Abstract
Background
Epicardial adipose tissue (EAT) has anatomic and functional proximity to the heart and is considered a novel diagnostic marker and therapeutic target in cardiometabolic diseases. The aim of this study was to evaluate whether EAT volume was associated with coronary artery calcification (CAC) in people living with diabetes, independently of confounding factors.
Methods
We included all consecutive patients with diabetes whose EAT volume and CAC score were measured using computed tomography between January 1, 2019 and September 30, 2020 in the Department of Diabetology-Endocrinology-Nutrition at Avicenne Hospital, France. Determinants of EAT volume and a CAC score ≥ 100 Agatston units (AU) were evaluated.
Results
The study population comprised 409 patients (218 men). Mean (± standard deviation) age was 57 ± 12 years, and 318, 56 and 35 had type 2 (T2D), type 1 (T1D), or another type of diabetes, respectively. Mean body mass index (BMI) was 29 ± 6 kg/m2, mean AET volume 93 ± 38 cm3. EAT volume was positively correlated with age, BMI, pack-year smoking history and triglyceridaemia, but negatively correlated with HDL-cholesterol level. Furthermore, it was lower in people with retinopathy, but higher in men, in Caucasian people, in patients on antihypertensive and lipid-lowering medication, in people with nephropathy, and finally in individuals with a CAC ≥ 100 AU (CAC < 100 vs CAC ≥ 100: 89 ± 35 vs 109 ± 41 cm3, respectively, p < 0.05). In addition to EAT volume, other determinants of CAC ≥ 100 AU (n = 89, 22%) were age, T2D, ethnicity, antihypertensive and lipid-lowering medication, cumulative tobacco consumption, retinopathy, macular edema and macrovascular disease. Multivariable analysis considering all these determinants as well as gender and BMI showed that EAT volume was independently associated with CAC ≥ 100 AU (per 10 cm3 increase: OR 1.11 [1.02–1.20]).
Conclusions
EAT volume was independently associated with CAC. As it may play a role in coronary atherosclerosis in patients with diabetes, reducing EAT volume through physical exercise, improved diet and pharmaceutical interventions may improve future cardiovascular risk outcomes in this population.
Keywords: Computed tomography, Coronary artery calcification, Diabetes, Epicardial adipose tissue, Epicardial fat tissue, Metabolic syndrome, Visceral fat
Background
Type 1 (T1D) and type 2 diabetes (T2D) are associated with an increased risk of cardiovascular disease, irrespective of improved multifactorial care [1, 2]. Epicardial adipose tissue (EAT) has recently been proposed as one determinant contributing to the pathophysiology of cardiovascular complications [3–6]. A recent meta-analysis showed that individuals with diabetes had higher EAT volumes than healthy controls, irrespective of T1D or T2D status and the method used to quantify EAT volume [3].
EAT is located between the myocardium and the visceral pericardium and is considered the heart’s visceral adipose tissue [3–6]. It secretes inflammatory factors and lipid metabolites, and has been suggested as a possible determinant of accelerated atherosclerosis [3–6]. However, at the clinical level, evidence that EAT is a marker of subclinical atherosclerosis in diabetic populations is still limited [5].
Coronary artery calcium (CAC) is considered a good marker of coronary risk [7]. The CAC score assesses the volume of coronary calcifications located in atherosclerotic plaques and provides a summary measure of atherosclerotic disease, reflecting the cumulative lifetime effect of risk factors and genetic and environmental factors [7]. The CAC score increases with cardiovascular risk in both the general population and in people with diabetes [8–10] and a threshold of 100 Agatston units (AU) is commonly used to identify the individuals at high risk [7–10]. To date, only four studies which have evaluated the association either between EAT volume [11–13] or EAT thickness [14] and CAC score in patients with diabetes have had mixed results: one study showed a positive association between EAT and CAC score in 333 patients without cardiovascular disease or renal impairment [11]; another only showed an association in the 38 individuals out of 95 studied who had early-onset T2D [13], while the remaining two studies reported no association whatsoever [12, 14].
In this context, the present study aimed to evaluate, in a large cohort of people living with diabetes, whether EAT volume was associated with CAC score, independently of confounding factors.
Methods
Study population
The present retrospective study involved each consecutive patient with diabetes admitted to the Department of Diabetology-Endocrinology-Nutrition, in Avicenne Hospital, Bobigny, France, between January 1, 2019 and September 30, 2020. All had a computed tomography (CT) scan during hospitalization to evaluate their CAC score (AU) and assess their cardiovascular risk [8, 10].
