Abstract
Background
Chronic rhinitis is a common condition generally treated with medical therapies. However, 10–22% of patients are refractory to medical therapies. A cryotherapy handheld device targeting the postganglionic nerve fibers of the posterior nasal nerve (PNN) now serves as an additional option for therapy. This study evaluates the efficacy of the cryosurgical ablation device of the PNN in the clinic setting.
Methods
This was a prospective single-arm trial of 24 adult patients at seven locations within a large health maintenance organization. Patients with chronic rhinitis that failed medical therapy were offered an in-office cryoablation of PNN. Patients completed the Total Nasal Symptom Score (TNSS) questionnaire consisting of 5 items reported based on the previous 12 hours and 2 weeks at the following time points: pre-treatment, 30 days, 90 days and 1 year post-treatment.
Results
Following cryoablation of the PNN, the TNSS 12-hour symptom score improved from 6.92 (±2.9) to 3.17 (±2.4, P < 0.001) at 30 days, 2.92 (±1.4, P < 0.001) at 90 days and 3.08 (±2.6, P < 0.001) at 1 year post treatment. Similar results were noted for the 2 weeks scores improving from 7.75 (±3.1) to 3.79 (±2.1, P < 0.001) at 30 days, 3.88 (±1.9, P < 0.001) at 90 days and 3.76 (±2.1, P < 0.001) at 1 year post-treatment. 64.7% of respondents stated the procedure decreased or eliminated nasal sprays.
Conclusions
Our independent evaluation of cryoablation of the PNN shows improvement in nasal symptoms over a 1 year period and is consistent with other published data.
Keywords: cryoablation, cryosurgery, cryosurgical ablation, posterior nasal nerve, rhinitis, rhinorrhea, congestion
Introduction
Chronic rhinitis is a common problem in the United States affecting roughly 60 million people.1,2 First line medical therapy typically includes saline irrigations, oral or topical antihistamines, nasal corticosteroid sprays, and anticholinergic nasal sprays. However, approximately 10–22% of these patients are refractory to medical treatment.3,4 Chronic rhinitis, much like chronic rhinosinusitis, is associated with significant reduction in quality of life measures.5–7
Surgical therapy for chronic rhinitis includes endoscopic vidian neurectomy and selective postganglionic pterygopalatine parasympathectomy. The goal of these surgeries is to disrupt the parasympathetic autonomic supply to the nasal mucosa thus resulting in decreased nasal drainage and congestion.8–11 Many patients are reluctant to pursue these surgical options as they are generally performed under general anesthesia and have potential risks including dry eye, soft palate hypoesthesia, oral and facial numbness, and postoperative bleeding among others.12–14
Cryotherapy or cryosurgery in medicine dates back to the early 1900s and is frequently used in other disciplines including dermatology, neurology, and urology. The mechanism of action in cryotherapy is believed to derive from the low temperature produced by liquid nitrogen. The formation of ice crystals induce cellular contraction and direct cell injury causing localized tissue damage.15,16
In 2017, a handheld, single patient-use, disposable cryosurgical device was approved for treatment of rhinitis (Stryker Corporation, ClariFixTM, Kalamazoo, MI, Manufacturer retail price $1,895 USD). Using the cryosurgical device, the postganglionic nerve fibers of the posterior nasal nerve (PNN) can be treated as an in-office procedure under local anesthesia for treatment of chronic rhinitis. To date, all outcome related literature of the device has been industry sponsored. The goal of this study was to perform an independent review of clinical symptomatic outcomes following cryosurgical ablation of the posterior nasal nerve.
Patients and Methods
This study was approved through the Southern California Kaiser Permanente Institutional Review Board. This was a prospective single-arm evaluation of 24 adult patients at seven locations within a large health maintenance organization (HMO) system. Inclusion criteria included age > 18, diagnosis of chronic rhinitis, and failure of medical therapy for a duration of at least 3 months. Patients were excluded from the evaluation for the following: active or chronic nasal/sinus infections, structural abnormalities restricting device from accessing the posterior middle meatus, cerebrospinal fluid leaks, rhinitis medicamentosa, confounding systemic conditions (ie granulomatosis with polyangiitis, Sjogren’s syndrome, cystic fibrosis, primary ciliary dyskinesia), active intranasal recreational drug use, recurrent history of epistaxis, coagulopathy, pregnancy, nasopharyngeal malignancy.
