Introduction
Polymorphous adenocarcinoma (PAC) is defined by the World Health Organization (WHO) classification1 as an infiltrative salivary gland carcinoma that is characterized by architectural diversity and cytologic uniformity. PAC is composed of a single type of uniform tumor cells that exhibit clear chromatin and inconspicuous nucleoli, resembling the nuclei of papillary thyroid carcinoma. The term “polymorphous” describes the various architectural patterns that may be seen in PAC.
Approximately 95% of PAC affects the minor salivary glands of upper aerodigestive tract, most commonly the palate (in approximately 60% of cases, range: 49%−87%)2–11. Other possible sites of origin include base of tongue, buccal mucosa, floor of mouth, lip, lateral tongue, retromolar trigone, sinonasal tract, oropharynx, and nasopharyx2–10. PAC may occasionally occur in major salivary glands, in particular the parotid gland, in approximately 3% (range: 0% to 9%) of cases2–10.
Clinically, PAC occurs more frequently in female with a reported female to male ratio of approximately 2:1 (range: 1.3:1 to 2.15:1)2–10. PAC may occur in a wide age range from 16 to 94-year-old, with a mean age of diagnosis in the 60s2–10.
Gross Features
Macroscopically, PAC typically presents as a submucosal nodule with or without surface ulceration. The tumor cross section manifests as an unencapsulated, multilobulated, firm, beige to tan mass with lobulated or infiltrative borders (Figure 1).
Figure 1. Macroscopic appearance of polymorphous adenocarcinoma (PAC).
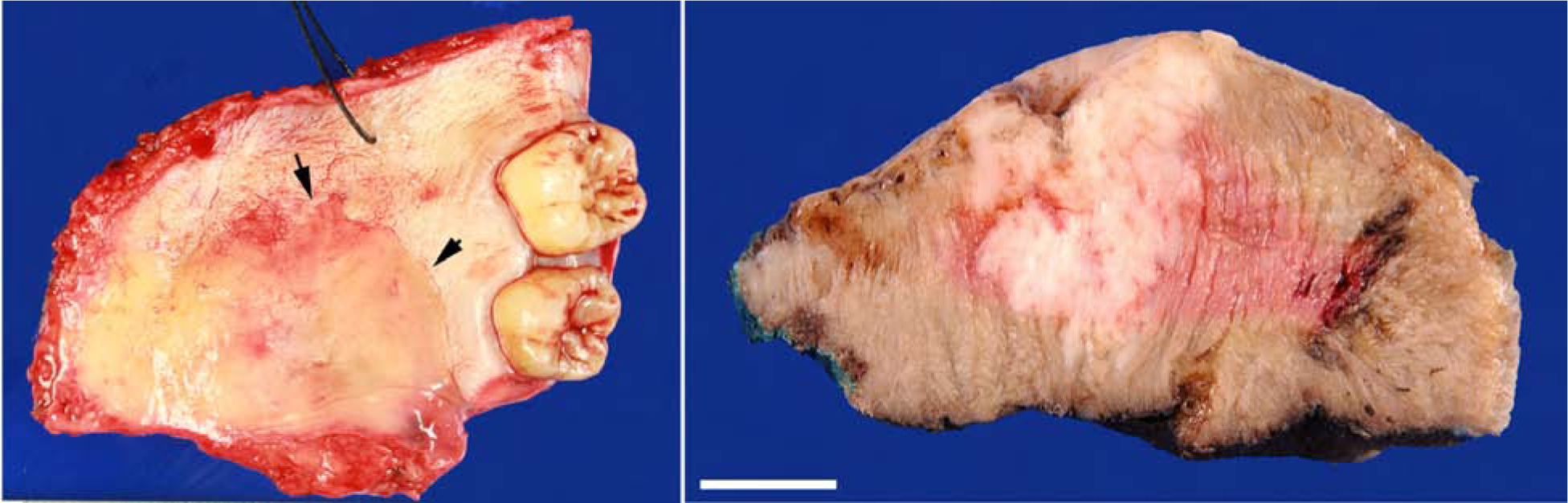
(Left) A palate PAC is present as an indurated and bulging submucosal nodule (arrows) without direct surface involvement. (Right) Cross section of a PAC/cribriform adenocarcinoma (CASG) originated from base of tongue shows an infiltrative multilobulated firm beige mass. Scale bar: 1 cm.
Microscopic Features
At low power, PAC typically presents as an infiltrative unencapsulated mass (Figure 2). The key diagnostic histologic features of PAC are its cytological uniformity and architectural diversity. Architectural patterns which may be variably present include single filing in which tumor cells aligned as single columns resembling lobular carcinoma of breast; tubules; elongated trabeculae; solid nests; anastomosing reticular or microcystic structures; papillary projection with or without fibrovascular cores; and cribriform structures. The extracellular matrix can be myxoid and/or hyalinized. Targetoid arrangement of tubules and trabeculae around nerves and vessels and streaming of tumor cells as single rows along nerve bundles are common histologic findings. Perineural invasion is frequent in PAC, being seen in 60–75% of cases3,12. Regardless of the architectural pattern, PAC is typically composed of one type of monotonous tumor cells that are characterized by open chromatin, and inconspicuous nucleoli, resembling the nuclei of papillary thyroid carcinoma (Box 1).
Figure 2. Histologic features of PAC.