CT imaging
CAC scores and EAT volume were calculated using ECG-gated cardiac CT without contrast injection. All CT scans were performed with GE (Healthcare Digital, France) or Siemens (Healthineers, France) scanners. CAC scores were calculated according to guidelines [15] using the dedicated tool available on Picture Archiving and Communication Systems (PACS) platforms (either from Carestream Health, Rochester, NY or Philips Healthcare, Best, the Netherlands). EAT volume was quantified with the software package AW VolumeShare 7 (GE Healthcare Digital). It was measured using a semi-automatic segmentation technique on every axial slice from the thoracic inlet to the beginning of the abdomen. The software automatically measured EAT volume (in cm3) by summing appropriate pixels using a CT Hounsfield unit, range − 150 to − 50 HU.
Data collection
Data were extracted from patients’ medical records and collected in a secure health database. For the present study, we focused on:
General data: current tobacco consumption and pack-year smoking history, diagnosed premature (before 55 years for men; before 65 years for women) coronary artery disease in first degree relatives; ethnicity (recorded as Caucasian, Arabic (Middle East, North Africa), Afro-Caribbean (African, African American, Caribbean), Asian (Asian continent), or other).
Medical history: routine treatments before admission, history of stroke, heart failure, or coronary artery disease. Hypertension and dyslipidaemia were self-reported and/or inferred from blood pressure- and lipid-lowering agents, respectively. Additionally, we collected data to measure possible overweightness (body mass index (BMI) ≥ 25 kg/m2) and obesity (BMI ≥ 30 kg/m2). BMI was calculated using the formula: weight (kg)/height2 (m2). Weight and height were measured within 24 h of hospital admission.
Biomarkers: HbA1c (high performance liquid chromatography variant); total and HDL cholesterol (colorimetric assay on homogenous phase and cholesterol dosage by cholesterol oxidase), triglycerides (colorimetric assay), and LDL-cholesterol (calculated using the Friedewald formula). All these measurements were performed on plasma from fasting individuals using a Cobas 6000 analyzer (Roche diagnostics). Serum creatinine was measured (colorimetry, Kone Optima, Thermolab System, Paris La Défense, France) and creatinine clearance estimated (using the Chronic Kidney Disease-Epidemiology Collaboration equation). The urinary albumin/creatinine ratio (UACR) was measured (laser immunonephelometry, BN100, Dade-Behring, Paris, France).
Diabetes-related complications: retinopathy (defined as any medical argument for a retinopathy); nephropathy [defined as renal failure (estimated creatinine clearance < 60 mL/min) and/or albuminuria (UACR > 3 mg/mmol)]; neuropathy (defined as any sign or symptoms of polyneuropathy); peripheral arterial occlusive disease (stenosis measured 50% by ultrasound examination); macroangiopathy (defined as peripheral arterial occlusive disease or history of stroke, heart failure or coronary artery disease).
Statistical analyses
Continuous variables were expressed as means ± standard deviation and compared using one-way ANOVA or the Mann–Whitney’s U test as appropriate. No data replacement procedure was used for missing data. Pearson’s and/or Spearman’s correlations were performed to identify the parameters associated with EAT. The χ2 test was used to measure significant differences between the proportion of patients with a CAC score < 100 and those with a score ≥ 100 AU. Logistic regression was performed for the multivariable analysis, which included only those parameters associated with a CAC score ≥ 100 AU, as well as BMI and gender. Odds ratios with 95% confidence intervals (95 CI) for the risk of a CAC score ≥ 100 AU were calculated. Statistical analyses were performed using SPSS software (SPSS, Chicago, IL). The level of significance for all tests was p < 0.05.
Results
Patient characteristics
Of the 410 patients who met study inclusion criteria, 1 was not included as the EAT volume could not be measured. The characteristics of the 409 included patients are shown in Table 1.
Table 1.
