Abstract
Context
Shoulder pain is the main cause of missed or modified training in competitive swimmers. Shoulder musculoskeletal maladaptations occur to some extent as a consequence of training loads during swimming that may increase the risk of shoulder injury. Further evidence is needed to understand the training intensities at which these maladaptations occur.
Objective
To determine the acute effect of training intensity on the shoulder musculoskeletal physical qualities associated with shoulder injury in competitive swimmers.
Design
Cross-sectional study.
Setting
Indoor swimming pool.
Patients or Other Participants
Sixteen asymptomatic national- and regional-level swimmers (7 females, 9 males; age = 14.6 ± 3.9 years, height = 160.5 ± 12.7 cm, mass = 55.3 ± 12.5 kg).
Main Outcome Measure(s)
Bilateral active shoulder-rotation range of motion (ROM), joint position sense, latissimus dorsi length, combined elevation test, and shoulder-rotation isometric peak torque and handgrip peak force normalized to body weight were measured before and immediately after low- and high-intensity swim-training sessions. The intensity of the sessions was determined by the distance swum over or at the pace threshold and confirmed by the swimmer's rating of perceived exertion.
Results
After the high-intensity training session, shoulder external-rotation ROM (dominant side: P < .001, change = −7.8°; d = 1.10; nondominant side: P = .002, change = −6.5°, d = 1.02), internal-rotator isometric peak torque (dominant side: P < .001, change = −11.4%, d = 0.42; nondominant side: P = .03, change = −6.6%, d = 0.20), and external-rotator isometric peak torque (dominant side: P = .004, change = −8.7%, d = 0.27; nondominant side: P = .02, change = −7.6%, d = 0.25) were reduced. No changes were found in any of the outcome measures after the low-intensity session.
Conclusions
Shoulder active external-rotation ROM and rotation isometric peak torque were decreased immediately after a high-intensity training session, possibly increasing the risk of injury during subsequent training. Monitoring these variables may help practitioners adjust and manage training loads to decrease the risk of shoulder injury.
Keywords: shoulder pain, shoulder injury, fatigue, training loads
Key Points
The intensity of the swim-training session, which can be easily measured using the rating of perceived exertion, may be an important factor that can lead to maladaptive changes in the physical qualities of the shoulder.
Active shoulder external-rotation range of motion and rotation isometric peak torque were immediately decreased after a high-intensity but not after a low-intensity training session, with predominant changes on the dominant side.
Maladaptive changes in the physical qualities of the shoulder after a high-intensity training session probably increase the risk of shoulder injury during the training that follows.
The shoulder is the most commonly injured body part in swimmers, accounting for 31% to 39% of all injuries.1,2 This might be explained by the fact that 90% of the propulsive forces during swimming are generated by the upper limbs.3 In addition, competitive swimmers swim approximately 10 000 to 14 000 m/day 6 or 7 times per week.3 This amount of training volume combined with the repetitive nature of the sport predisposes athletes to many shoulder overuse injuries.1,2 The prevalence of shoulder pain in competitive swimmers has been reported to be between 26% and 91%.4–6 Despite this high prevalence, most swimmers do not discontinue training because of shoulder pain.4 This is reflected in the low amount of time loss from training and competition reported as a consequence of shoulder concerns.2,5 Therefore, shoulder pain might interfere with training and competition performance, leading to the development of chronic injuries and in some cases to retirement from sport participation.4
The cause of musculoskeletal injuries in sport is dynamic and multifactorial.7 Emerging evidence8 has indicated that inadequate management of training loads is a major risk factor for injury. In their workload-injury etiology model, Windt and Gabbett9 suggested that the risk of injury changes dynamically as a result of the training loads applied and their effects on modifiable risk factors. Training loads can cause positive physiological adaptations (eg, fitness) that alter modifiable risk factors positively, decreasing the risk of injury. However, training loads can also cause negative physiological effects (eg, fatigue), altering modifiable risk factors and increasing the injury risk during subsequent training.9 The authors suggested the importance of understanding the interactions between training loads and modifiable risk factors for decreasing the risk of injury.9 This is supported by the complex-systems approach to sports injuries proposed by Bittencourt et al,7 who emphasized understanding the interactions among risk factors so as to identify injury risk profiles of an athlete or group of athletes. Several potential modifiable risk factors for shoulder pain, such as alterations in the physical qualities of the shoulder (eg, range of motion [ROM], flexibility, and strength), have been identified in swimmers. Regarding ROM and flexibility, reduced internal-rotation (IR) ROM,6 increased10,11 and decreased external-rotation (ER) ROM,11 reduced latissimus dorsi (LD) length,6 and reduced pectoralis minor length6 have been reported. Furthermore, reduced shoulder internal-rotator force6,12 and external-rotator endurance13 have been found in swimmers with shoulder pain.
Other physical qualities, such as shoulder joint position sense (JPS), results of the combined elevation test (CET), and handgrip force (HGF), are also considered important when clinicians examine swimmers. Although these have not been reported as risk factors for shoulder pain in this population, they are regularly used in clinical practice. Joint position sense is a submodality of proprioception and is defined as the ability to consciously recognize the position of a joint in space.14 Proprioception is essential for the practice of sport-related activities, providing neuromuscular control and joint stability.14 The CET is a screening tool used to assess the strength and mobility of the upper limb and thoracic spine.15 The movement performed during the CET is essential for achieving a high elbow position during a swimming stroke.15 This is important, because a dropped elbow has been suggested as a sign of potential shoulder injury.3 Finally, the HGF provides an objective indicator of the functional status of the upper limb and has also been proposed as an indirect assessment of posterior cuff function.16 Considering that training intensity is an important component of training loads,9 it is important to understand the effects of training intensity on these physical qualities.