Patients that fulfilled the above criteria were offered an in-office cryosurgical ablation of the PNN under local anesthesia. Patients completed a variation of the Total Nasal Symptom Score (TNSS) questionnaire consisting of 5 items (rhinorrhea, nasal congestion, nasal itching, sneezing, difficulty sleeping due to nasal symptoms) reported based on the previous 12 hours and 2 weeks at the following time points: pre-treatment, 30 days, 90 days, and 1 year post-treatment. The procedure was performed in the clinic setting using endoscopic visualization and local anesthesia as previously described by Hwang et al.17
Patients were tracked for adverse outcomes immediately after the procedure and during the remainder of the evaluation period. The subjects followed up at 30 days, 90 days and 1 year post-procedure and were evaluated with TNSS (total nasal symptom score) questionnaire (Figure 1). The data was then centrally collated. Statistical analysis was performed using the paired t-test examining changes from baseline at the different time intervals. Unless otherwise specified data is expressed as means ± standard deviation.
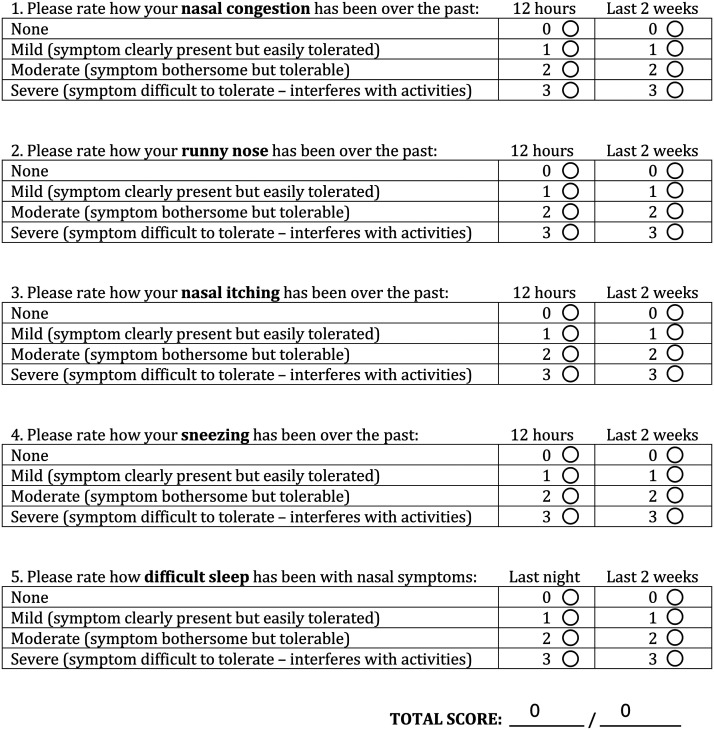
Figure 1.
TNSS questionnaire. Items evaluated include nasal congestion, runny nose, nasal itching, sneezing and difficulty sleeping throughout the past 12 hours and 2 weeks.
Results
Twenty-four patients were included in this study with equal distribution between males and females. Average age at the time of treatment was 60 years (range 25–91), and notably 63% of patients were age 70 or older. Patients evaluated were classified in three categories of rhinitis based on clinical symptomatology and regional radioallergosorbent panel: non-allergic (66.6%, n = 16, mean age 68 years), mixed (20%, n = 5, mean age 68 years) and allergic rhinitis (12.5%, n = 3, mean age 57 years). Previous medical therapy prior to the procedure included saline irrigations (20.8%), anticholinergic nasal sprays (54%), antihistamines (37.5%) and intranasal corticosteroids (58.3%) (Table 1). All patients were preoperatively evaluated by nasal endoscopy and completed their baseline TNSS questionnaire prior to the procedure. Medical therapy was continued, decreased or stopped based on post-cryoablation symptomatology. All procedures were well tolerated and able to be completed without major technical difficulties or device malfunction.