Patient characteristics
| Available data | Total | |
|---|---|---|
| Clinical characteristics | ||
| Age (years) | n = 409 | 57.1 ± 12.4 |
| Gender (Male/Female) | n = 409 | 218/191 |
| Body mass index (kg/m2) | n = 403 | 29.1 ± 5.9 |
| Overweight or obese | n = 403 | 325 (79.5) |
| Obese | n = 403 | 158 (38.6) |
| Ethnicity | n = 408 | |
| Caucasian | 88 (21.5) | |
| Sub-Saharan Africa—Antilles | 103 (25.2) | |
| Middle East, North Africa | 146 (35.7) | |
| Asia | 57 (14.0) | |
| Other | 14 (3.4) | |
| Diabetes | ||
| Type | n = 409 | |
| Type 1 | 56 (13.7) | |
| Type 2 | 318 (77.8) | |
| Other | 35 (8.6) | |
| Treatment | ||
| Oral antidiabetic agents | n = 408 | 295 (72.1) |
| Glucagon-like peptid 1 agonists | n = 408 | 75 (18.3) |
| Insulin | n = 408 | 252 (61.6) |
| Time since diagnosis (years) | n = 409 | 14.0 ± 10.2 |
| HbA1c, % | n = 403 | 9.0 ± 2.3 |
| Retinopathy | n = 392 | 160 (39.1) |
| Macular edema | n = 382 | 44 (10.8) |
| Nephropathy | n = 390 | 148 (36.2) |
| Albuminuria | n = 389 | 135 (33.0) |
| Renal failure | n = 409 | 39 (9.5) |
| Neuropathy | n = 401 | 163 (39.9) |
| Peripheral arterial occlusive disease | n = 409 | 37 (9.0) |
| History of stroke | n = 409 | 13 (3.2) |
| History of heart failure | n = 356 | 5 (1.2) |
| Coronary artery disease (CAD) | n = 409 | 4 (1.0) |
| Additional cardiovascular risk factors | ||
| Family history of premature CAD | n = 149 | 33 (8.1) |
| Hypertension | n = 408 | 224 (54.8) |
| Systolic blood pressure (mmHg) | n = 408 | 135 ± 18 |
| Diastolic blood pressure (mmHg) | n = 408 | 77 ± 13 |
| Dyslipidemia | n = 408 | 238 (58.2) |
| Total cholesterol (mmol/L) | n = 409 | 4.4 ± 1.2 |
| HDL cholesterol (mmol/L) | n = 409 | 1.2 ± 0.4 |
| Triglycerides (mmol/L) | n = 409 | 1.7 ± 1.2 |
| LDL cholesterol (mmol/L) | n = 406 | 2.4 ± 0.9 |
| Non HDL cholesterol (mmol/L) | n = 409 | 3.2 ± 1.1 |
| Current smoking | n = 409 | 71 (17.4) |
| Smoking history (pack-year) | n = 386 | 8.2 ± 16.6 |
| Computed tomography results | ||
| Epicardial adipose tissue volume (cm3) | n = 409 | 92 ± 37 |
| CAC score (Agatston unit) | n = 409 | 116 ± 332 |
Data are n (%) or mean ± standard deviation
CAC coronary artery calcium score, CAD coronary artery disease
Parameters associated with EAT
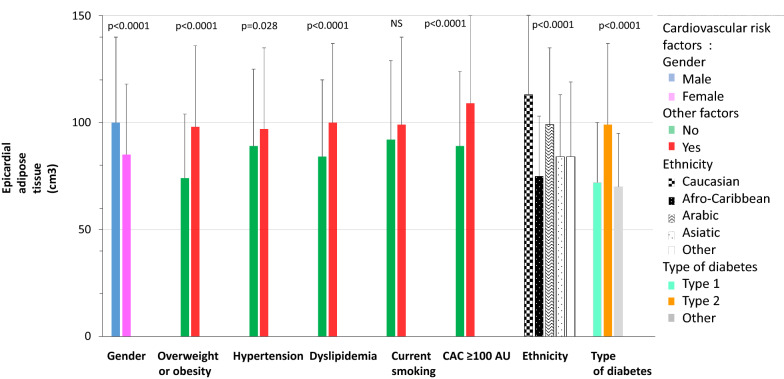
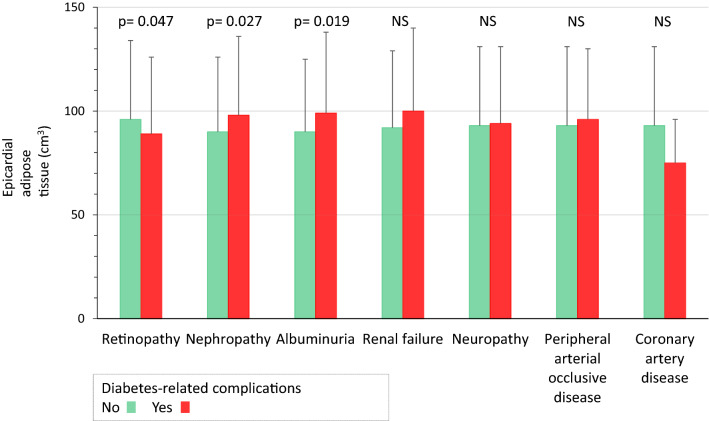
EAT volume was positively associated with age, BMI, triglyceride levels, cumulative tobacco consumption and CAC score. It was negatively correlated with creatinine clearance and HDL-cholesterol level (Table 2). EAT volume was higher in males, overweight and obese persons, those categorized as Caucasian, patients with T2D, those with hypertension, individuals with dyslipidaemia and people with a CAC score ≥ 100 AU (Fig. 1, Additional file 1: Table S1). It was also higher in participants with nephropathy and albuminuria, but lower in patients with retinopathy (Fig. 2, Additional file 1: Table S2).
Table 2.