To date, the effect of swim-training loads on the physical qualities of the shoulder in competitive swimmers has been investigated in only 2 studies.17,18 Matthews et al18 found a bilateral decrease in ER ROM and an increase in JPS error in the dominant extremity after swim training in 17 national youth swimmers. In a later study, Higson et al17 observed reduced ER ROM and pectoralis minor length and increased JPS errors after swim training in 16 elite swimmers. Based on the current evidence, shoulder maladaptation occurs immediately after swim training, which may increase the risk of shoulder injury. However, these researchers measured the effect of only 1 type of training and, thus, only 1 training intensity. No one has investigated the effect of different training intensities on the physical qualities of the shoulder. Understanding how the physical qualities of the shoulder are affected by training intensity could help inform researchers and clinicians on the appropriate management of training loads. The aim of appropriate load management is to maximize adaptation and performance while minimizing the risk of injury.8 This includes adequate prescription, monitoring, and adjustment of training loads.8 Our study may provide information about which physical qualities need to be monitored. Monitoring might help to inform researchers on the appropriate timing of high-intensity training for enhancing load capacity and performance without increasing the detrimental effects on these physical qualities. It may also help to identify postswim deficits and permit early interventions to reduce the susceptibility to shoulder injury. Furthermore, considering the multifactorial nature of sport injuries, assessment of more physical qualities is needed to support the current findings. To our knowledge, no authors have addressed the effect of training loads on LD length, CET, and HGF in swimmers. Therefore, the aim of our study was to determine the acute effect of training intensity on the musculoskeletal physical qualities associated with shoulder injury in competitive swimmers.
METHODS
Participants
We conducted this cross-sectional study among a swimming squad to assess the effects of swim-training intensity on the physical qualities of the shoulder. Sixteen regional- and national-level swimmers were part of a convenience sample. According to an a priori power analysis (version 3.1.9.2; G*Power, Heinrich-Heine-Universität, Düsseldorf, Germany) using the t test for means (1 group), a sample size of 15 participants would be required to detect a large effect size (0.8) after swim training, with a power of 0.80 and an α level of .05. The sample consisted of 7 female and 9 male participants (age = 14.6 ± 3.9 years [range = 11–20 years], height = 160.5 ± 12.7 cm, mass = 55.3 ± 12.5 kg). All swimmers trained in the same group during the year and completed the same practices regularly, regardless of age and level of competition. The participants had a mean of 6 years of regular swimming experience (range = 4–8 years), performed a mean of 5.5 days of swim training per week (range = 5–6 days), and completed a swimming volume of 35 000 ± 5000 m/week. All swimmers were regularly active in regional and national championships. The exclusion criteria were a history of shoulder surgery, shoulder pain at the time of the study, and any pain in the 2 weeks before the study that interfered with the ability to train or compete fully.17 All participants provided written informed consent. For participants <18 years old, parental or guardian signed consent was obtained. The study was approved by our university's ethics board.
Procedures
All tests were performed by the same researcher (M.Y.), who had 8 years of clinical experience. For each swimmer, measurements were recorded before and after low- and high-intensity training sessions. On the testing day, general demographic information of participants, such as sex, age, limb dominance, height, mass, and forearm length, were recorded. Limb dominance was determined by asking participants if they were right- or left-hand dominant. Before the testing, participants performed a standardized land-based warm-up consisting of multiplanar shoulder movements using an elastic band that was supervised by the tester. The warm-up consisted of 10 repetitions of ER and IR (0° of shoulder abduction) with a yellow TheraBand (The Hygenic Corporation, Akron, OH). Immediately after the warm-up, baseline measurements were recorded in the following order: shoulder-rotation ROM, shoulder JPS, shoulder-rotation isometric peak torque, LD length, CET, and handgrip peak force. All tests were standardized, and the dominant side was assessed first. Three trials of each test were performed on both limbs, and the results were averaged for further analysis. Immediately after completion of the training, swimmers exited the pool and repeated the baseline testing. The testing was conducted over 8 weeks because of the availability of only 1 researcher, and participants completed both sessions at least 8 times. Data were collected on the same days each week to ensure that the swimming sessions were the same. The tests were performed in block order: the high-intensity session data were collected on Wednesday afternoons, whereas the low-intensity session data were collected on Friday afternoons of the same week. All swimmers completed an aerobic-kick–focused session on Thursday morning between sessions. No weight training was performed before or after the testing sessions.
Instrumentation and Outcome Measures
The Goniometer Pro (5fuf5 Co, Bloomfield, NJ) digital inclinometer application for the iPhone (Apple, Inc, Cupertino, CA) was used to measure shoulder ROM, JPS, LD length, and CET. Mobile telephone applications are widely used in clinical practice. They have been shown to be reliable and valid when compared with the criterion-standard universal goniometer in patients with symptomatic shoulders.19 A detailed description of each measurement can be found in Supplemental Table 1 (see Supplemental Table 1, available online at http://dx.doi.org/10.4085/1062-6050-0357-19.S1). We measured ROM actively because it reflects the ability of swimmers to use their available movement. To assess IR ROM, we performed scapular stabilization, which has been shown to be more reliable than other methods.20 For ER ROM assessment, the end range was determined using the available range without any stabilization.21 We followed the protocol of Herrington and Horsley22 to assess JPS. The test was performed in the midrange position (20% of the available ER ROM) because feedback in this position relies more on the musculotendinous structures.23 Latissimus dorsi length assessment was based on the protocol of Herrington and Horsley22 using pressure biofeedback (model Pressure Biofeedback Stabilizer; Chattanooga Group, Hixson, TN) to supervise the posterior pelvic tilt movement during the procedure. Finally, the CET was performed using the protocol of Blanch15 in a swimmer population.