Table 1.
Demographics, Previous Treatment and Rhinitis Type.
| Total | N = 24 |
|---|---|
| Gender | |
| Male | 12 (50%) |
| Female | 12 (50%) |
| Age (Y) | |
| Mean | 60.04 |
| SD | 16.7 |
| Min | 25 |
| Max | 90 |
| Previous treatment | |
| Saline | 5 (20.8%) |
| Anticholinergic | 13 (54.1%) |
| Intranasal corticosteroid | 14 (58.3%) |
| Antihistamine (oral and systemic) | 9 (37.5%) |
| Rhinitis type | |
| Non-allergic | 16 (66.6%) |
| Subgroup mean age (Y) | 68 |
| Mixed | 5 (20.8%) |
| Subgroup mean age (Y) | 68 |
| Allergic | 3 (12.5%) |
| Subgroup mean age (Y) | 57 |
N: Number. Y: Years.
The TNSS questionnaire (Figure 1) asked patients to recall symptoms over the past 12 hours and 2 weeks at 4 sequential time points: prior to the procedure and following cryoablation of the PNNs at 30 days, 90 days and 1 year. Items evaluated included nasal congestion, runny nose, nasal itching, sneezing and difficulty sleeping due to nasal issues.
The total mean TNSS showed statistically significant improvement at all time points when compared to pre-procedure baseline (Figure 2). The mean 12-hour score improved from a baseline of 6.92 (±2.8) to 3.17 (±2.4, P < 0.001) at 30 days post-procedure, 2.92 (±1.4, P < 0.001) at 90 days and 3.08 (±2.6, P < 0.001) at 1 year. A similar trend was observed with the 2-week TNSS, improving from 7.75 (±3.1) at base line to 3.79 (±2.1, P < 0.001), 3.88 (±1.8, P < 0.001) and 3.76 (±2.1, P < 0.001) at 30, 90 days and 1 year post-treatment respectively.
Figure 2.
12 hour and 2-week mean TNSS score pre-procedure, 30 days, 90 days, and 1 year post-procedure. Error bars represent standard deviation, *represents statistical significance (P < 0.05).
Data was analyzed within each category of rhinitis (Figure 3). Non-allergic rhinitis showed the most prominent improvement with mean 12-hour TNSS improving from 7.1 (±3.1) to 3.0 (±2.0, P < 0.001) at 30 days, to 3.5 (±1.0 < 0.001) at 90 days and to 3.13 (±3.0 P < 0.001) at 1 year post treatment. The mean 2-week TNSS also demonstrated improvement from baseline (7.75 ± 3.6) at 30 days (4.21 ± 1.7; P < 0.001), 90 days (4.56 ± 1.7, P < 0.001), and 1 year (3.94 ± 2.4; P < 0.001) post-treatment.
Figure 3.
A, Non-allergic, mixed and allergic rhinitis 12 hour mean TNSS at baseline (pre-procedure), 30 days, 90 days, and 1 year post-procedure. B, Non-allergic, mixed and allergic rhinitis 2-week mean TNSS at baseline (pre-procedure), 30 days, 90 days, and 1 year post-procedure. Error bars represent standard deviation, *represents statistical significance (P < 0.05).
Most of the mixed rhinitis time points showed statistically significant improvement with exception of the 12-hour mean time point at 30 days and 1 year. The 12-hour TNSS improved from 6.4 (±2.1, baseline) to 4.0 (±3.6, P = 0.06), 2.0 (±1.2, P = 0.01), and 3.2 (±2.2, P = 0.06) at 30 days, 90 days and 1 year respectively. The 2-week TNSS decreased from 7.2 (±1.6) to 3.4 (±3.0, P = 0.003) at 30 days, 3.3 (±0.8, P = 0.01) at 90 days and 4.0 (±1.4, P = 0.04) at the 1 year time point.