Correlation of epicardial adipose tissue volume with quantitative data
| R | p-value | |
|---|---|---|
| Age, years | 0.324 | < 0.0001 |
| Body mass index, kg/m2 | 0.288 | < 0.0001 |
| HbA1c, % | − 0.093 | 0.142 |
| Urinary albumin/creatinin ratio, mg/mmol | 0.059 | 0.260 |
| Creatinine clearance, mL/min | − 0.237 | < 0.0001 |
| Systolic blood pressure, mmHg | − 0.022 | 0.656 |
| Diastolic blood pressure, mmHg | − 0.035 | 0.483 |
| Total cholesterol, mmol/L | − 0.057 | 0.250 |
| HDL cholesterol, mmol/L | − 0.190 | < 0.0001 |
| Triglycerides, mmol/L | 0.150 | 0.002 |
| LDL cholesterol, mmol/L | − 0.070 | 0.158 |
| Non HDL cholesterol, mmol/L | 0.005 | 0.925 |
| Smoking history (pack-year) | 0.233 | < 0.0001 |
| Coronary artery calcium score, Agatston unit | 0.287 | < 0.0001 |
Fig. 1.
Epicardial adipose tissue volume according to cardio-vascular risk factors. Data are mean ± SD. AU Agatston unit, CAC coronary artery calcification score
Fig. 2.
Epicardial adipose tissue volume according to diabetes-related complications. Data are mean ± SD
Additional file 1: Table S3 also shows that the use of the following treatments was associated with a higher EAT volume: metformin, sulfonylurea, glucagon-like peptide 1 receptor agonists, beta-blockers, statins and aspirin.
Parameters associated with CAC
Individuals with a CAC score ≥ 100 AU (versus < 100 AU) were older, more likely to be Caucasian (versus Afro-Caribbean, Arabic and Asian), more likely to have T2D (versus T1D), and more likely to have retinopathy, macular edema, nephropathy, macrovascular disease, hypertension and dyslipidaemia. Furthermore, they had had diabetes for a longer time and had higher pack-year smoking history (Table 3). In the multivariable analysis, after adjustment for all these parameters and both gender and BMI, EAT was independently associated with a CAC score ≥ 100 AU (Table 4).
Table 3.
Patient characteristics according to coronary artery calcium scores < or ≥ 100 Agatston units
| CAC < 100 AU | CAC ≥ 100 AU | OR [95%CI] | p | |
|---|---|---|---|---|
| n = 320 | n = 89 | |||
| Clinical characteristics | ||||
| Age (years) | 54.7 ± 12.2 | 65.7 ± 8.7 | < 0.0001 | |
| Gender (Male/Female) | 164/156 | 54/35 | 0.7 [0.4–1.1] | 0.120 |
| Body mass index (kg/m2) | 29.3 ± 6.0 | 28.2 ± 5.5 | 0.119 | |
| Ethnicity | ||||
| Caucasian | 57(17.9) | 31(34.8) | REF | |
| Afro-Caribbean | 88(27.6) | 15(16.9) | 0.3 [0.2–0.6] | 0.001 |
| Arabic | 116(36.4) | 30(33.7) | 0.5 [0.3–0.9] | 0.014 |
| Asia | 47(14.7) | 10(11.2) | 0.4 [0.2–0.9] | 0.023 |
| Other | 11(3.4) | 3(3.4) | 0.5 [0.1–1.9] | 0.316 |
| Diabetes | ||||
| Type | ||||
| Type 1 | 52(16.2) | 4(4.5) | REF | |
| Type 2 | 239(74.7) | 79(88.8) | 4.3 [1.5–12.3] | 0.006 |
| Other | 29(9.1) | 6(6.7) | 2.7 [0.7–10.3] | 0.149 |
| Time since diagnosis (years) | 12.9 ± 9.8 | 17.7 ± 10.8 | < 0.0001 | |
| HbA1c (%) | 9.0 ± 2.3 | 8.7 ± 2.1 | 0.280 | |
| Diabetes-related complication | ||||
| Retinopathy | 116(37.8) | 44(51.8) | 1.8[1.1–2.9] | 0.025 |
| Macular edema | 28(9.3) | 16(19.5) | 2.4 [1.2–4.6] | 0.018 |
| Nephropathy | 100(33.0) | 48(55.2) | 2.5 [1.5–4.1] | < 0.0001 |
| Neuropathy | 120(38.5) | 43(48.3) | 1.5 [0.9–2.4] | 0.112 |
| Macrovascular disease | 30(9.4) | 32(36.0) | 5.4 [3.1–9.6] | < 0.0001 |
| Additional cardiovascular risk factors | ||||
| Family history of premature CAD | 27(21.8) | 6(24.0) | 1.1 [0.4–3.1] | 0.795 |
| Hypertension | 163(50.9) | 61(69.3) | 2.2 [1.3–3.6] | 0.002 |
| Dyslipidaemia | 171(53.4) | 67(76.1) | 2.8 [1.6–4.8] | < 0.0001 |
| Current smoking | 52(16.2) | 19(21.3) | 1.4 [0.8–2.5] | 0.270 |
| Cumulative tobacco consumption (pack-year) | 6.4 ± 14.1 | 14.7 ± 22.8 | < 0.0001 | |
Data: mean ± standard deviation
AU Agatston unit, CAD coronary artery disease, OR odds ratio, 95% CI 95% confidence interval
Table 4.
