Abstract
The lack of vasculogenesis often hampers the survivability and integration of newly engineered tissue grafts within the host. Autologous endothelial cells (ECs) are an ideal cell source for neovascularization, but they are limited by their scarcity, lack of proliferative capacity, and donor site morbidity upon isolation. The objective of this study was to determine whether differentiation of human dental pulp stem cells (DPSCs) into the endothelial lineage can be enhanced by recombinant ETV2 overexpression. DPSCs were extracted from fresh dental pulp tissues. ETV2 overexpression in DPSCs was achieved by lentiviral infection and cellular morphological changes were evaluated. The mRNA and protein expression levels of endothelial-specific markers were assessed through quantitative real-time polymerase chain reaction, western blot, immunofluorescence staining, and flow cytometry. The tube formation assay and Matrigel plug assay were also performed to evaluate the angiogenic potential of the ETV2-transduced cells in vitro and in vivo, respectively. Additionally, proteomic analysis was performed to analyze global changes in protein expression following ETV2 overexpression. After lentiviral infection, ETV2-overexpressing DPSCs showed endothelial-like morphology. Compared with control DPSCs, significantly higher mRNA and protein expression levels of endothelial-specific genes, including CD31, VE-Cadherin, VEGFR1, and VEGFR2, were detected in ETV2-overexpressing DPSCs. Moreover, ETV2 overexpression enhanced capillary-like tube formation on Matrigel in vitro, as well as neovascularization in vivo. In addition, comparative proteomic profiling showed that ETV2 overexpression upregulated the expression of vascular endothelial growth factor (VEGF) receptors, which was indicative of increased VEGF signaling. Taken together, our results indicate that ETV2 overexpression significantly enhanced the endothelial differentiation of DPSCs. Thus, this study shows that DPSCs can be a promising candidate cell source for tissue engineering applications.
Keywords: vascular biology, transcription factors, tissue engineering, proteomics, cell differentiation, receptors
Introduction
Tissue engineering approaches have been extensively investigated for the repair or replacement of damaged tissues or organs. However, the lack of vasculogenesis remains an intractable problem and often leads to dysfunction and lack of integration of newly engineered tissue grafts after transplantation into the host. For example, lack of sufficient blood vessels in tissue-engineered bone constructs often results in cell death within the central regions of the implanted grafts and could impede bone defect healing, particularly in the case of critical-sized bone defects1. Therefore, prevascularization could be a promising approach to promote vasculogenesis prior to the implantation of newly engineered tissue grafts2. As one of the major components in vasculature development, endothelial cells (ECs) form the inner lining of blood vessels of the entire circulatory system, and they are indispensable for prevascularization. Some research studies focusing on EC-based therapies revealed that autologous ECs can make a significant contribution to enhancing neovascularization in the treatment of vascular injuries or ischemic diseases3,4. Nevertheless, the shortage of host tissue sources for cell extraction and the limited proliferative capacity of primary ECs hinder the clinical application of these cells on a large scale.
Embryonic stem cells (ESCs) have been shown to possess the potential to generate the EC lineage5,6. However, ESCs are not suitable for large-scale production of ECs, due to their low differentiation efficiency and controversial ethical issues pertaining to their clinical application. Meanwhile, many studies have also utilized induced pluripotent stem cells (iPSCs) to generate ECs7–9. However, the time-consuming process and the risk of tumorigenicity limit the broader clinical application of iPSCs. Adult stem cells (ASCs) possess the potential to differentiate into various cell types and can be a promising candidate for tissue engineering. Previous studies reported that ASCs can be isolated from various tissues, such as the amnion10, adipose tissue11, bone marrow12, and dental pulp13. The focus of this study will be on dental pulp stem cells (DPSCs), which is one of the most widely utilized cell types in tissue engineering applications, as these cells can be readily isolated from teeth extracted during dental treatment.
Various approaches have been investigated to promote endothelial lineage differentiation, including co-culture with other cell types14, treatment with growth factors15, modulation of signaling pathways16, and regulation of expression of transcription factors17. ETV2/ER71/Etsrp, a transcription factor of the Ets family, has been reported by previous studies to play a pivotal role in endothelial differentiation18,19. This study aims to provide insights into the effect of ETV2 overexpression on endothelial differentiation of DPSCs and perform the related proteomic analysis, so as to develop a novel strategy for vascular tissue engineering.
Materials and Methods
Cell Culture
This study was performed with the approval of the Medical Ethics Committee of the School of Stomatology, Shandong University. Written informed consent was obtained from all participants. Human dental pulp tissues were derived from third molars extracted from healthy patients aged 18 to 22 years. After being washed and minced, the pulp tissues were digested with a mixture of collagenase I (Invitrogen, Carlsbad, CA, USA) and dispase II (Sigma-Aldrich, St. Louis, USA). After filtration and centrifugation, the cells were resuspended in alpha minimum essential medium (α-MEM; Biological Industries, Israel) supplemented with 10% (v/v) fetal bovine serum (FBS; Biological Industries, Israel) and 1% ((w/v) penicillin/streptomycin (Invitrogen). Human umbilical vein endothelial cells (HUVECs) were purchased from ScienceCell (Invitrogen) and were cultured in endothelial cell growth medium (EGM; Cat#CC-3162, Lonza, Walkersville, MD, USA).
To evaluate the colony-forming potential of DPSCs, approximately 1000 cells were cultured in a 100-mm dish. After 10 days, the cells were stained with crystal violet. Additionally, flow cytometry was used to assess the expression of cell surface markers. Anti-CD24-FITC, anti-29-FITC, anti-CD34-PE, anti-CD44-FITC, anti-CD45-FITC, anti-CD73-FITC, anti-CD90-PE, and anti-CD105-PE antibodies (eBioscience, San Diego, CA, USA) were utilized in our study. The trilineage differentiation capacity of DPSCs was evaluated through osteogenic, adipogenic, and chondrogenic differentiation assay kits (Cyagen, USA, HUXDP-90021, HUXDP-90031, and HUXMA-9004), following the manufacturer’s protocols.