The allergic subgroup demonstrated a trend towards improvement in all time points analyzed, nevertheless, these were not statistically significant due to a small sample size (n = 3) except for the 1 year time point of the 2-week TNSS. Pre-procedure 12-hour TNSS improved from a baseline of 6.67 (±3.2) to 2.67 (±2.5, P = 0.13) at 30 days, to 1.33 (±1.5, P = 0.09) at 90 days and to 2.6 (±0.6, P = 0.09) at 1 year. Mean 2-week TNSS improved from a baseline of 8.67 (±2.5) to 2.33 (±2.5, P = 0.08) at 30 days, to 1.67 (±2.0, P = 0.06) at 90 days and 3.3 (±1.1, P = 0.04) at 1 year post-treatment.
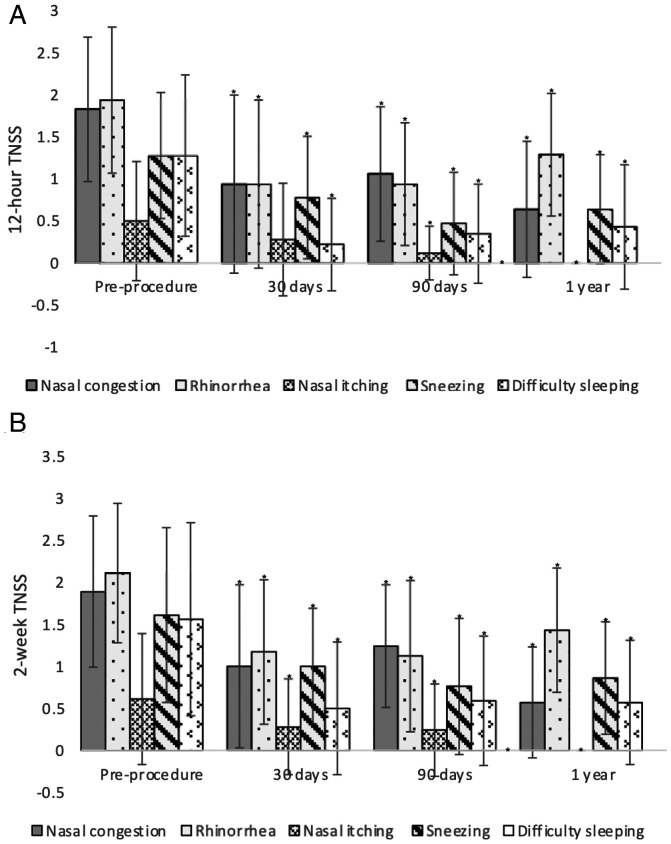
Marked decrease in TNSS was observed in all subdomain symptoms evaluated within the questionnaire (Figure 4). Nasal congestion improved from a baseline 12-hour TNSS mean of 1.83 (±0.9) to 0.94 (±1.0, P = 0.003) at 30 days post-procedure, 1.06 (±0.8, P = 0.02) at 90 days and to 0.64 (±0.8, P < 0.001) following cryosurgical ablation. The 2-week mean TNSS improved from a baseline of 1.89 (±0.9) to 1.0 (±0.9, P = 0.01) at 30 days, 1.24 (±0.7, P = 0.03) at 90 days and to 1.89 (±0.6, P < 0.001) at 1 year post treatment.
Figure 4.
A, 12 hour mean TNSS subdomain symptoms evaluated at baseline (pre-procedure), 30 days, 90 days, and 1 year post-procedure. B, 2-week mean TNSS subdomain symptoms evaluated at baseline (pre-procedure), 30 days, 90 days, and 1 year post-procedure. Error bars represent standard deviation, *represents statistical significance (P < 0.05).
A similar improvement was seen in rhinorrhea scores where the mean 12-hour TNSS score decreased from 1.94 (±0.8) to 0.94 (±1.0, P < 0.001) at 30 days post-procedure and was sustained at 0.94 (±0.7, P < 0.001) and 1.29 (±0.9, P = 0.03) at 90 days and 1 year respectively. The mean 2-week TNSS also decreased significantly from a baseline of 2.11 (±0.8) to 1.17 (±0.8, P < 0.001), 1.12 (±0.9, P < 0.001) and 1.43 (±0.7, P = 0.01) at 30 days, 90 days and 1 year respectively following cryosurgical ablation.
