Abstract
Our study aimed to investigate the levels and time-course of systemic inflammatory and hemostasis markers in the early postoperative period in patients undergoing total hip replacement (THR). The study included 70 patients of both sexes, average age 68.4 ± 10.9 years. Levels of inflammatory and hemostasis markers were measured before surgery (POD 0), a day after the surgery (POD 1) and 5 days after surgery (POD 5). In the postoperative period inflammatory markers increased. The operation provoked a significant increase of CRP on POD 1 in comparison to POD 0 (68.5 ± 5.4 vs 6.8 ± 2.2 μg/mL, p < 0.001) and the additional increase was registered on POD 5 (87.5 ± 8.1 vs 68.5 ± 5.4 μg/mL, p < 0.001). Interleukin-6 significantly increased on POD 1 (251.5 ± 21.6 vs 14.6 ± 7.1 μg/mL, p < 0.001) and after that (POD 5) decreased. After surgery leukocyte count, neutrophil/lymphocyte ratio and platelet/lymphocyte ratio were significantly higher compared to POD 0. Activation of coagulation in the postoperative period was shown by increased peak thrombin on POD 5 in comparison to POD 0 (185 ± 27 vs. 124 ± 31 nM, p < 0.001). D-dimer was increased on POD 1 and an additional rise was observed on POD 5. vWF also progressively increased in the observed period. Results of our study showed that after THR systemic inflammatory markers increased and coagulation function was enhanced. Determination of inflammatory and procoagulant markers could help identify patients at risk for cardiovascular thromboembolic events.
Keywords: total hip replacement, perioperative stress response, systemic inflammatory markers, cardiovascular complications, thromboembolic complications
Introduction
Surgical procedures including total hip replacement (THR) are associated with perioperative complications. Most frequent perioperative complications include cardiovascular, thromboembolic events and respiratory problems. Operative procedures cause a significant inflammatory systemic reaction.1 Increased inflammatory response as a consequence of oxidative stress is involved in the pathogenesis of most perioperative adverse events. The increased systemic inflammatory response in the perioperative period could be a risk factor for thromboembolic complications. Further, oxidative stress leads to deterioration of cardiovascular homeostasis including endothelial dysfunction, which is most probably involved in venous thromboembolic complications.2
There is increasing evidence, which points to an extensive cross-talk between inflammation and coagulation, where inflammation leads to activation of coagulation, and coagulation also considerably affects inflammatory activity.3 Proinflammatory cytokines and other mediators are capable of activating the coagulation system and down-regulating physiologic anticoagulant pathways. Furthermore, an activated coagulation system with increased thrombin generation downregulates anticoagulant mechanisms and endogenous fibrinolysis.3 This indicates that perioperative complications depend on the intensity of the systemic inflammatory response.
The presence of a hypercoagulable state caused by tissue injury and systemic inflammatory response plays an important role in predisposing to venous thromboembolism (VTE) and other thromboembolic complications after THR.4 Therefore, it would be helpful to identify patients who are at high risk of VTE immediately after the surgical procedures and provide this group of patients with more adequate prophylaxis. Many attempts have been made to establish a relationship between systemic inflammatory markers, coagulation- activation markers and risk for development of postoperative VTE but definite results are still not available.
Therefore, the aim of our study was to investigate levels of systemic inflammatory and hemostatic markers and their time course in the early postoperative period.
Methods
Study Description and Patient Characteristics
This prospective observational study included consecutive osteoarthritis patients who underwent an elective THR under spinal anesthesia from January till June 2017 in a tertiary orthopedic medical center. Out of 145 patients treated in observed period 70 eligible patients were included and the sample size was calculated with the help of the Glimmpse online calculator. The study was approved by the National Medical Ethics Committee and was conducted in compliance with the Declaration of Helsinki. All subjects provided written informed consent. The study was registered at https://ClinicalTrials.gov (NTC-03014765).
Excluded were patients who suffered acute coronary syndrome, cerebrovascular incident or acute venous thrombosis/pulmonary embolism within the last 6 months, acute inflammatory disease within 3 months or who were on permanent therapy with glucocorticoids. Also, patients with known liver failure, advanced chronic kidney disease (a severe reduction in glomerular filtration rate (GFR < 30 mL/min)), active malignant disease or use of immunosuppression after organ transplantation, pregnancy or patients who declined to participate were excluded from the study. Extensive history and physical exam included registration of diabetes mellitus, arterial hypertension, hypercholesterolemia, obesity, and smoking. In addition, the data on the preoperative medications, including nonsteroidal anti-inflammatory drugs, aspirin, and anticoagulants were collected.
Operative Procedure
All patients were operated under spinal anesthesia, which was performed using plain bupivacaine 0.5% (Marcaine Spinal Heavy; Astra Zeneca, Lund, Sweden). The surgery was performed in a lateral decubital position. In all patients anterolateral (modified Watson-Jones) approach for a hip replacement was used, all THRs were cementless. Before the induction of spinal anesthesia all patients received 2 g of Cefazolin in 100 mL of saline over 15 min intravenously (i.v.) and tranexamic acid 1 g i.v., after which an infusion of lactated Ringer’s solution (5 mL/kg/h i.v.) was started.