Lentivirus Transduction
ETV2-encoding lentiviral particles or control lentiviral particles were prepared by the GeneCopoeia Company (Guangzhou, China). For lentiviral infection, DPSCs were incubated with lentiviral particles and 8 μg/ml polybrene for 12 h. Then, the infection medium was replaced with a fresh culture medium. Transduction efficiency was assessed by quantitative real-time polymerase chain reaction (qRT-PCR), western blot analysis, and immunofluorescence staining.
qRT-PCR
The infected DPSCs were cultured at a density of 3 × 104 cells/cm2 in EGM for 0, 7, and 14 days. Total RNA from infected DPSCs at different time points was extracted with Trizol reagent (Invitrogen). After concentration determination, the extracted RNA was reverse-transcribed into cDNA following the manufacturer’s instructions using a PrimeScript RT reagent Kit (TaKaRa Biotech, Tokyo, Japan). PCR was performed with a Roche 480 instrument, and quantitative analysis of the gene transcripts was performed using the 2− △△ Ct method. All gene expression levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. The primer sequences are listed in Table 1.
Table 1.
Sequences of the Primers.
| Gene | Primers. | Sequence |
|---|---|---|
| ETV2 | Forward | ACGTCTCGGAAAATTCCCCC |
| Reverse | CATCCCAGTTCCACAGGTCC | |
| CD31 | Forward | AAGCTGCCGGTTCTTAAATCC |
| Reverse | AACTTGGTGGAAGGAGGGTATG | |
| VE-Cadherin | Forward | ATGAGATCGTGGTGGAAGCG |
| Reverse | ATGTGTACTTGGTCTGGGTGA | |
| VEGFR1 | Forward | TCCACCAAGATCTAAATCCAAACA |
| Reverse | CTGTCACAGGTGGTTTGCGTAT | |
| VEGFR2 | Forward | CGGTCAACAAAGTCGGGAGA |
| Reverse | CAGTGCACCACAAAGACACG | |
| GAPDH | Forward | TGCACCACCAACTGCTTAGC |
| Reverse | GGCATGGACTGTGGTCATGAG |
Western Blot Analysis
After the infected DPSCs were cultured in EGM for 0, 7, and 14 days, total protein lysates were extracted with radioimmunoprecipitation assay lysis buffer containing 1% (w/v) phenylmethylsulfonyl fluoride (Solarbio, Beijing, China). Then, the detection of protein concentrations was performed with a bicinchoninic acid protein assay kit (Solarbio), followed by protein denaturation using sodium dodecyl sulfate (SDS). Next, the protein samples were separated on SDS-polyacrylamide gels and transferred onto polyvinylidene fluoride membranes (Invitrogen). After blocking with 5% (w/v) skimmed milk, the membranes were probed with various antibodies overnight at 4 °C. The following primary antibodies were used in this study, which were specific for ETV2 (Cat#Ab181847, Abcam, MA, USA), CD31 (Cat#Ab32457, Abcam), VE-Cadherin (Cat#Ab33168, Abcam), VEGFR1 (Cat#Ab9540, Abcam), VEGFR2 (Cat#Ab134191, Abcam), and GAPDH (Cat#Ab181602, Abcam). Subsequently, the membranes were incubated with the secondary antibody (Zsbio, Beijing, China) and a chemiluminescent substrate ECL kit (Millipore, Billerica, MA, USA) was used to visualize the bands. The ImageJ software was used to quantify the densitometric intensities of the bands, and GAPDH was set as an internal control standard.
Immunofluorescence Staining
Immunofluorescence staining was performed after culturing the infected DPSCs or HUVECs in EGM for 7 days. The cells were treated with trypsin and seeded at a density of 1 × 104 cells/cm2 within a 24-well plate. After 24 h, the cells were fixed with 4% (v/v) paraformaldehyde for 30 min, followed by treatment with 0.25% (w/v) Triton X-100 for 10 min. After blocking with 3% (w/v) bovine serum albumin (BSA) for 60 min, the cells were incubated with primary antibodies against ETV2 (Cat#Ab181847, Abcam) and VE-Cadherin (Cat#Ab33168, Abcam) overnight at 4 °C. Then, the cells were incubated with a fluorescence-conjugated secondary antibody (Beyotime, Shanghai, China) for 60 min at room temperature. Diamidinophenylindole staining was performed for 5 min before examination under fluorescence microscopy.
Flow Cytometry
After 7 days of endothelial differentiation, the infected DPSCs or HUVECs were trypsinized, followed by resuspension in PBS. Next, the cells were incubated for 30 min at 4 °C with APC-conjugated monoclonal antibody against VE-Cadherin (17-1449-42, eBioscience). The isotypic control used was APC-IgG1 (17-4714-81, eBioscience). The analysis of the stained cells was performed with a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
In Vitro Tube Formation Assay on Matrigel
To evaluate the angiogenic potential of DPSCs in forming tube-like structures, the infected cells were trypsinized and resuspended in EGM. Subsequently, the cells were plated in a 96-well plate pre-coated with 50 µl of growth factor-reduced Matrigel (Cat#354230; BD Biosciences) at a density of 2 × 104 cells per well. Images were captured under an inverted phase-contrast microscope. Analysis of tube formation was performed using the ImageJ software.
In Vivo Matrigel Plug Assay
All animal experiment protocols were approved by the Animal Care and Use Committee of Shandong University. To evaluate the capacity for capillary formation by the infected DPSCs or HUVECs in vivo, 20 male 8-week-old athymic nude mice were utilized in this study and were divided randomly into the following 4 groups (n = 5 per group): (1) the HUVECs group: HUVECs embedded in Matrigel; (2) the ETV2 group: ETV2-overexpressing DPSCs embedded in Matrigel; (3) the control group: control DPSCs embedded in Matrigel; (4) the blank group: Matrigel without cells. A total of 1 × 106 cells were suspended in 500 µl of growth factor-reduced Matrigel. Then, the mixture was injected into the mice subcutaneously within the dorsal area with a 25-gauge needle. Fourteen days later, the Matrigel plugs were taken out and photographed. Then, the plugs were fixed in 4% (w/v) paraformaldehyde, embedded in paraffin, and sectioned for hematoxylin and eosin staining and immunohistochemistry with anti-human CD31 antibody (Cat#Ab32457, Abcam). All images were captured by a light microscope (BX51, Olympus, Tokyo, Japan).
