Abstract
Background:
After anterior cruciate ligament reconstruction, biomechanical asymmetries during gait are highly prevalent, persistent, and linked to posttraumatic knee osteoarthritis. Quadriceps strength is an important clinical measure associated with pre-operative gait asymmetries and post-operative function and is a primary criterion for return-to-sport clearance. Evidence relating symmetry in quadriceps strength with gait biomechanics is limited to pre-operative and early rehabilitation time points prior to return-to-sport training.
Purpose/Hypothesis:
To determine the relationship between symmetry in isometric quadriceps strength and gait biomechanics after return-to-sport training in athletes after anterior cruciate ligament reconstruction. We hypothesized that as quadriceps strength symmetry increases, athletes will demonstrate more symmetric knee joint biomechanics including tibiofemoral joint loading during gait.
Study Design:
Cross-sectional study.
Methods:
Seventy-six of 79 athletes enrolled in ACL-SPORTS were participants for this study after completing post-operative rehabilitation and 10 return-to-sport training sessions (7.1 ± 2.0 months after ACLR). All participants completed biomechanical walking gait analysis and isometric quadriceps strength assessment using an electromechanical dynamometer. Quadriceps strength was calculated using a limb symmetry index (involved limb value/ uninvolved limb value x 100%). The biomechanical variables of interest included: peak knee flexion angle (PKFA), peak knee internal extension moment, sagittal plane knee excursion at weight acceptance and mid-stance, quadriceps muscle force at PKFA, and peak medial compartment contact force. Spearman’s rho (ρ) correlation coefficients were used to determine the relationship between limb symmetry indexes in quadriceps strength and each biomechanical variable separately; alpha was set to 0.05.
Results:
Of the 76 participants, 27 (35%) demonstrated asymmetries in quadriceps strength, defined by quadriceps strength symmetry <90% (n=23) or >110% (n=4) (QI range: 56.9%-131.7%). For the biomechanical variables of interest, 67% demonstrated asymmetry in peak knee flexion angle, 68% and 83% in knee excursion during weight acceptance and midstance, respectively, 74% in internal peak knee extension moment, 57% in medial compartment contact force, and 74% in quadriceps muscle force. There were no significant correlations between quadriceps strength index and limb symmetry indexes for any biomechanical variable following return-to-sport training (p>0.129).
Conclusion:
Among those who completed return-to-sport training after ACL reconstruction, subsequent quadriceps strength symmetry is not correlated with the persistent asymmetries in gait biomechanics. After reaching a threshold of quadriceps strength, restoring strength alone may not ameliorate gait asymmetries and current clinical interventions and return-to-sport training may not adequately target gait.
Keywords: anterior cruciate ligament reconstruction, gait, biomechanics, rehabilitation
Brief summary:
Work from @elanna_arhos, @doclsmack, @JacobCapin suggests that in those well rehabilitated after ACLR, quadriceps strength is not related to symmetric gait mechanics. Developing gait-specific interventions may be a critical step to mitigating aberrant gait mechanics after ACLR.
Introduction:
After anterior cruciate ligament reconstruction (ACLR), quadriceps muscle inhibition and weakness is ubiquitous.41,54,59,63 Quadriceps weakness after ACL injury has been associated with pre-operative gait asymmetries49 and post-operative function.51,58 The quadriceps work eccentrically during the early stance phase of gait to control the amount of knee flexion and attenuate shock through the limb as it accepts body weight. When the function of the quadriceps during gait is impaired, individuals tend to reduce the amount of knee flexion and the internal knee extensor moment of the involved limb relative to the uninvolved limb.49 This gait pattern has been termed ‘quadriceps avoidance gait,’ referring to the individual’s adopted movement strategy to decrease full quadriceps activation.
Gait asymmetries and quadriceps weakness have both been suggested to impact the development of posttraumatic knee osteoarthritis.4,5,24,28,32,42,43,45,50,61 Quadriceps weakness can lead to posttraumatic osteoarthritis by reducing the ability of the knee to properly attenuate force during weight acceptance of gait.28 Quadriceps weakness is associated with lower internal knee extensor moment, which may in turn decrease the compressive forces within the tibiofemoral and patellofemoral joint, termed “underloading.”27,60 Underloading the medial tibiofemoral joint is a highly prevalent alteration in gait biomechanics after ACLR and related to the development of posttraumatic osteoarthritis4,32,42,60, though conflicting evidence exists on the mechanism of knee joint loading and the development of idiopathic knee osteoarthritis.25,26 Due to the role of the quadriceps in dynamically controlling the knee during movement including gait, quadriceps weakness may play an important role to the development of posttraumatic osteoarthritis assessed via radiographs32,61, blood biomarkers,22,44 and magnetic resonance imaging13,42,46,50 after ACLR.
Individuals early after ACL rupture exhibit gait abnormalities consistent with quadriceps avoidance including lower knee flexion excursion during weight acceptance, peak vertical ground reaction force, and knee extensor moments on the involved relative to the contralateral limb.49 A flexed knee gait strategy persists up to six months after ACLR,23,47,55 suggesting surgical reconstruction and early post-operative rehabilitation does not restore gait mechanics early after ACL reconstruction. During the course of rehabilitation and in the first two years after ACLR, joint mechanics and quadriceps strength improve.6,11,15 Yet clinically meaningful gait asymmetries56 persist well beyond return-to-sport clearance, up to five years after ACLR.11,23,31,32 While quadriceps strength is associated with aberrant gait mechanics early after ACLR,35 it is not clear if that relationship persists after return-to-sport training, or if other factors drive persistent gait asymmetries. The association between symmetry in isometric quadriceps strength and aberrant gait mechanics among individuals who have undergone rehabilitation and return-to-sport training after ACLR is unknown.
Quadriceps strength is a primary component of evidence-based return-to-sport criteria.2,21,33 Isometric measurements of quadriceps strength are widely used clinically after ACLR, and a gold standard of assessing strength.3,38,53 While isokinetic testing may also be used to assess the quadriceps after ACLR, isometric testing is a highly relevant clinical measurement.1 Isometric strength testing provides a measure of symmetry, and achieving symmetrical quadriceps strength is often used to clear athletes for return-to-sport.3,21 A relationship between isometric quadriceps strength and gait deficits has been established early after ACL rupture.35,47 While clinicians may assume that symmetrical walking mechanics are also restored at the time of return-to-sport, evidence relating decreased quadriceps strength with aberrant gait mechanics is limited to pre-operative and early rehabilitation time points (i.e. before 6 months).
Differences exist among evidence regarding the relationship between quadriceps strength and post-operative biomechanics. Roewer et al.47 reported that biomechanical asymmetries in noncopers (i.e. those with dynamic knee instability after ACL rupture) persisted at 6 months and 2 years after ACLR, despite restoration of quadriceps strength by 6 months, suggesting there are additional neuromuscular considerations in restoring mechanics. Another study, however, suggests that patients with more symmetrical quadriceps strength demonstrate more symmetry in peak knee flexion angle and external knee flexion moment during hop testing at return-to-sport clearance.51 The relationship of symmetry in quadriceps strength with gait mechanics after individuals have undergone structured rehabilitation and return-to-sport training is unknown; it is important to determine whether current rehabilitation strategies (e.g., strengthening) or more targeted gait training interventions are needed to address gait asymmetries. The purpose of this study was to determine the relationship between quadriceps strength symmetry and symmetry in knee joint kinematics (sagittal plane angles and excursions), knee kinetics (sagittal and frontal plane moments), quadriceps muscle forces, and tibiofemoral loading during gait after return-to-sport training (i.e., post-training) in athletes after ACLR. We hypothesized that as quadriceps strength symmetry increases, athletes will demonstrate more symmetric knee joint biomechanics including tibiofemoral joint loading during gait.