Regarding force assessment, a handheld dynamometer (model Hoggan MicroFET2; Scientific LLC, Salt Lake City, UT) was used to measure shoulder-rotation isometric peak torque, which has been shown to be reliable and valid in different populations compared with the criterion-standard isokinetic dynamometry.24 The testing position of 90° of shoulder abduction was used to recreate the mid–pull-through and recovery phases performed during the stroke.25 Force was converted into torque (in newton meters) by multiplying the absolute force (in newtons) by the lever arm length (in meters) of the dominant and nondominant sides. Next, torque was normalized to body weight (Nm/kg) and expressed as the percentage of change between sessions. For HGF assessment, a hand dynamometer (model T.K.K.5001 Grip-A; Takei Scientific Instruments Co, Ltd, Nigata City, Japan) was used. Hand dynamometers are the criterion-standard tool for assessing HGF and have been shown to be reliable in several populations and positions.26 Furthermore, HGF was normalized to body weight and expressed as the percentage of change between sessions.
Training Intensity Definition
Training intensity can be categorized into relative zones (ie, low, moderate, high) based on the stimulus from the training load.27 Training load has been defined as “the cumulative amount of stress placed on an individual from a single or multiple training sessions (structure or unstructured) over a period of time.”8(pg1) According to consensus statements on training loads,8,27 the recommendation is that a combination of external (amount of work performed by the athlete) and internal (athlete's response to external load) training loads should be used to monitor an athlete's response to training. The intensity of the training sessions was based on the external training loads and categorized as low or high. Considering that each session lasted 1 hour and consisted of comparable total volumes of 3 km, the intensity was determined by the volume swum at or above the threshold pace. Threshold pace was previously determined by the coach, and all athletes were familiar with and had experience swimming at this intensity (a hard sustainable pace).
During the low-intensity training session, 0% of the swimming was completed at or above the threshold pace. The session was evenly balanced among the 4 swimming strokes, with the athletes instructed to complete the entire volume at a low-intensity recovery pace. Conversely, during the high-intensity training session, one-third of the volume was dedicated to performing the athlete's number 1 stroke at or above the threshold pace. The remaining swim volume was designated for warm-up, dedicated skill practice, and swim down. A detailed description of each session can be found in Supplemental Table 2 (see Supplemental Table 2, available online at http://dx.doi.org/10.4085/1062-6050-0357-19.S1). The intensity of the session was confirmed by the swimmer's perception of intensity (internal load). Internal loads were quantified by the rating of perceived exertion (RPE) based on the modified version of the category-ratio scale of Borg.28 Immediately after completing the training, the swimmers were asked, “How hard was your workout?” The RPE is a valid and simple measurement for assessing training intensity in athletes and is commonly used to monitor athletes' physiological stress during or after training or competition.28 The RPE method has also been shown to be consistent with objective physiological indices, such as heart rate, in athletes.29
Reliability of Measurements
Before data collection, we performed a pilot study with 10 participants to assess the test-retest reliability of each measurement. We took each measurement before and after a 2-hour period. The rationale for this time frame was that a normal swimming session lasts around 2 hours.17 The intraclass correlation coefficient, standard error of measurement (SEM), and minimal detectable change (MDC) with 95% confidence interval for each physical quality were calculated. These results provided information to enable us to determine whether the changes in the shoulder physical qualities after a swim-training session were real or due to measurement error. The results of this pilot study are shown in Table 1.
Table 1.
Two-Hour Test-Retest Reliability for the Outcome Measures Calculated From the Pilot Study (N = 10)
| Test |
Side |
Intraclass Correlation Coefficient (3,3)a (95% Confidence Interval) |
Standard Error of Measurementb |
Minimal Detectable Changec |
| External-rotation range of motion, ° | Dominant | 0.980 (0.922, 0.995) | 2.39 | 6.61 |
| Nondominant | 0.990 (0.919, 0.998) | 1.70 | 4.72 | |
| Internal-rotation range of motion, ° | Dominant | 0.903 (0.602, 0.976) | 2.17 | 6.02 |
| Nondominant | 0.877 (0.536, 0.969) | 2.28 | 6.33 | |
| Joint position sense, ° | Dominant | 0.943 (0.498, 0.988) | 1.72 | 4.75 |
| Nondominant | 0.886 (0.570, 0.971) | 1.89 | 5.25 | |
| External-rotator force, Nm/kg | Dominant | 0.992 (0.905, 0.998) | 0.02 | 0.05 |
| Nondominant | 0.999 (0.994, 1.000) | 0.01 | 0.02 | |
| Internal-rotator force, Nm/kg | Dominant | 0.982 (0.925, 0.996) | 0.03 | 0.07 |
| Nondominant | 0.997 (0.990, 0.999) | 0.01 | 0.03 | |
| Latissimus dorsi length, ° | Dominant | 0.965 (0.858, 0.991) | 2.43 | 6.74 |
| Nondominant | 0.975 (0.898, 0.994) | 1.99 | 5.51 | |
| Combined elevation test, ° | Dominant | 0.950 (0.791, 0.998) | 2.14 | 5.92 |
| Nondominant | NA | NA | NA | |
| Handgrip force, kg/body mass | Dominant | 0.980 (0.919, 0.995) | 0.02 | 0.05 |
| Nondominant | 0.987 (0.948, 0.997) | 0.01 | 0.04 |
Abbreviation: NA, not applicable.
