Abstract
A rapid and reliable assessment of the dabigatran effect is desirable in dabigatran treated patients with uncontrolled bleeding or before acute surgery. The aim of this study was to study the anticoagulant effects of dabigatran in patients with atrial fibrillation (AF) as assessed by the whole blood assays ROTEM, and how data from these methods correlate to plasma dabigatran concentrations measured by Hemoclot. ROTEM was performed with ROTEM Gamma (Pentapharm GmbH, Munich, Germany). The assays used in our study were Ex-tem and In-tem assay. Plasma dabigatran concentrations were determined by hemoclot thrombin inhibitor assay (Hyphen BioMed, France) at trough and post-dose in 27 patients on dabigatran 150 mg BID. Median plasma dabigatran concentrations at trough were 74 ng/mL (11.2–250) and post-dose (2 h after ingestion) 120 ng/mL (31–282). The ROTEM clotting time (CT) and maximum clot firmnes (MCF) correlated strongly with dabigatran concentrations when activated with the reagents Ex-tem (p < 0.0001) and In-tem (p < 0.0001). In summary, in our study, we have found that the ROTEM variable CT and MCF, when activated with triggers Ex-tem and In-tem, has a strong and highly significant correlation with the plasma dabigatran concentration in a real-life population of AF-patients and could thereby be an alternative to estimate dabigatran concentration in emergency situations. However, additional studies are needed to further validate these findings.
Keywords: dabigatran, hemostasis, monitoring, rotation thromboelastometry
Introduction
Dabigatran is a direct thrombin inhibitor belonging to direct oral anticoagulants (DOACs), which are rapidly replacing warfarin as oral anticoagulants in nonvalvular atrial fibrillation (NVAF) or venous thromboembolism.1–4 One major advantage with dabigatran and other DOACs compared to warfarin is that no routine testing is required. Nevertheless, the degree of anticoagulation should be determined in cases of severe bleeding or urgent surgery. Routine coagulation tests (i.e. PT-INR, aPTT) have proven to be inadequate for sensitive determination of the anticoagulant effect of dabigatran.5–7 Actual dabigatran concentrations can be determined directly with high sensitivity through liquid chromatography with tandem mass spectrometry (LC-MS/MS), but also indirectly through special plasma-based functional coagulation assays (e.g. diluted thrombin time (Hemoclot), Ecarin clotting time—ECT).5,6 However, with these methods it may take 30 min to several hours before a test result can be delivered to the clinician with the risk of delaying important clinical interventions. Rotation thromboelastometry (ROTEM) is a new viscoelastometric point-of-care-test for the complex evaluation of changes in hemostasis introduced to clinical practice. This assay allows quick complex testing of hemostasis in 1 blood sample.8 As we have already mentioned, dabigatran inhibits thrombin, which has a key role in hemostasis with effects not only on plasma coagulation, but also on platelets.9,10 Therefore, it is important to investigate the hemostatic effect of dabigatran on a whole blood sample. ROTEM is a method that allows this. It has not been thoroughly evaluated for patients with oral anticoagulants but there are studies that indicate their usefulness.11–15 This observational study aimed to assess the anticoagulant effects of dabigatran in patients with NVAF as assessed by the whole blood assays ROTEM, and how data from these methods correlate to plasma dabigatran concentrations measured by Hemoclot.
Material and Methods
The local Ethical Committee of the Jessenius Faculty of Medicine in Martin approved this study (EK 1702/2015). All study participants agreed to participate in the project and signed a written informed consent in accordance with the Declaration of Helsinki.
We recruited patients treated with dabigatran 150 mg BID due to NVAF. All patients were on continuous treatment with dabigatran since >2 weeks. Dabigatran was administrated twice daily (at 7:00 pm and 7:00 am). Blood samples were taken 12 hours after a previous drug dose administration (sample 1, at 7:00 am) for the assessment of the dabigatran trough level and 2 hours after the next drug dose administration (sample 2, at 9:00 am) for the assessment of the dabigatran peak level. To be sure that the drug was administered at the right time, we implemented following measures. First, the drug was administered to the patients by a physician who was involved in this study. Second, treatment with dabigatran was monitored by hemoclot thrombin inhibitor assay (Hyphen BioMed, France) according to the manufacturer’s instructions. This dilute thrombin time assay is certified in Europe and USA for the quantitative determination of dabigatran plasma levels. None reported concomitant use of anti-platelet medication. Patients were weighed, measured and thromboembolic risk was calculated using the CHA2DS2-Vasc score.
