Abstract
Vegan diets are gaining popularity, also in families with young children. However, the effects of strict plant‐based diets on metabolism and micronutrient status of children are unknown. We recruited 40 Finnish children with a median age 3.5 years—vegans, vegetarians, or omnivores from same daycare centers—for a cross‐sectional study. They enjoyed nutritionist‐planned vegan or omnivore meals in daycare, and the full diets were analyzed with questionnaires and food records. Detailed analysis of serum metabolomics and biomarkers indicated vitamin A insufficiency and border‐line sufficient vitamin D in all vegan participants. Their serum total, HDL and LDL cholesterol, essential amino acid, and docosahexaenoic n‐3 fatty acid (DHA) levels were markedly low and primary bile acid biosynthesis, and phospholipid balance was distinct from omnivores. Possible combination of low vitamin A and DHA status raise concern for their visual health. Our evidence indicates that (i) vitamin A and D status of vegan children requires special attention; (ii) dietary recommendations for children cannot be extrapolated from adult vegan studies; and (iii) longitudinal studies on infant‐onset vegan diets are warranted.
Keywords: development, metabolism, nutrition, vegan, vitamin
Subject Categories: Metabolism
Nutritional and metabolic effects of strict vegan diets in young children are poorly understood. This first cross‐sectional study of preschool children reports that vegan diet remodels metabolome and lipidome and raises concern of their vitamin A and D statuses and essential amino acids.
The paper explained.
Problem
The metabolic and nutritional consequences of a vegan diet in children are insufficiently known. We studied the metabolic and nutritional status and diet of Finnish daycare children between 1 and 7 years of age with vegan, vegetarian, and omnivore diets. The special value of the study is in the setting: The children enjoyed nutritionist‐planned diets in daycares, designed to meet nutritional recommendations.
Results
Dietary assessment showed that the vegans had higher intake of folate and received smaller proportions of energy from protein and from saturated fatty acids than omnivores. Metabolomics and nutritional status biomarker analyses indicated that the statuses of transthyretin, essential amino acids, retinol‐binding protein, vitamin D, docosahexaenoic acid, and cholesterol (including total, LDL and HDL) of vegan children were lower than those of omnivores. Their lipidomic and bile acid patterns were also distinct.
Impact
Our evidence indicates that vegan children show remarkable metabolic differences compared to omnivores. The data indicate that strict vegan diet affects metabolism of healthy children. Our study indicates the importance of detailed longitudinal and cross‐sectional studies on the nutritional and health effects of a vegan diet before it can be recommended for infants or children.
Introduction
A vegan diet is gaining popularity among Western societies, and a growing number of children are born from vegan mothers (Baldassarre et al, 2020). However, knowledge on the metabolic consequences of a strict vegan diet in infants and children is scarce which may currently lead to conflicting views between healthcare professionals and families in need of healthcare interventions (Farella et al, 2020).
Abstaining from any food of animal origin can be motivated by ecological, ethical, health‐related, or religious reasons (Janssen et al, 2016). Positive health effects of vegan diets include lower body mass index (BMI), non‐HDL cholesterol, and fasting blood glucose, based on studies on vegan adults, or conclusions extrapolated from studies on vegetarians (Dinu et al, 2017). Adults following a vegan diet have been reported to have a reduced risk for ischemic heart disease, type‐2 diabetes, and all cancers combined, but an increased risk for bone fractures and brain hemorrhages (Appleby & Key, 2016; Tong et al, 2019). Furthermore, metabolic profiling in adults indicated that consuming a vegan diet results in a distinct metabolomics footprint (Schmidt et al, 2015; Schmidt et al, 2016). The nutrients considered as potentially critical include protein, vitamin B12, iodine, vitamin D, calcium, iron, zinc, long‐chain n‐3 fatty acids, riboflavin, and vitamin A (Kristensen et al, 2015; Schürmann et al, 2017; Baldassarre et al, 2020). Perhaps surprisingly, studies in infants and children are limited to anthropometric studies suggesting diminished average growth, yet still within normal range, and case reports of life‐threatening micronutrient deficiencies from poorly planned vegan diets (Sanders, 1988; Pawlak, 2017).
Children require more energy and nutrients per body weight unit than adults to ensure normal growth and development of neural, endocrine, and immunological systems (Heird, 2012). Hence, dietary recommendations for children cannot be extrapolated of conclusions from adult vegan studies. Furthermore, studies on vegetarian children provide limited information on vegans, as including animal‐based foods in the diet shifts the intake of several nutrients and the plasma metabolic profile significantly toward those of omnivores (Schmidt et al, 2015).
Finland is a high‐income country with public daycares of excellent quality, available for the families for the six first years of life of children. Up to 75% of Finnish children attend public daycares (Haapamäki & Ranto, 2015), which offer standardized nutritionist‐planned daily meals free of charge (Korkalo et al, 2019). Such a uniform system offers an excellent opportunity to study effects of vegan diet in young children. Here, we use targeted and untargeted metabolomics approach to comprehensively compare the nutritional and metabolic statuses of Finnish daycare children following vegan and omnivore diet.
Results
Participants
All vegan participants had followed a vegan diet since birth and were breastfed for 13–50 months by vegan mothers. All vegan participants had been weaned from breastfeeding more than a year before the study, and none of the participants were breastfed at the time of the study. All participants were of Finnish origin and were apparently healthy with no reported systemic medication according to the questionnaires. Table 1 presents the characteristics of the participants.
Table 1.
Characteristics of the participants.
| Omnivore (OMN) | Vegetarian (VGTR) | Vegan (VGN) | P‐value † | Total | |||
|---|---|---|---|---|---|---|---|
| VGN vs OMN | VGN vs VGTR | VGTR vs OMN | |||||
| n | 24 | 10 | 6 | NA | NA | NA | 40 |
| Age – years a |
3.89 [1.42 to 7.07] |
3.37 [1.73 to 5.67] |
3.32 [1.75 to 6.34] |
NA | NA | NA |
3.96 [1.42 to 7.07] |
| Sex – F:M | 12:12 | 4:6 | 3:3 | NA | NA | NA | 19:21 |
| Number of sibling pairs | 0 | 1 | 1 | NA | NA | NA | 2 |
| Height – z‐score b |
−0.44 [−3.52 to 1.09] |
−0.88 [−2.44 to 0.41] |
0.04 [−1.03 to 0.69] |
0.48 | 1.00 | 0.13 |
−0.46 [−3.52 to 1.10] |
| BMI – standard deviation score b |
0.27 [−2.59 to 3.06] |
0.54 [−1.18 to 1.83] |
−0.05 [−1.57 to 2.15] |
0.88 | 0.11 | 0.37 |
0.40 [−2.59 to 3.06] |
| MUAC – z‐score c |
0.24 [−0.96 to 1.17] |
0.24 [−0.87 to 1.26] |
0.17 [−0.84 to 2.07] |
0.85 | 0.50 | 0.91 |
0.22 [−0.96 to 2.07] |
Statistical distributions are described as medians [minimum – maximum]. BMI = body mass index, MUAC = Mid‐Upper Arm Circumference.
Age is presented as that at the time of anthropometric measurements.
Z‐score for height and SDS‐score for BMI was calculated from Finnish population growth data (Saari et al, 2011).
