Abstract
Objective:
There is evidence that females have a better outcome in intensive care units (ICUs) when compared with males. The aim of the present study was to compare hospital course and physiologic markers between severely burned pediatric females and males.
Summary Background Data:
One-hundred eighty-nine children sustaining a ≥40% total body surface area burn were divided into females (n = 76) and males (n = 113).
Methods:
Patient demographics, clinical parameters, and mortality were noted. Muscle protein synthesis was determined by stable isotope technique. Resting energy expenditure (REE) was measured by indirect calorimetry and body composition by dual x-ray absorptiometry. Serum hormones, proteins, and cytokines were determined. Cardiac function and liver size were determined by repeated ultrasound measurements.
Results:
There were no significant differences between females and males for mortality, demographics, burn size, nutritional intake, or concomitant injuries. ICU stay was in females: 29 ± 3 days whereas the stay in males was 38 ± 3 days, P < 0.05. Females had a significant attenuated loss in muscle protein net balance (females: −0.028 ± 0.001% vs. males: −0.05 ± 0.007%) and an increase in lean body mass (Δ females: 5 ± 4% vs. Δ males: − 1 ± 3%), P < 0.05. Percent-predicted REE was significantly decreased in females compared with males, P < 0.05. Systemic inflammatory markers and stress hormone levels were significantly decreased in females, P < 0.05. Cardiac and liver dysfunction were significantly attenuated in females compared with males, P < 0.05.
Conclusions:
Female burned patients exert an attenuated inflammatory and hypermetabolic response compared with males. This decrease is reflected in improved muscle protein net balance and preservation of lean body mass, which are associated with shortened hospital stay.
Gender differences in the outcome of critically ill patients have been discussed in many studies and are the center of many ongoing studies. There is a notion in the literature that female critically ill patients have a better survival and are more resistant to infections compared with males. However, there are also studies showing that there is no difference between males and females. We have recently shown that female burned patients have an attenuated postburn response with associated increased endogenous anabolic hormone levels, decreased serum inflammatory markers, and decreased stay in the intensive care unit (ICU) when compared with males. Over the last years, many experimental and clinical studies looked at differences in the outcome of female and male patients.1,2 Although some studies found improved survival for female patients, others could not find any differences in the outcome.3,4 We further conducted 2 clinical studies in which the main outcome measure was survival from a severe burn. The study in 1995 showed a significant better outcome for females, but a recent study showed no difference between males and females in terms of survival. The current study was conducted to determine whether severely burned females have a better acute clinical course compared with males and further to determine possible mechanisms.
The stress response to burn injury is similar to any critical illness or severe trauma only differing by its severity and duration. The hypermetabolic response after major burn is characterized by a hyperdynamic response with increased body temperature, oxygen and glucose consumption, CO2 production, glycogenolysis, proteolysis, lipolysis, and futile substrate cycling.5 This response begins on the fifth day postinjury and continues up to 9 months postburn, causing erosion of lean body mass, muscle weakness, immunodepression, and poor wound healing.6 In no other disease or trauma is the hypermetabolic response as severe as it is after a thermal injury. The increased metabolic requirements in patients with major burns can cause a major tissue breakdown leading to nitrogen loss and a potentially lethal depletion of essential protein stores.7 The energy requirements are met by the mobilization of proteins and amino acids. Increased protein turnover, degradation and negative nitrogen balance are all characteristic of this severe critical illness.7,8 As a consequence, the structure and function of essential organs, such as liver, skeletal muscle, skin, immune system, and cellular membrane transport functions are compromised.9 An increased and prolonged action of the proinflammatory acute phase response would enhance protein degradation and catabolism and may be associated with increased incidence of multiorgan system failure and ongoing sepsis.10 Protein degradation and negative nitrogen balance have been associated with resistance and decreased production of anabolic hormones.11 Based on the pathophysiologic response after burn and previous studies from our group,12–14 we asked the question whether the hypothesis holds true that there is a gender difference, and if so, by which mechanisms do these differences occur.
PATIENTS AND METHODS
Thermally injured children from 1996–2006 with the following inclusion criteria were prospectively placed in a cohort study and divided into females and males: 1–16 years of age and burns covering more than 40% total body surface area (TBSA) with a third-degree component of >10%, which required a minimum harvesting of 1 donor site for skin grafting. None of the patients in this cohort received anabolic agents.
Patients were resuscitated according to the Galveston formula with 5000 mL/m2 TBSA burned plus 2000 mL/m2 TBSA lactated Ringer’s solution given in increments over the first 24 hours.15 Within 48 hours of admission, all patients underwent total burn wound excision, and the wounds covered with available autograft skin with allograft used to cover any remaining open areas. After the first operative procedure, it took 5–10 days until the donor site was healed and patients were then taken back to the operation theater. This procedure was repeated until all open wound areas were covered with autologous skin.
All patients underwent the same nutritional treatment to a standardized protocol. The initial assessment for nutritional need is calculated by the Curreri formula, which is an accepted standard for estimating basal energy expenditure. This formula calls for 25 kcal/kg/d plus 40 kcal/% TBSA burned/d. It provides the needs, plus the additional caloric needs of the burn wounds. In children, formulas based on body surface area are more appropriate because of greater body surface per kilogram. For children, we used the Galveston formulas, Galveston Infant, Galveston Revised, Galveston Adolescent. The formulas change with age, based on the body surface alterations that occur with growth. Roughly the intake is calculated as 1500 kcal/m2 body surface plus 1500 kcal/m2 area burn.16 The composition of the nutritional supplement is also important. The optimal dietary composition contains 1–2 g/kg/d of protein, which provides a calorie to nitrogen ratio at around 100:1 with the suggested caloric intakes.16 Nonprotein calories can be given either as carbohydrate or as fat with clinical advantages for the carbohydrates. The diet was delivered by enteral nutrition, if possible, in all our patients.
Patient demographics (age, date of burn and admission, sex, burn size, and depth of burn) and concomitant injuries, such as inhalation injury and sepsis, morbidity, and mortality were recorded. Sepsis was defined as a blood culture identifying the pathogen during hospitalization or at autopsy, in combination with leukocytosis or leukopenia, hyperthermia or hypothermia and tachycardia. Wound healing was evaluated from time of donor site healing, and thus, time between operative interventions.
Indirect Calorimetry
As part of our routine clinical practice, all patients underwent resting energy expenditure (REE) measurements within 1 week after hospital admission and weekly thereafter during their acute hospitalization. To adjust for the ebb and flow phase of the hypermetabolic response, the first and second metabolic study was averaged and the results were defined as the acute study. This and subsequent measurements of REE were performed between midnight and 5:00 am while the patients were asleep and receiving continuous feeding. The amount of feeding was calculated as mentioned above. Resting energy expenditure was measured using a Sensor-Medics Vmax 29 metabolic cart (Yorba Linda, CA) as published previously.17 For statistical comparison, energy expenditure was expressed both as absolute REE and as the percentage of the basal metabolic rate predicted by the Harris-Benedict equation.