The Data Recorded in the Perioperative Period
The following data were recorded during the perioperative period: duration of surgery, fluid balance (calculated at the end of procedure, at release from post-anesthesia care unit (PACU) and 24 h after the surgery), length of stay (LOS) in the intensive care unit and total hospital LOS, amount of perioperative blood loss, received blood products, type, and duration of antithrombotic prophylaxis. The total amount of bleeding was estimated based on volume (mL) of blood in the suction tank and the number of gauze in the operation room (10 immersed gauzes were considered as 200 mL of blood) based on the anesthesiologist’s evaluation at the end of surgery. Additionally, blood loss through the drain was recorded. All postoperative complications following orthopedic procedures including fever (defined as a body temperature above 38°C), reoperation due to local infection, dehiscence or bleeding, as well as sepsis, pneumonia, venous thromboembolism, and acute coronary or cerebrovascular events or readmission in the first 30 days after surgery were recorded.
Postoperative Management of Patients
Patients were routinely mobilized on the first day after surgery and a standardized rehabilitation protocol was started. All patients wore graduated thigh-high compression stockings after the surgery until discharge. Prophylactic anticoagulant therapy was provided according to the American College of Chest Physicians Evidence-Based Clinical Practice Guidelines.5 Patients were administered dalteparin 5000 IU once-daily subcutaneous or rivaroxaban 10 mg orally once per day, beginning 8-12 h after surgery and were treated for 35 days, respectively, at the discretion of a treating orthopedic surgeon. All patients received additional doses of Cefazolin 2 g i.v. 8 and 16 h postoperatively.
Laboratory Parameters
Blood for laboratory analysis was collected a day before surgery (POD 0), up to 24 hours (POD 1) and 5 days after the surgery (POD 5), always in the morning after a 12h-overnight fast. Samples were drawn from the antecubital vein with minimal venous stasis firstly into 4.5 mL vacuum tubes containing 0.11 mol/L sodium citrate (9:1 v/v) (Becton Dickinson, Vacutainer System Europe, Heidelberg, Germany), followed by a 4 mL serum-separator tube and 2 mL K3-EDTA tube (both Vacutube, Laboratorijska tehnika Burnik, Kranj, Slovenia). From the K3-EDTA whole blood the complete blood counts, which included total white blood cells, neutrophils, lymphocytes, and platelets, were measured using a KX-21 N hematology analyzer (Sysmex, Japan). Neutrophil-to-lymphocyte (NLR) and platelet-to-lymphocyte ratio (PLR) were calculated as the ratio of neutrophils to lymphocytes and the platelets to lymphocytes, respectively, obtained from the blood samples that were taken at the fasting state. Serum was prepared by 15-minute centrifugation at 2,000 x g and citrated plasma by 20-minute centrifugation at 2,000 x g, immediately frozen and stored at −70°C for further analysis.
Plasma levels of CRP and IL-6 were measured by using an enzyme-linked immunosorbent assay (ELISA) method (R&D Laboratories, Minneapolis, MN, USA).
Serum cortisol levels were determined using cortisol ELISA kit according to the manufacturer’s protocol (LDN GmbH & Co., Nordhorn, Germany). Absorbance was measured using a spectrometer.
The calibrated automated thrombin method was used to assess thrombin generation over time. Further, peak thrombin which represents the highest concentration of thrombin in plasma sample at a given time was determined.6
D-dimer testing: venous blood was collected by venipuncture into 3.2% sodium citrate to a final ratio of 9:1 using Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ, USA.). Each specimen was centrifuged for 10 minutes at 3000 RPM. D-dimer levels were tested using Innovance DD (Date Behring, Marburg, Germany).
Commercially available ELISA kits were used to measure levels of vWF (Hyphen BioMed, Neuville-Sur-Oise, France) and microparticle—tissue factor (Hyphen BioMed, Neuville-Sur-Oise, France).
Statistical Analysis
To assess the normal distribution of variables we used the Shapiro-Wilks test. Normally distributed continuous variables were presented with the arithmetic mean and standard deviation. Non-normally distributed continuous variables were presented as a median and first to the third quartile. Categorical variables were presented as a count and percentage. Differences in the inflammatory and coagulation parameters were compared with baseline among the 2 postoperative periods (baseline, POD 1 and POD 5) by repeated-measures analysis of variance (ANOVA). One-way ANOVA with repeated measures in longitudinal variables was performed and also the ANOVA modification was used for repeated measure analysis. Post hoc analysis with Bonferroni correction was used when significant differences were observed.
Differences between different time points were compared by unpaired t-test or the Wilcoxon rank-sum test as appropriate. Differences in categorical variables were calculated using a chi-square test (and Fisher’s exact test). Statistical significance was considered a 2-tailed p < 0.05. Data were analyzed using STATA 2017 statistical software: release 15. College Station, Texas, USA: StataCorp LP.
Results
The study included 70 patients in whom elective THR was performed; their average age was 68.4 ± 10.9 years (Table 1). Most of the patients had one or more cardiovascular risk factors. The characteristics of surgical procedures and complications are shown in Table 2. The median duration of operation was 90 min (65-100 min), perioperative blood loss accounted for a median 300 mL (200-400 mL), 37% of patients developed mild postoperative side-effects like fever, postoperative nausea, and vomiting. Postoperative complications within 30 days after surgery were rare, one patient suffered ischemic cerebrovascular ischemia that has not caused significant clinical consequences. However, none of the patients was diagnosed with deep venous thrombosis (DVT).
