Abstract
This study was designed to clarify whether Shikonin causes proliferation, apoptosis, and invasion in cholangiocarcinoma cells and to investigate the mechanism of action. QBC939 cells were cultured with different doses of Shikonin, and then 3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium assay was used to detect cell viability. Apoptosis of cells was detected using flow cytometry with Annexin V/propidium iodide (PI) assay after being stained with Hoechst 33242. The role of Shikonin on the invasive and metastasis ability was detected using Transwell invasion assay. Real-time polymerase chain reaction and Western blotting were used to detect the expression of caspase-3, caspase-8, epidermal growth factor receptor (EGFR), and matrix metalloproteinase (MMP)-9. Shikonin inhibited proliferation and invasive ability of QBC939 cells in a dose-dependent manner; at the same time, apoptosis of cells was also observed in a concentration-dependent fashion. Moreover, Annexin V/PI assay and Transwell invasion assay results indicated that Shikonin induced apoptosis and invasion inhibitory probably due to upregulation of caspase-3 and caspase-8 expression and downregulation of MMP-9 and EGFR expression in a concentration-dependent fashion. Shikonin could enhance apoptosis and inhibit proliferation and invasion of QBC939 cells; such biological behaviors mainly occurred via upregulating the expression of caspase-3 and caspase-8 and downregulating the expression of MMP-9 and EGFR.
Keywords: Shikonin, cholangiocarcinoma, apoptosis, proliferation, invasion
Introduction
Cholangiocarcinoma (CCA) is a primary liver cancer with features of cholangiocyte differentiation, the epithelial cell lining, and the intrahepatic and extrahepatic portions of the biliary tree. Over the last 15 years, CCA’s incidence has steadily increased worldwide, and nowadays, it represents the second most common type of primary malignancy in the liver (15% to 20% of cases) after hepatocellular carcinoma1. The overall incidence of CCA from 2001 to 2015 in the USA was 1.26 per 100,000 people per year2. Radical surgical resection is the only potentially curative treatment for CCA. However, CCA is usually asymptomatic in the early stages and is often diagnosed when advanced (locally advanced/unresectable or metastatic). CCA hardly answers to chemotherapy, chemoradiotherapy, or targeted therapies, while locoregional treatments such as transarterial chemoembolization (TACE), selective internal radiation therapy (SIRT), radiofrequency ablation (RFA), and photodynamic therapy (PDT) has been investigated over the last few years, but no evidence had been shown yet if there would be any benefits3. Thus, new therapeutic strategies are still urgently needed to improve the treatment outcome of CCA.
Shikonin, a natural red naphthoquinone compound, is a major component of Zicao (purple gromwell). It comes from the dried root of Lithospermum erythrorhizon Sieb. et Zucc, Arnebia euchroma (Royle) Johnst, or Arnebia. Chemical constitution is 1,4-naphthoquinone including a benzene moiety (ring A) and a fully conjugated cyclic diketone (ring B), which were linearly fused, among which the carbonyl groups are placed in para-orientation to combine a chiral 6-carbon side chain (Fig. 1a). Moreover, Shikonin is considered a kind of polyphenols which consist of a C6 to C4 skeleton having 10 carbon atoms. Shikonin has been shown antitumor activity in varieties of human cancers, such as hepatocellular carcinoma, melanoma, glioma, mammary cancer, nonsmall cell lung cancer4–8.
Figure 1.
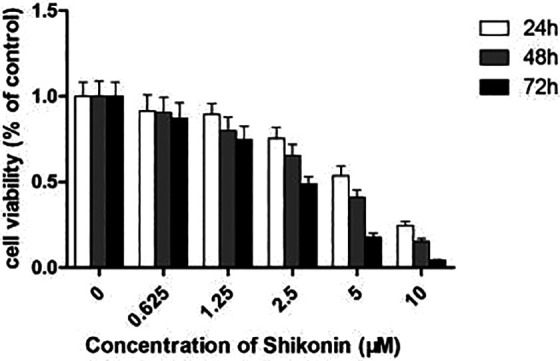
Shikonin inhibited the proliferation of QBC939 cells. Cultured QBC939 cells were incubated with an increasing dose of Shikonin (0 to 10 µM) for 24, 48, and 72 h, and cell viability was assessed using the 3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium assay.
In our study, a hypermetastatic CCA cell line, QBC939, is used to investigate the antitumor activity of Shikonin on CCA.
Materials and Methods
Culture of Cells
Human CCA cell line QBC939 was incubated in Dulbecco’s modified Eagle’s medium (DMEM; high glucose; Thermo. USA) medium with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 U/ml streptomycin in an incubator with 5% carbon dioxide (CO2) at 37 °C.