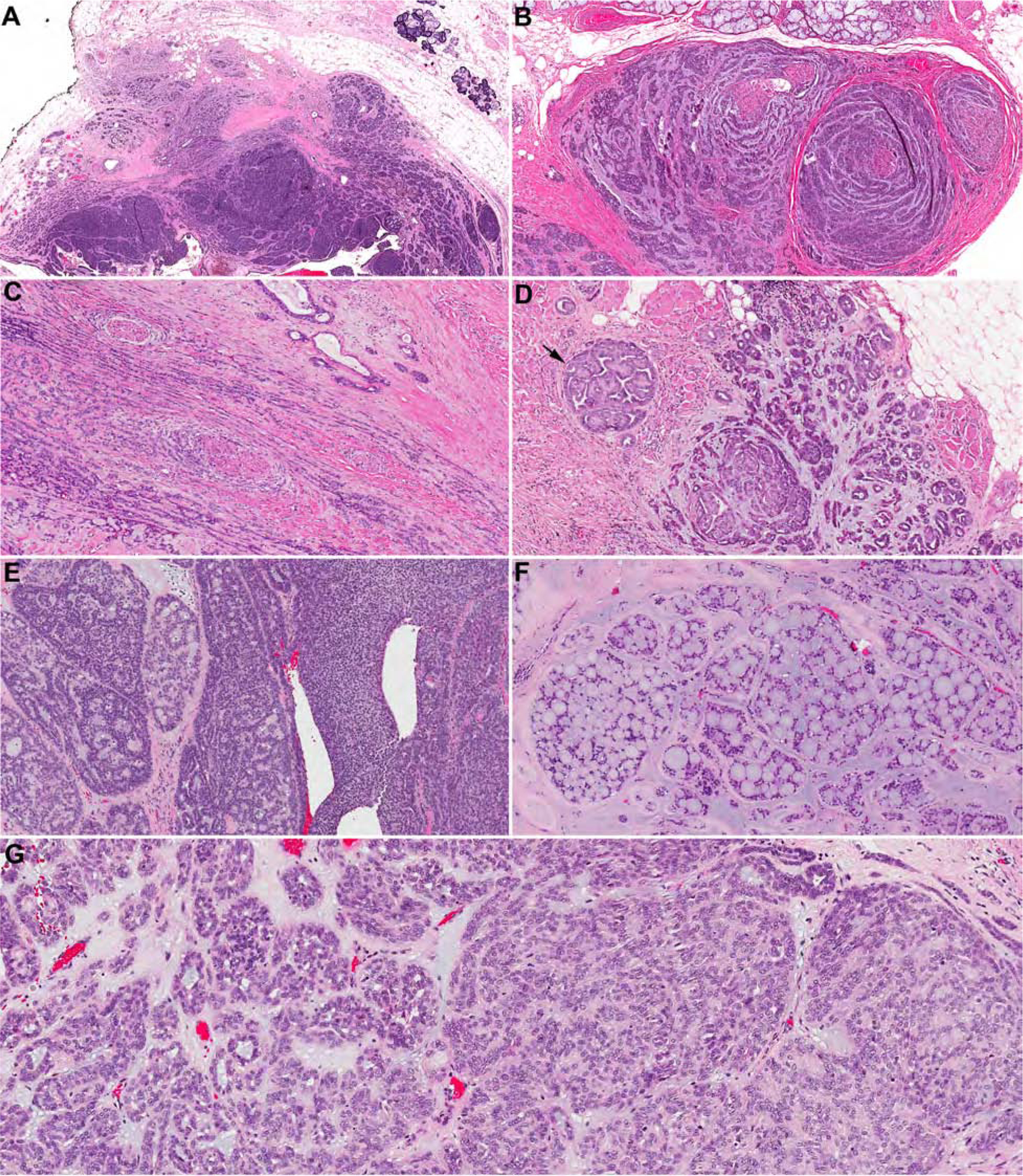
(A) At low power, PAC is present as an infiltrative tumor with various architectural patterns. Streaming of tubules and nests may be evident at the periphery. (B and C) Perineural invasion with tumor cells arranged as single rows, tubules, and trabecular streaming around and between nerve bundles forming a targetoid appearance. Other architectural patterns that may be seen in PAC include papillary (D, arrow), tubules (D, right), trabecular-interlacing reticular (E, left), solid (E, right), and cribriform pattern (F). (G) Regardless of the diverse architectural patterns, PAC is composed of one type of tumor cells typically with oval nuclei, pale fine chromatin, and inconspicuous nucleoli.
Box 1. Key pathologic features of PAC.
- PAC is characterized by cytologic uniformity and architectural diversity.
- PAC is composed of a single type of tumor cells with pale nuclei.
- PAC may contain multiple architectural patterns, which gives its polymorphous appearance.
Of note, certain histologic features, although infrequent, may be identified (usually focally) in PAC and the presence of these features does not exclude the diagnosis of PAC. These features include oncocytic changes, clear cell changes, mucocytes, foamy cells/sebaceous differentiation, coarse calcification, psammoma bodies, prominent myxoid stroma, and tumor necrosis (Figure 3). Metaplastic changes such as oncocytic or clear cell alterations can occasionally give the false impression that there are two cell types within the tumor.
Figure 3. Uncommon histologic features that may be seen in PAC.