Table 1.
Baseline Demographic and Clinical Characteristics of Patients Who Underwent Total Hip Replacement (n = 70).a
| Variable | Value |
|---|---|
| Basic demographic characteristics | |
| Age (years) | 68.4 ± 10.9 |
| Male | 37 (53%) |
| Weight (kg) | 79 (70-90) |
| Body mass index (kg/m2) | 28.5 (25.0-30.9) |
| Risk factors | |
| Arterial hypertension | 44 (63%) |
| Diabetes mellitus type 2 | 14 (20%) |
| Hyperlipidemia | 20 (29%) |
| Smoking | 7 (10%) |
| Medication | |
| Aspirin | 14 (20%) |
| Nonsteroidal anti-inflammatory drugs | 33 (47%) |
| Antihypertension drugs | 44 (63%) |
| Antidiabetic drugs | 13 (19%) |
| Statins | 20 (29%) |
a Data are expressed as mean ± standard deviation, median (interquartile range) or number (percentage), respectively.
Table 2.
Features of Surgical Procedures and Perioperative Complications in 70 Patients Who Underwent a Total Hip Replacement.a
| Variable | Value |
|---|---|
| Operative and early postoperative period | |
| Duration of surgery (min) | 90 (65-100) |
| The total length of hospital stay (days) | 7 (7-8) |
| Perioperative blood loss (mL) | 300 (200-400) |
| Amount of received crystalloids (mL) | 1000 (550-1450) |
| Amount of received colloids (mL) | 500 (300-750) |
| Fever after surgery | 26 (37%) |
| Postoperative nausea and vomiting | 32 (46%) |
| Revision of operative wound | 1 (1%) |
| 30 days after discharge | |
| Readmission | 3 (4%) |
| Surgical wound infection | 1 (1%) |
| Wound dehiscence | 0 |
| Sepsis | 0 |
| Pneumonia | 0 |
| Venous thromboembolism | 0 |
| Acute coronary syndrome | 0 |
| Ischemic Cerebrovascular Incident | 1 (1%) |
a Data are expressed as median (interquartile range) or number (percentage), respectively.
There was a correlation between patients’ characteristics and duration of the operative procedure: age was positively correlated with the duration of operation (r = 0.41, p < 0.05) and hospital LOS (r = 0.45, p < 0.001). The duration of operation was positively related to operative blood loss (r = 0.406, p = 0.005).
After the operation, the decrease of hemoglobin and platelets were found (Table 3). At POD 1, leukocyte count significantly increased which was related to neutrophil increase. However, the number of lymphocytes decreased. The highest mean peak for relations between inflammatory cells (neutrophils/lymphocytes and platelets/lymphocytes) was at POD 1 after the operative procedure (Table 3). The relationship between neutrophil/lymphocyte ratio (NLR) and age, body mass index (BMI) and D-dimer was found (Table 4). Analysis of relationship between blood loss and changes in blood count (decrease of platelets and increase of leukocytes) showed that changes in blood cell count were not caused by blood loss.
Table 3.
Changes in the Complete Blood Count With Differential and Blood Count Indices in the Perioperative Period (N = 70).
| Parameters | Before operation (0) |
24 h after operation (1) |
5 days after operation (2) |
P 0 vs 1 |
P 1 vs 2 |
P 2 vs 0 |
|---|---|---|---|---|---|---|
| Hemoglobin (g/L) | 138.0 (129.0-148.0) |
110.0 (103.5-121.0) |
108.0 (101.5-114.5) |
<0.001 |
1.000 | <0.001 |
| Leukocytes (×109) | 6.4 (5.4-7.3) |
8.4 (6.7-9.5) |
6.9 (6.0-7.7) |
<0.001 |
0.002 | 0.264 |
| Platelets (×109) | 215.0 (168.0-282.0) |
180.0 (140.0-207.5) |
235.0 (173.5-334.0) |
<0.001 | <0.001 | 0.001 |
| Lymphocytes (×109) | 1.6 (1.3-2.0) |
0.9 (0.6-1.3) |
1.2 (0.9-1.3) |
<0.001 |
0.047 | <0.001 |
| Neutrophils (×109) | 4.1 (3.2-4.9) |
6.5 (5.5-7.5) |
4.9 (4.1-5.8) |
<0.001 | <0.001 | 0.019 |
| Neutrophil/ lymphocyte ratio | 2.3 (2.0-3.6) |
6.9 (4.9-10.6) |
3.1 (2.3-4.0) |
<0.039 |
0.059 | 0.07 |
| Platelet/lymphocyte ratio | 53.1 (42.3-62.6) |
165.0 (120.2-265.5) |
136.4 (105.3-181.9) |
0.020 |
0.215 | <0.001 |
Table 4.
The Correlation Between Levels of Some Inflammatory Parameters and Other Patients’ Characteristics 24 h After the Operation (POD 1).
| Parameters | R | P |
|---|---|---|
| NLR vs age | 0.47 | 0.001 |
| NLR vs BMI | 0.36 | 0.015 |
| Leukocytes vs hospital stay | 0.28 | 0.031 |
| NLR vs D-dimer | 0.44 | 0.001 |
| PLR vs D-dimer | −0.33 | 0.028 |
NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; BMI, body mass index.