Cell Viability Assay
The inhibitory effect of the proliferation of Shikonin (Tauto Biotech Co., Ltd, Shanghai, China) against QBC939 cells was assessed using the 3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium (MTT) (Sigma, USA) dye uptake method. QBC939 cells with a density of 1 × 105/ml were cultured in a 96-well plate. After overnight growth, the cells were incubated with different concentrations of Shikonin (0 to 10 µM) for 24 h, 48 h, or 72 h. MTT (0.5%; 20 µl) was added into cells and continued culturing for 4 h near the end of the experiment. The supernatant was discarded, and the formation was dissolved in 0.2 ml dimethyl sulfoxide (DMSO). Optical density (A) was measured at 490 nm of the wavelength in a plate reader to calculate the survival rate and inhibitory rate.
Hoechst 33342 Staining
A concentration of cells (2 × 105 cells/ml) was seeded in 24-well plates for 24 h before treatment with 0.1% DMSO (v/v, as a control) or Shikonin (2.5 µM and 5 µM). Twenty-four hours after treatment, the living cells were stained with 10 µg/ml Hoechst 33342 (Sigma, USA) for 10 min. The cells were gently washed with phosphate-buffered saline (PBS) and photographed using a fluorescence microscope.
Flow Cytometry
A concentration of QBC939 cells (2 × 105 cells/ml) was seeded in 6-well plates for 24 h. Cells were harvested and washed in cold PBS twice after being treated by the DMSO control group and Shikonin (2.5 µM, 5 µM) group for 48 h. Then the cells were mixed in 100 of 1× binding buffer (1×, 100 µl) was used to mix the cells, and the Annexin V/propidium iodide (PI) double staining solution (Sigma, USA) was used to stained the cells for 15 min at 37 °C. The apoptosis percentage of the cells after staining was detected using flow cytometry, and the necrotic cells were analyzed using ModFitLT software (Verity Software House, Topsham, ME, USA).
Transwell Cell Invasion Assay
The invasion of cancer cells was detected in Transwell chambers (Falcon, BD, USA) loaded with 60 µl of a 1:5 diluted material (BD, USA) in cold DMEM. A concentration of QBC939 cells (5 × 104 cells/ml) was placed in the upper chamber containing DMEM with 1% FBS, and the lower chamber containing DMEM with 10% FBS after treating with different concentrations of Shikonin for 24 h. The cells which invaded the lower side of the filter after incubation for 24 h were stained with crystal violet and observed by an inverted microscope.
Real-Time Polymerase Chain Reaction (PCR) Assay
The total RNA was extracted from QBC939 cells after incubating with Shikonin for 24 h using TRIzol reagent (Invitrogen, USA) according to the manufacturers’ instruction. The total RNA (1.0 μg) was reverse-transcribed directly according to the manufacturer’s protocol of RevertAid First Strand cDNA Synthesis Kit (Fermentas). Quantitative real-time reverse transcription-PCR (qRT-PCR) was conducted using SYBR Premix Ex Taq Kit (TaKaRa) with forward and reverse primers for caspase-3, caspase-8, matrix metalloproteinase (MMP)-9, epidermal growth factor receptor (EGFR), and β-actin. The expression level of β-actin was selected as internal reference. Primers for PCR detection were designed and synthesized according to the information of target gene sequences, as shown in Table 1. The amplifications were performed in a 96-well plate at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Each sample was run in triplicate. The relative RNA expression was expressed using the 2−ΔΔCt method.
Table 1.
