Abstract
This review was conducted to assess the capacity of the public sector to prevent and control noncommunicable diseases (NCDs) in low-and middle-income countries (LMIC) based on WHO-PEN standards. A PRISMA systematic search appraisal of PubMed, Scopus, and Embase was conducted during May-2020 for original articles conducted in LMIC and reported the capacity of the public sector to prevent and control NCDs. The country readiness score was calculated as the mean score of items for each domain. The indices were compared to an agreed cutoff at 80% the WHO optimal target of availability of affordable essential medicines and basic technologies required to treat NCDs. The literature search yielded 5 original studies, conducted in twelve countries, and surveyed 304 public health facilities. All countries failed to reach the WHO optimal target of availability of affordable essential medicines and basic technologies. The readiness index score according to WHO-PEN standards among countries in terms of essential medicines, diagnostic investigations, and basic equipment were range from 13.5% to 51%, 0.0% to 59.4%, and 29.2% to 51.2% respectively. This review revealed critical gaps in the twelve LMIC public sector capacity to prevent and control of NCDs in terms of essential medicines, basic equipment, and diagnostic investigations.
Keywords: Health facilities, low- and middle-income countries, non-communicable diseases, public sector, a systematic review
Background
Noncommunicable Diseases (NCDs) or chronic diseases are health conditions produced by a combination of genetic, physiological, environmental, and behavioral factors.1,2 NCDs are identified as a major public health concern worldwide, and increasing rapidly in low and middle-income countries (LMIC).1,2 The World Health Organization (WHO) estimated that NCDs - primarily cardiovascular diseases, cancer, diabetes, and chronic respiratory diseases were responsible for 71% of the world death yearly, and accountable for 41 million deaths yearly, the burden of these diseases is increasing disproportionately in LMIC; most of the NCDs deaths (74%) and the majority of premature deaths (86%) occur in LMIC.3 By 2030, around 80% those deaths come from LMIC, with predicting about 52 million deaths yearly.2-4 Effective approaches to reducing the NCDs burden in LMIC include a mix of population- wide and individuals interventions, and methods for early detection of NCDs and their diagnoses using low-cost technologies, non-pharmacological and pharmacological, the effective delivery of these interventions, can lead to future savings in terms of reduced medical costs, improved quality of life and productivity.5 The efficient use of restricted health care resources, access to basic diagnostics, and essential medicines, such care can be delivered equitably only through health systems based on primary care.5 The availability of affordable essential medicines and basic technologies (diagnostic tests, and basic equipment) for NCDs in health systems are essential in addressing the increasing burden of NCDs.2 In the Global Action Plan (2013-2020) for prevention and control of NCDs, the WHO included a target of ⩾ 80% affordable availability of essential medicines and basic technologies required to treat major NCDs in public and private facilities.2 The WHO established a package of essential NCDs interventions (WHO-PEN) for health systems based on primary care in low-resource settings, the WHO-PEN is the minimum standard for NCDs to strengthen countrywide capacity to integrate and scale-up care of NCDs in low resource settings.5 To the best of our knowledge, there was no systematic review that assessed public-sector capacity to prevent and control NCDs in LMIC based on WHO-PEN standards in terms of essential medicines, basic equipment, and diagnostic investigations. We, therefore, sought to review and summarize available data on the capacity of public-sector facilities, in terms of essential medicines, basic equipment, and diagnostic investigations to implement basic interventions for prevention and control of NCDs in LMIC based on WHO-PEN standards.
Methods
Types of studies and data sources
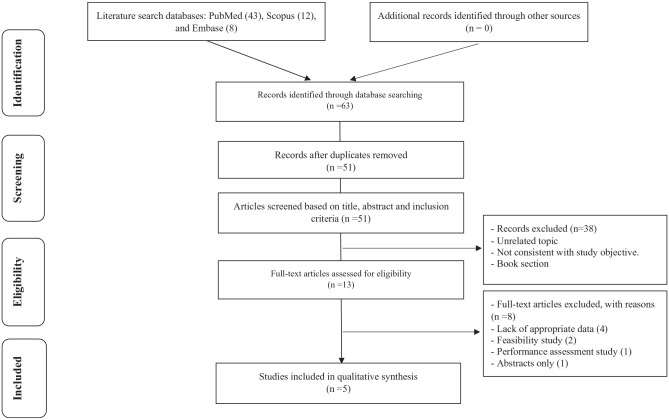
A PRISMA systematic search appraisal was conducted. A systematic literature search of PubMed, Scopus, and Embase was conducted during May 2020. All published studies of any type of design that reported data about the public-sector capacity to prevent and control NCDs in LMIC, based on WHO-PEN standards in terms of essential medicines, basic equipment, and diagnostic investigations were considered. All selected papers’ references were checked to increase the comprehensive of our search. Google Scholar was also used to find relevant articles and documents. A preliminary search was performed to identify common keywords that were used in the articles. In total, the researchers identified 63 records, of which 5 fulfilled the eligibility criteria and were therefore included in the review (Figure 1).
Figure 1.
Results of the systematic literature search.
Inclusion and exclusion criteria
The present systematic review included the articles that were written in English language, used any keywords mentioned in Box 1, The review was focus on studies of any design type that included information about the public-sector capacity to prevent and control NCDs in LMIC based on WHO-PEN standards in terms of essential medicines, basic equipment, and diagnostic investigations. In contrast, studies that included information about the public-sector capacity to prevent and control NCDs in LMIC based on other than WHO-PEN standards, high-income countries, conferences, book sections, review articles, and abstracts without details were excluded. The studies selected were required to meet the following criteria: (1) a study conducted in LMIC; (2) a study assessed the capacity of the public-sector based on WHO-PEN standards; (3) the target/reference population clearly defined; and (4) a study with sufficient information about sample size.