Proteomic Analysis
DPSCs derived from the 3 different donors were infected with ETV2-overexpressing lentiviral particles or control lentiviral particles. Protein samples were harvested after 10 days of endothelial differentiation. Proteome profiling was performed by the iProteome platform of the Fudan University Human Phenome Institute according to the user guide. Proteins with fold change >2 were considered to be differentially expressed. Functional enrichment analysis of identified proteins was accomplished through Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis, and Reactome pathway analysis.
Statistical Analysis
Statistical analysis was conducted using the Prism 6 software (GraphPad Software, La Jolla, CA, USA). All experiments were carried out in triplicates, and all data were presented as mean ± SD. Statistical comparisons were performed by Student’s t-test, one-way analysis of variance, or two-way analysis of variance. P values of less than 0.05 were considered to be statistically significant.
Results
Characterization of Human DPSCs and Detection of ETV2 Expression
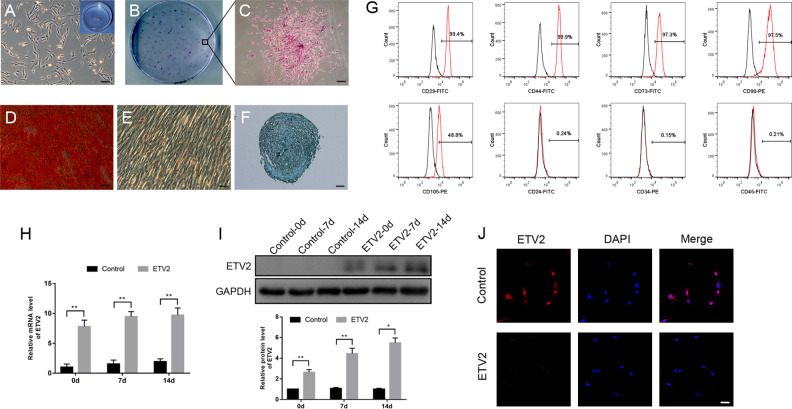
At the third culture passage, the DPSCs exhibited a typical spindle-shape morphology (Fig. 1A) and had the ability to form colonies (Fig. 1B, C). Fig. 1D–F show that after the cells were cultured in osteogenic, adipogenic, and chondrogenic differentiation media for 21 days, the multilineage differentiation potential of DPSCs was validated by the positive results of Oil Red O, Alcian Blue, and Alizarin red S staining, respectively. Meanwhile, flow cytometry analysis (Fig. 1G) showed that the cells displayed positive expression of canonical mesenchymal stem cell markers CD29, CD44, CD73, CD90, and CD105, but they were negative for CD24, CD45, and CD34.
Figure 1.
Characterization of human DPSCs and confirmation of ETV2 overexpression efficiency. (A) Cell morphology of DPSCs. Scale bar: 50 μm. (B, C) The colony-forming potential of DPSCs was confirmed through crystal violet staining. Scale bar: 200 μm. (D to F) Multilineage differentiation potential of DPSCs was verified through osteogenic, adipogenic, and chondrogenic induction assays. Scale bar: 50 μm. (G) Flow cytometric characterization of DPSCs showed that the cells were positive for CD29, CD44, CD73, CD90, and CD105 and negative for CD24, CD45, and CD34. (H to J) The results of quantitative real-time polymerase chain reaction (H), Western blot analysis (I), and immunofluorescence staining (J) showed that the mRNA and protein expression levels of ETV2 were significantly upregulated in the infected group. Scale bar: 50 μm, *P < 0.05, **P < 0.01. DPSCs: dental pulp stem cells.
After lentiviral infection, the mRNA and protein expression levels of ETV2 were quantified. The results of qRT-PCR (Fig. 1H) showed that ETV2 expression at the mRNA level in the ETV2-infected cells was more than 7-fold higher relative to that in the control cells. Similarly, the protein expression level of ETV2 was also significantly upregulated in the ETV2-infected cells, as compared to the control cells, according to the results of western blot (Fig. 1I) and immunofluorescence (Fig. 1J) analyses.
ETV2 Overexpression Upregulated the mRNA and Protein Expression Levels of Endothelial Markers
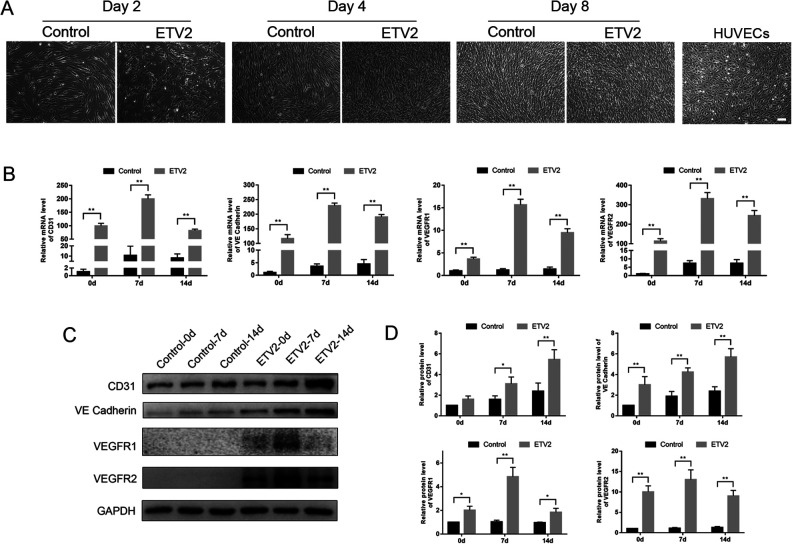
From day 2 onward after ETV2 lentiviral infection, the cells exhibited a cobblestone-like morphology, a typical feature of ECs. In comparison, the control cells retained a fibroblast-like morphology and were typically spindle shaped (Fig. 2A).
Figure 2.