Primers for Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction Analysis of Gene Transcript Expression.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Caspase-3 | 5′-AGAGGGGATCGTTGTAGAAGTC-3′ | 5′-ACAGTCCAGTTCTGTACCACG-3′ |
| Caspase-8 | 5′-ATGTTGGAGGAAAGCAATCTGT-3′ | 5′-ATTTGAGCCCTGCCTGGTGTCT-3′ |
| MMP-9 | 5′-AGACCTGGGCAGATTCCAAAC-3′ | 5′-CGGCAAGTCTTCCGAGTAGT-3′ |
| EGFR | 5′-CCCACTCATGCTGTACAACCC-3′ | 5′-TCGCACTTCTTACACTTGCGG-3′ |
| β-actin | 5′-GGAGTCCTGTGGCATCCACG-3′ | 5′-CTAGAAGCATTTGCGGTGGA-3′ |
Western Blot Analysis
The cells were cultured with Shikonin for 24 h. They were harvested and washed with PBS, and then laid in radioimmunoprecipitation assay buffer (KeyGEN BioTECH, Nanjing, China) with the protease inhibitor. After centrifugation at 12,000 rpm, the supernatant was used to determine protein concentration by bicinchoninic acid assay (Beyotime). Total proteins (50 μg) were extracted from tissue and were sampled. Eight percent of concentrated gel and 15% of isolated gel were prepared, respectively, to isolate proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Objective and internal proteins were transferred to polyvinylidene difluoride membrane, closed with 5% skimmed milk powder sealing fluid for 1 h at room temperature, incubated with antibodies against caspase-3 (1:500; Cell Signaling), caspase-8 (1:500; Cell Signaling), MMP-9 (1:1000; Abcam), and β-actin (1:2000; Santa Cruz) at 37 °C for 1 h, washed 4 times with Tris-buffered saline with Tween 20. Peroxidase conjugated secondary antibody (1:1000) was added and incubated at 37 °C for 1 h and then washed 3 times with PBS with Tween 20 again. At last, the color was developed with ECL luminescent solution, protein bands were exposed by gel image analysis system, and images were photographed and quantitatively analyzed.
Statistical Analysis
For each variable, at least 3 independent experiments were carried out. The data were expressed as mean ± SD. The results from treated and untreated control cells were analyzed by Student’s t-test, and differences were considered significant at P < 0.05.
Results
Shikonin Inhibited the Proliferation of QBC939 Cells
QBC939 cells were cultured with varying concentrations (0 to 10 µM) of Shikonin for 24 h, 48 h, and 72 h in order to investigate the effect of the proliferation of Shikonin. Cell survival was assessed by the MTT assay. As shown in Fig. 1, proliferation and survival were restrained in a concentration-dependent fashion. The half-maximal inhibitory concentration (IC50) values were approximately 4.43 µM, 3.39 µM, and 2.20 µM for 24 h, 48 h, and 72 h treatment, respectively. These results indicate that Shikonin has potent antiproliferative effects in CCA cells.
Shikonin Induced Apoptosis in QBC939 Cells
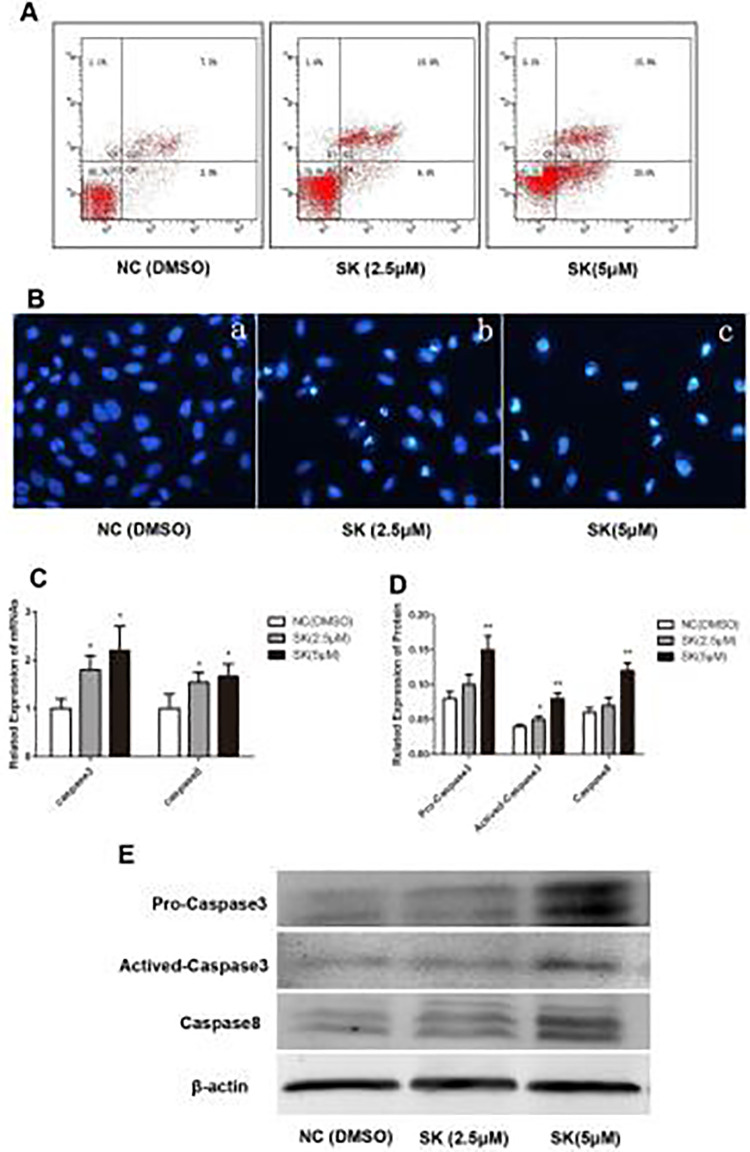
Annexin V/PI assay was applied to investigate whether Shikonin induces apoptosis in QBC939 cells. QBC939 cells were treated with 0, 2.5, and 5 µM of Shikonin for 48 h. It was observed that apoptosis of QBC939 cells was in a concentration-dependent fashion (Fig. 2A). Hoechst 33242 staining indicated that the QBC939 cells treated by Shikonin displayed typical morphological features of apoptosis: condensed chromatin, gradual disintegration of the nuclear membrane, and pyknotic (shrunken and dark) nuclei (Fig. 2B).