Z‐score for mid‐upper arm circumference (MUAC) was calculated from the WHO arm circumference‐for‐age growth standards for 1‐ to 5‐year‐olds and from extended growth standard tables by Mramba et al (2017) for children older than 5 years old (World Health Organization, 2007).
P‐values for pairwise comparisons between vegans and omnivores (primary analysis) and between vegetarians and other dietary groups (secondary analysis) were calculated using age‐ and sex‐adjusted exact permutation tests with n = 47,500 permutations. NA = not applicable.
Anthropometrics and diet
Figure 1A illustrates the heights and BMIs of the participants compared to the current Finnish growth references. No differences were indicated between the diet groups in the z‐scores of height, BMI, or mid‐upper arm circumference (Table 1). The children on the vegan diet had lower intake (percentage of energy, E%) of protein and saturated fatty acids, and higher intake of mono‐ and polyunsaturated fatty acids than the omnivorous children (Fig 1B, Table EV1). The calculated intakes of linoleic acid (LA) and alpha‐linoleic acid (ALA) were higher in vegans than in omnivores. The vegan diets included only trace amounts of cholesterol, and no eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA). Fiber and folate intakes were higher in vegans than in omnivores. Vitamin B12 intake was similar in the diet groups, with the main food sources for vegans being fortified drinks and brewer's yeast flakes. We found no difference in the total intake of vitamin A in retinol activity equivalents (RAE) between the groups. The only foods containing retinoids in the reported vegan diets were margarines fortified with vitamin A, which contributed 33% of the total RAE in the diet of this group. The corresponding proportions in vegetarians and omnivores were 41 and 60%, respectively. Most participants, including all vegans, took vitamin D supplements, and all but one vegan child took vitamin B12 supplements (Table EV1). Main food sources of energy and macronutrients are presented in Tables [Link], [Link].
Figure 1. Anthropometric measurements and dietary intake of omnivores, vegetarians, and vegans.
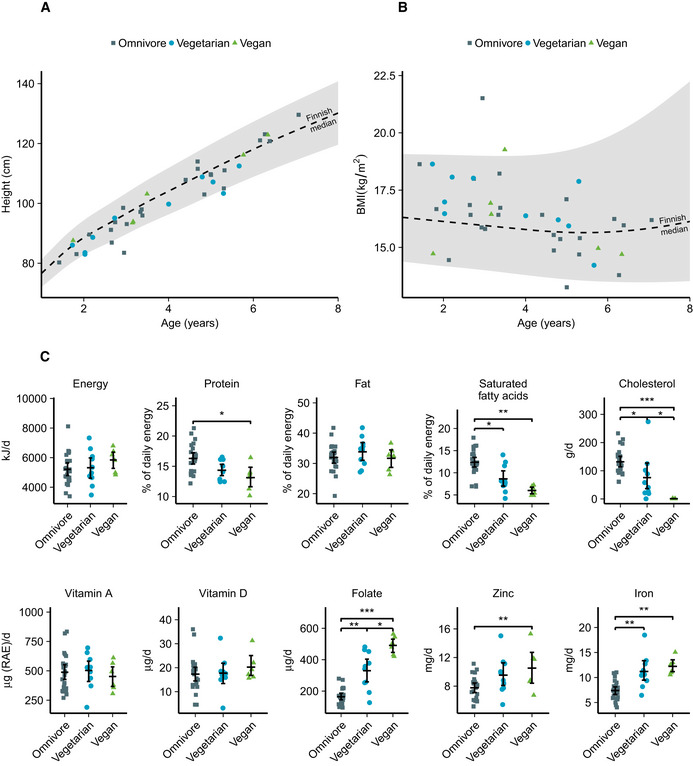
-
A, BHeight (A) and body mass index (B) of the participants by age in the diet groups compared to 2 SD intervals (shaded area) and median in the Finnish reference population.
-
CDietary intake of selected macro‐ and micronutrients of participants.
Data information: The summary bar is presented as mean ± SEM. Differences between diet groups were evaluated with age‐ and sex‐adjusted permutation tests with Benjamini–Hochberg correction for multiple testing. Only significances P < 0.05 are displayed. n = 24 omnivores, 10 vegetarians, six vegans. *P < 0.05, **P < 0.01, ***P < 0.001. Exact P‐values are provided in Table EV1.
Source data are available online for this figure.
Cholesterol metabolism
The plasma total cholesterol, LDL cholesterol (LDL‐C), and HDL cholesterol (HDL‐C) concentrations were significantly lower in vegans than in omnivores. The cholesterol absorption biomarkers showed higher values in vegans than in omnivores, while there was no difference in cholesterol biosynthesis biomarkers (Fig 2B, Table EV6).
Figure 2. Biomarkers of micronutrient statuses and cholesterol metabolism in omnivores, vegetarians, and vegans.
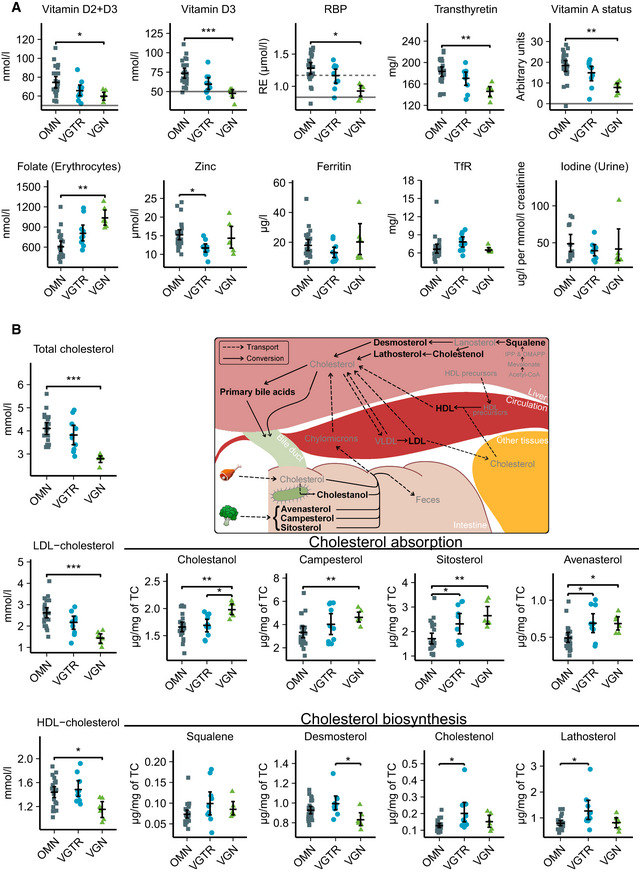
- Serum, plasma, or urine levels of vitamin D, vitamin A, folate, zinc, iron, and iodine status biomarkers in diet groups. Solid lines indicate cut‐offs for deficiency, and dashed lines indicate cut‐offs for insufficiency. The RBP limits for vitamin A insufficiency and deficiency are validated for the method at 1.17 and 0.83 µmol/l, respectively. Vitamin A status was calculated based on RBP, transthyretin, and CRP (Talsma et al, 2015).