Muscle Protein Synthesis
The degree of protein catabolism was quantified using stable isotope tracers. Protein kinetic studies were performed between 5:00 am and 7:00 am, on postoperative day 5 after the first excision and grafting procedure. All stable isotope studies consisted of a 5-hour infusion of 2H5-phenylalanine, as described previously.18 Because phenylalanine is neither synthesized nor degraded in the peripheral tissues (it is metabolized only in the liver), measurement across the leg reflects the net balance of protein synthesis and breakdown. Blood samples were taken simultaneously from an ipsilateral femoral artery and vein for this determination. Indocyanine green was used to determine leg blood flow. The blood concentration of unlabeled phenylalanine was determined by gas chromatography-mass spectrometry (GCMS) using the internal standard approach and the tert-butyldimethylsilyl esters, as described previously.18 Indocyanine green concentrations were determined spectrophotometrically at λ = 805 mm on a Spectronic 1001 (Bausch and Lomb, Rochester, NY). As phenylalanine is neither synthesized nor degraded in the periphery, the difference in concentration of this substrate in the femoral arterial and venous plasma pools reflects the net balance of leg skeletal muscle protein synthesis and breakdown. The net balance was calculated and standardized for leg volume by the following equation: Net balance = (CA − CV) × BF, where CA and CV are the blood free amino acid concentrations of the femoral artery and vein, and BF is leg blood flow in mL/min/100 mL leg. Leg blood flow was determined from a modification of Fick’s equation. BF was normalized for each patient by leg volume. Subject weight, leg circumference at prescribed points relative to anatomic landmarks, and the distances between these points were used to mathematically model leg volume.18
Muscle Fractional Synthetic Rate
Muscle tissue samples were ground, and intracellular free amino acids and muscle proteins were extracted as described previously.18 Muscle intracellular free concentration and enrichment of phenylalanine was determined by GCMS. Mixed muscle protein-bound phenylalanine enrichment was analyzed by GCMS after protein hydrolysis and amino acid extraction. We calculated the fractional synthetic rate of mixed muscle proteins by measuring the incorporation rate of the phenylalanine tracer into the proteins (ΔEp/t) and using the precursor-product model to calculate the synthesis rate:
Fractional Synthetic Rate = (ΔEp/t)/[EM(1) + EM(2)/2] × 60 × 100 where ΔEp is the increment in protein-bound phenylalanine enrichment between 2 sequential biopsies, t is the time between the 2 sequential biopsies, and EM(1) + EM(2) are the phenylalanine enrichments in the free intracellular pool in the 2 sequential biopsies.18 Data are expressed as percent per hour.
Body Composition
Height and body weight were determined clinically 5 days after admission and at discharge. Total body lean mass, fat, bone mineral density, and bone mineral content were measured by dual energy x-ray absorptiometry (DEXA). A hologic model QDR-4500W DEXA (Hologic Inc, Waltham, MA) was used to measure body composition as published previously.19,20
Serum Hormones, Proteins, and Cytokines
Blood was collected from the burned patients at the time of admission, preoperatively, and 5 days postoperatively for 4 weeks for serum hormone, protein, and cytokine analysis (Fig. 1). Blood was drawn in a serum-separator collection tube and centrifuged for 10 minutes at 1320 rpm; the serum was removed and stored at −70°C until assayed. Serum hormones and acute phase proteins were determined using high performance liquid chromatography and enzyme-linked immunosorbent assay techniques. The Bio-Plex Human Cytokine 17-Plex panel was used with the Bio-Plex Suspension Array System (Bio-Rad, Hercules, CA) to profile expression of 17 inflammatory mediators [IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, IL-17, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony stimulating factor (GM-CSF), interferon-gamma (IFN-γ), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1-beta (MIP-1β), and TNF]. The assay was performed according to the manufacturer’s instructions as published previously.21,22 Urine cortisol was determined by standard laboratory techniques, counting for urine amount, creatinine and creatinine clearance.
FIGURE 1.
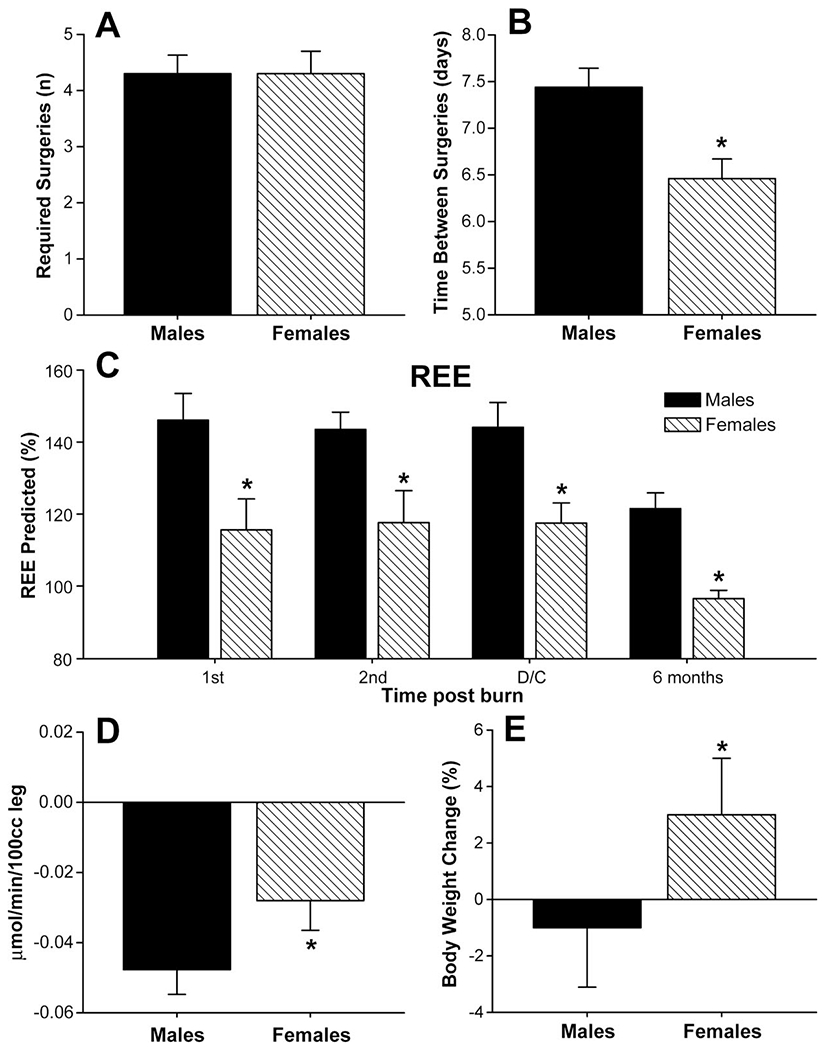
(A) Female and male patients required similar amounts of surgeries throughout the acute hospital stay. (B) The time between operations was significantly shorter in females when compared with males. *Significant difference between males versus females, P < 0.05. (C) Females had a significant decreased predicted REE acutely, at discharge and 6 months post burn when compared with males. Normal predicted REE 80–90%. *Significant difference between males versus females, P < 0.05. (D) Muscle protein net balance determined by stable isotope infusion. Females (n = 50) have an attenuated negative net muscle protein balance when compared with males (n = 52). *Significant difference between males versus females, P < 0.05. (E) Females gained approximately 3% of their body weight from admission to discharge, whereas males lost 1% of their body weight during the same time period. *Significant difference between males versus females, P < 0.05.
