Abstract
Patients with extensive burns rely on the use of tissue engineered skin due to a lack of sufficient donor tissue, but it is a challenge to identify reliable and economical scaffold materials and donor cell sources for the generation of a functional skin substitute. The current review attempts to evaluate the performance of the wide range of biomaterials available for generating skin substitutes, including both natural biopolymers and synthetic polymers, in terms of tissue response and potential for use in the operating room. Natural biopolymers display an improved cell response, while synthetic polymers provide better control over chemical composition and mechanical properties. It is suggested that not one material meets all the requirements for a skin substitute. Rather, a composite scaffold fabricated from both natural and synthetic biomaterials may allow for the generation of skin substitutes that meet all clinical requirements including a tailored wound size and type, the degree of burn, the patient age, and the available preparation technique. This review aims to be a valuable directory for researchers in the field to find the optimal material or combination of materials based on their specific application.
Keywords: natural biomaterials, skin regeneration, skin substitutes, synthetic biomaterials, tissue engineering
1. Introduction
The skin is the largest organ in the body by surface area and is exposed to injury more than any other part of the body. These injuries vary from small cuts which can be healed naturally in a couple of days, to severe third-degree burns which can threaten a patient’s life if medical intervention is not successful.[1,2] The normal wound healing response and intrinsic tissue regeneration of the skin are not sufficient in cases like acute and large area wounds and burns, or in elderly and diabetic patients.[1,3–5] While autologous grafts are considered to be the gold-standard for skin regeneration,[6–8] limitations in the donor site availability and complications associated with reharvesting donor sites necessitate the investigation of skin substitutes.[2,9,10] Currently, different skin substitutes are being used in clinic. For example, Integra, the most commonly used skin substitute in the burn clinics, is mainly made of bovine collagen covered by a silicon layer, that lacks the epidermal component and has a risk of fluid buildup leading to infection.[3,11] The silicone layer will be removed after about 2–3 weeks and replaced by a split-thickness graft (provided enough donor site is available). Hence Integra is just a dermal substitute and a preliminary step for split-thickness grafting that does not eliminate the need for a graft. Some other products that are clinically available are derived from human. An example is Alloderm which is associated with a high cost and a risk of infectious disease transmission and requires multiple surgeries.[3,11] With advances in tissue engineering and the development of biomaterials and scaffold fabrication methods for different organs, the availability of ideal skin substitutes that can heal wounds and fully regenerate skin, especially for extensive burn wounds, is more attainable than ever.[12,13]
Skin is comprised of two layers: the epidermis and dermis (Figure 1). The outermost layer of the skin, the epidermis, is constructed from packed sheets of keratinocytes that provide a barrier to the external environment and control the loss of water. The epidermis is built up on top of the basement membrane, a meshed network of the proteins collagen IV and laminin.[14] Keratinocytes adjacent to basement membrane are proliferative while the outermost cells in the epidermis are no longer living.[15] It has been shown that the surface topography of the basement membrane affects keratinocyte phenotype.[16] The extracellular matrix (ECM) of the inner dermis layer has a fibrous structure made of mainly rigid collagen fibers as well as rubber-like elastin fibers.[17] This fibrous matrix is filled with nonfibrillar proteins such as hyaluronan, or hyaluronic acid (HA), and chondroitin sulfate that generates a negatively charged, hydrophilic, and dynamic space capable of hydration of the tissue and dispersing forces.[18] These properties of the ECM imparts the dermis with physical strength and stress absorbing character. Fibroblasts, macrophages, and adipocytes are the main cells that reside in the dermis.[19–21] Full-thickness skin substitutes should consider both epidermis and dermis structural and functional properties and requirements to better recapitulate the native skin.[22] While epidermis may be regenerated quickly in adults provided a dermal substrate is present, dermis regeneration may be slow or nonexistent in severe burns.[23] Consequently, dermal regeneration should be the first step to speed up the formation of new skin.
Figure 1.
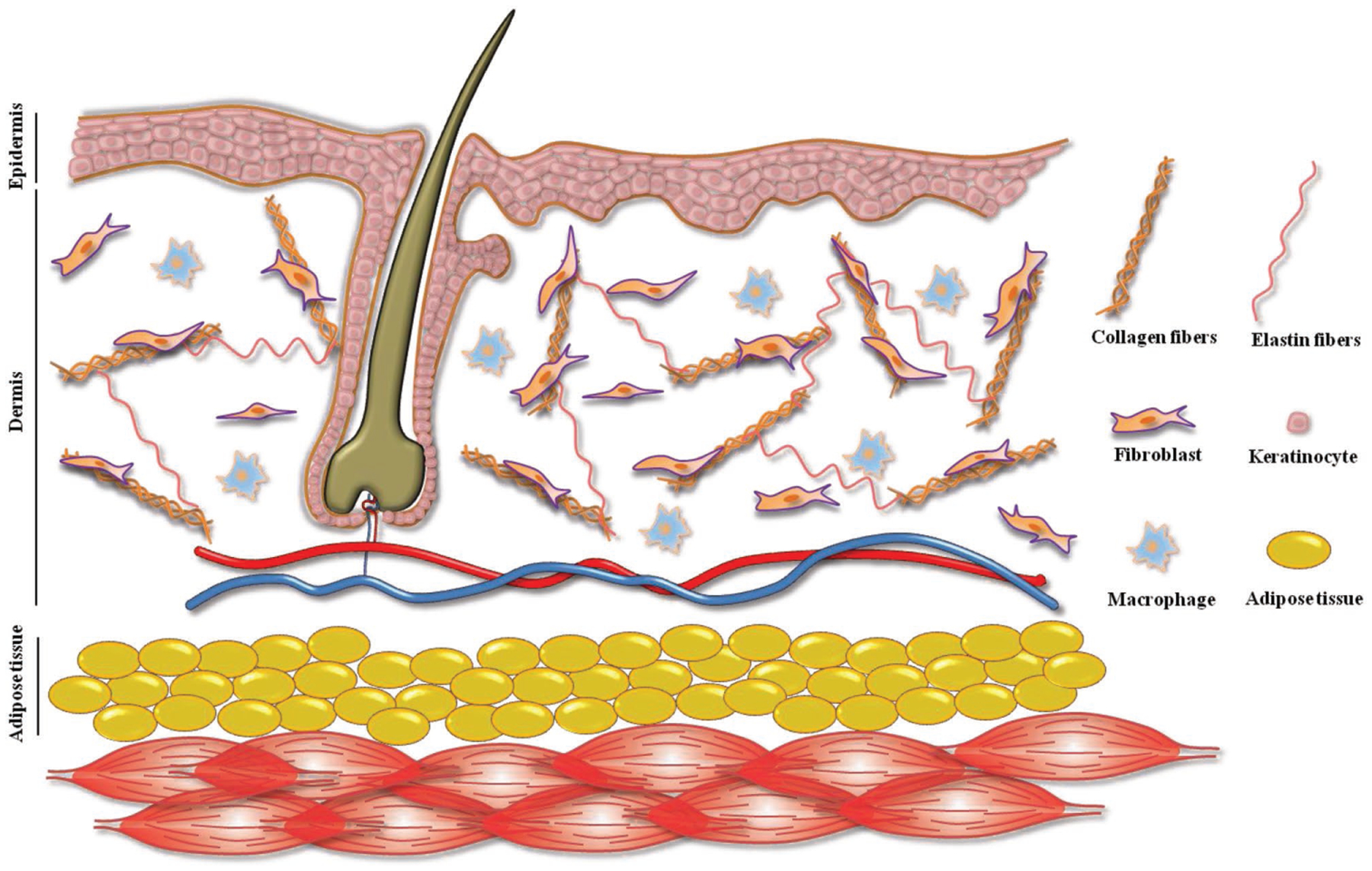
Human Skin structure in cluding epidermis (including keratinocytes) and dermis (with fibroblasts and macrophages as residing cells and collagen and elastin fibers as the main ECM components).
Several skin substitutes (cellularized or acellular, containing dermal and/or epidermal components) are commercially available.[24–26] However, due to their inefficiency for treating severe or large wounds and burns, research is still in progress for developing new and improved skin substitutes.[27,28] Different tissue engineering strategies are considered to address current skin substitutes’ drawbacks, and a large number of papers reviewing current strategies have been published recently,[2,6,7,24–27,29–48] However, a more comprehensive review focused on biomaterials used for skin tissue engineering scaffolds for the treatment of severe burns is still missing. Other than the fabrication of scaffolds using available biomaterials, decellularized tissues, and cell sheets are also being used for skin regeneration and scaffolding. Our focus in the current review is to summarize the biomaterials that have been used for skin tissue engineering for treatment of burns. We will discuss the advantages and disadvantages of both natural and synthetic polymers in terms of tissue response, ease of fabrication, and clinical applicability.
2. Natural Biomaterials
Tissue engineering scaffolds are designed to mimic the function of the natural ECM in tissue, and as such, researchers have explored using ECM proteins as materials for tissue engineering scaffolds, such as collagen, gelatin (a hydrolyzed form of collagen), elastin, and HA. These materials are reviewed and evaluated here in terms of their performance as a skin tissue engineering scaffold material, along with additional natural polymers fibrin (blood clotting protein) and pullulan (a natural biopolymer produced by a fungus).
2.1. Collagen
As the most abundant protein in the body and one of the major components of the ECM,[49,50] collagen has been widely used for different biomedical applications including wound healing and skin regeneration.[51,52] Three polypeptide helices, each containing about 1000 amino acids, wrap around each other to form a molecule of collagen with a triple helical structure. Loosely interwoven, large, wavy, randomly oriented bundles of fibrillar collagen, mainly of type I collagen, form 70–80% of dry weight of dermal matrix.[30,53] Types III and V collagens which have a fibrillar structure are also detected in dermis.[54] Collagen matrix in dermis cushions body skin against mechanical forces and is responsible for skin’s inherent tensile strength.[54]
Collagen can be extracted from any animal tissue, and its properties depend on both the animal and the tissue of extraction.[51] Available collagens for use in tissue engineering scaffolds are commonly from bovine skin and tendons, porcine skin, intestine, or bladder mucosa, and rat tail.[51,55] As animal-derived collagen has the potential for pathogen transmission, alternative options such as collagen produced by heterologous expression in other species or collagen mimetic peptides are being studied.[56] It is also reported that fish collagen has less risk of disease transmission compared with bovine and porcine counterparts.[57] Collagen scaffolds are mainly made by lyophilization,[23] electrospinning,[58] and bio-printing.[59] To enhance integrity and stability of scaffolds fabricated from collagen, chemical, UV light, and enzyme-based crosslinking methods are employed. The degree of crosslinking can decrease the rate of degradation of the scaffold.[51] Collagen scaffolds and hydrogels shrink after contact with aqueous media which can hinder their performance.[60,61] This issue can be minimized by selecting proper drying and crosslinking methods, such as using crosslinking reagent in ethanol, by forming a composite of collagen with other biomaterials,[62,63] or by introducing a reinforcing structure (e.g., a crosslinked meshwork within a sponge).[64]
2.1.1. Collagen–Chondroitin 6-Sulfate
A summary of recent studies in which collagen was used as the base material for skin tissue engineering scaffolds is shown in Table 1. Freeze-drying is the major methods for making collagen skin substitutes, likely due to the low-cost, wide availability and simplicity of this method. To improve its physical and mechanical properties, degradation rate, cell-interaction, and in vitro and in vivo function, collagen is normally used with other ECM proteins. Gelatin, elastin, chitosan (CS), and glycosaminoglycans (GAGs) like chondroitin 6-sulfate (C6S) and HA, are among the natural biomaterials that have been introduced in collagen scaffolds. C6S is the most frequent component added to collagen composites. A collagen–C6S scaffold was designed and developed using freeze-drying followed by dehydrothermal crosslinking by Yannas and Burke in 1980.[23,61,65–68] This composite scaffold was later commercially introduced to the market as Integra. The presence of C6S in the collagen scaffold is known to improve elasticity and ultimate tensile strength of the scaffolds as well as decrease the rate of degradation by increasing its resistance to collagenase degradation.[65] This scaffold has been shown to promote regeneration rather than wound contraction by disrupting the formation of a contractile cell capsule at the edges of the wound.[69] It has been shown that while the acellular collagen–C6S scaffold could only delay the onset of wound contraction for 10 d, the scaffold seeded with dermal and epidermal cells led to the formation of a mature epidermis and a nearly physiological dermis without hair follicles, and reversed contraction by regenerating new skin.[23] To better mimic the skin, a nonporous layer of the same composition has been laminated on the scaffold to provide a substrate for keratinocytes which restricts them to the outer layer of the substitute.[70] To slow its degradation, glutaraldehyde and (1-ethyl-3-(3-dimethylaminopropyl) ([EDC) carbodiimide hydrochloride) were used for crosslinking collagen–C6S scaffolds.[71–73]
Table 1.
Collagen-based scaffolds for skin regeneration application.
| Biomaterial | Fabrication method | Cell(s) | Performance | Ref. |
|---|---|---|---|---|
| Collagen | Freeze-drying + DHTa) crosslinking | Acellular | Wound contraction is disrupted in the favor of skin regeneration | [69] |
| Collagen | Electrospinning + GA vapor crosslinking | NHOK, NHEK | Cell attachment is not sufficient unless ECM proteins added to the scaffold | [94] |
| Collagen | Freeze-drying vs electrospinning (DHT + EDC crosslinking) | HDF, HEK | Electrospun scaffold led to less wound contraction | [58] |
| Matriderm (collagen + elastin) | LaBP (Laser-assisted bioprinting) | NIH-3T3 and HaCaT keratinocytes | Multilayered epidermis formation with angiogenesis from wound bed and edges | [59] |
| Collagen–C6S | Freeze-drying + DHT crosslinking | HDF, HEK | Formation of a mature epidermis and a nearly physiological dermis without hair follicles with delayed wound contraction compared with acellular scaffold | [23] |
| Collagen–C6S | Freeze-drying + DHT crosslinking + a laminated layer | HEK | HEK were restricted to the outer layer of the substitute | [70] |
| Collagen–C6S | Freeze-drying + chemical crosslinking | HDF, HEK | Improved degradation and wound contraction | [73] |
| Collagen–HA | freeze-drying + EDC crosslinking | HDF | Improved HDF attachment in vitro with no effect on wound contraction in vivo | [74] |
| Collagen–gelatin | SCPL + Freeze-drying | DF | Improved DF infiltration and reduced inflammatory response | [103] |
| Collagen–elastin | elastin hydrolysate-coated collagen scaffold | Autologous dermal fibroblasts | Lower degradation rate and reduced migration and/or proliferation of subcutaneous fibroblasts in the wound tissue compared with acellular scaffold | [78] |
| Collagen–elastin | Commercial collagen–elastin membrane | Keratinocyte | Promoted dermal vascularization and basement membrane formation | [79] |
| Collagen–chitosan | Freeze-drying | HDF, HEK | Promoted rapid remodeling of ECM nearly similar to normal dermis as well as organization of elastin deposits in thin fibrils after 90 d | [85,80] |
| Collagen–chitosan | Freeze-drying | HDF, HEK, HUVEC | Capillary-like structures were formed without addition of external angiogenesis promoting agents | [90] |
| Collagen–chitosan | Freeze-drying | HDF, HEK | Nerve fibers were observed four months after transplantation | [91] |
| Collagen–chitosan-laminin | Freeze-drying | HDF, HEK | Enhanced sensory perception recovery | [92] |
| Collagen–chitosan | Freeze-drying + GA crosslinking | HDF | Chitosan reduced biodegradation of the scaffold | [93] |
| Chitosan–collagen | Freeze-drying | HDF | Improved HDF proliferation and decreased contraction | [89] |
DHT crosslinking: dehydrothermal crosslinking, C6S: chondroitin 6-sulfate, HDF: human dermal fibroblast, HEK: human epidermal keratinocyte, SCPL: solvent casting-particulate leaching, DF: dermal fibroblast, GA: glutaraldehyde, HA: Hyaluronic acid, BM-MSCs: bone marrow mesenchymal stem cells, NHOK: Normal human oral keratinocytes, NHEK: Normal human epidermal keratinocytes, LaBP: Laser-assisted bioprinting, HaCaT cells: human keratinocyte cell line.
