Abstract
Severe burn causes a pronounced hypermetabolic response characterized by catabolism and extensive protein wasting. We recently found that this hypermetabolic state is driven by a severe inflammatory response. We characterized in detail the kinetics of serum levels of a panel of cytokines in a rat model, which may serve as reference for the development of therapeutic interventions applicable to humans. Male Sprague-Dawley rats (n = 8) received a full-thickness burn of 60% total body surface area. Serum was harvested 1, 3, 6, 12, 24, 48, 96, and 168 h after burn. Eight serum cytokines commonly used to assess the inflammatory response in humans, such as IL-1β, IL-6, IL-10, TNF, vascular endothelial growth factor, and monocyte chemotactic protein 1, and the rat-specific cytokines cytokine-induced neutrophil chemoattractant (CINC) 1, CINC-2, and CINC-3 were measured by enzyme-linked immunosorbent assay technique and were compared with controls (n = 4). Statistical analysis was conducted using the t test, with P < 0.05 considered as significantly different. Thermal injury resulted in significantly increased serum levels of IL-1β, IL-6, IL-10, monocyte chemotactic protein 1, CINC-1, CINC-2, and CINC-3 when compared with the concentrations detected in nonburned rats (P < 0.05). Serum levels of TNF-α and vascular endothelial growth factor in burned rats were not found to be significantly different to controls. Burn causes a profound inflammatory response in rats. Specific cytokines known to increase in humans postburn such as IL-1 β, IL-6, IL-10, MCP-1, and IL-8 (CINC-1, CINC-2, and CINC-3 in the rat) were also observed in our rat burn model, which now allows us to study new anti-inflammatory treatment options.
Keywords: Burn, inflammation, cytokines, CINC, IL-6
INTRODUCTION
The trauma of severe burn injury induces a distinct systemic inflammatory response that has been well described in human subjects. Cytokines are the primary mediators of this inflammatory reaction to injury (1). They constitute a group of proteins with autocrine and endocrine activities that provide communication among different types of cells, including those that mediate immune functions, angiogenesis, cell proliferation, and apoptosis. Active in minute quantities, cytokines regulate homeostasis and cellular repair through effects on cell growth and differentiation via receptor activation. Various cytokines such as IL-1, IL-6, and TNF have been used as markers of the severity of burn injury (2–4). Recently, we showed in a pediatric cohort that 16 of 17 measured cytokines were significantly elevated throughout the first week after burn when compared with healthy children (5). It is also known that increased proinflammatory cytokine synthesis posttrauma leads to hypermetabolism and catabolism (6, 7). As a consequence, the structure and function of essential tissues such as muscle, skin, heart, immune system, and liver are compromised and contribute to multiorgan failure and mortality (2, 8–10). Because changes in cytokine levels occur before alterations in metabolism, it may be possible to modulate the hypermetabolic response postburn by exogenously modulating cytokine expression levels. Animal models are a valuable tool to assess whether such a manipulation of the expression profile of cytokines postinjury might represent a viable alternative for the management of hypermetabolism and catabolism. Thus, it is imperative to determine whether the animals used display a similar inflammatory response postburn to that of humans (5).
A broad variety of animal models are used to mimic trauma (11). Although models have been developed in rats, dogs, pigs, and primates, rodents are particularly attractive because of the availability of genetically homogeneous individuals, low cost, and ease of handling (11). Importantly, rodents are known to respond to trauma with the secretion of various proinflammatory and anti-inflammatory cytokines. Rodents lack a homolog of human IL-8 (12), but they express a recently characterized cytokine with similar properties and functions termed cytokine-induced neutrophil chemoattractant (CINC) (13). These rat CINCs were grouped into four isoforms (CINC-1, CINC-2α, CINC-2β, and CINC-3) and have been identified as potent chemotactic factors belonging to the IL-8 family (14), acting as proinflammatory agents for cytokines such as IL-1 and TNF-α (15).
Here, we studied the kinetics of alterations in the serum cytokine profiles of a rat burn model immediately postburn and throughout a period of 7 days. Evaluation of the expression patterns of the cytokines studied here, which included the CINCs, allowed us to examine whether the rat burn model is adequate for the investigation of novel therapeutic strategies to decrease hypermetabolism and catabolism in human patients.
MATERIALS AND METHODS
All animal manipulations were approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch at Galveston. The National Institutes of Health’s Guidelines for the Care and Use of Experimental Animals were met.