Results of inflammatory and coagulation markers are presented in Table 5 and Figures 1 -4.
Table 5.
Results of Laboratory Analysis Before and After the Operative Procedure of Patients With Total Hip Replacement.a
| Parameters | Before operation (POD 0) (0) (N = 70) |
24 h after operation (POD 1) (1) (N = 70) |
5 days after operation (POD 5) (2) (N = 70) |
P 0 vs 1 |
P 1 vs 2 |
P 2 vs 0 |
|---|---|---|---|---|---|---|
| Glucose (mmol/L) | 5.5 (4.9-6.5) |
6.0 (5.4-7.4) |
6.2 (5.3-7.5) |
0.011 | 1.000 | 0.077 |
| Urea (mmol/L) | 5.9 (4.4-7.0) |
5.4 (4.3-6.6) |
5.8 (4.6-7.8) |
0.252 | 0.261 | 1.000 |
| Potassium (mmol/L) | 4.3 (4.0-4.7) |
4.1 (3.9-4.3) |
4.0 (3.9-4.5) |
0.252 | 0.261 | 1.000 |
| Sodium (mmol/L) | 142.0 (139.0-144.0) |
138.0 (136.5-141.0) |
143.0 (139.5-144.0) |
0.006 | <0.001 | 0.437 |
| Creatinine (µmol/L) | 71.8 (59.8-80.3) |
56.3 (47.7-66.3) |
62.0 (53.9-76.2) |
<0.001 | <0.001 | 0.004 |
| D-dimer (ng/mL) | 800 ± 214 | 2245 ± 368 | 2980 ± 341 | <0.001 | <0.001 | <0.001 |
| Peak thrombin (nM) | 124 ± 31 | 135 ± 30 | 185 ± 27 | 0.035 | <0.001 | <0.001 |
| Cortisol (ng/mL) | 215 ± 78 | 248 ± 81 | 96 ± 47 | 0.015 | <0.001 | <0.001 |
| Interleukin-6 (pg/mL) | 14.6 ± 7.1 | 251.5 ± 21.6 | 51.7 ± 15.4 | <0.001 | <0.001 | <0.001 |
| C-reactive protein (µg/mL) | 6.8 ± 2.2 | 68.5 ± 5.4 | 87.5 ± 8.1 | <0.001 | <0.001 | <0.001 |
| Von Willebrand factor (%) | 170 ± 10 | 213 ± 11 | 295 ± 18 | <0.001 | <0.001 | <0.001 |
| Microparticle tissue factor (pg/mL) | 7.8 ± 1.2 | 5.6 ± 0.9 | 5.1 ± 1.1 | <0.001 | 0.004 | <0.001 |
a Data are expressed as median (interquartile range) or mean (standard deviation), respectively.
Figure 1.
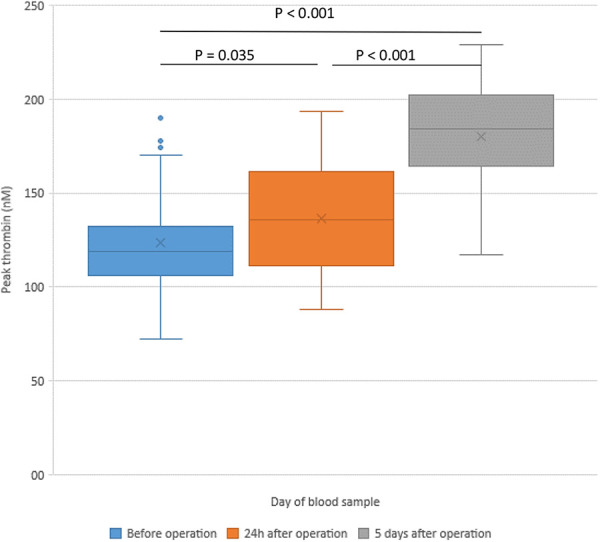
Changes of peak thrombin in patients with hip replacement—before the operation (POD 0) and in the postoperative period.
Figure 2.
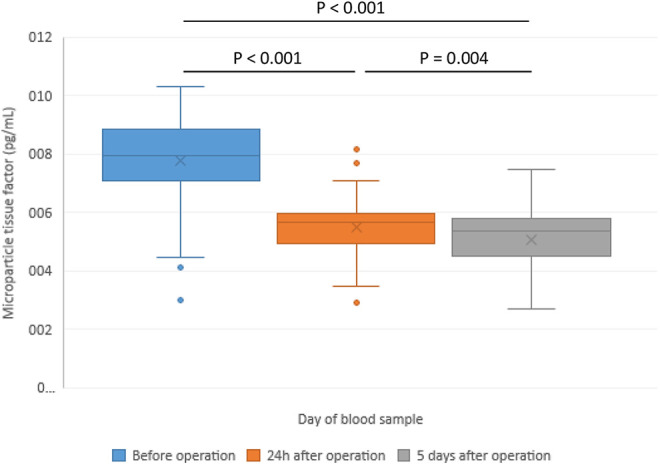
Changes of microparticle tissue factor in patients with hip replacement—before the operation (POD 0) and in the postoperative period.
Figure 3.