Data information: The summary bar is presented as mean ± SEM. Differences between diet groups were evaluated with age‐ and sex‐adjusted permutation tests with Benjamini–Hochberg correction for multiple testing. Only significances P < 0.05 are displayed. n = 24 omnivores, 10 vegetarians, six vegans except for panel (A) iodine, where n = 13 omnivores, nine vegetarians and six vegans. *P < 0.05, **P < 0.01, ***P < 0.001. Exact P‐values are provided in Table EV6. DMAPP, dimethylallyl pyrophosphate; HDL, high‐density lipoprotein; IPP, isopentenyl pyrophosphate; LDL, low‐density lipoprotein; OMN, omnivore; RBP, retinol‐binding protein; VGN, vegan; VGTR, vegetarian; VLDL, very low‐density lipoprotein.
Source data are available online for this figure.
Transthyretin and micronutrient biomarkers
Serum concentrations (Fig 2A, Table EV6) of transthyretin, RBP, 25‐hydroxyvitamin D3 (25(OH)D3), and total‐25(OH)D were lower in vegans than in omnivores. The RBP of all children in the vegan diet group fell below the insufficiency cut‐off level, and the levels of two of the children fell below the deficiency cut‐off level, while the regression model of vitamin A status classified only one omnivore as vitamin A deficient. Vegans had more erythrocyte folate than omnivores. We found no differences between vegans and omnivores in serum ferritin, transferrin receptor, zinc, or spot urine sample iodine concentrations. Secondary statistical analysis between all groups suggests a lower zinc concentration in the vegetarian group than in omnivores. Serum concentrations of transcobalamin‐bound vitamin B12 were adequate in all groups. Four (67%) vegans, nine (90%) vegetarians, and 22 (92%) omnivores had transcobalamin‐bound vitamin B12 above the linear detection limit of 128 pmol/l. The smallest measured concentration was 77 pmol/l, and the cut‐off for further clinical examination is 70 pmol/l.
Bile acid metabolism
Pathway analysis from untargeted metabolomics (Fig 3A, Appendix Table S3) highlighted bile acid synthesis as highly different in vegans compared to omnivores. Direct investigation of serum bile acids revealed higher steady‐state levels of unconjugated primary bile acids and a lower taurine to glycine conjugation ratio of bile acids in vegans than in omnivores (Fig 3B, Table EV6). Serum levels of bile acids in total (Appendix Table S2) and bile acid synthesis biomarker 7‐alpha‐hydroxy‐4‐cholesten‐3‐one (Table EV6) did not differ among diet groups.
Figure 3. Analysis of untargeted flow injection TOF‐MS metabolomics data and further targeted analysis of serum bile acid concentrations.
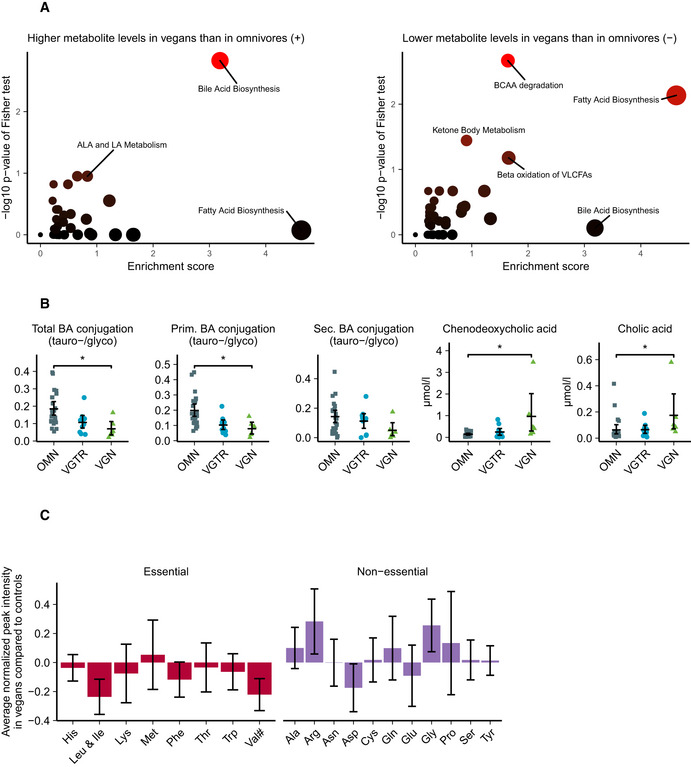
- Pathway analysis from 872 detected untargeted metabolites between omnivores and vegans.
- Targeted analysis of bile acid concentrations in serum of omnivores, vegetarians and vegans.
- Amino acid levels in untargeted metabolomics. Numeric means and standard deviations in this figure can be found in Appendix Table S4. #Valine and betaine are two major components under the same m/z peak, causing uncertainty in the interpretation of valine levels.
Data information: Pathway analysis in subfigure (A) was performed with a gene‐set enrichment analysis (GSEA)–based method. The summary bar of subfigures (B, C) is presented as mean ± SEM. Differences between diet groups in subfigure (B) were evaluated with age‐ and sex‐adjusted permutation tests with Benjamini–Hochberg correction for multiple testing. Only significances P < 0.05 are displayed. n = 24 omnivores, 10 vegetarians, six vegans in panel (B). *P < 0.05. Exact P‐values for panel (A) are provided in Appendix Table S2 and for panel (B) in Table EV6. ALA, alpha‐linolenic acid; BCAA, branched‐chain amino acid; BA, bile acid; LA, linoleic acid; OMN, omnivore; VGTR, vegetarian; VGN, vegan, VLCFA, very long‐chain fatty acid.
Source data are available online for this figure.
Amino acids and fatty acids
Untargeted metabolomics indicated a pattern of overall lower concentrations of circulating essential amino acids (Fig 3C) in vegans. Only a few individual amino acids showed significant differences. The largest differences in essential amino acids were seen in branched‐chain amino acid levels. Analysis of fatty acid compartments (Appendix Table S4) in serum indicated lower levels of DHA, higher levels of ALA (Fig 4A) and long‐chain fatty acid carnitines (Fig 4B), higher lysophosphatidylcholine (lysoPC) per lysophosphatidylethanolamine (lysoPE) ratio (Fig 4C), and higher level of triglycerides with total carbon atom number correlating to medium‐chain fatty acid tails (Fig 4D) in vegans than in omnivores.
Figure 4. Fatty acid analysis from untargeted MS metabolomics in omnivores, vegetarians, and vegans.
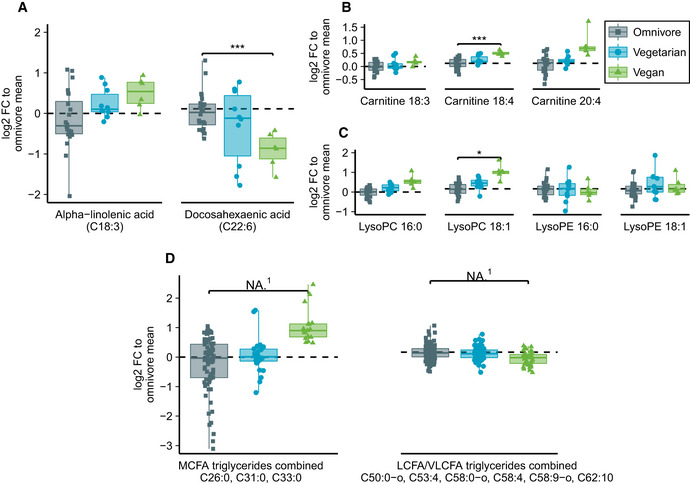
- Fatty acid analysis from untargeted MS metabolomics including free alpha‐linolenic acid (ALA) and docosahexaenoic acid (DHA).