2.1.2. Collagen–HA
Hyaluronic acid is another GAG (present in the native dermis) that may be combined with collagen for generating skin substitutes.[74–76] When HA is mixed with collagen at different ratios and crosslinked by EDC, human dermal fibroblast (HDF) attachment improved in vitro. However, the addition of HA did not show any effect on wound contraction in vivo.[74]
Elastin is one of the major components of connective tissue and is responsible for skin elasticity. It has been added to collagen for reduced wound contraction and improved skin regeneration.[77–82,84] In such applications, elastin hydrolysate is normally used to coat and modify the collagen scaffold, a strategy used in the commercial product Matriderm[77,78,81] The elastin-modified scaffold has been shown to improve skin regeneration compared to a split-thickness skin graft.[77,78]
2.1.3. Collagen–Chitosan
Chitosan is an abundant antibacterial biopolymer that has been incorporated into collagen scaffolds for skin substitutes.[85–89] Collagen–chitosan scaffolds prepared by freeze-drying have been seeded with fibroblasts and keratinocytes.[85] It was illustrated that the scaffold could promote rapid remodeling of ECM at a rate similar to the native dermis. The authors also reported organization of elastin deposits in thin fibrils after 90 d, a process which normally takes several years in human.[80] When the scaffolds were seeded with fibroblasts, keratinocytes, and human vascular endothelial cells (HUVECs), capillary-like structures were formed without the addition of external angiogenesis promoting agents.[90] Nerve fibers were observed four months after transplantation.[91] Moreover, incorporating laminin to the same scaffold enhanced sensory perception recovery.[92] The presence of chitosan in collagen has been shown to reduce biodegradation of the scaffold.[93]
2.1.4. Electrospinning of Collagen
Electrospinning is another method that has been widely employed to make collagen scaffolds for skin substitutes, despite their intrinsically smaller pore size and resultantly decreased cell infiltration compared with freeze-dried sponges.[58,87,94–102] The use of organic solvents for electrospinning collagen and the subsequent chemical crosslinking of the scaffold may lead to its denaturation and subsequent reduced keratinocyte attachment.[94] However, a further modification of electrospun fibers with ECM proteins like collagen I or laminin can improve cell–scaffold interaction.[94] Collagen scaffolds prepared by freeze-drying versus electrospinning were compared by Boyce and co-workers[58] Although cell proliferation, surface hydration, cellular organization as well as basement membrane and blood vessel formation were similar for both preparation methods, electrospun scaffold led to a reduced wound contraction (Figure 2).
Figure 2.

Immunohistological images of FCSS A–D) and ECSS E–H) showing cell nuclei A,E), human involucrin B,F), human collagen type IV C,G), and merged images D,H). Epidermis and dermis are denoted by e and d, respectively. The dashed line indicates the dermal–epidermal junction. Scale bar = 100 mm. Reproduced with permission.[58] Copyright 2017, Elsevier.
In summary, collagen can be used as the basis for scaffolds with a good wound healing response (minimal wound contraction, fast tissue integration, and formation of vascular beds), and can be used in multiple fabrication methods including freeze-drying sponge formation, electrospinning, and bio-printing. It is the most prevalent component of skin substitute biomaterials being used in the clinic in products like Integra, OrCel, Promogran, and PurAply.[11] Additional ECM molecules may be used with collagen for improved cell behavior, some of which are described in Table 1.
2.2. Gelatin
Gelatin is a denatured form of collagen that is obtained by controlled hydrolysis of fibrous collagen and so retains most of the collagen chemical functionality.[104,105] Similar to collagen, gelatin’s structure is mostly composed of triple amino acids of glycine, proline, and hydroxyproline. Gelatin has been added to various biomaterials to enhance cell–scaffold interactions through its Arg-Gly-Asp (RGD) motifs that are recognized by integrin receptors in cell membranes and positive charges from lysine and arginine amino acids that facilitate attachment of negatively charged cell membrane.[106,107] It is shown that gelatin has a lower antigenicity than collagen,[105] which becomes crucial in the case of applications for wounds with a high risk of infection. Furthermore, gelatin has higher solubility in most solvents and is less expensive than collagen. Due to the aforementioned advantages, gelatin has a great potential for application in skin tissue engineering scaffolds. Freeze-drying,[108] electrospinning,[109,110] and UV-crosslinking have been the main methods used for developing gelatin skin substitutes. Gelatin is soluble in aqueous medium and as a result, it needs to be crosslinked in order to enhance its stability for application in tissue engineering. Similar to collagen, several crosslinking techniques from physical to chemical methods have been used for gelatin crosslinking.[111]
Studies in which gelatin-based scaffolds for skin substitutes were explored are summarized in Table 2. Gelatin has been made in the form of mono[108] and bilayer scaffolds[112,113] and is used for both dermal[108,114] and epidermal[115,116] regeneration. Compared with acellular scaffolds, pre-seeding the gelatin with cells like fibroblasts, keratinocytes, and hair follicle stem cells results in improved skin regeneration and wound healing in terms of better reepithelialization,[108,112,113] enhanced graft take,[112,113] formation of a well-developed dermal–epidermal junction basement membrane,[112,113] and promoted vascularization.[117] It has been employed in combination with HA,[112,113,117] C6S,[112,113,118,117] and fibrinogen.[114] Gelatin has excellent potential for electrospinning due to its high solubility.[109,110] It is demonstrated that interfiber distances between 5 and 10 μm result in the best dermis and epidermis organization.[109] While smaller pore size prevents enough cell infiltration to the bulk, larger interfiber distances lead to the initial sinking of fibroblasts, preventing the formation of a packed layer of fibroblasts and thus inability of keratinocytes to attach and form a well-stratified epithelium.
Table 2.
Gelatin-based scaffolds for skin regeneration application.
| Biomaterial | Fabrication method | Cell(s) | Performance | Ref. |
|---|---|---|---|---|
| Gelatin | SCPLa) + Freeze-drying + EDC crosslinking | HDF | Pre-seeding with fibroblasts led to a better performance in reepithelialization | [108] |
| Gelatin | Electrospinning + DHT and EDC crosslinking | HDF, HEK | Interfiber distances between 5 and 10 μm results in the best dermis and epidermis organization | [109] |
| Gelatin | Needleless electrospinning + GA crosslinking | BM-MSCs, HDF, HEK | Faster wound closure, enhanced epithelialization, increased depth of granulation tissue and density of myofibroblasts in the wound area for gelatin vs PCL | [110] |
| Gelatin-C6S-HA | Freeze-drying + EDC crosslinking | Foreskin dermal fibroblasts and keratinocytes | Enhanced wound healing and graft take compared to the acellular scaffolds, with a well-developed epidermis and dermal–epidermal junction basement membrane after 4 weeks | [112,113] |
| Gelatin-C6S-HA | EDC crosslinking + Freeze-drying | VEGF165-modified rHFSCs | Promotes vascularization in the scaffold and enhances wound healing | [117] |
| Gelatin–fibrinogen | GA crosslinking + Freeze-thawing | Primary HDF | Cell proliferation and infiltration were affected by GA crosslinker, mostly lower than Integra | [114] |
| Gelatin, collagen (bilayer) | Freeze-thawing, gelation | Acellular | Promote reepithelialization and wound healing | [115] |
SCPL: solvent casting-particulate leaching, DHT crosslinking: Dehydrothermal crosslinking, GA: glutaraldehyde, BM-MSCs: Bone marrow mesenchymal stem cells, C6S: chondroitin 6-sulfate, HA: hyaluronic acid, VEGF165-modified rHFSCs: VEGF165 gene-modified rat Hair follicle stem cells, VEGF: vascular endothelial growth factor.
In summary, gelatin has similar biocompatibility and processing flexibility as collagen, but with increased economic feasibility due to its high solubility and lower cost. As with collagen, it is likely to be combined with other naturally occurring molecules of the ECM to improve its biological performance.
2.3. Elastin
Elastin constitutes 2–4% of skin,[119] and elastin fibers are responsible for the skin’s elasticity. In vivo, it presents in the form of water-soluble tropoelastin monomers that highly crosslink to insoluble elastin.[120] Most of the skin substitutes in the market are mainly made of collagen and do not include elastin. Therefore, they suffer from low elasticity, high contraction during wound healing, and scar tissue formation. In wound healing, elastin formation is very slow (4–5 years), its distribution is compromised and the morphology is not fibrous which leads to lack its native function (Figure 3).[121–123,119] In addition, elastin is rare in scar tissue. The presence of elastin in an engineered skin substitute not only helps decrease the aforementioned problems but also enhances elastin formation upregulation during wound healing and skin formation.[123] Elastin hydrolysate,[77] recombinant human tropoelastin,[124] and α-elastin[125] are the elastin-related biomaterials used in the literature to make or modify scaffolds such as collagen for skin substitutes. As mentioned, collagen dermal substitutes coated with elastin hydrolysate, showed decreased wound contraction in vivo, and tissue regeneration improved during the first 2 weeks of wound healing phase.[77,78]
Figure 3.
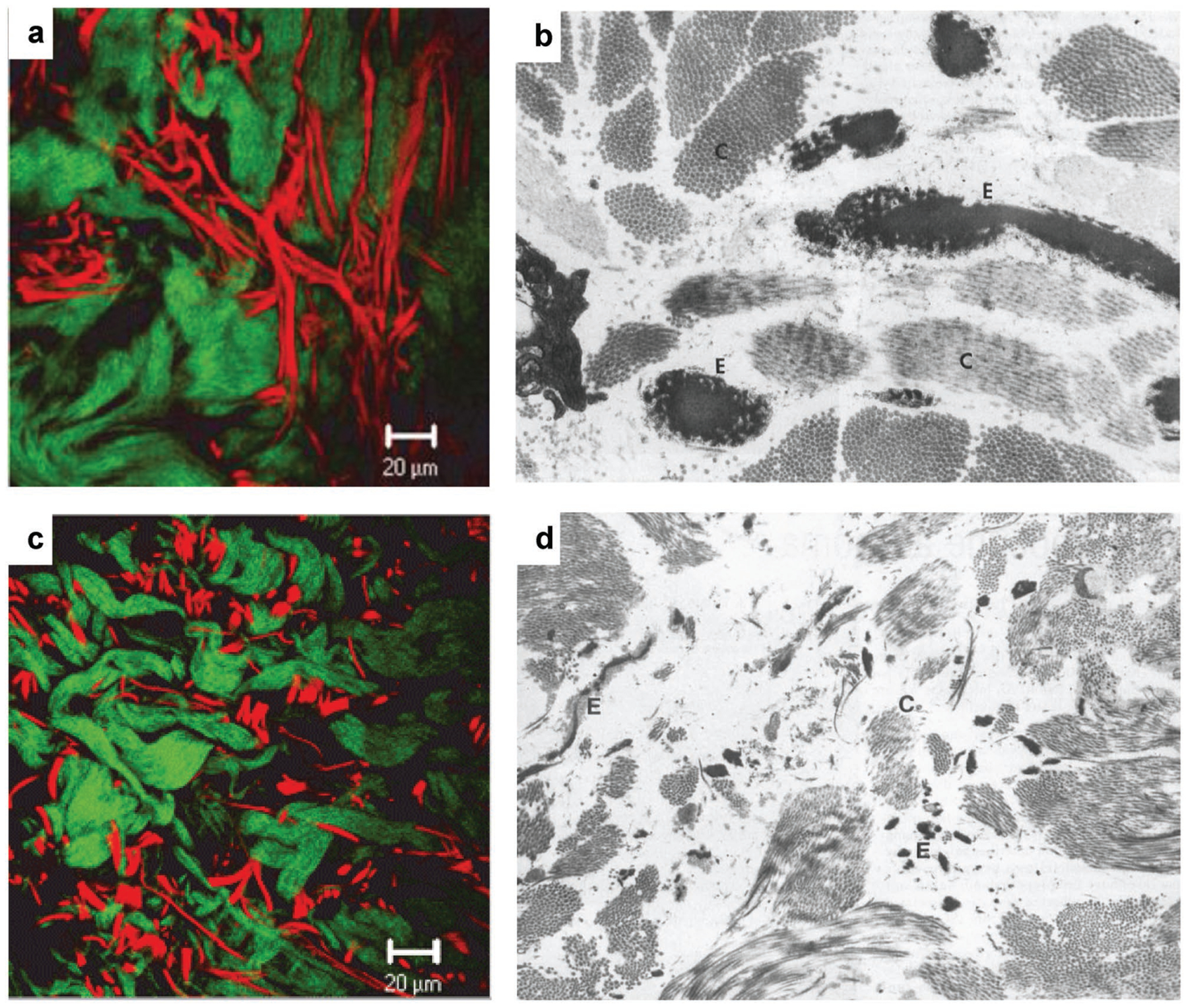
Collagen and elastin fibers in normal dermis a,b) and hypertrophic scar tissue c,d). In two-photon-excited fluorescence images a,c) collagen bundles are green and elastin fibers are red. In transmission electron microscopy images b,d) collagen (letter C) is light gray and elastin (letter E) is dark gray. While thick and long elastin fibers are visible among collagen bundles in the normal dermis, in the hypertrophic scar elastin is scattered as thin fibers among collagen bundles. Reproduced from ref. [121] and ref. [122] with permission from Wiley journals.