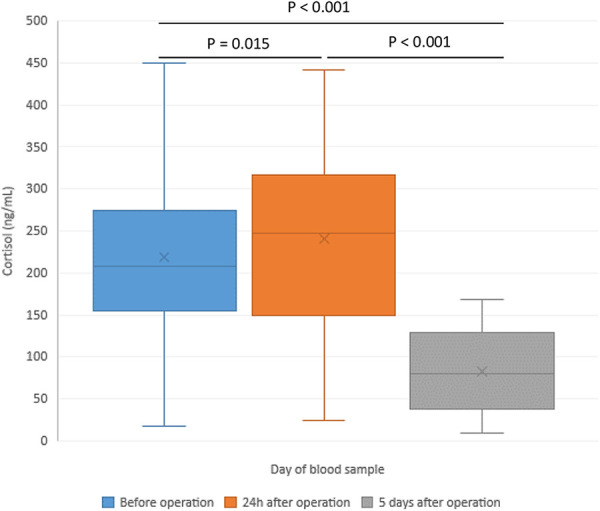
Changes of cortisol in patients with hip replacement—before the operation (POD 0) and in the postoperative period.
Figure 4.
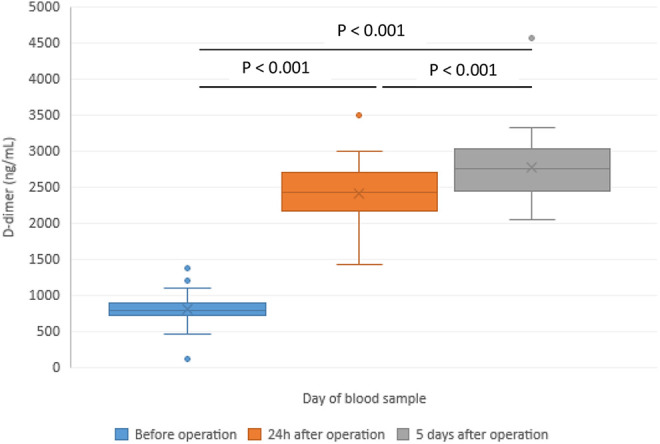
Changes of peak D-dimer in patients with hip replacement—before the operation (POD 0) and in the postoperative period.
C-reactive protein (CRP) was at POD 1 significantly higher than before operation (68.5 ± 5.4 vs 6.8 ± 2.2 µg/mL, p < 0.001) and an additional increase of its value was found at POD 5 (87.5 ± 8.1 µg/mL). In the early postoperative period, there was a significant increase in interleukin-6 (IL-6) that at POD 5 gradually decreased. However, IL-6 average values were at POD 5 after the surgery still significantly higher than in the preoperative period. Preoperatively, patients with diabetes had insignificantly higher levels of CRP. However, increase of inflammatory markers was not associated with the presence of risk factors of atherosclerosis.
Peak thrombin was significantly increased at POD 5 in comparison to POD 0 and POD 1 (Figure 1). Microparticle tissue factor (MP-TF) was immediately after the operation significantly decreased and an additional decrease was registered in the follow-up period at POD 5 compared with baseline (Figure 2). Cortisol levels increased at POD 1 (248 ± 81 ng/mL vs 215 ± 78 ng/mL, p = 0.015) and a significant decrease was registered at POD 5 (96 ± 47 ng/mL vs 215 ± 78 ng/mL, p < 0.001) (Figure 3). A significant increase in D-dimer was registered at POD 1 and it additionally increased on POD 5 (Figure 4). vWF activity levels increased at POD 1 compared with baseline (213 ± 11% vs 170 ± 10%, p < 0.001) and it additionally increased at POD 5 (295 ± 18% vs 213 ± 11%, p < 0.001).
Discussion
Characteristics of our study population represent typical patients with osteoarthritis, namely, they were overweight and aged around 70 years.7
The most important finding of our study was an increased systemic inflammatory response and enhanced coagulation function caused by the operative procedure.
Inflammatory Markers
An increased inflammatory response was most probably provoked by increased operative stress related to operative procedure which was showed by increased cortisol level in the peri-operative period registered 24-hours after the surgery. As there is a cross-talk and bidirectional communication between inflammation and the endocrine system, activation of both systems was expected.
In our study the highest cortisol levels were registered on POD 1 and on POD 5 they were significantly lower than on POD 0. In most studies, cortisol level rises rapidly and usually returns to baseline values within 24-48 hours.8 However, minimally invasive surgeries do not cause a peri-operative cortisol peak. Moreover, cortisol actually decreases in comparison to presurgical measurements. This phenomenon could be explained by the fact that lower stress is counteracted by other factors, such as anesthesia and sedation. Anesthetic agent etomidate is known inhibitor of cortisol synthesis and can influence the level of cortisol.9 Lower levels of cortisol on POD 5 in our study could be also the consequence of drop of corticosteroid binding globulin (CBG) after surgery, particularly after major procedures. Further, epidural as well as spinal anesthesia techniques may lead to blunted cortisol response.10 The drop in cortisol in our patients could be explained by the fact that in all of them the surgical procedure was performed under spinal anesthesia.