Box 1.
Electronic search strategy.
| PubMed, May 2020, Results: 43 |
| (((“(Non communicable Diseases/classification”[Mesh] OR “Non communicable Diseases/drug therapy”[Mesh] OR “Non communicable Diseases/organization and administration”[Mesh] OR “Non communicable Diseases/prevention and control”[Mesh] OR “Non communicable Diseases/therapy”[Mesh]))) OR “NCDs/diagnostic investigations”) OR “NCDs/basic equipment’s”) OR “NCDs/Essential medicines”) OR “NCDs” OR “Non communicable diseases”))) AND (((“Package of essential non communicable diseases interventions”) OR “WHO-PEN”) OR “World Health Organization-Package of essential non communicable diseases interventions”))) |
| Embase, May 2020, Results: 9 |
| #1 (“Non communicable diseases/classification,” OR #2 “Non communicable diseases/drug therapy,” OR #3 “Non communicable diseases/organization and administration,” OR #4 “Non communicable diseases/prevention and control,” OR #5 “Non communicable diseases/therapy,” OR #6 “NCDs/diagnostic investigations,” OR #7 “NCDs/basic equipment’s”, OR #8 “NCDs/essential medicines” OR #9 “NCDs”, OR #10 “Non communicable diseases”), AND # 11 “package of essential non communicable diseases interventions” OR #12 “who-pen,” OR #13 “world health organization-package of essential non communicable diseases interventions”) #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 AND #11 OR #12 OR #13 |
| Scopus, May 2020, Results: 12 |
| (TITLE-ABS-KEY (“NCDs” OR “Non communicable diseases”)) OR (TITLE-ABS-KEY ((“Non communicable Diseases-classification”), OR (“Non communicable Diseases-drug therapy”), OR (“Non communicable Diseases-organization and administration”), OR (“Non communicable Diseases-prevention and control”))) AND (TITLE-ABS-KEY ((“Package of essential non communicable diseases interventions”) OR (“WHO-PEN”) OR (“World Health Organization-Package of essential non communicable diseases interventions”))) |
Data extraction
Three Authors (AHA, AA, and AHB) followed separately the defined search strategy, after that, the authors screened the articles by titles and abstracts and regained relevant full-text articles. Furthermore, the reevaluation of the full-text article was performed based on inclusion and exclusion criteria. Selected were reviewed, and the data extraction was accomplished based on extraction form, including (1) first author’s and second author’s last name, (2) country, (3) year of publication, (4) study design, (5) sample size, (5) world bank income class, and (6) public facilities type.
Statistical analysis
Descriptive statistics were used to present the data as a number and percentage for different categorical variables. The readiness index score for the country was calculated for all public-sector facilities and primary healthcare centers (PHCs) in each study. In addition, the country readiness scores were calculated for the 3 domains separately (essential medicines, basic equipment, and diagnostic investigations). For each domain, 2 indexes were calculated as the mean score of items expressed as a percentage. The first index was calculated as the mean score of WHO-PEN items included in the study for each domain; the second index was calculated as the mean score of all items recommended by WHO-PEN standards for each domain. For example, if there were 6 diagnostic investigations items on the study, and the country had only 5 diagnostic investigations available, that means, the readiness of the diagnostic capacity index for WHO-PEN items included in the study for that country was 5/6 × 100 = 83.3%, but the WHO-PEN standard recommended at least 7 diagnostic investigations. Therefore, the country’s second index was 5/7 × 100 = 71.4. These indices were compared to an agreed cutoff at (80%) the WHO optimal target of availability of affordable essential medicines and basic technologies required to treat NCDs.2 All statistical analysis was performed using SPSS version 22.
Results
A total of 63 of recorded was retrieved via database search, of which 43 were from PubMed, 12 were from Scopus, and 8 were from Embase. According to the PRISMA flow chart, 12 of the articles, after duplicated, were removed, 38 of the articles were excluded because their title and abstracts not related to the capacity of the public sector based on WHO-PEN strategy, not consistent with the study objective and book section; while 13 of full-text articles were assessed for eligibility criteria. Finally, only 5 articles were included in the systematic review Figure 1. After qualified papers were determined, data were extracted according to a standard protocol. To critical appraisal and improve accuracy, data extraction was conducted by 3 independents researchers, and disagreements between researchers were resolved through consensus.
Characteristics of the included studies
Five studies were identified,6-10 these were included in the final synthesis, and all of the included studies were quantitative studies (cross-sectional). The characteristics of the studies included in this systematic review were displayed in Table 1. While low, lower-middle, upper-middle-income countries were present in the final list of studies. Studies were published during 2012-2018, 1 study was conducted in 8 low-upper-middle-income countries (Benin, Eritrea, Sudan, Bhutan, Sri Lanka, Vietnam, Suriname, and Syria), and other studies were conducted in low-lower middle-income countries (Ghana, Nepal, Uganda, Zambia). Besides, only 173 (56.9%) of 304 public sector facilities included in the studies were classified as PHCs.
Table 1.