ROTEM was performed with ROTEM Gamma (Pentapharm GmbH, Munich, Germany). Citrated plasma samples (300 mL) were used for the examination. The measuring part comprises a cylindrical cup, in which a whole blood sample was placed. A pin was then immersed into the cup with the pin connected to a detector. The cup and the pin make an oscillation of each other at angle of 4.75°. Between pin and cup, the previously present gap of 1 mm, bridged by the blood, was subsequently formed. It was not able to test the blood viscosity or detect the initiation of blood clotting, when the first signals occurred after pin was connected by the first fibrin fibers filling the entire distance to the cup wall.16 This kinetics were detected mechanically and calculated by the computer to the curves and parameters. The assays used in our study were Ex-tem and In-tem. The measures, recorded from 2 channels, performing EXTEM and INTEM tests simultaneously were: clotting time (CT), clot formation time (CFT), and maximum clot firmnes (MCF). CT may be regarded as a standard coagulation time, CFT is defined as the time required for the clot to reach a fixed firmness, and MCF represents the maximal amplitude of the tracing. All ROTEM analyses were performed at 37°C within 30 min after sampling and in accordance with the manufacturer’s recommendations
Blood was collected in 3.2% citrate tubes for determination of dabigatran concentrations, ROTEM measurements, fibrinogen levels, PT and aPTT; in EDTA tubes for measurements of hemoglobin concentrations and platelet counts; and in lithium heparin tubes for analysis of creatinine levels. Glomerular filtration rate was estimated (eGFR) using the Cockcroft-Gault formula.
Data are expressed as median and range and non-parametric tests used for statistical analysis. Correlations between variables are expressed as Pearson’s correlation coefficients. All tests were 2-tailed, and P values < .05 were considered statistically significant. All statistical analyses were performed using SPSS version 25 (IBM Inc., USA).
Results
In total 27 patients (16 woman), were sampled twice (54 sampling occasions). The patients’ age were 70 years (47–87), weight 80 kg (58–130) and they had a CHA2DS2-Vasc score of 4 (2–6) (data presented as median and range). The eGFR according to the Cockcroft-Gault formula was in median 80 mL/min (55–110) and classified as normal (n = 14), mildly (n = 12) or moderately (n = 1) impaired. Median plasma dabigatran concentrations at trough were 74 ng/mL (11.2–250) and post-dose (2 h after ingestion) 120 ng/mL (31–282). All patients had haemoglobin concentrations (140 g/L (123–165)), platelet counts (189 × 109/L (165–340)) and plasma fibrinogen levels (2.9 g/L (2.5–3.1)) within reference ranges.