Methods:
This study is a secondary analysis (level of evidence: III) of prospectively collected data from a clinical trial (NCT01773317). The University of Delaware Institutional Review Board approved this study, and rights of subjects were protected. Level I and II18 athletes participated in this study between December 2011 and March 2017 at the University of Delaware. Participants provided written informed consent, and additional consent and assent were obtained from parents/guardians and minors.
Seventy-nine athletes who were 7.1 ± 2.0 (mean +/− SD) months after a primary, unilateral ACLR participated in the ACL-SPORTS trial.62 Of these 79, 76 individuals had complete quadriceps strength, kinematic, and kinetic data, and 68 individuals had full modeling data sets to calculate quadriceps muscle force and peak medial compartment contact force; analyses were run separately for these 2 variables. Modeling analyses for 8 subjects failed due to poor EMG quality or failed model tuning or predictions during the patient-specific musculoskeletal modeling.19 Participants were excluded if they had concomitant grade III ligamentous injury, >1 cm2 full thickness chondral defects assessed via MRI or arthroscopy, or a previous history of serious injury or surgery to either lower extremity.
To initiate return-to-sport training, patients had to be at least 3 months after ACLR (range 3-10 months; mean=5.4 ± 1.9 months [mean ± SD]) and to have met the following criteria: full knee range of motion, trace or no effusion, and 80% quadriceps strength index.62 Athletes then completed 10 sessions of return-to-sport training over the course of 7.4 ± 1.4 (mean ± SD) weeks; training consisted of agility drills, plyometric training, and secondary prevention exercises, all of which were conducted bilaterally, in addition to continued strengthening. Half of the cohort was randomized to a group that also received the addition of perturbation training; however, analyses showed no difference in outcomes between the two groups,6–8,10,11,14,15 so groups were collapsed for the purpose of this analysis. After return-to-sport training, participants underwent comprehensive clinical and biomechanical testing including isometric quadriceps strength index (QI), a series of four hop tests, gait analysis, and patient-reported outcome measures. Participants who did not achieve return-to-sport criteria (i.e., ≥ 90% QI, 90% limb symmetry index [LSI] on four hop tests,40 and 90% on the Knee Outcome Survey-Activities of Daily Living Scale [KOS-ADLS])29 continued additional rehabilitation and were not cleared to return to sport until they achieved these criteria. The vast majority of patients reached these return-to-sport milestones and subsequently were cleared to begin a return-to-sports progression (pending clearance from the surgeon); all returned to sports within two years, most to the same sport level.10,11
Quadriceps strength, kinetic and kinematic data were analyzed from 76 participants (38 women; ages 21.2 ± 7.7 years) following return-to-sport training (approximately 7.1 ± 2.0 months after ACLR), at which time quadriceps strength index had the largest spread (Table 1). Sixty-eight participants had full modeling data sets and data were analyzed separately for quadriceps force and peak medial compartment contact force (n=68; 32 women; ages 21.9 ± 7.7 years) following return-to-sport training (approximately 7.0 ± 1.9 months after ACLR).
TABLE 1.
Participant demographics at post-training timepoint (n=76). Values are mean ± standard deviation, or number (%).
| Time Since Surgery (months) | 7.1 ± 2.0 | |
| Age at Timepoint (years) | 21.2 ± 7.7 | |
| BMI (kg/m2) | 26.1 ± 3.1 | |
| Sex (female) | 38 (50) | |
| Pre-Injury Level | 69 Level I (91) 7 Level II (9) |
|
| QI (%) | 93.4 ± 12.1 | |
| Graft Type | Allograft | 17 (22.4) |
| BPTB | 22 (28.9) | |
| Hamstring | 37 (48.7) | |
| Medial Meniscus Pathology* | None | 44 (57.9) |
| Partial Meniscectomy | 13 (17.1) | |
| Repair | 12 (15.8) | |
| Lateral Meniscus Pathology* | None | 36 (47.4) |
| Partial Meniscectomy | 25 (32.9) | |
| Repair | 8 (10.5) | |
Abbreviations: RTS, return to sport; BMI, body mass index; QI, quadriceps index; BPTB, bone patellar tendon bone
7 participants did not have operative reports, therefore no meniscal data were available for these participants.
Quadriceps Strength
Quadriceps strength was measured using an electromechanical dynamometer (Kin-com, DJO Global, Chula Vista, CA, USA; or System 3, Biodex, Shirley, NY, USA) during a maximal voluntary isometric contraction and reported using a quadriceps strength symmetry index (involved quadriceps maximal voluntary isometric contraction/uninvolved quadriceps maximal voluntary isometric contraction x 100%). Participants sat with their knees and hips flexed to 90 degrees, and the dynamometer’s axis of rotation was aligned to the axis of rotation of the knee joint. The participants’ legs were strapped in at the pelvis, thigh, and shank to minimize accessory motion during testing. Approximately three trials each were completed, first on the uninvolved limb and then on the involved limb. The highest value obtained for each limb was used in the quadriceps strength index (QI) calculation.
Gait Analysis
Electromyography (EMG) electrodes (MA-300 EMG System; Motion Lab Systems, Baton Rouge, LA) were placed over seven muscles: rectus femoris, vastus lateralis, vastus medialis, medial and lateral hamstrings, and medial and lateral gastrocnemii after abrading the skin. EMG data were band-passed filtered (20-500 Hz) prior to sampling, then sampled at 1080 Hz. Highest physiological values for the maximal voluntary isometric contraction of each muscle group were used to normalize the EMG signals, as previously described.12 EMG data were high-pass filtered at 30 Hz using a 2nd order Butterworth filter, rectified, and low-pass filtered at 6 Hz to create a linear envelope.
Thirty-nine retroreflective markers were affixed to the pelvis and bilateral lower extremities. Kinetic (1080 Hz) and kinematic (120 Hz) data were captured with an 8-camera motion analysis system (VICON, Oxford, UK) as participants walked over an embedded force platform (Bertec Corporation, Columbus, OH). Participants completed over-ground gait analysis at self-selected walking speeds, which were recorded and held consistent within individuals across trials (± 5%). Commercially available software (Visual 3D, C-Motion, Germantown, MD) was used to calculate kinematic and kinetic variables. Marker trajectories were low-pass filtered using a zero-lag, fourth order Butterworth filter with a 6 Hz cut-off frequency. Ground reaction force data were low-pass filtered at 50 Hz.62 Biomechanical data were normalized to percent stance phase of gait, averaged across three trials per limb, and reported as limb symmetry indexes (LSI). Kinetic data were normalized to mass and height (kg x m).39 Data were processed using Visual 3D, and knee kinetics were calculated via inverse dynamics.