Two-way mixed model. A coefficient ≥0.90 was considered excellent reliability; ≤0.89 to ≥0.80, good reliability; ≤0.79 to ≥0.70, moderate reliability; and <0.70, low reliability.
Standard deviation × 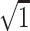 – intraclass correlation coefficient.
– intraclass correlation coefficient.
Calculated as standard error of measurement × 1.96 ×  .
.
Statistical Analysis
For the statistical analysis, SPSS (version 25; IBM Corp, Armonk, NY) was used. The Shapiro-Wilk test was performed to determine if the variables had a normal distribution. The analysis was conducted separately for the dominant and nondominant sides. To determine if a difference in the shoulder physical qualities was present before and after a training session, we used the Wilcoxon signed rank test if the sample was not normally distributed and the paired t test if it was normally distributed. The α level was set at ≤.05. We calculated the Cohen d effect size to determine the magnitude of any difference among measurements. The following effect size values were considered: >0.8 (large), 0.5–0.79 (moderate), 0.49–0.20 (small), and <0.2 (trivial).30
RESULTS
Sixteen swimmers were analyzed before and after the low- and high-intensity training sessions. All swimmers completed both sessions with no dropouts. Regarding the RPE, we observed a difference between sessions (P < .001). Swimmers demonstrated RPE averages of 2.44 ± 1.2 (minimum–maximum = 1–4) and 7.44 ± 1.3 (minimum–maximum = 5–9) for the low- and high-intensity session, respectively. No swimmers experienced shoulder pain during either session. The comparison between pre- and postswim tests for both the low- and high-intensity training sessions are shown in Tables 2 and 3.
Table 2.
High- and Low-Intensity Training Sessions: Preswim and Postswim Rotational Range of Motion, Joint Position Sense, Latissimus Dorsi Length, and Combined Elevation Test (N = 16)
| Session Intensity: Test |
Side |
Mean ± SD |
Mean Difference |
Effect Size |
P Valuea |
|
| Preswim |
Postswim |
|||||
| High intensity | ||||||
| External-rotation range of motion, ° | Dominant | 101.0 ± 6.5 | 93.2 ± 7.5 | −7.8 | 1.10 | <.001b |
| Nondominant | 101.3 ± 7.2 | 94.8 ± 5.5 | −6.5 | 1.02 | .002b | |
| Internal-rotation range of motion, ° | Dominant | 57.5 ± 5.8 | 59.7 ± 7.4 | +2.3 | 0.33 | .19 |
| Nondominant | 59.9 ± 8.6 | 61.5 ± 5.7 | +1.6 | 0.22 | .36 | |
| Joint position sense, ° | Dominant | 5.9 ± 3.1 | 6.1 ± 3.4 | +0.2 | 0.06 | .83 |
| Nondominant | 6.2 ± 3.2 | 6.1 ± 3.1 | −0.1 | 0.03 | .92 | |
| Latissimus dorsi length, ° | Dominant | 134.1 ± 8.5 | 132.3 ± 8.4 | −1.7 | 0.21 | .24 |
| Nondominant | 137.4 ± 8.8 | 135.0 ± 9.3 | −2.4 | 0.27 | .12 | |
| Combined elevation test, ° | Dominant | 2.9 ± 5.4 | 2.1 ± 4.2 | −0.8 | 0.17 | .28 |
| Nondominant | NA | NA | NA | NA | NA | |
| Low intensity | ||||||
| External-rotation range of motion, ° | Dominant | 98.8 ± 7.8 | 100.5 ± 8.1 | +1.7 | 0.21 | .19 |
| Nondominant | 97.2 ± 7.3 | 96.7 ± 5.8 | −0.5 | 0.08 | .66 | |
| Internal-rotation range of motion, ° | Dominant | 59.6 ± 6.2 | 59.0 ± 6.1 | −0.6 | 0.10 | .60 |
| Nondominant | 59.1 ± 7.9 | 61.9 ± 5.7 | +2.8 | 0.41 | .12 | |
| Joint position sense, ° | Dominant | 5.7 ± 2.3 | 7.4 ± 4.1 | +1.7 | 0.53 | .21 |
| Nondominant | 6.4 ± 2.8 | 6.6 ± 3.6 | +0.3 | 0.06 | .73 | |
| Latissimus dorsi length, ° | Dominant | 137.3 ± 12.2 | 135.6 ± 10.2 | −1.7 | 0.15 | .39 |
| Nondominant | 138.2 ± 10.2 | 136.6 ± 9.9 | −1.6 | 0.16 | .39 | |
| Combined elevation test, ° | Dominant | 2.8 ± 3.8 | 2.9 ± 4.5 | +0.1 | 0.02 | .83 |
| Nondominant | NA | NA | NA | NA | NA | |
Abbreviation: NA, not applicable.
Calculated from independent-samples t tests comparing the average number of preswim and postswim scores obtained in each test.
Indicates difference (P < .05).
Table 3.