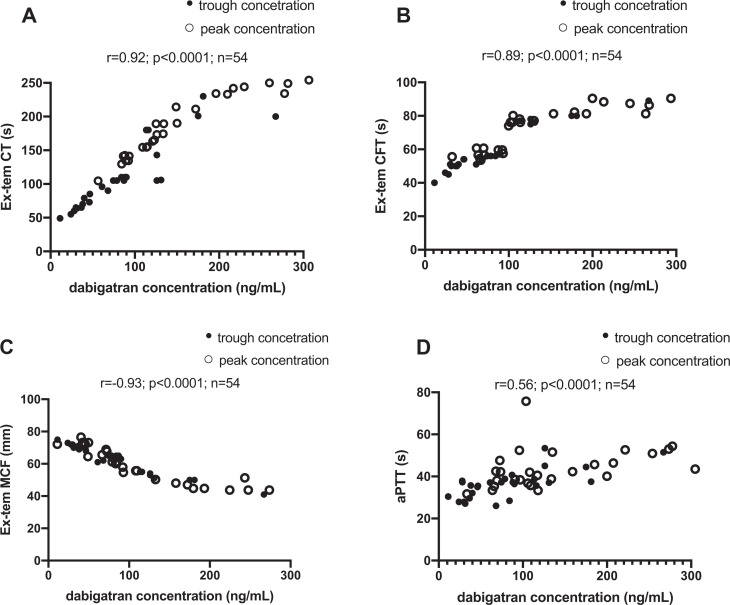
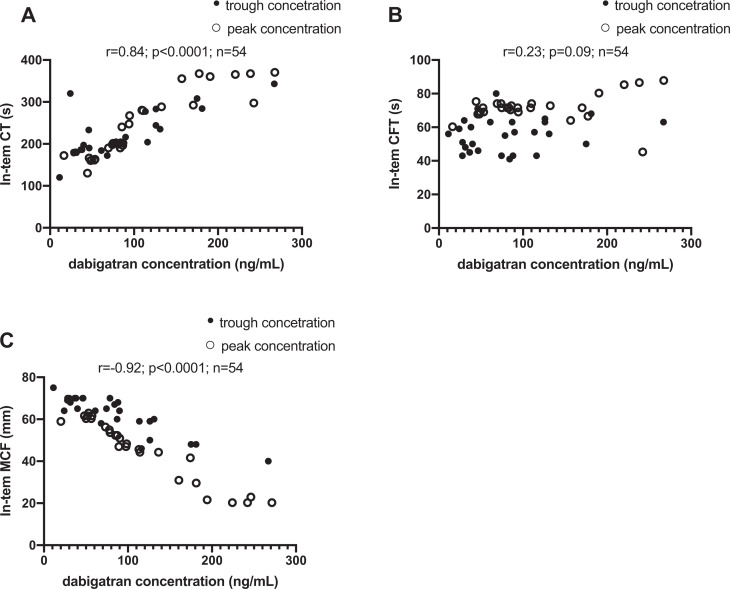
In time points for trough and peak dabigatran plasma concentrations, we investigated relationships between functional coagulation assays (hemoclot thrombin inhibitor assay) and selected ROTEM tests. Using the ROTEM triggers Ex-tem, correlations between CT and dabigatran concentrations were strong and linear (Figure 1A: r = 0.92, p < 0.0001). Correlations between dabigatran concentrations and CT using In-tem were weaker (Figure 2A: r = 0.84, p < 0.0001).
Figure 1.
Correlations Between Dabigatran Plasma Concentrations and Ex-tem (A, B, C). Between Trough Plasma Dabigatran Concentration and aPTT (D). All sampling occasions included (n = 54).
Figure 2.
Correlations Between Dabigatran Plasma Concentrations and In-tem (A, B, C). All Sampling Occasions included (n = 54).
Correlations between dabigatran concentrations and CFT using In-tem (Figure 2B: r = 0.23, p = 0.09) were insignificant. However, the results were significant by using the Extem method (Figure 1B: r = 0.89, p < 0.0001).
We observed similar results as CT for MCF. Using the Ex-tem, correlations between MCF and dabigatran concentrations were strong and linear (Figure 1C: r = -0.93, p < 0.0001). Correlations between dabigatran concentrations and MCF using In-tem were weaker (Figure 2C: r = -0.92, p < 0.0001). All results are summarized in the Figures 1–2 and Table 1.
Table 1.
Correlation Between Plasma Dabigatran Concentration Measured by Dilute Thrombin Time Assay and Selected Rotem Tests and aPTT.
| TESTS | Pearson’s correlation coefficient | ||
|---|---|---|---|
| all samples (n = 54) | samples with trough concentration (n = 27) | samples with peak concentration (n = 27) | |
| ROTEM Ex-tem CT (n = 54) | 0.92* | 0.89* | 0.94* |
| ROTEM Ex-tem CFT (n = 54) | 0.89* | 0.93* | 0.84* |
| ROTEM Ex-tem MCF (n = 54) | -0.93* | -0.85* | -0.9* |
| ROTEM In-tem CT (n = 54) | 0.84* | 0.74* | 0.9* |
| ROTEM In-tem CFT (n = 54) | 0.23 | 0.2 | 0.27 |
| ROTEM In-tem MCF (n = 54) | -0.92* | -0.84* | -0.94* |
| aPTT | 0.56 | 0.7 | 0.4 |
* p < 0.0001.
Correlations between dabigatran concentrations and ROTEM variables and aPTT are presented in Table 1. The strongest and linear correlation is in CT and MCF variables measured by Ex-tem or In-tem tests.