Burn injury
A well-established method was used to induce a full-thickness scald burn in our model (16). Sprague-Dawley rats weighing 325 to 350 g were allowed to acclimate for 1 week before experiments. Rats were housed in an institutional animal care facility and received a high-protein diet (Ensure) and water ad libitum throughout the study. Ensure was administered 7 days before the study to adjust the animals to the liquid diet. Animals were anesthetized with general anesthesia (40 mg/kg body weight ketamine and 5 mg/kg body weight xylazine, both i.p.) and received analgesia (0.05 mg/kg body weight buprenorphine, s.c.). The dorsum of the trunk and the abdomen were shaved, and a 60% total body surface area burn was administered by placing the animals in a mold exposing defined areas of the skin of the back and abdomen under general anesthesia and analgesia. The mold was placed in 96°C to 98°C water, scalding the back for 10 s and the abdomen for 2 s. This method delivers a full-thickness cutaneous burn as confirmed by histologic section. Lactated Ringer solution (40 mL/kg body weight, i.p.) was administered immediately after the burn for resuscitation. After burn and resuscitation, animals were observed, received oxygen, and were then placed into cages. Unburned (sham) animals received the same treatment except for the scald burn.
Cytokine determinations
Animals were euthanized by decapitation without anesthesia 1, 3, 6, 12, 24, 48, 96, and 168 h after burn injury or sham procedures. Blood was collected immediately after decapitation and stored on ice until serum preparation. Serum was separated by centrifugation at 4,500 rpm for 3 min at 4°C and stored at −80°C until analyzed. The levels of IL-1α, IL-6, IL-10, TNF-β, vascular endothelial growth factor (VEGF), CINC-1, CINC-2, CINC-3, and monocyte chemotactic protein (MCP) 1 were determined by double-sandwich, enzyme-linked immunosorbent assays (R&D Systems Inc., Minneapolis, Minn) according to the protocol of the manufacturer. Each serum cytokine was determined by optical density, which was measured in a microplate reader (TECAN Austria GmbH, Grodig, Austria) set to 450 nm wave length.
Statistical analysis
Results are presented as mean ± SEM (n = 8 rats per burn group and n = 4 rats per sham group at each time point). The data were analyzed using t test or Mann-Whitney rank sum test. Differences were considered significant at a P value less than 0.05.
RESULTS
No mortality occurred within the groups during the experiment. To determine whether the observed alterations in the levels of the cytokines measured resulted in a hypermetabolic state in the rat, we measured total body weight of the animals in our experimental and control groups (Table 1). We found that burn led to a significant reduction in weight during the duration of our study: burned animals underwent a weight reduction of approximately 10% of their original weight, whereas control animals basically maintained their original body weight.
Table 1.
Weights of experimental and control groups
| Start weight, g | End weight, g | |
|---|---|---|
| Sham group | 360.1 ± 2.9 | 354.0 ± 3.6 |
| Bum group | 358.4 ± 2.0 | 323.3 ± 4.9 (P< 0.001) |
Data are presented as means ± SEM.
During the 168-h study period, significant increases were found in the serum levels of certain cytokines. Thermal injury resulted in augmented serum CINC-1 and CINC-2 concentrations up to 24- and 16-fold, respectively, as well as IL-6 and MCP-1 levels up to 5- and 10-fold, respectively, over control values. IL-1β, CINC-3, and IL-10 were also found to be significantly increased after burn when compared with the concentrations detected in nonburned rats. Serum levels of TNF-α and VEGF in burned rats were not significantly different in nonburned rats.
IL-1β showed a significant increase 3 h postburn injury. These levels peaked at 12 h, remained significantly elevated for up to 48 h, and then rapidly decreased to basal levels at 96 and 168 h (Fig. 1A).
Fig. 1. Cytokines commonly used to evaluate the inflammatory response are elevated in the rat burn model.