Effects of ETV2 overexpression on mRNA and protein expression levels of endothelial-specific gene markers. (A) Observation of morphological changes showed that ETV2 overexpressing DPSCs displayed cobblestone-like morphology as early as day 2 of induction. Scale bar: 100 μm. (B) mRNA expression levels of CD31, VE-Cadherin, VEGFR1, and VEGFR2 on days 0, 7, and 14 of endothelial differentiation. (C) Protein expression levels of CD31, VE-Cadherin, VEGFR1, and VEGFR2 on days 0, 7, and 14 of endothelial differentiation, as detected by Western blot analysis. (D) Relative quantification of the protein expression levels was shown by the column chart. *P < 0.05, **P < 0.01. DPSCs: dental pulp stem cells.
As shown in Fig. 2B, on day 0 of endothelial differentiation, the results of qRT-PCR on day 0 revealed a significant increase in the expression of endothelial markers, including CD31, VE-Cadherin, VEGFR1, and VEGFR2, in the ETV2-infected group, as compared with the control group. After 7 and 14 days of induction, the expression levels of endothelial-specific genes displayed the same trend.
Consistently, western blot analysis on days 0, 7, and 14 showed significantly upregulated protein expression levels of CD31, VE-Cadherin, VEGFR1, and VEGFR2 in the ETV2-infected group, as compared with the control group (Fig. 2C, D), but the difference in CD31 expression between groups on day 0 was not statistically significant (P > 0.05).
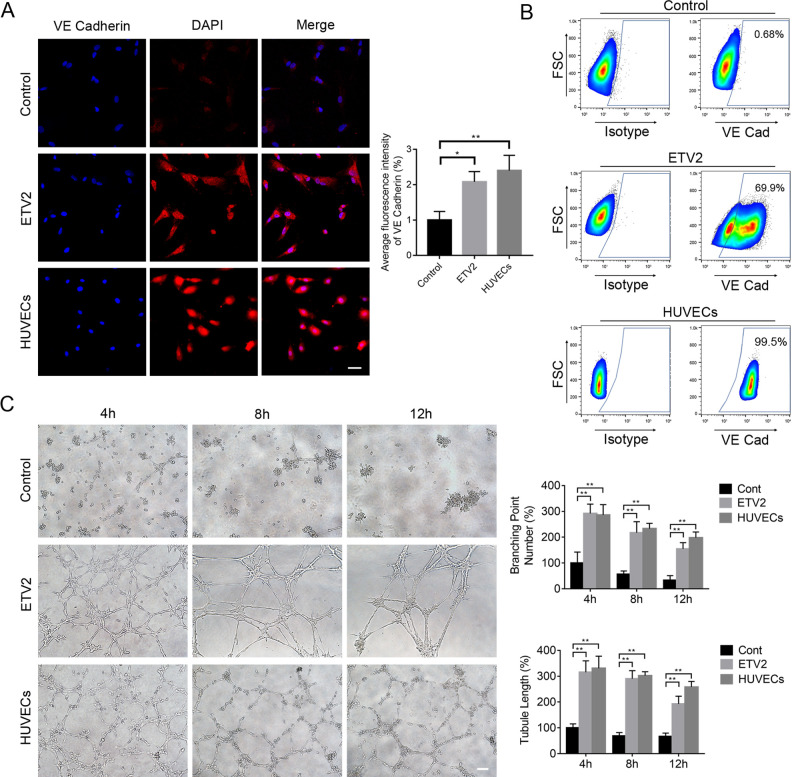
Immunofluorescence staining analysis further showed that after endothelial induction for 7 days, the ETV2 group yielded significant enhancement in the expression of VE-Cadherin, as compared with the control group (Fig. 3A).
Figure 3.
ETV2 overexpression enhanced endothelial differentiation of DPSCs. (A) Effects of ETV2 overexpression on the protein expression levels of VE-Cadherin, as detected by immunofluorescence staining and quantitative analysis of fluorescence intensity. Scale bar: 50 μm. (B) Evaluation of endothelial-specific marker expression by flow cytometry. (C) Results of tube formation assay in vitro and quantification of tubule length and branching point numbers. Scale bar: 50 μm, *P < 0.05, **P < 0.01. DPSCs: dental pulp stem cells.
Additionally, we also used flow cytometry to detect the protein expression levels of VE-Cadherin at 7 days after induction. Fig. 3B showed elevated VE-Cadherin expression in most of the ETV2-infected cells (69.90% ± 8.58%), implying that the majority of induced cells in the ETV2-infected group displayed characteristics of the endothelial lineage. In contrast, the control cells only expressed low levels of VE-Cadherin (0.68 ± 0.14). Thus, recombinant ETV2 overexpression could stimulate the transformation of DPSCs to early EC-like cells.
ETV2 Overexpression Promoted Tube Formation by DPSCs
To compare functional capacities between groups, the tube formation assay on Matrigel was performed. As shown in Fig. 3C, after being incubated for 24 h, the cells in the ETV2 group displayed tube-like structures and formed networks after 3 h to 12 h of culture on Matrigel in vitro. In contrast, the cells in the control group exhibited very limited network formation. Furthermore, image analysis showed that tubule lengths and branching point numbers were significantly enhanced in the ETV2 group relative to the control group, thus indicating that ETV2 overexpression could induce the expression of endothelial characteristics by DPSCs.