Inflammatory blood cells (leukocytes and neutrophils) increased in the first 24 h after the operation. It indicates increased inflammatory response provoked by THR. Another newly developed indicator of inflammation is the NLR, which shows the relationship between the absolute neutrophil and lymphocyte counts, is a marker of the systemic inflammatory response.11 In our patient group, NLR significantly increased 24 hours after the operation, and after 5 days values were comparable to preoperative values. Wasko et al. showed a 1.2-fold increase in NLR on day 3 after the procedure—values did not exceed normal values (NLR < 5) in the perioperative period, however, they did not measure it immediately after the procedure.12
As CRP values in our study were high even at POD 5, NLR could potentially be a better and immediate biomarker to follow postoperative inflammation or early infection after THR. Barker and co-workers followed changes in NLR after unilateral total knee replacement and in patients with unicompartmental knee arthroplasty and concluded that NLR increased in both groups, however, its increase was greater following total knee replacement.13 In our study, the relationship between the increase in NLR and age, BMI and D-dimer was found. It indicates that older patients and patients with increased BMI are prone to a more intense inflammatory response.
Another indicator of systemic inflammation is PLR.14 In our patients, 24 hours after operative procedure PLR was significantly increased because of lower lymphocyte count. Higher PLR may reflect underlying inflammation.15 Therefore, PLR may be a surrogate marker of systemic inflammation and as such provide an explanation for increased cardiovascular risk and venous thromboembolic complications in the perioperative period. These assumptions are partly confirmed by findings of Yao, et al. who found that postoperative PLR values were significantly associated with DVT after joint arthroplasty.16 In our study, no clinically evident DVT was identified. However, we have not performed systematical screening of DVT, therefore subclinical thrombosis was not identified.
In our study also CRP levels significantly increased 24 hours after the operation and 5 days later an additional increase was registered. Increased CRP in the postoperative period again confirmed that operative procedure induces tissue damage, which leads to a systemic inflammatory response. Hepatic synthesis of CRP peaks at around 48 hours.17
Our study also showed that operation provokes IL-6 increase with peak concentration 24 hours after the procedure which is followed by a significant decrease in the following days. IL-6 is one of the pro-inflammatory cytokines which is involved in the progression of atherosclerosis,18 and in the pathogenesis of the venous thromboembolic disease.19 Different studies showed that some interleukins, including IL-6, are associated with a 2-to-6-fold increase in the risk of deep venous thrombosis.20
Procoagulant Markers
D-dimer which represents a degradation product of cross-linked fibrin by plasmin may be elevated in any conditions where clots form. In our patients, a significant increase of D-dimer with the highest values were found at POD 5. This indicated that operative procedures provoke activation of coagulation system in the early postoperative period. Studies showed that elevated soluble fibrin and D-dimer represent a useful tool in risk assessment of postoperative DVT in patients with hip arthroplasty or knee arthroplasty.21 It was also shown that values of D-dimer were higher after total knee replacement than after THR.22 However, levels of D-dimer in those patients without clinically manifest DVT were not significantly different from patients with DVT. Some data indicate that duration of D-dimer elevation after surgery may be predictive for DVT.23 Further, some cut-off levels of D-dimer may provide reference for the absence of DVT.24 Similarly, also thrombin generation and peak thrombin were in our patients significantly increased on POD 5. Values were significantly higher relative to plasma samples collected at POD 0 or on POD 1.
Excessive thrombin generation can result in thromboembolic complications, so identifying biomarkers of thrombin generation could play a major role in the prevention and management of these complications. The Longitudinal Investigation of Thromboembolism Etiology (LITE) study showed that basal peak thrombin generation was associated with a 74% increased risk of VTE.25 Preoperative (POD 0) and POD 1 values of peak thrombin were lower than in samples of healthy controls. Lower baseline values could be caused by drugs taken by patients preoperatively: aspirin and statins. Lower POD 1 values may be caused also by heparin treatment and heparin-antithrombin effect, while on POD 5 patients were treated mostly with rivaroxaban. The study of Green and co-workers indicated that in the postoperative period thrombin generation was increased but was blocked by dabigatran.26 Otherwise, thrombin generation is associated with the risk of thrombotic complications and may be useful predictive marker for evaluation of thrombosis on an individual basis.27
One of the most interesting findings of our study was a progressive increase in vWF in the early postoperative period. vWF is an important player in hemostasis and thrombosis. It mediates adherence of platelets to the damaged vessel wall and promotes thrombus formation. An increase of vWF in our patients indicates that in the early postoperative period there is an enhanced coagulation function. As elevated plasma levels of vWF may predict thrombosis, inhibition of vWF could be a novel way of VTE prophylaxis.28
All these markers: increased D-dimer, peak thrombin generation and vWF in early postoperative period indicate that operative stress stimulates thrombotic cascade and enhances coagulation function. Increased inflammatory and procoagulant factors may be involved in perioperative, particularly thromboembolic complications in a patient with THR.