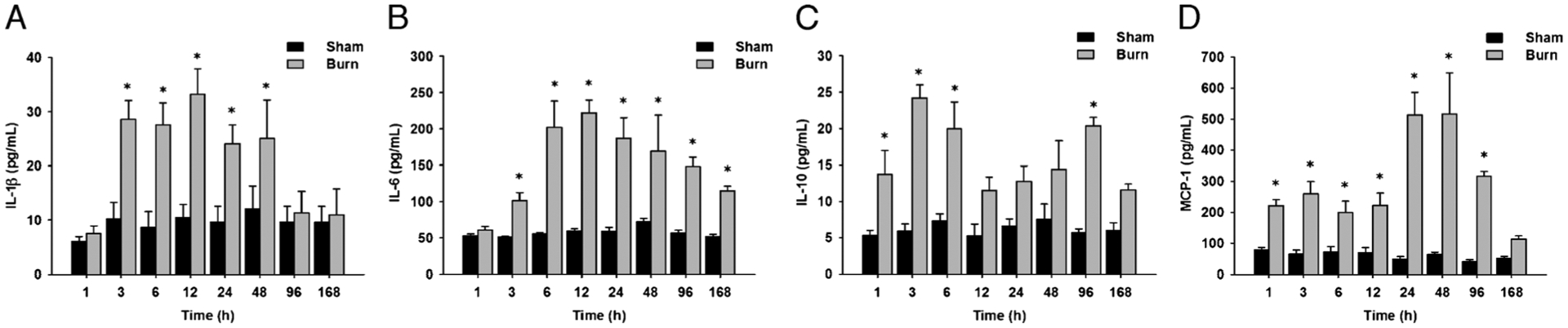
A, Serum IL-1β levels are significantly increased up to 48 days postburn compared with controls. B, Serum IL-6 levels are significantly increased up to 168 days postburn compared with controls. C, Serum IL-10 levels are significantly increased up to 96 days postburn compared with controls. D, Serum MCP-1 levels are significantly increased up to 96 days postburn compared with controls. Throughout the figure, histograms depict serum concentrations of the respective cytokine at steady-state levels. Results shown represent eight different animals per group, as indicated in the main text. Bars represent means; error bars correspond to SEM. Asterisks denote statistical significance: P < 0.05 for every comparison between groups.
The concentrations of IL-6 increased immediately after burn injury. However, IL-6 levels peaked at 6 h and then gradually decreased for the remaining of the study, although they remained significantly increased compared with the control group (Fig. 1B).
IL-10 showed a biphasic progression during the time course studied, with a dramatic increase upon burn injury. IL-10 serum concentrations peaked at 3 h after burn injury and gradually decreased subsequently. IL-10 levels showed an additional increase at 96 h postinjury before decreasing to normal levels at the end of the period studied (Fig. 1C)
Analysis of the rates at which the various CINCs increased revealed that there was no lag phase for the observed increment of CINC-1 after burn trauma. Cytokine-induced neutrophil chemoattractant 1 concentrations increased immediately after burn injury, with a peak at 12 h. During the remaining time of the study, CINC-1 levels decreased gradually but remained significantly elevated until the end of the investigation (Fig. 2A). Unlike CINC-1, the levels of CINC-2 did not dramatically increase immediately after burn injury. Instead, CINC-2 levels displayed relatively constant values for up to 24 h after burn. At 48 h, a dramatic increase in the levels of this cytokine was observed, with a further increase at 96 h that was maintained for the remainder of the study (Fig. 2B). The serum concentrations of CINC-3 followed a biphasic progression during the time line studied. Levels of this particular chemoattractant increased significantly at 3 h postburn injury, with a peak at 6 h. After a second dramatic decrease at 12 and 24 h, CINC-3 levels increased again with a late peak at 96 h and maintained significantly elevated for the remainder of the study (Fig. 2C).
Fig. 2. Cytokine-induced neutrophil chemoattractant family members are elevated in the rat burn model.
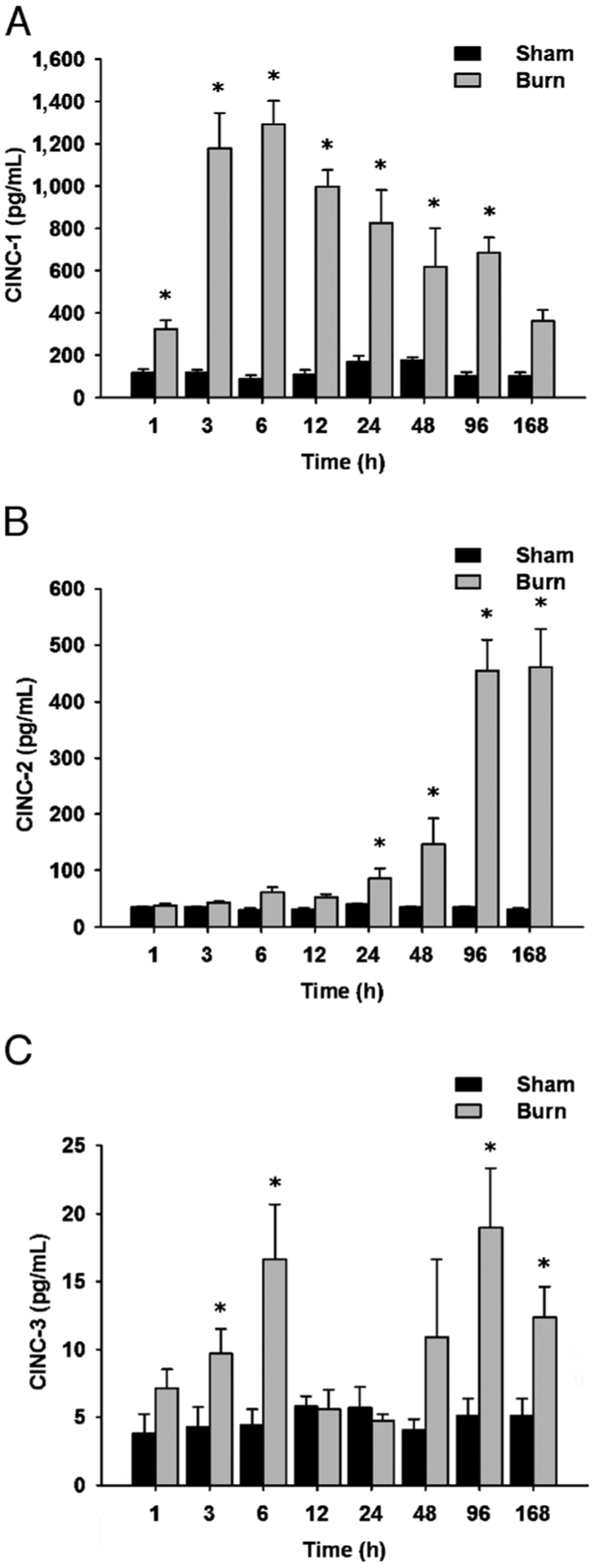