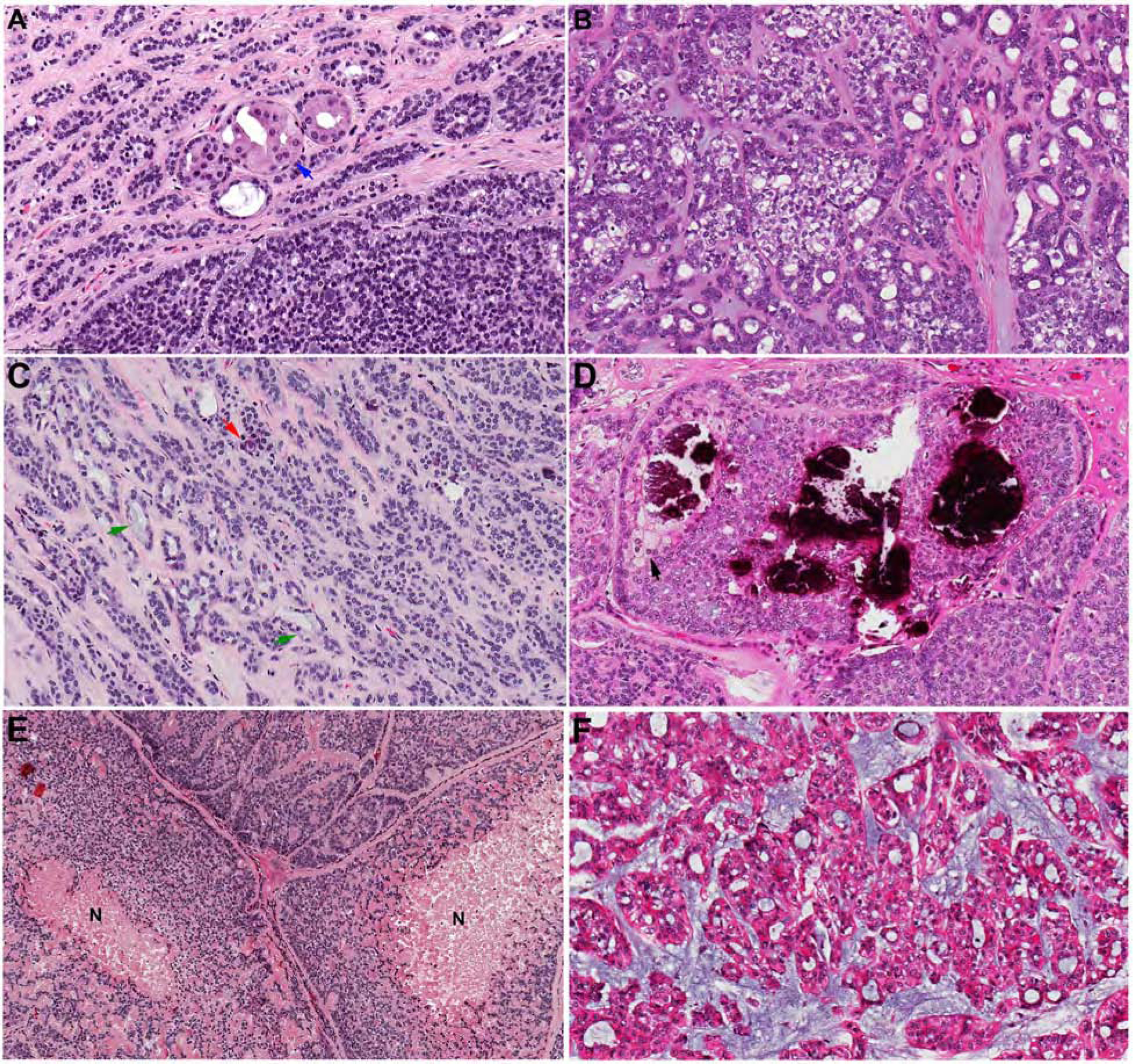
(A) oncocytic changes (blue arrow). (B) clear cell changes. (C) mucocytes (green arrows) and psammoma bodies (red arrow). (D) Coarse calcification and foamy/sebaceous cells (black arrow). (E) Tumor necrosis (N). (F) Prominent myxoid stroma may be seen in a proportion of PAC/CASG.
High grade transformation is a rare phenomenon that has been reported in PAC. It is characterized by marked nuclear atypia, prominent nucleoli, high mitotic count and tumor necrosis13,14.
Ancillary studies, diagnostic
Immunohistochemistry
Typically, PAC is diffusely and strongly positive for S10010,15–17, SOX1018, CAM 5.210,12, and CK710. Focal immunoexpression of myoepithelial markers, e.g. smooth muscle actin (SMA), muscle specific actin (MSA), and glial fibrillary acidic protein (GFAP), can be seen in PAC10,16,17. The Ki-67 proliferation index is typically low (less than 10%)3,10,12. However, elevated Ki-67 index may be seen in 10% −20% of cases3,10,12. Recently, several studies have reported that PAC usually has a p63 positive/p40 negative immunoprofile3,19,20. Notably, the p63 positivity is quite variable, and can be focal/weak, or diffuse and strong. Also important, the p63 staining pattern is typically diffuse or random, not biphasic, in contrast to some histologic mimickers (see differential diagnosis). It must also me noted that the characteristic p63/p40 staining patterns appear to be dependent on laboratory conditions; occasional studies have not found this pattern to be as consistent as others.3,19
Molecular testing
In 2014, Weinreb et al. were the first to report the molecular signature of PAC21,22. The majority (73–89%) of PAC harbors PRKD1 E710D hotspot mutation2,15,22,23, whereas 6–11% contain fusions involving PRKD1, PRKD2 or PRKD3 genes2,15,21. The presence of PRKD alterations is highly specific for PAC/cribriform adenocarcinoma of salivary gland (CASG, see section below) and has not been reported in other salivary gland neoplasms22,23, rendering it a useful molecular diagnostic tool for PAC.
Cribriform adenocarcinoma of salivary gland (CASG)
In 1999, Michal et al. first coined the terminology “cribriform adenocarcinoma of the tongue” to describe a salivary gland carcinoma with a propensity to posterior tongue, lobulated architecture, a predominant solid, microcystic and cribriform growth pattern, and a uniform type of tumor cells with ground-glass optically-cleared nuclei24. Subsequent studies show that although cribriform adenocarcinoma most frequently occurs in posterior tongue/base of tongue, it may also affect palate, buccal mucosa, lip, tonsil, sinonasal tract, and rarely major salivary glands2,3,15,21,25–27. Therefore, a revised terminology of CASG has been suggested.