Liver and Cardiac Changes
Ultrasound measurements in this study were made with the HP Sonos 100 CF echocardiogram (Hewlett Packard Imaging Systems, Andover, MA). The liver was scanned using an Eskoline B-scanner, a modified HP diagnostic sounder 7214A, and a modified 3.5 MHz transducer probe. To obtain the ultrasound liver weight, a 3.5-MHz transducer was placed directly below midline of the rib cage on the right upper quadrant on a vertical line running through the right nipple with the patient in the supine position. Once the liver was visualized, measurements were made by scanning in a plane perpendicular to the base of the liver. The base of the liver, and the free edge hepatic dome, was marked on the display screen and the distance between these 2 points was automatically measured. The formula used for estimating liver weight from the single longitudinal scan along the right nipple line was WT = (1.15l)3d where l3 represents the volume of a cube cut in half diagonally to visualize the approximate shape of the normal liver in situ. A factor of 1.15 was used to correct for the portion of the liver (15%) lateral to the left nipple line and representing the most inferior point of the liver. This correction was estimated from the liver at autopsy. The density (d) of the liver was measured on several sections by water displacement. Determining the right nipple line was not problematic unless the nipple was obliterated by a severe burn to the thorax. In these cases, an approximation was made and recorded as such. Actual size was then compared with predicted size.
M-Mode echocardiograms were completed as follows: at the time of the study, none of the patients presented with or previously suffered from other concomitant diseases affecting cardiac function, such as diabetes mellitus, coronary artery disease, long standing hypertension, or hyperthyroidism. Study variables included: resting cardiac output, cardiac index, stroke volume, resting heart rate and left ventricular ejection fraction. Stroke volume and cardiac output were adjusted for body surface area and expressed as indexes. All ultrasound measurements were made with the SonoSite Titan echocardiogram, with a 3.5 MHz transducer. Recordings were performed with the subjects in a supine position and breathing freely. M-Mode tracings were obtained at the level of the tips of the mitral leaflets in the parasternal long axis position, and measurements were performed according to the American Society of Echocardiography recommendations. Left ventricular volumes determined at end diastole and end systole were used to calculate ejection fraction, stroke volume, cardiac output, and cardiac index. Three measurements were performed and averaged for data analysis.
Ethics and Statistics
The study was reviewed and approved by the Institutional Review Board of the University Texas Medical Branch, Galveston, Texas. The Institutional Review Board approved all interventions and determinations presented in this study. Before the study, each subject, parent or child’s legal guardian had to sign a written informed consent form. Analysis of variance with post hoc Bonferroni correction, paired and unpaired Student t test, χ2 analysis, and Mann-Whitney tests were used where appropriate. Data are expressed as mean ± SD or standard error of the mean, where appropriate. Significance was accepted at P < 0.05.
Role of Funding Source
The funding source had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
RESULTS
Demographics
One-hundred eighty-nine severely burned children were included in the present study. Patients’ demographics are shown in Table 1. None of the patients received anabolic agents. There was no significant difference in age, TBSA and third-degree burn, and nutritional intake (Table 1). The average time from burn to hospital admission was 6 to 8 days in both groups. There were no differences in caloric intake or distribution between groups. It is important to notice that caloric intake was 92% from the calculated need (Table 1). There was a significant difference in length of ICU stay for survivors. Although male survivors stayed 35 days, female survivors stayed only 26 days, P < 0.05 (Table 1).
TABLE 1.
Patient Demographics
| Female (n = 76) | Male (n = 113) | P (M vs. F) | |
|---|---|---|---|
| Age (yrs) | 8 ± 5 | 8 ± 5 | 0.6 |
| TBSA (%) | 55 ± 17 | 54 ± 19 | 0.9 |
| 3rd degree (%) | 41 ± 23 | 45 ± 24 | 0.4 |
| Flame burn (%) | 90 | 87 | 0.8 |
| Electrical burn (%) | 1 | 4 | 0.6 |
| Scald burn (%) | 9 | 9 | 0.9 |
| Burn to admission (d) | 8 ± 13 | 6 ± 10 | 0.4 |
| Length of ICU stay survivors (d) | 26 ± 17 | 35 ± 25* | 0.02 |
| Time to death (days) | 36 ± 7 | 38 ± 8 | 0.8 |
| Nutritional intake | |||
| Caloric intake (% calculated) | 93 ± 10 | 91 ± 12 | 0.7 |
| Protein (% of total calories) | 77 ± 16 | 78 ± 14 | 0.7 |
| Fat (% of total calories) | 19 ± 10 | 19 ± 9 | 0.7 |
Data presented as mean ± SD or percentages.
Significant difference between male vs. female, P < 0.05.
Female and male patients required similar amounts of surgeries throughout the acute hospital stay (Fig. 1A). The time between operations was significantly shorter in female burned victims (6.3 days) when compared with male burned victims (7.4 days) indicating a faster wound healing in females, P < 0.05 (Fig. 1B).
Table 2 shows relative risks and the 95% confidence interval for mortality, inhalation injury, and incidence of minor and major infections between males and females. There was no difference in mortality and incidence of infections between the 2 groups.
TABLE 2.
Outcome Variables
| Female (n = 76) | Male (n = 113) | Relative Risk* | 95% Confidence Interval | |
|---|---|---|---|---|
| Inhalation injury (%) | 35 | 42 | 0.86 | 0.57–1.26 |
| Incidence of infection (%) | 28 | 32 | 0.88 | 0.56–1.32 |
| Major (%) | 14 | 13 | 1.06 | 0.58–1.67 |
| Minor (%) | 14 | 18 | 0.86 | 0.46–1.4 |
| Mortality (%) | 8 | 7 | 1.07 | 0.46–1.84 |
Data presented as percentages.
Relative risk <1 means females are less at risk than males.
Indirect Calorimetry
Predicted resting energy expenditure was increased during acute hospitalization and at discharge in females and males. Females had a significant decreased predicted REE acutely, at discharge and 6 months postburn when compared with males, P < 0.05 (Fig. 1C).
Muscle Protein Synthesis
Stable isotope infusions were used to measure muscle protein synthesis and breakdown to determine net protein balance. Both females and males have a negative muscle net balance 3–4 weeks after the burn (Fig. 1D). However, females have an attenuated negative net muscle protein balance when compared with males, P < 0.05 (Fig. 1D).
Body Composition
There were no differences in body composition determined by DEXA scan during the acute stay between males and females. However, females gained approximately 3% of their body weight from admission to discharge, whereas males lost 1% of their body weight during the same time period, P < 0.05 (Fig. 1E).
Serum Hormones, Proteins, and Cytokines
Confirming previous studies, we found that females had significantly higher serum IGF-I levels during the acute hospital stay compared with males, P < 0.05 (Fig. 2A). Serum IGFBP-3 was not significantly different between females and males (Fig. 2B). Constitutive hepatic proteins were decreased in males and females after burn and increased over time. Females had significantly higher prealbumin levels compared with males (P < 0.05, Fig. 2C), but there were no difference between gender for serum transferrin (Fig. 2D). Acute phase proteins increased after the burn injury. We found that females have significantly lower acute phase protein levels for serum α2-macroglobulin, Haptoglobin, α1-acid glycoprotein, and C-reactive protein when compared with males, P < 0.05 (Fig. 2E, F, G). There were no differences between males and females for complement C3, apolipoprotein A1 and B (Fig. 2). However, females had significantly lower serum-free fatty acid levels beginning 21 days postburn when compared with males, P < 0.05 (Fig. 2M).