A, Cytokine-induced neutrophil chemoattractant 1 levels are significantly increased up to 96 days postburn compared with controls. B, Cytokine-induced neutrophil chemoattractant 2 levels are significantly increased up to 168 days postburn compared with controls. C, Cytokine-induced neutrophil chemoattractant 3 levels are significantly increased up to 168 days postburn compared with controls. Throughout the figure, histograms depict serum concentrations of the respective CINC protein at steady-state levels. Results shown represent eight different animals per group, as indicated. Bars represent means; error bars correspond to SEM. Asterisks denote statistical significance: P < 0.05 for every comparison between groups.
The levels of MCP-1 showed a significant increase immediately after injury. These levels were maintained for up to 12 h. A peak at 48 h was observed, with subsequent decrease in the levels of this cytokine at 96 and 168 h (Fig. 1D).
DISCUSSION
Severe burn trauma induces a distinct systemic inflammatory reaction in patients (2–4). Release of proinflammatory mediators postburn is associated with protein wasting and organ dysfunction (17, 18), which contributes to increased incidence of infection and sepsis, factors that augment the risk of multiple organ failure and death (5). Alterations in the levels of cytokines postburn occur before the observed metabolic abnormalities. Thus, it may be possible to design therapeutic interventions that attenuate the hypermetabolic response by decreasing the expression of cytokines associated with it. The aim of this study was to assess the cytokine profile of a rat burn model that can be used for the characterization in alterations of cytokine expression patterns after severe burn injury during the acute and postacute inflammatory phases. Indeed, clinically applicable models have been developed in rodents for the study of the inflammatory response after trauma and pulmonary sepsis (19).
As summarized in Figure 3, our results showed that proinflammatory and anti-inflammatory cytokines, including IL-1β, IL-6, IL-10, CINC-1, CINC-2, and CINC-3, as well as MCP-1, are significantly elevated in rodents up to 7 days after severe burn injury when compared with nonburned animals. Significantly, this general behavior of the cytokines in our model was similar to the cytokine response in burned patients (5). Most cytokines recently measured in a pediatric cohort were elevated during the first week after burn and decreased significantly over time to approach concentrations of normal, unburned children (5). Similarly, in our study, the levels of most cytokines measured were augmented during the first days postburn. Serum levels of TNF-α and VEGF in burned rats were not significantly different to nonburned rats under our experimental conditions.
Fig. 3. Heat map comparing normal and burn serum cytokine protein expression profiles.