Proteomic Analysis of ETV2-Overexpressing DPSCs
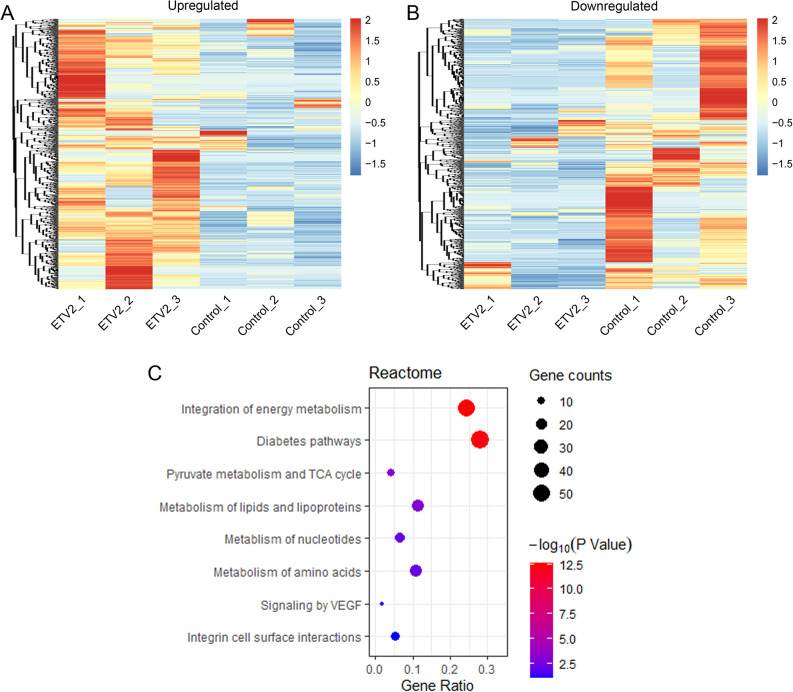
To analyze the global changes in protein expression following ETV2 overexpression, proteomic profiling was performed. In total, 4079-5128 proteins were identified in 6 samples. Then, the proteomes of ETV2-overexpressing DPSCs were compared with control DPSCs. Proteins with expression levels differing by more than 2-fold in at least 2 donors were regarded as differentially expressed proteins. Among the differentially expressed proteins, 941 were upregulated while 883 were downregulated in the ETV2-overexpressing cells compared with the control cells (Fig. 4A, B). Specifically, vascular endothelial growth factor (VEGF) receptors, including VEGFR1/FLT1, VEGFR2/FLK1/KDR, and NRP2, were in the list of upregulated proteins. GO enrichment analysis was used to annotate the identified proteins according to their functional features and the differentially expressed proteins were classified by the biological process or molecular function. As shown in Supplemental Fig. 1A and B, proteins associated with the electron transport chain and NADH dehydrogenase (ubiquinone) activity exhibited the most significant changes in the biological process (BP) and molecular function (MF) of the upregulated proteins, respectively. For downregulated proteins, mitotic cell cycle and adenyl ribonucleotide binding were the top enriched terms of BP and MF, respectively (Supplemental Fig. 1C, D). The enriched KEGG pathway terms in the top 20 of the lists are shown in Supplemental Fig. 2. Additionally, the Reactome pathway enrichment analysis is presented in Fig. 4C and Supplemental Tables S1. Particularly, as shown in Fig. 4C, among the limited pathway terms related to upregulated proteins, the term “signaling by VEGF” (4.7-fold) has been described in angiogenesis, which is related to the regulation of the development of ECs. The mass spectrometry proteomics data have been deposited into ProteomeXchange via the iProX partner repository with the dataset identifier PXD018138.
Figure 4.
Proteome analysis of control and ETV2-overexpressing DPSCs. (A, B) Heat map analysis identified 941 upregulated protein (A) and 883 downregulated proteins (B) in the ETV2 group, as compared with the control group. (C) Reactome pathway enrichment analysis related to upregulated proteins.
ETV2 Overexpression Enhanced Angiogenesis Within Matrigel Plugs In Vivo
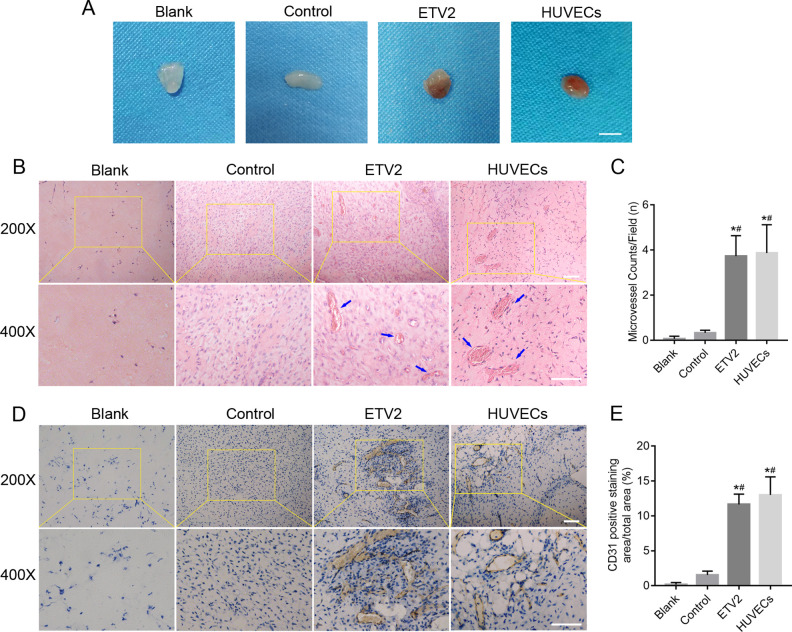
Additionally, to investigate whether ETV2 overexpression can exert proangiogenic effects in vivo, the Matrigel plug assay was performed in mice. At 14 days postimplantation, the implanted Matrigel plugs were taken out and analyzed. General observation of the plugs revealed that the implants were nearly transparent in the blank and control groups, while reddish cell masses with macroscopic vessels were observed in the ETV2 group (Fig. 5A). After hematoxylin and eosin staining, capillaries containing red blood cells can be observed in the ETV2 group under microscopy (Fig. 5B). Also, a significant increase in the blood vessel counts was observed in the ETV2 group, as compared with the other 2 groups (Fig. 5C). Meanwhile, Fig. 5D and E show that the positive staining area for CD31 was markedly increased in the ETV2 group, as compared with the blank or control group. Altogether, our results showed that ETV2 possessed the capacity to stimulate angiogenesis in vitro and in vivo.
Figure 5.
Effects of ETV2 overexpression on angiogenesis in vivo. (A) Representative photographs of Matrigel plugs under general observation. (B) Histological characterization of Matrigel plugs by hematoxylin and eosin staining and (C) quantification of blood vessels within the plugs. The blue arrow shows capillaries containing red blood cells. (D) Representative immunohistochemistry images after staining with anti-CD31 antibody and (E) quantitative analysis of CD31 positively-stained area. Scale bar: 50 μm, *P < 0.05, **P < 0.01.