Tropoelastin is the main precursor to make elastin skin substitutes and electrospinning has been the major method of fabrication.[120,124,126–128] Larger pores can be obtained by employing higher feed rates, which allows for deeper HDF infiltration and greater deposition of collagen I and fibronectin compared with a tropoelastin hydrogel.[124] Using elastin scaffolds gives rise to only moderate scaffold contraction in vitro that can be attributed to the elasticity or signaling events contributed by elastin.[126] Upon the addition of 20% collagen, the electrospun elastin scaffold ease of handling and pore size was increased, and cell infiltration and proliferation and blood vessel formation were improved.[128] In some studies, the pore size in elastin hydrogel scaffolds was increased by making the hydrogel under high-pressure CO2.[125,129]
Elastin may be a beneficial component of skin scaffolds to decrease contraction of the scaffold during healing, however, it is more likely used as an additive to other scaffold materials such as collagen due to its poor mechanical strength and availability.
2.4. Fibrin
Fibrin hydrogels are normally formed through rapid polymerization of fibrinogen activated by protease thrombin and are available commercially as purified allogenic materials.[130] After damage to blood vessels, a fibrous network comprised primarily of fibrin forms spontaneously in vivo during hemostasis, providing a provisional scaffold for attachment and migration of cells involved in the early stage of wound healing (e.g., neutrophils, macrophages, and fibroblasts).[131,132] It binds to several vital factors released into the blood during wound healing such as fibronectin, growth factors, and protease inhibitors.[132] However, when formed in vitro, pure fibrin scaffolds suffer from significant shrinkage, weak mechanical properties, and fast degradation.[130] Cross-linking with other biomolecules has been used to improve its low mechanical properties and decrease the rate of degradation.[133]
Fibrin has been widely used as a scaffold material (in both mono-[134] and bilayer form) for skin substitutes. It has a great potential for vascularizing the scaffolds due to its special protein-binding character mentioned above.[132] Vascularization seen in the scaffold is reported to be implemented by enhancing VEGF secretion and promoting vascular endothelial cell migration to the scaffold[135] or differentiating residing stem cells to a vascular phenotype and developing a microvascular network.[133] Fibrin scaffolds and gels have been seeded with both HDF and keratinocytes[134,136] (capable of forming stratified epithelium with early expression of basement membrane proteins) as well as stem cells (while preserving their differentiation capacity).[137] Fibrin has also been utilized to prevascularize a dermo-epidermal skin graft, forming blood and lymphatic capillaries in vitro.[138]
Fibrin may have the most beneficial role by acting as a keratinocyte cell delivery scaffold to develop the epidermal layer at the graft site,[139,140] as fibrin has been shown to be important in epithelialization of the wound through facilitating keratinocyte migration.[120,135–138] Its presence in the scaffold as an additive to other biomaterials can harness its specific capacity to promote vascularization in the scaffold. However, control over its structure and mechanical properties is limited.
2.5. Hyaluronic Acid
Hyaluronic acid, or hyaluronan, is a nonsulfated GAG and a linear polymer of glucuronic acid and N-acetylglucosamine disaccharide, present in most ECM tissues. HA enhances both fibroblast and keratinocyte proliferation, so it has been used for dermal and epidermal reconstruction.[141,142] It readily dissolves in water, thus crosslinking is necessary for applications in tissue engineering.[143]
HYAFF-11 and Laserskin are two important HA-based biomaterials that have been used to culture dermal fibroblasts and epidermal keratinocytes, respectively and employed to make bilayer dermal–epidermal skin substitute.[141,144–146] Coculturing endothelial cells with fibroblasts on HYAFF-11 led to the organization of endothelial cells within the scaffold into lumen structures. Keratinocytes were later seeded on this scaffold to make a bilayer skin graft.[145,146] HA has been used to make mono-[147–149] and bilayer[150] scaffolds. Its negatively charged nature makes it an interesting option to be combined with positively charged polymers like poly-l-lysine (PLL)[151] and chitosan.[148,149] For example, spray-assisted layer-by-layer (LBL) assembly was employed to make an HA-based bilayer via of HA and positively charged PLL as an epidermal layer on a porous HA scaffold acting as a dermal layer.[150] Culturing keratinocytes showed that keratinocytes grew as a monolayer on the sprayed coating and did not invade to the dermal component.[150] This is necessary for the formation of an epidermal layer on the skin substitutes. The negative charge of HA can be utilized to enhance scaffold hydrophilicity and cell infiltration.[152] For example, the presence of HA in poly(ε-caprolactone) (PCL)–silk fibroin–HA electrospun scaffolds led to decreased collagen I deposition and suppressed macrophage adhesion as well as fibrosis of the scaffold after in vivo transplantation.[152] In summary, HA has a great potential for application in skin regeneration, especially when combined with other biomaterials.
2.6. Chitosan
CS is a linear polysaccharide derived from chitin, the second most abundant biopolymer after cellulose.[153] Amino groups in chitosan provide positive charges making it the only naturally occurring positively charged polysaccharide. This enables chitosan to interact with negatively charged species like GAGs and red blood cells. Terminal hydroxyl groups act as functionalization terminals for chitosan. Chitosan is an antibacterial, antifungal, mucoadhesive, analgesic, and hemostatic biomaterial that does not trigger inflammation after transplanting.[153,154] Chitosan degradation in vivo is mainly done through enzymatic degradation and lysozyme is the main enzyme responsible for its degradation.[155] Biodegradation products are nontoxic oligosaccharides of variable length. Chitosan is normally crosslinked to decrease its degradation rate.[153] All above mentioned properties make chitosan a potential biomaterial for tissue engineering. However, chitosan is poorly soluble in aqueous solutions except in acidic medium[153] which can limit the application of chitosan for 3D cell encapsulation. It is normally used with other biomaterials rather than in pure form.
Chitosan alone and in combination with other materials has been widely used for wound dressings[156–169] and skin substitutes.[148,149,170–174] It has been used in the form of both freeze-dried sponges[171,172] and hydrogels,[174] mainly in combination with other biomaterials like collagen, gelatin, and fibrin.[172] It is reported that cellularizing the scaffold with HDF and primary human hair follicle stem cells before transplanting in vivo leads to an organized epithelium or mature epidermis.[172,174] The presence of fibrin has been shown to enhance blood clotting and platelet activation, as well as promote collagen deposition.[172] It is also reported that, compared with pure chitosan films or sponges, electrospun chitosan membrane improves cell adhesion and proliferation in vitro and promotes blood vessel formation in vivo. While collagen deposition was observed in vivo for the electrospun membranes, chitosan sponges induced foreign body granuloma.[175]
2.7. Silk
Silk is another natural biomaterial with slow degradation rate,[176] suitable for skin tissue engineering, that can be obtained from either Bombyx mori cocoons or Nephila silk glands.[177] They can be used to make silk films, foams as well as woven or nonwoven scaffolds. It is shown that silk scaffolds support fibroblast and keratinocyte adhesion and proliferation.[178–184] They have also been used in combination with other biomaterials such as chitin[185,186] and chitosan.[183,187] It is reported that presence of 5% chitosan in the silk fibroin/HA scaffold improves angiogenesis and collagen deposition.[187] It is shown that silk dressings promote wound healing.[188,189] Keratin which is normally used for making skin dressing[190] has also been introduced into the silk scaffolds which led to improvement in HDF adhesion and proliferation.[191]
3. Synthetic Biomaterials
Synthetic polymers are generated in the lab from hydrocarbon building blocks. Though they may lack the inherent cell-interaction moieties present in naturally occurring biopolymers, their ability to be controlled precisely in terms of composition and reproducibility and be used as composites with naturally occurring biomaterials make them very interesting for skin regeneration applications. In the proceeding sections, synthetic biomaterials that have been used for skin substitute production are reviewed.
3.1. Poly(Lactic Acid) (PLA)
PLA is an aliphatic, biodegradable polyester derived from materials like rice and corn, approved by the US Food and Drug Administration (FDA) for many biomedical applications. Despite disadvantages including poor cell-interaction, slow degradation rate, shrinkage,[192] acidic (proinflammatory) degradation products, low elongation, and hydrophobicity, which can elicit an inflammatory response and impede some of its biomedical applications, PLA has been extensively employed for tissue engineering scaffold applications.[193] Lactic acid has two isomers: l-lactic acid (LLA) and d-lactic acid (DLA) which can be used to make four materials: crystalline PDLA, hemicrystalline PLLA, amorphous poly-DL-lactic acid (PDLLA), and meso-PLA. PLA scaffolds may be fabricated through processing techniques like gas foaming[194] and electrospinning.[192]
Pure[195] and modified[194] PLA has also been used to deliver cells to the wound site. However, due to its hydrophobicity that hinders cell attachment,[192] further modification of PLA is advantageous.[194,192] For example modification with RGD improves cells’ function on PLA and is shown to promote vascularization in a dermal wound model.[194] Also, the electrospun scaffold of a blend of PDLLA and poly(ethylene glycol) (PEG) showed improvement in HDF infiltration, decreased dimensional shrinkage, and a degradation pattern change from surface erosion to bulk degradation.[192] In summary, due to its earlier stated disadvantages, PLA has limited value as a material for skin substitutes unless incorporated as a modification to other biomaterials.
3.2. Poly(Lactic-co-Glycolic Acid) (PLGA)
PLGA is a biodegradable polyester copolymer that has also been used as a skin substitute scaffold material. PLGA produces lactic and glycolic acid upon degradation, and as is the case with PLA is known to be proinflammatory. Electrospinning has been one of the main methods of making PLGA scaffolds for skin regeneration.[196–201] Efforts to improve scaffold properties like cell viability, migration, and infiltration, as well as collagen deposition, have been pursued by making looser fibers and larger pores[199] or providing a heterogeneous environment (by hybrid electrospinning nano- and microfibers[202]). Also, cellularizing PLGA scaffold improves in vivo performance of the scaffolds. It is reported that PLGA knitted mesh seeded with HDF and human keratinocytes show comparable wound contraction with an autograft control, while acellular PLGA mesh results in larger wound contraction along with interyarn spaces with no cells.[203,204]
In summary, PLGA on its own, similar to PLA, has limited application for skin substitute. But as an additive, it can be a potential candidate to improve physical and mechanical characteristics of natural biomaterials.
3.3. Poly(ε-Caprolactone)
PCL is a polyester with slower degradation rate than PLA and PLGA. PCL has been used in numerous studies for skin tissue engineering since its first application in 2001.[205] Electrospinning is the main method used for making PCL scaffolds for skin regeneration, which allows for control over the fiber size and alignment. Faster wound closure is an important goal in wound healing. To pursue this, radially aligned PCL electrospun fibers seeded with dural fibroblasts. It was observed that the cells migrate faster along the fibers compared with randomly oriented fibers.[206] However, the potential for promoting differentiation of fibroblasts to myofibroblasts which can lead to scarring is not investigated. On the other hand, the role of keratinocyte attachment, proliferation, and spreading over the surface to close the wound is ignored. Pore size is another parameter of electrospun scaffolds which contributes significantly to their success in vivo. It is reported that pore size has a greater impact relative to fiber size on the cell proliferation.[207] Also, HDF cell can only bridge over pore size 6.5 μm while stretch along the fibers when the pore size is >20 μm.[207] Smaller pores give rise to faster ECM production while larger pores lead to better cell infiltration.[207] So there always should be a balance between these two phenomena based on different applications. In another study, the slow degradation rate of PCL was harnessed to battle wound contraction. In this work, degradable, elastomeric poly(l-lactide-co-ε-capro-lactone) (PLCL) copolymer electrospun scaffold with micrometer-sized fibers demonstrated decreased myofibroblast formation compared to an in vitro hypertrophic scar (HSc) contraction model (fibroblast populated collagen lattice assay) and led to significantly less HSc contraction compared with Integra in a validated immune-competent mouse model (Figure 4).[208] This observation highlights the crucial role of degradation rate and mechanical properties of skin substitute in scarring.
Figure 4.
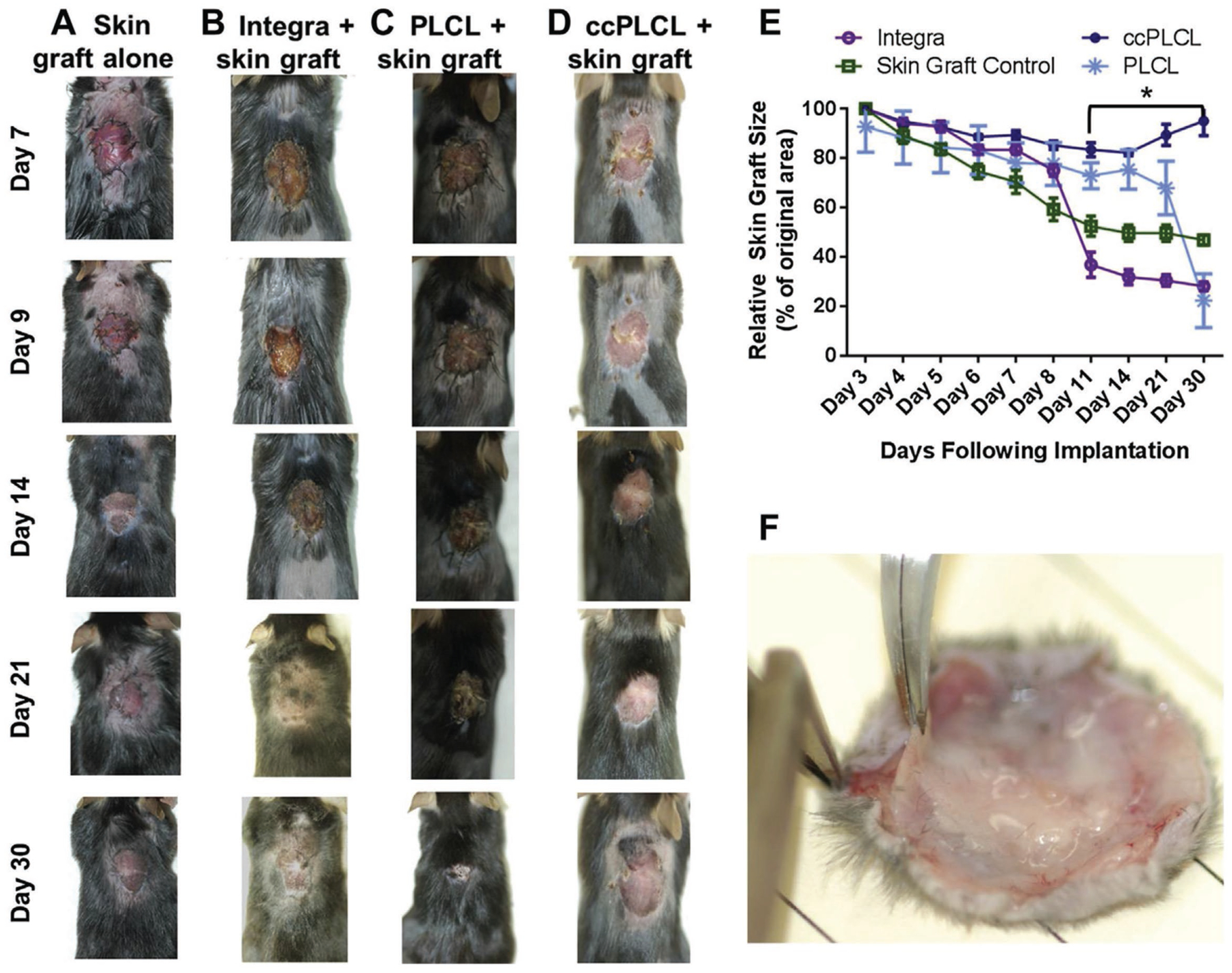
Wound contraction and scaffold incorporation at day 30. Gross appearance of wounds treated with A) skin graft alone. B) Integra beneath skin graft. C) PLCL scaffold. D) ccPLCL scaffold, at days 7, 9, 14, 21, and 30 following surgery. E) Wound contraction curves derived from measurements of wounds shown in (A–D). F) ccPLCL scaffolds beneath skin graft immediately following excision from wound bed on d30. Reproduced with permission.[208] Copyright 2017, Elsevier.