Musculoskeletal Modeling
A validated, patient-specific, EMG-driven musculoskeletal model was used to estimate quadriceps muscle forces and joint contact forces bilaterally from EMG data during walking.9,36 Subject models were scaled using data collected during a standing reference trial, and the linear envelopes from each muscle were normalized to peak values obtained from isometric and dynamic maximum effort trials. This previously validated36 musculoskeletal model uses a Hill-type muscle fiber in series with an elastic tendon and applies a simulated annealing process to fit a forward dynamics knee flexion moment curve to the knee flexion moment curve derived through inverse dynamics. The derived forward dynamics knee flexion moment is varied through several muscle parameters and coefficients varying within ± 2 standard deviations of physiological norms. This process minimizes the root mean square error between the forward and inverse dynamics knee flexion moment curves. The process is completed for 5 walking trials per limb per participant. Each trial is then predicted using the derived muscle parameters and coefficients. Three trials are selected by maximizing the R2 values and minimizing the root mean square error of the predicted trials. Muscle forces are individually calculated for each predicted trial, and medial and lateral tibiofemoral joint contact forces are estimated using the Winby frontal plane moment algorithm.64 Quadriceps muscle forces and joint contact forces were normalized by bodyweight (BW). Further details including step by step methodology for the musculoskeletal modeling have been published previously.9,32,36
Modeling variables of interest included quadriceps muscle forces at peak knee flexion angle and peak medial compartment contact force of the tibiofemoral joint. The key variables of interest included QI, peak knee flexion angle, peak internal knee extension moment, and sagittal plane knee excursion at weight acceptance and mid-stance during the stance phase of gait, quadriceps muscle force at peak knee flexion angle, and peak medial compartment contact force. All peak variables during gait, including peak knee flexion angle, peak internal knee extension moment, quadriceps muscle force, and peak medial compartment contact force, were constrained to the first 50% of stance.
Statistical Analysis
Biomechanical variables of interest were expressed as limb symmetry indexes (involved limb value/uninvolved limb value x 100%). Quadriceps strength index and all biomechanical variables were assessed for normality using the Shapiro-Wilk test. While quadriceps strength index was normally distributed, biomechanical variables were not normally distributed. Therefore, we used Spearman rho’s (ρ) correlation coefficients to determine the relationship between quadriceps strength index and symmetry of our biomechanical variables of interest. We ran secondary Spearman rho’s (ρ) correlation coefficients to determine if there was a relationship between quadriceps strength symmetry and gait biomechanics when grouping by quadriceps asymmetry by dividing individuals into symmetric (QI≥90 and ≤110) and asymmetric (QI<90 or >110). Of note, only 4 individuals had QI >110, and Spearman rho’s (ρ) were run with and without these individuals included. We also ran secondary Spearman rho’s (ρ) to determine if there was a relationship between our variables of interest when grouped by sex. A power analysis calculated with G*Power 3.1 (Universität Düsseldorf, Düsseldorf, Germany) using the Correlation: Bivariate normal model statistical test; β was set at 0.80, α at 0.05, and a large effect size of 0.517 revealed that 29 total individuals were required to reach sufficient power. Alpha was set at 0.05 a priori. Analyses were computed with SPSS Version 25.0 (IBM Corporation, Armonk, NY).
Results:
Of the 76 participants, 27 (35%) demonstrated asymmetries in quadriceps strength, defined by quadriceps strength symmetry <90% (n=23) or >110% (n=4) (QI range: 56.9%-131.7%). For the biomechanical variables of interest, 67% demonstrated asymmetry in peak knee flexion angle, 68% and 83% in knee excursion during weight acceptance and midstance, respectively, 74% in internal peak knee extension moment, 57% in medial compartment contact force, and 74% in quadriceps muscle force. There was no significant correlation between quadriceps strength symmetry and any of the biomechanical variables of interest (expressed as limb symmetry indexes), including peak knee flexion angle, peak knee extension moment, knee excursion during weight acceptance and midstance, quadriceps muscle force at peak knee flexion angle, and peak medial compartment contact force ((p=0.13-0.91); Figures 1–3, Table 2, Table 3). Similarly, there was no significant correlation between quadriceps strength symmetry and any biomechanical variables of interest when groups were dichotomized both by asymmetry values (symmetric group: p=0.25-0.87; asymmetric group: p=0.19-0.67) and by sex (women: p=0.19-0.87; men: p=0.22-0.96).
FIGURE 1.
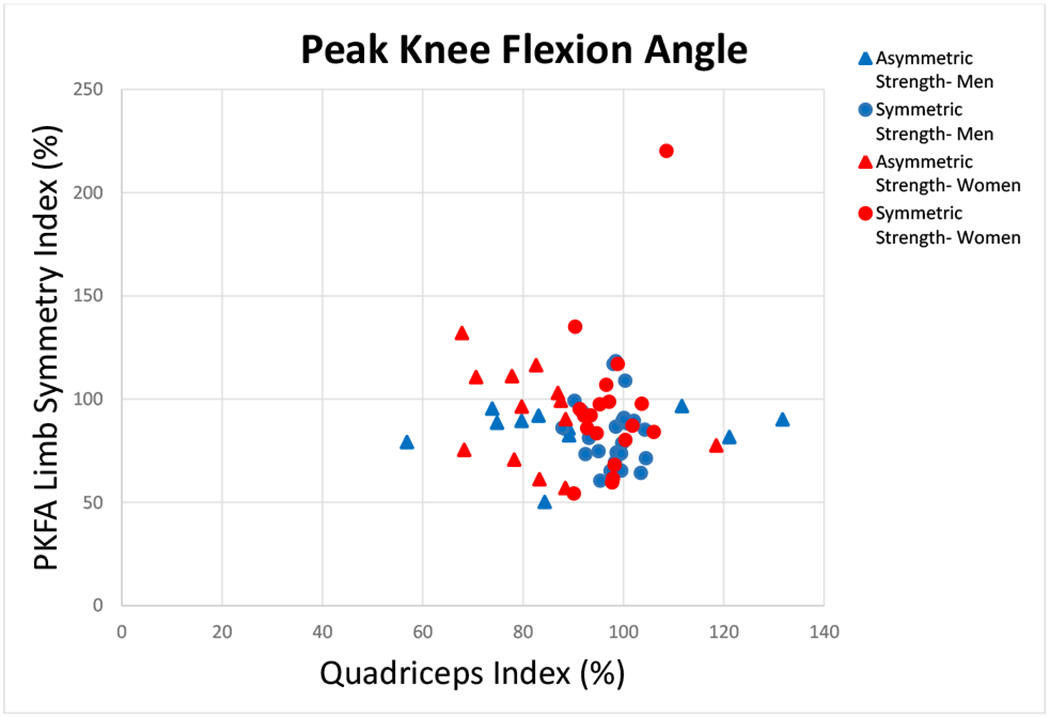
There was no relationship between quadriceps index and peak knee flexion angle (PKFA) (Spearman’s ρ −0.045; p=0.698). Groups are based on quadriceps index symmetry (Symmetric Strength-Men, Symmetric Strength- Women: QI≥90 and ≤110) and asymmetry (Asymmetric Strength- Men, Asymmetric Strength- Women: QI<90 or >110).
FIGURE 3.