CASG shares certain histologic, immunophenotypic and molecular similarity with PAC. A comparison between PAC and CASG is provided in Table 1. Histologically, both tumors are composed of one type of cells with pale optically-cleared nuclei (Figure 4). Unlike classic PAC which usually shows an infiltrative growth and various architectural patterns, CASG tends to have lobulated growth separated by fibrous septa, and relatively uniform architecture predominantly composed of solid, microcystic, and/or cribriform patterns. Peripheral palisading and clefts, hemorrhage, and glomeruloid-like structures are common. The immunoprofile of CASG is indistinguishable from PAC.
Table 1.
Comparison between polymorphous adenocarcinoma (PAC) and cribriform adenocarcinoma of salivary gland (CASG).
| PAC | CASG | |
|---|---|---|
| Primary site | Palate (49%−87%), MSG (13–19%), BOT (0–9%), major salivary gland (0–9%) | BOT (24–61%), MSG (26–29%), palate (1357%), major salivary gland (0–10%) |
| Growth pattern | Infiltrative, often with targetoid arrangement around nerves and vessels | Lobulated architecture separated by fibrous septa |
| Architecture | Highly variable, including single file arrangement, tubular, trabecular, reticular, solid, cribriform, and papillary | Relatively uniform, enriched in solid, microcystic, cribriform, and papillary growth |
| Cytologic features | One type of tumor cells with uniform pale open nuclei resembling papillary thyroid carcinoma | |
| Perineural invasion | Common (60–75%) | uncommon (38%) |
| Immunohistochemistry | Typically positive for: S100, CK7, SOX10, and p63 Occasionally positive for: GFAP, SMA, MSA, and EMA Typically negative for: p40 |
|
| PRKD1 E710D hotspot mutation | 73–89% | 0–20% |
| PRKD1/2/3 fusion | 6–11% | 70–94% |
| Nodal metastasis at presentation | 4–6% | 7–100% |
BOT: base of tongue/posterior tongue; MSG: minor salivary gland outside of palate and BOT.
Note that a subset (25 to 30%) of PAC/CASG spectrum shows indeterminate histologic features and may be difficult if not impossible to classified as PAC or CASG
Figure 4. Cribriform adenocarcinoma of salivary glands (CASG).
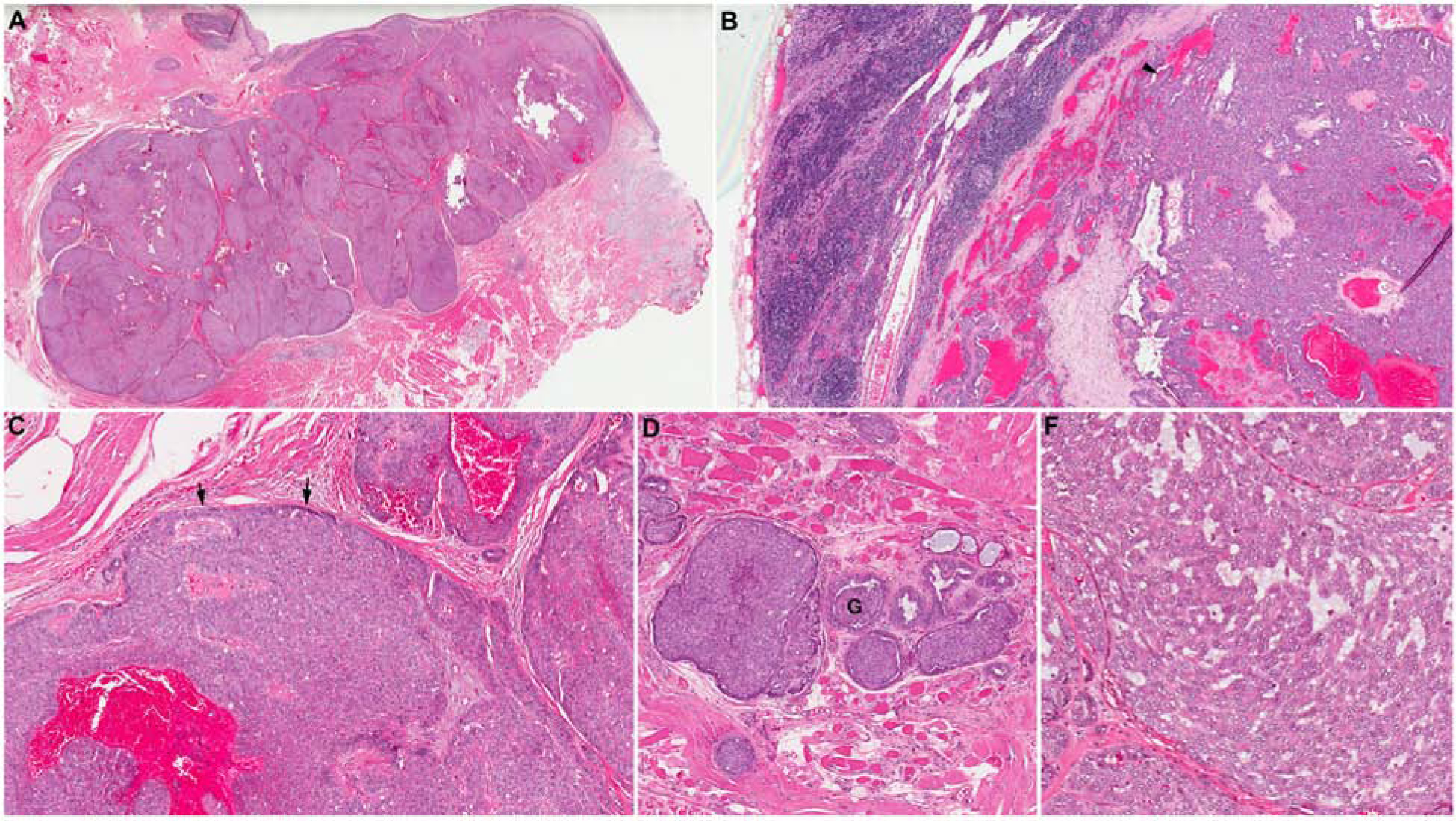
(A) A CASG originated from base of tongue is composed of multiple tightly packed tumor nodules separated by fibrous septa. (B) The tumor is associated with nodal metastases at presentation. (C) CASG typically shows solid growth pattern with peripheral palisading (black arrows). Pools of blood (“blood lake”) are a common histologic finding within the tumor. (D) CASG often has peripheral clefts within the tumor nests, giving the glomeruloid (G) appearance. (E) Microcystic architecture is common. The tumor is composed of a single type of tumor cells with optically clear nuclei.