Characteristics of the included studies.
| Authors name | Year of publication | Country | World bank income class | Study design | Sample size | Facilities type | ||
|---|---|---|---|---|---|---|---|---|
| PHC N (%) | Other N (%) | |||||||
| 1. | Mendis et al6 | 2012 | Benin | Low income | Cross sectional | 12 | 12 (100) | 0 (0.00) |
| Eritrea | Low income | Cross sectional | 6 | 6 (100) | 0 (0.00) | |||
| Sudan | Lower middle | Cross sectional | 12 | 12 (100) | 0 (0.00) | |||
| Syria | Low income | Cross sectional | 14 | 14 (100) | 0 (0.00) | |||
| Bhutan | Lower middle | Cross sectional | 7 | 7 (100) | 0 (0.00) | |||
| Sri Lanka | Upper-middle | Cross sectional | 14 | 14 (100) | 0 (0.00) | |||
| Vietnam | Lower middle | Cross sectional | 15 | 15 (100) | 0 (0.00) | |||
| Suriname | Upper middle | Cross sectional | 10 | 10 (100) | 0 (0.00) | |||
| 2. | Nyarko et al7 | 2016 | Ghana | Lower middle | Cross sectional | 23 | 18 (78.3) | 3 (13.0)* |
| 2 (8.7)** | ||||||||
| 3. | Aryal et al10 | 2018 | Nepal | Low income | Cross sectional | 92 | 8 (8.7) | 3 (3.3)* |
| 81 (88.0)*** | ||||||||
| 4. | Mutale et al9 | 2018 | Zambia | Lower middle | Cross sectional | 46 | 43 (93.5) | 3 (6.5)**** |
| 5. | Rogers et al8 | 2018 | Ugandan | Low income | Cross sectional | 53 | 14 (26.4) | 26 (49.0)***** |
| 13 (24.5)** | ||||||||
The World Bank. Country and Lending Groups by Income, available at: http://data.worldbank.org/about/country-classifications/country-and-lending-groups.
Abbreviation: PHC, primary health care.
Other: *District hospital.
Regional Hospitals.
Health post.
1sth level hospitals.
General Hospital.
Essential medicines
For the implementation of basic NCDs interventions based on WHO-PEN standards, a core set of 33 essential medicines need to be available.5,11 Table 2 revealed the percentage availability of essential medicines in health facilities in defined areas in the twelve countries and readiness indexes scores by country. The result revealed that none of the surveyed countries reported that their public sector facilities having all the essential medicines recommended by WHO-PEN standards, and none of the surveyed countries had scored a mean index higher than the 80% cut off. Also, the readiness index score according to the recommendation of WHO-PEN standard among countries ranged from 13.5% to 51%. The highest readiness index scores were found in Suriname (51.0%), followed by Ghana (39.8%), and the lowest readiness index scores were found in Nepal (10.9%), followed by Syria (13.5%).
Table 2.
Percentage availability of essential medicines in health facilities in defined areas in twelve countries and readiness index score by country.
| Variables | Benin (n = 12) | Eritrea (n = 6) | Sudan (n = 12) | Syria (n = 14) | Bhutan (n = 7) | Sri Lanka (n = 14) | Vietnam (n = 15) | Suriname (n = 10) | Ghana (n = 23) | Nepal (n = 92) | Zambia (n 4 6) | Ugandan (n = 53) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Adrenaline | NA | NA | NA | NA | NA | NA | NA | NA | 47.8 | 34.8 | 71.7 | NA |
| 2. Atenolol | 8.3 | 0.0 | 42.9 | 14.3 | 0.0 | 69.2 | 93.3 | 100.0 | 26.1 | 65.2 | 28.3 | 64.2 |
| 3. Enalapril | 33.3 | 0.0 | 28.6 | 14.3 | 0.0 | 69.2 | 60.0 | 30.0 | 26.1 | 0.0 | 6.5 | 67.9 |
| 4. Amlodipine | 58.3 | 0.0 | 50.0 | 0.0 | 0.0 | 69.2 | 86.7 | 80.0 | 65.2 | 65.2 | 23.9 | 90.6 |
| 5. Hydrochlorothiazide | 75.0 | 100.0 | 35.7 | 14.3 | 85.7 | 69.2 | 40.0 | 90.0 | 26.1 | 35.9 | NA | 83.0 |
| 6. Isosorbide dinitrate | 0.0 | 0.0 | 0.0 | 21.4 | 0.0 | 69.2 | 60.0 | 90.0 | 17.4 | NA | NA | 73.6 |
| 7. Frusemide (other | 83.3 | 50.0 | 85.7 | 92.9 | 14.3 | 69.2 | 86.7 | 100.0 | 34.8 | NA | 58.7 | 73.6 |
| 8. Simvastatin or lovastatin | 8.3 | 0.0 | 35.7 | 7.1 | 0.0 | 23.1 | 46.7 | 50.0 | 13.0 | 0.0 | 0.0 | NA |
| 9. Insulin long acting | 0.0 | 0.0 | 21.4 | 21.4 | 0.0 | 30.8 | 0.0 | 80.0 | 17.4 | NA | 17.4 | 52.8 |
| Insulin soluble | 0.0 | 0.0 | 28.6 | 21.4 | 0.0 | 30.8 | 0.0 | 80.0 | 21.7 | NA | 21.7 | 52.8 |