Parameters explaining a coronary artery calcium score ≥ 100 Agaston units in multivariable analysis
| Odds ratio | 95% confidence interval | p-value | |
|---|---|---|---|
| Epicardial adipose tissue (per 10 cm3) | 1.13 | 1.04–1.23 | 0.004 |
| Age (per year) | 1.08 | 1.05–1.12 | < 0.001 |
| Body mass index (per kg/m2) | 0.94 | 0.89–1.00 | 0.051 |
| Male vs Female | NS | ||
| Type 2 vs Type 1 diabetes | NS | ||
| Other diabetes types vs Type 1 diabetes | NS | ||
| Afro-Caribbean vs Caucasian | NS | ||
| Arabic vs Caucasian | NS | ||
| Asia vs Caucasian | NS | ||
| Other vs Caucasian | NS | ||
| Hypertension | NS | ||
| Dyslipidaemia | NS | ||
| Diabetes duration | NS | ||
| Cumulative Tobacco consumption (per pack-year) | 1.03 | 1.01–1.04 | 0.002 |
| Retinopathy | 1.89 | 0.99–3.58 | 0.05 |
| Macular edema | NS | ||
| Nephropathy | NS | ||
| Macrovascular disease | 3.94 | 1.92–8.07 | < 0.001 |
Discussion
Our cohort study results show that EAT volume was independently associated with CAC in people with diabetes. This reflects results from another study with a mix of individuals with and without diabetes [16]. Similarly, Yerramasu et al. found that EAT volume was an independent marker of both the presence and severity of CAC burden in 333 asymptomatic patients with T2D [11]. However, the latter finding goes against results from three others studies where no such association was found [12–14]. There are several possible reasons for this. First, these three studies had less statistical power than Yerramasu’s and ours, as they only included between 95 and 200 patients [12–14]. Second, one of the three (Christensen et al.) measured EAT thickness not volume [14]. Third, inclusion criteria differed between the three studies: only patients with T2D and elevated urinary albumin excretion rate were recruited in Christensen et al.’s study [14], while only young Native Americans with T2D were included in Reinhardt et al.’s study [13]. Our results in diabetic persons are clinically relevant as we were able to show that the association remained significant even after adjustment for numerous confounding factors.
Parameters associated with EAT volume, to be considered as confounders
First, EAT volume was positively correlated with male gender and older age. Elsewhere, EAT volume has been associated with all the components of metabolic syndrome in people with T2D [16, 17] and those with T1D [18, 19]. Similarly, we found an association between EAT volume and higher BMI, increased triglycerides levels, lower HDL-cholesterol levels and antihypertensive treatment. This result is in line with previous studies which showed a higher EAT volume in individuals with T2D than those with T1D [12, 19]. Additionally, cumulative tobacco consumption, which is associated with insulin resistance and metabolic syndrome [20], was positively correlated with EAT volume in our study. Finally, we also found that EAT volume was higher in individuals of Caucasian ethnicity. Similarly, the difference in EAT thickness between persons with and without metabolic syndrome was more evident in Caucasians [17, 21]. Other studies have also found that EAT levels differ according to racial/ethnic group in the general population [22–25].
EAT and subclinical atherosclerosis, including CAC
The association between EAT and CAC in our study suggests that EAT might play a role in subclinical atherosclerosis in diabetes. There are arguments to support this hypothesis. First, increased EAT volume/thickness has been associated with markers of subclinical atherosclerosis other than CAC score, such as coronary artery disease and cardiac dysfunction [5]. Second, prospective studies have shown that high EAT volume/thickness is associated with more cardiovascular events in the general population [26], in patients with T2D without participant-selection study criteria [27] and T2D patients with microalbuminuria [14]. Third, EAT identifies individuals at increased risk of CAC progression [11]. Fourth, whereas EAT is physiologically cardioprotective—as it provides mechanical protection and energy to the myocardium and has anti-inflammatory properties—, abnormally increases in EAT volume are proinflammatory [3–6]. Furthermore, EAT secretes vasoactive factors that regulate coronary endothelial function and facilitate free fatty acid influx [3–6]. Therefore, the positive association between EAT and CAC may reflect early pathophysiological effects of EAT on local atherosclerosis. EAT volume could therefore be considered as a surrogate of coronary atherosclerosis. Similarly, EAT volume has been associated with plaque vulnerability, which may contribute to acute coronary syndrome [28]. Finally, EAT might in addition directly promote calcification processes in atheroma [29].