FIGURE 2.
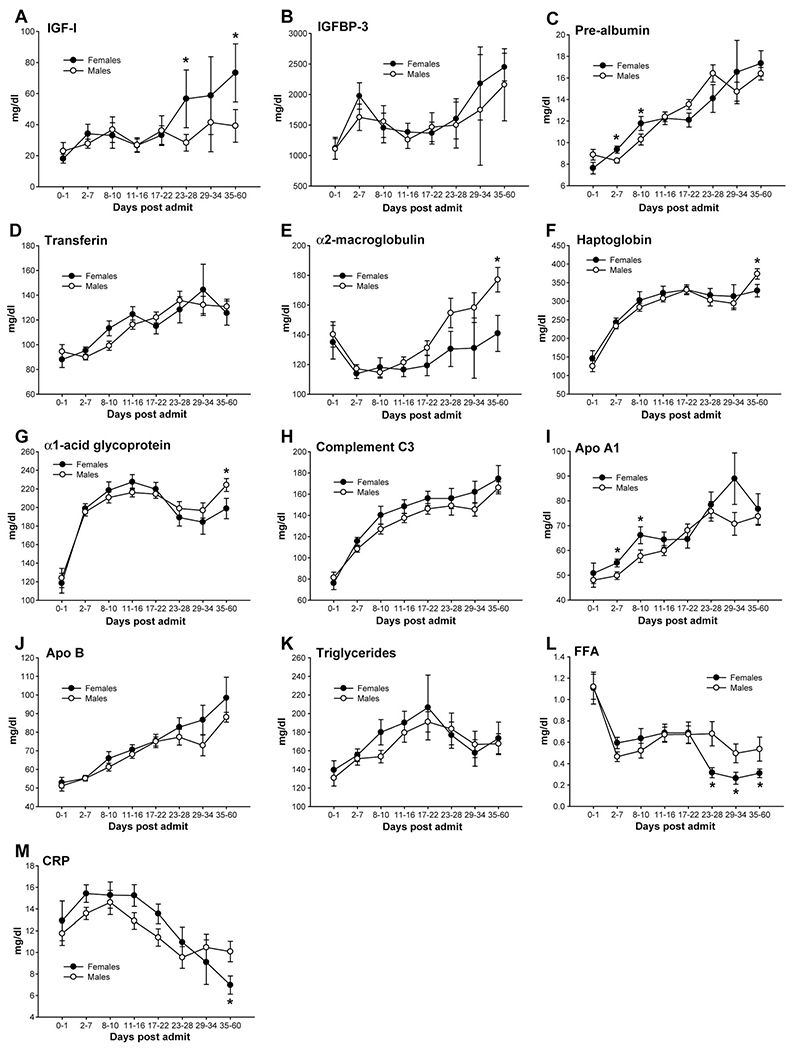
(A) Females had significantly higher serum IGF-I levels during the acute hospital stay compared with males, P < 0.05. (B) Serum IGFBP-3 was not significantly different between females and males. (C) Females had significantly higher prealbumin levels compared with males, P < 0.05. (D) There were no differences between genders for serum transferrin. Females have significantly lower acute phase protein levels, serum α2-macroglobulin (E), Haptoglobin (F), α1-acid glycoprotein, and C-reactive protein (G) when compared with males, P < 0.05. There were no differences between males and females for complement C3 (H), apolipoprotein A1 (I) and B (K). However, females had significantly lower serum free fatty acid levels (M) at multiple time points when compared with males, P < 0.05.
Serum estradiol levels were significantly higher in females up to 7 days after the burn compared with males, P < 0.05 (Fig. 3A). There were no significant differences beginning 8 days postburn for estradiol for the 2 groups. Serum testosterone was significantly higher in males immediately after burn, but levels dropped, and no differences between males and females could be detected anymore, P < 0.05 (Fig. 3B). We could not find any differences between both genders in serum progesterone (Fig. 3C). Serum cortisone was found significantly decreased in females at various time points compared with males, P < 0.05 (Fig. 3D).
FIGURE 3.
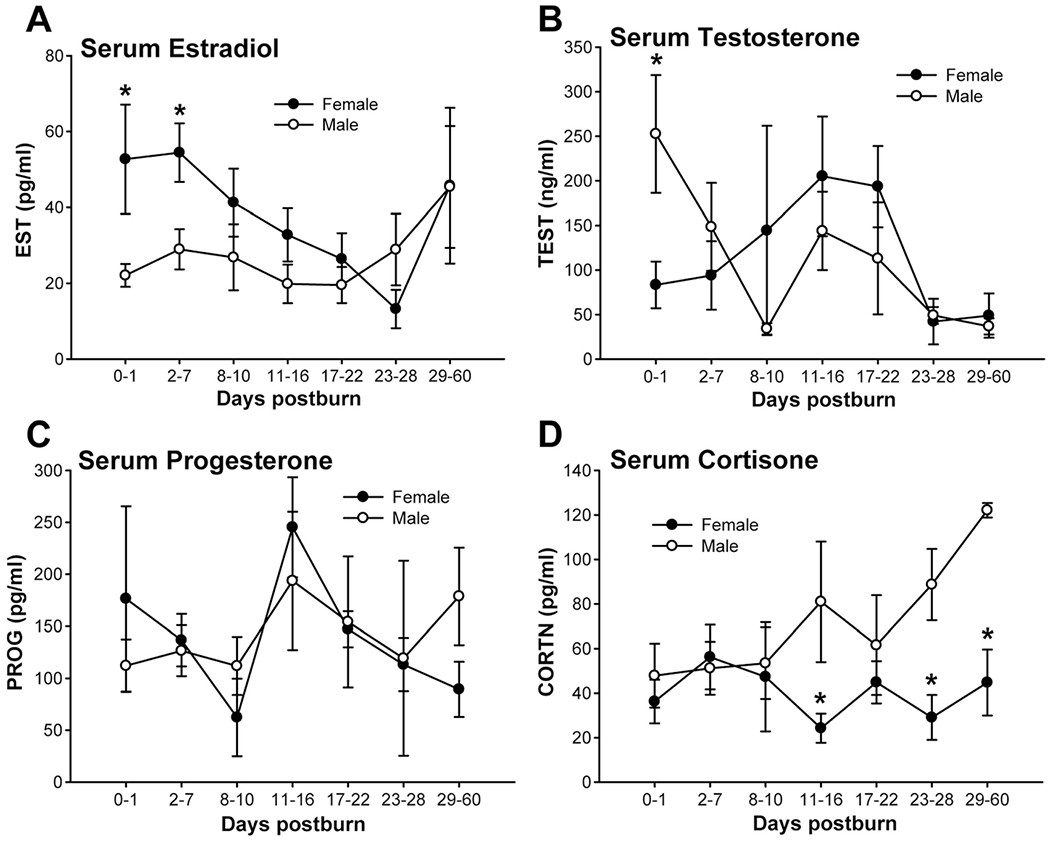
(A) Estradiol levels were significantly higher in females up to 7 days after the burn compared with males, P < 0.05. (B) Testosterone was significantly higher in males immediately after burn, but levels dropped and no differences between males and females could be detected anymore, P < 0.05. We could not find any difference between both genders in serum progesterone (C). Serum cortisone was found significantly decreased in females at various time points compared with males, P < 0.05 (D).