In summary, considering the slow degradation time of PCL and its acidic degradation products, it can be considered as an interesting option to modify natural biopolymers for a slower degradation and enhanced mechanical properties.
3.4. Poly(Ethylene Glycol)
As a synthetic biopolymer, PEG, has advantages over natural biopolymers such as control over structural and compositional properties; however, it lacks cell-interactive character due to its bio-inert nature.[209] Accordingly, it is normally modified through the addition of ECM peptide motifs or mixing with ECM biomaterials. For example poly(ethylene glycol-terephthalate)–poly (butylenes terephthalate) prepared by solvent casting-particulate leaching method was used as a dermal substitute in which the scaffold was seeded with dermal fibroblasts, and then fibroblast-populated collagen was used to fill the pores and prevent keratinocytes from infiltration to the scaffold.[210] In the last step, keratinocytes were seeded on the scaffold and formed fully differentiated stratified epidermis and basement membrane.
In summary, PEG as a blank template can be modified different moieties to pass different requirements of a skin substitute like cell adhesion, short-term degradation, and minimum inflammation. So it can be used to decouple the role of each of the mentioned factor on the skin substitute performance.
3.5. Polyurethane (PU)
PUs are a vast family of polymers with many established applications in biomedical fields. Polyurethanes have been used mainly for wound dressing fabrication.[211–214] Also, HydroDerm as a commercial PU has been used for keratinocyte delivery to the full thickness burn sites.[215,216] It can be employed to improve mechanical properties of natural biopolymers. It is shown that collagen coated electrospun PU (ccPU) results in less contraction and αSMA expression than a collagen scaffold. Also in a murine HSc model, the ccPU scaffold lead to significantly less HSc contraction than Integra.[217] In summary, like other synthetic biomaterials, PU is a good option to improve degradation and weak mechanical properties of natural biopolymers.
3.6. Self-Assembling Peptides
Self-assembling peptides are synthetic nano-biomaterials that self-assemble to different structures in response to a change in pH, temperature or ionic concentration. These materials are composed of amino acids which are found all over in the body and can be synthesized precisely in the lab.[218] Self-assembling peptides have also been used for wound dressing fabrication[219,220] and skin substitute application.[221] It was shown that functionalizing RADA16 peptide with a collagen I amino acid motif enhances skin keratinocyte and fibroblast proliferation and migration.[221] Self-assembling peptide hydrogels tend to have low mechanical properties compared to other biomaterials which limit their application for skin substitutes.
4. Natural and Synthetic Composite Scaffolds
Using a unique combination of both natural and synthetic materials to fabricate scaffolds for tissue engineering has been explored by many investigators as a way to exploit the inherent biocompatibility of natural materials while compensating for their lack of sufficient mechanical strength. Using different formulations of synthetic versus natural components also allows for more control over degradation rate and chemistry. In the following section, common scaffolds made of composites of natural and synthetic materials will be reviewed and evaluated.
4.1. Collagen/Synthetic Composites
Synthetic biomaterials can be used to improve mechanical properties and degradation rate of collagen scaffolds. PCL is one of the biomaterials that is used in many cases in combination with collagen for skin regeneration[96,222] (Table 3). PCL–collagen scaffolds can be fabricated through impregnation of freeze-dried collagen mats in PCL solution,[223] coating collagen–PCL electrospun membranes with collagen gel to form a secondary network of finer fibers,[224] and electrospinning a collagen–PCL blend[97] or core–shell electrospinning.[225] Fibroblasts,[225] keratinocytes,[226] and bone-marrow mesenchymal stem cells[97] have been cultured on the collagen–PCL scaffolds. It is reported that PCL (core)–collagen (shell) electrospun scaffolds show a better HDF proliferation than pure PCL and collagen-coated PCL fibers.[225] This was attributed to a higher availability of collagen binding sites for the cells. It has been shown that immobilization of epidermal growth factor on PCL–collagen fibers enhanced differentiation ability of keratinocytes in vitro.[226] Moreover, the presence of an ultrafine collagen fibrous network within a collagen–PCL membrane significantly enhanced cell migration through activation of integrin-β1.[224] Electrospun scaffolds can be further modified by introducing large micropores into them mechanically.[99,100] The presence of micropores was shown to enhance HDF infiltration, support keratinocytes proliferation, and differentiation, and help a faster wound closure and dermal regeneration with the more blood vessel formation and hair follicle development.[99]
Table 3.
Collagen Composite Scaffolds.
| Biomaterial | Fabrication method | Cell(s) | Performance | Ref. |
|---|---|---|---|---|
| Collagen–PCL | Electrospinning + PCL coating | HDF | Supports HDF attachment and proliferation | [222] |
| Collagen–PCL | Electrospinning + EDC crosslinking | HDF, HEK | No significant change in mechanical properties. Worse cell viability, lack of basal cell layer and poor epidermal formation at some PCL concentrations | [96] |
| Collagen–PCL | Electrospinning (non-cross-linked) + micro pores | Neonatal foreskin fibroblast and keratinocyte | Faster wound closure and dermal regeneration with more blood-vessel formation and hair follicle development | [99] |
| Collagen–PCL | Electrospinning (non-cross-linked) + micro pores | DF | Pre-seeded scaffolds with fibroblasts promoted greater wound-healing than acellular scaffolds | [100] |
| collagen/PLLA-co-PCL | Electrospinning (non-crosslinked) | BM-MSCs | Promote differentiation of BM-MSCs to keratinocyte-like cell | [97] |
| Collagen–PCL | Electrospinning + EDC crosslinking | acellular | Induced inflammatory response in vivo. Neutrophils destroyed the scaffold and invaded the surrounding dermis. Keratinocytes did not grow in the scaffold and rather grew under the scaffold | [98] |
| PLLA/collagen PLLA/gelatin | Freeze-drying on PLLA woven mesh | HDF | Improved HDF behavior in vitro. Less wound contraction than collagen sponge in vivo | [232] |
| PLGA–collagen | Freeze-drying on PLGA woven mesh | HDF | Enhanced skin fibroblast distribution. Dermal tissues were formed after 2 weeks and became epithelialized after 4 weeks | [230] |
| PLGA–collagen | Coating PLGA mesh | Fibroblast, keratinocyte | Enhanced blood vessel formation and epithelialization on rabbits | [231] |
| PLGA–collagen | Electrospinning | HDF | Improved HDF attachment, proliferation and extracellular matrix deposition | [200] |
| PLGA–collagen | Electrospinning | BM-MSCs | Faster epithelialization on mouse | [228] |
| PCL–collagen | Freeze-drying + Solvent casting | 3T3, NHEK | The composite films support growth of HDF and NHEK | [223] |
| PCL–collagen | Core-shell Electrospinning | HDF | Better HDF proliferation than pure PCL and collagen-coated PCL fibers | [225] |
| PCL/collagen-collagen | Electrospinning + collagen gel coating | Human keratinocyte | Presence of ultrafine collagen fibrous network significantly enhance keratinocyte migration | [224] |
| PCL–collagen | Electrospinning | HDF | Aligned fibers promoted elongated cell morphology, accelerated migration and differentiated to myofibroblasts | [227] |
Modification of collagen scaffolds with PCL sometimes fails to enhance the viability of cells seeded on the scaffold[96] and also may promote an inflammatory response.[98] Also, it was shown that membranes with aligned fibers of PCL–collagen (made by layer-by-layer method) seeded with HDFs promoted cell alignment and accelerated migration along the fibers, exhibiting enhanced differentiation to the myofibroblast phenotype.[227]
Collagen has also been used in combination with PLGA either through blend electrospinning[200,228,229] or through the formation of a secondary scaffold in between PLGA knitted mesh to improve scaffold strength.[230,231] The presence of collagen sponges in the PLGA structure was shown to enhance skin fibroblast distribution.[230] It was further reported that such scaffolds can promote the formation of an epithelium in 2–4 weeks.[230,231]
In summary, PCL, PLLA, and PLGA are the main synthetic biomaterials have been added to collagen. Presence of these materials in combination with collagen have been utilized to improve mechanical properties, degradation rate, shrinking, and in vivo contraction of the collagen.
4.2. Gelatin/Synthetic Composites
PCL,[233–235] dextran,[236] and PLLA[232] are among the biomaterials that have been used in combination with gelatin to make skin substitutes (Table 4). Due to gelatin’s poor mechanical strength (which sometimes leads to scaffold damage during engraftment by surgeons) and its quick degradation rate, PCL is commonly added to gelatin to make a scaffold for wound healing and skin tissue engineering. Both of these materials have the potential for electrospinning.[233–235] Coaxial electrospinning can be used to improve the mechanical properties of gelatin by formulating a PCL core and a gelatin shell to provide the excellent cell-interaction offered by pure gelatin fibers.[233] Another way of strengthening gelatin scaffolds is forming gelatin sponge on PLLA woven mesh. This has been shown to decrease wound contraction.[232]
Table 4.
Gelatin Composite Scaffolds.
| Biomaterial | Fabrication method | Cell(s) | Performance | Ref. |
|---|---|---|---|---|
| Gelatin–PCL | Core-shell electrospinning + EDC crosslinking | HDF, HEK | Promotes cell adhesion and metabolism as well as dermal/epidermal layer stratification to a level equivalent to pure gelatin fibers while offering significantly improved mechanical properties | [233] |
| Gelatin–PCL | Electrospinning (non-crosslinked) | GFP-mouse fibroblast, HaCaT cells | Phase separation during electrospinning affects the GFP-mouse fibroblasts and HaCaT cells adhesion and proliferation | [235] |
| Gelatin–dextran, PCL–Poloxamer (bilayer) | Gelation, Electrospinning | ADSCs | The bilayer scaffold supports cell viability | [236] |
| GelMA | Cell-laden + UV crosslinking | HaCaT | Controlling hydrogel stiffness, improved degradation, stratification of keratinocytes, decreased water loss rate and enhanced electrical resistance vs collagen scaffold | [116] |
In another study, gelatin methacrylamide (GelMA) as a photo-crosslinkable hydrogel was used to encapsulate keratinocytes for epidermal reconstruction.[116] Employing HaCaT cell-laden GelMA provided controlling hydrogel stiffness, prolonged degradation of the scaffold in collagenase solutions, stratification of keratinocytes, decreased water loss rate, and enhanced electrical resistance compared with the control collagen scaffold (Figure 5).
Figure 5.

Expression of proteins of reconstructed epidermis on hydrogel scaffolds. Examples dermis thickness exists. Furthermore, the of ki 67 (red, proliferation maker), involucrin (green, differentiation marker), and DAPI (blue, hydrogel nature endows GelMA with ready nuclei) stained sections of reconstructed epidermis on A) GelMA and B) collagen scaffolds modification of its chemical and physical after 2 weeks (i) and 6 weeks (ii) of culture at ALI and C) human epidermis. Scale bar = 100 μm. properties (e.g., incorporation or conjugation D) Quantifi cation of the number of epidermis layers of the reconstructed epidermis at different times of culture at ALI and human epidermis. Reproduced permission.[116]
So similar to the collagen, synthetic polymers have been employed mainly to compensate low mechanical properties and fast degradation of gelatin scaffolds.
4.3. Chitosan/Synthetic Composites
PLGA,[196] poly(vinyl alcohol) (PVA),[196,237] HA,[238] PCL,[238–240] PLA,[241,242] and collagen[243] are some of the materials have been used in combination with chitosan. Electrospinning has been one of the major methods for fabrication of chitosan composite skin substitutes. Coelectrospinning and coaxial electrospinning of PLGA/chitosan membranes improve cell adhesion and viability of PLGA membranes.[244,245] The positive charge of chitosan can be exploited for mixing with negatively charged polymers in a LBL method. HA can be an option due to its negatively charged nature. Toward this end, chitosan and HA were coated on PCL using the LBL method and showed support of keratinocyte attachment and growth.[238] It has also been shown that adding chitosan to PCL electrospun fibers increases the hydrophilicity of the scaffold and enhances cell adhesion, spreading, and proliferation.[239,240]
4.4. Dextran/Synthetic Composites
Dextran is another polysaccharide that has been used for wound healing[246–249] and skin reconstruction.[4,28,236,250,251] It can be electrospun[250] (for example, in a blend with PLGA[250]) or used to make a composite synthetic hydrogel. Dextran scaffolds are normally crosslinked using photocrosslinking the dextran[250] or the other component of the composite like PEGDA.[4] A dextran-based material, dextran-allyl isocyanate-ethylamine, blended with PEGDA polymer and UV crosslinked to form a hydrogel.[4] The hydrogel facilitated early inflammatory cell infiltration leading to degradation of the scaffold, which promoted endothelial cell infiltration to the hydrogel. This led to neovascularization by day 7 (Figure 6), forming a mature epithelial layer with hair follicles and sebaceous glands by day 21 and promoting new hair growth after 5 weeks. When tried on the porcine third-degree burn, the scaffolds promoted rapid wound closure, better reepithelialization as well as enhanced extracellular matrix remodeling and nerve reinnervation.[28]
Figure 6.