Discussion
Many different approaches have been investigated to overcome the problem of insufficient vasculogenesis in tissue engineering14– 17. The results of this study provide new evidence for the stimulatory effects of ETV2 on endothelial differentiation. After being infected with ETV2-encoding lentiviral vectors, DPSCs displayed EC-like morphology. Upon recombinant overexpression of ETV2, the expression levels of other endothelial-specific genes and proteins were also significantly upregulated. Moreover, the enhanced formation of vascular networks in vitro and increased formation of functional blood vessels in vivo further validated the proangiogenic effects of ETV2. Overall, our results confirmed that DPSCs can be a promising cell candidate for therapeutic applications, as well as the development of a novel strategy for vascular tissue engineering.
Considering the limited availability of autologous ECs, autologous mesenchymal stem cells (MSCs) may potentially be a readily available alternative cell source for neovascularization of newly engineered tissue grafts. As a subtype of ASCs, MSCs possess multilineage differentiation potential20. DPSCs, which are in fact dental-derived MSCs originating from the dental pulp tissue, exhibit typical fibroblast-like morphology. The differentiation potential of these cells into osteoblasts, adipocytes, and chondroblasts was validated in this study. However, it must be noted that the differentiation potential of MSCs into the endothelial lineage is still controversial, although several previous studies have reported about the induction of MSCs into the endothelial lineage21– 23. For example, Roobrouck et al.24 reported that the expression levels of endothelial markers, including CD31, vWF, and Tie2, remained relatively constant, or were even repressed in human bone marrow-derived MSCs (hBMSCs) after 14 days of induction with VEGF. Moreover, functional assays showed that MSCs failed to develop functional vessels in vivo. These observations were similar to the results reported by Fan et al.21, which showed that there was no significant increase in the expression of endothelial gene markers, such as CD31, VEGFR2, and vWF, in hBMSCs induced with EC differentiation medium. Furthermore, Otsu et al.25 reported that MSCs at high cell densities negatively modulated the generation of neocapillary networks. In this study, the control DPSCs displayed increased mRNA expression levels of CD31, VE-Cadherin, and VEGFR2, as well as upregulated protein expression levels of CD31 and VE-Cadherin after 14 days of endothelial differentiation. Despite this finding, the control group showed few capillaries following subcutaneous implantation in vivo. Overall, our results indicate that DPSCs may yield limited vascular regeneration with regular endothelial induction.
Various strategies have been attempted to enhance the differentiation of MSCs into the endothelial lineage. According to a study by Zhang et al.26, the activation of Wnt signaling positively modulated the vasculogenic differentiation of DPSCs and stem cells from human exfoliated deciduous teeth (SHED). Meanwhile, Xu et al.16 showed that inhibition of transforming growth factor-beta signaling could upregulate the differentiation of SHED into the endothelial lineage. Additionally, growth factors also play a vital role in the process of endothelial differentiation. For example, concentrated growth factors, which are extracted from autologous plasma, exert a stimulatory effect on the angiogenic process of DPSCs, as reported in a study by Jin et al22. Moreover, noncoding RNAs, such as miRNA-12627 and lncRNA TUG123, have also been shown to exert positive effects on the endothelial differentiation of adipose-derived stem cells (ADSCs). However, the endothelial induction efficiency was still not high enough to meet the requirements for clinical application.
The modulation of transcription factor activity could possibly yield a more efficient differentiation of MSCs into the endothelial lineage. The Ets family of transcription factors has been shown to play pivotal roles in hematopoiesis and vasculogenesis. In particular, ETV2 has attracted much attention among the various Ets factors28. Accumulating evidence indicates that ETV2 alone or combined with other transcription factors possesses the ability to reprogram the fibroblasts or MSCs into endothelial cells. According to the research by Han et al.29, the combination of transcription factors Etv2/Er71, Foxo1, Klf2, Tal1, and Lmo2 could convert mouse adult skin fibroblasts into ECs. Meanwhile, Wong WT et al.30 reported that 4 endothelial transcription factors (ETV2, FLI1, GATA2, and KLF4) possess the potential to efficiently transdifferentiate human neonatal fibroblasts into ECs. At the same time, some studies of human fibroblasts revealed that ETV2 alone is sufficient to reprogram cells, such as human postnatal dermal fibroblasts31 or primary human adult skin fibroblasts, into ECs32. In addition, Yan et al.29 reported that after infection with ETV2-encoding lentiviral vectors, 5 different cell types, including ADSCs and umbilical cord-derived MSCs, exhibited endothelial-like morphology, and the protein expression level of the endothelial marker VE-Cadherin was observed to be markedly upregulated with flow cytometry. As a subpopulation of MSCs, human DPSCs represent potentially promising candidate cell sources for tissue engineering due to their characteristics, like the ease of isolation with minimal invasiveness, ready availability, and immunocompatibility arising from their autologous origin. Our flow cytometry data showed that nearly 70% of ETV2-overexpressing DPSCs also expressed VE-Cadherin. Additionally, the ETV2-overexpressing DPSCs exhibited significantly upregulated levels of various vascular-related gene and protein markers in vitro and markedly stronger capacity for capillary formation in vivo. Although the formation of functional blood vessels in vivo was observed after ETV2 overexpression in this study, the long-term stability of the newly formed vessels is still uncertain, and a longer experimental period may be needed for verification. Morita et al.32 found that, on day 28 after Matrigel implantation, endothelial nitric oxide synthase expression was observed in some functional vascular endothelial cells induced by ETV2, and these cells may possess the ability to form stable vasculature. Meanwhile, it has been reported that after the formation of the initial lumen, blood flow is required for stabilization of functional vessels33. Overall, our findings suggest that ETV2-infected DPSCs have the potential to substitute autologous endothelial cells for clinical applications. However, there are underlying safety issues associated with the viral vectors used in this study. Techniques that do not involve genetic modification, for example, the utilization of small molecule compounds that can upregulate ETV2 expression, maybe a better option. What’s more, long-term overexpression of ETV2 could lead to excessive blood vessel formation. To avoid generating excessive vasculature, the doxycycline-dependent inducible expression systems are often adopted to regulate the expression of the specific transcription factor ETV2.