The serum cytokine profile was distinctly different between females and males. Females had significant increases in IL-2 (Fig. 4A), IL-4 (Fig. 4B), IL-13 (Fig. 4M), IL-17 (Fig. 4N) and G-CSF (Fig. 4O) compared with males, P < 0.05. Females had significantly decreased levels of IL-6 (Fig. 4C), interleukin-1 beta (IL-1β) (Fig. 4I), IL-5 (Fig. 4J), MCP-1 (Fig. 4P) and MIP1β (Fig. 4Q) when compared with males, P < 0.05. There were no differences for IL-8, IL-10, GM-CSF, IFN-γ, TNF, and IL-12p70 between groups (Fig. 4).
FIGURE 4.
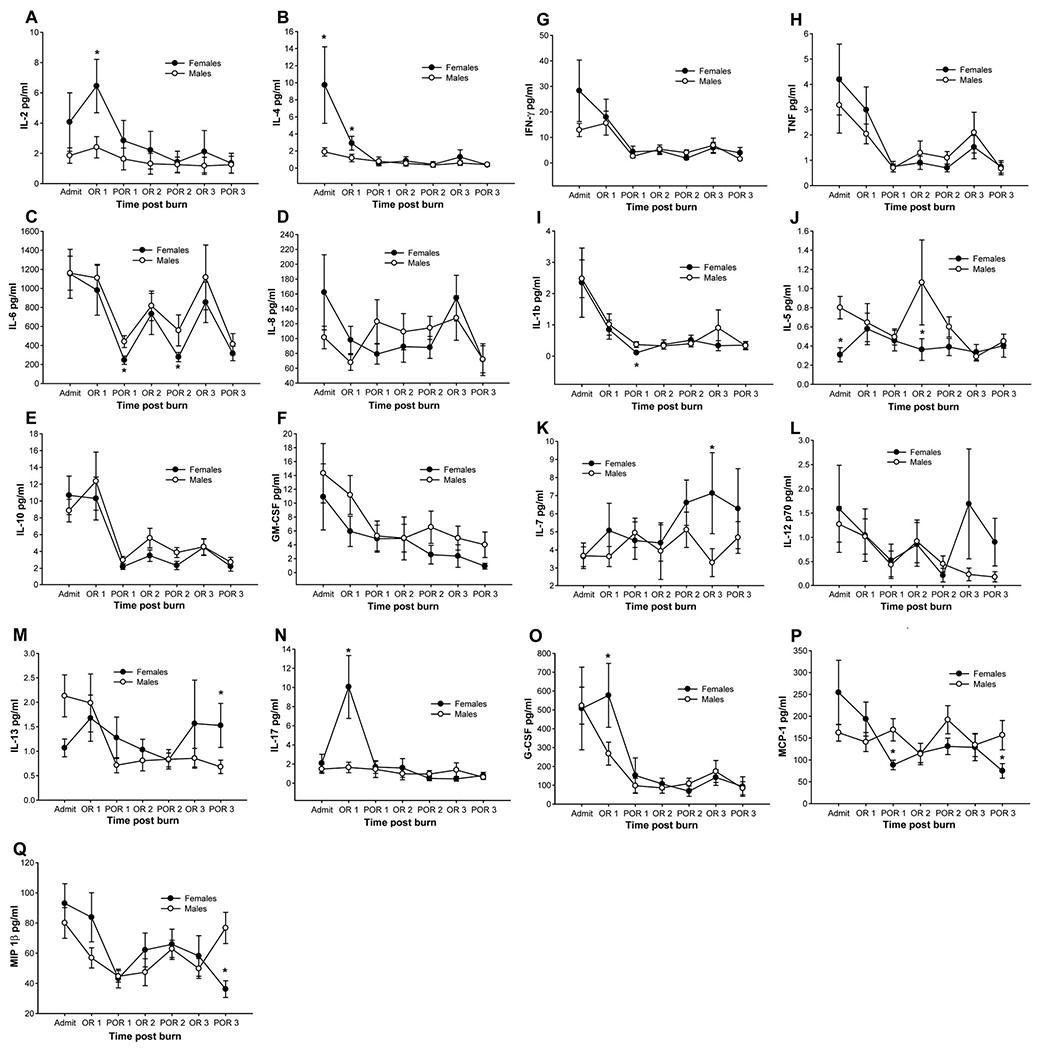
Females had significant increases in IL-2, IL-4, IL-13, IL-17, and G-CSF compared with males. Females had significantly decreased levels of IL-6, IL-1β, IL-5, MCP-1, and MIP1β when compared with males. There were no differences for IL-8, IL-10, GM-CSF, IFN-γ, TNF, and IL-12p70 between groups. *Significant difference between males versus females, P < 0.05.
Urine Cortisol
Urine cortisol was increased during the acute stay in both groups. Females had significant decreased urine cortisol concentrations at all time points measured in this study when compared with males, P < 0.05 (Fig. 5). Urine norepinephrine and VME were significantly decreased at 0–20 days and 21–40 days postburn compared with males, P < 0.05 (data not shown). There were no differences between males and females in urine dopamine and epinephrine.
FIGURE 5.
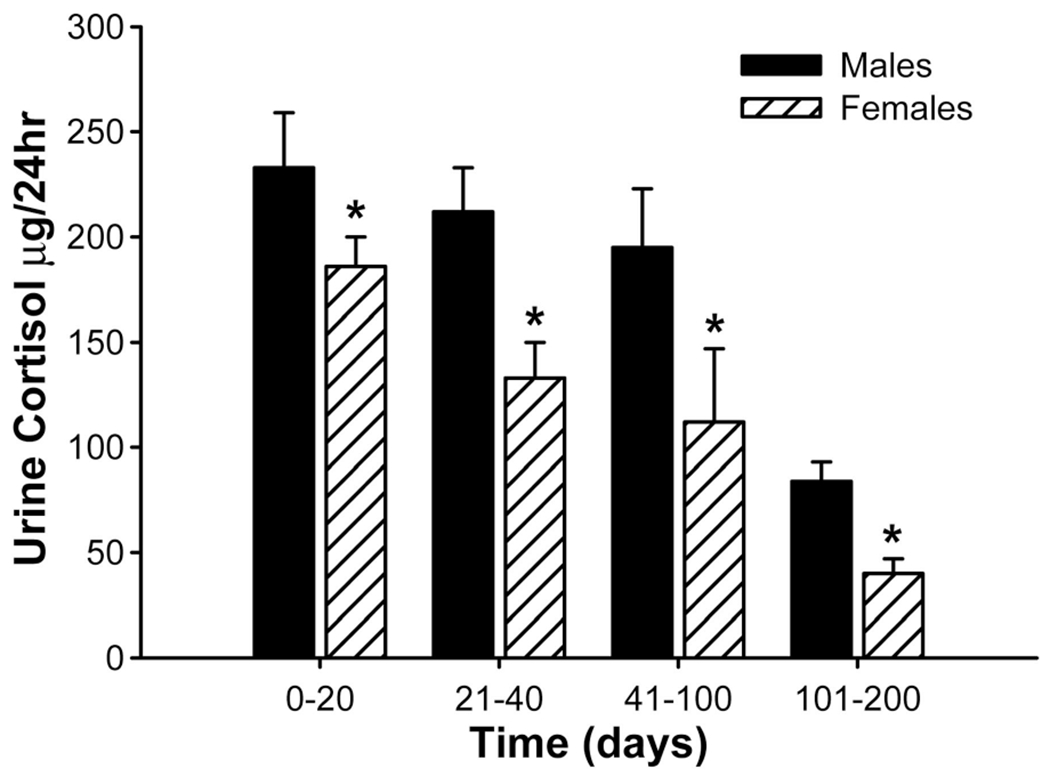
Females had significant decreased urine cortisol concentrations at all time points measured in this study when compared with males. *Significant difference between males versus females, P < 0.05.
