Abstract
Objective
To estimate the prevalence of objectively confirmed olfactory and gustatory dysfunction in US adults reporting chronic rhinosinusitis (CRS) symptoms in a nationally representative database.
Study Design
Cross-sectional epidemiologic analysis.
Setting
Data were analyzed from the smell and taste component of the 2013-2014 NHANES data set (National Health and Nutrition Examination Survey).
Methods
Individuals reporting the presence of ≥2 cardinal CRS symptoms (nasal blockage, sinus pain, discolored mucus, and dysosmia) were identified as patients with a potential diagnosis of CRS. Associations were examined between the presence of CRS symptoms and both self-reported and objectively measured smell and taste.
Results
One-third (33%) of adults who have ≥2 CRS symptoms report subjective olfactory impairment, though only 18% of these adults have quantifiable olfactory dysfunction on objective testing. Of these adults, 27% report subjective taste impairment, but just 17% have quantifiable gustatory dysfunction on objective testing. The presence of ≥2 CRS symptoms was not significantly associated with objective olfactory or gustatory dysfunction, although the individual symptoms of subjective dysosmia and discolored mucus were associated with objectively confirmed olfactory dysfunction.
Conclusion
The prevalence of objective olfactory and gustatory dysfunction was higher among adults reporting the presence of ≥2 CRS symptoms, but the differences were not statistically significant. Specific sinonasal symptoms, including discolored mucus and subjective smell dysfunction, were significantly associated with objective smell impairment.
Keywords: chronic rhinosinusitis, taste, olfaction, hyposmia, NHANES
Chronic rhinosinusitis (CRS) is a common condition estimated to affect between 2% and 14% of people in the United States.1-3 Olfactory dysfunction has long been considered one of the cardinal symptoms of CRS, and CRS is thought to be one of the leading causes of olfactory dysfunction.4,5
Impairment of smell has been shown to have direct and indirect effects on various aspects of health, including appetite, nutrition, and environmental safety.5,6 It can have negative effects on mood, social functioning, and overall quality of life.7,8 A thorough understanding of olfactory dysfunction as a symptom and a potential target for treatment is critical in the diagnosis and treatment of patients with CRS.
In addition to problems with smell, patients with CRS often report issues with taste, with studies showing a higher prevalence of gustatory dysfunction in patients with CRS as compared with the general population.9,10 However, the exact effect of CRS on taste is hard to distinguish, as the experiences of smell and taste are closely related.11 It is unclear if issues with taste in CRS are entirely secondary to abnormal olfaction. Quantitative investigations of gustatory function in CRS have been limited.
While olfactory dysfunction is acknowledged as one of the cardinal symptoms of CRS, the true prevalence of olfactory dysfunction among patients with CRS is unknown. Estimates in literature range from 30% to 83%, with wide variation in subjective versus objective smell impairment and the methods of objective assessment used.12-15 Additionally, the majority of studies represent patients seeking treatment in a clinical setting and potentially exclude a larger subset of community-dwelling patients with CRS who have milder or less symptomatic disease. To our knowledge, there has not been an epidemiologic study examining the prevalence of objective olfactory and gustatory dysfunction in CRS among the general US population. We hypothesize that the prevalence of chemosensory dysfunction among community dwelling adults with a potential diagnosis of CRS will differ from previously reported estimates.
The aim of this study was to estimate the prevalence of objectively measured olfactory dysfunction among community-dwelling adults in a nationally representative sample who report having ≥2 cardinal CRS symptoms. As a secondary aim, we sought to examine the prevalence of gustatory dysfunction within the same study sample.
Methods
Study Participants
The analytic cohort was composed of 3519 adults participating in the 2013-2014 National Health and Nutrition Examination Survey (NHANES) who had complete data on smell and taste function. NHANES is a database collected by the Centers for Disease Control and Prevention to assess the nutritional and health status of the noninstitutionalized civilian population in the United States. Each cross-sectional study cycle utilizes a stratified multistage probability sampling design. Analysis accounting for the complex survey design yields results that are generalizable to the US population. The study was deemed exempt from approval by the Keck School of Medicine of the University of Southern California Institutional Review Board as the data had been deidentified and are publicly available.