Figure 2.
Effect of Shikonin on cell apoptosis. (A) Apoptosis and necrosis of QBC939 were measured by flow cytometry with Annexin V/propidium iodide assay. (B) Morphological changes visualized under the fluorescence microscope with Hoechst 33342 staining (magnification 400×). (C) Changes of caspase-3 and caspase-8 mRNA expression were analyzed by real-time polymerase chain reaction (*P < 0.05, compared with the control). (D, E) Expression of pro-caspase-3, activated-caspase-3, and caspase-8 analyzed by Western Blot. (*P < 0.05, **P < 0.01, compared with the control).
Activation of caspases has been considered as a hallmark of apoptosis, while caspase-8 activation constitutes the extrinsic pathway. The expression of both caspase-8 and caspase-3 increased after being treated by Shikonin (Fig. 2C–E) which was detected by real-time PCR and western blot, indicating Shikonin induces apoptosis in CCA cells by the extrinsic pathway.
Shikonin Inhibit the Invasion Behavior of QBC939 Cells
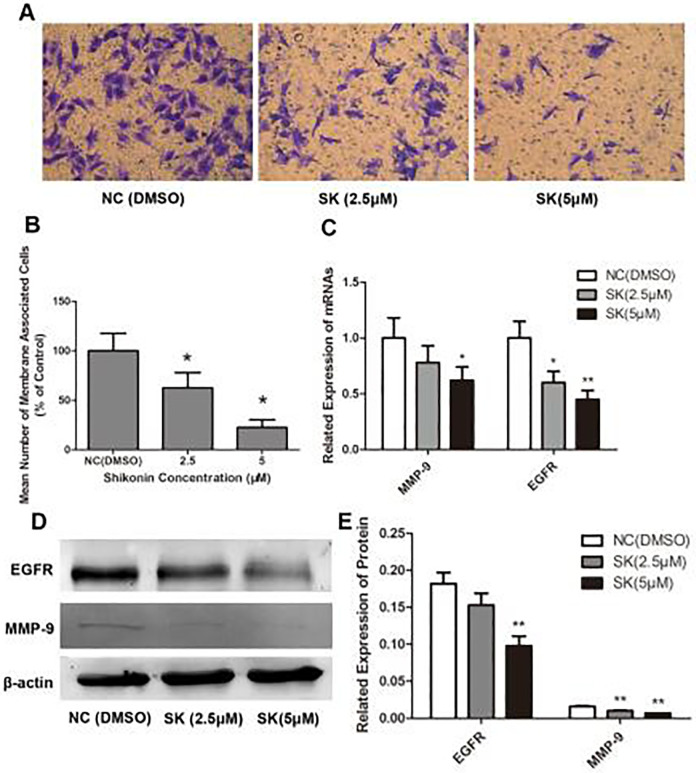
Transwell invasion assay was performed in order to investigate the influence of Shikonin on the invasive ability of QBC939 cells. Different Shikonins (0, 2.5, and 5 µM) were administrated in QBC939 cells for 24 h and placed in the upper chamber.
After 24 h, a light microscope was used to calculate the number of cells which moved to the underside of the coated membrane. It was shown in Fig. 3A that the number of cells that invaded the lower chamber was obviously decreased after administration of Shikonin for 24 h. The reduction was showed to be concentration dependent (Fig. 3B).
Figure 3.