- Carnitine‐bound fatty acids commonly found in serum that were detected in mass spectrometry.
- Lysophosphatidylcholine and lysophosphatidylethanolamine with C16:0 (palmitic acid) and C18:1 (oleic acid).
- Combined analysis of all found triglycerides corresponding to medium‐chain fatty acid lengths and long or very long‐chain fatty acid lengths.
Data information: Box plot center represents group median, hinge covers 25th to 75th percentile and whiskers cover interval from minimum to maximum. Zero‐level represents omnivore mean to which each individual is compared in log2 scale. Differences between diet groups in subfigures (A–C) were evaluated with Student's t‐test (unequal variance, two‐sided) with Benjamini–Hochberg correction for multiple testing. Only significances P < 0.05 are displayed. Exact P‐values are provided in Appendix Table S8. In subfigures (A–C), n = 24, 10, 6 for omnivores, vegetarians, and vegans, respectively. In subfigure (D), multiple‐related metabolites (three MCFAs and six LCFAs/VLCFAs) are combined to same box plot yielding n = 72, 30, and 18 in MCFA subfigure and n = 144, 60, and 36 in (V)LCFA subfigure for omnivores, vegetarians, and vegans, respectively. *P < 0.05, ***P < 0.001, NA, not applicable. Cxx:y, fatty acid of xx carbon atoms and y double bonds; FC, fold change; LysoPC, lysophosphatidylcholine; LysoPE, lysophosphatidylethanolamine; MCFA, medium‐chain fatty acid; LCFA, long‐chain fatty acid; VLCFA, very long‐chain fatty acid. (1) Student's t‐test is not suitable for testing of multiple dependent metabolites in a single test. Of single metabolites included in subfigure (D), Benjamini–Hochberg adjusted t‐test was significant (α = 0.05) for C50:0‐o, C53:4, and C58:0‐o.
Source data are available online for this figure.
Hierarchical clustering
Hierarchical clustering (Fig EV1) of participants based on the untargeted metabolomics data resulted in four major clusters. 80% of vegan participants (5/6) clustered together, forming 56% (5/9) of cluster B. Vegetarian participants were most dispersed among clusters with 4/10 vegetarians forming 44% of cluster B and 6/10 vegetarians clustering together with most of the omnivores to clusters A and D.
Figure EV1. Hierarchical clustering of participants based on untargeted biomarkers in blood.
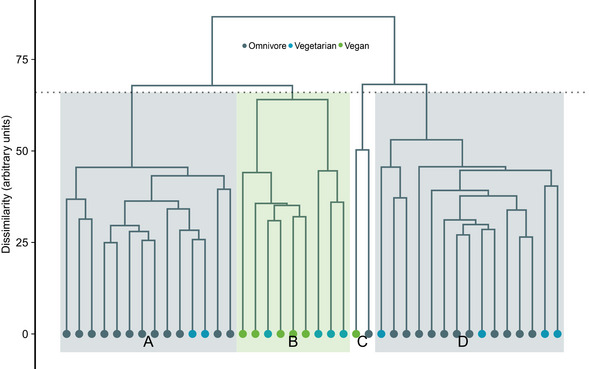
Hierarchical clustering of participants based on the detected 872 metabolites in untargeted metabolomics from serum. Hierarchical clustering was performed using Ward's method and Euclidean distance as the measure of dissimilarity. n = 24 omnivores, 10 vegetarians, six vegans.
Source data are available online for this figure.
Discussion
Here, we report that diet markedly modifies the metabolism of young children. The sample was homogenous and unique: The children were of Finnish origin, had a median age of less than four years, and consumed meals that were centrally planned to fulfill dietary recommendations. The children who followed the vegan diet from birth showed a metabolic profile and nutrient status distinct from those of lacto‐ovo‐vegetarians and omnivores, indicating that only relatively little animal source foods are enough to shift the metabolism of children. The main findings in vegan children included very low cholesterol concentrations and modified bile acid metabolism, as well as their markedly low fat‐soluble vitamin status despite their nutrient intakes matching current national recommendations fairly well. Despite of the adequate estimated vitamin A intake, the RBP results of vegan children in our sample indicated insufficient vitamin A status. Their vitamin D levels were low although the samples were taken during and after summer with expectedly high sunlight exposure and vitamin D storage. Our evidence indicates that special attention is needed to ensure adequate status of these important micronutrients for children on a vegan diet.
Children on a vegan diet showed strikingly low plasma HDL‐C and LDL‐C as well as total cholesterol levels, with a median total cholesterol level of 2.85 mmol/l. The value was markedly lower than the median total cholesterol level of 3.7 mmol/l in Finnish adults following a vegan diet (Elorinne et al, 2016). Low non‐HDL cholesterol in vegans has been reported in different studies (Elorinne et al, 2016; Benatar & Stewart, 2018). This may reflect the cholesterol‐lowering elements (Mach et al, 2019) in well‐planned vegan diets such as the negligible amount of dietary cholesterol, the dietary fatty acid profile that is low in saturated fatty acids and high in unsaturated fatty acids, and a high fiber intake. The few children in our sample with high LDL‐C and total cholesterol belonged to the omnivore group. The endogenous hepatic cholesterol biosynthesis markers were similar between the vegan and omnivore children. These data suggest that endogenous cholesterol biosynthesis does not show a compensatory response to lack of dietary cholesterol.
The low cholesterol levels resulting from adult vegan diet have mostly been linked to positive cardiovascular health effects (Appleby & Key, 2016; Elorinne et al, 2016), although a recent study also suggested an increased risk for stroke (Tong et al, 2019). The markedly low cholesterol in vegan infants and children in our study raises the question of whether such levels are healthy, as cholesterol is essential for cellular growth, division, and development of physiological systems due to its major role in the synthesis of cell membranes, steroid hormones, bile acids, and brain myelin. Early studies on LDL receptors suggested that the physiological concentration of blood LDL‐C may be as low as 0.65–1.6 mmol/l (vegan children in our study ranged from 1.0 to 1.8 mmol/l) (Brown & Goldstein, 1986; O'Keefe et al, 2004). However, longitudinal studies on the health effects of consuming a strict vegan diet since birth have not been conducted.
The main route of cholesterol excretion from the body is through bile acids, the biosynthesis of which occurs in the liver. Our metabolomics analysis indicated that bile acid biosynthesis was the pathway that differed most significantly between the diet groups. In vegans, direct measurement revealed higher primary bile acids, cholic acid, and chenodeoxycholic acid, which were previously reported to increase upon fasting in children (Barbara et al, 1980), and a lower taurine to glycine ratio in bile salt conjugation than omnivores. Vegan diets contain only little taurine, and the relatively low taurine‐conjugation compared to glycine conjugation of bile salts in vegan children is in accordance with previous adult studies (Ridlon et al, 2016). In addition to the role of bile acids in digestion and absorption of fat‐soluble components from the diet, recent studies have elucidated their diverse roles in endocrine and metabolic signaling and gut–microbiome–brain interactions (De Aguiar Vallim, 2013; Ridlon et al, 2016; Kiriyama & Nochi, 2019). What physiological consequences such findings indicate in children following a strict vegan diet remains to be studied. Our evidence indicates that vegan diet remarkably modifies bile acid homeostasis in young children.