In the nasal itching subgroup, the mean 30 day 12-hour TNSS improvements did not reach statistical significance decreasing from a baseline of 0.5 (±0.7) to 0.28 (0.6, P = 0.15) at 30 days. Nevertheless, it did reach statistical significance at 90 days and 1 year, improving to 0.12 (±0.3, P = 0.01) and 0 (±0, P < 0.004) respectively. The 2-week nasal itching scores improved from a baseline of 0.61 (±0.7) to 0.28 (±0.5, P = 0.03), 0.24 (±0.5, P = 0.02) and 0 ((±0, P < 0.001) at 30, 90 days and 1 year respectively post-procedure. Of note, none of the evaluated patients reported nasal itching symptomatology at the 1 year time point.
The sneezing subdomain demonstrated a significant reduction in TNSS at all time points post-procedure. The 12-hour mean TNSS within this group improved from a baseline of 1.28 (±0.7) to 0.78 (±0.7, P = 0.01), 0.47 (±0.6, P < 0.001) and 0.64 (±0.6, P = 0.01) at 30 days, 90 days and 1 year respectively post-procedure. The 2-week mean TNSS improved from a pre-procedure baseline of 1.61 (±1.0) to 1.0 (±0.6, P = 0.02), 0.76 (±0.8, P = 0.004) and 0.86 (±0.6, P = 0.01) at 30 days, 90 days and 1 year respectively.
The difficulty sleeping subdomain also demonstrated a significant decrease at all time points post-procedure. The 12-hour TNSS mean decreased from 1.28 (±0.9) to 0.22 (±0.5, P < 0.001), 0.35 (±0.5 P = 0.003) and 0.43 (±0.7, P < 0.001) at 30 days 90 days and 1 year respectively post-procedure. The 2-week TNSS mean decreased from a baseline of 1.56 (±1.1) to 0.5 (±0.7, P = 0.004), 0.59 (±0.7, P = 0.003) and 0.57 (±0.7, P = 0.002) at 30 days, 90 days and 1 year respectively following the cryoablation procedure.
At the end of the trial, 66.7% of patients (12/18) had eliminated or reduced the use of medication to manage their rhinitis when compared to their preoperative baseline. When analyzing each rhinitis subgroup individually, 66.6% (8/12) of the non-allergic rhinitis, 100% (3/3) of the mixed rhinitis and 33.3% (1/3) of the allergic rhinitis patients decreased or eliminated the of medications. Postoperative treatment was tailored individually for every patient. 77.8% of patients (14/18) responded that they felt the procedure was effective and would do it again if needed. At 1 year, of 24 patients, six patients were lost to follow up.
Discussion
For many patients affected by chronic rhinitis, medical therapy has failed to alleviate their symptoms and improve their quality of life. In addition, some patients are unable to comply with consistent medical therapy or tolerate side effects of intranasal sprays including epistaxis and nasal irritation. In the past, patients’ options were limited to medical therapy or undergoing surgical procedures with variable side effects and outcomes. In our independent study, we find that cryoablation of the PNN appears to be a safe and effective in-office treatment of non-allergic, allergic, and mixed rhinitis. The TNSS questionnaire used in this study evaluated both patients’ recall of nasal symptoms over the past 12 hours as well as the past 2 weeks, which were well correlated as expected. Similar to previous studies, the largest improvements are seen in the rhinorrhea and nasal congestion subdomains though improvements in nasal sneezing, nasal itching, and sleep quality are also seen.3,17 Of particular note, this evaluation showed extended improvement in the subdomain of difficulty sleeping due to nasal issues which was not addressed in previous studies.