Proteome-wide analyses of control DPSCs and ETV2-overexpressing DPSCs were performed in this study. Our results showed that the protein expression levels of VEGF receptors VEGFR1/FLT1, VEGFR2/KDR/FLK1, and NRP2 were upregulated and that this upregulation was related to increased signaling by VEGF upon ETV2 overexpression. Similarly, ETV2 ChIP-Seq analysis by Liu et al.34 showed that VEGF receptors, including Flk1, Flt1, Nrp1, and Nrp2, were directly upregulated by ETV2 and that the activity of the VEGF signaling pathway could be amplified by ETV2 overexpression. Likewise, Yan et al.29 performed a transcriptome-wide analysis of ETV2 expression in HSkMCs and found that the VEGF-VEGFR2 pathway was significantly upregulated in ETV2-HSkMCs. Notably, VEGF signaling, in particular VEGFR2 signaling, plays a vital role in regulating the development of ECs. Moreover, the ETV2-FLK1 feed-forward mechanism34 amplifies the markedly upregulated expression of VEGFR2/KDR/FLK1, which in turn further enhances endothelial differentiation. Altogether, our results provide additional insights into how differentiation into the endothelial lineage is enhanced upon ETV2 overexpression.
In summary, ETV2-transduced DPSCs display proangiogenic effects both in vitro and in vivo. Furthermore, these findings confirm that DPSCs can be a novel potential cell source for clinical application in tissue engineering.
Supplemental Material
Supplemental Material, sj-pdf-1-cll-10.1177_0963689720978739 for Upregulation of ETV2 Expression Promotes Endothelial Differentiation of Human Dental Pulp Stem Cells by Jing Li, Youming Zhu, Na Li, Tao Wu, Xianyu Zheng, Boon chin Heng, Duohong Zou and Jianguang Xu in Cell Transplantation
Footnotes
Ethical Approval: The study was approved by Ethics Committee of the School of Stomatology, Shandong University.
Statement of Human and Animal Rights: All animal experiment protocols were approved by the Animal Care and Use Committee of Shandong University.
Statement of Informed Consent: Written informed consent was obtained from all participants prior to research participation.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Natural Science Foundation of Anhui Province (CN) (1908085MH255).
ORCID iD: Jianguang Xu  https://orcid.org/0000-0001-8773-1241
https://orcid.org/0000-0001-8773-1241
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Liu Y, Chan JK, Teoh SH. Review of vascularised bone tissue-engineering strategies with a focus on co-culture systems. J Tissue Eng Regen Med. 2015;9(2):85–105. [DOI] [PubMed] [Google Scholar]
- 2. Laschke MW, Menger MD. Prevascularization in tissue engineering: current concepts and future directions. Biotechnol Adv. 2016;34(2):112–121. [DOI] [PubMed] [Google Scholar]
- 3. Hagensen MK, Raarup MK, Mortensen MB, Thim T, Nyengaard JR, Falk E, Bentzon JF. Circulating endothelial progenitor cells do not contribute to regeneration of endothelium after murine arterial injury. Cardiovasc Res. 2012;93(2):223–231. [DOI] [PubMed] [Google Scholar]
- 4. Losordo DW, Kibbe MR, Mendelsohn F, Marston W, Driver VR, Sharafuddin M, Teodorescu V, Wiechmann BN, Thompson C, Kraiss L, Carman T, et al. A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circ Cardiovasc Interv. 2012;5(6):821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richards M, Tan S, Fong CY, Biswas A, Chan WK, Bongso A. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells. 2003;21(5):546–556. [DOI] [PubMed] [Google Scholar]
- 6. Zhao H, Li M, Ouyang Q, Lin G, Hu L. VEGF promotes endothelial cell differentiation from human embryonic stem cells mainly through PKC-ε/η pathway. Stem Cells Dev. 2020;29(2):90–99. [DOI] [PubMed] [Google Scholar]
- 7. Xu B, Kurachi M, Shimauchi-Ohtaki H, Yoshimoto Y, Ishizaki Y. Transplantation of iPS-derived vascular endothelial cells improves white matter ischemic damage. J Neurochem. 2020;153(6):759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Atchison L, Abutaleb NO, Snyder-Mounts E, Gete Y, Ladha A, Ribar T, Cao K, Truskey GA. iPSC-derived endothelial cells affect vascular function in a tissue-engineered blood vessel model of hutchinson-gilford progeria syndrome. Stem Cell Reports. 2020;14(2):325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rose M, Gao K, Cortez-Toledo E, Agu E, Hyllen AA, Conroy K, Pan G, Nolta JA, Wang A, Zhou P. Endothelial cells derived from patients’ induced pluripotent stem cells for sustained factor VIII delivery and the treatment of hemophilia A. Stem Cells Transl Med. 2020;9(6):686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohara M, Ohnishi S, Hosono H, Yamamoto K, Yuyama K, Nakamura H, Fu Q, Maehara O, Suda G, Sakamoto N. Extracellular vesicles from amnion-derived mesenchymal stem cells ameliorate hepatic inflammation and fibrosis in rats. Stem Cells Int. 2018;2018(3):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shan X, Choi JH, Kim KJ, Lee YJ, Ryu YH, Lee SJ, Moon SH, Rhie JW. Adipose stem cells with conditioned media for treatment of acne vulgaris scar. Tissue Eng Regen Med. 2018;15(1):49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perugini V, Meikle ST, Guildford AL, Santin M. Hyperbranched poly(ϵ-lysine) substrate presenting the laminin sequence YIGSR induces the formation of spheroids in adult bone marrow stem cells. PLoS One. 2017;12(12):e0187182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yasui T, Mabuchi Y, Morikawa S, Onizawa K, Akazawa C, Nakagawa T, Okano H, Matsuzaki Y. Isolation of dental pulp stem cells with high osteogenic potential. Inflamm Regen. 2017;37(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joddar B, Kumar SA, Kumar A. A contact-based method for differentiation of human mesenchymal stem cells into an endothelial cell-phenotype. Cell Biochem Biophys. 2018;76(1-2):187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Orlova VV, van den Hil FE, Petrus-Reurer S, Drabsch Y, Ten Dijke P, Mummery CL. Generation, expansion and functional analysis of endothelial cells and pericytes derived from human pluripotent stem cells. Nat Protoc. 2014;9(6):1514–1531. [DOI] [PubMed] [Google Scholar]
