Accumulation of senescent cells has been implicated in the pathogenesis of organ fibrosis and might be therapeutically targeted using senolytic agents. A recent first-in-humans, open-label trial of senolytic therapy using dasatinib plus quercetin in idiopathic pulmonary fibrosis (IPF) showed that functional clinical improvement was associated with reduced levels of circulating proteins, microRNAs, and cytokines related to senescence-associated secretory phenotype (SASP) (1). Similarly to IPF, systemic sclerosis (SSc) is commonly complicated by fibrotic interstitial lung disease (ILD) and presently lacks approved therapies. To address the potential pathogenic role of cellular senescence in SSc-associated ILD (SSc-ILD), we reexamined our results from a recent single-arm clinical trial of dasatinib (2), in which 12 patients with SSc-ILD received treatment for 169 days. We sought to investigate changes in skin SASP gene signature across clinical improvers (defined as a decrease of >5 points or >20% from baseline in the modified Rodnan skin thickness score (3), a validated measure of SSc skin involvement) and in non-improvers.
For this analysis, we retrieved a set of 77 genes annotated to SASP from Reactome (4), 66 of which were present in our skin biopsy-based gene expression data set. While a hallmark database (5) was previously used, in the present analysis we used the Reactome as a more appropriate database, since the former does not contain SASP and senescence gene sets. Despite using different databases, we found that the SASP gene set significantly overlapped (false discovery rate [FDR] <5%) with several hallmarks we identified previously, such as HYPOXIA, TNFA_SIGNALING_VIA_NFKB, P53_PATHWAY, INFLAMMATORY_RESPONSE, PI3K_AKT_MTOR_SIGNALING, and IL6_JAK_STAT3_SIGNALING. For each gene from the Reactome SASP gene set, we created its centroid by averaging its expression across baseline and posttreatment biopsies from 3 dasatinib improvers and 9 non-improvers. The results showed that the SASP gene signature was significantly decreased from baseline in dasatinib-treated improvers; in marked contrast, no change from baseline was seen in non-improvers. We also found that SASP levels were significantly higher at baseline, and significantly lower posttreatment, in improvers compared to non-improvers (Figure 1A). Among the 66 SASP signature genes, 53 (80.3%) showed a decrease in expression posttreatment in improvers, compared to only 35 (53.0%) in non-improvers (P = 0.0015 by Fisher’s exact test).
Figure 1.
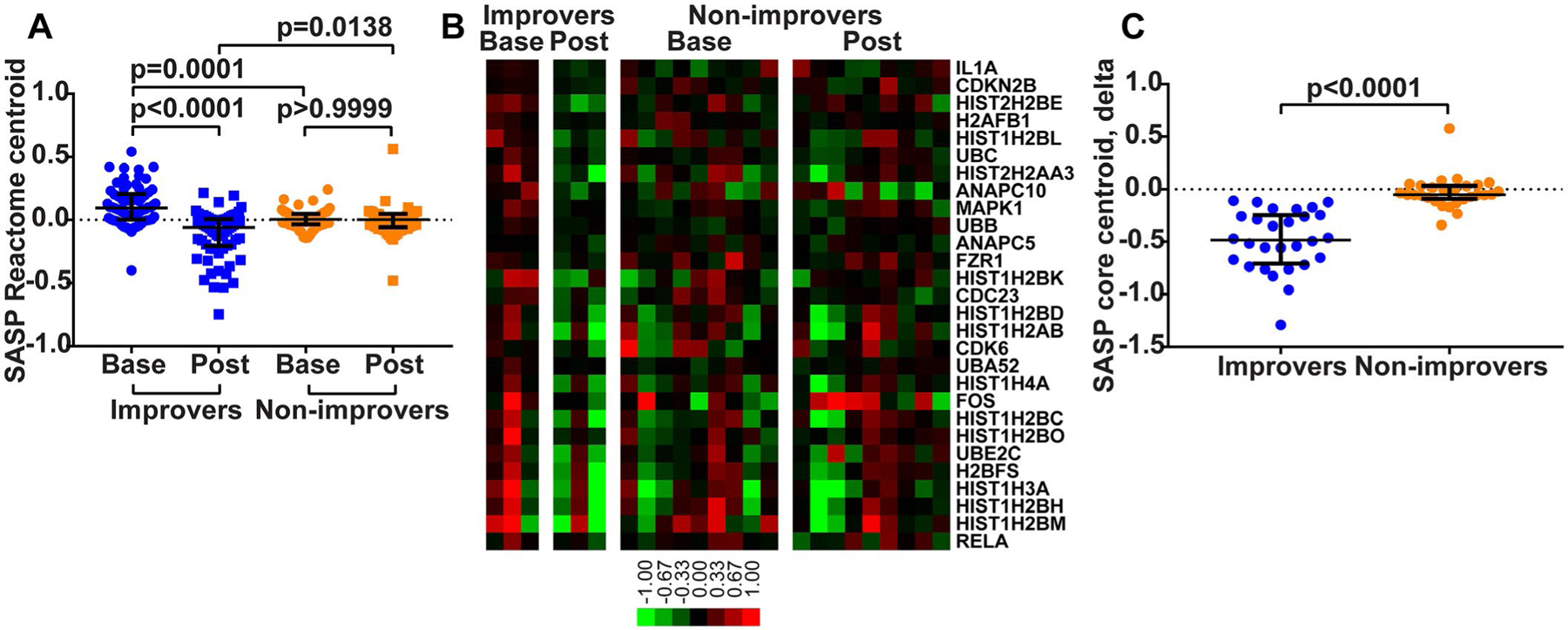
Skin senescence-associated secretory phenotype (SASP) gene signature across dasatinib improvers and non-improvers. A, Centroids of genes in the Reactome SASP gene set, created by averaging expression values for component genes across baseline (base) and posttreatment (post) biopsy samples from improvers and non-improvers. P values were determined by Kruskal-Wallis test with Dunn’s multiple comparison test. B, Gene expression heatmap of 28 SASP pathway genes that significantly contributed to SASP enrichment in baseline biopsy samples from improvers. Genes are ordered by rank metric score (i.e., IL1A was the largest contributor to SASP enrichment). Color bar shows the log2-transformed median-centered fold change represented by each color in the heatmap. C, Comparison of the degree of change (calculated as posttreatment minus baseline expression level) for 28 SASP pathway core enrichment genes between improvers and non-improvers. P value was determined by Mann-Whitney test. In A and C, each symbol represents a specific gene; bars show the median and interquartile range.