| 10. Metformin | 25.0 | 0.0 | 42.9 | 14.3 | 0.0 | 69.2 | 53.3 | 100.0 | 26.1 | 47.8 | 45.7 | 92.3 |
| 11. Glibenclamide | 41.7 | 0.0 | 71.4 | 21.4 | 14.3 | 69.2 | 33.3 | 100.0 | 26.1 | 0.0 | 45.7 | 81.1 |
| 12. Beclometasone inhaler | 33.3 | 16.7 | 21.4 | 28.6 | 0.0 | 15.4 | 6.7 | 80.0 | 17.4 | NA | NA | NA |
| 13. Prednisolone | 0.0 | 0.0 | 42.9 | 7.1 | 0.0 | 69.2 | 93.3 | 90.0 | 39.1 | NA | 95.7 | NA |
| 14. Salbutamol inhaler | 33.3 | 100.0 | 71.4 | 78.6 | 0.0 | 30.8 | 20.0 | 90.0 | 39.1 | NA | 73.9 | NA |
| Salbutamol tablets | 66.7 | 100.0 | 85.7 | 14.3 | 100.0 | 69.2 | 93.3 | 90.0 | 65.2 | 92.4 | NA | NA |
| Salbutamol injectable | NA | NA | NA | NA | NA | NA | NA | NA | 17.4 | NA | NA | NA |
| 15. Ipratropium bromide | 0.0 | 0.0 | 14.3 | 14.3 | 0.0 | 30.8 | 0.0 | 20.0 | 4.3 | NA | NA | NA |
| 16. Aspirin | 100.0 | 100.0 | 100.0 | 21.4 | 0.0 | 69.2 | 100.0 | 100.0 | 82.6 | 65.2 | 67.4 | NA |
| 17. Paracetamol | 100.0 | 100.0 | 92.0 | 0.0 | 100.0 | 69.2 | 100.0 | 100.0 | 100.0 | NA | 78.3 | NA |
| 18. Ibuprofen | 83.3 | 100.0 | 100.0 | 0.0 | 100.0 | 69.2 | 73.3 | 80.0 | 100.0 | NA | 78.3 | NA |
| 19. Morphine (oral) | 0.0 | 0.0 | 14.3 | 0.0 | 0.0 | 15.4 | 0.0 | 20.0 | 8.7 | NA | NA | NA |
| Morphine injection | 8.3 | 0.0 | 14.3 | 0.0 | 0.0 | 38.5 | 0.0 | 20.0 | 13.0 | NA | NA | NA |
| 20. Codeine | 8.3 | 0.0 | 35.7 | 0.0 | 0.0 | 15.4 | 33.3 | 60.0 | 13.0 | NA | NA | NA |
| 21. Glucose solution for injection | 91.7 | 0.0 | 0.0 | 0.0 | 57.1 | 53.8 | 66.7 | 80.0 | 86.9 | NA | 82.6 | NA |
| 22. Sodium chloride infusion | 91.7 | 100.0 | 92.9 | 35.7 | 0.0 | 53.8 | 100.0 | 60.0 | 91.3 | NA | 84.8 | NA |
| 23. Benzathine penicillin | 50.0 | 100.0 | 92.9 | 57.1 | 30.8 | 0.0 | 60.0 | 100.0 | 69.6 | NA | 95.7 | NA |
| 24. Erythromycin | NA | NA | NA | NA | NA | NA | NA | NA | 73.9 | NA | 95.7 | NA |
| 25. Amoxicillin | NA | NA | NA | NA | NA | NA | NA | NA | 100 | NA | 95.7 | NA |
| 26. Hydrocortisone (steroids) | NA | NA | NA | NA | NA | NA | NA | NA | 91.3 | NA | 95.7 | NA |
| 27. Heparin | NA | NA | NA | NA | NA | NA | NA | NA | 21.7 | NA | NA | NA |
| 28. Diazepam | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 29. Magnesium sulphate | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 73.6 |
| 30. Promethazine | NA | NA | NA | NA | NA | NA | NA | NA | 82.6 | NA | 91.3 | NA |
| 31. Senna | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 32. Glyceryl trinitrate | NA | NA | NA | NA | NA | NA | NA | NA | 8.7 | NA | 6.5 | NA |
| 33. Spironolactone | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Readiness index score for WHO-PEN items included in the study | 39.9 | 34.8 | 48.9 | 20.0 | 20.1 | 43.9 | 52.3 | 75.6 | 44.6 | 40.7 | 59.2 | 73.23 |
| Readiness index score, WHO-PEN standard | 27.0 | 23.4 | 33.0 | 13.5 | 13.6 | 29.7 | 35.3 | 51.0 | 39.8 | 10.9 | 36.8 | 21.8 |
Data are expressed as percentage for different categorical variables.
Abbreviation: NA: Not available in the study survey.
Diagnostic tests
For the implementation of basic NCDs interventions based on WHO-PEN standards, a core set of 7 diagnostic investigations need to be available.11 Table 3 displayed the percentage availability of basic diagnostic investigations (Urine albumin, urine glucose, urine ketones, blood glucose, blood cholesterol, serum creatinine, and serum troponin), and readiness indexes scores by country. None of the surveyed countries had scored a mean index higher than the 80% cut off. The readiness index score according to the recommendation of WHO-PEN standards, among countries ranged from 0.0% to 59.4%. Furthermore, from the surveyed countries, all basic diagnostic investigations are not available in facilities surveyed in Vietnam. Besides, the highest readiness indexes scores were found in Sudan (59.4%), followed by Benin (54.2%), and the lowest readiness indexes scores were found in Vietnam (0.0%), followed by Ghana (22.4%). Moreover, studies in Zambia and Nepal not clarified the diagnostic tests surveyed.
Table 3.