The biomarkers for fat‐soluble vitamins A and D showed markedly low levels in the Finnish children following a vegan diet, although there were no indications of compromised absorption of fat‐soluble dietary compounds. The total fat intake in vegan group was similar, and cholesterol absorption biomarkers showed higher levels than those of omnivores. Vitamin D insufficiency is a well‐established concern in Northern countries with restricted exposure to sunlight (Itkonen et al, 2020). The seasonal variation was observed in vitamin D status in Danish children from 2 to 14 years of age. The high peak levels in autumn were between 11 and 19 nmol/l higher than during the lowest season in spring for supplement users and slightly greater for non‐supplemented individuals (Hansen et al, 2018). Vegan children in our sample had lower status of vitamin D than omnivores despite all vegan families reporting daily use of supplements that reached the daily vitamin D intake recommendations (THL, 2019), and the blood samples having been collected during the high peak of seasonal variation in vitamin D status. Different forms of vitamin D fortification may play a role in low status of vitamin D in vegan children. Vegan supplements contain “vegan‐friendly” vitamin D3, whereas vegan food products, such as soymilk, are often fortified with vitamin D2. Vitamin D3 has been suggested to be more effective than D2 at raising total 25(OH)D concentrations, especially in the wintertime (Tripkovic et al, 2017). The vegan children in our study had levels of the endogenous and animal‐based form D3 between 33 and 53 nmol/l, and total vitamin D between 53 and 67 nmol/l, when the clinical cut‐off of insufficiency of total vitamin D level 50 nmol/l. Additionally, lower vitamin A intake in vegan adults has been suggested previously (Kristensen et al, 2015). The calculated intake of vitamin A in the different diet groups of our sample was similar. Despite this similarity, based on the RBP levels reflecting the actively available vitamin A, the vitamin A status of all vegans was insufficient and in two vegan children RBP concentrations were below the deficiency cut‐off. Notably, RBP is considered reliable in group level analysis of vitamin A status and the assessment on individual level has some pitfalls (Tanumihardjo et al, 2016). The linear model for vitamin A status considering inflammatory status did not classify any vegans as vitamin A deficient, but showed significantly lower status for vegans than omnivores, in agreement with RBP alone. RBP synthesis shows complex regulation together with hepatic vitamin A, zinc and iron levels, and overall protein and energy intake (Tanumihardjo et al, 2016). According to our data, the energy intake and zinc and iron status did not differ between vegans and omnivores. Lower protein intake, transthyretin levels, and essential amino acid levels in vegans compared to omnivores may affect the protein status in vegans and therefore the interpretation of RBP levels as vitamin A biomarker. Our results indicate, however, that the vitamin D and A statuses of children following a vegan diet require special attention. Direct measurements of serum retinol, clinical measurement of vitamin A status such as dark adaptation tests and comparison of vegan vitamin D status at winter season are required for further evaluation of vitamin A and D statuses in vegan children.
The vitamin B12, zinc, iron, and iodine statuses, previously found to be challenged in adult vegans (Craig, 2009; Elorinne et al, 2016), did not differ between the diet groups. Intakes of zinc and iron were in fact significantly higher in vegans than in omnivores. Vegans had higher folate intake and concentration than omnivores, and four out of six vegans had levels above the reference range 208–972 nmol/l. Although high folate status is traditionally considered to have positive health effects, recent studies have raised concerns on possible adverse effects of high folate status combined to low vitamin B12 status on neurocognitive health and birth outcomes (Maruvada et al, 2020).
The dietary data of vegan children in our sample indicated protein intake of 10–16 E%, which is in line with recommendations (THL, 2019). However, the untargeted metabolomics suggested that their overall circulating essential amino acid pools were systematically lower than those of omnivores, specifically those of branched‐chain amino acids. Similar findings have been reported in adult vegans (Schmidt et al, 2016; Lindqvist et al, 2019). Serum transthyretin has a short half‐life and is sensitive to the availability of essential amino acids and vitamin A in the liver (Dellière & Cynober, 2017). The transthyretin concentration was also lower in vegans than in omnivores, albeit still in the reference range. Further correlation analysis (Appendix Table S5) showed that branched‐chain amino acids correlated positively to serum transthyretin levels, and lysine negatively with standardized MUAC. The source of different patterns of circulating amino acids in children is not well known. Increased circulating branched‐chain amino acid concentrations are associated with obesity and the risk of insulin resistance in both adults and children (Zhao et al, 2016), whereas undernourished children show chronically low circulating essential amino acid concentrations (Semba et al, 2016). Our evidence of low transthyretin and essential amino acid levels invites attention to dietary protein quality, not only proportional intake measured as E%, in growing children following a vegan diet. Follow‐up studies, specifically focusing on amino acid quantities, will enlighten the aspect further.
Vegan diets are rich in the essential fatty acids ALA and LA, but practically devoid of the ALA derivatives DHA and EPA, long‐chain n‐3 fatty acids of which DHA is needed for visual process and synaptic functioning (Sanders, 2009). Accordingly, we found high intake of ALA and low intake of EPA and DHA in the diet vegan children. Untargeted metabolomics suggested consistent findings, high ALA and low DHA, in serum levels. This correlates well to findings in vegan adults (Sanders, 2009). Vegan children have not been found to have compromised declined visual function linked to primary DHA deficiency (Sanders, 2009). However, DHA and active vitamin A are both important for eyesight (Lien & Hammond, 2011), and the low statuses of both in children may raise a concern for the visual health.
Vegan children show widespread differences to omnivores and vegetarians also in other serum fatty acid compartments. In accordance with our results, higher circulating long‐chain fatty acid carnitine levels, and higher lysoPC/lysoPE ratio have earlier been associated with diets with lower dairy intake and higher unsaturated/saturated fat ratio (Playdon et al, 2017). Recent studies have increased our knowledge on the signaling potential of circulating lysophospholipids (Makide et al, 2014). The intracellular role of carnitines and medium‐chain fatty acids are well known for mitochondrial energy production (Schönfeld & Wojtczak, 2016). However, the current understanding on the roles and significance of extracellular circulating different fatty acid carriers for health is scarce and particularly insufficient in children.
The unsupervised hierarchical clustering of untargeted metabolomics data indicated the clustering of vegans separate from omnivores, indicating the major effect of diet to metabolism of healthy children. However, the vegetarians showed heterogeneous clustering, 60% clustering with omnivores, and the rest with the vegans. Most of the measured biomarkers demonstrated a similar but more subtle trend in the vegetarian group than in vegans compared to omnivores. Our vegetarian group consisted of children who consumed fully vegan meals in daycare and pesco‐/lacto‐ovo‐vegetarian diet at home. In full‐time care, the daycare meals of Finnish daycare children account for approximately 50–60% of the daily intake of energy and most of the macro‐ and micronutrients during weekdays (Korkalo et al, 2019). The evidence indicates that even part‐time consumption of lacto‐ovo‐vegetarian products in an otherwise strict vegan diet may substantially alleviate the risk to nutrient deficiencies in children. Our data indicate the importance of studying vegan children to enable evidence‐based nutritional recommendations.