Recently, it was reported that 75–94% of CASG harbor fusions involving PRKD1, PRKD2 or PRKD3 genes2,15,21, with the fusion partners being ARID1A or DDX3X2,21. Although Weinreb et al. only reported PRKD fusions in CASG, PRKD1 E710D hotspot mutations is subsequently detected in a small subset (13–20%) of CASG2,15.
In the initial series of 8 cases reported by Michal et al., all patients had lymph node metastasis at presentation, which suggests a more aggressive clinical behavior of CASG. However, subsequent studies have reported a wide range of frequency of nodal metastasis at presentation from 7% to 65%3,27,28. The great variation of nodal metastasis frequency reported in CASG may in part be attributed to the rarity of this tumor and the capability of a pathologist to recognize these tumors. A recent study has shown that only a fair interobserver agreement is achieved among expert head and neck pathologists in diagnosing CASG15. Nevertheless, the risk of nodal metastasis in CASG appears to be overall higher compared with 4–6% risk observed in patients with PAC3,7,10,11.
Interestingly, tumors with PRKD fusion, regardless of the histologic classification of PAC or CASG, are associated with a 50% initial risk of lymph node metastasis compared with 0% risk in tumors with PRKD1 mutation2. Therefore, in challenging cases that are difficult to be further classified as PAC or CASG, molecular testing for PRKD genes may provide additional prognostic information in term of risk of nodal metastasis. On the other hand, the high rate of metastasis may also be partly a function of the frequent oropharyngeal location of CASG. It is well established that squamous cell carcinomas of the oropharynx frequently metastasize to cervical lymph nodes due to the unique microanatomy of this location, and other salivary gland tumor types show a higher rate of regional metastases when arising in the oropharynx.29
Currently, CASG is considered as a variant of PAC by the WHO classification1. However, this is controversial as some authors consider CASG and PAC as two separate entities given the differences in histologic features and underlying molecular alterations30. Recent data suggest that CASG and PAC may represent a morphologic spectrum of the same tumor. Although the classic cases of PAC and CASG can be distinguished histologically, there is a subset of 25 to 30% of cases showing indeterminate histologic features that are difficult if not impossible to be labelled definitively as either conventional PAC or CASG2,3,15,21. The interobserver agreement even among expert head and neck pathologists in further classifying these tumors are fair to moderate at most15. Taken together, the findings suggest that PAC/CASG may represent a spectrum with classic PAC and PRKD1 hotspot mutation at one end, CASG and PRKD fusions at the other, and indeterminate neoplasms with overlapping histology and molecular alterations in the middle.
Differential Diagnosis
Adenoid cystic carcinoma
Table 2 provides a comparison between adenoid cystic carcinoma and PAC. Similar to PAC, adenoid cystic carcinoma predominantly occurs in minor salivary glands31–34. Approximately 21–26% occur in the palate31–34. Histologically, unlike PAC/CASG which exhibits one cell type, adenoid cystic carcinoma is biphasic and is composed of two cell types: ductal (epithelial) and myoepithelial. This feature is particularly important to differentiate adenoid cystic carcinoma from CASG as both may show a predominant cribriform pattern with myxoid stroma and perineural invasion. The myoepithelial cells of adenoid cystic carcinoma typically contain angulated dark nuclei, giving adenoid cystic carcinoma its basaloid appearance. Both PAC and adenoid cystic carcinoma are commonly infiltrative, display various architectural patterns, and may contain myxoid stroma and hyalinized globules. However, adenoid cystic carcinoma is characterized with tubular, cribriform and solid growth, whereas lacks other patterns that may be seen in PAC (e.g. single filing arrangement, papillary architecture) and seems less architecturally diverse. Given the biphasic nature of adenoid cystic carcinoma, the immunohistochemical profile is usually more variable, showing patchy rather than diffuse staining for S100 and CK7. Recent reports have shown that adenoid cystic carcinoma is commonly p63 (+)/p40 (+), compared to p63 (+)/p40 (−) that is observed in PAC19,20. Moreover, the pattern of p63 and p40 in adenoid cystic carcinoma is clearly biphasic, while p63 staining in PAC is haphazard. In challenging cases, molecular testing for MYB, MYBL1 or NFIB gene fusions may be of use as 60–90% of adenoid cystic carcinoma carries signature fusions, in particular t(6,9) MYB-NFIB fusion31,35,36. Clinically, adenoid cystic carcinoma is associated with a dismal long-term outcome, compared with an excellent prognosis of PAC31–34.
Table 2.