Percentage availability of basic diagnostic tests in health facilities in defined areas in twelve countries and readiness index score by country.
| Variables | Benin (n = 12) | Eritrea (n = 6) | Sudan (n = 12) | Syria (n = 14) | Bhutan (n = 7) | Sri Lanka (n = 14) | Vietnam (n = 15) | Suriname (n = 10) | Ghana (n = 23) | Nepal (n = 92) | Zambia (n = 46) | Ugandan (n = 53) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Urine albumin | 100 | 67 | 92 | 64 | 100 | 46 | 0.0 | 70 | 34.8 | NA | NA | 13.2 |
| 2. Urine glucose | 92 | 67 | 92 | 71 | 100 | 54 | 0.0 | 70 | 34.8 | NA | NA | 92.5 |
| 3. Urine ketones | 42 | 33 | 58 | 79 | 0.0 | 0.0 | 0.0 | 40 | 26.1 | NA | NA | 92.5 |
| 4. Blood glucose | 67 | 17 | 75 | 93 | 0.0 | 0.0 | 0.0 | 90 | 26.1 | NA | NA | 92.5 |
| 5. Blood cholesterol | 25 | 0.0 | 33 | 14 | 0.0 | 8.0 | 0.0 | 20 | 17.4 | NA | NA | 28.3 |
| 6. Serum creatinine | 33 | 0.0 | 58 | 0.0 | 0.0 | 8.0 | 0.0 | 10 | 17.4 | NA | NA | 43.4 |
| 7. Serum troponin | 8.0 | 0.0 | 8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | NA | NA | NA |
| Readiness index score for WHO-PEN items included in the study | 52.4 | 26.3 | 59.4 | 45.9 | 28.6 | 16.6 | 0.0 | 42.9 | 22.4 | NA | 74.2 | 60.4 |
| Readiness index score, WHO-PEN standard | 52.4 | 26.3 | 59.4 | 45.9 | 28.6 | 16.6 | 0.0 | 42.9 | 22.4 | NA | NA | 51.8 |
Data are expressed as percentage for different categorical variables.
Abbreviation: NA: Not available in the study survey.
Basic equipment
For the implementation of the basic NCDs interventions based on WHO-PEN standards, a core set of 13 basic equipment need to be available.11 Table 4 displayed the percentage availability of basic equipment in health facilities in defined areas, in the twelve countries and readiness indexes scores by country. Our data revealed that none of the surveyed countries had scored a mean index higher than the 80% cut off. The readiness indexes score according to the recommendation of WHO-PEN standards among countries ranged from 29.2% to 51.2%. The highest readiness indexes scores were found in Ghana (51.2%), followed by Nepal (50.8%), and the lowest readiness index score was found in Uganda (29.2%). Zambia survey did not clarify the basic equipment included in the study. Also, Mendis et al6 survey in Benin, Eritrea, Sudan, Bhutan, Sri Lanka, Vietnam, Suriname, and Syria demonstrated the availability of each item of the basic equipment’s in the 8 countries together.
Table 4.
Percentage availability of basic equipment in health facilities in defined areas in twelve countries and readiness index score by country.
| Equipment | Benin (n = 12) | Eritrea (n = 6) | Sudan (n = 12) | Syria (n = 14) | Bhutan (n = 7) | Sri Lanka (n = 14) | Vietnam (n = 15) | Suriname (n = 10) | Ghana (n = 23) | Nepal (n = 92) | Zambia (n = 46) | Ugandan (n = 53) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Blood pressure devices (Mercury) | 100 | 100 | 97.8 | NA | 39.6 | |||||||
| 2. Oxygen cylinders or masks | 44 | 26.1 | 10.9 | NA | NA | |||||||
| 3. Weighing machines | 99 | 100 | 98.9 | NA | 56.6 | |||||||
| 4. Stethoscope | NA | 100 | 98.9 | NA | 58.5 | |||||||
| 5. Thermometer | NA | 100 | 98.9 | NA | 54.7 | |||||||
| 6. Measuring tape | 63 | 87.0 | 90.2 | NA | 50.9 | |||||||
| 7. Health education material | NA | 43.5 | NA | NA | 15.1 | |||||||
| 8. Nebulizers | 37 | 26.1 | 35.9 | NA | 15.1 | |||||||
| 9. Electrocardiographs | 28 | 17.4 | 7.6 | NA | 15.1 | |||||||
| 10. Peak flow meters | 20 | 13.0 | NA | NA | 1.9 | |||||||
| 11. Pulse oximeter | 2 | 26.1 | 13.0 | NA | NA | |||||||
| 12. Spacer | NA | 0.0 | 19.6 | NA | 9.4 | |||||||
| 13. Glucometer | NA | 26.1 | 89.1 | NA | 62.3 | |||||||
| Readiness index score for WHO-PEN items included in the study | 49.1 | 51.2 | 60.1 | 57.6 | 34.5 | |||||||
| Readiness index score, WHO-PEN standard | 30.2 | 51.2 | 50.8 | NA | 29.2 | |||||||
Data are expressed as percentage for different categorical variables.
Abbreviation: NA: Not available in the study survey.
Primary healthcare center capacity
Only 173 (56.9%) of 304 public sector facilities included in the studies were classified as PHCs. Table 5 shows that, the readiness indexes score according to the WHO-PEN standards for the included PHCs, in terms of essential medicines were range from 13.5% in Syria to 51.0% in Suriname. In terms of diagnostic tests were range from 0.0% in Vietnam to 59.4 in Sudan; while in terms of basic equipment’s were range from 69.2% in Nepal to 26.4% in Uganda.
Table 5.