NHANES Smell and Taste Protocol
Smell and taste functions were assessed by objective tests and self-report for the first and only time in the 2013-2014 NHANES cycle. The NHANES Chemosensory Protocol was created in collaboration among researchers, clinicians, and epidemiologist in chemosensation, designed to generate population-based estimates of chemosensory function among US adults aged ≥40 years.16 The protocol was adapted from previously published chemosensory assessment tools and content validated by a national team of chemosensory experts with good to excellent test-retest reliability.17
Smell Assessments
Smell function was assessed via objective test and self-report. Objective smell impairment was defined by the NHANES Pocket Smell Test (score range, 0-8; Sensonics International). Participants were asked to identify 8 odorants: smoke, leather, grape, soap, natural gas, onion, strawberry, and chocolate. Participants scoring ≤5 were categorized as having objective smell impairment as previously defined.16 Subjective smell impairment was defined as reporting a problem with the ability to smell during the past 12 months, worse sense of smell since age of 25 years, or phantosmia as previously defined.16
Taste Assessments
Taste function was assessed via objective test and self-report. Objective taste examination included tongue-tip and whole-mouth taste tests.16 Participants were presented with 2 tastes (1mM quinine as a bitter taste and 1M NaCl as a salt taste) and asked to identify from choices including salty, bitter, sour, some other taste, or no taste. Mouth was rinsed with tap water between all taste examinations. Objective taste impairment was defined as not being able to identify quinine or NaCl from tongue-tip and whole-mouth taste tests as previously defined. Subjective taste impairment was defined as reporting yes to the question “During the past 12 months, have you had a problem with your ability to taste sweet, sour, salty or bitter foods and drinks?”16
Sinonasal Symptoms
Participants were asked about a series of sinonasal symptoms as part of the smell and taste questionnaire.16 Following the question “Do you now have any of the following problems with your nose?” participants marked whether they had “completely blocked up nose” (nasal blockage), “green, yellow or brown mucus discharge” (discolored nasal mucus), and/or “sinus pain.” Participants were also asked whether they had a problem with smell in the past 12 months (dysosmia). Similar to Bhattacharyya et al, we identified adults with a potential diagnosis of CRS based on symptomology from the CRS guidelines, specifically respondents reporting ≥2 of the following symptoms: nasal blockage, discolored nasal mucus, sinus pain, and dysosmia.1
Other Study Measures
Demographic factors included in the NHANES included age, gender, race (White, Black, or other), education (high school graduate or less, some college or more), income (<$45,000, ≥$45,000). Medical history included hypertension, stroke, diabetes mellitus, smoking status, heavy alcohol use, and cardiovascular disease (myocardial infarction, congestive heart failure, angina pectoris, or coronary artery disease). Categories for demographic factors were collapsed a priori to account for low counts among participants with ≥2 CRS symptoms. Other olfaction-related medical history included ever having a broken nose or other serious injury to face or skull. The Patient Health Questionnaire–9 (score range, 0-27) was used to assess depressive disorders. Those who answered “more than half the day” or “nearly every day” and had a score ≥10 were categorized as having major depressive disorder.18
Statistical Analysis
Baseline characteristics of the study participants were compared with the Student t test and Pearson chi-square test. The prevalence of smell and taste impairment was assessed while accounting for the complex sampling design according to the NHANES analytic guidelines.19 The association between smell/taste impairment and sinonasal symptoms was investigated with logistic regression models. Multivariate models were sequentially adjusted for demographics and medical comorbidities. P < .05 was considered significant based on 2-tailed t test. STATA version 16.0 (StataCorp) was used for all analyses.
Results
A cohort of 3519 adults from the 2013-2014 NHANES database who had complete data on smell and taste function were included in this study. All demographic variables and comorbidities are summarized in Table 1 . Using the complex sampling design according to the NHANES analytic guidelines, we estimate that 3.0% (95% CI, 2.2%-3.9%) of US adults aged ≥40 years have ≥2 CRS symptoms, with 7.7% (95% CI, 6.2%-9.5%) having nasal blockage, 3.3% (95% CI, 2.4%-4.5%) sinus pain, 1.9% (95% CI, 1.6%-2.1%) discolored mucus, and 8.0% (95% CI, 6.4%-9.8%) subjective smell impairment.
Table 1.
Unweighted Sample Characteristics of Participants.