To conclude, our study demonstrates exceptional clustering of metabolic readouts in different diet groups of young Finnish children, enjoying centrally planned daycare diets designed to meet dietary requirements. Our data of lower status of several biomarkers in vegan children compared to omnivores, in the relatively low number of study subjects, calls for larger studies before early‐life vegan diet can be recommended as a healthy and fully nourishing diet for young children, despite its many health‐promoting effects in adults. We suggest that the metabolic effects of vegan diet in adults cannot be generally extrapolated to children. Long‐term follow‐up studies are needed to clarify the causes and consequences of lower levels of vitamin D, RBP, transthyretin, essential amino acids, total cholesterol, and DHA in vegan children.
Materials and Methods
Study design and participants
This study was approved by the coordinating ethics committee of Helsinki University Hospital and followed the guidelines of the Declaration of Helsinki and the U.S. Department of Health and Human Services Belmont Report. Parents of each child provided written informed consent.
This cross‐sectional study was conducted while the City of Helsinki initiated a trial to provide a vegan diet option in 20 of its municipal daycare centers. Daycare centers offered an omnivore/vegan breakfast, lunch, and afternoon snack 5 days a week using standardized recipes. All menus were planned by a nutritionist to ensure compliance with dietary recommendations (THL, 2019).
During the recruitment period (flow chart in Appendix Fig S1) between May and October 2017, out of the 20 daycares with the vegan option, the parents of 31 children at 9 daycare centers had chosen a vegan diet for their child. Invitation letters were given to parents of all the children on vegan diet at daycare. We asked the daycare personnel of the same nine daycares to provide an invitation letter to parents of 161 children, who were selected based on the criteria that they were not receiving any special diet at daycare and they belonged to the same age group as the recruited vegan children. Parents of 25 children (81% of invited) receiving a vegan diet at daycare and 34 omnivore children (21% of invited) agreed to participate.
Our original plan was to study vegans and omnivores only. However, questionnaire data revealed that most participants consuming vegan food at daycare consumed either a vegetarian or an omnivorous diet at home. Therefore, we formed three diet groups: (i) vegans, (ii) “vegetarians” including lactovegetarians, lacto‐ovo‐vegetarians, and pescatarians who consumed vegan food at daycare, and (iii) omnivores. We excluded children who followed an omnivorous diet at home but a vegan diet at daycare because these participants (n = 2) did not fit into any of the diet groups. A further criterion for exclusion from the analysis was the lack of a blood sample. The sample for this article included 40 children (hereinafter participants): six strict vegans, 10 vegetarians, and 24 omnivores.
Diet and anthropometry
The parents filled in questionnaires on dietary habits and basic health information and the dietary intake was calculated from an estimated 4‐day food record filled in by the parents and daycare personnel between May and October 2017 (details in Appendix Supplementary Methods). The details of nutrient intake calculations are described in the Appendix Supplementary Methods. Anthropometric measurements were conducted according to the World Health Organization (WHO) procedures with two exceptions: Height was measured in the standing position for children under the age of two, and the same scale was used for children of all ages (WHO Expert committee, 1995). For height, we used Seca 213 portable stadiometer assembled and propped according to the manufacturer's instructions. No calibration rod was used. Weight was measured with Seca 878 Mobile flat scales and MUAC with Seca 201 circumference measuring tape.
Blood and urine samples
Blood and urine samples were collected between June and October 2017. Venous blood samples of 15 ml were collected after overnight fasting and analyzed immediately in the clinical laboratory, the remaining sample material was pseudonymized and cooled to 4°C, and the serum was separated and stored at −80°C.
Single random midstream urine samples were obtained from all participants by the families at home according to written instructions. The samples were pseudonymized and aliquoted to a 10 ml sterile trace element tube (Sarstedt item no. 62.554.502) and stored at −80°C.
Blood and urine examinations
Standard laboratory tests and targeted analysis of micronutrients, inflammation biomarkers, blood lipids, glucose, and transthyretin were performed by accredited clinical laboratories. Serum cholestanol, plant sterols, and cholesterol biosynthesis intermediates were used as biomarkers of cholesterol absorption and biosynthesis and they were analyzed by gas–liquid chromatography (GLC) (Miettinen et al, 1990). Serum bile acids were analyzed by high‐performance liquid chromatography–mass spectrometry (HPLC‐MS/MS) (Xiang et al, 2010). Iodine and creatinine were measured in the urine samples of six vegans, nine vegetarians, and 13 omnivores. The samples for this analysis were chosen to cover whole age group in the study and equally include both sexes. We present two different cut‐offs for non‐sufficient RBP level, and we use the term insufficiency for the higher (1.17 µmol/l) and deficiency for the lower (0.83 µmol/l) limit (Tanumihardjo et al, 2016). Concentrations of transcobalamin II‐bound vitamin B12 were measured up to 128 pmol/l. Appendix Table S1 presents a comprehensive list of methods with references, measurements, and clinical cut‐offs.
Untargeted metabolomics and lipidomics
Untargeted metabolomics analysis of serum samples was performed by flow injection‐time‐of‐flight (TOF)‐MS on an Agilent 6550 QTOF instrument in negative mode exactly as described by Fuhrer et al (Fuhrer et al, 2011). Putative annotations were created based on Human Metabolome Database v.3.6 using both accurate mass per charge (tolerance 0.001 m/z) and isotopic correlation patterns, allowing the detection of 872 metabolites. The approach is sufficient to assign molecular formulas in most cases, but does not distinguish between isomers. Full list of possible annotations of each detected metabolite is provided in Appendix Table S6. Untargeted metabolomics data were used in metabolic pathway analysis and analyses of amino acids and fatty acids that are considered abundant compared to alternative molecules matching the same m/z peak and that showed biologically relevant patterns in multiple metabolites in our data. The Appendix Supplementary Methods present the extraction protocols and comprehensive lists of the measured metabolites.
Statistical analysis and figures
All statistical analyses and images were produced using R version 3.6.0 (code in Appendix Supplementary Methods). Anthropometric data were transformed to z‐scores based on the Finnish growth standards (Saari et al, 2011), with the exception of mid‐upper arm circumference, for which the WHO growth standards extended by Mramba et al (2017) were used (World Health Organization, 2007). Vitamin A status was assessed by retinol‐binding protein (RBP) and a validated regression model of RBP, transthyretin, and C‐reactive protein (CRP) (Talsma et al, 2015). Statistical analyses of targeted metabolites, intake, and anthropometrics between diet groups were conducted using age‐ and sex‐adjusted permutation tests with probability index as test statistic. Statistical analyses of untargeted metabolomics were conducted using two‐tailed Student's t‐test assuming unequal variance. Benjamini–Hochberg correction was applied to take multiple comparisons in account. Comparisons between vegans and omnivores were considered as primary analyses, and comparisons of vegetarian group to omnivores and vegans were conducted as secondary analyses. To reveal the relation of vegetarian group to other two diet groups, hierarchical clustering of participants based on untargeted metabolomics data using agglomerative Ward's method with squared Euclidean distance as dissimilarity measure were conducted. Pathway analysis between omnivores and vegans was based on gene‐set enrichment analysis (GSEA). Because untargeted metabolomics provides semi‐quantitative results instead of concentrations, differences in individual amino acids and fatty acids were analyzed in logarithmic fold changes. The Behrens–Fisher approach was used for the 95% confidence intervals of group differences in levels of amino acids. The Appendix Supplementary Methods presents the construction of the permutation tests, pathway analysis, and vitamin A status markers.