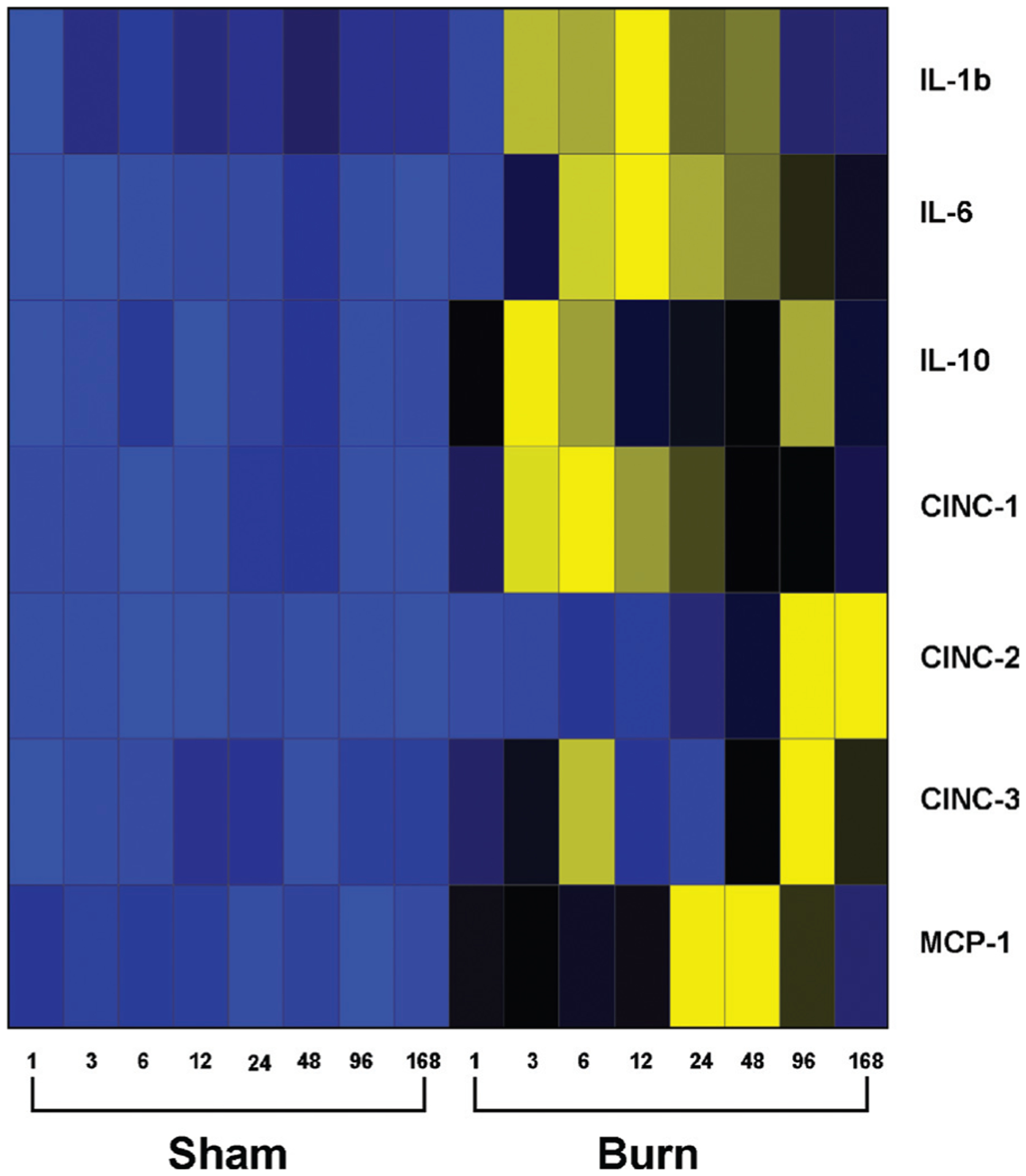
Values (average cytokine concentration; picograms per milliliter), with blue indicating lowest levels, yellow indicating highest levels, and black in the middle. Gray squares indicate that no expression was detected.
In humans, early markers of inflammation include IL-1β, IL-6, IL-8, IL-10, and TNF-α. IL-1 and IL-8 typically display the highest levels at the time of patient admission to the hospital (20). IL-1 seems to be a key component of the inflammatory mediator cascade, regulating the host response to infection, injury, and inflammation (21). For example, it has been shown to be critical for the maintenance of a persistent inflammatory state in the lungs of patients with acute respiratory distress syndrome (22). In the rat, there is a true homolog of IL-1, which in this study was observed to be immediately increased after burn injury.
IL-8 is a key cytokine mediator of the acute-phase response to injury and infection (23–25). However, IL-8 does not exist as such in rodents (26). Instead, rats express CINC-1, CINC-2, and CINC-3 proteins, which may be thought of as functional isoforms of IL-8 (26). Indeed, CINCs have been found to be potent chemotactic factors for neutrophils in acute lung inflammation induced by LPS (27). However, their kinetic behavior after burn injury has not been characterized in detail (28). In our study, the expression profiles of CINC cytokines were not similar among themselves. Cytokine-induced neutrophil chemoattractant 1 was observed to increase immediately after burn in a manner similar to that of IL-8 in humans. Cytokine-induced neutrophil chemoattractant 2 showed a considerable lag before displaying a significant increase. Finally, there were two peaks associated with CINC-3 expression: one immediately postburn and a second one near the end of our study.