High- and Low-Intensity Training Sessions: Preswim and Postswim Isometric Peak Torque and Handgrip Force Normalized to Body Weight (N = 16)
| Session Intensity: Test |
Side |
Mean ± SD |
Mean Difference |
% Change, Mean ± SDa |
Effect Size |
P Valueb |
|
| Preswim |
Postswim |
||||||
| High intensity | |||||||
| External-rotator torque, Nm/kg | Dominant | 0.40 ± 0.11 | 0.37 ± 0.11 | −0.03 | −8.7 ± 9.4 | 0.27 | .004c |
| Nondominant | 0.37 ± 0.12 | 0.34 ± 0.11 | −0.03 | −7.6 ± 11.6 | 0.25 | .02c | |
| Internal-rotator torque, Nm/kg | Dominant | 0.46 ± 0.13 | 0.41 ± 0.12 | −0.05 | −11.4 ± 8.6 | 0.42 | <.001c |
| Nondominant | 0.44 ± 0.16 | 0.41 ± 0.14 | −0.03 | −6.6 ± 10.2 | 0.20 | .03c | |
| Handgrip force, kg/body mass | Dominant | 0.43 ± 0.09 | 0.43 ± 0.10 | 0 | 0.3 ± 11.2 | 0 | .92 |
| Nondominant | 0.43 ± 0.10 | 0.41 ± 0.10 | −0.02 | −3.3 ± 11.5 | 0.20 | .23 | |
| Low intensity | |||||||
| External-rotator torque, Nm/kg | Dominant | 0.44 ± 0.15 | 0.42 ± 0.13 | −0.02 | −1.8 ± 10.0 | 0.14 | .15 |
| Nondominant | 0.40 ± 0.12 | 0.39 ± 0.11 | −0.01 | −3.1 ± 9.1 | 0.08 | .16 | |
| Internal-rotator torque, Nm/kg | Dominant | 0.49 ± 0.15 | 0.49 ± 0.14 | 0 | −0.8 ± 8.4 | 0 | .89 |
| Nondominant | 0.50 ± 0.16 | 0.48 ± 0.14 | −0.02 | −1.7 ± 9.5 | 0.13 | .36 | |
| Handgrip force, kg/body mass | Dominant | 0.44 ± 0.12 | 0.43 ± 0.11 | −0.01 | −0.5 ± 15.7 | 0.08 | .59 |
| Nondominant | 0.44 ± 0.10 | 0.43 ± 0.09 | −0.01 | −2.9 ± 5.2 | 0.10 | .02c | |
Change value between sessions expressed as a percentage of body weight.
Calculated from independent-samples t tests comparing the average number of preswim and postswim scores obtained in each test.
Indicates difference (P < .05).
High-Intensity Training Session
We observed changes in ER ROM and rotation isometric peak torque that were different. Box plots showing the differences between the low- and high-intensity sessions for ER ROM and isometric peak torque are displayed in Figures 1 and 2, respectively. Decreases were present in ER ROM, with large effect sizes for the dominant (P < .001; change = −7.8°; d = 1.10) and nondominant (P = .002; change = −6.5°; d = 1.02) sides. Based on the pilot study results, the values of change in ER ROM on the dominant and nondominant sides exceeded the SEM and MDC. A decrease in ER ROM below 93° has been reported as a cutoff value for the development of shoulder pain in swimmers.11 After the training session, 8 of 16 (50%) and 7 of 16 (43.8%) swimmers exhibited a decrease in ER ROM below this value on the dominant and nondominant sides, respectively.
Figure 1.

Box plots showing the change in shoulder-rotation range of motion after low- and high-intensity swimming sessions. A, Dominant shoulder. B, Nondominant shoulder. The lower and upper edges of the box indicate the 25th and 75th percentiles of the sample, respectively. The height of the box indicates the interquartile range, and the line inside the box shows the median. The X inside the box represents the mean. The whiskers represent extreme data points that are no more than 1.5 times the interquartile range from the lower and upper edges of the box. The circles beyond the whiskers represent outliers.
Figure 2.

Box plots showing the percentage of change in shoulder isometric peak torque after low- and high-intensity swimming sessions. A, External rotators in the dominant shoulder. B, External rotators in the nondominant shoulder. C, Internal rotators in the dominant shoulder. D, Internal rotators in the nondominant shoulder. Force was converted into torque by multiplying the absolute force by the lever arm. Torque was normalized to body weight and expressed as a percentage of change between sessions. The lower and upper edges of the box indicate the 25th and 75th percentiles, respectively. The height of the box indicates the interquartile range, and the line inside the box shows the median. The X inside the box represents the mean. The whiskers represent extreme data points that are no more than 1.5 times the interquartile range from the lower and upper edges of the box. The circles beyond the whiskers represent outliers.
Regarding isometric peak torque, we found decreases in the internal rotators, with small effect sizes for the dominant (P < .001; d = 0.42) and nondominant (P = .03; d = 0.20) sides. The changes represented mean decreases of 11.4% (0.05 Nm/kg) and 6.6% (0.03 Nm/kg) in body weight for the dominant and nondominant sides, respectively. For both sides, the value of change exceeded the SEM but not the MDC. With respect to external-rotator isometric peak torque, we observed a decrease for the dominant side, with a small effect size (P = .004; d = 0.27). The change represented a mean decrease of 8.7% (0.03 Nm/kg) of body weight. The value of change exceeded the SEM but not the MDC. Regarding the nondominant side, external-rotator isometric peak torque decreased, with a small effect size (P = .02; d = 0.25). The change represented a mean decrease of 7.6% (0.03 Nm/kg) of body weight. In this case, the value of change exceeded the SEM and MDC. We observed no differences between preswim and postswim measurements for the IR ROM, JPS, LD length, CET, or HGF outcomes.
Low-Intensity Training Session
After the session, only the HGF on the nondominant side decreased, with a trivial effect size (P = .02; d = 0.10). The change represented a mean decrease of 2.9% (0.01 kg/body mass) in body weight. The change did not exceed the SEM or the MDC, probably indicating that it was due to chance or random error. We noted no differences between preswim and postswim measurements in any of the other measurements. Regarding ER ROM, 1 of 16 (6.2%) and 4 of 16 (25%) swimmers displayed decreases below 93° on the dominant and nondominant sides, respectively.