- 16. Xu JG, Gong T, Wang YY, Zou T, Heng BC, Yang YQ, Zhang CF. Inhibition of TGF-β signaling in SHED enhances endothelial differentiation. J Dent Res. 2018;97(2):218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elcheva I, Brok-Volchanskaya V, Slukvin I. Direct induction of hemogenic endothelium and blood by overexpression of transcription factors in human pluripotent stem cells. J Vis Exp. 2015;(106):e52910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kataoka H, Hayashi M, Nakagawa R, Tanaka Y, Izumi N, Nishikawa S, Jakt ML, Tarui H, Nishikawa S. Etv2/ER71 induces vascular mesoderm from Flk1+PDGFRα+ primitive mesoderm. Blood. 2011;118(26):6975–6986. [DOI] [PubMed] [Google Scholar]
- 19. Sumanas S, Choi K. ETS transcription factor ETV2/ER71/Etsrp in hematopoietic and vascular development. Curr Top Dev Biol. 2016;118:77–111. [DOI] [PubMed] [Google Scholar]
- 20. Bronckaers A, Hilkens P, Martens W, Gervois P, Ratajczak J, Struys T, Lambrichts I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 2014;143(2):181–196. [DOI] [PubMed] [Google Scholar]
- 21. Fan W, Crawford R, Xiao Y. The ratio of VEGF/PEDF expression in bone marrow mesenchymal stem cells regulates neovascularization. Differentiation. 2011;81(3):181–191. [DOI] [PubMed] [Google Scholar]
- 22. Jin R, Song G, Chai J, Gou X, Yuan G, Chen Z. Effects of concentrated growth factor on proliferation, migration, and differentiation of human dental pulp stem cells in vitro. J Tissue Eng. 2018;9:2041731418817505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xue YN, Yan Y, Chen ZZ, Chen J, Tang FJ, Xie HQ, Tang SJ, Cao K, Zhou X, Wang AJ, Zhou JD. LncRNA TUG1 regulates FGF1 to enhance endothelial differentiation of adipose-derived stem cells by sponging miR-143. J Cell Biochem. 2019;120(11):19087–19097. [DOI] [PubMed] [Google Scholar]
- 24. Roobrouck VD, Clavel C, Jacobs SA, Ulloa-Montoya F, Crippa S, Sohni A, Roberts SJ, Luyten FP, Van Gool SW, Sampaolesi M, Delforge M, et al. Differentiation potential of human postnatal mesenchymal stem cells, mesoangioblasts, and multipotent adult progenitor cells reflected in their transcriptome and partially influenced by the culture conditions. Stem Cells. 2011;29(5):871–882. [DOI] [PubMed] [Google Scholar]
- 25. Otsu K, Das S, Houser SD, Quadri SK, Bhattacharya S, Bhattacharya J. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood. 2009;113(18):4197–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Z, Nör F, Oh M, Cucco C, Shi S, Nör JE. Wnt/β-catenin signaling determines the vasculogenic fate of postnatal mesenchymal stem cells. Stem Cells. 2016;34(6):1576–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xie H, Wang F, Chen X, Ying H. miR-126 is essential for endothelial phenotype expression during endothelial differentiation in adipose-derived stem cells. Mol Med Rep. 2018;17(1):442–446. [DOI] [PubMed] [Google Scholar]
- 28. Lammerts van Bueren K, Black BL. Regulation of endothelial and hematopoietic development by the ETS transcription factor Etv2. Curr Opin Hematol. 2012;19(3):199–205. [DOI] [PubMed] [Google Scholar]
- 29. Yan G, Yan R, Chen C, Chen C, Zhao Y, Qin W, Veldman MB, Li S, Lin S. Engineering vascularized skeletal muscle tissue with transcriptional factor ETV2-induced autologous endothelial cells. Protein Cell. 2019;10(3):217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong WT, Cooke JP. Therapeutic trans-differentiation of human fibroblasts into endothelial cells using forced expression of lineage-specific transcription factors. J Tissue Eng. 2016;7:2041731416628329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee S, Park C, Han JW, Kim JY, Cho K, Kim EJ, Kim S, Lee SJ, Oh SY, Tanaka Y, Park IH, et al. Direct reprogramming of human dermal fibroblasts into endothelial cells using ER71/ETV2. Circ Res. 2017;120(5):848–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morita R, Suzuki M, Kasahara H, Shimizu N, Shichita T, Sekiya T, Kimura A, Sasaki K, Yasukawa H, Yoshimura A. ETS transcription factor ETV2 directly converts human fibroblasts into functional endothelial cells. Proc Natl Acad Sci U S A. 2015;112(1):160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Y, Kaiser MS, Larson JD, Nasevicius A, Clark KJ, Wadman SA, Roberg-Perez SE, Ekker SC, Hackett PB, McGrail M, Essner JJ. Moesin1 and Ve-cadherin are required in endothelial cells during in vivo tubulogenesis. Development. 2010;137(18):3119–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu F, Li D, Yu YY, Kang I, Cha MJ, Kim JY, Park C, Watson DK, Wang T, Choi K. Induction of hematopoietic and endothelial cell program orchestrated by ETS transcription factor ER71/ETV2. EMBO Rep. 2015:16(5):654–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-cll-10.1177_0963689720978739 for Upregulation of ETV2 Expression Promotes Endothelial Differentiation of Human Dental Pulp Stem Cells by Jing Li, Youming Zhu, Na Li, Tao Wu, Xianyu Zheng, Boon chin Heng, Duohong Zou and Jianguang Xu in Cell Transplantation