Similarly to IL-8, IL-6 plays a pivotal role in mediating the acute-phase response. However, prolonged and excessive elevations of circulating IL-6 and IL-8 levels in patients after trauma, severe burn, and elective surgery have been highly associated with complications and mortality (29–32). However, the role of IL-6 during inflammation remains controversial because it has both proinflammatory and anti-inflammatory properties. On the one hand, it has been shown that IL-6 stimulates inflammation by modulating the functional repertoire of mature polymorphonuclear neutrophils, immune cells that seem to be important effectors in the genesis of postinjury inflammation and tissue injury (31). On the other hand, as part of its anti-inflammatory effects, IL-6 reflects its ability to induce the production in the liver of a wide spectrum of acute-phase proteins known to be essentially protective and to limit the inflammatory process (31). Here, we found that IL-6 increased immediately after burn and remained elevated throughout the length of the study. A similar result was observed for this cytokine in our recent study of burned pediatric patients (5). Cruickshank et al. (33) found that IL-6 rises within 2 to 4 h and peaks at 6 to 12 h in a group of patients undergoing a variety of procedures. Plackett et al. (34) observed a peak of IL-6 in liver tissue 12 h after burn injury in a mice burn model. In addition, IL-6 is known to enhance the release of anti-inflammatory mediators such as IL-10 (35). Consistent with this observation, we found here that the levels of IL-10 in the rat were also elevated immediately postburn and showed fluctuation throughout the study, although a significant increase was detected at the end of the period measured. The ratio of IL-6 to IL-10 has been reported to predict mortality in critically ill patients with systemic inflammatory syndrome (30). Schneider et al. (36) found that IL-10 is a critical mediator of immunosuppression after traumatic injury. Studies by Lyons et al. (37) indicated that increased IL-10 production correlates with subsequent septic events, and in the burn mouse, IL-10 seems to induce decreased resistance to infection.
Monocyte chemotactic protein 1 is a member of the β-chemokines and plays a crucial role in the trafficking and recruitment of effector leukocytes to primary sites of immune responses and inflammation (38, 39). New in vitro data demonstrate that MCP-1 has the ability to induce insulin resistance in adipocytes and skeletal muscle cells (40). In the rat, we found that the expression of MCP-1 was elevated immediately postburn, with a significant increase at 24 and 48 h after burn trauma. A similar result was observed for this cytokine in our recent study of burned pediatric patients (5): after an early peak postburn, plasma concentrations of MCP-1 started decreasing 6 days postburn injury.
Vascular endothelial growth factor is one of the most potent mediators of vascular regulation in angiogenesis and vascular permeability and has been shown to be elevated in severely burned patients, mainly in association with adult respiratory distress syndrome or systemic inflammatory response syndrome (41). TNF is a cytokine produced mainly by macrophages and monocytes and primarily increased in burn patients with sepsis (42). In burn rats, VEGF and TNF serum levels were not significantly different to nonburned rats under our experimental conditions. These findings correlate with the profile of these particular cytokines in severely burned children without inhalation injury or sepsis (5).
In addition to determining the profiles of the major cytokines associated with inflammation postburn, we wanted to ascertain that our burn treatment indeed led to a hypermetabolic state, as is observed in human patients. The hypermetabolic response after a major burn is characterized by a hyperdynamic response with increments in a wide variety of metabolic outcomes, including body temperature, oxygen and glucose consumption, carbon dioxide production, and muscle proteolysis (16). This response begins on the 5th day postinjury and continues up to 24 months postburn, leading to a significant loss of lean body mass, muscle weakness, and poor wound healing (16, 43, 44). In the present study, we measured the total body weight of animals in our experimental and control groups. Burn led to a significant decrease in total body weight, indicating that the observed alterations in circulating cytokines indeed led to the generation of a hypermetabolic state.
In summary, we analyzed a panel of serum cytokines in rats over time, known to increase in humans postburn that may serve as reference for future development of therapeutic interventions. Various cytokines were observed in our rat burn model to follow a similar kinetic profile to that of humans. Because rats provide a cost-effective, easily manipulatable, and genetically homogeneous model system, we believe that this model is an adequate system to explore the mechanisms underlying the pathophysiology of the acute and postacute phases of burn injury. Our model may be useful in exploring therapeutic interventions directed at modifying the expression of cytokines, thus decreasing the hypermetabolic state associated with increased morbidity and mortality in human patients.
Acknowledgments
This study was supported by the American Surgical Association, Shriners Hospitals for Children (grant no. 8660), and the National Institute of General Medical Sciences (grant nos. R01-GM56687, T32 GM008256, and P50 GM60338).