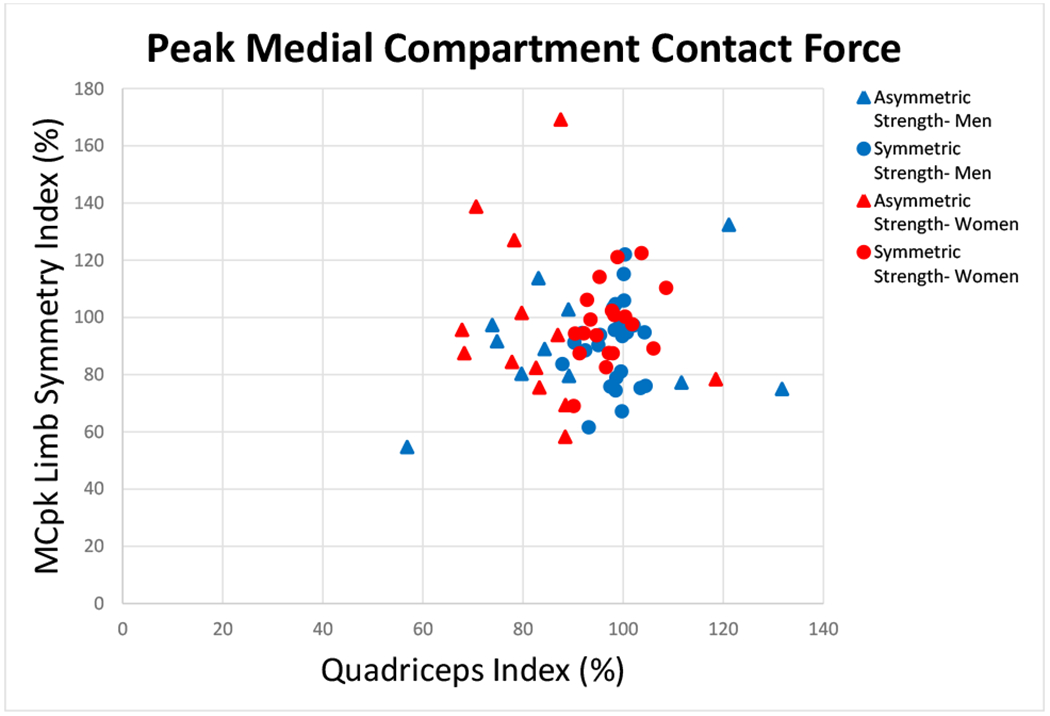
There was no relationship between quadriceps index and peak medial compartment contact force (MCpk) (Spearman’s ρ=0.235; p=0.129). Groups are based on quadriceps index symmetry (Symmetric Strength-Men, Symmetric Strength- Women: QI≥90 and ≤110) and asymmetry (Asymmetric Strength- Men, Asymmetric Strength- Women: QI<90 or >110).
TABLE 2.
Descriptive values of biomechanical variables of interest during the first 50% of the stance phase of gait (expressed as median and interquartile ranges for involved and uninvolved limbs).
| Involved (Interquartile Range) | Uninvolved (Interquartile Range) | |
|---|---|---|
| Peak Knee Flexion Angle | 18.4° (15.5- 22.8) | 22.2° (18.7- 25.6) |
| Peak Knee Extension Moment | 0.38 Nm/kg·m (0.31- 0.50) | 0.49 Nm/kg·m (0.41- 0.58) |
| Knee Excursion-Weight Acceptance | 14.4° (11.5- 16.5) | 18.0° (14.9- 20.1) |
| Knee Excursion-Midstance | 12.9° (8.6- 16.8) | 19.6° (15.3- 23.2) |
| Peak Medial Compartment Contact Force | 2.7 BW (2.3-3.0) | 2.9 BW (2.7- 3.2) |
| Quadriceps Muscle Force at PKFA | 2.0 BW (1.7- 2.5) | 2.6 BW (2.3- 2.9) |
Abbreviations: SD, standard deviation; BW, bodyweight
TABLE 3.
Spearman’s rho correlation coefficients (and p-values) for key biomechanical variables during gait and QI. All relationships were analyzed using limb symmetry indexes.
| LSI of biomechanical variables: | Peak Knee Flexion Angle (n=76) |
Peak Knee Extension Moment (n=76) |
Knee Excursion-Weight Acceptance (n=76) |
Knee Excursion-Midstance (n=76) |
Peak Medial Compartment Contact Force (n=68) |
Quadriceps Muscle Force at PKFA (n=68) |
|---|---|---|---|---|---|---|
| QI | −0.045 (p=0.698) |
0.075 (p=0.521) |
−0.013 (p=0.914) |
0.071 (p=0.544) |
0.235 (p=0.129) |
0.094 (p=0.547) |
Abbreviations: QI, quadriceps index; PKFA, peak knee flexion angle
Discussion:
The purpose of this study was to determine the relationship between symmetry in quadriceps strength and gait biomechanics in athletes who had undergone ACLR and criterion-based post-operative rehabilitation and achieved 80% QI and subsequently participated in specialized return-to-sport training. Our findings refute our hypothesis and indicate that among those after ACLR who had undergone criterion-based rehabilitation, attained at least 80% QI, and completed return-to-sport training, quadriceps strength symmetry was not related to gait asymmetries. Of the 76 participants, 27 (35%) demonstrated asymmetries in quadriceps strength, defined by having quadriceps strength symmetry <90% (n=23) or >110% (n=4). For the biomechanical variables of interest, 67% demonstrated asymmetry in peak knee flexion angle, 68% and 83% in knee excursion during weight acceptance and midstance, respectively, 74% in internal peak knee extension moment, 57% in medial compartment contact force, and 74% in quadriceps muscle force. Our findings suggest that once individuals have undergone criterion-based rehabilitation and return-to-sport training, isometric quadriceps strength symmetry may not be the primary driver of biomechanical gait asymmetries. Further, our results reinforce that current clinical interventions may not be successful in targeting restoration of gait mechanics post-operatively.
Our cohort received evidence-based, progressive post-operative rehabilitation and the addition of 10 return-to-sport training sessions, extending beyond the scope of traditional rehabilitation. Meeting our enrollment criteria of 80% quadriceps strength symmetry is a high standard, as most patients in the “real world” do not meet this threshold prior to return-to-sport clearance.30 Following return-to-sport training, however, there was more variability in quadriceps strength symmetry (56.9%-131.7%; 35% demonstrating symmetry values <90% or >110%). The return-to-sport training protocol emphasized both the involved and uninvolved limbs, whereas traditional rehabilitation typically focuses on the involved limb only. As a result, some participants may re-gain strength on the deconditioned uninvolved limb more quickly than the involved limb during return-to-sport training, leading to quadriceps strength symmetry of less than 80% in some individuals. While all participants in the ACL-SPORTS trial ultimately met all our return-to-sport criteria including a QI ≥ 90% prior to receiving return-to-sport clearance, patients in the “real world” often do not. While the ACL-SPORTS trial specifically assesses the impact of return-to-sport training, most athletes complete some type of return-to-sports training outside of rehabilitation prior to full, unrestricted return-to-play. Our findings highlight the potential need for interventions beyond traditional strengthening and sport-specific training to address biomechanical asymmetries during gait that persist widely among even the most rehabilitated patients.
Previous literature has suggested a relationship between quadriceps function and aberrant gait mechanics early after ACLR.35,58 Quadriceps weakness in patients after ACLR is related to smaller angles and moments in the involved knee during early stance in both walking and jogging, while those with symmetric quadriceps strength have demonstrated mechanics similar to uninjured individuals.35 The mean quadriceps strength in these previous results, however, is low with the involved side being <80% of the uninvolved side. Similarly, individuals are within the first 6 months after ACLR. In our present study, the mean quadriceps strength symmetry was 93.4% (min 56.9, max 131.7) and all participants had achieved a QI of at least 80% prior to enrollment. Our results suggest that while quadriceps strength deficits may be implicated in aberrant mechanics early post-operatively, at the return-to-sport time point (≥ 7 months post-operatively), quadriceps weakness had been resolved in 65% of individuals. Further, our data suggests a lack of relationship between our strength measures and gait mechanics at the return-to-sports timepoint in both those with symmetric and asymmetric quadriceps strength.