DISCUSSION
Our study was conducted to determine the effect of 2 training intensities on the physical qualities of the shoulder in competitive swimmers. After high-intensity sessions, active ER ROM and rotation isometric peak torque were reduced, but IR ROM, JPS, LD length, CET score, and HGF did not change. However, after the low-intensity session, we identified no changes in any of the physical qualities. Considering the changes in certain physical qualities after the high-intensity session, it was important to establish whether these changes were clinically meaningful. Clinical meaningfulness was determined by the magnitude of the change (ie, effect size) and whether the change values exceeded the SEM and MDC.31 For ER ROM, we observed large effect sizes, with change values that exceeded the MDC, whereas isometric peak torque had small effect sizes, with only the external-rotator isometric peak torque of the nondominant side exceeding the MDC (a detailed explanation of the clinical meaningfulness of each variable is provided in the following subsection). We showed that musculoskeletal adaptations varied in response to training intensity over a short period (ie, 1 training session). This suggests that some physical qualities are in constant fluctuation due to the training loads being applied. Bittencourt et al7 proposed that athletes are open and dynamic systems that interact with the environment and evolve over time. Thus, our results provided information about the short-term interaction between training intensity and the physical qualities of the shoulder in competitive swimmers. We suggest that the intensity of the swim training may be an important factor that influences acute changes in the physical qualities of the shoulder and, therefore, dynamically modifies the potential risk of injury.
In addition to the mean decreases in ER ROM and isometric peak torque after the high-intensity training, the variability of the responses among swimmers was important (Figures 1 and 2). Windt and Gabbett9 proposed that a specific external load elicits different internal responses. Our results support this concept: the same training intensity produced different responses among swimmers. Thus, the shoulder physical qualities need to be regularly monitored, and training loads need to be progressed individually.8
Training Intensity
The intensity of the sessions was defined by the coach and determined by the volume swum at or above the pace threshold. The swimmers exhibited higher RPE values after the high-intensity session (7.44 ± 1.3) than the low-intensity session (2.44 ± 1.2). Based on the modified version of the category-ratio scale of Borg,28 the low-intensity session was perceived as easy, whereas the high-intensity session was perceived as very hard. A mean RPE value of 7 ± 1.3 has been associated with the onset of blood lactate accumulation in female distance runners.32 Hence, the high-intensity session would probably result in the accumulation of blood lactate, leading to fatigue. This might explain the negative effects on ER ROM and rotation isometric peak torque after the high-intensity but not the low-intensity session.
Shoulder-Rotation ROM
Internal-rotation ROM was not affected after the high- or low-intensity training session. These results are in accordance with those of Matthews et al18 and Higson et al,17 who reported no changes in IR ROM after a swim-training session. In contrast, acute reductions in IR ROM of the dominant side have been described after tennis33 and baseball34 training. Researchers33 have indicated that the high levels of eccentric stress placed on the external rotators to decelerate the throwing or striking motion may increase posterior rotator cuff stiffness and consequently decrease IR ROM. The lack of changes found in the studies of swimmers might be explained by the low activation level of the external rotators during the freestyle stroke35 combined with the endurance nature of the sport. Regarding ER ROM, we observed reductions after the high-intensity but not the low-intensity training session. After the high-intensity session, ER ROM decreased by 7.8° on the dominant side and 6.5° on the nondominant side with large effect sizes (dominant side: d = 1.10; nondominant side: d = 1.02). An effect size of 1.0 indicates that the mean of the postsession is at the 84th percentile of the presession; thus, a swimmer with an average score in the postsession had a lower ER ROM score than 84% of the swimmers in the presession.36 Also, the probability of correctly guessing if a swimmer performed a low- or high-intensity session was 69% based on the ER ROM score alone.36 Furthermore, postswim changes in ROM exceeded the MDC on both sides. Therefore, we can be 95% confident that the changes in ER ROM after a high-intensity training session were attributable to the swim training and not due to measurement error. The large effect sizes reported and the values exceeding the MDC confirmed the clinical meaningfulness of the changes in ER ROM.
Authors of 2 studies17,18 of swimmers have noted reductions in ER ROM after a training session. Matthews et al18 found ER ROM decreases of 5.29° on the dominant side and 3.18° on the nondominant side after a fatiguing protocol consisting of 8 sets of a 100-m swim. The effect sizes were moderate for the dominant side (d = 0.75) and small for the nondominant side (d = 0.42). The larger effect sizes in our study may be explained by the greater total training volume (3000 m versus 1000 m). However, given the different definitions of training intensity and measures used to confirm fatigue, it is difficult to compare studies. Matthews et al18 set the swimming intensity at 85% of the swimmers' best 100-m times, and blood lactate levels were used as an objective measure to confirm fatigue. In contrast, in our study, the intensity was set in relation to the threshold pace, and RPE was used as a subjective measure of fatigue. In a later study, Higson et al17 demonstrated a decrease in ER ROM of 3.4°, with a moderate effect size (d = 0.34) after a 2-hour training session. Higson et al17 defined the external training load only in terms of time (2 hours), without specifying the distance or intensity. Furthermore, the internal loads were not measured; therefore, the swimmers' response to the training was unknown. Consensus statements on training loads and injury8,27 recommended combining internal and external training loads to monitor an athlete's response to training. Moreover, subjective measures of internal loads, such as the RPE, could be preferable because they are easily used in the clinical setting.8
The acute reductions in ER ROM after swim training may be explained by the biomechanics of the stroke. The repetitive forces during swimming can lead to hypertrophic changes and muscular tightness of the internal rotators, consequently decreasing ER ROM.17 Deficits in shoulder ER ROM have been shown to be a potential risk factor for shoulder pain in competitive swimmers.11 In a 1-year prospective study, Walker et al11 found that competitive swimmers with ER ROM values <93° measured actively at the beginning of the season were at 12.5 times greater risk of developing shoulder pain that resulted in missed or modified training. The authors11 suggested that limited ER ROM during the recovery phase may contribute to shoulder pathomechanics. Interestingly, after the high-intensity training session, half of our swimmers (8/16) decreased their ER ROM to <93° in the dominant limb. In contrast, after the low-intensity session, only 1 swimmer had an ER ROM of <93° on the dominant side. After a high-intensity training session, active ER ROM decreased to values associated with the risk of shoulder injury in a significant number of swimmers.