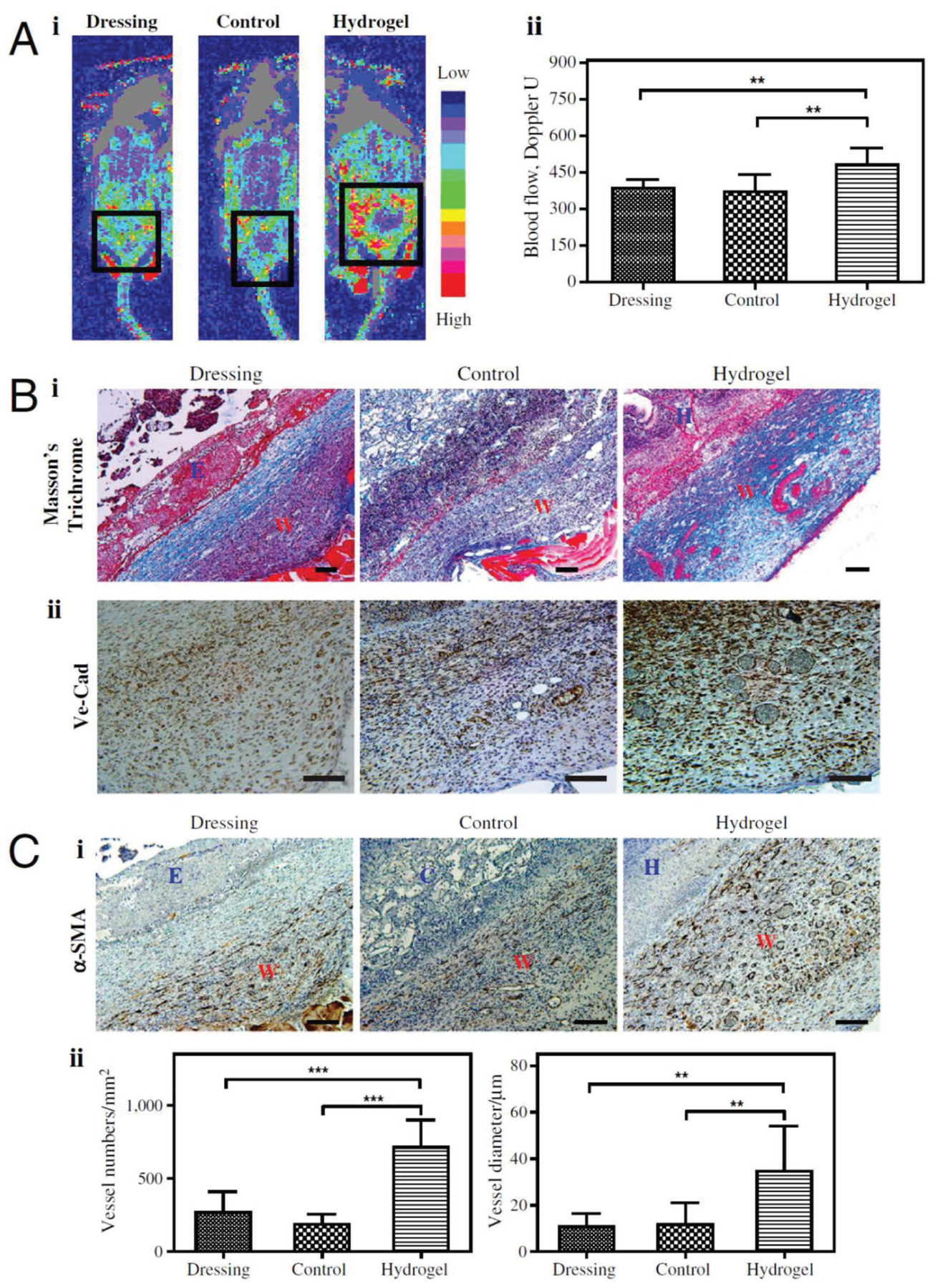
Angiogenic response in day 7. A) Doppler images of angiogenic response to wound injuries (i), and quantification (ii).The square indicates the wound area under Doppler. B) Masson’s staining (i) and VE-Cad staining (ii) of wound sites. Collagen layers were formed on the control (untreated) wounds, whereas no such layers formed on control scaffold-treated and hydrogel-treated wounds by day 7; we observed functional blood cells in the hydrogel-treated wounds. C) Photo of α-SMA staining (i) and quantification based on α-SMA staining (ii) of the wound areas. W, wound area; E, eschar; H, hydrogel scaffold; D, dressing; C, control scaffold. Significance levels were set at: *p < 0.05,**p < 0.01, and ***p < 0.001. Values shown are means ± SD. Scale bars, 100 μm. Reproduced permission.[4] Copyright 2017, National Academy of Sciences.
4.5. Pullulan/Synthetic Composites
Pullulan is a neutral, linear, antioxidant, biodegradable, and nontoxic polysaccharide produced by the fungus Aureoba-sidium pullulans.[252–254] Pullulan can be further crosslinked and functionalized through its hydroxyl functional groups. Pullulan scaffolds are most commonly fabricated via solvent casting and porogen leaching followed by sodium trimetaphosphate (STMP) crosslinking. They are normally used in combination with collagen or gelatin in order to enhance their cell-interaction.[252–255] A pullulan–collagen scaffold has been shown to be able to deliver MSCs in vivo while the scaffold protects MSCs from the harsh conditions in the high-oxidative-stress wounds and leads to better cell viability and significantly enhanced wound healing and angiogenesis.[253] Recently, a pullulan-gelatin (PG-1) scaffold fabricated via solvent casting and porogen leaching seeded with fibroblasts and keratinocytes was used to form a bilayer skin substitute and transplanted in vivo (Figure 7).[254] Application of the epidermal-dermal PG-1 led to a significantly thicker neo-dermis formation after 14 d in vivo as well as reduced macrophage infiltration while supporting increased angiogenesis. While pullulan is still in the early stages of the investigation, its unique anti-inflammatory properties confer it with much potential for future application in wound healing.
Figure 7.

Scanning electron microscopic images of pullulan-gelatin hydrogel at 27× magnification (left) and 75× magnification (right). Reproduced permission.[254] Copyright 2017, Mary Ann Liebert, Inc.
5. Vascularization in Skin Substitutes
Vascularization is an important qualifier of any skin substitute for successful take. This relies on the vasculogenesis within the scaffold and sprouting capillaries from the adjacent tissue.[256] Skin grafts from donor sites will integrate to the wound bed faster due to the presence of blood vessel residues.[256] This highlights the important role of prevascularization and to a lesser amount precellularizing the skin substitutes.[123] Fibrin is involved in angiogenesis,[256] stimulating vascularization,[256,131] and accelerating graft revascularization due to improved adherence to the wound bed when used as glue.[257] Normally, infiltration of the inflammatory cells such as macrophages into the scaffold causes degradation of the scaffold and promotes endothelial cell infiltration to the hydrogel.[4] This will facilitate vascularization in the scaffold. However, there is no strong evidence of excellence in vascularization for a specific biomaterial compared to others. In the meantime several strategies have been recruited for prevascularization of the skin substitutes[138,258] or to enhance their vascularization in vivo[259] which mainly include employing endothelial[90,138,194,260,261] as well as adipose-derived stem cells,[261] manipulating the scaffold’s pore size[99] and using angiogenic growth factors.[258] As a rule of thumb, larger pore size leads to an improved vascularization; however, it should be considered that cells cannot bridge the pores and fill them with ECM if the pore sizes are too large.[99,254] Considering the very harsh condition of burn wounds, growth factors may not last long to be effective. So works on the scaffold biomaterial, pore size and using the cells that promote angiogenesisshould be considered for future research.
6. Conclusion
Despite the wide range of available materials, many of which have been reviewed here, only collagen, gelatin, and chitosan have been used extensively in the laboratories or for commercial products. Nevertheless, substantial complications such as wound contraction, insufficient vascularization, scar formation,[262] and high cost are associated with these products. In Table 5, the main reviewed biomaterials are summarized and evaluated based on their performance as skin substitutes in vitro, in vivo and in the clinic, and their corresponding pros and cons are highlighted. As can be seen in the table, mainly natural biomaterials have been investigated in vivo and led to clinical products which can be due to their higher biocompatibility and similarity to the native skin. However, natural polymers suffer from low mechanical properties and fast degradation which impede their application even in the clinic. In contrast, the majority of synthetic biomaterials have good mechanical and physical properties (which can be controlled during production), with less ideal cell interaction and very long degradation rate compared with natural biomaterials. So, most of the aforementioned problems can be addressed through intelligently designing composite biomaterials containing both natural and synthetic polymers. From a material point of view, among different natural biomaterials, gelatin is the least expensive option with a fair similarity to collagen as the main component of skin. However, due to its low mechanical properties, there is no reported clinical outcome so far. So as an example, a mixture of an elastic and biodegradable synthetic biomaterial with gelatin can be a potential candidate for a successful and economical skin substitute that can eliminate drawbacks of currently available substitutes on the market. In order to follow with similar proposed solutions, further advancements in materials design and development, cell biology of skin and in situ fabrication technologies like 3D printing or cell electrospinning is necessary. The interdisciplinary approaches are essential for tissue regeneration and skin regeneration is not an exception. It needs the close partnership of biomaterial scientists, mechanical and microfluidic scientists, cell biologists and clinicians. Research institutes need to pave this forms of partnership/collaboration and funding agencies may allocate funds for this multidisciplinary approaches. This review calls for the need to expand multidisciplinary research on the use of biomaterials for skin substitutes.
Table 5.
Comparison of reviewed biomaterials.
| Material | In vitroa) | In vivo | Clinical | Pros | Cons | Cell adherence | Nontoxic, noninflammatory | Biodegradable | Mechanical properties | Cost-effective | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Application | Products | ||||||||||
| Collagen | *** | *** | *** | Integra, OrCel, Promogran, PurAply | Highest similarity to dermis ECM | Needs crosslinking, expensive, acidic pH of aqueous solutions | *** | *** | *** | ** | * |
| Gelatin | ** | ** | – | Cheap, very similar structure to collagen fibers in dermis | Needs crosslinking, forms gel at physiologic temperature | *** | *** | *** | ** | *** | |
| Elastin | ** | ** | * | As a modification for collagen in MatriDerm | Mimicking elastic fibers in native dermis | Needs crosslinking, expensive | *** | *** | *** | ** | * |
| Fibrin | ** | ** | * | As tissue glue or sealant (like TISSEEL, EVICEL, Beriplast) and for cell spraying | Possibility to have autologous fibrin toward preventing immune response | Needs crosslinking, significant shrinkage, hard to control its structure, weak mechanical properties, fast degradation, expensive | *** | *** | *** | * | * |
| HA | ** | ** | ** | HYAFF, Laserskin, Hayalomatrix | Mimicking HA present in native dermis | Needs crosslinking, weak mechanical properties, expensive | *** | *** | *** | * | * |
| PLA | * | * | – | Reproducible physical and mechanical properties | Slow degradation rate, acidic degradation products, low elongation, hydrophobicity | ** | ** | ** | *** | *** | |
| PLGA | * | * | – | Faster degradation rate than PLA, reproducible physical and mechanical properties | Acidic degradation products | ** | ** | ** | *** | *** | |
| PCL | ** | * | – | Reproducible physical and mechanical properties | Slower degradation rate (even than PLA and PLGA) | ** | ** | ** | *** | *** | |
| PEG | * | – | – | Reproducible physical and mechanical properties | No cell interaction, very slow degradation | * | ** | ** | ** | *** | |
| PU | * | * | – | Reproducible physical and mechanical properties, high elasticity | Slow degradation | ** | ** | ** | *** | *** | |
| SA Peptides | * | * | – | Reproducible physical and mechanical properties | weak mechanical properties, expensive | ** | *** | ** | * | * | |
| Chitosan | ** | * | – | Antibacterial, antifungal, mucoadhesive, analgesic and hemostatic, cheap | Needs crosslinking, | ** | *** | *** | ** | *** | |
| Dextran | * | * | – | Needs crosslinking, expensive | ** | ** | *** | ** | *** | ||
| Pullulan | * | * | – | Antioxidant, biodegradable | Weak cell interaction, Needs crosslinking, | * | ** | *** | ** | *** | |
Frequency of application (in vitro or in vivo) and biomaterial score in different criteris are shown with a range from low (*) to high (***).
Acknowledgements
The authors appreciate funding from Canadian Institutes of Health Research (CIHR-MGJ), National Institutes of Health (NIH-MGJ), and a donation from Toronto Hydro (MGJ and SAN) and EMHSeed award (MGJ and SAN) as well as Natural Sciences and Engineering Research Council of Canada (NSERC-MEW) to carry out this research.
Biographies

Mohammadali Sheikholeslam is a post-doctoral fellowat Sunnybrook Research Institute, University of Toronto in Canada. He holds a Ph.D. degree (2015) in Chemical Engineering (Nanotechnology) from University of Waterloo in Canada. In Waterloo he worked on the application of self-assembling peptides and carbon nanotubes for biosensor development and tissue engineering and cancer research. His research at Sunnybrook and in collaboration with researchers at IBBME (University of Toronto) is in the field of translational tissue engineering and devoted to developing biodegradable skin substitutes especially for burn patients.

Marc G. Jeschke has been caring for burn patients for nearly 20 years and is a global leader in burn care, research, and education. Dr. Jeschke was appointed Director of the Ross Tilley Burn Centre at Sunnybrook Health Sciences Centre in 2010. He is a Surgeon Scientist and Professor in the Departments of Surgery and Immunology at the University of Toronto.

Saeid Amini-Nik is an Assistant Professor in the department of surgery and the department of lab medical pathobiology at the University of Toronto and a junior scientist at Sunnybrook Research Institute. After receiving his M.D., he received a Ph.D. in cancer biology from the University of Leuven in Belgium and accomplished postdoctoral training in the SickKids hospital in Toronto. During his fellowship, he generated several transgenic animals to verify and characterize the cell of origin during skin healing. At Sunnybrook, Dr. Amini-Nik focused on skin healing and regeneration by employing several different biological and tissue engineering approaches in order to manufacture/3D print of skin.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Mohammadali Sheikholeslam, Sunnybrook Research Institute, University of Toronto, Toronto, ON, Canada; Department of Surgery, Division of Plastic and Reconstructive Surgery, University of Toronto, Toronto, ON, Canada.
Meghan E. E. Wright, Institute of Biomaterials & Biomedical Engineering, University of Toronto, Toronto, ON, Canada
Marc G. Jeschke, Sunnybrook Research Institute, University of Toronto, Toronto, ON, Canada Department of Surgery, Division of Plastic and Reconstructive Surgery, University of Toronto, Toronto, ON, Canada; Institute of Medical Science, University of Toronto, Toronto, ON, Canada.
Saeid Amini-Nik, Sunnybrook Research Institute, University of Toronto, Toronto, ON, Canada; Department of Surgery, Division of Plastic and Reconstructive Surgery, University of Toronto, Toronto, ON, Canada; Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, ON, Canada.