Roewer et al.47 found in a cohort of non-copers (i.e., individuals who experience dynamic knee instability after ACL injury) that although quadriceps strength normalized by 6 months after ACLR, asymmetric knee angles and moments persisted at 6 months. These findings suggest that resolving quadriceps strength does not restore gait asymmetries at 6 months after surgery and neuromuscular impairments are not resolved by addressing only quadriceps weakness. Our findings expand upon prior results, noting similar findings in a more diverse cohort of individuals who have undergone ACLR, post-operative rehabilitation with neuromuscular training, and structured return-to-sports training and are 7.1 ± 2.0 months after surgery. In our sample, however, athletes did not all demonstrate symmetrical quadriceps strength after return-to-sports training; 35% of individuals had quadriceps strength asymmetry. While individuals in Roewer et al.47 underwent criterion-based postoperative rehabilitation including progressive strength and functional training,37 individuals in our cohort also underwent 10 sessions of return-to-sports training sessions, focused on prevention exercises, quadriceps strengthening and agility exercises. In addition, our sample size powered us to determine specific relationships between quadriceps strength and biomechanical variables using a correlational analysis. Collectively, these data suggest current rehabilitation is not addressing neuromuscular deficits underlying changes in gait mechanics.
Lewek et al.35 demonstrated that patients with weak quadriceps (QI <80%; mean QI 67.6%) less than 6 months after ACLR had lower knee flexion angles and internal knee extensor moments during stance than healthy individuals, consistent with a quadriceps avoidance gait strategy. In contrast, individuals with symmetric quadriceps strength (quadriceps strength symmetry >90%) demonstrated appropriate eccentric quadriceps control during weight acceptance.35 There is a suggested relationship between quadriceps weakness and aberrant gait mechanics early after ACLR,35,47 however the point at which quadriceps strength no longer relates to persistent biomechanical asymmetries during rehabilitation remains unknown. Our data suggest that after progressive postoperative rehabilitation and return-to-sport testing, quadriceps strength symmetry, or lack thereof, is no longer a primary factor related to asymmetric gait mechanics that persist in this cohort. Gait-specific components to ACL rehabilitation may therefore be necessary to restore gait symmetry.
In a cohort of individuals 6-10 months after ACLR, Shi et al.52 found that asymmetrical quadriceps strength was significantly related to asymmetry in knee flexion angle and knee extensor moment during the stance phase of gait. This cohort, however, included individuals 6-10 months after ACLR without quadriceps strength symmetry restoration (isometric quadriceps strength of involved limb: 0.94 ± 0.28 Nm/kg/m, uninvolved limb: 1.26 ± 0.28 Nm/kg/m; p<0.001). The average quadriceps strength symmetry, calculated using the same methods as our data, was 74.6%, indicating the range of quadriceps strength symmetry was significantly lower compared to our cohort (mean=93.4%). This low quadriceps strength symmetry mean value seen in Shi et al.52 indicates these individuals demonstrate substantial quadriceps weakness that may relate to biomechanical asymmetries during gait. These data indicate that quadriceps weakness may lead to gait asymmetries. In our cohort, asymmetries continue to persist among those whose have met evidence-based return-to-sport criteria (enrollment criteria for our study), even though 35% of individuals in our sample exhibit quadriceps strength asymmetry.
Individuals after ACLR whose quadriceps strength has been restored continue to exhibit interlimb asymmetry during functional tasks such as squatting.48 Chan and Sigward16 showed that individuals after ACLR have loading strategies similar to ‘learned nonuse’ that occurs in survivors of stroke57, which may underlie the persistence of certain interlimb asymmetries. In their cohort, Chan and Sigward16 provided evidence that individuals 3 months after ACLR have a mismatch in their natural loading behaviors and their true ability to meet the demands of the tasks. These data suggest individuals have the capacity to meet the demands of the task and restore symmetry, though they may self-select an asymmetric loading strategy to complete the task due to learned behaviors. It is possible this ‘learned nonuse’ exists in gait as well, and future research should examine individual’s abilities to walk symmetrically after ACLR.
Our cohort received evidence-based post-operative rehabilitation and the addition of return-to-sport training, which improved all functional outcome measures including self-reported function, hop testing, and quadriceps strength.6,7,10,14 Despite these improvements, aberrant gait biomechanics persisted. Our results suggest current rehabilitation after ACLR may not be adequately targeting gait. There may be sensorimotor control deficits that extend beyond restoring strength and clinically measured function that current clinical interventions are not addressing. Post-operative rehabilitation should include gait specific components to address these lingering biomechanical asymmetries. Our current study did not assess eccentric strength, which may play a role in adequate rehabilitation of quadriceps strength and knee mechanics. Recent evidence suggests eccentric strengthening may be effective in restoring quadriceps strength via neuromuscular training.20,34 Training the quadriceps eccentrically would allow for task-specific strengthening of how the quadriceps work during the loading response of the gait cycle. Clinicians should consider incorporating gait-specific training in rehabilitation to mitigate post-operative gait asymmetries in this clinical population.
There are several limitations to consider when interpreting our results. The cross-sectional analysis precluded cause and effect conclusions. As this is a secondary analysis, there was no statistical analysis published prior to conducting this study; however, our a priori power analysis indicated our analyses were adequately powered. Our findings are limited to walking, a relatively low-demand task. Inferences to higher level tasks that require greater quadriceps strength cannot be made, and inclusion of biomechanical analyses of higher level RTS tasks may provide additional insight. Our range of quadriceps strength symmetries, however, allows us to capture a wide range of possible relationships between strength and mechanics at the post-training timepoint (Figure 1). Our strength and biomechanical data are reported as limb symmetry indexes, which is a symmetry measure and therefore we cannot draw conclusions about the involved limb specifically. Similarly, our strength measurements are isometric, and isokinetic strength including eccentric measurements may provide additional insights in future studies. All variables of interest were expressed as limb symmetry indexes; therefore, the involved limb was only compared to the uninvolved limb. Future studies could also compare quadriceps strength in the involved limb to a control group, as well as analyze EMG data for quadriceps activation deficits during gait. Finally, our data do not include pre-operative or early post-operative rehabilitation time-points, where prior literature suggests a relationship between quadriceps strength and gait mechanics exists.
Conclusion:
Among those who meet rehabilitation milestones including 80% QI and subsequently undergo return-to-sport training, quadriceps strength symmetry is not correlated with biomechanical gait asymmetries, which remain prevalent. Current clinical interventions may not be sufficiently targeting gait and achieving quadriceps strength alone during rehabilitation, while important, is not in-and-of-itself sufficient to restore symmetrical gait mechanics. Evaluating and incorporating task-specific gait and neuromuscular training is a critical direction for future rehabilitation research.
FIGURE 2.