Shikonin impairs the invasiveness of QBC939 cells. (A) QBC939 cells were treated with various concentrations of Shikonin for 24 h and detected by Transwell invasion assays. Representative photomicrographs of the membrane-associated cells (purple part) were assayed using crystal violet staining (magnification 400×). (B) Semiquantitative analysis of the anti-invasion effects of Shikonin on QBC939 cells (*P < 0.01, compared with the control). (C) Changes of epidermal growth factor receptor (EGFR) and matrix metalloproteinase (MMP)-9 mRNA expression were analyzed by real-time PCR (*P < 0.05, **P < 0.01, compared with the control). (D, E) Expression of EGFR and MMP-9 analyzed by Western Blot (**P < 0.01, compared with the control).
MMP-9 and EGFR play critical roles in cancer cell invasiveness. Thus, we detected the influence of Shikonin on the expression of MMP-9 and EGFR. As showed in Fig. 3C–E, Shikonin reduced both MMP-9 and EGFR expression in a concentration-dependent fashion.
Discussion
CCA is one of the highly aggressive tumors with a dismal prognosis. The low incidence compared to other digestive tumors, heterogeneity of the disease, poor patient’s performance status, and recurrent episodes of biliary tract complications (requiring palliative but invasive procedures) are some of the reasons that have limited the scientific development in this field3.
It had been demonstrated that Zicao extracts possess multiple pharmacological activities including anti-inflammation, antioxidative stress, antivirus, antibacteria, and anticancer9. Multiple mechanisms involved in the anticancer influence on Shikonin, including the inhibition of cell growth, migration, and invasion, and the induction of cell death. Despite being confirmed by numerous in vitro and in vivo results, the molecular mechanisms including the signaling pathways and cellular targets in the antitumor activity of Shikonin appear to be complex and remained elusive. Wang9 reviewed the synthesis, biological function, and evaluation of Shikonin in cancer therapy early this year, showing diverse signaling pathways are involved, such as the PKM2-dependent tumor glycolysis, the rat sarcoma virus/rapidly accelerated fibrosarcoma/mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) pathway, the Janus kinase (JNK) pathway, the phosphoinositide 3-kinase/protein kinase B/ mammalian target of rapamycin/phosphatase and tensin homolog pathway, c-Myc, reactive oxygen species (ROS) generation, etc.
Here we report the role of Shikonin on biological behaviors in the human CCA cell line QBC939 in a concentration-dependent fashion and QBC939 cells in a time-dependent and dose-dependent manner. It has been reported that Shikonin can restrain breast cancer cell proliferation by declining tumor-derived exosomal miR-12810 and inhibit EGFR signaling to modulate cell proliferation in human epidermoid carcinoma cells since it decreased phosphorylated levels of EGFR, ERK1/2, and protein tyrosine kinases while increasing phosphorylated JNK1/2 levels11. Our study also identified that Shikonin reduced EGFR expression in a concentration-dependent fashion, suggesting that EGFR signaling pathway is involved in the inhibition of proliferation of QBC939 cells induced by Shikonin.
Also, we identified that Shikonin induced apoptosis in QBC939 cells. Hochest 33342 staining and flow cytometry confirmed qualitatively and quantitatively that Shikonin induces apoptosis. Apoptosis is accomplished by the activation of caspases, and the characters include cell membrane swelling, cell contraction, karyorrhexis, chromatin condensation, and DNA fragmentation. The activation of caspases is a hallmark of apoptosis. While caspase-9 activation constitutes the intrinsic pathway, the extrinsic pathway requires caspase-8 activation. In this study, we demonstrated that the activity of caspase-8 and caspase-3 was significantly increased in a concentration-dependent fashion in QBC939 cells, indicating the apoptosis was induced by Shikonin in CCA cells by the extrinsic pathway. Zhou12 also reported that Shikonin inhibited cell viability and induced apoptosis of CCA cells, effects enhanced by tumor necrosis factor-related apoptosis-inducing ligand treatment via activation of caspase-3, caspase-8, and caspase-9. In the meantime, it was also noticed that Shikonin promotes adriamycin-induced apoptosis via upregulating caspase-3 and caspase-8 in osteosarcoma13, and the apoptosis effect in human glioma cells was in a concentration-dependent fashion by enhancing the intracellular levels of ROS, which are mostly generated by mitochondrial complex II, lipoxygenase, and NADPH oxidase14.
Invasion and metastasis are the main characteristics of a malignant tumor. Shikonin had been confirmed to inhibit invasions in several cancer cells, such as thyroid cancer cells, lung cancer cells, glioblastoma cells, gastric cancer cells, and breast cancer cells15–18. Here we report that Shikonin inhibits the invasion behavior of QBC939 cells in a concentration-dependent manner.