References
- [1].Atiyeh BS, Gunn SW, Hayek SN, World J Surg. 2005, 29, 131. [DOI] [PubMed] [Google Scholar]
- [2].Blais M, Parenteau-Bareil R, Cadau S, Berthod FB, Stem Cells Transl. Med 2013, 2, 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nicholas MN, Jeschke MG, Amini-Nik S, Cell. Mol. Life Sci 2016, Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sun G, Zhang X, Shen Y, Sebastian R, Dickinson LE, Fox-talbot K, Reinblatt M, Steenbergenc C, Harmonb JW, Gerecht S, Proc. Natl. Acad. Sci. USA 2011, 108, 20976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bielefeld KA, Amini-Nik S, Alman BA, Cell. Mol. Life Sci 2013, 70, 2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Metcalfe AD, Ferguson MWJ, Biomaterials 2007, 28, 5100. [DOI] [PubMed] [Google Scholar]
- [7].Jayarama Reddy V, Radhakrishnan S, Ravichandran R, Mukherjee S, Balamurugan R, Sundarrajan S, Ramakrishna S, Wound Repair Regen. 2013, 21, 1. [DOI] [PubMed] [Google Scholar]
- [8].Arno AI, Amini-Nik S, Blit PH, Al-Shehab M, Belo C, Herer E, Tien C, Jeschke MG, Stem Cell Res. Ther 2014, 5, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schulz III JT, Tompkins RG, Burke JF, Annu. Rev. Med 2000, 51, 231. [DOI] [PubMed] [Google Scholar]
- [10].Bakhtyar N, Jeschke MG, Mainville L, Herer E, Amini-Nik S, Front. Physiol 2017, 8, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Arno AI, Jeschke MG, Wound Repair Regen 2014, 22, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Abdullahi A, Amini-Nik S, Jeschke MG, Cell. Mol. Life Sci 2014, 71, 3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Amini-Nik S, Yousuf Y, Jeschke MG, Adv. Drug Delivery Rev 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].LeBleu VS, MacDonald B, Kalluri R, Exp. Biol. Med 2007, 232, 1121. [DOI] [PubMed] [Google Scholar]
- [15].Viswanathan P, Guvendiren M, Chua W, Telerman SB, Liakath-Ali K, Burdick JA, Watt FM, Integr. Biol 2016, 8, 21. [DOI] [PubMed] [Google Scholar]
- [16].Parkinson LG, Rea SM, Stevenson AW, Wood FM, Fear MW, Tissue Eng. Part A 2012, 18, 703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bielefeld KA, Amini-Nik S, Whetstone H, Poon R, Youn A, Wang J, Alman BA, J. Biol. Chem 2011, 286, 27687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tracy LE, Minasian R. a., Caterson EJ, Adv. Wound Care 2016, 5, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Amini-Nik S, Cambridge E, Yu W, Guo A, Whetstone H, Nadesan P, Poon R, Hinz B, Alman BA, J. Clin. Invest 2014, 124, 2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shah A, Amini-Nik S, Int. J. Mol. Sci 2017, 18, 1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Amini-Nik S, Glancy D, Boimer C, Whetstone H, Keller C, Alman BA, Stem Cells 2011, 29, 1371. [DOI] [PubMed] [Google Scholar]
- [22].Jeschke MG, Sadri A-R, Belo C, Amini-Nik S, Tissue Eng., Part C 2017, 23, 237. [DOI] [PubMed] [Google Scholar]
- [23].Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF, Proc. Natl. Acad. Sci. USA 1989, 86, 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].MacNeil S, Mater. Today 2008, 11, 26. [Google Scholar]
- [25].Böttcher-Haberzeth S, Biedermann T, Reichmann E, Burns 2010, 36, 450. [DOI] [PubMed] [Google Scholar]
- [26].Kamel RA, Ong JF, Eriksson E, Junker JPE, Caterson EJ, J. Am. Coll. Surg 2013, 217, 533. [DOI] [PubMed] [Google Scholar]
- [27].Chua AWC, Khoo YC, Tan BK, Tan KC, Foo CL, Chong SJ, Burns Trauma 2016, 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shen Y, Song HG, Papa AE, Burke JA, Volk SW, Gerecht S, J. Invest. Dermatol 2015, 135, 2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Clark RAF, Ghosh K, Tonnesen MG, J. Invest. Dermatol 2007, 127, 1018. [DOI] [PubMed] [Google Scholar]
- [30].Metcalfe AD, Ferguson MWJ, Soc JR. Interface 2007, 4, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].MacNeil S, Nature 2007, 445, 874. [DOI] [PubMed] [Google Scholar]
- [32].Mansbridge J, J. Biomater. Sci. Polym. Ed 2008, 19, 955. [DOI] [PubMed] [Google Scholar]
- [33].Shevchenko RV, James SL, James SE, Soc JR. Interface 2010, 7, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Huang S, Fu X, J. Controlled Release 2010, 142, 149. [DOI] [PubMed] [Google Scholar]
- [35].Annabi N, Nichol JW, Zhong X, Ji C, Koshy S, Khademhosseini A, Dehghani F, Tissue Eng., Part B 2010, 16, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Groeber F, Holeiter M, Hampel M, Hinderer S, Schenke-Layland K, Adv. Drug Delivery Rev 2011, 63, 352. [DOI] [PubMed] [Google Scholar]
- [37].Wong VW, Levi B, Rajadas J, Longaker MT, Gurtner GC, Int. J. Biomater 2012, 2012, 926059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yildirimer L, Thanh NTK, Seifalian AM, Trends Biotechnol. 2012, 30, 638. [DOI] [PubMed] [Google Scholar]
- [39].Bi H, Jin Y, Burns Trauma 2013, 1, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pereira R, Barrias C, Granja P, Bartolo P, Nanomedicine 2013, 8, 603. [DOI] [PubMed] [Google Scholar]
- [41].Hu MS, Maan ZN, Wu J-C, Rennert RC, Hong WX, Lai TS, Cheung ATM, Walmsley GG, Chung MT, McArdle A, Longaker MT, Lorenz HP, Ann. Biomed. Eng 2014, 42, 1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Madaghiele M, Sannino A, Ambrosio L, Demitri C, Burns Trauma 2014, 2, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sun BK, Siprashvili Z, Khavari PA, Science 2014, 346, 941. [DOI] [PubMed] [Google Scholar]
- [44].Vyas K, Vasconez H, Healthcare 2014, 2, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Varkey M, Ding J, Tredget E, J. Funct. Biomater 2015, 6, 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Debels H, Hamdi M, Abberton K, Morrison W, Plast. Reconstr. Surg. Glob. Open 2015, 3, e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chandika P, Ko SC, Jung WK, Int. J. Biol. Macromol 2015, 77, 24. [DOI] [PubMed] [Google Scholar]
- [48].Norouzi M, Boroujeni SM, Omidvarkordshouli N, Soleimani M, Adv. Healthcare Mater 2015, 4, 1114. [DOI] [PubMed] [Google Scholar]
- [49].Drury JL, Mooney DJ, Biomaterials 2003, 24, 4337. [DOI] [PubMed] [Google Scholar]
- [50].Amini-Nik S, Kraemer D, Cowan ML, Gunaratne K, Nadesan P, Alman BA, Miller RJD, PLoS One 2010, 5, e13053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chattopadhyay S, Raines RT, Biopolymers 2014, 101, 821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ruszczak Z, Adv. Drug Delivery Rev 2003, 55, 1595. [DOI] [PubMed] [Google Scholar]
- [53].Waller JM, Maibach HI, Ski Res. Technol 2006, 12, 145. [DOI] [PubMed] [Google Scholar]
- [54].Cen L, Liu WEI, Cui LEI, Zhang W, Cao Y, L RSW, People S, Tong SJ, Pediatr. Res 2008, 63, 492. [DOI] [PubMed] [Google Scholar]
- [55].Badylak SF, Transplant Immunol. 2004, 12, 367. [DOI] [PubMed] [Google Scholar]
- [56].Olsen D, Adv. Drug Delivery Rev 2003, 55, 1547. [DOI] [PubMed] [Google Scholar]
- [57].Chandika P, Ko S, Oh G, Heo S, Nguyen V-T, Jeon Y-J, Lee B, Jang CH, Kim G, Park WS, Chang W, Choi I, Jung W, Int. J. Biol. Macromol 2015, 81, 504. [DOI] [PubMed] [Google Scholar]
- [58].Powell H, Supp D, Boyce S, Biomaterials 2008, 29, 834. [DOI] [PubMed] [Google Scholar]
- [59].Michael S, Sorg H, Peck C-T, Koch L, Deiwick A, Chichkov B, Vogt PM, Reimers K, PLoS One 2013, 8, e57741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chen PY, Hsieh HJ, Huang LLH, J. Biomed. Mater. Res., Part A 2014, 102, 4581. [DOI] [PubMed] [Google Scholar]
- [61].Dagalakis N, Flink J, Stasikelis P, Burke JF, Yannas IV, J. Biomed. Mater. Res 1980, 14, 511. [DOI] [PubMed] [Google Scholar]
- [62].Barnes CP, Pemble CW, Brand DD, Simpson DG, Bowlin GL, Tissue Eng. 2007, 13, 1593. [DOI] [PubMed] [Google Scholar]
- [63].Meng L, Arnoult O, Smith M, Wnek GE, J. Mater. Chem 2012, 22, 19412. [Google Scholar]
- [64].Seo YK, Youn HH, Park CS, Song KY, Park JK, Biotechnol. Bioprocess Eng 2008, 13, 745. [Google Scholar]
- [65].Yannas IV, Burke JF, J. Biomed. Mater. Res 1980, 14, 65. [DOI] [PubMed] [Google Scholar]
- [66].Yannas IV, Burke JF, Gordon PL, Huang C, Rubenstein RH, J. Biomed. Mater. Res 1980, 14, 107. [DOI] [PubMed] [Google Scholar]
- [67].Maugh T, Science 1982, 217, 522. [DOI] [PubMed] [Google Scholar]
- [68].Tzeranis DS, Soller EC, Buydash MC, So PTC, Yannas IV, Ann. Biomed. Eng 2016, 44, 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Soller EC, Tzeranis DS, Miu K, So PTC, Yannas IV, Biomaterials 2012, 33, 4783. [DOI] [PubMed] [Google Scholar]
- [70].Boyce ST, Christianson DJ, Hansbrough JF, J. Biomed. Mater. Res 1988, 22, 939. [DOI] [PubMed] [Google Scholar]
- [71].Harriger MD, Supp AP, Warden GD, Boyce ST, J. Biomed. Mater. Res 1997, 35, 137. [DOI] [PubMed] [Google Scholar]
- [72].Powell HM, Boyce ST, Biomaterials 2006, 27, 5821. [DOI] [PubMed] [Google Scholar]
- [73].Powell HM, Boyce ST, Biomaterials 2007, 28, 1084. [DOI] [PubMed] [Google Scholar]
- [74].Park SN, Lee HJ, Lee KH, Suh H, Biomaterials 2003, 24, 1631. [DOI] [PubMed] [Google Scholar]
- [75].Wang W, Zhang M, Lu W, Ph D, Yu C, Ph D, Jin Y, Ph D, Tissue Eng., Part C 2010, 16, 269. [DOI] [PubMed] [Google Scholar]
- [76].Wang HM, Chou YT, Wen ZH, Wang ZR, Chen CH, Ho ML, PLoS One 2013, 8, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lamme EN, de Vries HJ, van Veen H, Gabbiani G, Westerhof W, Middelkoop E, J. Histochem. Cytochem 1996, 44, 1311. [DOI] [PubMed] [Google Scholar]
- [78].Lamme EN, Van Leeuwen RTJ, Jonker A, Van Marle J, Middelkoop E, J. Invest. Dermatol 1998, 111, 989. [DOI] [PubMed] [Google Scholar]
- [79].Hafemann B, Ensslen S, Erdmann C, Niedballa R, Zühlke A, Ghofrani K, Kirkpatrick CJ, Burns 1999, 25, 373. [DOI] [PubMed] [Google Scholar]
- [80].Berthod F, Germain L, Li H, Xu W, Damour O, Auger FA, Matrix Biol. 2001, 20, 463. [DOI] [PubMed] [Google Scholar]
- [81].Keck M, Haluza D, Lumenta DB, Burjak S, Eisenbock B, Kamolz LP, Frey M, Burns 2011, 37, 626. [DOI] [PubMed] [Google Scholar]
- [82].Attia-Vigneau J, Terryn C, Lorimier S, Sandre J, Antonicelli F, Hornebeck W, J. Invest. Dermatol 2014, 134, 58. [DOI] [PubMed] [Google Scholar]
- [83].Hafemann B, Ghofrani K, Gattner HG, Stieve H, Pallua N, J. Mater. Sci. Mater. Med 2001, 12, 437. [DOI] [PubMed] [Google Scholar]
- [84].Klein B, Schiffer R, Hafemann B, Klosterhalfen B, Zwadlo-Klarwasser G, J. Mater. Sci. Mater. Med 2001, 12, 419. [DOI] [PubMed] [Google Scholar]
- [85].Li H, Berthod F, Xu W, Damour O, Germain L, a Auger F, J. Surg. Res 1997, 73, 143. [DOI] [PubMed] [Google Scholar]
- [86].Kellouche S, Martin C, Korb G, Rezzonico R, Bouard D, Benbunan M, Dubertret L, Soler C, Legrand C, Dosquet C, Biochem. Biophys. Res. Commun 2007, 363, 472. [DOI] [PubMed] [Google Scholar]
- [87].Chen J-P, Chang G, Chen J, Colloids Surf., A 2008, 313–314, 183. [Google Scholar]
- [88].Ding CM, Zhou Y, He YN, Tan WS, Process Biochem. 2008, 43, 287. [Google Scholar]
- [89].Romanova OA, Grigor’ev TE, Goncharov ME, Rudyak SG, Solov’yova EV, Krasheninnikov ST, Saprykin VP, Sytina EV, Chvalun SN, Pal’tsev MA, Panteleev AA, Bull. Exp. Biol. Med 2015, 159, 557. [DOI] [PubMed] [Google Scholar]
- [90].Black AF, Berthod F, L’heureux N, Germain L, Auger FA, FASEB J. 1998, 12, 1331. [DOI] [PubMed] [Google Scholar]