| ≥2 CRS symptoms, No. (%) | ||||
|---|---|---|---|---|
| Characteristic | Overall (N = 3519) | Yes (n = 106) | No (n = 3416) | P value |
| Age, y, mean ± SD | 59.0 ± 12.0 | 59.1 ± 11.7 | 59.0 ± 12.1 | .921 |
| Sex: female a | 1840 (52.3) | 61 (57.6) | 1779 (52.1) | .271 |
| Race/ethnicity | .354 | |||
| White | 1564 (44.4) | 54 (50.9) | 1510 (44.2) | |
| Black | 727 (20.7) | 21 (19.8) | 706 (20.7) | |
| Other | 1228 (34.9) | 31 (29.3) | 1197 (35.1) | |
| Education | .905 | |||
| High school graduate or less | 1604 (45.6) | 50 (47.2) | 1554 (45.3) | |
| Some college or more | 1912 (54.3) | 56 (52.8) | 1856 (54.4) | |
| Income, $ | .126 | |||
| <45,000 | 1623 (46.1) | 58 (54.7) | 1565 (45.9) | |
| ≥45,000 | 1602 (45.5) | 38 (35.6) | 1564 (45.8) | |
| Unknown | 294 (8.4) | 10 (9.4) | 282 (8.3) | |
| Hypertension a | 1637 (46.6) | 65 (61.3) | 1572 (46.1) | .002 |
| Cardiovascular disease a,b | 411 (11.7) | 19 (17.9) | 392 (11.5) | .042 |
| Diabetes a | 673 (19.1) | 30 (28.3) | 643 (18.8) | .015 |
| Stroke a | 174 (5.0) | 7 (6.6) | 167 (4.9) | .426 |
| Smoking | .006 | |||
| Never | 1878 (53.4) | 44 (41.5) | 1834 (53.8) | |
| Former | 999 (28.4) | 31 (29.3) | 968 (28.4) | |
| Current | 641 (18.2) | 31 (29.3) | 610 (17.9) | |
| Heavy alcohol use | 517 (18.7) | 33 (37.5) | 484 (18.1) | <.001 |
| History of head injury | 490 (14.0) | 32 (30.5) | 458 (13.4) | <.001 |
| Nasal fracture | 503 (14.3) | 30 (28.3) | 473 (13.9) | <.001 |
| Major depressive disorder | 257 (7.9) | 21 (20.4) | 236 (7.5) | <.001 |
Abbreviation: CRS, chronic rhinosinusitis.
Binary variable.
Cardiovascular disease includes any history of congestive heart failure, coronary artery disease, angina pectoris, or myocardial infarction.
Olfactory Function
We estimate that 67% (95% CI, 54%-77%) of adults meeting with ≥2 CRS symptoms report subjective impairment in smell, while 18% (95% CI, 11%-28%) have quantifiable olfactory dysfunction as measured by the NHANES Pocket Smell Test (score ≤5), with 13.0% (95% CI, 6.5%-24.5%) having mild hyposmia (score, 4 or 5) and 5.2% (95% CI, 2.5%-10.5%) having severe hyposmia/anosmia (score ≤3; Figure 1 ). The presence of ≥2 CRS symptoms was significantly associated with subjective smell impairment (odds ratio [OR], 6.80; 95% CI, 3.88-11.89) but not with objectively measured olfactory dysfunction in the univariate and multivariate models ( Table 2 ). When the association of objective smell impairment with individual CRS symptoms was analyzed, self-reported subjective smell impairment (OR, 4.26; 95% CI, 2.86-6.35) and discolored mucus (OR, 2.13; 95% CI, 1.01-4.46) were significantly associated with objectively measured olfactory dysfunction. Nasal blockage and sinus pain were not significantly associated with objective smell impairment ( Table 3 ). The presence of more sinonasal symptoms was associated with lower scores on objective olfactory testing (OR, −0.24; 95% CI, −0.37 to −0.11; Supplemental Table S1, available online).
Figure 1.
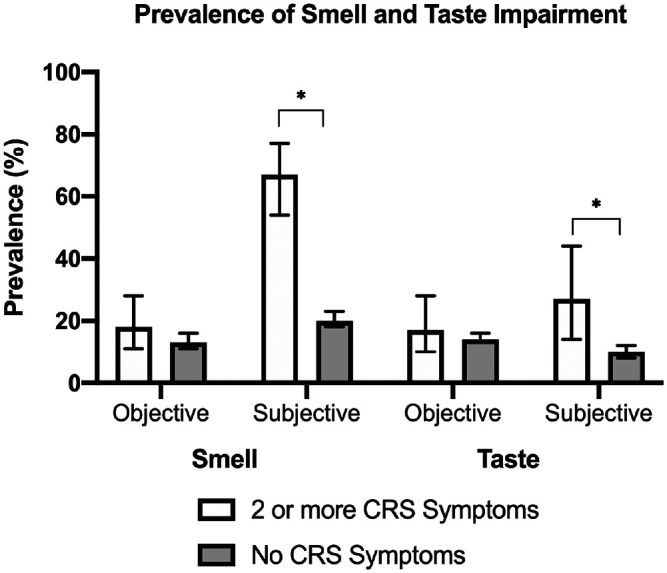
Estimated prevalence of smell and taste impairment among adults with ≥2 chronic rhinosinusitis (CRS) symptoms. Error bars indicate 95% CI. *P < .05.
Table 2.