Next, in order to characterize whole-genome transcriptional changes in an unbiased manner, we performed a gene set enrichment analysis (GSEA) (6,7) using the entire Reactome pathway gene set database. The SASP gene set was significantly enriched in baseline biopsy samples (FDR 1.5%; top 3 result in terms of statistical significance), and decreased posttreatment in improvers. Core enrichment genes from SASP (28 of 66) followed the same trends as the overall SASP pathway (Figure 1B), and their degree of change (calculated as posttreatment minus baseline expression level) was significantly lower in improvers compared to non-improvers (Figure 1C).
Four additional senescence-related gene sets were significantly enriched in baseline biopsy samples from improvers and decreased posttreatment (cellular senescence, DNA damage/telomere stress-induced senescence, oncogene-induced senescence, and oxidative stress-induced senescence; all FDRs <5%). In fact, these gene sets were overrepresented among gene sets significantly enriched in improvers at baseline, compared to all other gene sets enriched in improvers (5 of 23 gene sets with FDR <5% versus 0 of 755 gene sets with FDR > 5%; P < 0.0001 by Fisher’s exact test). In contrast, we found that in non-improvers the senescence-related gene sets showed no change from baseline to posttreatment (lowest FDR for senescence-related gene set 26.9%, FDR for SASP 78.0%). Baseline GSEA comparison between improvers and non-improvers revealed that while SASP was nonsignificantly enriched in baseline biopsy specimens from improvers (FDR 11.7%), the DNA damage/telomere stress-induced senescence gene set was very significantly enriched in improvers compared to non-improvers (FDR 0.6%; top 2 result in terms of statistical significance).
In summary, a reanalysis of the results from a recent open-label trial of the senolytic agent dasatinib in patients with SSc-ILD demonstrated that a decrease in skin expression of SASP and other senescence-related gene sets was associated with treatment, and correlated with clinical improvement. Additionally, baseline skin biopsy specimens from clinical improvers showed higher expression of these senescence-associated gene sets. These results can be viewed as consistent with a clinically beneficial senolytic effect, suggesting that dasatinib-mediated clearance of pathogenic senescent cells in lesional tissue and consequent reduction in the systemic senescence burden may have mitigated the fibrotic process and led to clinical improvement. Indeed, this mechanistic scenario closely parallels recent experimental observations in an animal model of bleomycin-induced lung fibrosis, where pharmacologic clearance of senescent cells using dasatinib plus quercetin resulted in functional attenuation of the lung fibrosis (8). While the potential of a tissue SASP gene signature as a biomarker of clinical response to antifibrosis therapy requires further study, the present results provide conceptual support for senolytic therapy in SSc-ILD.
Acknowledgments
Drs. Martyanov and Whitfield’s work was supported by grant P50-AR-060780 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and a grant from the Scleroderma Research Foundation. No other disclosures relevant to this article were reported.
Contributor Information
Michael L. Whitfield, Geisel School of Medicine at Dartmouth, Hanover, NH.
John Varga, Northwestern University, Chicago, IL.
References
- 1.Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 2019;40:554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martyanov V, Kim GJ, Hayes W, Du S, Ganguly BJ, Sy O, et al. Novel lung imaging biomarkers and skin gene expression subsetting in dasatinib treatment of systemic sclerosis-associated interstitial lung disease. PLoS One 2017;12:e0187580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clements P, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol 1995;22:1281–5. [PubMed] [Google Scholar]
- 4.Fabregat A, Jupe S, Matthews L, Sidiropoulos K, Gillespie M, Garapati P, et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res 2018;46:D649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 2015;1:417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1 α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003;34:267–73. [DOI] [PubMed] [Google Scholar]
- 7.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005;102:15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 2017;8:14532. [DOI] [PMC free article] [PubMed] [Google Scholar]