Discussion
ROTEM is a point-of-care test that has an established role in perioperative and trauma settings as it provides rapid assessment of hemostasis and can guide the clinician in acute situations complicated by bleeding.17 In a previous study ROTEM variable CT correlated to PT-INR in warfarin treated patients.18 In this study we demonstrate its potential utility to estimate dabigatran concentrations, which we believe would be of great value in emergency situations. The variable CT and MCF using the triggers Ex-tem and In-tem, correlates to a high degree with the plasma dabigatran concentrations (Table 1 & Figures 1-2). To the best of our knowledge, this is the first study in which ROTEM variables have been compared to dabigatran plasma concentrations as measured with hemoclot diluted thrombin time. Since ROTEM data are available within minutes from sampling, a ROTEM based approach to determine dabigatran plasma concentrations could prove to be of great clinical value. In contrast, the analyses available today to determine plasma dabigatran concentrations, such as LC-MS/MS, Hemoclot and ECT, take 30 min to several hours to complete. In addition, the standard coagulation assay aPTT, which also require up to 1 h until data can be obtained, have been shown to be unreliable in the determination of dabigatran concentrations (Table 1 & Figure 1D). In earlier studies, it was observed that CT was the most sensitive ROTEM variable in Ex-tem test to detect dabigatran treatment.11,19–27 We found in our research very strong association between dabigatran concentration and MCF. MCF represents the maximal amplitude of the tracing and it is possible that dabigatran might change the clot formation and therefore affect this parameter.
Limitation of the Study
There are several limitations to be acknowledged in the present study. The main limitations of our study include the low number of patients investigated, and with only 4 observation of very low plasma dabigatran concentrations (i.e. <30 ng/mL). ROTEM is greatly affected by preanalytical issues; thus, interpretation of hemostasis changes might be adversely influenced by prestudy factors (e.g. thrombocytopenia, anemia, low fibrinogen, platelet inhibitor…).
An expanded and more thorough validation of the method is necessary before this test could be used routinely in the clinic.
Conclusion
In summary, in our study, we have found that the ROTEM variable CT and MCF, when activated with triggers Ex-tem and In-tem, has a strong and highly significant correlation with the plasma dabigatran concentration in a real-life population of NVAF-patients and could thereby be an alternative to estimate dabigatran concentration in emergency situations. However, additional studies are needed to further validate these findings.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by grants VEGA 1/0187/17 and APVV-17-0054.
ORCID iD: Frantisek Nehaj  https://orcid.org/0000-0003-3070-5776
https://orcid.org/0000-0003-3070-5776
References
- 1. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. [DOI] [PubMed] [Google Scholar]
- 2. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342–2352. [DOI] [PubMed] [Google Scholar]
- 3. Slavik L, Jacova J, Friedecky D, et al. Evaluation of the DOAC-stop procedure by LC-MS/MS assays for determining the residual activity of dabigatran, Rivaroxaban, and Apixaban. Clin Appl Thromb Hemost. 2019;25:1076029619872556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost. 2009;15(Suppl 1):9S–16S. [DOI] [PubMed] [Google Scholar]
- 5. Skeppholm M, Hjemdahl P, Antovic JP, et al. On the monitoring of dabigatran treatment in “real life” patients with atrial fibrillation. Thromb. Res. 2014;134(4):783–789. [DOI] [PubMed] [Google Scholar]
- 6. Antovic JP, Skeppholm M, Eintrei J, et al. Evaluation of coagulation assays versus LC-MS/MS for determinations of dabigatran concentrations in plasma. Eur J Clin Pharmacol. 2013;69(11):1875–1881. [DOI] [PubMed] [Google Scholar]
- 7. Helin TA, Pakkanen A, Lassila R, Joutsi-Korhonen L. Laboratory assessment of novel oral anticoagulants: method suitability and variability between coagulation laboratories. Clin Chem. 2013;59(5):807–814. [DOI] [PubMed] [Google Scholar]
- 8. Luddington RJ. Thromboelastography/thromboelastometry. Clin Lab Haem. 2005;27(2):81–90. [DOI] [PubMed] [Google Scholar]