Stepwise Multivariate Logistic Regression of the Association Between ≥2 CRS Symptoms and Smell/Taste Impairment.
| Smell impairment | Taste impairment | |||||||
|---|---|---|---|---|---|---|---|---|
| Objectively measured | Subjectively measured | Objectively measured | Subjectively measured | |||||
| Logistic regression models | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value |
| ≥2 CRS symptoms | ||||||||
| Base | 1.44 (0.82-2.52) | .186 | 7.91a (4.82-12.96) | <.001 | 1.25 (0.63-2.48) | .494 | 3.24a (1.49-7.03) | .006 |
| Base + demographics | 1.55 (0.98-2.46) | .062 | 7.87a (4.67-13.26) | <.001 | 1.20 (0.59-2.44) | .588 | 3.08a (1.46-6.47) | .006 |
| Base + demographics + medical comorbidities | 1.53 (0.95-2.46) | .075 | 6.80a (3.88-11.89) | <.001 | 1.20 (0.59-2.43) | .599 | 2.38b (1.07-5.29) | .035 |
Abbreviations: CRS, chronic rhinosinusitis; OR, odds ratio.
P < .01.
P < .05.
Table 3.
Stepwise Multivariate Logistic Regression of an Association Between Individual CRS Symptoms and Objectively Measured Smell and Taste Impairment.
| Objectively measured impairment (binary) | ||||
|---|---|---|---|---|
| Smell | Taste | |||
| Logistic regression models | OR (95% CI) | P value | OR (95% CI) | P value |
| Nasal blockage | ||||
| Base | 0.82 (0.53-1.27) | .343 | 1.03 (0.66-1.60) | .892 |
| Base + demographics | 0.81 (0.54-1.24) | .311 | 1.00 (0.64-1.56) | .996 |
| Base + demographics + medical comorbidities | 0.81 (0.52-1.27) | .339 | 0.98 (0.61-1.55) | .912 |
| Sinus pain | ||||
| Base | 1.12 (0.56-2.23) | .735 | 0.21a (0.06-0.67) | .012 |
| Base + demographics | 1.27 (0.68-2.39) | .426 | 0.21a (0.06-0.67) | .012 |
| Base + demographics + medical comorbidities | 1.32 (0.69-2.56) | .377 | 0.23a (0.07-0.73) | .016 |
| Discolored mucus | ||||
| Base | 2.08a (1.18-3.65) | .015 | 1.04 (0.43-2.54) | .918 |
| Base + demographics | 2.11a (1.09-4.07) | .029 | 1.06 (0.44-2.56) | .890 |
| Base + demographics + medical comorbidities | 2.13a (1.01-4.46) | .047 | 1.13 (0.46-2.80) | .776 |
| Dysosmia | ||||
| Base | 3.71b (2.47-5.56) | <.001 | 1.31 (0.88-1.96) | .173 |
| Base + demographics | 4.02b (2.77-5.84) | <.001 | 1.33 (0.89-1.99) | .152 |
| Base + demographics + medical comorbidities | 4.26b (2.86-6.35) | <.001 | 1.38 (0.87-2.20) | .159 |
Abbreviations: CRS, chronic rhinosinusitis; OR, odds ratio.
P < .05.
P < .01.
Gustatory Function
Among those with ≥2 CRS symptoms, 27% (95% CI, 14%-44%) reported subjective impairment in taste function, though only 17% (95% CI, 10%-18%) had quantifiable taste dysfunction on objective testing ( Figure 1 ). In multivariate models accounting for demographics and medical comorbidities, the presence of ≥2 cardinal CRS symptoms was significantly associated with self-reported subjective taste impairment (OR, 2.38; 95% CI, 1.07-5.29) but not with objectively measured taste impairment (OR, 1.20; 95% CI, 0.59-2.43; Table 2 ). When the association between objective taste impairment and individual CRS symptoms was analyzed, sinus pain was significantly associated with a decreased risk of objective taste impairment (OR, 0.23; 95% CI, 0.07-0.73). Nasal blockage, discolored mucus, and dysosmia were not significantly associated with objective taste impairment ( Table 3 ). The presence of more sinonasal symptoms was not significantly associated with objective taste impairment (Supplemental Table S1, available online).
Olfactory and Gustatory Function
In this cohort, objective smell impairment was significantly associated with subjective taste impairment (OR, 2.18; 95% CI, 1.65-2.89), but there was no association between objective smell and objective taste impairment (OR, 1.26; 95% CI, 0.86-1.87).