REFERENCES
- 1.Nyhlen K, Gautam C, Andersson R, Srinivas U: Modulation of cytokine-induced production of IL-8 in vitro by interferons and glucocorticosteroids. Inflammation 28(2):77–88, 2004. [DOI] [PubMed] [Google Scholar]
- 2.de Bandt JP, Chollet-Martin S, Hernvann A, Lioret N, du Roure LD, Lim SK, Vaubourdolle M, Guechot J, Saizy R, Giboudeau J, et al. : Cytokine response to burn injury: relationship with protein metabolism. J Trauma 36(5):624–628, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Ueyama M, Maruyama I, Osame M, Sawada Y: Marked increase in plasma interleukin-6 in burn patients. J Lab Clin Med 120(5):693–698, 1992. [PubMed] [Google Scholar]
- 4.Endo S, Inada K, Yamada Y, Kasai T, Takakuwa T, Nakae H, Kikuchi M, Hoshi S, Suzuki M, Yamashita H, et al. : Plasma tumour necrosis factor-alpha (TNF-alpha) levels in patients with burns. Burns 19(2):124–127, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, Queiroz DM, Rocha AM, Jeschke MG: Cytokine expression profile over time in severely burned pediatric patients. Shock 26(1):13–19, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Frost RA, Lang CH, Gelato MC: Transient exposure of human myoblasts to tumor necrosis factor-alpha inhibits serum and insulin-like growth factor-I stimulated protein synthesis. Endocrinology 138(10):4153–4159, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Van den Berghe G, de Zegher F, Veldhuis JD, Wouters P, Awouters M, Verbruggen W, Schetz M, Verwaest C, Lauwers P, Bouillon R, et al. : The somatotropic axis in critical illness: effect of continuous growth hormone (GH)-releasing hormone and GH- releasing peptide-2 infusion. J Clin Endocrinol Metab 82(2):590–599, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Wray CJ, Mammen JM, Hasselgren PO: Catabolic response to stress and potential benefits of nutrition support. Nutrition 18(11–12):971–977, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Herndon DN: Total Burn Care. New York, NY: WB Saunders, 2002. [Google Scholar]
- 10.Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, Hinds CJ: Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med 341(11):785–792, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Parker SJ, Watkins PE: Experimental models of gram-negative sepsis. Br J Surg 88(1):22–30, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Cugini D, Azzollini N, Gagliardini E, Cassis P, Bertini R, Colotta F, Noris M, Remuzzi G, Benigni A: Inhibition of the chemokine receptor CXCR2 prevents kidney graft function deterioration due to ischemia/reperfusion. Kidney Int 67(5):1753–1761, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Ramos CD, Heluy-Neto NE, Ribeiro RA, Ferreira SH, Cunha FQ: Neutrophil migration induced by IL-8-activated mast cells is mediated by CINC-1. Cytokine 21(5):214–223, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa H, Ikesue A, Kato H, Debuchi H, Watanabe K, Tsurufuji S, Naganawa M, Mitamura M: Changes in the levels of rat interleukin 8/CINC and gelatinase in the exudate of carrageenin-induced inflammation in rats. J Pharmacobiodyn 15(9):461–466, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe K, Koizumi F, Kurashige Y, Tsurufuji S, Nakagawa H: Rat CINC, a member of the interleukin-8 family, is a neutrophil-specific chemoattractant in vivo. Exp Mol Pathol 55(1):30–37, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Herndon DN, Wilmore DW, Mason AD Jr: Development and analysis of a small animal model simulating the human postburn hypermetabolic response. J Surg Res 25(5):394–403, 1978. [DOI] [PubMed] [Google Scholar]
- 17.Yeh FL, Shen HD, Fang RH: Deficient transforming growth factor beta and interleukin-10 responses contribute to the septic death of burned patients. Burns 28(7):631–637, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Jeschke MG, Einspanier R, Klein D, Jauch KW: Insulin attenuates the systemic inflammatory response to thermal trauma. Mol Med 8(8):443–450, 2002. [PMC free article] [PubMed] [Google Scholar]
- 19.Davis KA, Santaniello JM, He LK, Muthu K, Sen S, Jones SB, Gamelli RL, Shankar R: Burn injury and pulmonary sepsis: development of a clinically relevant model. J Trauma 56(2):272–278, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Vindenes HA, Ulvestad E, Bjerknes R : Concentrations of cytokines in plasma of patients with large burns: their relation to time after injury, burn size, inflammatory variables, infection, and outcome. Eur J Surg 164(9):647–656, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan E, Dinarello CA, Gelfand JA: Interleukin-1 and the response to injury. Immunol Res 8(2):118–129, 1989. [DOI] [PubMed] [Google Scholar]