- [91].Gingras M, Paradis I, Berthod F, Biomaterials 2003, 24, 1653. [DOI] [PubMed] [Google Scholar]
- [92].Caissie R, Gingras M, Champigny MF, Berthod F, Biomaterials 2006, 27, 2988. [DOI] [PubMed] [Google Scholar]
- [93].Ma L, Gao C, Mao Z, Zhou J, Shen J, Hu X, Han C, Biomaterials 2003, 24, 4833. [DOI] [PubMed] [Google Scholar]
- [94].Rho KS, Jeong L, Lee G, Seo B, Park YJ, Hong S, Roh S, Cho JJ, Park WH, Min B, Biomaterials 2006, 27, 1452. [DOI] [PubMed] [Google Scholar]
- [95].Yeo I, Oh J, Jeong L, Lee TS, Lee SJ, Park WH, Min B, Biomacromolecules 2008, 9, 1106. [DOI] [PubMed] [Google Scholar]
- [96].Powell HM, Boyce ST, Tissue Eng., Part A 2009, 15, 2177. [DOI] [PubMed] [Google Scholar]
- [97].Jin G, Prabhakaran MP, Ramakrishna S, Acta Biomater. 2011, 7, 3113. [DOI] [PubMed] [Google Scholar]
- [98].Gilmartin DJ, Alexaline MM, Thrasivoulou C, Phillips ARJ, Jayasinghe SN, Becker DL, Adv. Healthcare Mater 2013, 2, 1151. [DOI] [PubMed] [Google Scholar]
- [99].Bonvallet PP, Culpepper BK, Bain JL, Schultz MJ, Thomas SJ, Bellis SL, Tissue Eng., Part A 2014, 20, 2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bonvallet PP, Schultz MJ, Mitchell EH, Bain JL, Culpepper BK, Thomas SJ, Bellis SL, PLoS One 2015, 10, e0122359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Jayasinghe SN, Auguste J, Scotton CJ, Adv. Mater 2015, 27, 7794. [DOI] [PubMed] [Google Scholar]
- [102].Aktürk Ö, Keskin D, Turk. J. Biol 2016, 40, 380. [Google Scholar]
- [103].Koide M, Osaki K, Konishi J, Oyamada K, Katakura T, Takahashi A, Yoshizato K, J. Biomed. Mater. Res 1993, 27, 79. [DOI] [PubMed] [Google Scholar]
- [104].Kozlov PV, Burdygina GI, Polymer 1983, 24, 651. [Google Scholar]
- [105].Choi YS, Hong SR, Lee YM, Song KW, Park MH, Nam YS, Biomaterials 1999, 20, 409. [DOI] [PubMed] [Google Scholar]
- [106].Lee SB, Jeon HW, Lee YW, Lee YM, Song KW, Park MH, Nam YS, Ahn HC, Biomaterials 2003, 24, 2503. [DOI] [PubMed] [Google Scholar]
- [107].Su K, Wang C, Biotechnol. Lett 2015, 37, 2139. [DOI] [PubMed] [Google Scholar]
- [108].Lee SB, Kim YH, Chong MS, Hong SH, Lee YM, Biomaterials 2005, 26, 1961. [DOI] [PubMed] [Google Scholar]
- [109].Powell HM, Boyce ST, J. Biomed. Mater. Res., Part A 2008, 84, 1078. [DOI] [PubMed] [Google Scholar]
- [110].Dubsky M, Kubinova S, Sirc J, Voska L, Zajicek R, Zajicova A, Lesny P, Jirkovska A, Michalek J, Munzarova M, Holan V, Sykova E, J. Mater. Sci. Mater. Med 2012, 23, 931. [DOI] [PubMed] [Google Scholar]
- [111].Gorgieva S, Kokol V, in Biomaterials Applications for Nanomedicine (Ed: Pignatello R), InTech UK; 2011, pp. 17–51. [Google Scholar]
- [112].Wang TW, Sun JS, Wu HC, Tsuang YH, Wang WH, Lin FH, Biomaterials 2006, 27, 5689. [DOI] [PubMed] [Google Scholar]
- [113].Wang TW, Wu HC, Huang YC, Sun JS, Lin FH, Artif.Organs 2006, 30, 141. [DOI] [PubMed] [Google Scholar]
- [114].Dainiak MB, Allan IU, Savina IN, Cornelio L, James ES, James SL, Mikhalovsky SV, Jungvid H, Galaev IY, Biomaterials 2010, 31, 67. [DOI] [PubMed] [Google Scholar]
- [115].Huang S, Zhang Y, Tang L, Deng Z, Lu W, Feng F, Xu X, Jin Y, Tissue Eng., Part A 2009, 15, 2617. [DOI] [PubMed] [Google Scholar]
- [116].Zhao X, Lang Q, Yildirimer L, Lin ZY, Cui W, Annabi N, Ng KW, Dokmeci MR, Ghaemmaghami AM, Khademhosseini A, Adv. Healthcare Mater 2016, 5, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Quan R, Zheng X, Xu S, Zhang L, Yang D, Stem Cell Res. Ther 2014, 5, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Pezeshki-Modaress M, Mirzadeh H, Zandi M, Mater. Sci. Eng., C 2015, 48, 704. [DOI] [PubMed] [Google Scholar]
- [119].Daamen W, Veerkamp J, Vanhest J, Vankuppevelt T, Biomaterials 2007, 28, 4378. [DOI] [PubMed] [Google Scholar]
- [120].Almine JF, Bax DV, Mithieux SM, Nivison-Smith L, Rnjak J, Waterhouse A, Wise SG, Weiss AS, Chem. Soc. Rev 2010, 39, 3371. [DOI] [PubMed] [Google Scholar]
- [121].Tsuji T, Sawabe M, J. Cutaneous Pathol 1987, 14, 106. [DOI] [PubMed] [Google Scholar]
- [122].Chen G, Chen J, Zhuo S, Xiong S, Zeng H, Jiang X, Chen R, Xie S, Br. J. Dermatol 2009, 161, 48. [DOI] [PubMed] [Google Scholar]
- [123].Rnjak J, Wise SG, Mithieux SM, Weiss AS, Tissue Eng., Part B Rev 2011, 17, 81. [DOI] [PubMed] [Google Scholar]
- [124].Rnjak J, Li Z, Maitz PKM, Wise SG, Weiss AS, Biomaterials 2009, 30, 6469. [DOI] [PubMed] [Google Scholar]
- [125].Annabi N, Mithieux SM, Weiss AS, Dehghani F, Biomaterials 2009, 30, 1. [DOI] [PubMed] [Google Scholar]
- [126].Nivison-Smith L, Rnjak J, Weiss AS, Acta Biomater. 2010, 6, 354. [DOI] [PubMed] [Google Scholar]
- [127].Rnjak-Kovacina J, Wise SG, Li Z, Maitz PKM, Young CJ, Wang Y, Weiss AS, Biomaterials 2011, 32, 6729. [DOI] [PubMed] [Google Scholar]
- [128].Rnjak-Kovacina J, Wise SG, Li Z, Maitz PKM, Young CJ, Wang Y, Weiss AS, Acta Biomater. 2012, 8, 3714. [DOI] [PubMed] [Google Scholar]
- [129].Annabi N, Mithieux SM, Boughton EA, Ruys AJ, Weiss AS, Dehghani F, Biomaterials 2009, 30, 4550. [DOI] [PubMed] [Google Scholar]
- [130].Ahmed TAE, Dare EV, Hincke M, Tissue Eng., Part B. Rev 2008, 14, 199. [DOI] [PubMed] [Google Scholar]
- [131].Weigel PH, Fuller GM, LeBoeuf RD, J. Theor. Biol 1986, 119, 219. [DOI] [PubMed] [Google Scholar]
- [132].Janmey PA, Winer JP, Weisel JW, Soc JR. Interface 2009, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Natesan S, Zamora DO, Wrice NL, Baer DG, Christy RJ, J. Burn Care Res 2013, 34, 18. [DOI] [PubMed] [Google Scholar]
- [134].Meana A, Iglesias J, Del Rio M, Larcher F, Madrigal B, Fresno MF, Martin C, San Roman F, Tevar F, Burns 1998, 24, 621. [DOI] [PubMed] [Google Scholar]
- [135].Hojo M, Inokuchi S, Kidokoro M, Fukuyama N, Tanaka E, Tsuji C, Miyasaka M, Tanino R, Nakazawa H, Plast. Reconstr. Surg 2003, 111, 1638. [DOI] [PubMed] [Google Scholar]
- [136].Sun T, Haycock J, MacNeil S, Biomaterials 2006, 27, 3459. [DOI] [PubMed] [Google Scholar]
- [137].Kober J, Gugerell A, Schmid M, Kamolz L-P, Keck M, Biomed. Res. Int 2015, 2015, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Marino D, Luginbühl J, Scola S, Meuli M, Reichmann E, Sci. Transl. Med 2014, 6, 1. [DOI] [PubMed] [Google Scholar]
- [139].Hunyadi J, Farkas B, Bertenyi C, Olah J, Dobozy A, J. Dermatol. Surg. Oncol 1988, 14, 75. [DOI] [PubMed] [Google Scholar]
- [140].Ronfard V, Broly H, Mitchel V, Galizia JP, Hochart D, Chambon E, Pellerin P, Huart J, Burns 1991, 17, 181. [DOI] [PubMed] [Google Scholar]
- [141].Zacchi V, Soranzo C, Cortivo R, Radice M, Brun P, Abatangelo G, J. Biomed. Mater. Res 1998, 40, 187. [DOI] [PubMed] [Google Scholar]
- [142].Harris P, di Francesco F, Barisoni D, Leigh I, Navsaria H, Lancet 1999, 353, 35. [DOI] [PubMed] [Google Scholar]
- [143].Price RD, Berry MG, Navsaria HA, J. Plast. Reconstr. Aesthetic Surg 2007, 60, 1110. [DOI] [PubMed] [Google Scholar]
- [144].a Hollander D, Soranzo C, Falk S, Windolf J, J. Trauma 2001, 50, 1125. [DOI] [PubMed] [Google Scholar]
- [145].Tonello C, Vindigni V, Zavan B, Abatangelo S, Abatangelo G, Brun P, Cortivo R, Faseb J 2005, 19, 1. [DOI] [PubMed] [Google Scholar]
- [146].Scuderi N, Onesti MG, Bistoni G, Ceccarelli S, Rotolo S, Angeloni A, Marchese C, Biomaterials 2008, 29, 1620. [DOI] [PubMed] [Google Scholar]
- [147].Ghosh K, Ren XD, Shu XZ, Prestwich GD, Clark RA, Tissue Eng. 2006, 12, 601. [DOI] [PubMed] [Google Scholar]
- [148].Mao JS, Liu HF, Yin YJ, Yao KD, Biomaterials 2003, 24, 1621. [DOI] [PubMed] [Google Scholar]
- [149].Liu H, Mao J, Yao K, Yang G, Cui L, Cao Y, J. Biomater. Sci. Polym. Ed 2004, 15, 25. [DOI] [PubMed] [Google Scholar]
- [150].Monteiro IP, Shukla A, Marques AP, Reis RL, Hammond PT, J. Biomed. Mater. Res., Part A 2015, 103, 330. [DOI] [PubMed] [Google Scholar]
- [151].Khademhosseini A, Suh KY, Yang JM, Eng G, Yeh J, Levenberg S, Langer R, Biomaterials 2004, 25, 3583. [DOI] [PubMed] [Google Scholar]
- [152].Li L, Qian Y, Jiang C, Lv Y, Liu W, Zhong L, Cai K, Li S, Yang L, Biomaterials 2012, 33, 3428. [DOI] [PubMed] [Google Scholar]
- [153].Croisier F, Jérôme C, Eur. Polym. J 2013, 49, 780. [Google Scholar]
- [154].Mao J, Zhao L, De Yao K, Shang Q, Yang G, Cao Y, J. Biomed. Mater. Res. A 2003, 64, 301. [DOI] [PubMed] [Google Scholar]
- [155].Kim I, Seo S, Moon H, Yoo M, Park I, Kim B, Cho C, Biotechnol. Adv 2008, 26, 1. [DOI] [PubMed] [Google Scholar]
- [156].Spasova M, Paneva D, Manolova N, Radenkov P, Rashkov I, Macromol. Biosci 2008, 8, 153. [DOI] [PubMed] [Google Scholar]
- [157].Zhou Y, Yang D, Chen X, Xu Q, Lu F, Nie J, Biomacromolecules 2008, 9, 349. [DOI] [PubMed] [Google Scholar]
- [158].Muzzarelli RAA, Carbohydr. Polym 2009, 76, 167. [Google Scholar]
- [159].Jayakumar R, Prabaharan M, Kumar PTS, Nair SV, Tamura H, Biotechnol. Adv 2011, 29, 322. [DOI] [PubMed] [Google Scholar]
- [160].Dai T, Tanaka M, Huang Y, Expert Rev. Anti-Infect. Ther 2011, 9, 857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Gholipour-Kanani A, Bahrami SH, Samadi-Kochaksaraie A, Ahmadi-Tafti H, Rabbani S, Kororian A, Erfani E, IET Nanobiotechnol. 2012, 6, 129. [DOI] [PubMed] [Google Scholar]
- [162].Gu BK, Park SJ, Kim MS, Kang CM, Il Kim J, Kim CH, Carbohydr. Polym 2013, 97, 65. [DOI] [PubMed] [Google Scholar]
- [163].Xie Z, Paras CB, Weng H, Punnakitikashem P, Su LC, Vu K, Tang L, Yang J, Nguyen KT, Acta Biomater 2013, 9, 9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Bertoncelj V, Pelipenko J, Kristl J, Jeras M, Cukjati M, Kocbek P, Eur. J. Pharm. Biopharm 2014, 88, 64. [DOI] [PubMed] [Google Scholar]
- [165].Naseri N, Algan C, Jacobs V, John M, Oksman K, Mathew AP, Carbohydr. Polym 2014, 109, 7. [DOI] [PubMed] [Google Scholar]
- [166].Li Z, Yuan B, Dong X, Duan L, Tian H, RSC Adv. 2015, 5, 94248. [Google Scholar]
- [167].Azuma K, Izumi R, Osaki T, Ifuku S, Morimoto M, J. Funct. Biomater 2015, 6, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Antunes BP, Moreira AF, Gaspar VM, Correia IJ, Carbohydr. Polym 2015, 130, 104. [DOI] [PubMed] [Google Scholar]
- [169].Guang S, An Y, Ke F, Zhao D, Shen Y, Xu H, J. Appl. Polym. Sci 2015, 42503, 1. [Google Scholar]
- [170].Adekogbe I, Ghanem A, Biomaterials 2005, 26, 7241. [DOI] [PubMed] [Google Scholar]
- [171].Chen K, Liao W, Kuo S, Tsai F, Chen Y, Biomacromolecules 2009, 10, 1642. [DOI] [PubMed] [Google Scholar]
- [172].Bakar A, Hilmi M, Halim AS, Jaafar H, Asiah AB, Hassan A, Biomed Res. Int 2013, 2013, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Hilmi Mohd AB, Halim AS, Hassan A, Lim CK, Noorsal K, Zainol I, Springerplus 2013, 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Kumar PTS, Praveen G, Raj M, Chennazhi KP, Jayakumar R, RSC Adv. 2014, 4, 65081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Tchemtchoua VT, Atanasova G, Aqil A, Fil P, Garbacki N, Vanhooteghem O, Deroanne C, Christine J, Nusgens B, Poumay Y, Colige A, Biomacromolecules 2011, 12, 3194. [DOI] [PubMed] [Google Scholar]
- [176].Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL, Biomaterials 2003, 24, 401. [DOI] [PubMed] [Google Scholar]
- [177].Reimers K, Liebsch C, Radtke C, Kuhbier JW, Vogt PM, Biotechnol. Bioeng 2015, 112, n/a. [DOI] [PubMed] [Google Scholar]