Discussion
In this study, we estimate that 3% of the general US population aged ≥40 years have ≥2 CRS symptoms at any given time and carry a potential diagnosis of CRS based on symptomology. Among those adults, 67% report subjective olfactory impairment, while 18% have objectively confirmed olfactory impairment (5%, anosmia or severe hyposmia; 13%, mild hyposmia). Similarly, 27% of these adults reported subjective impairment in taste function, but only 17% had objectively confirmed impairments in taste. The presence of ≥2 CRS symptoms was associated with subjective smell impairment, though there was no association with objective smell or taste impairment. Discolored mucus alone was associated with objective smell impairment, while sinus pain was associated with a decreased risk of objective taste impairment. Moreover, patients who had more sinonasal symptoms had greater odds of objective olfactory dysfunction but not objective taste impairment. To our knowledge, this is the first epidemiologic study of quantifiable olfactory and gustatory dysfunction in a nationally representative sample of US adults who carry a potential diagnosis of CRS based on sinonasal symptoms.
These results suggest that the prevalence of olfactory dysfunction among community-dwelling adults in the United States with a potential diagnosis of CRS may be lower than previously published data among cohorts of patients with CRS.14,20,21 A recent meta-analysis estimated that 30% to 78% of patients with CRS had objective olfactory dysfunction, depending on the type of olfaction testing used.12 A key difference in the studies in the meta-analysis is that their cohorts comprised patients with a confirmed diagnosis of CRS who were specifically seeking treatment for their CRS symptoms, thereby possibly representing a subset of patients with CRS who had more severe symptomatic disease. The true prevalence of objectively confirmed CRS among the general US adult population is unclear, with a wide range of estimates in previous epidemiologic studies due to different methods of identifying patients with CRS within national databases.1,22-24 The population of adults reporting symptoms consistent with a potential diagnosis of CRS in this study may represent a population of potentially undiagnosed patients with CRS within the community. It is possible that the prevalence of objective olfactory dysfunction among community-dwelling adults with CRS is lower than in those presenting to a clinical setting for treatment. Additionally, it is possible that olfactory dysfunction may be a key driver in motivating patients with CRS to seek treatment.
We found that subjective reports of smell impairment are significantly associated with quantifiable olfactory dysfunction on objective testing, though prior studies examining the correlation between subjective olfactory dysfunction and objective assessments have been mixed. Many studies examining olfactory complaints in the general population have showed poor correlation between subjective complaints and objective measures,25-28 though others show better correlation in patients with more severe olfactory loss.29,30 Studies among patients seen within the setting of an otolaryngology clinic have similarly reported mixed reliability in self-assessment of olfactory function.31-34 These variable results may be due to the fluctuating nature of smell and inflammation in sinonasal disease. These data suggest that while self-assessed screenings of olfactory functions can be useful in evaluation of patients, self-screening alone cannot reliably identify patients with true olfactory loss.
This study found a significantly higher prevalence of subjective taste impairment among patients with ≥2 symptoms of CRS, although there was no significant difference in the prevalence of objectively measured taste impairment. Specific investigations into the effects of CRS on taste function have been limited, though some studies have shown an increased prevalence of subjective complaints and objectively measured taste dysfunction. However, these studies report poor correlation between perceived taste dysfunction and objective measures of taste or smell.9,10 It is not clear if the observed complaints regarding gustatory function in patients with CRS is entirely secondary to olfactory changes or if CRS exhibits independent effects on gustatory function. However, our study found no associations between the presence of ≥2 CRS symptoms and objectively confirmed taste dysfunction, suggesting that sinonasal symptoms affect only the subjective experience of taste. Further investigation is needed into the effects of CRS on quantifiable taste dysfunction.
A strength of this study is that it uses data from a nationally representative sample of the general adult population in the United States with symptoms compatible with a potential diagnosis of CRS. The majority of literature regarding CRS uses data collected on care-seeking patients. However, it is likely that there is a proportion of adults in the community with a potential diagnosis of CRS who have not yet sought care for their disease. Using a nationally representative sample of adults in the United States allows the ability to potentially capture a subset of patients with CRS symptoms who have not yet been formally diagnosed and would not be available in studies of patients in ambulatory care. The NHANES database used in this study utilizes a weighted analysis of a large representative national sample, increasing the generalizability of the findings to noninstitutionalized US adults aged ≥40 years. This epidemiologic analysis may present a more generalizable estimate of olfactory dysfunction among community-dwelling adults with sinonasal symptoms and utilizes objective assessments of olfactory and gustatory function, which had not been reported in previous epidemiologic studies.35
The major limitation of this study is that this cohort with ≥2 CRS symptoms lacked objective confirmation of a CRS diagnosis (eg, imaging or endoscopy). Admittedly, the symptoms of CRS can overlap with other respiratory disorders, and the presence of such symptoms does not always translate into a formal diagnosis of CRS with endoscopy or imaging. The cohort in this study reflects a population of US adults who carry a potential diagnosis of CRS based on sinonasal symptomology and may include individuals who may not meet objective criteria for a diagnosis of CRS. Similarly, because these data lack objective examination findings, we cannot account for objective examination findings that have been shown to affect olfaction and taste.36,37 For example, a prior, population-based study among adults from Sweden showed a 2.1-times increased risk of olfactory dysfunction in those with nasal polyps.27 Furthermore, there may be other potentially confounding variables that were not accounted for in this analysis, such as history of other respiratory and nonrespiratory disorders that may affect chemosensation (eg, allergic disease, neurologic headache, medication side effects).21,38 Also, this database was limited to adults aged ≥40 years, potentially excluding a large subset of younger patients with CRS. Other large-scale epidemiologic studies have reported a high prevalence of sinusitis in adults aged <40 years.2,24 For instance, a large-scale epidemiologic study examining the prevalence of CRS in a Canadian population found that CRS was most prevalent among adults in their 30s.24 It is well known that age is an independent risk factor for worse olfactory function, and other studies of olfaction in CRS have reported associations with age and poorer smell.12,14,27,39,40 It is possible that the older population represented in this study may overestimate the prevalence of olfactory dysfunction.