- 9. Nehaj F, Sokol J, Mokan M, Ivankova J, Mokan M. Thrombin receptor agonist peptide-induced platelet aggregation is reduced in patients receiving dabigatran. Clin Appl Thromb Hemost. 2018;24(2):268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sokol J, Nehaj F, Ivankova J, Mokan M, Mokan M, Stasko J. Dabigatran affects thrombin-dependent platelet aggregation after a week-long therapy. Scand Cardiovasc J. 2018;52(4):227–231. [DOI] [PubMed] [Google Scholar]
- 11. Taune V, Wallén H, Ågren A, et al. Whole blood coagulation assays ROTEM and T-TAS to monitor dabigatran treatment. Thromb Res. 2017;153:76–82. [DOI] [PubMed] [Google Scholar]
- 12. Seyve L, Richarme C, Polack B, Marlu R. Impact of four direct oral anticoagulants on rotational thromboelastometry (ROTEM). Int J Lab Hematol. 2018;40(1):84–93. [DOI] [PubMed] [Google Scholar]
- 13. Taune V, Skeppholm M, Ågren A, et al. Rapid determination of anticoagulating effects of dabigatran in whole blood with rotational thromboelastometry and a thrombin-based trigger. J Thromb Haemost. 2018;16(12):2462–2470. [DOI] [PubMed] [Google Scholar]
- 14. Kyriakou E, Ikonomidis I, Stylos D, et al. Laboratory assessment of the anticoagulant activity of dabigatran. Clin Appl Thromb Hemost. 2015;21(5):434–445. [DOI] [PubMed] [Google Scholar]
- 15. Takeshita S, Tanaka KA, Sawa T, Sanda M, Mizobe T, Ogawa S. Whole blood point-of-care testing for incomplete reversal with idarucizumab in supratherapeutic dabigatran. Anesth Analg. 2020;130(2):535–541. [DOI] [PubMed] [Google Scholar]
- 16. Tynngard N, Lindahl TL, Ramstrom S. Assays of different aspects of haemostasis—what do they measure? Thromb J. 2015;13:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wikkelso A, Wetterslev J, Moller AM, Afshari A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst Rev. 2016;2016(8):CD007871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmidt DE, Holmstrom M, Majeed A, Naslin D, Wallen H, Agren A. Detectionof elevated INR by thromboelastometry and thromboelastography in warfarin treated patients and healthy controls. Thromb Res. 2015;135(5):1007–1011. [DOI] [PubMed] [Google Scholar]
- 19. Herrmann R, Thom J, Wood A, Phillips M, Muhammad S, Baker R. Thrombin generation using the calibrated automated thrombinoscope to assess reversibility of dabigatran and rivaroxaban. Thromb Haemost. 2014;111(5):989–995. [DOI] [PubMed] [Google Scholar]
- 20. Eller T, Busse J, Dittrich M, et al. Dabigatran, rivaroxaban, apixaban, argatroban and fondaparinux and their effects on coagulation POC and platelet function tests. Clin Chem Lab Med. 2014;52(6):835–844. [DOI] [PubMed] [Google Scholar]
- 21. Dinkelaar J, Patiwael S, Harenberg J, Leyte A, Brinkman HJ. Global coagulation tests: their applicability for measuring direct factor Xa- and thrombin inhibition and reversal of anticoagulation by prothrombin complex concentrate. Clin Chem Lab Med. 2014;52(11):1615–1623. [DOI] [PubMed] [Google Scholar]
- 22. Grottke O, van Ryn J, Spronk HM, Rossaint R. Prothrombin complex concentrates and a specific antidote to dabigatran are effective ex-vivo in reversing the effects of dabigatran in an anticoagulation/liver trauma experimental model. Crit Care. 2014;18(1):R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Honickel M, Treutler S, van Ryn J, et al. Reversal of dabigatran anticoagulation ex vivo: porcine study comparing prothrombin complex concentrates and idarucizumab. Thromb. Haemost. 2015;113(4):728–740. [DOI] [PubMed] [Google Scholar]
- 24. Honickel M, Maron B, van Ryn J, et al. Therapy with activated prothrombin complex concentrate is effective in reducing dabigatran-associated blood loss in a porcine polytrauma model. Thromb. Haemost. 2016;115(2):271–2S84. [DOI] [PubMed] [Google Scholar]
- 25. Comuth WJ, Henriksen LØ, van de Kerkhof D, et al. Comprehensive characteristics of the anticoagulant activity of dabigatran in relation to its plasma concentration. Thromb Res. 2018;164:32–39. [DOI] [PubMed] [Google Scholar]
- 26. Henskens YMC, Gulpen AJW, van Oerle R, et al. Detecting clinically relevant rivaroxaban or dabigatran levels by routine coagulation tests or thromboelastography in a cohort of patients with atrial fibrillation. Thromb J. 2018;16:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vedovati MC, Mosconi MG, Isidori F, et al. Global thromboelastometry in patients receiving direct oral anticoagulants: the RO-DOA study. J Thromb Thrombolysis. 2020;49(2):251–258. [DOI] [PubMed] [Google Scholar]