Shoulder-Rotation Isometric Peak Torque
Isometric peak torque decreased for both the internal and external rotators after the high-intensity but not the low-intensity session. After the high-intensity session, torque decreased between 6.6% and 11.4% of body weight. In spite of the changes, the effect sizes were small, ranging from 0.20 to 0.42. This indicated that a swimmer with an average score in the postsession had less rotation torque than 58% to 66% of the swimmers in the presession.36 Furthermore, the probability of correctly guessing if a swimmer performed a low- or high-intensity session was between 54% and 58% based on test score alone.36 Only the changes in the external rotators of the nondominant side exceeded the MDC. Therefore, we can be 95% confident that the changes were attributable to the swim training and not to measurement error. The changes in the internal-rotator torque on both sides and external-rotator torque on the dominant side exceeded the SEM but not the MDC. Hence, we can be confident only 68% of the time that the changes were not due to an error. The interpretation of these results indicated that the small effect sizes for isometric peak torque might weaken their clinical meaningfulness. Furthermore, only the changes in the external rotators on the nondominant side exceeded the MDC and, consequently, reflected clinical meaningfulness.
Matthews et al18 were the sole researchers to investigate the effect of swim training on shoulder isometric force, and they reported contradictory findings. Although fatigue was confirmed by blood lactate levels, rotation isometric force did not change after a swim-training session in 17 national-level swimmers.18 Given the different training protocols performed, it is difficult to explain the variable findings between studies. Considering that the participants' ages and levels of competition were similar, the different testing positions might have influenced the results. We assessed force in the supine position, whereas Matthews et al18 measured it in the standing position. Authors37 have suggested that upper limb strength assessments performed in the standing position are influenced by the synergistic effects of the lower limb muscles. The lack of change in shoulder force described by Matthews et al18 may have been due to compensation of the lower limbs.
The acute decrease in internal-rotator torque that we noted may be explained by the predominant internal-rotator forces that occur during swimming.35 Because of the repetitive internal-rotator forces, the subscapularis muscle is constantly active during all stroke phases, stabilizing the glenohumeral joint.35 However, this constant activity may render the subscapularis muscle susceptible to fatigue.35 Deficits in internal-rotator forces have been shown to be a potential risk factor for shoulder pain in swimmers.6,12 Bak and Magnusson12 and Tate et al6 identified decreases in internal-rotator force in the injured shoulders of competitive swimmers, suggesting that internal-rotator deficits may affect stroke dynamics. These findings are supported by Scovazzo et al,38 who used electromyography to demonstrate decreased subscapularis activity during the midrecovery phase in the painful shoulders of swimmers. Regarding external-rotator torque, we reported decreases after the high-intensity session on both the dominant and nondominant sides. The infraspinatus muscle is mainly active during the midrecovery phase to control the internal-rotator forces of the subscapularis muscle, whereas the teres minor muscle controls the internal-rotator forces of the pectoralis major muscle during the pull phase.35 With respect to the relationship between external-rotator weakness and risk of shoulder injury in swimmers, Beach et al13 determined that swimmers with shoulder pain displayed decreased external-rotator endurance as measured using isokinetic dynamometry. Investigators39 have indicated that decreased infraspinatus activity led to glenohumeral instability, which may result in functional impingement. However, given the cross-sectional designs of studies addressing the relationship between shoulder pain and rotator force, whether the force deficits seen were due to pain inhibition or a compensatory strategy to remain pain free is unknown. In addition to ER ROM, we found greater mean reductions in rotation isometric peak torque on the dominant than the nondominant side. An explanation for these findings may be that during swimming, the dominant limb is mainly used for propulsion and the nondominant limb for control and support.17 Despite the greater mean reductions on the dominant side, the changes on the nondominant side were more variable (Figures 1 and 2).
Limitations
Our study had limitations. Although we calculated the necessary sample size, it was small for the competitive swimmer population and probably limits the generalization of the results. The large age range could also have been a limitation because it might not have represented the adaptations of a specific age group. A history of shoulder pain was a nonmodifiable risk factor for shoulder pain in swimmers.1,6 We excluded only swimmers with shoulder pain at the time of the study or any pain in the 2 weeks before the study that had interfered with the ability to train or compete fully and did not exclude swimmers with a history of shoulder pain. A history of shoulder pain might have been a confounding factor that affected the results. However, studying swimmers without a history of shoulder pain is challenging because most describe either a history of shoulder pain or shoulder symptoms at the time of testing.17 Another limitation of our study was that all swimmers were not all measured on the same day because only 1 researcher was available. To mitigate this, the measurements were taken on the same days and at the same times every week. Yet other uncontrollable factors could have influenced the results. Despite the pre- and postswim differences in rotation torque and values exceeding the SEM, the reader must be aware of the small effect sizes. This might be a problem with respect to determining a true difference between pre- and postswim scores. Another possible limitation was that swimmers were not randomized to the different intensity sessions. Instead, we performed the tests in block order: the high-intensity session on Wednesday and the low-intensity session on Friday of the same week. It is possible that the results of the Friday sessions could have been affected by the Wednesday sessions. Still, no changes occurred in the Friday sessions; therefore, carryover effects did not appear to have influenced the Friday sessions, regardless of the activity on Thursday. In addition, we focused only on the acute postswim adaptations as a result of training intensity without including other training-load variables, such as time and volume. Finally, we assessed only the interactions between training loads and musculoskeletal risk factors. Bittencourt et al7 suggested that the athlete should be analyzed as a complex system, with a focus on multilevel risk factors, including biomechanical, behavioral, psychological, and physiological factors.