Tumor cells permeate the extracellular matrix (ECM) and basement membrane and then diffuse into the surrounding and distant organs. MMPs are neutral proteinases which degrade ECM and appear to play important roles in the process of tumor invasion and metastasis19. It is well established that MMPs induce cancer cell invasion and metastatic spread by degrading the ECM and other barriers; their role in cancer progression is related to their involvement in the ECM degradation and in the regulation and processing of adhesion and cytoskeletal proteins, growth factors, chemokines, and cytokines20. In hepatocellular carcinoma, Liao21 reported that hepatocellular carcinoma cell invasion and migration are modulated by the genes RHOC, MMP-2, and MMP-9, while Min22 reported Shikonin inhibited tumor invasion via downregulating the expression of MMP-9 in human high-metastatic adenoid cystic carcinoma cells. Previous studies also have confirmed that MMP-9 expression in CCA was obviously higher than in normal tissues23. Here we indicate that Shikonin declined MMP-9 expression in a concentration-dependent fashion.
EGFR also plays an important role in tumor-associated neo-angiogenesis, which could bind to vascular endothelial growth factor (VEGF) and contribute to providing oxygen, nutrition, and a route for metastasis24. It has been reported that EGFR and VEGF expression was relatively common in CCA, and EGFR expression was regarded as an independent prognostic factor since it was significantly correlated with shorter survival of patients25–27. Epithelial-mesenchymal transition (EMT) is a cellular process involved in cancer progression. The first step of EMT consists of the disruption of E-cadherin-mediated adherence junctions. It has been identified that the EGF/EGFR axis triggers EMT in CCA cells highlighting the key role of this pathway in CCA progression28. It had been demonstrated that Shikonin could inhibit several major players in the metastatic process and the EMT, including MMP-2, MMP-9, MMP-13, SRC, FAK, integrin β1, etc29. In this study, despite MMP-9, we also identified Shikonin’s reduced EGFR expression in a concentration-dependent manner, suggesting Shikonin could reduce the invasiveness of CCA and inhibit CCA angiogenesis by downregulating MMP-9 and EGFR expression.
Shikonin is being considered as a potential antitumor drug since numerous literature had mentioned its antitumor effect in vitro; even more, Shikonin-related drugs also were considered as potential chemopreventive drugs due to their considerable antiproliferative activity in vitro and in vivo 30. However, only a few researches proved its anticancer activity in vivo. Shikonin has poor water solubility and bioavailability, which limited its clinical application. Therefore, it is important to improve the solubility in an aqueous solution and tumor-specific accumulation for Shikonin.
RGD-modified Shikonin-loaded liposomes (RGD-SSLs-SHK) were carried out to improve the physical and chemical properties of Shikonin31. It had shown that a combination of Shikonin and thermosensitive nano-micelles could enhance the cytotoxicity for breast cancer cells32. Polyethylene glycosylated (PEGylated) liposomes were also considered to be used for loading Shikonin33. These findings truly improve Shikonin’s use in clinical practice. Researches on the mechanisms of shikonin’s anti-CCA behavior are needed and in the meantime, it is worth paying more attention to the synergistic interactions of shikonin with chemotherapy, immunotherapy, and other therapy modalities.
In this study, we found that Shikonin inhibited the proliferation and invasive ability of QBC939 cells in a dose-dependent manner; at the same time, apoptosis of cells was also observed in a concentration-dependent fashion. In addition, Annexin V/PI assay and Transwell invasion assay results indicated that Shikonin induced apoptosis and invasion inhibitory probably due to the upregulation of caspase-3 and caspase-8 expression and downregulation of MMP-9 and EGFR expression, which indicated that Shikonin could treat cholangiocarcinoma through this mechanism. The cell death by Shikonin alone or in combination with other drugs needs further study.
Conclusions
In conclusion, our data indicate that Shikonin not only could increase apoptosis but also inhibit proliferation and invasion of QBC939 cells; such biological behaviors mainly occurred via upregulating the expression of caspase-3 and caspase-8 and downregulating the expression of MMP-9 and EGFR. In this case, Shikonin may be a feasible chemotherapeutic treatment of cholangiocarcinoma.