Comparison between PAC and adenoid cystic carcinoma.
| PAC | Adenoid cystic carcinoma | |
|---|---|---|
| Primary site | Minor salivary gland: > 90%
|
Minor salivary gland: 61–63%
|
| Architecture | Highly variable Single filing arrangement, tubular, trabecular, reticular, solid, cribriform, and papillary |
Variable Tubular, cribriform, and solid |
| Cell composition | Cytologic uniformity: one type of tumor cells only | Biphasic showing ductal (epithelial) and myoepithelial differentiation |
| Nuclear features | Pale nuclei and open chromatin | Dark angulated nuclei |
| Perineural invasion | Common: 60–75% | Common: 88% |
| Immunohistochemistry | S100: diffuse and strong CK7: diffuse and strong p63 positive/p40 negative |
S100: patchy, in myoepithelial or ductal cells CK7: patchy, predominantly in ductal cells p63 positive/p40 positive in myoepithelial cells only |
| Molecular profile |
PRKD1 E710D mutation: 73–89% PRKD1/2/3 fusion: 6–11% |
Fusion involving MYB, MYBL1, or NFIB genes, most common being MYB-NFIB fusion: 60–90% |
| Outcome | Excellent 10-year DSS: 94–99% Risk of distant metastasis: 0–3% |
Poor 5-year DSS: 55–89% Risk of distant metastasis: 8–46% |
DSS: disease specific survival
Other S100-positive salivary gland neoplasms
As PAC is diffusely positive for S100, its differential diagnosis, especially in small biopsy materials, includes other S100-positive salivary gland neoplasms such as secretory carcinoma, myoepithelial carcinoma, and epithelial-myoepithelial carcinoma.
Myoepithelial carcinoma and PAC share certain histologic and immunophenotypic similarities. Both tumors are composed of one type of cells, contain myxoid stroma, and may be positive for S100 and myoepithelial markers, e.g. GFAP, p63, SMA, and MSA. However, myoepithelial carcinoma typically shows expansile lobulated growth pattern and lacks the architectural diversity observed in PAC. Perineural invasion is uncommon in myoepithelial carcinoma37,38. Myoepithelial carcinomas that are positive for p63 are usually also positive for p40, in contrast to PAC which is usually p63 positive but p40 negative. In challenging cases, molecular testing for PRKD alteration may serve as a useful ancillary diagnostic tool.
Epithelial myoepithelial carcinoma is not typically as infiltrative as PAC and is composed of ductal and myoepithelial cells instead of one cell type. Moreover, S100 is not diffuse and stain the myoepithelial component in epithelial myoepithelial carcinoma. Both p63 and p40 are positive in the myoepithelial cell component of epithelial myoepithelial carcinoma, resulting in a biphasic staining pattern.
Compared with PAC, secretory carcinoma more often shows microcystic and papillary-cystic architecture, and does not commonly show cribriform or single cell patterns. Secretory carcinoma harbors ETV6 fusions39,40. GATA3, mammaglobin, and CK7 can be positive in both PAC and secretory carcinoma10,18,41,42.
Diagnosis
In typical cases, histology alone is sufficient for the diagnosis. A diagnosis of PAC can be rendered using the following 3 characteristics:
Typical histologic features of PAC characterized by architectural diversity and cytologic uniformity with pale nuclei and fine chromatin.
An immunoprofile of diffuse and strong S100 and CK7 positivity.
The presence of PRKD alterations, in particular PRKD1 E710D hotspot mutation.
Prognosis
PAC has an overall excellent prognosis with a 10-year disease specific survival of 94%−99%4,5,28 and a 10-year recurrence free survival of 83–88%4,28. Distant metastasis is rare in PAC but may occur in up to 3% of cases3,5. Histologic architecture with ≥10% papillary pattern or ≥30% cribriform pattern has been shown to be an independent adverse prognostic factor associated with decreased disease free survival3. As discussed above, CASG and PRKD1/2/3 fusion are associated with an increased risk of lymph node metastasis2,24,27.
Summary
PAC is a salivary gland carcinoma that often occurs in minor salivary gland location, in particular palate. It is characterized by cytologic uniformity, architectural diversity, an immunoprofile with S100 (+)/CK7 (+)/p63 (+)/p40 (−), PRKD1 E710D hotspot mutation and an excellent outcome. CASG shares histologic and immunohistochemical features with PAC and has a propensity to base of tongue location. CASG is characterized by lobulated growth pattern, predominant solid and cribriform architectures, high frequency of PRKD fusions and an increased risk of nodal metastasis. Regardless of the diagnosis of PAC or CASG, tumors with ≥10% of papillae or ≥30% cribriform architecture associated with decreased disease specific survival.
KEY POINTS.
Polymorphous adenocarcinoma (PAC) is characterized with cytologic uniformity, architectural diversity, and frequent PRKD1 hotspot mutations.
Cribriform adenocarcinoma of salivary gland (CASG) is a tumor with lobulated growth pattern, predominant solid/microcystic/cribriform architecture, peripheral palisading, glomeruloid structures, and high frequency of PRKD1, PRKD2, or PRKD3 fusion.
PAC and CASG may represent tumors in the same morphologic spectrum; however, some pathologists regard them as two separate entities.
Although PAC and CASG commonly affect minor salivary glands, they may occur in major salivary glands.
Tumors with ≥30% cribriform or ≥10% papillary architectural patterns are associated with decreased disease free survival.
SYNOPSIS.
Polymorphous adenocarcinoma (PAC) is typically originated from the minor salivary glands and is characterized by cytology uniformity and architectural diversity. PAC commonly harbors PRKD1 E710D mutation. PAC has an excellent prognosis. However, ≥10% papillary or ≥30% cribriform pattern is an independent adverse prognostic factor. Cribriform adenocarcinoma of salivary gland (CASG) is a controversial entity that is considered within the same histologic spectrum of PAC in current classification schemes; however, it is regarded by some pathologists as a separate entity. CASG shows a propensity to base of tongue location, a lobulated growth pattern, a predominant solid/cribriform architecture, and a high frequency of PRKD1/2/3 fusion.
Clinics Care Points.