Liver and Cardiac Changes
Analysis of cardiac output, stroke volume, and heart rate showed that females have significantly lower cardiac output at admission, lower percent predicted cardiac output, stroke volume, and percent predicted stroke volume than those of males, P < 0.05 (Table 3). No differences were found for heart rate and predicted heart rate. There were no significant differences between males and females at discharge except percent-predicted stroke volume which was significantly increased in females compared with males, P < 0.05 (Table 3). There were no differences in central venous pressure between groups.
TABLE 3.
Cardiac Function in Females and Males at Admission and Discharge
| Females (n = 74) |
Males (n = 112) |
|||||
|---|---|---|---|---|---|---|
| Admit | Discharge | Δ | Admit | Discharge | Δ | |
| CO | 4.6 ± 0.3* | 4.5 ± 0.3 | −7.5 ± 7.4 | 6.6 ± 0.3 | 5.1 ± 0.3 | −14 ± 8 |
| % pred CO | 130 ± 9* | 124 ± 8 | −7.5 ± 7.4 | 151 ± 8 | 112 ± 5 | −14 ± 8 |
| SV | 33 ± 2* | 35 ± 3 | 5 ± 9 | 48 ± 2 | 45 ± 2 | 5 ± 7 |
| % pred SV | 79 ± 6* | 82 ± 7* | 5 ± 9 | 86 ± 5 | 79 ± 3 | 5 ± 7 |
| HR | 143 ± 4 | 133 ± 5 | −7 ± 3 | 140 ± 4 | 113 ± 3 | −19 ± 3 |
| % pred HR | 165 ± 6 | 152 ± 6 | −7 ± 3 | 184 ± 5 | 149 ± 4 | −19 ± 3 |
Data presented as mean ± SEM or percentages.
Significant difference between male vs. female, P < 0.05.
CO indicates cardiac output; SV, stroke volume; HR, heart rate; % pred, percent predicted; Δ, change from admission to discharge.
Immediately after burn, the liver increased by almost 100% in both groups. Although the liver of males further increased to 140%, the liver of females is significantly decreased during acute hospitalization and at discharge when compared with males, P < 0.05 (Fig. 6).
FIGURE 6.
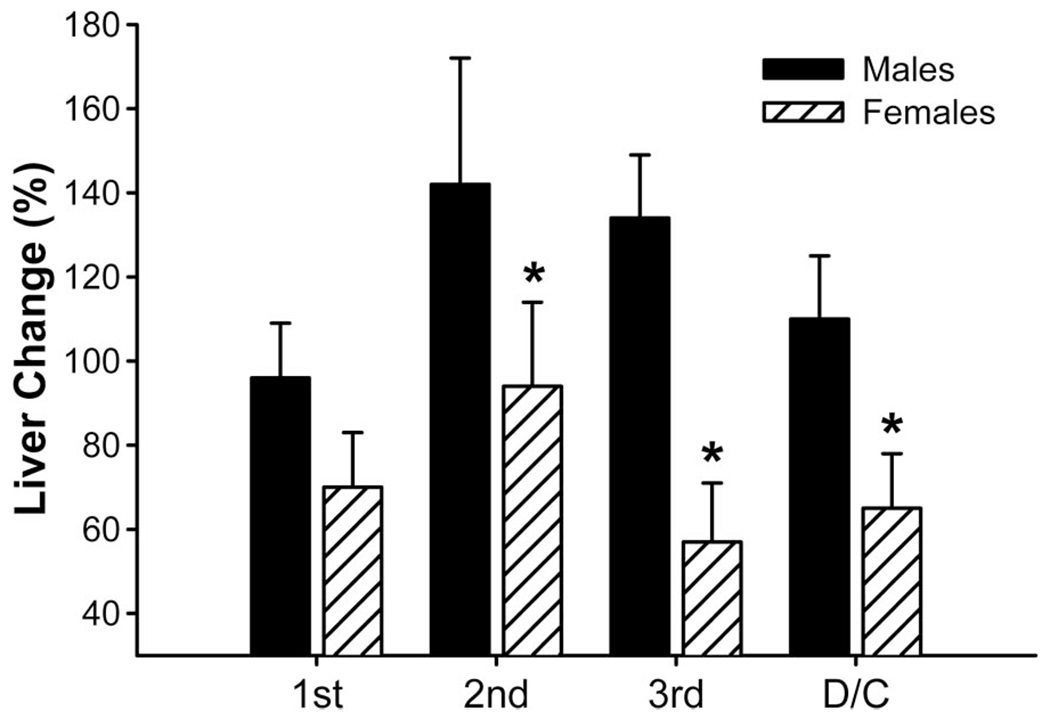
Liver changes during acute hospital stay. *Significant difference between males versus females, P < 0.05.
DISCUSSION
Gender differences in the outcome of critically ill patients have been discussed in many studies and are the foci of many ongoing clinical and basic science studies. There is a tendency that female critically ill patients have a better survival and are more resistant to infections compared with males. Over the last years, many experimental and clinical studies looked at differences in the outcome of female and male patients.1,2 Although some studies found improved survival for female patients, others could not find any differences in outcome.3,4 We have recently shown that female pediatric burned patients have an attenuated postburn response with associated increased endogenous anabolic hormone levels, decreased serum inflammatory markers, and decreased stay in the ICU when compared with males.14 We further conducted 2 clinical studies in which the main outcome measure was survival from a severe burn. The study in 1995 showed a significantly better outcome for females,12 but a recent study showed no difference between males and females in terms of survival.13 The current study was conducted to determine whether gender in severely burned pediatric patients really matters and whether severely burned female children have a better morbidity and clinical course compared with severely burned male children, and further to determine possible mechanisms.