Author contributions
AS, ME, RF, LK, PI, and TH devised the study concept and design. TH, LK, AS, ME, and RF drafted the manuscript. All authors critically revised the manuscript for important intellectual content. TH, LK, ES, and JN did the statistical analysis. LK, ES, RF, and TH measured the anthropometrics and conducted the urine sample collection. NZ acquired and preprocessed the untargeted metabolomics, HG conducted cholesterol biomarker measurements, and MN acquired targeted bile acid measurements. MN conducted the targeted bile acid, and HG the targeted cholesterol biomarker measurements. All authors analyzed and interpreted data, and provided administrative, technical, and material support. AS and TH had full access to original data, and all authors to the results. AS and ME supervised the study.
Conflict of interest
TH, RF, ES, PI, MN, JN, and HG have nothing to disclose. LK was a board member of the company TwoDads at the time of the study. ME and LK disclose author's fee from Finnish Medical Journal Duodecim. NZ is the founder and scientific advisor of General Metabolics. AS has obtained speaker fees from Orion Pharma and is part of Khondrion SAB, unrelated to the current study.
For more information.
National Nutrition Council / Finnish Food Authority. Recommendations for vegan diet: https://www.ruokavirasto.fi/en/themes/healthy‐diet/nutrition‐and‐food‐recommendations/vegan‐diet/
Supporting information
Appendix
Expanded View Figures PDF
Table EV1
Table EV2
Table EV3
Table EV4
Table EV5
Table EV6
Source Data for Expanded View
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Acknowledgements
We thank the families who participated in this study; Markus Innilä for the management of the samples; the City of Helsinki, especially the daycare personnel and Tarja Heikkinen from the City of Helsinki Service Center, for their collaboration; Dr. Jürgen Erhardt of VitMinLab for measuring and interpreting data related to vitamin A status, iron status, and inflammation. The authors also wish to thank the following funding sources: Sigrid Jusélius Foundation, Academy of Finland, Helsinki University Hospital and University of Helsinki (for AS), Finnish Cultural Foundation, and Finnish Society for Nutrition Research (for LK).
EMBO Mol Med (2021) 13: e13492.
See also: AE Allen & JW Locasale (February 2021)
Data availability
All R code used in statistical analysis and building figures for this article are open access in GitHub: https://github.com/topihovinen/mirahelsinki.
This study includes no data deposited in external repositories.
References
- Appleby PN, Key TJ (2016) The long‐term health of vegetarians and vegans. Proc Nutr Soc 75: 287–293 [DOI] [PubMed] [Google Scholar]
- Baldassarre ME, Panza R, Farella I, Posa D, Capozza M, Di Mauro A, Laforgia N (2020) Vegetarian and vegan weaning of the infant: how common and how evidence‐based? A population‐based survey and narrative review. Int J Environ Res Public Health 17: 4835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara L, Lazzari R, Roda A, Aldini R, Festi D, Sama C, Morselli AM, Collina A, Bazzoli F, Mazzella G et al (1980) Serum bile acids in newborns and children. Pediatr Res 14: 1222–1225 [DOI] [PubMed] [Google Scholar]
- Benatar JR, Stewart RAH (2018) Cardiometabolic risk factors in vegans; a meta‐analysis of observational studies. PLoS One 13: e0209086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL (1986) A receptor‐mediated pathway for cholesterol homeostasis. Science 232: 34–47 [DOI] [PubMed] [Google Scholar]
- Craig WJ (2009) Health effects of vegan diets. Am J Clin Nutr 89(suppl): 1627S–1633S [DOI] [PubMed] [Google Scholar]
- De Aguiar Vallim TQ, Tarling EJ, Edwards PA (2013) Pleiotropic roles of bile acids in metabolism. Cell Metab 17: 657–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellière S, Cynober L (2017) Is transthyretin a good marker of nutritional status? Clin Nutr 36: 364–370 [DOI] [PubMed] [Google Scholar]
- Dinu M, Abbate R, Gensini GF, Casini A, Sofi F (2017) Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta‐analysis of observational studies. Crit Rev Food Sci Nutr 57: 3640–3649 [DOI] [PubMed] [Google Scholar]
- Elorinne AL, Alfthan G, Erlund I, Kivimäki H, Paju A, Salminen I, Turpeinen U, Voutilainen S, Laakso J (2016) Food and nutrient intake and nutritional status of Finnish vegans and non‐vegetarians. PLoS One 11: e0148235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farella I, Panza R, Baldassarre ME (2020) The difficult alliance between vegan parents and pediatrician: a case report. Int J Environ Res Public Health 17: 6380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrer T, Heer D, Begemann B, Zamboni N (2011) High‐throughput, accurate mass metabolome profiling of cellular extracts by flow injection ‐time‐of‐flight mass spectrometry. Anal Chem 83: 7074–7080 [DOI] [PubMed] [Google Scholar]
- Haapamäki E, Ranto S (2015) Varhaiskasvatus ja lasten hoidon tuet Helsingissä. Helsingin kaupungin tietokeskuksen tilastoja: 11. http://www.hel.fi/hel2/tietokeskus/julkaisut/pdf/15_03_31_Tilastoja_11_Ranto_Haapamaki.pdf [In Finnish].
- Hansen L, Tjønneland A, Køster B, Brot C, Andersen R, Cohen AS, Frederiksen K, Olsen A (2018) Vitamin D status and seasonal variation among danish children and adults: a descriptive study. Nutrients 10: 1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heird WC (2012) Nutritional requirements of infants and children In Modern Nutrition in Health and Disease, Ross CA, Caballero B, Cousins RJ, Tucker KL, Ziegler TR (eds), 11th edn, pp 712–733. Wolters Kluwer Health Adis; https://jhu.pure.elsevier.com/en/publications/modern‐nutrition‐in‐health‐and‐disease‐eleventh‐edition [Google Scholar]
- Itkonen ST, Andersen R, Björk AK, Konde ÅB, Eneroth H, Erkkola M, Holvik K, Madar AA, Meyer HE, Tetens I et al (2020) Vitamin D status and current policies to achieve adequate vitamin D intake in the Nordic countries. Scand J Public Health 10.1177/1403494819896878 [DOI] [PubMed] [Google Scholar]
- Janssen M, Busch C, Rödiger M, Hamm U (2016) Motives of consumers following a vegan diet and their attitudes towards animal agriculture. Appetite 105: 643–651 [DOI] [PubMed] [Google Scholar]
- Kiriyama Y, Nochi H (2019) The biosynthesis, signaling and neurological functions of bile acids. Biomolecules 9: 232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkalo L, Nissinen K, Skaffari E, Vepsäläinen H, Lehto R, Kaukonen RE, Koivusilta L, Sajaniemi N, Roos E, Erkkola M (2019) The contribution of preschool meals to the diet of Finnish preschoolers. Nutrients 11: 1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen NB, Madsen ML, Hansen TH, Allin KH, Hoppe C, Fagt S, Lausten MS, Gøbel RJ, Vestergaard H, Hansen T et al (2015) Intake of macro‐ and micronutrients in Danish vegans. Nutr J 14: 115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien EL, Hammond BR (2011) Nutritional influences on visual development and function. Prog Retin Eye Res 30: 188–203 [DOI] [PubMed] [Google Scholar]