Conclusion
Despite these limitations, we present an epidemiologic analysis of quantifiable chemosensory testing among those in the general US population with a potential diagnosis of CRS based on sinonasal symptomology. As compared with the general US adult population, the prevalence of objective olfactory and gustatory dysfunction was higher among adults with ≥2 CRS symptoms, though the differences were not statistically significant. Specific sinonasal symptoms, including discolored mucus and subjective smell dysfunction, were significantly associated with objective smell impairment.
Author Contributions
James H. Kim, study design, data collection and analysis, manuscript preparation; Janet Choi, study design, data collection and analysis, manuscript preparation; Sophie S. Jang, data collection, analysis, manuscript preparation; Bozena B. Wrobel, study design, manuscript preparation; Elisabeth H. Ference, study design, data analysis, manuscript preparation.
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: None.
Supplemental Material
Supplemental material, sj-docx-1-opn-10.1177_2473974X20986756 for Smell and Taste Impairment in a Nationwide Sample of US Adults With Chronic Rhinosinusitis Symptoms by James H. Kim, Janet Choi, Sophie S. Jang, Bozena B. Wrobel and Elisabeth H. Ference in OTO Open: The Official Open Access Journal of the American Academy of Otolaryngology-Head and Neck Surgery Foundation
Footnotes
Supplemental Material: Additional supporting information is available at http://journals.sagepub.com/doi/suppl/10.1177/2473974X20986756
References
- 1. Bhattacharyya N, Gilani S. Prevalence of potential adult chronic rhinosinusitis symptoms in the United States. Otolaryngol Head Neck Surg. 2018;159(3):522-525. doi:10.1177/019459981 8774006 [DOI] [PubMed] [Google Scholar]
- 2. Pleis JR, Lethbridge-Cejku M. Summary health statistics for US adults: national health interview survey, 2006. Vital Health Stat 10 2007;(235):1-153. [PubMed] [Google Scholar]
- 3. Benninger M, Ferguson B, Hadley J. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129(3):S1-S32. doi: 10.1016/S0194-5998(03)01397-4 [DOI] [PubMed] [Google Scholar]
- 4. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis executive summary. Otolaryngol Head Neck Surg. 2015;152(4):598-609. doi: 10.1177/0194599815574247 [DOI] [PubMed] [Google Scholar]
- 5. Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life—an updated review. Chemical Senses. 2014;39(3):185-194. doi: 10.1093/chemse/bjt072 [DOI] [PubMed] [Google Scholar]
- 6. Pence TS, Reiter ER, DiNardo LJ, Costanzo RM. Risk factors for hazardous events in olfactory-impaired patients. JAMA Otolaryngol Head Neck Surg. 2014;140(10):951. doi: 10.1001/jamaoto.2014.1675 [DOI] [PubMed] [Google Scholar]
- 7. Neuland C, Bitter T, Marschner H, Gudziol H, Guntinas-Lichius O. Health-related and specific olfaction-related quality of life in patients with chronic functional anosmia or severe hyposmia. Laryngoscope. 2011;121(4):867-872. doi: 10.1002/lary.21387 [DOI] [PubMed] [Google Scholar]
- 8. Brämerson A, Nordin S, Bende M. Clinical experience with patients with olfactory complaints, and their quality of life. Acta Otolaryngol. 2007;127(2):167-174. doi:10.1080/0001648 0600801357 [DOI] [PubMed] [Google Scholar]
- 9. Othieno F, Schlosser RJ, Rowan NR, et al. Taste impairment in chronic rhinosinusitis: taste impairment in CRS. Int Forum Allergy Rhinol. 2018;8(7):783-789. doi: 10.1002/alr.22113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wolf A, Renner B, Tomazic PV, Mueller CA. Gustatory function in patients with chronic rhinosinusitis. Ann Otol Rhinol Laryngol. 2018;127(4):229-234. doi: 10.1177/0003489418754583 [DOI] [PubMed] [Google Scholar]