The exact pathophysiology of rhinitis is still not completely understood but postulated to be secondary to autonomic nervous system (ANS) dysfunction. Several small case control studies have consistently demonstrated objective evidence of ANS dysfunction in patients with both allergic and non-allergic rhinitis as compared to age matched controls.15,16 The target of in-office cryotherapy is the PNN which is a branch of the vidian nerve that carries parasympathetic innervation to the nasal mucosa.8,18 The device applies targeted and precise cooling of the mucosa to cause axonotmesis of the PNN without compromising blood supply of the mucosa. The technique results in a low post-procedural inflammatory reaction and faster healing of the nasal mucosa. Standardization of the ablation procedure with this device allows for consistent results even across multiple surgeons and multiple centers as seen in our study.
When comparing rhinitis subtype, the non-allergic subgroup showed the most prominent improvement in TNSS scores as well as reduction or elimination of medications post-procedure. The decreased improvement in the allergic rhinitis subgroup can possibly be explained by the differing pathophysiology. The pathophysiology of allergic rhinitis is characterized by the secretion of multiple proinflammatory mediators in response to an offending allergen (type I hypersensitivity reaction) clinically manifesting as nasal obstruction and rhinorrhea. These local mechanisms which are not addressed by cryoablation of the PNNs could possibly explain the lower response of allergic subtype patients.
In-office cryoablation of the PNN offers several advantages over selective postganglionic pterygopalatine parasympathectomy or vidian neurectomy. The cryoablation procedure was well tolerated by all treated patients who experienced only minimal discomfort during and post-procedure. Patients were able to return to work or their usual activities the following day without significant recovery time. No patients developed epistaxis, palate numbness or dry eye complications. The majority of the treated patients were older with several comorbidities and preferred not to undergo surgery due to risks of general anesthesia. Anecdotally, the procedure was successfully performed on two patients without stopping their anticoagulation medication. No cardiac events were reported with topical lidocaine application or injected lidocaine with epinephrine during the procedure.
Financially, our patients’ copay for an in-office procedure was significantly less than undergoing a surgical procedure in the operating room. In our closed health system, transferring procedures to the office setting also improves operating room access and decreases system costs. Two thirds of the patients also noted cessation or reduced use of prescription nasal sprays and allergy medications after treatment also decreasing overall system costs. However, for practitioners in other settings we acknowledge that some of these benefits are not applicable. Due to the novelty of this device and lack of a specific billing code, insurances may be reimbursing the office procedure at varying rates.
The ability to successfully perform in-office cryoablation of the PNN is limited by visualization and patient anatomy. Candidate selection is important. Patients with severe septal deviation or with significant turbinate hypertrophy in addition to rhinorrhea may benefit from a surgical approach to address these issues in addition to PNN resection. In addition, patients with primary symptoms of postnasal drip and throat irritation over rhinorrhea and nasal congestion should be considered for a trial of anti-reflux therapy prior to consideration of cryoablation of the PNN.
Our study from a multi-site community practice has several limitations. Primary limitations of the study include small sample size and short follow up time. However, this independent non-sponsored evaluation is consistent with prior sponsored studies showing sustained benefit post-procedure for up to a 1 year.3,17 The rhinitis type subset analysis is limited by the relatively few pure allergic rhinitis patients. Future studies will be needed to demonstrate longer term improvement and sustained results without use of additional medications.
Conclusion
Our independent non-sponsored evaluation of in-office cryoablation of the PNN is consistent with other published literature and shows improvement in multiple domains of patient symptom outcome scores after a 1 year period with no significant adverse events.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by our institutional review board.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Statement of Human and Animal Rights: The study adhered to good clinical practices and ethical standards.
Statement of Informed Consent: All the participants received verbal and/or written information and provided informed consent.