EAT and diabetic microangiopathic complications
We found that EAT volume was positively associated with albuminuria—without correlation with renal failure—whereas such a correlation was not found previously in the diabetic population [11, 12]. Albuminuria is a known marker of poor cardiovascular prognosis in people with diabetes [8, 10]. We might hypothesize that the association between EAT and albuminuria only reflects EAT-induced coronary endothelial dysfunction through paracrine effects [30]. On the contrary, EAT volume was lower in case of retinopathy. In the study by Yerramasu et al., the percentage of retinopathy also decreased by increasing EAT tertiles (34, 27 and 23%, respectively) but statistical significance was not reached (p = 0.18) [11]. Whether EAT would play a systemic role on eyes need further specific studies. Finally, as previously reported [11], EAT volume did not differ by presence of neuropathy.
Clinical and therapeutic implications
Our results suggest that measuring EAT volume might improve assessment of cardiovascular prognosis, but whether the prognostic value of EAT is additional to the one of CAC score is unknown [14, 26, 27]. There are also therapeutic implications of our work. EAT volume can be modified by lifestyle such as diet and/or exercise, bariatric surgery and pharmaceutical interventions [31, 32]. For example, thiazolidinediones, dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors have all been reported to reduce EAT volume [33]. On the contrary, CAC cannot regress.
Limitations and strengths of the study
Our study has several limitations. Its design was observational, which prevented us from being able to draw conclusions about causal relationships between EAT volume and CAC. Neither were we able to make a conclusion about the role of therapy on EAT volume. Furthermore, in order to evaluate the prognostic value of EAT volume, we used a marker of subclinical atherosclerosis (i.e., CAC) instead of measuring the incidence of cardiovascular events. Moreover, we only included patients who had been admitted to our hospital department and who had their CAC score measured. Therefore, our results may not be representative of all patients with diabetes. Finally, we did not have data on waist circumference, which could have influenced the association between EAT volume and CAC. However, we did adjust for several confounders, including BMI.
The main strength of our study is that we measured EAT and not pericardial (or total cardiac) adipose tissue. EAT lies between the myocardium and the visceral layer of the pericardium and is different from pericardial fat which is located externally to the myocardium. There is no fascia separating EAT and myocardium. Therefore both tissues are in direct contact [3–6, 31]. To date, EAT is the only type of cardiac adipose tissue which has been observed to predict incident cardiovascular events in T2D patients [27]. Furthermore, we applied a robust methodology—CT acquisition and assessment following standard methods—as well as cardiac software to automatically quantify EAT. Only 1 EAT measure out of the 410 performed (study population) could not be interpreted. CT scans are considered the gold standard for EAT as, unlike echography, they measure EAT volume not thickness [4, 14].
Conclusions
We showed that in individuals with diabetes, EAT volume was higher in males, persons of Caucasian ethnicity and those who met the criteria for the components of metabolic syndrome. More importantly, we demonstrated that EAT was independently associated with subclinical coronary atherosclerosis in our study population. This suggests that EAT quantification might improve early assessment of cardiovascular prognosis. Furthermore, the anatomic proximity of EAT and adjacent myocardial and vascular tissues might provide an opportunity for therapeutic interventions.
Supplementary Information
Additional file 1: Table S1. Epicardial adipose tissue volume according to cardio-vascular risk factors. Table S2. Epicardial adipose tissue volume according to diabetes-related complications. Table S3. Epicardial adipose tissue volume according to treatment.
Acknowledgements
We thank Sylvain Guillaume and Adeline Digard from GE Healthcare, France, for providing the software to calculate EAT volume. Our thanks also to Jude Sweeney (Milan, Italy) for the English editing and revision of the manuscript.
Abbreviations
- AU
Agatston unit
- BMI
Body mass index
- CAC
Coronary artery calcium
- CT
Computed tomography
- EAT
Epicardial adipose tissue
- PACS
Picture Archiving and Communication Systems
- T1D
Type 1 diabetes
- T2D
Type 2 diabetes
- UACR
Urinary albumin/creatinine ratio
Authors’ contributions
EC and HB conceived and designed this study and had full access to all the study data. EC is responsible for the integrity of the data and the accuracy of the data analyses. IR and PYB collected the data. EC drafted the paper, with help from MTN, IR, ST, PYB and HB. MTN performed the analyses. All authors critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding
Internal funding was provided by Assistance Publique—Hôpitaux de Paris and Paris 13 University.
Availability of data and materials
Data for the present analysis can be provided from the first author on reasonable request.
Ethics approval and consent to participate
In Avicenne hospital and in general in the various Public Assistance Hospitals in Paris, all patients are informed at admission that their medical records may be used for research, unless they indicate their opposition. For the present study, no patient indicated opposition. Approval for the use of patient data was provided by the local ethics committee (Approval number: CLEA-2020-148). Data were analyzed anonymously. No patient opposed.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Emmanuel Cosson, Email: emmanuel.cosson@aphp.fr.
