaceutical manufacturer programs utilized to help your patients obtain their oral chemotherapy? |
| ○ 0 or never |
| ○ 1-2 patients a year |
| ○ 1-2 patients every 6 months |
| ○ 1-2 patients a month |
| ○ 1-2 patients a week |
| ○ 1-2 patients a day |
| ○ I do not know |
| • How often are disease-based assistance grants utilized to help your patients obtain their oral chemotherapy? |
| ○ 0 or never |
| ○ 1-2 patients a year |
| ○ 1-2 patients every 6 months |
| ○ 1-2 patients a month |
| ○ 1-2 patients a week |
| ○ 1-2 patients a day |
| ○ I do not know |
| • How often are pharmaceutical manufacturer co-pay reduction coupons utilized to help your patients obtain their oral chemotherapy? |
| ○ 0 or never |
| ○ 1-2 patients a year |
| ○ 1-2 patients every 6 months |
| ○ 1-2 patients a month |
| ○ 1-2 patients a week |
| ○ 1-2 patients a day |
| ○ I do not know |
| 7. Thank You Message |
| Thank you so much for completing this survey! Your time and input is very much appreciated! |
References
- Abbott, R., Edwards, S., Edwards, J., Dranitsaris, G., & McCarthy, J. (2011). Oral anti-cancer agents in the community setting: A survey of pharmacists in Newfoundland and Labrador. Canadian Pharmacists Journal/Revue des Pharmaciens du Canada, 144(5), 220–226. 10.3821/1913-701X-144.5.220 [DOI] [Google Scholar]
- American Cancer Society (2016). Cancer Facts & Figures. Retrieved from http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf [Google Scholar]
- American Medical Association (2017). AMA prior authorization physician survey. Retrieved from https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/arc/prior-auth-2017.pdf [Google Scholar]
- American Medical Association (2018a). Anthem, Inc, AMA collab to create positive change to health system. Retrieved from https://www.ama-assn.org/press-center/press-releases/anthem-inc-ama-collab-create-positive-change-health-system
- American Medical Association (2018b). Consensus statement on improving the prior authorization process. Retrieved from https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/arc-public/prior-authorization-consensus-statement.pdf [Google Scholar]
- Association of Community Cancer Centers (2016). Steps to success: Implementing oral oncolytics. Retrieved from https://www.accc-cancer.org/docs/projects/resources/pdf/implementing-oral-oncolytics-final [Google Scholar]
- Butt, F., & Ream, E. (2016). Implementing oral chemotherapy services in communities pharmacies: A qualitative study of chemotherapy nurses’ and pharmacists’ views. International Journal of Pharmacy Practice, 24(3), 140–159. 10.1111/ijpp.12237 [DOI] [PubMed] [Google Scholar]
- Chavez-MacGregor, M., Clarke, C. A., Lichtensztajn, D. Y., & Giordano, S. H. (2016). Delayed initiation of adjuant chemotherapy among patients with breast cancer. JAMA Oncology, 2(3), 322–329. 10.1001/jamaoncol.2015.3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, A. L., Sanoff, H. K., & Duetzina, S. B. (2017). Possible insufficiency of generic price competition to contain prices for orally administered anticancer therapies. JAMA Internal Medicine, 177(11), 1679–1680. 10.1001/jamainternmed.2017.2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusetzina, S. B., Huskamp, H. A., Winn, A. N., Basch, E., & Keating, N. L. (2018). Out-of-pocket and health care spending changes for patients using orally administered anticancer therapy after adoption of state parity laws. JAMA Oncology, 4(6), e173598 10.1001/jamaoncol.2017.3598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusetzina, S. B., Muluneh, B., Khan, T., Richards, K. L., & Keating, N. L. (2014). Obstacles to affordable cancer treatment. North Carolina Medical Journal, 75(4), 257–260. 10.18043/ncm.75.4.257 [DOI] [PubMed] [Google Scholar]
- Gallagher, C. M., More, K., Kamath, T., Masaquel, A., Guerin, A., Ionescu-Ittu, R.,… Barnett, B. (2016). Delay in initiation of adjuvant trastuzumab therapy leads to decreased overall survival and relapse-free survival in patients with HER2-positive non-metastatic breast cancer. Breast Cancer Research and Treatment, 157, 145–156. 10.1007/s10549-016-3790-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenapple, R. (2012). Emerging trends in cancer care: Health plans’ and pharmacy benefit managers’ perspective on changing care models. American Health & Drug Benefits, 5(4), 242–253. [PMC free article] [PubMed] [Google Scholar]
- Lee, M. J., Khan, M. M., & Salloum, R. G. (2018). Recent trends in cost-related medication nonadherence among cancer survivors in the United States. Journal of Managed Care & Specialty Pharmacy, 24(1), 56–64. 10.18553/jmcp.2018.24.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, P., & Figgins, B. (2016). Oral anticancer therapy: A comprehensive assesment of patient perceptions and challenges. Journal of Community and Supportive Oncology, 14(3), 112–116. 10.12788/jcso.0226 [DOI] [PubMed] [Google Scholar]
- Neugut, A. I., Subar, M., Wilde, E. T., Stratton, S., Brouse, C. H., Hillyer, G. C.,… Hershman, D. L. (2011). Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. Journal of Clinical Oncol, 29(18), 2534–2542. 10.1200/JCO.2010.33.3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noens, L., Van Lierde, M. A., De Bock, R., Verhoef, G., Zachee, P., Berneman, Z.,…Abraham, I. (2009). Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patietns with chronic myeloid leukemia: The ADAGIO study. Blood, 113(22), 5401–5411. 10.1182/blood-2008-12-196543 [DOI] [PubMed] [Google Scholar]
- O’Bryant, C. L., & Crandell, B. C. (2008). Community pharmacists’ knowledge of and attitudes toward oral chemotherapy. Journal of the American Pharmacists Association, 48(5), 632–639. 10.1331/JAPhA.2008.07082 [DOI] [PubMed] [Google Scholar]
- Olszewski, A. J., Zullo, A. R., Nering, C. R., & Huynh, J. P. (2018). Use of charity assistance to pay for novel oral anticancer agents. Journal of Oncology Practice, 14(4), e221–e228. 10.1200/JOP.2017.027896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raborn, M. L., Pelletier, E. M., Smith, D. B., & Reyes, C. M. (2012). Patient out-of-pocket payments for oral oncolytics: Results from a 2009 U.S. claims data analysis. Journal of Oncology Practice, 8(3), 9s–15s. 10.1200/JOP.2011.000516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy, K., Mayer, E., & Partridge, A. (2009). Patient adherence and persistence with oral anticancer treatment. CA: A Cancer Journal for Clinicians, 59(1), 56–66. 10.3322/caac.20004 [DOI] [PubMed] [Google Scholar]
- Streeter, S. B., Schwartzberg, L., Husain, N., & Johnsrud, M. (2011). Patient and plan characteristics affecting abandonment of oral oncolytic prescriptions. American Journal of Managed Care, 17(5), 38–44. 10.1200/JOP.2011.000316 [DOI] [PubMed] [Google Scholar]
- Vachon, E. (2017). Educating patients about oral oncolytic agents. Retrieved fromhttps://www.oncnursingnews.com/publications/oncology-nurse/2017/june-2017/educating-patients-about-oral-oncolytic-agents [Google Scholar]