The metabolic rate in burns is extremely high and energy requirements are immense and met by the mobilization of proteins and amino acids.6 Increased protein turnover, degradation, and negative nitrogen balance are characteristics of this severe critical illness.7 As a consequence, the structure and function of essential organs are compromised, such as skeletal muscle, skin, immune system, and cellular membrane transport functions.6,9 This compromise can lead to multi organ dysfunction or even death.6 In the present study, we found that female burn patients demonstrated decreased catecholamine and stress hormone levels which were associated with decreased hypermetabolism and catabolism. Females had significantly decreased resting energy expenditure, body weight loss, and net muscle protein balance when compared with males. Thus, female pediatric burn patients are less hypermetabolic and catabolic during the postburn response compared with males. We propose that an attenuated hypermetabolism is the underlying mechanism why pediatric female burn patients have a muscle mass preservation whereas their male counterparts loose muscle mass. Further indicators that females are less hypermetabolic than males are decreased acute phase proteins and increased constitutive hepatic proteins. Female burn patients exert a distinctly different acute phase response when compared with males. Females have higher constitutive hepatic proteins and lower acute phase proteins when compared with males. In conjunction with an attenuated organ hypertrophy, it appears that a decreased inflammatory and hypermetabolic response is associated with an improved liver function and structure in female pediatric burn patients. In addition, by using time between operations as a crude measurement for dermal and epidermal regeneration, we found that females had a shorter time between operations indicating improved donor site healing time. Faster donor site healing time implies improved wound healing in females compared with males.
Catecholamines, cytokines, and proinflammatory mediators are known via cellular mediators to block and, therefore, decrease endogenous anabolic agents.23,24 We showed, in the present study, that female pediatric burn patients demonstrated a different cytokine expression profile compared with males. Females had significantly decreased proinflammatory cytokine and increased anti-inflammatory cytokine levels when compared with males. This anti-inflammatory effect was reflected in higher endogenous IGF-I levels when compared with males. Multiple cytokines, eg, IL-1β was shown to inhibit the anabolic IGF-I axis. IGF-I was shown to exert anabolic effects on skeletal muscle protein synthesis, to attenuate the hepatic acute phase response, and to improve dermal and epidermal regeneration.25–27 Furthermore, decreased growth hormone and IGF-I levels lead to a deficit in transmembrane amino acid transport and compromised immune system.28–30
In the present study, we included children as young as 1-year-old and the majority of the population has not undergone any of the hormonal changes of puberty. The questions arise as to whether the “estrogen effect” occurs at such a young age, whether one would not expect to see a much greater survival effect after puberty when hormones are intense, and whether a difference before and after puberty can be found. In the present study, we looked at burned children at all ages. We did not conduct a subgroup analysis as we wanted our patient number to remain high for statistical power. However, we looked at estrogen and testosterone levels in patients from 0–8 years and 9–16 years and found that these levels are only different at admission whereas there was no difference between males and females throughout hospital course. This indicates that estrogen, at admission, has this beneficial effect or some other gender-related factor.
Some critical points need to be mentioned. First, there is a lot of controversy about the validity of the DEXA to determine lean body mass or water content. We conducted a study in which we correlated DEXA data postburn with the K-count and found that DEXA correlate very well, indicating that DEXA indeed detects protein and lean body mass rather than water (unpublished data). There, we suggest that our DEXA measurements are valid. Second, an attenuated muscle mass loss in females may be due to the fact that females had less muscle to begin with. It is certainly true that females have less muscle compared with males and therefore loose less body mass, however, hypermetabolism was significantly reduced in females which also can account for an improvement in body composition.
Recent laboratory studies have demonstrated that immune responses differ between male and female rodents, and some clinical studies have suggested gender differences regarding incidence and mortality from sepsis.1 The differences appear because of both deleterious testosterone and beneficial estrogen effects; clinical trials of testosterone blockage and/or estrogen administration for male subjects have been suggested.3,31 In the present study, we showed that females had higher serum estradiol levels shortly after burn compared with males, but levels decreased beginning 3 days postburn and we found no difference between groups anymore. Serum testosterone was significantly increased in males only at admission with decreasing levels in both genders over time. The question that arises is: can the initial difference in serum estradiol and testosterone account for the beneficial outcome in females? In a recent study, Chaudry et al showed that a one-time application of estrogen in a hemorrhagic shock model improves mitochondrial function and survival (personal communication).2 It, therefore, could be possible that the benefit observed in this study is due to initial high estrogen levels; however, we suggest that the exact role of hormones in burn patients is not defined and needs further investigation. We showed in this study that females who had higher estrogen levels also had better liver structure and function as well as cardiac function and cardiac work. It may well be that higher estrogen levels for only a short period is associated with these beneficial effects, but it may be that the differences between males and females are due to their difference in the genome. However, improved acute hospital morbidity in terms of preserved lean body mass, attenuated inflammatory, acute phase, and stress response, as well as hypermetabolism are not reflected in an improved mortality. In addition, we would like to add that not all studies in trauma and burn patients show a favorable outcome for females. Sharma, et al32 found in a large cohort in Kuwait that gender had no effect on mortality, similar to Barrow, et al13 in pediatric burn patients. There are, however, 2 large trials that demonstrate that female adult patients have a less favorable outcome when compared with male adult patients. The first one by McGwin, et al33 showed that women less than 60 years of age who sustain burn injuries have an increased risk of death compared with males. The second study by O’Keefe, et al34 provided a detailed method for estimating the risk of mortality after burn trauma, based on a large, contemporary cohort of patients. These estimates were validated on a second sample and proved to predict mortality accurately, and they identified a 2-fold increase mortality risk in women of 30 to 59 years of age. If estrogens are not the underlying hormone improving morbidity postburn, then we would like to raise the question: which factor causes an improved postburn response? Identification of this factor or hormone would certainly be interesting as it may represent a new therapeutic approach to improve burn-related outcomes.
CONCLUSIONS
Based on our findings, we suggest that female pediatric burn patients have a different inflammatory and hypermetabolic response to a severe burn compared with male burn pediatric patients. Female patients showed higher levels of endogenous anabolic hormones, higher estrogen, attenuated stress hormones, and inflammatory markers when compared with those of male pediatric patients. Hence catabolism, hypermetabolism, and compromise of the structure and function of essential organs are attenuated in severely burned female patients. Given the pathophysiology that protein catabolism, inflammation, and hypermetabolism leads to prolonged need for ventilation, delay in mobilization, compromised immune system and delay in wound healing, higher concentration of anabolic hormones, and lower levels of stress hormones and markers should have beneficial effects in terms of improving clinical outcome. In fact, we found that female patients had a significant shorter stay on the ICU when compared with males. Improved clinical outcome was not reflected in mortality. Others35 and we13 found no differences in mortality between males and females. This leads to suggest that female pediatric burn patients have a beneficial acute clinical course with attenuated inflammation and hypermetabolism, but the differences are not significant enough that they would lead to an improvement in survival. However, by identifying the mechanisms by which female pediatric burn patients have a better clinical outcome, we may initiate new therapeutic strategies to attenuate hypermetabolism and inflammation in male pediatric burn patients.
ACKNOWLEDGMENTS
The authors thank David L. Chinkes, PhD, for his statistical assistance. They also thank Eileen Figueroa and Steven Schuenke for their help in editing this manuscript.
This study was supported by the American Surgical Association Foundation, Shriners Hospitals for Children 8490, 8660, 8640, 8760, and 9145, National Institutes of Health R01-GM56687, T32 GM008256, and P50 GM60338, and NIDRR H133A020102.