Readiness index score, WHO-PEN standard for primary healthcare centers.
| Country | Primary healthcare centers N (%) | Essential medicines | Basic diagnostic tests | Basic equipment’s |
|---|---|---|---|---|
| Benin | 12.0 (100) | 27.0 | 52.4 | 30.2 |
| Eritrea | 6.0 (100) | 23.4 | 26.3 | 30.2 |
| Sudan | 12.0 (100) | 33.0 | 59.4 | 30.2 |
| Syria | 14.0 (100) | 13.5 | 45.9 | 30.2 |
| Bhutan | 7.0 (100) | 13.6 | 28.6 | 30.2 |
| Sri Lanka | 14.0 (100) | 29.7 | 16.6 | 30.2 |
| Vietnam | 15.0 (100) | 35.3 | 0.0 | 30.2 |
| Suriname | 10.0 (100) | 51.0 | 42.9 | 30.2 |
| Ghana | 18.0 (78.3) | 31.5 | 6.3 | 41.4 |
| Nepal | 8.0 (8.7) | 17.0 | NA | 69.2 |
| Zambia | 43.0 (93.5) | NA | NA | NA |
| Ugandan | 14.0 (26.4) | 21.2 | 42.9 | 26.4 |
Data are expressed as percentage for different categorical variables.
Abbreviation: NA: Not available in the study survey.
Discussion
There is a shortage of comprehensive data concerning the public sector capacity in LMIC to respond to the requirements of people with NCDs. In this work, we have revised systematically the literature available in different databases, comprehensive assessment of public sector capacity to prevent and control of NCDs in twelve LMIC based on WHO-PEN standards in terms of essential medicines, basic equipment, and diagnostic investigations. The results of this review showed critical gaps in public sector capacity in the twelve countries in terms of essential medicines, basic equipment, and diagnostic investigations, based on WHO-PEN standards recommendations.
In the current systematic review, in terms of essential medicines, variation was reported in the number of essential medicines recommended by WHO-PEN included in the studies. The number of essential medicines ranged from 10 drugs in Ugandan and Nepal to 29 drugs in Ghana. Furthermore, the readiness index score for WHO-PEN items included in each study ranged from 20.0% in Syria to 75.6% in Suriname. Also, the readiness index score according to the recommendation of WHO-PEN standards among countries ranged from 13.5% to 51%.
WHO reported in the 2017 global survey conducted in 194 countries to assess the national capacity for the prevention and control of noncommunicable diseases that the ten essential medicines for the treatment of major NCDs were available in the majority of countries surveyed, but variations were reported between countries based on the income class, for instance, Insulin is available in 98% of high-income countries compared to 39% in low-income countries, and statins is available in 98% of high-income countries compared to 16% in low-income countries.12 A study based on surveys conducted among forty developing countries demonstrated that fifteen drugs required to treat NCDs were suboptimal across the different countries especially in the public sector compared to the private sector which ranged from 36% availability in the public sector to 54.7% in the private sector.13
World Health organization estimations that the public sector covers only one-third of needs in terms of essential medicines and the private sector covers about two thirds.14 Globally, the availability of essential medicines for NCDs in the public sector remains sub-optimal based on the WHO optimal target of availability of essential medicines required to treat NCDs.15-17 Cameron et al,13,18 demonstrated that low availability of medicines in general, medicines for NCDs are less available than medicines for acute diseases, and the availability in the private sector was higher than the public sector. The possible factors leading to poor availability of essential drugs in the LMIC public sector compared to the private sector are insufficient financial resources for medicines purchase, failure to forecast the requirements accurately, ineffective purchasing, incompetent distribution systems, the prices of medicines in public sectors lower than the prices in private sectors; the public spending on health in a lot of LMIC is inadequate to achieve a comprehensive set of NCDs interventions and in many of LMIC is the abuse of drugs by doctors, pharmacists, and patients.18-20
In terms of diagnostic investigations, our review showed that most of the included studies, surveyed all diagnostic investigations need to be available in health facility according to WHO-PEN standards except Zambia, Nepal, and Ugandan. The studies in Zambia and Nepal did not clarify the diagnostic tests surveyed. The readiness index score for WHO-PEN items included in each study and the readiness index score according to the recommendation of WHO-PEN standards were the same for Benin, Eritrea, Sudan, Syria, Bhutan, Sri Lanka, Vietnam, Suriname, and Ghana, which ranged from 0.0% in Vietnam to 59.4 % in Sudan. For the Uganda study, one of the recommended tests not available in the study survey (Serum troponin, Besides, the readiness index score for WHO-PEN items included in Uganda was 60.4% and the readiness index score according to the recommendation of WHO-PEN standard was 51.8%. According to WHO 2017 global survey, the diagnostic tests were reported as being generally available, but variations were reported between countries based on the income class, for instance, total cholesterol test is available in around 90% of high-income countries compared to less than 20% in low-income countries and blood glucose tests are available in around 98% of high-income countries compared to less than 50% in low-income countries.12 A recent study conducted in the Gaza Strip, Palestine among 52 PHCs demonstrated that the readiness index score in terms of diagnostic investigations recommended by the WHO-PEN standards was 62.2%.21 In contrast, In Saudi Arabia that classified as a High-income country, a study conducted among 41 PHCs demonstrated that all basic diagnostic investigations were available in all the centers with 100%.22 Concerning basic equipment, variation was reported in the number of basic equipment recommended by WHO-PEN included in the studies. The number of basic equipment ranged from 8 in (Benin, Eritrea, Sudan, Bhutan, Sri Lanka, Vietnam, Suriname, and Syria) to 13 in Ghana; the rest of the other studies were 11 in Nepal and Uganda. One study conducted in Zambia did not clarify the basic equipment surveyed. Mendis et al6 survey in Benin, Eritrea, Sudan, Bhutan, Sri Lanka, Vietnam, Suriname, and Syria demonstrated the availability of each item of the basic equipment in the 8 countries together. The readiness index score for WHO-PEN items included in each study ranged from 34.5% in Uganda to 60.1% in Nepal. Moreover, the readiness index score according to the recommendation of WHO-PEN standard among countries ranged from 29.2% in Uganda to 51.2% in Ghana.