Limitations of the Study
Most of patients were treated before operative procedure with aspirin (20%) or non-steroidal anti-inflammatory drugs, which could influence inflammatory response and prothrombotic state immediately after the operation. Also, postoperative treatment with dalteparin or rivaroxaban probably influenced coagulation markers, which were measured in our study. Observation period was relatively short (5-7 days after operation). Therefore, it was impossible to calculate outcome and long-term prognosis on the base of elevated inflammatory markers. Inflammatory and immune response to surgery could be protective for the body in early postoperative period.29 However, the magnitude of the postoperative systemic inflammatory response is also independent risk factor for postoperative complications.30
Conclusion
Surgical trauma and anesthesia are considered major stresses for the human body which provoke inflammation and procoagulant state resulting in the development of perioperative complications. Most frequent and serious events represent cardiovascular and venous thromboembolic complications. Our study showed that in patients undergoing THR immediately after procedure circulating markers of inflammation are increased. On the first postoperative day, inflammatory cells were significantly higher than in the preoperative period. Further, CRP and IL-6 significantly increased 24 hours after the operation. As systemic inflammation is related to cardiovascular and venous thromboembolic complications, the results of our study indicated that increased risk for perioperative complications is most probably caused by inflammatory response related to the operative procedure. The systemic inflammatory response was accompanied by activation of coagulation which was shown by an increased D-dimer, vWF and peak thrombin. Determination of these markers may help in the identification of patients with osteoarthritis undergoing THR who are at high risk for thromboembolic complications. The time course of inflammatory and procoagulant factors indicates that the risk of complications is the highest in the first days after the surgery.
Abbreviations
- ANOVA
analysis of variance
- BMI
body mass index
- CBG
corticosteroid binding globulin
- CRP
C-reactive protein
- DVT
deep venous thrombosis
- ELISA
enzyme-linked immunosorbent assay
- GFR
glomerular filtration rate
- LOS
length of hospital stay
- MP-TF
microparticle tissue factor
- NLR
neutrophil/lymphocyte ratio
- PACU
post-anesthesia care unit
- PLR
platelet/lymphocyte ratio
- POD
postoperative day
- POD 0
before surgery
- POD 1
1 day after the surgery
- POD 5
5 days after the surgery
- THR
total hip arthroplasty
- VTE
venous thromboembolism
- vWF
von Willebrand factor
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Peter Poredos  https://orcid.org/0000-0001-5258-443X
https://orcid.org/0000-0001-5258-443X
Pavel Poredos  https://orcid.org/0000-0002-9913-1419
https://orcid.org/0000-0002-9913-1419
Mateja K. Jezovnik  https://orcid.org/0000-0002-5317-0148
https://orcid.org/0000-0002-5317-0148
Jawed Fareed  https://orcid.org/0000-0003-3465-2499
https://orcid.org/0000-0003-3465-2499
References
- 1. Sedlár M, Kudrnová Z, Trca S, et al. Inflammatory response in patients undergoing hip surgery due to osteoarthrosis or different types of hip fractures. Osteoarthr Cartil. 2008;16(1):26–33. PubMed PMID: 17689272; doi:10.1016/j.joca.2007.05.023 [DOI] [PubMed] [Google Scholar]
- 2. Poredoš P. Interrelationship between venous and arterial thrombosis. Int Angiol. 2017;36(4):295–298. PubMed PMID: 28263045; doi:10.23736/S0392-9590.17.03820-2 [DOI] [PubMed] [Google Scholar]
- 3. Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med. 2010;38(2 Suppl):S26–34. PubMed PMID: 20083910; doi:10.1097/CCM.0b013e3181c98d21 [DOI] [PubMed] [Google Scholar]
- 4. Cofrancesco E, Cortellaro M, Corradi A, Ravasi F, Bertocchi F. Coagulation activation markers in the prediction of venous thrombosis after elective hip surgery. Thromb Haemost. 1997;77(2):267–269. PubMed PMID: 9157579. [PubMed] [Google Scholar]
- 5. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S–e325S. PubMed PMID: 22315265; PubMed Central PMCID: PMCPMC3278063; doi:10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Veen JJ, Gatt A, Makris M. Thrombin generation testing in routine clinical practice: are we there yet? Br J Haematol. 2008;142(6):889–903. PubMed PMID: 18564356; doi:10.1111/j.1365-2141.2008.07267.x [DOI] [PubMed] [Google Scholar]
- 7. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–1330. PubMed PMID: 24553908; doi:10.1136/annrheumdis-2013-204763 [DOI] [PubMed] [Google Scholar]
- 8. Prete A, Yan Q, Al-Tarrah K, et al. The cortisol stress response induced by surgery: a systematic review and meta-analysis. Clin Endocrinol (Oxf). 2018;89(5):554–567. doi:10.1111/cen.13820. PMID: 30047158 [DOI] [PubMed] [Google Scholar]
- 9. Fellows IW, Byrne AJ, Allison SP. Adrenocortical suppression with etomidate. Lancet. 1983;2(8340):54–55. doi:10.1016/s0140-6736(83)90043-0 [DOI] [PubMed] [Google Scholar]
- 10. Liu S, Carpenter RL, Neal JM. Epidural anesthesia and analgesia. Their role in postoperative outcome. Anesthesiology. 1995;82(6):1474–1506. PMID: 7793661; doi:10.1097/00000542-199506000-00019 [DOI] [PubMed] [Google Scholar]