PAC has a propensity to minor salivary gland, in particular the palate. However, PAC may affect major salivary glands on occasion.
Compared with biphasic salivary gland tumors e.g. adenoid cystic carcinoma, pleomorphic adenoma, and epithelial-myoepithelial carcinoma, PAC is composed of one type of tumor cells with pale nuclei.
PAC is among one of the three salivary gland carcinomas exhibiting diffuse and strong S100 positivity and one cell type; the others are secretory carcinoma and myoepithelial carcinoma.
The presence of focal uncommon features, e.g. oncocytes, mucocytes, foamy cells/sebaceous differentiation and calcification, does not exclude PAC.
A typical case of CASG can be recognized by its lobulated growth pattern, predominant solid and cribriform architecture, peripheral clefting, and glomeruloid structure. Neck dissection may be considered as CASG is associated with a relatively high frequency of nodal metastasis.
Acknowledgments
Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
The Authors have nothing to disclose.
References
- 1.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. World Health Organization Classification of Tumours: pathology and genetics of head and neck tumours (4th edition). Lyon: International Agency for Research on Cancer (IARC); 2017. [Google Scholar]
- 2.Sebastiao APM, Xu B, Lozada JR, et al. Histologic spectrum of polymorphous adenocarcinoma of the salivary gland harbor genetic alterations affecting PRKD genes. Mod Pathol. 2020;33(1):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu B, Aneja A, Ghossein R, Katabi N. Predictors of Outcome in the Phenotypic Spectrum of Polymorphous Low-grade Adenocarcinoma (PLGA) and Cribriform Adenocarcinoma of Salivary Gland (CASG): A Retrospective Study of 69 Patients. Am J Surg Pathol. 2016;40(11):1526–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elhakim MT, Breinholt H, Godballe C, et al. Polymorphous low-grade adenocarcinoma: A Danish national study. Oral Oncol. 2016;55:6–10. [DOI] [PubMed] [Google Scholar]
- 5.Patel TD, Vazquez A, Marchiano E, Park RC, Baredes S, Eloy JA. Polymorphous low-grade adenocarcinoma of the head and neck: A population-based study of 460 cases. Laryngoscope. 2015;125(7):1644–1649. [DOI] [PubMed] [Google Scholar]
- 6.Seethala RR, Johnson JT, Barnes EL, Myers EN. Polymorphous low-grade adenocarcinoma: the University of Pittsburgh experience. Arch Otolaryngol Head Neck Surg. 2010;136(4):385–392. [DOI] [PubMed] [Google Scholar]
- 7.Evans HL, Luna MA. Polymorphous low-grade adenocarcinoma: a study of 40 cases with long-term follow up and an evaluation of the importance of papillary areas. Am J Surg Pathol. 2000;24(10):1319–1328. [DOI] [PubMed] [Google Scholar]
- 8.Evans HL, Batsakis JG. Polymorphous low-grade adenocarcinoma of minor salivary glands. A study of 14 cases of a distinctive neoplasm. Cancer. 1984;53(4):935–942. [DOI] [PubMed] [Google Scholar]
- 9.Vincent SD, Hammond HL, Finkelstein MW. Clinical and therapeutic features of polymorphous low-grade adenocarcinoma. Oral surgery, oral medicine, and oral pathology. 1994;77(1):41–47. [DOI] [PubMed] [Google Scholar]
- 10.Castle JT, Thompson LD, Frommelt RA, Wenig BM, Kessler HP. Polymorphous low grade adenocarcinoma: a clinicopathologic study of 164 cases. Cancer. 1999;86(2):207–219. [PubMed] [Google Scholar]
- 11.Kimple AJ, Austin GK, Shah RN, et al. Polymorphous low-grade adenocarcinoma: a case series and determination of recurrence. Laryngoscope. 2014;124(12):2714–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Ordonez B, Linkov I, Huvos AG. Polymorphous low-grade adenocarcinoma of minor salivary glands: a study of 17 cases with emphasis on cell differentiation. Histopathology. 1998;32(6):521–529. [DOI] [PubMed] [Google Scholar]
- 13.Simpson RH, Pereira EM, Ribeiro AC, Abdulkadir A, Reis-Filho JS. Polymorphous low-grade adenocarcinoma of the salivary glands with transformation to high-grade carcinoma. Histopathology. 2002;41(3):250–259. [DOI] [PubMed] [Google Scholar]
- 14.Pelkey TJ, Mills SE. Histologic transformation of polymorphous low-grade adenocarcinoma of salivary gland. American journal of clinical pathology. 1999;111(6):785–791. [DOI] [PubMed] [Google Scholar]
- 15.Xu B, Barbieri AL, Bishop JA, et al. Histologic Classification and Molecular Signature of Polymorphous Adenocarcinoma (PAC) and Cribriform Adenocarcinoma of Salivary Gland (CASG): An International Interobserver Study. Am J Surg Pathol. 2020;44(4):545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gnepp DR, Chen JC, Warren C. Polymorphous low-grade adenocarcinoma of minor salivary gland. An immunohistochemical and clinicopathologic study. Am J Surg Pathol. 1988;12(6):461–468. [DOI] [PubMed] [Google Scholar]
- 17.Regezi JA, Zarbo RJ, Stewart JC, Courtney RM. Polymorphous low-grade adenocarcinoma of minor salivary gland. A comparative histologic and immunohistochemical study. Oral surgery, oral medicine, and oral pathology. 1991;71(4):469–475. [DOI] [PubMed] [Google Scholar]