- 22.Goodman RB, Pugin J, Lee JS, Matthay MA: Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev 14(6):523–535, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Greenberger MJ, Kunkel SL, Strieter RM, Lukacs NW, Bramson J, Gauldie J, Graham FL, Hitt M, Danforth JM, Standiford TJ: IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J Immunol 157(7):3006–3012, 1996. [PubMed] [Google Scholar]
- 24.van der Poll T, Keogh CV, Guirao X, Buurman WA, Kopf M, Lowry SF: Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis 176(2):439–444, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Vindenes H, Ulvestad E, Bjerknes R: Increased levels of circulating interleukin-8 in patients with large burns: relation to burn size and sepsis. J Trauma 39(4):635–640, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Hata J, Aoki K, Mitsuhashi H, Uno H: Change in location of cytokine-induced neutrophil chemoattractants (CINCs) in pulmonary silicosis. Exp Mol Pathol 75(1):68–73, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Mitsuhashi H, Hata J, Asano S, Kishimoto T: Appearance of cytokine-induced neutrophil chemoattractant isoforms and immunolocalization of them in lipopolysaccharide-induced acute lung inflammation in rats. Inflamm Res 48(11):588–593, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Kovacs EJ, Schwacha MG, Chaudry IH, Choudhry MA: Acute alcohol intoxication increases interleukin-18Ymediated neutrophil infiltration and lung inflammation following burn injury in rats. Am J Physiol Lung Cell Mol Physiol 292(5):L1193–L1201, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Yamada Y, Endo S, Inada K: Plasma cytokine levels in patients with severe burn injury—with reference to the relationship between infection and prognosis. Burns 22(8):587–593, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Taniguchi T, Koido Y, Aiboshi J, Yamashita T, Suzaki S, Kurokawa A: The ratio of interleukin-6 to interleukin-10 correlates with severity in patients with chest and abdominal trauma. Am J Emerg Med 17(6):548–551, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Biffl WL, Moore EE, Moore FA, Peterson VM: Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg 224(5): 647–664, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hack CE, Hart M, van Schijndel RJ, Eerenberg AJ, Nuijens JH, Thijs LG, Aarden LA: Interleukin-8 in sepsis: relation to shock and inflammatory mediators. Infect Immun 60(7):2835–2842, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cruickshank AM, Fraser WD, Burns HJ, Van Damme J, Shenkin A: Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci (Lond) 79:161–165, 1990. [DOI] [PubMed] [Google Scholar]
- 34.Plackett TP, Colantoni A, Heinrich SA, Messingham KA, Gamelli RL, Kovacs EJ: The early acute phase response after burn injury in mice. J Burn Care Res 28(1):167–172, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK: IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 285(2):E433–E437, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Schneider CP, Schwacha MG, Chaudry IH: The role of interleukin-10 in the regulation of the systemic inflammatory response following trauma-hemorrhage. Biochim Biophys Acta 1689(1):22–32, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Lyons A, Kelly JL, Rodrick ML, Mannick JA, Lederer JA: Major injury induces increased production of interleukin-10 by cells of the immune system with a negative impact on resistance to infection. Ann Surg 226(4):450–460, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luster AD: Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med 338(7):436–445, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Kim CH, Broxmeyer HE: Chemokines: signal lamps for trafficking of T and B cells for development and effector function. J Leukoc Biol 65(1):6–15, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Sell H, Eckel J: Monocyte chemotactic proteinY1 and its role in insulin resistance. Curr Opin Lipidol 18(3):258–262, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Infanger M, Faramarzi S, Grosse J, Kurth E, Ulbrich C, Bauer J, Wehland M, Kreutz R, Kossmehl P, Paul M, et al. : Expression of vascular endothelial growth factor and receptor tyrosine kinases in cardiac ischemia/reperfusion injury. Cardiovasc Pathol 16(5):291–299, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Khalil AA, Hall JC, Aziz FA, Price P: Tumour necrosis factor: implications for surgical patients. ANZ J Surg 76(11):1010–1016, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Pereira C, Murphy K, Jeschke M, Herndon DN: Post burn muscle wasting and the effects of treatments. Int J Biochem Cell Biol 37(10):1948–1961, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Przkora R, Barrow RE, Jeschke MG, Suman OE, Celis M, Sanford AP, Chinkes DL, Mlcak RP, Herndon DN: Body composition changes with time in pediatric burn patients. J Trauma 60(5):968–971, 2006. [DOI] [PubMed] [Google Scholar]