ORCID iD: Rohit Garg https://orcid.org/0000-0002-5672-1689
References
- 1.Settipane RA. Epidemiology of vasomotor rhinitis. World Allergy Organ J. 2009; 2(6):115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Settipane RA. Rhinitis: a dose of epidemiological reality. Allergy Asthma Proc. 2003; 24(3):147–154. [PubMed] [Google Scholar]
- 3.Chang MT, Song S, Hwang PH. Cryosurgical ablation for treatment of rhinitis: a prospective multicenter study. Laryngoscope. 2020; 130(8):1877–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lieberman P, Kaliner MA, Wheeler WJ. Open-label evaluation of azelastine nasal spray in patients with seasonal allergic rhinitis and nonallergic vasomotor rhinitis. Curr Med Res Opin. 2005; 21(4):611–618. doi:10.1185/030079905X41408 [DOI] [PubMed] [Google Scholar]
- 5.Alt JA, Sautter NB, Mace JC, Detwiller KY, Smith TL. Antisomnogenic cytokines, quality of life, and chronic rhinosinusitis: a pilot study: antisomnogenic cytokines and CRS. Laryngoscope. 2014; 124(4):E107–E114. doi:10.1002/lary.24412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alt JA, Smith TL, Mace JC, Soler ZM. Sleep quality and disease severity in patients with chronic rhinosinusitis: sleep quality and disease severity in patients with chronic rhinosinusitis. Laryngoscope. 2013; 123(10):2364–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meltzer EO. Allergic rhinitis: burden of illness, quality of life, comorbidities, and control. Immunol Allergy Clin North Am. 2016; 36(2):235–248. [DOI] [PubMed] [Google Scholar]
- 8.Bleier BS, Schlosser RJ. Endoscopic anatomy of the postganglionic pterygopalatine innervation of the posterolateral nasal mucosa. Int Forum Allergy Rhinol. 2011; 1(2):113–117. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi T, Hyodo M, Nakamura K, Komobuchi H, Honda N. Resection of peripheral branches of the posterior nasal nerve compared to conventional posterior neurectomy in severe allergic rhinitis. Auris Nasus Larynx. 2012; 39(6):593–596. [DOI] [PubMed] [Google Scholar]
- 10.Li S, Cheng J, Yang Jet al. Efficacy of posterior nasal neurectomy for allergic rhinitis combined with chronic rhinosinusitis with nasal polyps. Acta Otolaryngol (Stockh). 2019; 139(10):890–894. doi:10.1080/00016489.2019.1654132 [DOI] [PubMed] [Google Scholar]
- 11.Ma Y, Tan G, Zhao Z, Li W, Huang L, Liu G. Therapeutic effectiveness of endoscopic vidian neurectomy for the treatment of vasomotor rhinitis. Acta Otolaryngol (Stockh ) 2014; 134(3):260–267. [DOI] [PubMed] [Google Scholar]
- 12.Halderman A, Sindwani R. Surgical management of vasomotor rhinitis: a systematic review. Am J Rhinol Allergy. 2015; 29(2):128–134. [DOI] [PubMed] [Google Scholar]
- 13.Yan CH, Hwang PH. Surgical management of nonallergic rhinitis. Otolaryngol Clin North Am. 2018; 51(5):945–955. [DOI] [PubMed] [Google Scholar]
- 14.Marshak T, Yun WK, Hazout C, Sacks R, Harvey RJ. A systematic review of the evidence base for vidian neurectomy in managing rhinitis. J Laryngol Otol. 2016; 130 Suppl 4:S7–S28. [DOI] [PubMed] [Google Scholar]
- 15.Kompelli AR, Janz TA, Rowan NR, Nguyen SA, Soler ZM. Cryotherapy for the treatment of chronic rhinitis: a qualitative systematic review. Am J Rhinol Allergy. 2018; 32(6):491–501. [DOI] [PubMed] [Google Scholar]
- 16.Erinjeri JP, Clark TWI. Cryoablation: mechanism of action and devices. J Vasc Interv Radiol. 2010; 21(8):S187–S191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang PH, Lin B, Weiss R, Atkins J, Johnson J. Cryosurgical posterior nasal tissue ablation for the treatment of rhinitis: endoscopic cryosurgical treatment of rhinitis. Int Forum Allergy Rhinol. 2017; 7(10):952–956. doi:10.1002/alr.21991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahara D, Takeno S, Hamamoto T, Ishino T, Hirakawa K. Management of intractable nasal hyperreactivity by selective resection of posterior nasal nerve branches. Int J Otolaryngol. 2017; 2017:1907862. doi:10.1155/2017/1907862 [DOI] [PMC free article] [PubMed] [Google Scholar]