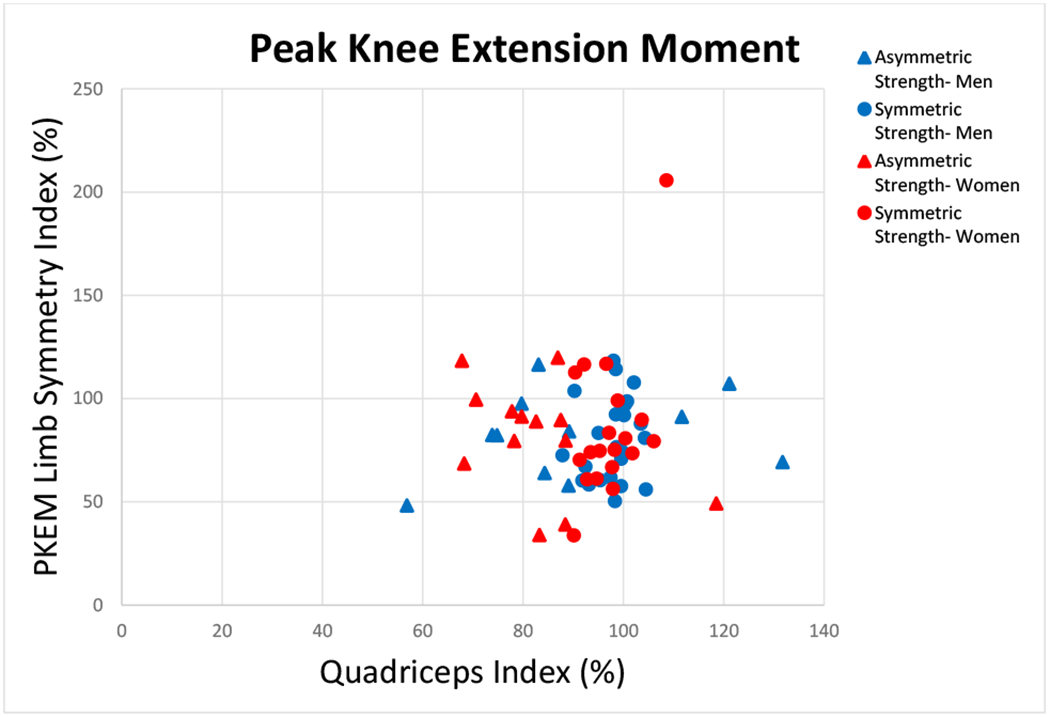
There was no relationship between quadriceps index and peak knee extension moment (PKEM) (Spearman’s ρ=0.075; p=0.521). Groups are based on quadriceps index symmetry (Symmetric Strength-Men, Symmetric Strength- Women: QI≥90 and ≤110) and asymmetry (Asymmetric Strength- Men, Asymmetric Strength- Women: QI<90 or >110).
Clinical Relevance:
Among those approximately 7 months after ACL reconstruction who completed return-to-sport training, subsequent quadriceps strength symmetry was not related to gait asymmetries. Developing and incorporating task-specific gait interventions may be a critical step to mitigating aberrant gait mechanics after ACL reconstruction.
What is known about the subject:
Gait asymmetries are highly prevalent pre-operatively and after ACL reconstruction, persisting up to 5 years post ACL rupture. Both pre-operatively and early post-operatively (up to 5 months), these asymmetries are related to quadriceps weakness. These aberrant gait mechanics are implicated in the development of posttraumatic osteoarthritis after ACL reconstruction. Current clinical interventions have not been successful in restoration of gait asymmetries.
What this study adds to existing knowledge:
This study examines the relationship between quadriceps strength and gait asymmetries in a cohort of individuals who have undergone evidence-based, progressive rehabilitation and 10 return-to-sport training sessions and are about 7 months after ACL reconstruction. Clinicians often assume that once function is restored at this point in rehabilitation, and return-to-sport is being considered, gait asymmetries are also normalized. The present study suggests that other neuromuscular impairments contribute to aberrant post-operative gait mechanics beyond quadriceps strength and suggests our clinical interventions do not currently suffice for reducing aberrant gait mechanics.
Acknowledgments:
The authors would like to acknowledge Kathleen Cummer, Amelia J.H. Arundale, P. Michael Eckrich, Georgia Gagianas, Naoaki Ito, Martha Callahan and the Delaware Rehabilitation Institute Research Core, and Angela H. Smith and the University of Delaware Physical Therapy Clinic for their assistance in data collection, processing, and management. Thank you to Ashutosh Khandha and Kurt Manal for their assistance with musculoskeletal modeling.
One or more of the authors has declared a potential conflict of interest as specified in the AJSM Conflict of Interest statement: This work was supported by the National Institutes of Health (R37 HD037985, R01 AR048212, R01 HD087459, F30 HD096830, F32 AG066274).
Footnotes
Investigation performed at the University of Delaware, Newark, DE, USA.
References:
- 1.Adams D, Logerstedt D, Hunter-Giordano A, Axe MJ, Snyder-Mackler L. Current concepts for anterior cruciate ligament reconstruction: A criterion-based rehabilitation progression. J Orthop Sports Phys Ther. 2012;42(7):601–614. doi: 10.2519/jospt.2012.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams D, Logerstedt D, Hunter-Giordano A, Axe MJ, Snyder-Mackler L. Current Concepts for Anterior Cruciate Ligament Reconstruction: A Criterion-Based Rehabilitation Progression. J Orthop Sport Phys Ther. 2012;42(7):601–614. doi: 10.2519/jospt.2012.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams D, Logerstedt D, Hunter-Giordano A, Axe MJ, Snyder-Mackler L. Current Concepts for Anterior Cruciate Ligament Reconstruction: A Criterion-Based Rehabilitation Progression. J Orthop Sport Phys Ther. 2012;42(7):601–614. doi: 10.2519/jospt.2012.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andriacchi TP, Dyrby CO. Interactions between kinematics and loading during walking for the normal and ACL deficient knee. J Biomech. 2005;38(2):293–298. doi: 10.1016/j.jbiomech.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Andriacchi TP, Mündermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447–457. http://www.ncbi.nlm.nih.gov/pubmed/15095819. [DOI] [PubMed] [Google Scholar]
- 6.Arundale AJH, Capin JJ, Zarzycki R, Smith A, Snyder-Mackler L. Functional and Patient-Reported Outcomes Improve Over the Course of Rehabilitation: A Secondary Analysis of the ACL-SPORTS Trial. Sports Health. 2018. doi: 10.1177/1941738118779023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arundale AJH, Cummer K, Capin JJ, Zarzycki R, Snyder-Mackler L. Report of the Clinical and Functional Primary Outcomes in Men of the ACL-SPORTS Trial: Similar Outcomes in Men Receiving Secondary Prevention With and Without Perturbation Training 1 and 2 Years After ACL Reconstruction. Clin Orthop Relat Res. 2017;475(10):2523–2534. doi: 10.1007/s11999-017-5280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arundale AJH, Zarzycki R, Capin JJ, Snyder-Mackler L, Smith AH. Two Year Acl Reinjury Rate of 2.5%: Outcomes Report of the Men in a Secondary Acl Injury Prevention Program (Acl-Sports). Int J Sports Phys Ther. 2018;13(3):422–431. doi: 10.26603/ijspt20180422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchanan TS, Lloyd DG, Manal K, Besier TF. Neuromusculoskeletal modeling: Estimation of muscle forces and joint moments and movements from measurements of neural command. J Appl Biomech. 2004. doi: 10.1123/jab.20.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capin JJ, Failla M, Zarzycki R, et al. Superior 2-Year Functional Outcomes Among Young Female Athletes after ACL Reconstruction in Just 10 Return-to-Sport Training Sessions: Comparisons of ACL-SPORTS Randomized Control Trial to Delaware-Oslo and MOON Cohorts. Orthop J Sport Med. 2019;Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capin JJ, Khandha A, Zarzycki R, et al. Gait mechanics and tibiofemoral loading in men of the ACL-SPORTS randomized control trial. J Orthop Res. 2018;36(9):2364–2372. doi: 10.1002/jor.23895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capin JJ, Khandha A, Zarzycki R, Manal K, Buchanan TS, Snyder-Mackler L. Gait mechanics and second ACL rupture: Implications for delaying return-to-sport. J Orthop Res. 2017;35(9):1894–1901. doi: 10.1002/jor.23476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capin JJ, Williams JR, Neal K, et al. Slower Walking Speed Is Related to Early Femoral Trochlear Cartilage Degradation After ACL Reconstruction. J Orthop Res. 2020;38(3):645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capin JJ, Zarzycki R, Arundale A, Cummer K, Snyder-Mackler L. Report of the Clinical and Functional Primary Outcomes in Men of the ACL-SPORTS Trial: Similar Outcomes in Men Receiving Secondary Prevention With and Without Perturbation Training 1 and 2 Years After ACL Reconstruction. Clin Orthop Relat Res. 2017;475(10):2523–2534. doi: 10.1007/s11999-017-5280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capin JJ, Zarzycki R, Ito N, et al. Gait Mechanics in Women of the ACL-SPORTS Randomized Control Trial: Interlimb Symmetry Improves Over Time Regardless of Treatment Group. J Orthop Res. 2019:Accepted. doi: 10.1002/jor.24314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan MS, Sigward SM. Loading Behaviors Do Not Match Loading Abilities Postanterior Cruciate Ligament Reconstruction. Med Sci Sports Exerc. 2019. doi: 10.1249/MSS.0000000000001956. [DOI] [PubMed] [Google Scholar]