REFERENCES
- 1.Angele MK, Xu YX, Ayala A, et al. Gender dimorphism in trauma-hemorrhage-induced thymocyte apoptosis. Shock. 1999;12:316–322. [DOI] [PubMed] [Google Scholar]
- 2.Chaudry IH, Samy TS, Schwacha MG, et al. Endocrine targets in experimental shock. J Trauma. 2003;54:S118–S125. [DOI] [PubMed] [Google Scholar]
- 3.Croce MA, Fabian TC, Malhotra AK, et al. Does gender difference influence outcome? J Trauma. 2002;53:889–894. [DOI] [PubMed] [Google Scholar]
- 4.Mostafa G, Huynh T, Sing RF, et al. Gender-related outcomes in trauma. J Trauma. 2002;53:430–435. [DOI] [PubMed] [Google Scholar]
- 5.Barret JP, Herndon DN. Modulation of inflammatory and catabolic responses in severely burned children by early burn wound excision in the first 24 hours. Arch Surg. 2003;138:127–132. [DOI] [PubMed] [Google Scholar]
- 6.Takala J, Ruokonen E, Webster NR, et al. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341:785–792. [DOI] [PubMed] [Google Scholar]
- 7.Rennie MJ. Muscle protein turnover and the wasting due to injury and disease. Br Med Bull. 1985;41:257–264. [DOI] [PubMed] [Google Scholar]
- 8.Arnold J, Campbell IT, Samuels TA, et al. Increased whole body protein breakdown predominates over increased whole body protein synthesis in multiple organ failure. Clin Sci (Lond). 1993;84:655–661. [DOI] [PubMed] [Google Scholar]
- 9.Biolo G, Toigo G, Ciocchi B, et al. Metabolic response to injury and sepsis: changes in protein metabolism. Nutrition 1997;13:S52–S57. [DOI] [PubMed] [Google Scholar]
- 10.Hart DW, Wolf SE, Chinkes DL, et al. Beta-blockade and growth hormone after burn. Ann Surg. 2002;236:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boonen S, Mohan S, Dequeker J, et al. Down-regulation of the serum stimulatory components of the insulin-like growth factor (IGF) system (IGF-I, IGF-II, IGF binding protein [BP]-3, and IGFBP-5) in age-related (type II) femoral neck osteoporosis. J Bone Miner Res. 1999;14:2150–2158. [DOI] [PubMed] [Google Scholar]
- 12.Barrow RE, Herndon DN. Thermal burns, gender, and survival. Lancet. 1988;2:1076–1077. [DOI] [PubMed] [Google Scholar]
- 13.Barrow RE, Przkora R, Hawkins HK, et al. Mortality related to gender, age, sepsis, and ethnicity in severely burned children. Shock. 2005;23: 485–487. [PubMed] [Google Scholar]
- 14.Jeschke MG, Barrow RE, Mlcak RP, et al. Endogenous anabolic hormones and hypermetabolism: effect of trauma and gender differences. Ann Surg. 2005;241:759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warden GD. Fluid resuscitation and early management In: Herndon DN, ed. Total Burn Care. Philadelphia: Saunders, 2007:107–118. [Google Scholar]
- 16.Saffle JR, Graves C. Nutritional support of the burned patient In: Herndon DN, ed. Total Burn Care. Philadelphia: Saunders; 2007:398–419. [Google Scholar]
- 17.Mlcak RP, Jeschke MG, Barrow RE, et al. The influence of age and gender on resting energy expenditure in severely burned children. Ann Surg. 2006;244:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolfe RR. Regulation of skeletal muscle protein metabolism in catabolic states. Curr Opin Clin Nutr Metab Care. 2005;8:61–65. [DOI] [PubMed] [Google Scholar]
- 19.Przkora R, Barrow RE, Jeschke MG, et al. Body composition changes with time in pediatric burn patients. J Trauma. 2006;60:968–971. [DOI] [PubMed] [Google Scholar]
- 20.Przkora R, Jeschke MG, Barrow RE, et al. Metabolic and hormonal changes of severely burned children receiving long-term oxandrolone treatment. Ann Surg. 2005;242:384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finnerty CC, Herndon DN, Chinkes DL, et al. Serum cytokine differences in severely burned children with and without sepsis. Shock. 2007;27:4–9. [DOI] [PubMed] [Google Scholar]
- 22.Finnerty CC, Herndon DN, Przkora R, et al. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13–19. [DOI] [PubMed] [Google Scholar]
- 23.Delhanty PJ. Interleukin-1 beta suppresses growth hormone-induced acid-labile subunit mRNA levels and secretion in primary hepatocytes. Biochem Biophys Res Commun. 1998;243:269–272. [DOI] [PubMed] [Google Scholar]
- 24.Wang P, Li N, Li JS, Li WQ. The role of endotoxin, TNF-alpha, and IL-6 in inducing the state of growth hormone insensitivity. World J Gastroenterol. 2002;8:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herndon DN, Ramzy PI, DebRoy MA, et al. Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg. 1999; 229:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeschke MG, Barrow RE, Hawkins HK, et al. IGF-I gene transfer in thermally injured rats. Gene Ther. 1999;6:1015–1020. [DOI] [PubMed] [Google Scholar]
- 27.Jeschke MG, Barrow RE, Suzuki F, et al. IGF-I/IGFBP-3 equilibrates ratios of pro- to anti-inflammatory cytokines, which are predictors for organ function in severely burned pediatric patients. Mol Med. 2002;8: 238–246. [PMC free article] [PubMed] [Google Scholar]
- 28.Takagi K, Suzuki F, Barrow RE, et al. Recombinant human growth hormone modulates Th1 and Th2 cytokine response in burned mice. Ann Surg. 1998;228:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tessari P, Inchiostro S, Biolo G, et al. Differential effects of hyperinsulinemia and hyperaminoacidemia on leucine-carbon metabolism in vivo. Evidence for distinct mechanisms in regulation of net amino acid deposition. J Clin Invest. 1987;79:1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfe RR. Control of muscle protein breakdown: effects of activity and nutritional states. Int J Sport Nutr Exerc Metab. 2001;(11 suppl):S164–S169. [DOI] [PubMed] [Google Scholar]
- 31.Jarrar D, Kuebler JF, Wang P, et al. DHEA: a novel adjunct for the treatment of male trauma patients. Trends Mol Med. 2001;7:81–85. [DOI] [PubMed] [Google Scholar]
- 32.Sharma PN, Bang RL, Ghoneim IE, et al. Predicting factors influencing the fatal outcome of burns in Kuwait. Burns. 2005;31:188–192. [DOI] [PubMed] [Google Scholar]
- 33.McGwin G Jr, George RL, Cross JM, et al. Gender differences in mortality following burn injury. Shock. 2002;18:311–315. [DOI] [PubMed] [Google Scholar]
- 34.O’Keefe GE, Hunt JL, Purdue GF. An evaluation of risk factors for mortality after burn trauma and the identification of gender-dependent differences in outcomes. J Am Coll Surg. 2001;192:153–160. [DOI] [PubMed] [Google Scholar]
- 35.Kerby JD, McGwin G Jr, George RL, et al. Sex differences in mortality after burn Injury: results of analysis of the National Burn Repository of the American Burn Association. J Burn Care Res. 2006;27:452–456. [DOI] [PubMed] [Google Scholar]