Minh Tuan Nguyen, Email: mtuanng2001@yahoo.com.
Imen Rezgani, Email: imenrezgani8@gmail.com.
Sopio Tatulashvili, Email: sopio.tatulashvili@aphp.fr.
Meriem Sal, Email: meriem.sal@aphp.fr.
Narimane Berkane, Email: narimane.berkane@aphp.fr.
Lucie Allard, Email: lucie.allard@aphp.fr.
Pierre-Yves Brillet, Email: pierre-yves.brillet@aphp.fr.
Hélène Bihan, Email: helene.bihan@aphp.fr.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-021-01225-6.
References
- 1.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 2.Vergès B. Cardiovascular disease in type 1 diabetes: a review of epidemiological data and underlying mechanisms. Diabetes Metab. 2020;46:442–449. doi: 10.1016/j.diabet.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Liu B, Li Y, Jing X, Deng S, Yan Y, et al. Epicardial fat tissue in patients with diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Diabetol. 2019;18:3. doi: 10.1186/s12933-019-0807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villasante Fricke AC, Iacobellis G. Epicardial adipose tissue: clinical biomarker of cardio-metabolic risk. Int J Mol Sci. 2019;20:5989. doi: 10.3390/ijms20235989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen RH, von Scholten BJ, Lehrskov LL, Rossing P, Jørgensen PG. Epicardial adipose tissue: an emerging biomarker of cardiovascular complications in type 2 diabetes? Ther Adv Endocrinol Metab. 2020;11:2042018820928824. doi: 10.1177/2042018820928824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iacobellis G, Barbaro G. Epicardial adipose tissue feeding and overfeeding the heart. Nutrition. 2019;59:1–6. doi: 10.1016/j.nut.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72:434–447. doi: 10.1016/j.jacc.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 9.Fox CS, Golden SH, Anderson C, Bray GA, Burke LE, de Boer IH, et al. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2015;38:1777–1803. doi: 10.2337/dci15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valensi P, Belle EV, Cosson E, Emmerich J, Prevost G, Ferrières J, et al. Risk stratification and screening for coronary artery disease in asymptomatic patients with diabetes mellitus. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Yerramasu A, Dey D, Venuraju S, Anand DV, Atwal S, Corder R, et al. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis. 2012;220:223–230. doi: 10.1016/j.atherosclerosis.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 12.Zobel EH, Christensen RH, Winther SA, Hasbak P, Hansen CS, von Scholten BJ, et al. Relation of cardiac adipose tissue to coronary calcification and myocardial microvascular function in type 1 and type 2 diabetes. Cardiovasc Diabetol. 2020;19:16. doi: 10.1186/s12933-020-0995-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reinhardt M, Cushman TR, Thearle MS, Krakoff J. Epicardial adipose tissue is a predictor of decreased kidney function and coronary artery calcification in youth- and early adult onset type 2 diabetes mellitus. J Endocrinol Invest. 2019;42:979–986. doi: 10.1007/s40618-019-1011-8. [DOI] [PubMed] [Google Scholar]
- 14.Christensen RH, von Scholten BJ, Hansen CS, Heywood SE, Rosenmeier JB, Andersen UB, et al. Epicardial, pericardial and total cardiac fat and cardiovascular disease in type 2 diabetic patients with elevated urinary albumin excretion rate. Eur J Prev Cardiol. 2017;24:1517–1524. doi: 10.1177/2047487317717820. [DOI] [PubMed] [Google Scholar]
- 15.Hecht HS, Cronin P, Blaha MJ, Budoff MJ, Kazerooni EA, Narula J, et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Thorac Imaging. 2017;32:W54–66. doi: 10.1097/RTI.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 16.Wang C-P, Hsu H-L, Hung W-C, Yu T-H, Chen Y-H, Chiu C-A, et al. Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin Endocrinol. 2009;70:876–882. doi: 10.1111/j.1365-2265.2008.03411.x. [DOI] [PubMed] [Google Scholar]
- 17.Pierdomenico SD, Pierdomenico AM, Cuccurullo F, Iacobellis G. Meta-analysis of the relation of echocardiographic epicardial adipose tissue thickness and the metabolic syndrome. Am J Cardiol. 2013;111:73–78. doi: 10.1016/j.amjcard.2012.08.044. [DOI] [PubMed] [Google Scholar]