According to WHO 2017 global survey, the basic equipment were reported as being generally available, but variations were reported between countries based on the income class, for instance, heigh and weight measuring devices are available in around 98% of high-income countries compared to less than 80% in low-income countries and blood pressure measuring devices are available in around 98% of high-income countries compared to around 90% in low-income countries.12 In Gaza Strip /Palestine, the readiness index score in terms of basic equipment recommended by the WHO-PEN standards was 66%.21 On the other hand, In Saudi Arabia, the overall readiness index score in terms of basic equipment was 92%.22 The results of the current review consistent with other studies conducted in LMIC demonstrated sub-optimal availability of basic technologies (diagnostic tests, basic equipment) for NCDs, there are many LMIC does not able to provide basic NCDs interventions.5,23,24 A possible explanation of sub-optimal availability of basic technologies is in the present period of global fiscal restraint, governments are seeking ways of decreasing expenditures on social sectors, including health.24 Enhancing the access to essential medicines and basic technologies is important for the accomplishment of the Millennium Development Goals.25 Inadequate availability of medicines and basic technologies required to treat NCDs have harmful financial consequences. NCDs tend to put a substantial burden on households.26 The inadequate availability of essential medicines and basic technologies in the public sector may either prevent patients from having treatment or lead the patients to obtain the services in the private sector, where they are frequently extra expensive.25 In LMIC, World Health organization data reported that around half of households (41%-56%) utilized all their expenditure for health on medicines purchasing.27 Health policies must redesign to protect individuals especially NCDs patients from extra expenditure by health insurance schemes that cover and ensure the availability of essential medicines and basic technologies. A balance must be found between multisectoral policies to ensuring that the public health sector interventions provide NCDs patients with quality care.24 Inadequate financial resources, one of the major reason for gaps identified, the next stage that could be taken as the remedial stage encourages the efficiency of NCDs services delivery, which can be done throughput priorities for NCDs interventions based on equity, safety, and cost-effectiveness, healthcare providers must be adherence to prescription and investigations requests based on evidence-based and cost-effectiveness.5,23,28 If these prioritized interventions are delivered validly, it will enhance the chances of prevention and control of NCDs, and reducing health care budgets.29-31 The study demonstrated that the international community should assist the low-middle-income countries to NCDs control, including increasing access to essential medicines and basic technologies, and support local production of essential medicines.32 The study also identifies the urgent need for standardized tools to allow LMIC to regularly screening the availability of health services capacity and readiness, the absence of regular monitoring and evaluation of health system readiness and capacity may lead to main challenges to appropriate planning and distribution of limitable resources.8
Strengths and limitations
The main strength of our study was it’s being the first systematic review, which evaluated the public sector capacity to prevent and control NCDs in LMIC based on WHO-PEN standards in terms of essential medicines, basic equipment, and diagnostic investigations. The main limitations of the systematic review were the package of essential NCDs interventions was developed for the primary health care system in LMIC, while many of the included studies used it in hospitals and other public facilities not classified as PHCs. Also, the included studies had heterogeneity of results, time of the study, and sample sizes. Finally, all of the included studies were cross-sectional studies, which measured a one-time point assessment of essential medicines, basic equipment, and diagnostic investigations.
Conclusion
In the time of sustainable development goals, and states working to achieve universal health coverage, this systematic review revealed critical gaps in the twelve LMIC public sector capacity to prevent and control NCDs in terms of essential medicines, basic equipment, and diagnostic investigations. All countries failed to reach the WHO optimal target of availability of affordable essential medicines and basic technologies required to treat major NCDs based on WHO-PEN standards. These results should attract national and international policymakers to increase commitments to improve the availability of essential medicines, basic equipment, and diagnostic investigations in public sectors.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: AHA, AA, AHB, MT, AT, and AAS were responsible for the overall manuscript preparation, made equal contributions, and have read and approved the final manuscript.