- Lindqvist HM, Rådjursöga M, Malmodin D, Winkvist A, Ellegård L (2019) Serum metabolite profiles of habitual diet: evaluation by 1H‐nuclear magnetic resonance analysis. Am J Clin Nutr 110: 53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA et al (2019) ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J ehz455 [Google Scholar]
- Makide K, Uwamizu A, Shinjo Y, Ishiguro J, Okutani M, Inoue A, Aoki J (2014) Novel lysophospholipid receptors: their structure and function. J Lipid Res 55: 1986–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruvada P, Stover PJ, Mason JB, Bailey RL, Davis CD, Field MS, Finnell RH, Garza C, Green R, Gueant J‐L et al (2020) Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: a summary, and perspectives, from an NIH workshop. Am J Clin Nutr 112: 1390–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen TA, Tilvis RS, Kesäniemi YA (1990) Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol 131: 20–31 [DOI] [PubMed] [Google Scholar]
- Mramba L, Ngari M, Mwangome M, Muchai L, Bauni E, Walker AS, Gibb DM, Fegan G, Berkley JA (2017) A growth reference for mid upper arm circumference for age among school age children and adolescents, and validation for mortality: growth curve construction and longitudinal cohort study. BMJ 358: j3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DL, Cox MM (2012) Cholesterol, steroids and isoprenoids: Biosynthesis, regulation and transport, In Lehninger Principles of Biochemistry, Nelson DL, Cox MM (eds), 6th edn, pp 859–876, New York, NY: W.H. Freeman and Company; [Google Scholar]
- O'Keefe JH Jr, Cordain L, Harris WH, Moe RM, Vogel R (2004) Optimal low‐density lipoprotein is 50 to 70 mg/dl: lower is better and physiologically normal. J Am Coll Cardiol 43: 2142–2146 [DOI] [PubMed] [Google Scholar]
- Pawlak R (2017) To vegan or not to vegan when pregnant, lactating or feeding young children. Eur J Clin Nutr 71: 1259–1262 [DOI] [PubMed] [Google Scholar]
- Playdon MC, Moore SC, Derkach A, Reedy J, Subar AF, Sampson JN, Albanes D, Gu F, Kontto J, Lassale C et al (2017) Identifying biomarkers of dietary patterns by using metabolomics. Am J Clin Nutr 105: 450–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridlon JM, Wolf PG, Gaskins HR (2016) Taurocholic acid metabolism by gut microbes and colon cancer. Gut Microbes 7: 201–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risley JM (2002) Cholesterol biosynthesis: Lanosterol to cholesterol. J Chem Educ 79: 377–384 [Google Scholar]
- Saari A, Sankilampi U, Hannila M‐L, Kiviniemi V, Kesseli K, Dunkel L (2011) New Finnish growth references for children and adolescents aged 0 to 20 years: Length/height‐for‐age, weight‐for‐length/height and body mass index‐for‐age. Ann Med 43: 235–248 [DOI] [PubMed] [Google Scholar]
- Sanders TA (1988) Growth and development of British vegan children. Am J Clin Nutr 48: 822–825 [DOI] [PubMed] [Google Scholar]
- Sanders TA (2009) DHA status of vegetarians. Prostaglandins Leukot Essent Fatty Acids 81: 137–141 [DOI] [PubMed] [Google Scholar]
- Schmidt JA, Rinaldi S, Ferrari P, Carayol M, Achaintre D, Scalbert A, Cross AJ, Gunter MJ, Fensom GK, Appleby PN et al (2015) Metabolic profiles of male meat eaters, fish eaters, vegetarians and vegans from the EPIC‐Oxford cohort. Am J Clin Nutr 102: 1518–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JA, Rinaldi S, Scalbert A, Ferrari P, Achaintre D, Gunter MJ, Appleby PN, Key TJ, Travis RC (2016) Plasma concentration and intakes of amino acids in male meat‐eaters, fish‐eaters, vegetarians and vegans: a cross‐sectional analysis in the EPIC‐Oxford cohort. Eur J Clin Nutr 70: 306–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönfeld P, Wojtczak L (2016) Short‐ and medium‐chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res 57: 943–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmann S, Kersting M, Alexy U (2017) Vegetarian diets in children: a systematic review. Eur J Nutr 56: 1797–1817 [DOI] [PubMed] [Google Scholar]
- Semba RD, Shardell M, Sakr Ashour FA, Moaddel R, Trehan I, Maleta KM, Ordiz I, Kraemer K, Khadeer MA, Ferrucci L et al (2016) Child stunting is associated with low circulating essential amino acids. EBioMedicine 6: 246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talsma EF, Verhoef H, Brouwer ID, Mburu‐de Wagt AS, Hulshof PJM, Melse‐Boonstra A (2015) Proxy markers of serum retinol concentration, used alone and in combination, to assess population vitamin A status in Kenyan children: a cross‐sectional study. BMC Med 13: 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ (2016) Biomarkers of nutrition for development (BOND)—vitamin A review. J Nutr 146: 1816S–1848S [DOI] [PMC free article] [PubMed] [Google Scholar]
- THL (Finnish Institute for Health and Welfare) (2019) Eating together ‐ food recommendations for families with children, 2nd updated ed Finland: Helsinki; http://urn.fi/URN:ISBN:978‐952‐343‐264‐2 [Google Scholar]
- Tong TYN, Appleby PN, Bradbury KE, Perez‐Cornago A, Travis RC, Clarke R, Key TJ (2019) Risks of ischaemic heart disease and stroke in meat eaters, fish eaters, and vegetarians over 18 years of follow‐up: results from the prospective EPIC‐Oxford study. BMJ 366: I4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripkovic L, Wilson LR, Hart K, Johnsen S, de Lusignan S, Smith CP, Bucca G, Penson S, Chope G, Elliott R et al (2017) Daily supplementation with 15 μg vitamin D2 compared with vitamin D3 to increase wintertime 25‐hydroxyvitamin D status in healthy South Asian and white European women: a 12‐wk randomized, placebo‐controlled food‐fortification trial. Am J Clin Nutr 106: 481–490 [DOI] [PubMed] [Google Scholar]
- WHO Expert committee (1995) Physical status: the use and interpretation of anthropometry. WHO Technical Report Series 854 [PubMed]
- World Health Organization (2007) Construction of the arm circumference‐for‐age standards In WHO Child Growth Standards: Head Circumference‐for‐age, Arm Circumference‐for‐age, Triceps Skinfold‐for‐age and Subscapular Skinfold‐for‐age. Methods and Development, de Onis M et al (ed), pp 57–106. World Health Organization; https://apps.who.int/iris/handle/10665/43706 [Google Scholar]
- Xiang X, Han Y, Neuvonen M, Laitila J, Neuvonen PJ, Niemi M (2010) High performance liquid chromatography‐tandem mass spectrometry for the determination of bile acid concentrations in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 878: 51–60 [DOI] [PubMed] [Google Scholar]
- Zhao X, Gang X, Liu Y, Sun C, Han Q, Wang G (2016) Using metabolomic profiles as biomarkers for insulin resistance in childhood obesity: a systematic review. J Diabetes Res 2016: 8160545 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Table EV1
Table EV2
Table EV3
Table EV4
Table EV5
Table EV6
Source Data for Expanded View
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Data Availability Statement
All R code used in statistical analysis and building figures for this article are open access in GitHub: https://github.com/topihovinen/mirahelsinki.
This study includes no data deposited in external repositories.