- 18.Colom C, Viladés D, Pérez-Cuellar M, Leta R, Rivas-Urbina A, Carreras G, et al. Associations between epicardial adipose tissue, subclinical atherosclerosis and high-density lipoprotein composition in type 1 diabetes. Cardiovasc Diabetol. 2018;17:156. doi: 10.1186/s12933-018-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svanteson M, Holte KB, Haig Y, Kløw NE, Berg TJ. Coronary plaque characteristics and epicardial fat tissue in long term survivors of type 1 diabetes identified by coronary computed tomography angiography. Cardiovasc Diabetol. 2019;18:58. doi: 10.1186/s12933-019-0861-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cena H, Fonte ML, Turconi G. Relationship between smoking and metabolic syndrome. Nutr Rev. 2011;69:745–753. doi: 10.1111/j.1753-4887.2011.00446.x. [DOI] [PubMed] [Google Scholar]
- 21.Salami SS, Tucciarone M, Bess R, Kolluru A, Szpunar S, Rosman H, et al. Race and epicardial fat: the impact of anthropometric measurements, percent body fat and sex. Ethn Dis. 2013;23:281–285. [PubMed] [Google Scholar]
- 22.Willens HJ, Gómez-Marín O, Chirinos JA, Goldberg R, Lowery MH, Iacobellis G. Comparison of epicardial and pericardial fat thickness assessed by echocardiography in African American and non-Hispanic White men: a pilot study. Ethn Dis. 2008;18:311–316. [PubMed] [Google Scholar]
- 23.Apfaltrer P, Schindler A, Schoepf UJ, Nance JW, Tricarico F, Ebersberger U, et al. Comparison of epicardial fat volume by computed tomography in black versus white patients with acute chest pain. Am J Cardiol. 2014;113:422–428. doi: 10.1016/j.amjcard.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 24.El Khoudary SR, Shin C, Masaki K, Miura K, Budoff M, Edmundowicz D, et al. Ectopic cardiovascular fat in middle-aged men: effects of race/ethnicity, overall and central adiposity. The ERA JUMP study. Int J Obes. 2015;39:488–494. doi: 10.1038/ijo.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams DB, Narayan O, Munnur RK, Cameron JD, Wong DTL, Talman AH, et al. Ethnic differences in coronary plaque and epicardial fat volume quantified using computed tomography. Int J Cardiovasc Imaging. 2017;33:241–249. doi: 10.1007/s10554-016-0982-1. [DOI] [PubMed] [Google Scholar]
- 26.Mahabadi AA, Berg MH, Lehmann N, Kälsch H, Bauer M, Kara K, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2013;61:1388–1395. doi: 10.1016/j.jacc.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 27.Christensen RH, von Scholten BJ, Hansen CS, Jensen MT, Vilsbøll T, Rossing P, et al. Epicardial adipose tissue predicts incident cardiovascular disease and mortality in patients with type 2 diabetes. Cardiovasc Diabetol. 2019;18:114. doi: 10.1186/s12933-019-0917-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nerlekar N, Brown AJ, Muthalaly RG, Talman A, Hettige T, Cameron JD, et al. Association of epicardial adipose tissue and high-risk plaque characteristics: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6:e006379. doi: 10.1161/JAHA.117.006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakahara T, Dweck MR, Narula N, Pisapia D, Narula J, Strauss HW. Coronary artery calcification: from mechanism to molecular imaging. JACC Cardiovasc Imaging. 2017;10:582–593. doi: 10.1016/j.jcmg.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Cosson E, Pham I, Valensi P, Pariès J, Attali J-R, Nitenberg A. Impaired coronary endothelium-dependent vasodilation is associated with microalbuminuria in patients with type 2 diabetes and angiographically normal coronary arteries. Diabetes Care. 2006;29:107–112. doi: 10.2337/diacare.29.01.06.dc05-1422. [DOI] [PubMed] [Google Scholar]
- 31.Rafeh R, Viveiros A, Oudit GY, El-Yazbi AF. Targeting perivascular and epicardial adipose tissue inflammation: therapeutic opportunities for cardiovascular disease. Clin Sci. 2020;134:827–851. doi: 10.1042/CS20190227. [DOI] [PubMed] [Google Scholar]
- 32.Launbo N, Zobel EH, von Scholten BJ, Faerch K, Jørgensen PG, Christensen RH. Targeting epicardial adipose tissue with exercise, diet, bariatric surgery or pharmaceutical interventions: a systematic review and meta-analysis. Obes Rev. 2020 doi: 10.1111/obr.13136. [DOI] [PubMed] [Google Scholar]
- 33.Xourgia E, Papazafiropoulou A, Melidonis A. Effects of antidiabetic drugs on epicardial fat. World J Diabetes. 2018;9:141–148. doi: 10.4239/wjd.v9.i9.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Epicardial adipose tissue volume according to cardio-vascular risk factors. Table S2. Epicardial adipose tissue volume according to diabetes-related complications. Table S3. Epicardial adipose tissue volume according to treatment.
Data Availability Statement
Data for the present analysis can be provided from the first author on reasonable request.