ORCID iD: Abdel Hamid El Bilbeisi  https://orcid.org/0000-0001-8870-8326
https://orcid.org/0000-0001-8870-8326
References
- 1. Puoane T, Tsolekile L, Sanders D, Parker W. Chronic non-communicable diseases: primary health care: programme areas. S Afr Health Rev. 2008;2008:73-87. [Google Scholar]
- 2. World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013-2020. In: Follow-up to the political declaration of the high-level meeting of the General Assembly on the Prevention and Control of Non-communicable Diseases, World Health Organization, 2013. [Google Scholar]
- 3. World Health Organization. Global status report on noncommunicable diseases 2014. World Health Organization, 2014. [Google Scholar]
- 4. World Health Organization. Assessing national capacity for the prevention and control of noncommunicable diseases: report of the 2015 global survey. World Health Organization, 2016. [Google Scholar]
- 5. World Health Organization. Package of essential noncommunicable (PEN) disease interventions for primary health care in low-resource settings. World Health Organization, 2010. [Google Scholar]
- 6. Mendis S, Al Bashir I, Dissanayake L, et al. Gaps in capacity in primary care in low-resource settings for implementation of essential noncommunicable disease interventions. Int J Hypertens. 2012; 2012:584041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nyarko KM, Ameme DK, Ocansey D, Commeh E, Markwei MT, Ohene SA. Capacity assessment of selected health care facilities for the pilot implementation of Package for Essential Non-communicable Diseases (PEN) intervention in Ghana. Pan Afr Med J. 2016;25:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rogers HE, Akiteng AR, Mutungi G, Ettinger AS, Schwartz JI. Capacity of Ugandan public sector health facilities to prevent and control non-communicable diseases: an assessment based upon WHO-PEN standards. BMC Health Serv Res. 2018;18:606. doi: 10.1186/s12913-018-3426-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mutale W, Bosomprah S, Shankalala P, et al. Assessing capacity and readiness to manage NCDs in primary care setting: gaps and opportunities based on adapted WHO PEN tool in Zambia. PloS One. 2018;13:e0200994. doi: 10.1371/journal.pone.0200994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aryal BK, Daud M, Thapa A, Mahotra A, Magar SA, Malla CK. Assesssment of health facilities for implementation of non-communicable disease package. J Nepal Health Res Counc. 2018;16:149-155. [PubMed] [Google Scholar]
- 11. World Health Organization. Prevention and control of noncommunicable diseases: guidelines for primary health care in low resource settings-Sample Questionnaire. World Health Organization, 2010. [PubMed] [Google Scholar]
- 12. World Health Organization. Assessing national capacity for the prevention and control of noncommunicable diseases: report of the 2017 global survey. World Health Organization, 2018. [Google Scholar]
- 13. Cameron A, Roubos I, Ewen M, Mantel-Teeuwisse AK, Leufkens HG, Laing RO. Differences in the availability of medicines for chronic and acute conditions in the public and private sectors of developing countries. Bull World Health Organ. 2011;89:412-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Force MGT. Delivering on the global partnership for achieving the millennium development goals. United Nations Publications, 2008. [Google Scholar]
- 15. Ewen M, Zweekhorst M, Regeer B, Laing R. Baseline assessment of WHO’s target for both availability and affordability of essential medicines to treat non-communicable diseases. PloS One. 2017;12:e017128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vialle-Valentin CE, Serumaga B, Wagner AK, Ross-Degnan D. Evidence on access to medicines for chronic diseases from household surveys in five low-and middle-income countries. Health Policy Plan. 2015;30:1044-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khatib R, McKee M, Shannon H, et al. Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet. 2016;387:61-69. [DOI] [PubMed] [Google Scholar]
- 18. Cameron A, Ewen M, Ross-Degnan D, Ball D, Laing R. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet. 2009;373:240-249. [DOI] [PubMed] [Google Scholar]
- 19. Fattouh R, Abu Hamad B. Impact of using essential drug list: analysis of drug use indicators in Gaza Strip. East Mediterr Health J 2010;16:886-892. [PubMed] [Google Scholar]
- 20. World Health Organization. Prevention of cardiovascular disease. World Health Organization, 2007. [Google Scholar]
- 21. Albelbeisi AH, Albelbeisi A, El Bilbeisi AH, Takian A, Akbari-Sari A. Capacity of Palestinian primary health care system to prevent and control of non-communicable diseases in Gaza Strip, Palestine: a capacity assessment analysis based on adapted WHO-PEN tool. The Int J Health Plan Manag. 2020;35:1412-1425. [DOI] [PubMed] [Google Scholar]
- 22. Bawazir A, Al-Surimi K, Suwaidan SD, AlShehri AM, AlFarhan AI, Abolfotouh MA. Capacity and readiness of primary health care centers for implementation of the basic strategy for prevention and control of non-communicable diseases in Saudi Arabia. A case study from the Ministry of National Guard-Health Affairs, Riyadh, Saudi Arabia. Saudi Med J. 2019;40:614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kibirige D. Availability and affordability of essential medicines and diagnostic tests for diabetes mellitus in sub-Saharan Africa: A systematic review. Department of Endocrinology and Diabetes, Queen Mary University of London. [Google Scholar]
- 24. Mendis S. The policy agenda for prevention and control of non-communicable diseases. Br Medical Bull. 2010;96:23-43. [DOI] [PubMed] [Google Scholar]
- 25. Force MGT. The global partnership for development at a critical juncture. United Nations Publications, 2010. [Google Scholar]
- 26. Vita-Finzi L. Preventing chronic diseases: a vital investment. World Health Organization, 2005. [Google Scholar]
- 27. Faden L, Vialle-Valentin C, Ross-Degnan D, Wagner A. The role of health insurance in the cost-effective use of medicines in low-and middle-income countries. Health Policy. 2011;100:134-143. [DOI] [PubMed] [Google Scholar]
- 28. World Health Organization. The world health report: health systems financing: the path to universal coverage: executive summary. World Health Organization, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mendis S, Johnston SC, Fan W, Oladapo O, Cameron A, Faramawi MF. Cardiovascular risk management and its impact on hypertension control in primary care in low-resource settings: a cluster-randomized trial. Bull World Health Organ. 2010;88:412-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mendis S, Abegunde D, Yusuf S, et al. WHO study on Prevention of REcurrences of Myocardial Infarction and StrokE (WHO-PREMISE). Bull World Health Organ. 2005;83:820-829. [PMC free article] [PubMed] [Google Scholar]
- 31. World Health Organization. Prevention of recurrent heart attacks and strokes in low and middle income populations: evidence-based recommendations for policy-makers and health professionals. World Health Organization, 2003. [Google Scholar]
- 32. Force MGT. The global partnership for development: making rhetoric a reality. United Nations Publications, 2012. [Google Scholar]