- 11. Small DM, Prescott J. Odor/taste integration and the perception of flavor. Exp Brain Res. 2005;166(3-4):345-357. doi: 10.1007/s00221-005-2376-9 [DOI] [PubMed] [Google Scholar]
- 12. Kohli P, Naik AN, Harruff EE, Nguyen SA, Schlosser RJ, Soler ZM. The prevalence of olfactory dysfunction in chronic rhinosinusitis: olfactory dysfunction in chronic sinusitis. Laryngoscope. 2017;127(2):309-320. doi: 10.1002/lary.26316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haxel BR. Recovery of olfaction after sinus surgery for chronic rhinosinusitis: a review. Laryngoscope. 2019;129(5):1053-1059. doi: 10.1002/lary.27764 [DOI] [PubMed] [Google Scholar]
- 14. Litvack JR, Fong K, Mace J, James KE, Smith TL. Predictors of olfactory dysfunction in patients with chronic rhinosinusitis. Laryngoscope. 2008;118(12):2225-2230. doi: 10.1097/MLG.0b013e318184e216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soler ZM, Mace J, Smith TL. Symptom-based presentation of chronic rhinosinusitis and symptom-specific outcomes after endoscopic sinus surgery. Am J Rhinol. 2008;22(3):297-301. doi: 10.2500/ajr.2008.22.3172 [DOI] [PubMed] [Google Scholar]
- 16. Hoffman HJ, Rawal S, Li C-M, Duffy VB. New chemosensory component in the US National Health and Nutrition Examination Survey (NHANES): first-year results for measured olfactory dysfunction. Rev Endocr Metab Disord. 2016;17(2):221-240. doi: 10.1007/s11154-016-9364-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rawal S, Hoffman HJ, Honda M, Huedo-Medina TB, Duffy VB. The Taste and Smell Protocol in the 2011-2014 US National Health and Nutrition Examination Survey (NHANES): test-retest reliability and validity testing. Chem Percept. 2015;8(3):138-148. doi: 10.1007/s12078-015-9194-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. US Centers for Disease Control and Prevention. Specifying weighting parameters. Published 2013. https://www.cdc.gov/nchs/tutorials/nhanes/SurveyDesign/Weighting/intro.htm
- 20. Klimek L, Hummel T, Moll B, Kobal G, Mann WJ. Lateralized and bilateral olfactory function in patients with chronic sinusitis compared with healthy control subjects. Laryngoscope. 1998;108(1):111-114. doi: 10.1097/00005537-199801000-00021 [DOI] [PubMed] [Google Scholar]
- 21. Whitcroft KL, Cuevas M, Andrews P, Hummel T. Monitoring olfactory function in chronic rhinosinusitis and the effect of disease duration on outcome: monitoring olfaction in CRS. Int Forum Allergy Rhinol. 2018;8(7):769-776. doi: 10.1002/alr.22104 [DOI] [PubMed] [Google Scholar]
- 22. Min YG, Jung HW, Kim HS, Park SK, Yoo KY. Prevalence and risk factors of chronic sinusitis in Korea: results of a nationwide survey. Eur Arch Otorhinolaryngol. 1996;253(7):435-439. doi: 10.1007/bf00168498 [DOI] [PubMed] [Google Scholar]
- 23. Hastan D, Fokkens WJ, Bachert C, et al. Chronic rhinosinusitis in Europe—an underestimated disease. A GA2LEN study: chronic rhinosinusitis in Europe. Allergy. 2011;66(9):1216-1223. doi: 10.1111/j.1398-9995.2011.02646.x [DOI] [PubMed] [Google Scholar]
- 24. Chen Y, Dales R, Lin M. The epidemiology of chronic rhinosinusitis in Canadians. Laryngoscope. 2003;113(7):1199-1205. doi: 10.1097/00005537-200307000-00016 [DOI] [PubMed] [Google Scholar]
- 25. Philpott C, Wolstenholme C, Goodenough P, Clark A, Murty G. Comparison of subjective perception with objective measurement of olfaction. Otolaryngol Head Neck Surg. 2006;134(3):488-490. doi: 10.1016/j.otohns.2005.10.041 [DOI] [PubMed] [Google Scholar]
- 26. Landis BN. Ratings of overall olfactory function. Chem Senses. 2003;28(8):691-694. doi: 10.1093/chemse/bjg061 [DOI] [PubMed] [Google Scholar]