- 11. Grimnes G, Horvei LD, Tichelaar V, Brækkan SK, Hansen JB. Neutrophil to lymphocyte ratio and future risk of venous thromboembolism and mortality: the Tromsø Study. Haematologica. 2016;101(10): e401–e404. PubMed PMID: 27479821; PubMed Central PMCID: PMCPMC5046660; doi:10.3324/haematol.2016.145151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wasko MK, Struminski M, Bobecka-Wesolowska K, Kowalczewski J. Neutrophil-to-lymphocyte ratio shows faster changing kinetics than C-reactive protein after total hip and knee arthroplasty. J Orthop Translat. 2017;10:36–41. PubMed PMID: 29662758; PubMed Central PMCID: PMCPMC5822996; doi:10.1016/j.jot.2017.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barker T, Rogers VE, Henriksen VT, et al. Is there a link between the neutrophil-to-lymphocyte ratio and venous thromboembolic events after knee arthroplasty? A pilot study. J Orthop Traumatol. 2016;17(2):163–168. PubMed PMID: 26387114; PubMed Central PMCID: PMCPMC4882298; doi:10.1007/s10195-015-0378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arruda-Olson AM, Reeder GS, Bell MR, Weston SA, Roger VL. Neutrophilia predicts death and heart failure after myocardial infarction: a community-based study. Circ Cardiovasc Qual Outcomes. 2009;2(6):656–662. PubMed PMID: 20031905; PubMed Central PMCID: PMCPMC2850275; doi:10.1161/CIRCOUTCOMES.108.831024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smyth SS, McEver RP, Weyrich AS, et al. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7(11):1759–1766. PubMed PMID: 19691483; doi:10.1111/j.1538-7836.2009.03586.x [DOI] [PubMed] [Google Scholar]
- 16. Yao C, Zhang Z, Yao Y, Xu X, Jiang Q, Shi D. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio for acute deep vein thrombosis after total joint arthroplasty: a retrospective study. J Orthop Surg Res. 2018;13(1):40 PubMed PMID: 29482566; PubMed Central PMCID: PMCPMC5828483; doi:10.1186/s13018-018-0745-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–1812. PubMed PMID: 12813013; PubMed Central PMCID: PMCPMC161431; doi:10.1172/JCI18921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. PubMed PMID: 11877368. [DOI] [PubMed] [Google Scholar]
- 19. Poredos P, Jezovnik MK. In patients with idiopathic venous thrombosis, interleukin-10 is decreased and related to endothelial dysfunction. Heart Vessels. 2011;26(6):596–602. PubMed PMID: 21267581; doi:10.1007/s00380-010-0111-3 [DOI] [PubMed] [Google Scholar]
- 20. Fox EA, Kahn SR. The relationship between inflammation and venous thrombosis. A systematic review of clinical studies. Thromb Haemost. 2005;94(2):362–365. PubMed PMID: 16113826; doi:10.1160/TH05-04-0266 [DOI] [PubMed] [Google Scholar]
- 21. Niimi R, Hasegawa M, Sudo A, Shi D, Yamada T, Uchida A. Evaluation of soluble fibrin and D-dimer in the diagnosis of postoperative deep vein thrombosis. Biomarkers. 2010;15(2):149–157. PubMed PMID: 19903012; doi:10.3109/13547500903367276 [DOI] [PubMed] [Google Scholar]
- 22. Rafee A, Herlikar D, Gilbert R, Stockwell RC, McLauchlan GJ. D-Dimer in the diagnosis of deep vein thrombosis following total hip and knee replacement: a prospective study. Ann R Coll Surg Engl. 2008;90(2):123–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dindo D, Breitenstein S, Hahnloser D, et al. Kinetics of D-dimer after general surgery. Blood Coagul Fibrinolysis. 2009;20(5):347–352. PMID: 19474701; doi:10.1097/MBC.0b013e32832a5fe6 [DOI] [PubMed] [Google Scholar]
- 24. Jiang Y, Li J, Liu Y, Li YC, Zhang WG. Risk factors for deep vein thrombosis after orthopedic surgery and the diagnostic value of D-dimer. Ann Vasc Surg. 2015;29(4):675–681. PMID: 25728333; [DOI] [PubMed] [Google Scholar]
- 25. Lutsey PL, Folsom AR, Heckbert SR, Cushman M. Peak thrombin generation and subsequent venous thromboembolism: The Longitudinal Investigation of Thromboembolism Etiology (LITE) study. J Thromb Haemost. 2009;7(10):1639–1648. doi:10.1111/j.1538-7836.2009.03561.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Green L, Lawrie AS, Patel R, et al. The effect of total hip/knee replacement surgery and prophylactic dabigatran on thrombin generation and coagulation parameters. Thromb Res. 2012;130(5):775–779. PubMed PMID: 22245224; doi:10.1016/j.thromres.2011.12.023 [DOI] [PubMed] [Google Scholar]
- 27. Brummel-Ziedins KE, Vossen CY, Butenas S, Mann KG, Rosendaal FR. Thrombin generation profiles in deep venous thrombosis. J Thromb Haemost. 2005;3(11):2497–2505. PMID: 16241948; doi:10.1111/j.1538-7836.2005.01584.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shahidi M. Thrombosis and von Willebrand Factor. Adv Exp Med Biol. 2017;906:285–306. PubMed PMID: 27628010; doi:10.1007/5584_2016_122 [DOI] [PubMed] [Google Scholar]
- 29. Paruk F, Chausse JM. Monitoring the post surgery inflammatory host response. J Emerg Crit Care Med. 2019;3:47. [Google Scholar]
- 30. McSorley ST, Watt DG, Horgan PG, McMillan DC. Postoperative systemic inflammatory response, complication severity, and survival following surgery for colorectal cancer. Ann Surg Oncol. 2016;23(9):2832–2840. PMID: 27016295; doi:10.1245/s10434-016-5204-5 [DOI] [PMC free article] [PubMed] [Google Scholar]