Footnotes
Ethical Approval: Ethical approval was given by Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by National Natural Science Foundation of China (No. 81500487), and Siming Foundation of Shuguang Hospital affiliated to Shanghai University of Traditional Chinese Medicine (SGKJ-201801). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ORCID iD: Jie Shen  https://orcid.org/0000-0003-2741-3297
https://orcid.org/0000-0003-2741-3297
References
- 1. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3(12):1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel N, Benipal B. Incidence of cholangiocarcinoma in the USA from 2001 to 2015: a US cancer statistics analysis of 50 states. Cureus. 2019;11(1):e3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adeva J, Sangro B, Salati M, Edeline J, La Casta A, Bittoni A, Berardi R, Bruix J, Valle JW. Medical treatment for cholangiocarcinoma. Liver Int. 2019;39:123–142. [DOI] [PubMed] [Google Scholar]
- 4. Guo N, Miao R, Gao X, Huang D, Hu Z, Ji N, Nan Y, Jiang F, Gou X. Shikonin inhibits proliferation and induces apoptosis in glioma cells via downregulation of CD147. Mol Med Rep. 2019;19(5):4335–4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu Y, Kang X, Niu G, He S, Zhang T, Bai Y, Li Y, Hao H, Chen C, Shou Z, Li B. Shikonin induces apoptosis and prosurvival autophagy in human melanoma A375 cells via ROS-mediated ER stress and p38 pathways. Artif Cells Nanomed Biotechnol. 2019;47(1):626–635. [DOI] [PubMed] [Google Scholar]
- 6. Xu J, Koizumi K, Liu M, Mizuno Y, Suzaki M, Iitsuka H, Inujima A, Fujimoto M, Shibahara N, Shimada Y. Shikonin induces an anti-tumor effect on murine mammary cancer via p38-dependent apoptosis. Oncol Rep. 2019;41(3):2020–2026. [DOI] [PubMed] [Google Scholar]
- 7. Liu B, Jin J, Zhang Z, Zuo L, Jiang M, Xie C. Shikonin exerts antitumor activity by causing mitochondrial dysfunction in hepatocellular carcinoma through PKM2-AMPK-PGC1α signaling pathway. Biochem Cell Biol. 2019;97(4):397–405. [DOI] [PubMed] [Google Scholar]
- 8. Li B, Yuan Z, Jiang J, Rao Y. Anti-tumor activity of Shikonin against afatinib resistant non-small cell lung cancer via negative regulation of PI3K/Akt signaling pathway. Biosci Rep. 2018;38(6):BSR20181693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang F, Yao X, Zhang Y, Tang J. Synthesis, biological function and evaluation of Shikonin in cancer therapy. Fitoterapia. 2019;134:329–339. [DOI] [PubMed] [Google Scholar]
- 10. Nie Y, Yang Y, Zhang J, Cai G, Chang Y, Chai G, Guo C. Shikonin suppresses pulmonary fibroblasts proliferation and activation by regulating Akt and p38 MAPK signaling pathways. Biomed Pharmacother. 2017;95:1119–1128. [DOI] [PubMed] [Google Scholar]
- 11. Leticia VP, Yi Y, Cheng J, Lolita SP, Elvira C, Peter CK, Ilma SB. Lapatinib inhibits amphiregulin-induced bewo choriocarcinoma cell proliferation by reducing ERK1/2 and AKT signaling pathways. Anticancer Res. 2019, 39(5):2377–2383. [DOI] [PubMed] [Google Scholar]
- 12. Zhou G, Yang Z, Wang X, Tao R, Zhou Y. TRAIL enhances shikonin induced apoptosis through ROS/JNK signaling in cholangiocarcinoma cells. Cell Physiol Biochem. 2017;42(3):1073–1086. [DOI] [PubMed] [Google Scholar]
- 13. Yang Q, Li S, Fu Z, Lin B, Zhou Z, Wang Z, Hua Y, Cai Z. Shikonin promotes adriamycin-induced apoptosis by upregulating caspase-3 and caspase-8 in osteosarcoma. Mol Med Rep. 2017;16(2):1347–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang JT, Li ZL, Wu JY, Lu FJ, Chen CH. An oxidative stress mechanism of shikonin in human glioma cells. PLoS One. 2014;9(4):e94180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Y, Sun B, Huang Z, Zhao DW, Zeng Q. Shikonin inhibites migration and invasion of thyroid cancer cells by downregulating DNMT1. Med Sci Monit. 2018;24:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsieh YS, Liao CH, Chen WS, Pai JT, Weng MS. Shikonin inhibited migration and invasion of human lung cancer cells via suppression of c-met-mediated epithelial-to-mesenchymal transition. J Cell Biochem. 2017;118(12):4639–4651. [DOI] [PubMed] [Google Scholar]