- 17.Cohen J Statistical power for the social sciences. Hillsdale, NJ: Laurence Erlbaum Assoc; 1988. [Google Scholar]
- 18.Daniel DM, Stone M Lou, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured Patient: A Prospective Outcome Study. Am J Sports Med. 1993;22(5):632–644. [DOI] [PubMed] [Google Scholar]
- 19.Gardinier ES, Manal K, Buchanan TS, Snyder-Mackler L. Minimum detectable change for knee joint contact force estimates using an EMG-driven model. Gait Posture. 2013;38(4):1051–1053. doi: 10.1016/j.gaitpost.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gokeler A, Bisschop M, Benjaminse A, Myer GD, Eppinga P, Otten E. Quadriceps function following ACL reconstruction and rehabilitation: Implications for optimisation of current practices. Knee Surgery, Sport Traumatol Arthrosc. 2014. doi: 10.1007/s00167-013-2577-x. [DOI] [PubMed] [Google Scholar]
- 21.Grindem H, Snyder-Mackler L, Moksnes H, Engebretsen L, Risberg MA. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: The Delaware-Oslo ACL cohort study. Br J Sports Med. 2016;50(13):804–808. doi: 10.1136/bjsports-2016-096031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harkey MS, Luc BA, Golightly YM, et al. Osteoarthritis-related biomarkers following anterior cruciate ligament injury and reconstruction: A systematic review. Osteoarthr Cartil. 2015. doi: 10.1016/j.joca.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Hart HF, Culvenor AG, Collins NJ, et al. Knee kinematics and joint moments during gait following anterior cruciate ligament econstruction: A systematic review and meta-analysis. Br J Sports Med. 2016;50(10):597–612. doi: 10.1136/bjsports-2015-094797. [DOI] [PubMed] [Google Scholar]
- 24.Hart JM, Turman KA, Diduch DR, Hart JA, Miller MD. Quadriceps muscle activation and radiographic osteoarthritis following ACL revision. Knee Surgery, Sport Traumatol Arthrosc. 2011. doi: 10.1007/s00167-010-1321-z. [DOI] [PubMed] [Google Scholar]
- 25.Henriksen M, Creaby MW, Lund H, Juhl C, Christensen R. Is there a causal link between knee loading and knee osteoarthritis progression? A systematic review and meta-analysis of cohort studies and randomised trials. BMJ Open. 2014. doi: 10.1136/bmjopen-2014-005368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henriksen M, Hunter DJ, Dam EB, et al. Is increased joint loading detrimental to obese patients with knee osteoarthritis? A secondary data analysis from a randomized trial. Osteoarthr Cartil. 2013. doi: 10.1016/j.joca.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Herrington L, Alarifi S, Jones R. Patellofemoral Joint Loads During Running at the Time of Return to Sport in Elite Athletes With ACL Reconstruction. Am J Sport Med. 2017;45(12):2812–2816. [DOI] [PubMed] [Google Scholar]
- 28.Hurley M V The role of muscle weakness in the pathogenesis of osteoarthritis. Rheum Dis Clin North Am. 1999. doi: 10.1016/S0889-857X(05)70068-5. [DOI] [PubMed] [Google Scholar]
- 29.Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80(8):1132–1145. http://www.ncbi.nlm.nih.gov/pubmed/9730122. [DOI] [PubMed] [Google Scholar]
- 30.Ithurburn MP, Paljieg A, Thomas S, Hewett TE, Paterno M V., Schmitt LC. Strength and Function Across Maturational Levels in Young Athletes at the Time of Return to Sport After ACL Reconstruction. Sports Health. 2019. doi: 10.1177/1941738119849070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaur M, Ribeiro DC, Theis JC, Webster KE, Sole G. Movement Patterns of the Knee During Gait Following ACL Reconstruction: A Systematic Review and Meta-Analysis. Sport Med. 2016;46(12):1869–1895. doi: 10.1007/s40279-016-0510-4. [DOI] [PubMed] [Google Scholar]
- 32.Khandha A, Manal K, Wellsandt E, Capin J, Snyder-Mackler L, Buchanan TS. Gait mechanics in those with/without medial compartment knee osteoarthritis 5 years after anterior cruciate ligament reconstruction. J Orthop Res. 2017;35(3):625–633. doi: 10.1002/jor.23261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kyritsis P, Bahr R, Landreau P, Miladi R, Witvrouw E. Likelihood of ACL graft rupture: Not meeting six clinical discharge criteria before return to sport is associated with a four times greater risk of rupture. Br J Sports Med. 2016;50(15):946–951. doi: 10.1136/bjsports-2015-095908. [DOI] [PubMed] [Google Scholar]
- 34.Lepley LK, Lepley AS, Onate JA, Grooms DR. Eccentric Exercise to Enhance Neuromuscular Control. Sports Health. 2017. doi: 10.1177/1941738117710913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewek M, Rudolph K, Axe M, Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech. 2002. doi: 10.1016/S0268-0033(01)00097-3. [DOI] [PubMed] [Google Scholar]
- 36.Manal K, Buchanan TS. An Electromyogram-Driven Musculoskeletal Model of the Knee to Predict in Vivo Joint Contact Forces During Normal and Novel Gait Patterns . J Biomech Eng. 2013. doi: 10.1115/1.4023457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manal TJ, Snyder-Mackler L. Practice guidelines for anterior cruciate ligament rehabilitation: A criterion-based rehabilitation progression. Oper Tech Orthop. 1996;6(3):190–196. doi: 10.1016/S1048-6666(96)80019-X. [DOI] [Google Scholar]
- 38.Martin H, Yule V, Syddall H, Dennison E, Cooper C, Sayer A. Is hand-held dynamometry useful for the measurement of quadriceps strength in older people? A comparison with the gold standard Bodex dynamometry. Gerontology. 2006;52(3):154–159. [DOI] [PubMed] [Google Scholar]
- 39.Moisio KC, Sumner DR, Shott S, Hurwitz DE. Normalization of joint moments during gait: A comparison of two techniques. J Biomech. 2003. doi: 10.1016/S0021-9290(02)00433-5. [DOI] [PubMed] [Google Scholar]
- 40.Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by functional hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19:513–518. [DOI] [PubMed] [Google Scholar]