- 18.Adkins BD, Geromes A, Zhang LY, et al. SOX10 and GATA3 in Adenoid Cystic Carcinoma and Polymorphous Adenocarcinoma. Head Neck Pathol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atiq A, Mushtaq S, Hassan U, Loya A, Hussain M, Akhter N. Utility of p63 and p40 in Distinguishing Polymorphous Adenocarcinoma and Adenoid Cystic Carcinoma. Asian Pac J Cancer Prev. 2019;20(10):2917–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rooper L, Sharma R, Bishop JA. Polymorphous low grade adenocarcinoma has a consistent p63+/p40− immunophenotype that helps distinguish it from adenoid cystic carcinoma and cellular pleomorphic adenoma. Head Neck Pathol. 2015;9(1):79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinreb I, Zhang L, Tirunagari LM, et al. Novel PRKD gene rearrangements and variant fusions in cribriform adenocarcinoma of salivary gland origin. Genes Chromosomes Cancer. 2014;53(10):845–856. [DOI] [PubMed] [Google Scholar]
- 22.Weinreb I, Piscuoglio S, Martelotto LG, et al. Hotspot activating PRKD1 somatic mutations in polymorphous low-grade adenocarcinomas of the salivary glands. Nat Genet. 2014;46(11):1166–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andreasen S, Melchior LC, Kiss K, et al. The PRKD1 E710D hotspot mutation is highly specific in separating polymorphous adenocarcinoma of the palate from adenoid cystic carcinoma and pleomorphic adenoma on FNA. Cancer Cytopathol. 2018;126(4):275–281. [DOI] [PubMed] [Google Scholar]
- 24.Michal M, Skalova A, Simpson RH, et al. Cribriform adenocarcinoma of the tongue: a hitherto unrecognized type of adenocarcinoma characteristically occurring in the tongue. Histopathology. 1999;35(6):495–501. [DOI] [PubMed] [Google Scholar]
- 25.Michal M, Kacerovska D, Kazakov DV. Cribriform adenocarcinoma of the tongue and minor salivary glands: a review. Head Neck Pathol. 2013;7 Suppl 1:S3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laco J, Kamaradova K, Vitkova P, et al. Cribriform adenocarcinoma of minor salivary glands may express galectin-3, cytokeratin 19, and HBME-1 and contains polymorphisms of RET and H-RAS proto-oncogenes. Virchows Arch. 2012;461(5):531–540. [DOI] [PubMed] [Google Scholar]
- 27.Skalova A, Sima R, Kaspirkova-Nemcova J, et al. Cribriform adenocarcinoma of minor salivary gland origin principally affecting the tongue: characterization of new entity. Am J Surg Pathol. 2011;35(8):1168–1176. [DOI] [PubMed] [Google Scholar]
- 28.Mimica X, Katabi N, McGill MR, et al. Polymorphous adenocarcinoma of salivary glands. Oral Oncol. 2019;95:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navale P, Rooper LM, Bishop JA, Westra WH. Mucoepidermoid carcinoma of the oropharynx: a tumor type with a propensity for regional metastasis unrelated to histologic grade. Hum Pathol. 2019;93:1–5. [DOI] [PubMed] [Google Scholar]
- 30.Gnepp DR. Salivary gland tumor “wishes” to add to the next WHO Tumor Classification: sclerosing polycystic adenosis, mammary analogue secretory carcinoma, cribriform adenocarcinoma of the tongue and other sites, and mucinous variant of myoepithelioma. Head Neck Pathol. 2014;8(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu B, Drill E, Ho A, et al. Predictors of Outcome in Adenoid Cystic Carcinoma of Salivary Glands: A Clinicopathologic Study With Correlation Between MYB Fusion and Protein Expression. Am J Surg Pathol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moran JJ, Becker SM, Brady LW, Rambo VB. Adenoid cystic carcinoma. A clinicopathological study. Cancer. 1961;14:1235–1250. [DOI] [PubMed] [Google Scholar]
- 33.Spiro RH, Huvos AG, Strong EW. Adenoid cystic carcinoma of salivary origin. A clinicopathologic study of 242 cases. Am J Surg. 1974;128(4):512–520. [DOI] [PubMed] [Google Scholar]
- 34.Fordice J, Kershaw C, El-Naggar A, Goepfert H. Adenoid cystic carcinoma of the head and neck: predictors of morbidity and mortality. Arch Otolaryngol Head Neck Surg. 1999;125(2):149–152. [DOI] [PubMed] [Google Scholar]
- 35.Mitani Y, Li J, Rao PH, et al. Comprehensive analysis of the MYB-NFIB gene fusion in salivary adenoid cystic carcinoma: Incidence, variability, and clinicopathologic significance. Clin Cancer Res. 2010;16(19):4722–4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Persson M, Andren Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(44):18740–18744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong M, Drill EN, Morris L, et al. Prognostic factors in myoepithelial carcinoma of salivary glands: a clinicopathologic study of 48 cases. Am J Surg Pathol. 2015;39(7):931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu B, Mneimneh W, Torrence DE, et al. Misinterpreted Myoepithelial Carcinoma of Salivary Gland: A Challenging and Potentially Significant Pitfall. Am J Surg Pathol. 2019;43(5):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skalova A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34(5):599–608. [DOI] [PubMed] [Google Scholar]
- 40.Xu B, Haroon Al Rasheed MR, Antonescu CR, et al. Pan-Trk immunohistochemistry is a sensitive and specific ancillary tool for diagnosing secretory carcinoma of the salivary gland and detecting ETV6-NTRK3 fusion. Histopathology. 2020;76(3):375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz LE, Begum S, Westra WH, Bishop JA. GATA3 immunohistochemical expression in salivary gland neoplasms. Head Neck Pathol. 2013;7(4):311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bishop JA, Yonescu R, Batista D, Begum S, Eisele DW, Westra WH. Utility of mammaglobin immunohistochemistry as a proxy marker for the ETV6-NTRK3 translocation in the diagnosis of salivary mammary analogue secretory carcinoma. Hum Pathol. 2013;44(10):1982–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