- 17. Liu JP, Liu D, Gu JF, Zhu MM, Cui L. Shikonin inhibits the cell viability, adhesion, invasion and migration of the human gastric cancer cell line MGC-803 via the Toll-like receptor 2/nuclear factor-kappa B pathway. J Pharm Pharmacol. 2015;67(8):1143–1155. [DOI] [PubMed] [Google Scholar]
- 18. Jang SY, Lee JK, Jang EH, Jeong SY, Kim JH. Shikonin blocks migration and invasion of human breast cancer cells through inhibition of matrix metalloproteinase-9 activation. Oncol Rep. 2014;31(6):2827–2833. [DOI] [PubMed] [Google Scholar]
- 19. Conlon G A, Murray G I. Recent advances in understanding the roles of matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 2019;247(5):629–640. [DOI] [PubMed] [Google Scholar]
- 20. Gonzalez-Avila G, Sommer B, Mendoza-Posada DA, Ramos C, Garcia-Hernandez AA, Falfan-Valencia R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit Rev Oncol Hematol. 2019;137:57–83. [DOI] [PubMed] [Google Scholar]
- 21. Liao CG, Kong LM, Zhou P, Yang XL, Huang JG, Zhang HL, Lu N. miR-10b is overexpressed in hepatocellular carcinoma and promotes cell proliferation, migration and invasion through RhoC, uPAR and MMPs. J Transl Med. 2014;12:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Min R, Zun Z, Min Y, Wenhu D, Wenjun Y, Chenping Z. Shikonin inhibits tumor invasion via down-regulation of NF-kappaB-mediated MMP-9 expression in human ACC-M cells. Oral Dis. 2011;17(4):362–369. [DOI] [PubMed] [Google Scholar]
- 23. Bangchun M, Oncology D O. Expression levels of MMP-2 and MMP-9 in lung cancer tissues and their relationship with pathological parameters and metastasis. Int J Lab Med. 2018;39(22):2774–2777. [Google Scholar]
- 24. Cobiella D, Gram D, Santoro D. Noninvasive evaluation of vascular endothelial growth factor-A (VEGF-A) protein concentrations in the stratum corneum and serum of healthy and atopic dogs. Vet Dermatol. 2020, 31(2):102–e14. [DOI] [PubMed] [Google Scholar]
- 25. Tian Jj, Liu Xf, Xiu x, Jing Ph, Sa Na, Wang Hb, Xu W. Notch1 serves as a prognostic factor and regulates metastasis via regulating EGFR expression in hypopharyngeal squamous cell carcinoma. Onco Targets Ther. 2018;11:7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Padthaisong S, Thanee M, Techasen A, Namwat N, Yongvanit P, Liwatthakun A, Hankla K, Sangkhamanon S, Loilome W. Nimotuzumab inhibits cholangiocarcinoma cell metastasis via suppression of the epithelial-mesenchymal transition process. Anticancer Res. 2017;37(7):3591–3597. [DOI] [PubMed] [Google Scholar]
- 27. Sia D, Hoshida Y, Villanueva A, Roayaie S, Ferrer J, Tabak B, Peix J, Sole M, Tovar V, Alsinet C, Cornella H, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013;144(4):829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clapéron A, Mergey M, Nguyen Ho-Bouldoires TH, Vignjevic D, Wendum D, Chrétien Y, Merabtene F, Frazao A, Paradis V, Housset C, Guedj N, et al. EGF/EGFR axis contributes to the progression of cholangiocarcinoma through the induction of an epithelial-mesenchymal transition. J Hepatol. 2014;61(2):325–332. [DOI] [PubMed] [Google Scholar]
- 29. Boulos JC, Rahama M, Hegazy MF, Efferth T. Shikonin derivatives for cancer prevention and therapy. Cancer Lett. 2019;459:248–267. [DOI] [PubMed] [Google Scholar]
- 30. Wang R, Yin R, Zhou W, Xu D, Li S. Shikonin and its derivatives: a patent review. Expert Opin Ther Pat. 2012;22(9):977–997. [DOI] [PubMed] [Google Scholar]
- 31. Wen X, Li J, Cai D, Yue L, Wang Q, Zhou L, Fan L, Sun J, Wu Y. Anticancer efficacy of targeted shikonin liposomes modified with RGD in breast cancer cells. Molecules. 2018;23(2):268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Su Y, Huang N, Chen D, Zhang L, Dong X, Sun Y, Zhu X, Zhang F, Gao J, Wang Y, Fan K, et al. Successful in vivo hyperthermal therapy toward breast cancer by Chinese medicine shikonin-loaded thermosensitive micelle. Int J Nanomedicine. 2017;12:4019–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kontogiannopoulos KN, Tsermentseli SK, Assimopoulou AN, Papageorgiou VP. Sterically stabilized liposomes as a potent carrier for Shikonin. J Liposome Res. 2014;24(3):230–240. [DOI] [PubMed] [Google Scholar]