- [178].Min BM, Jeong L, Nam YS, Kim JM, Kim JY, Park WH, Int. J. Biol. Macromol 2004, 34, 223. [DOI] [PubMed] [Google Scholar]
- [179].Min BM, Lee G, Kim SH, Nam YS, Lee TS, Park WH, Biomaterials 2004, 25, 1289. [DOI] [PubMed] [Google Scholar]
- [180].Lei C, Zhu H, Li J, Li J, Feng X, Chen J, Polym. Eng. Sci 2015, 55, 907. [Google Scholar]
- [181].Jeong L, Yeo IS, Kim HN, Il Yoon Y, Jang DH, Jung SY, Min BM, Park WH, Int. J. Biol. Macromol 2009, 44, 222. [DOI] [PubMed] [Google Scholar]
- [182].Wendt H, Hillmer A, Reimers K, Kuhbier JW, Schäfer-Nolte F, Allmeling C, Kasper C, Vogt PM, PLoS One 2011, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Luangbudnark W, Viyoch J, Laupattarakasem W, Surakunprapha P, Laupattarakasem P, Sci. World J 2012, 2012, 697201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Bellas E, Seiberg M, Garlick J, Kaplan DL, Macromol. Biosci 2012, 12, 1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Park KE, Jung SY, Lee SJ, Min BM, Park WH, Int. J. Biol. Macromol 2006, 38, 165. [DOI] [PubMed] [Google Scholar]
- [186].Yoo CR, Yeo IS, Park KE, Park JH, Lee SJ, Park WH, Min BM, Int. J. Biol. Macromol 2008, 42, 324. [DOI] [PubMed] [Google Scholar]
- [187].Yan S, Zhang Q, Wang J, Liu Y, Lu S, Li M, Kaplan DL, Acta Biomater. 2013, 9, 6771. [DOI] [PubMed] [Google Scholar]
- [188].Zhang W, Chen L, Chen J, Wang L, Gui X, Ran J, Xu G, Zhao H, Zeng M, Ji J, Qian L, Zhou J, Ouyang H, Zou X, Adv. Healthcare Mater 2017, 6, 1. [DOI] [PubMed] [Google Scholar]
- [189].Chouhan D, Chakraborty B, Nandi SK, Mandal BB, Acta Biomater. 2017, 48, 157. [DOI] [PubMed] [Google Scholar]
- [190].Bhardwaj N, Sow WT, Devi D, Ng KW, Mandal BB, Cho N-J, Integr. Biol 2015, 7, 53. [DOI] [PubMed] [Google Scholar]
- [191].Hodgkinson T, Yuan X-F, Bayat A, J. Tissue Eng 2014, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Cui W, Zhu X, Yang Y, Li X, Jin Y, Mater. Sci. Eng. C 2009, 29, 1869. [Google Scholar]
- [193].Xiao L, Wang B, Yang G, Gauthier M, in Biomedical Science, Engineering and Technology, InTech, UK; 2012, pp. 247–282. [Google Scholar]
- [194].Kim KL, Han DK, Park K, Song SH, Kim JY, Kim JM, Ki HY, Yie SW, Roh CR, Jeon ES, Kim DK, Suh W, Biomaterials 2009, 30, 3742. [DOI] [PubMed] [Google Scholar]
- [195].Lafrance ML, Armstrong DW, Tissue Eng. 1999, 5, 153. [DOI] [PubMed] [Google Scholar]
- [196].Duan B, Yuan X, Zhu Y, Zhang Y, Li X, Zhang Y, Yao K, Eur. Polym. J 2006, 42, 2013. [Google Scholar]
- [197].Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK, J. Biomed. Mater. Res 2002, 60, 613. [DOI] [PubMed] [Google Scholar]
- [198].Kumbar SG, Nukavarapu SP, James R, Nair LS, Laurencin CT, Biomaterials 2008, 29, 4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Zhu X, Cui W, Li X, Jin Y, Biomacromolecules 2008, 9, 1795. [DOI] [PubMed] [Google Scholar]
- [200].Yang Y, Zhu X, Cui W, Li X, Jin Y, Macromol. Mater. Eng 2009, 294, 611. [Google Scholar]
- [201].Ru C, Wang F, Pang M, Sun L, Chen R, Sun Y, ACS Appl. Mater. Interfaces 2015, 7, 10872. [DOI] [PubMed] [Google Scholar]
- [202].Kim SJ, Jang DH, Park WH, Min BM, Polymer 2010, 51, 1320. [Google Scholar]
- [203].Ng KW, Khor HL, Hutmacher DW, Biomaterials 2004, 25, 2807. [DOI] [PubMed] [Google Scholar]
- [204].Ng KW, Hutmacher DW, Biomaterials 2006, 27, 4591. [DOI] [PubMed] [Google Scholar]
- [205].Ng K, Hutmacher D, Schantz J, Ng C, Too H, Lim T, Phan T, Teoh S, Tissue Eng. 2001, 7, 441. [DOI] [PubMed] [Google Scholar]
- [206].Xie J, Macewan MR, Ray WZ, Liu W, Siewe DY, Xia Y, ACS Nano 2010, 4, 5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Lowery JL, Datta N, Rutledge GC, Biomaterials 2010, 31, 491. [DOI] [PubMed] [Google Scholar]
- [208].Lorden ER, Miller KJ, Bashirov L, Ibrahim MM, Hammett E, Jung Y, a Medina M, Rastegarpour A, a Selim M, Leong KW, Levinson H, Biomaterials 2015, 43, 61. [DOI] [PubMed] [Google Scholar]
- [209].Zhu J, Biomaterials 2010, 31, 4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].El Ghalbzouri A, Lamme EN, Van Blitterswijk C, Koopman J, Ponec M, Biomaterials 2004, 25, 2987. [DOI] [PubMed] [Google Scholar]
- [211].Khil M-S, Cha D-I, Kim H-Y, Kim I-S, Bhattarai N, J. Biomed. Mater. Res., Part B 2003, 67, 675. [DOI] [PubMed] [Google Scholar]
- [212].Kim SE, Heo DN, Lee JB, Kim JR, Park SH, Jeon SH, Kwon IK, Biomed. Mater 2009, 4, 44106. [DOI] [PubMed] [Google Scholar]
- [213].Guo HF, Li ZS, Dong SW, Chen WJ, Deng L, Wang YF, Ying DJ, Colloids Surf., B 2012, 96, 29. [DOI] [PubMed] [Google Scholar]
- [214].Unnithan AR, Barakat NAM, Tirupathi Pichiah PB, Gnanasekaran G, Nirmala R, Cha YS, Jung CH, El-Newehy M, Kim HY, Carbohydr. Polym 2012, 90, 1786. [DOI] [PubMed] [Google Scholar]
- [215].Wright KA, Nadire KB, Busto P, Tubo R, McPherson JM, Wentworth BM, Burns 1998, 24, 7. [DOI] [PubMed] [Google Scholar]
- [216].Rennekampff HO, Hansbrough JF, Kiessig V, Abiezzi S, Diego S, Genter M, Surgery 1996, 120, 16. [DOI] [PubMed] [Google Scholar]
- [217].Lorden ER, Miller KJ, Ibrahim MM, Bashirov L, Hammett E, Chakraborty S, Quiles-Torres C, Selim MA, Leong KW, Levinson H, Acta Biomater 2016, 32, 100. [DOI] [PubMed] [Google Scholar]
- [218].Wu EC, Zhang S, Hauser CAE, Adv. Funct. Mater 2012, 22, 456. [Google Scholar]
- [219].Schneider A, Garlick JA, Egles C, PLoS One 2008, 3, e1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Meng H, Chen L, Ye Z, Wang S, Zhao X, J. Biomed. Mater. Res., Part B 2009, 89, 379. [DOI] [PubMed] [Google Scholar]
- [221].Bradshaw M, Ho D, Fear MW, Gelain F, Wood FM, Iyer KS, Sci. Rep 2014, 4, 6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Venugopal J, Ramakrishna S, Tissue Eng 2005, 11, 847. [DOI] [PubMed] [Google Scholar]
- [223].Dai NT, Williamson MR, Khammo N, Adams EF, Coombes AGA, Biomaterials 2004, 25, 4263. [DOI] [PubMed] [Google Scholar]
- [224].Fu X, Xu M, Liu J, Qi Y, Li S, Wang H, Biomaterials 2014, 35, 1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Zhang YZ, Venugopal J, Huang ZM, Lim CT, Ramakrishna S, Biomacromolecules 2005, 6, 2583. [DOI] [PubMed] [Google Scholar]
- [226].Gümüşderelioglu M, Dalkiranoğlu S, Aydin RST, Çakmak S, J. Biomed. Mater. Res., Part A 2011, 98 A, 461. [DOI] [PubMed] [Google Scholar]
- [227].Huang C, Fu X, Liu J, Qi Y, Li S, Wang H, Biomaterials 2012, 33, 1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Ma K, Liao S, He L, Lu J, Ramakrishna S, Chan CK, Tissue Eng. Part A 2011, 17, 1413. [DOI] [PubMed] [Google Scholar]
- [229].Liu SJ, Kau YC, Chou CY, Chen JK, Wu RC, Yeh WL, J. Membr. Sci 2010, 355, 53. [Google Scholar]
- [230].Chen G, Sato T, Ohgushi H, Ushida T, Tateishi T, Tanaka J, Biomaterials 2005, 26, 2559. [DOI] [PubMed] [Google Scholar]
- [231].Ananta M, Brown RA, Mudera V, Tissue Eng., Part A 2012, 18, 353. [DOI] [PubMed] [Google Scholar]
- [232].Lu H, Oh HH, Kawazoe N, Yamagishi K, Chen G, Sci. Technol. Adv. Mater 2012, 13, 64210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Blackstone BN, Drexler JW, Powell HM, Tissue Eng., Part A 2014, 20, 2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Gautam S, Chou C, Dinda AK, Potdar PD, Mishra NC, Mater. Sci. Eng., C 2014, 34, 402. [DOI] [PubMed] [Google Scholar]
- [235].Feng B, Duan H, Fu W, Cao Y, Zhang WJ, Zhang Y, J. Biomed. Mater. Res., Part A 2015, 103, 431. [DOI] [PubMed] [Google Scholar]
- [236].Pan J, Liu N, Sun H, Xu F, PLoS One 2014, 9, e112885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Sundaramurthi D, Vasanthan K, Kuppan P, Krishnan U, Sethuraman S, Biomed. Mater 2012, 7, 45005. [DOI] [PubMed] [Google Scholar]
- [238].Croisier F, Atanasova G, Poumay Y, Jérôme C, Adv. Healthcare Mater 2014, 3, 2032. [DOI] [PubMed] [Google Scholar]
- [239].Prasad T, Shabeena EA, Vinod D, Kumary TV, Anil Kumar PR, J. Mater. Sci. Mater. Med 2015, 26, 5352. [DOI] [PubMed] [Google Scholar]
- [240].Chen H, Huang J, Yu J, Liu S, Gu P, Int. J. Biol. Macromol 2011, 48, 13. [DOI] [PubMed] [Google Scholar]
- [241].Li Y, Chen F, Nie J, Yang D, Carbohydr. Polym 2012, 90, 1445. [DOI] [PubMed] [Google Scholar]
- [242].Shalumon KT, Sathish D, Nair SV, Chennazhi KP, Tamura H, Jayakumar R, J. Biomed. Nanotechnol 2012, 8, 405. [DOI] [PubMed] [Google Scholar]
- [243].Sarkar SD, Farrugia BL, Dargaville TR, Dhara S, J. Biomed. Mater. Res., Part A 2013, 101A, 3482. [DOI] [PubMed] [Google Scholar]
- [244].Duan B, Wu L, Yuan X, Hu Z, Li X, Zhang Y, Yao K, Wang M, J. Biomed. Mater. Res., Part A 2007, 83, 868. [DOI] [PubMed] [Google Scholar]
- [245].Wu L, Li H, Li S, Li X, Yuan X, Li X, Zhang Y, J. Biomed. Mater. Res., Part A 2010, 92, 563. [DOI] [PubMed] [Google Scholar]
- [246].Frank L, Lebreton-decoster C, Godeau G, Coulomb B, Jozefonvicz J, J. Biomater. Sci. Polym. Ed 2006, 17, 499. [DOI] [PubMed] [Google Scholar]
- [247].Yue XL, Kerros ME, Petit E, Kern P, J. Biomed. Mater. Res., Part A 2009, 90, 641. [DOI] [PubMed] [Google Scholar]
- [248].Hwang M, Kim JO, Lee JH, Il Kim Y, Kim JH, Chang SW, Jin SG, Kim JA, Lyoo WS, Han SS, Ku SK, Yong CS, Choi H, AAPS PharmSciTech 2010, 11, 1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Ribeiro MP, Morgado PI, Miguel SP, Coutinho P, Correia IJ, Mater. Sci. Eng., C 2013, 33, 2958. [DOI] [PubMed] [Google Scholar]
- [250].Pan H, Jiang H, Chen W, Biomaterials 2006, 27, 3209. [DOI] [PubMed] [Google Scholar]
- [251].Pan H, Jiang H, Chen W, Biomaterials 2008, 29, 1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Wong VW, Rustad KC, Galvez MG, Neofytou E, Glotzbach JP, Januszyk M, Major MR, Sorkin M, Longaker MT, Rajadas J, Gurtner GC, Tissue Eng., Part A 2011, 17, 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Wong VW, Rustad KC, Glotzbach JP, Sorkin M, Inayathullah M, Major MR, Longaker MT, Rajadas J, Gurtner GC, Macromol. Biosci 2011, 11, 1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Nicholas MN, Jeschke MG, Amini-Nik S, Tissue Eng., Part A 2016, 22, 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Rustad KC, Wong VW, Sorkin M, Glotzbach JP, Major MR, Rajadas J, Longaker MT, Gurtner GC, Biomaterials 2012, 33, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Potter MJ, Linge C, Cussons P, Dye JF, Sanders R, Plast. Reconstr. Surg 2006, 117, 1876. [DOI] [PubMed] [Google Scholar]
- [257].Brown DM, Barton BR, Young L, Pruitt BA, Arch. Surg 1992, 127, 404. [DOI] [PubMed] [Google Scholar]
- [258].Lugo LM, Lei P, Andreadis ST, Tissue Eng., Part A 2011, 17, 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Frueh FS, Menger MD, Lindenblatt N, Giovanoli P, Laschke MW, Crit. Rev. Biotechnol 2016, 8551, 1. [DOI] [PubMed] [Google Scholar]
- [260].Demidova-Rice TN, Durham JT, Herman IM, Adv. Wound Care 2012, 1, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Klar AS, Güven S, Biedermann T, Luginbühl J, Böttcher-Haberzeth S, Meuli-Simmen C, Meuli M, Martin I, Scherberich A, Reichmann E, Biomaterials 2014, 35, 5065. [DOI] [PubMed] [Google Scholar]
- [262].Arno AI, Amini-Nik S, Blit PH, Al-Shehab M, Belo C, Herer E, Tien CH, Jeschke MG, Stem Cell Res. Ther 2014, 5, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]