- 27. Bramerson A, Johansson L, Ek L, Nordin S, Bende M. Prevalence of olfactory dysfunction: the Skovde Population-Based Study. Laryngoscope. 2004;114(4):5. [DOI] [PubMed] [Google Scholar]
- 28. Knaapila A, Tuorila H, Kyvik KO, et al. Self-ratings of olfactory function reflect odor annoyance rather than olfactory acuity. Laryngoscope. 2008;118(12):2212-2217. doi: 10.1097/MLG.0b013e3181826e43 [DOI] [PubMed] [Google Scholar]
- 29. Rawal S, Hoffman HJ, Chapo AK, Duffy VB. Sensitivity and specificity of self-reported olfactory function in a home-based study of independent-living, healthy older women. Chem Percept. 2014;7(3-4):108-116. doi: 10.1007/s12078-014-9170-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Welge-Luessen A, Hummel T, Stojan T, Wolfensberger M. What is the correlation between ratings and measures of olfactory function in patients with olfactory loss? Am J Rhinol. 2005;19(6):567-571. [PubMed] [Google Scholar]
- 31. Haxel BR, Bertz-Duffy S, Fruth K, Letzel S, Mann WJ, Muttray A. Comparison of subjective olfaction ratings in patients with and without olfactory disorders. J Laryngol Otol. 2012;126(7):692-697. doi: 10.1017/S002221511200076X [DOI] [PubMed] [Google Scholar]
- 32. Nguyen DT, Nguyen-Thi P-L, Jankowski R. How does measured olfactory function correlate with self-ratings of the sense of smell in patients with nasal polyposis? Laryngoscope. 2012;122(5):947-952. doi: 10.1002/lary.23219 [DOI] [PubMed] [Google Scholar]
- 33. Delank KW, Stoll W. Olfactory function after functional endoscopic sinus surgery for chronic sinusitis. Rhinology. 1998;36(1):15-19. [PubMed] [Google Scholar]
- 34. Philpott CM, Rimal D, Tassone P, Prinsley PR, Premachandra DJ. A study of olfactory testing in patients with rhinological pathology in the ENT clinic. Rhinology. 2008;46(1):34-39. [PubMed] [Google Scholar]
- 35. Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinology. 2016;56(1):1-30. doi: 10.4193/Rhin16.248 [DOI] [PubMed] [Google Scholar]
- 36. Banglawala SM, Oyer SL, Lohia S, Psaltis AJ, Soler ZM, Schlosser RJ. Olfactory outcomes in chronic rhinosinusitis with nasal polyposis after medical treatments: a systematic review and meta-analysis. Olfactory outcomes in CRSwNP after treatments. Int Forum Allergy Rhinol. 2014;4(12):986-994. doi: 10.1002/alr.21373 [DOI] [PubMed] [Google Scholar]
- 37. Wu D, Bleier BS, Wei Y. Temporary olfactory improvement in chronic rhinosinusitis with nasal polyps after treatment. Eur Arch Otorhinolaryngol. 2018;275(9):2193-2202. doi: 10.1007/s00405-018-5066-5 [DOI] [PubMed] [Google Scholar]
- 38. Katotomichelakis M, Gouveris H, Tripsianis G, Simopoulou M, Papathanassiou J, Danielides V. Biometric predictive models for the evaluation of olfactory recovery after endoscopic sinus surgery in patients with nasal polyposis. Am J Rhinol Allergy. 2010;24(4):276-280. doi: 10.2500/ajra.2010.24.3476 [DOI] [PubMed] [Google Scholar]
- 39. Bhattacharyya N, Kepnes LJ. Contemporary assessment of the prevalence of smell and taste problems in adults: prevalence of smell and taste problems. Laryngoscope. 2015;125(5):1102-1106. doi: 10.1002/lary.24999 [DOI] [PubMed] [Google Scholar]
- 40. Liu G, Zong G, Doty RL, Sun Q. Prevalence and risk factors of taste and smell impairment in a nationwide representative sample of the US population: a cross-sectional study. BMJ Open. 2016;6(11):e013246. doi: 10.1136/bmjopen-2016-013246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-opn-10.1177_2473974X20986756 for Smell and Taste Impairment in a Nationwide Sample of US Adults With Chronic Rhinosinusitis Symptoms by James H. Kim, Janet Choi, Sophie S. Jang, Bozena B. Wrobel and Elisabeth H. Ference in OTO Open: The Official Open Access Journal of the American Academy of Otolaryngology-Head and Neck Surgery Foundation