- 41.østerăs H, Augestad LB, Tøndel S. Isokinetic muscle strength after anterior cruciate ligament reconstruction. Scand J Med Sci Sports. 1998. doi: 10.1111/j.1600-0838.1998.tb00483.x. [DOI] [PubMed] [Google Scholar]
- 42.Pfeiffer SJ, Spang J, Nissman D, et al. Gait Mechanics and T1ρ MRI of Tibiofemoral Cartilage 6 Months after ACL Reconstruction. Med Sci Sports Exerc. 2019;51(4):630–639. doi: 10.1249/MSS.0000000000001834. [DOI] [PubMed] [Google Scholar]
- 43.Pietrosimone B, Blackburn J, Harkey M, et al. Greater Mechanical Loading During Walking Is Associated With Less Collagen Turnover in Individuals With Anterior Cruciate Ligament Reconstruction. Am J Sport Med. 2016;44(2):425–432. [DOI] [PubMed] [Google Scholar]
- 44.Pietrosimone B, Loeser R, Blackburn J, et al. Biochemical markers of cartilage metabolism are associated with walking biomechanics 6-months following anterior cruciate ligament reconstruction. J Orthop Res. 2017;35(10):2288–2297. doi: 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pietrosimone B, Loeser RF, Blackburn JT, et al. Biochemical markers of cartilage metabolism are associated with walking biomechanics 6-months following anterior cruciate ligament reconstruction. J Orthop Res. 2017. doi: 10.1002/jor.23534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pietrosimone B, Pfeiffer SJ, Harkey MS, et al. Quadriceps weakness associates with greater T1ρ relaxation time in the medial femoral articular cartilage 6 months following anterior cruciate ligament reconstruction. Knee Surgery, Sport Traumatol Arthrosc. 2019;27(8):2632–2642. doi: 10.1007/s00167-018-5290-y. [DOI] [PubMed] [Google Scholar]
- 47.Roewer BD, Di Stasi SL, Snyder-Mackler L. Quadriceps strength and weight acceptance strategies continue to improve two years after anterior cruciate ligament reconstruction. J Biomech. 2011. doi: 10.1016/j.jbiomech.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roos PE, Button K, Van Deursen RWM. Motor control strategies during double leg squat following anterior cruciate ligament rupture and reconstruction: An observational study. J Neuroeng Rehabil. 2014. doi: 10.1186/1743-0003-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rudolph KS, Eastlack ME, Axe MJ, Snyder-Mackler L. 1998 Basmajian Student Award Paper Movement patterns after anterior cruciate ligament injury: A comparison of patients who compensate well for the injury and those who require operative stabilization. J Electromyogr Kinesiol. 1998. doi: 10.1016/S1050-6411(97)00042-4. [DOI] [PubMed] [Google Scholar]
- 50.Saxby DJ, Bryant AL, Van Ginckel A, et al. Greater magnitude tibiofemoral contact forces are associated with reduced prevalence of osteochondral pathologies 2–3 years following anterior cruciate ligament reconstruction. Knee Surgery, Sport Traumatol Arthrosc. 2019;27(3):707–715. doi: 10.1007/s00167-018-5006-3. [DOI] [PubMed] [Google Scholar]
- 51.Schmitt LC, Paterno MV., Hewett TE. The impact of quadriceps femoris strength asymmetry on functional performance at return to sport following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2012. doi: 10.2519/jospt.2012.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi H, Huang H, Ren S, et al. The relationship between quadriceps strength asymmetry and knee biomechanics asymmetry during walking in individuals with anterior cruciate ligament reconstruction. Gait Posture. 2019. doi: 10.1016/j.gaitpost.2019.07.151. [DOI] [PubMed] [Google Scholar]
- 53.Sinacore JA, Evans AM, Lynch BN, Joreitz RE, Irrgang JJ, Lynch AD. Diagnostic Accuracy of Handheld Dynamometry and 1-Repetition-Maximum Tests for Identifying Meaningful Quadriceps Strength Asymmetries. J Orthop Sport Phys Ther. 2017;47(2):97–107. doi: 10.2519/jospt.2017.6651. [DOI] [PubMed] [Google Scholar]
- 54.Snyder-Mackler L, Delitto A, Bailey SL, Stralka SW. Strength of the Quadriceps Femoris Muscle and Functional Recovery after Reconstruction of the Anterior Cruciate Ligament. J Bone Jt Surg. 1995;77-A(8):1166–1173. [DOI] [PubMed] [Google Scholar]
- 55.Di Stasi SL, Hartigan EH, Snyder-Mackler L. Sex-specific gait adaptations prior to and up to six months after ACL reconstruction. J Orthop Sport Phys Ther. 2016;45(3):207–214. doi: 10.2519/jospt.2015.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Stasi SL, Snyder-Mackler L. The effects of neuromuscular training on the gait patterns of ACL-deficient men and women. Clin Biomech. 2012. doi: 10.1016/j.clinbiomech.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taub E, Uswatte G, Mark VW, Morris DM. The learned nonuse phenomenon: Implications for rehabilitation. Eura Medicophys. 2006. [PubMed] [Google Scholar]
- 58.Troy Blackburn J, Pietrosimone B, Harkey MS, Luc BA, Pamukoff DN. Quadriceps Function and Gait Kinetics after Anterior Cruciate Ligament Reconstruction. Med Sci Sports Exerc. 2016. doi: 10.1249/MSS.0000000000000963. [DOI] [PubMed] [Google Scholar]
- 59.Urbach D, Nebelung W, Becker R, Awiszus F. Effects of reconstruction of the anterior cruciate ligament on voluntary activation of quadriceps femoris. J Bone Jt Surg - Ser B. 2001. doi: 10.1302/0301-620X.83B8.11618. [DOI] [PubMed] [Google Scholar]
- 60.Wellsandt E, Gardinier ES, Manal K, Axe MJ, Buchanan TS, Snyder-Mackler L. Decreased knee joint loading associated with early knee osteoarthritis after anterior cruciate ligament injury. Am J Sport Med. 2016;44(1):143–151. doi: 10.1177/0363546515608475.Decreased. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wellsandt E, Gardinier ES, Manal K, Axe MJ, Buchanan TS, Snyder-Mackler L. Decreased Knee Joint Loading Associated With Early Knee Osteoarthritis After Anterior Cruciate Ligament Injury. Am J Sports Med. 2016;44(1):143–151. doi: 10.1007/BF00612995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White K, Di Stasi SL, Smith AH, Snyder-Mackler L. Anterior cruciate ligament-specialized post-operative return-to-sports (ACL-SPORTS) training: A randomized control trial. BMC Musculoskelet Disord. 2013;14(1):1. doi: 10.1186/1471-2474-14-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilk KE, Romaniello WT, Soscia SM, Arrigo CA, Andrews JR. The relationship between subjective knee scores, isokinetic testing, and functional testing in the ACL-reconstructed knee. J Orthop Sports Phys Ther. 1994. doi: 10.2519/jospt.1994.20.2.60. [DOI] [PubMed] [Google Scholar]
- 64.Winby CR, Lloyd DG, Besier TF, Kirk TB. Muscle and external load contribution to knee joint contact loads during normal gait. J Biomech. 2009. doi: 10.1016/j.jbiomech.2009.06.019. [DOI] [PubMed] [Google Scholar]