Further research is needed to analyze the adaptations in different age groups and levels of competition. Also, larger sample sizes will allow swimmers to be subdivided into groups according to their training responses so that we can understand specific group adaptations. It may also be necessary to investigate how other components of training loads, such as training time and volume, affect these physical qualities. Furthermore, it is important to evaluate the cumulative effects of training loads on these physical qualities. Ideally, longitudinal research should be done to monitor ER ROM and isometric peak torque, which will allow us to understand changes over time and their relationship with the development of shoulder pain. Additional work is needed to evaluate the recovery time of these variables after a high-intensity session. Finally, investigating the interactions of training loads with psychological and behavioral factors may also be necessary.
CONCLUSIONS
Our results demonstrated that the intensity of a training session may be an important factor that leads to maladaptive changes in the physical qualities of the shoulder. A high-intensity training session immediately decreased shoulder active ER ROM and rotation isometric peak torque in competitive swimmers, particularly on the dominant side. However, we observed no changes in any of the physical qualities after the low-intensity session. We showed that these physical qualities changed dynamically as a result of the training load applied. This provides information about the short-term interaction between training intensity and the physical qualities of the shoulder in competitive swimmers. Shoulder ER ROM and rotator force have been described as potential modifiable risk factors for shoulder pain in this population; hence, their maladaptive changes may increase the risk of shoulder injury in subsequent training. Considering this, the application of appropriate training loads may be required to minimize the risk of injury associated with these changes. High training loads are necessary to increase load capacity and tolerate further loads9; nevertheless, it is essential to know when to train hard. Understanding the appropriate timing of a strenuous training session can enhance load capacity and performance without increasing the detrimental effects on shoulder physical qualities. Clinically, our findings suggested the importance of individual in-season monitoring of ER ROM and rotation isometric peak torque. Regular monitoring can ensure that swimmers have restored these qualities before or after undertaking high-intensity training. If these qualities are impaired before a high-intensity session, practitioners and coaches can adjust the training loads to avoid further maladaptations and reduce the potential risk of injury. Furthermore, identifying deficits in postswim rotation torque and ER ROM may permit early interventions and serve as a practical way to reduce the athlete's susceptibility to shoulder injury. In addition, an individualized regular exercise program to maintain ER ROM and improve shoulder-rotation torque should be performed to minimize these postswim adaptations. Finally, training intensity can be easily quantified in clinical practice by the RPE, which provides an individual perspective of the training load.
Supplementary Material
REFERENCES
- 1.Chase KI, Caine DJ, Goodwin BJ, Whitehead JR, Romanick MA. A prospective study of injury affecting competitive collegiate swimmers. Res Sports Med. 2013;21(2):111–123. doi: 10.1080/15438627.2012.757224. [DOI] [PubMed] [Google Scholar]
- 2.Kerr ZY, Baugh CM, Hibberd EE, Snook EM, Hayden R, Dompier TP. Epidemiology of National Collegiate Athletic Association men's and women's swimming and diving injuries from 2009/2010 to 2013/2014. Br J Sports Med. 2015;49(7):465–471. doi: 10.1136/bjsports-2014-094423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pink MM, Tibone JE. The painful shoulder in the swimming athlete. Orthop Clin North Am. 2000;31(2):247–261. doi: 10.1016/s0030-5898(05)70145-0. [DOI] [PubMed] [Google Scholar]
- 4.Hibberd EE, Myers JB. Practice habits and attitudes and behaviors concerning shoulder pain in high school competitive club swimmers. Clin J Sport Med. 2013;23(6):450–455. doi: 10.1097/JSM.0b013e31829aa8ff. [DOI] [PubMed] [Google Scholar]
- 5.Sein ML, Walton J, Linklater J, et al. Shoulder pain in elite swimmers: primarily due to swim-volume-induced supraspinatus tendinopathy. Br J Sports Med. 2010;44(2):105–113. doi: 10.1136/bjsm.2008.047282. [DOI] [PubMed] [Google Scholar]
- 6.Tate A, Turner GN, Knab SE, Jorgensen C, Strittmatter A, Michener LA. Risk factors associated with shoulder pain and disability across the lifespan of competitive swimmers. J Athl Train. 2012;47(2):149–158. doi: 10.4085/1062-6050-47.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bittencourt NFN, Meeuwisse WH, Mendonça LD, Nettel-Aguirre A, Ocarino JM, Fonseca ST. Complex systems approach for sports injuries: moving from risk factor identification to injury pattern recognition—narrative review and new concept. Br J Sports Med. 2016;50(21):1309–1314. doi: 10.1136/bjsports-2015-095850. [DOI] [PubMed] [Google Scholar]
- 8.Soligard T, Schwellnus M, Alonso JM, et al. How much is too much? (Part 1) International Olympic Committee consensus statement on load in sport and risk of injury. Br J Sports Med. 2016;50(17):1030–1041. doi: 10.1136/bjsports-2016-096581. [DOI] [PubMed] [Google Scholar]
- 9.Windt J, Gabbett TJ. How do training and competition workloads relate to injury? The workload-injury aetiology model. Br J Sports Med. 2017;51(5):428–435. doi: 10.1136/bjsports-2016-096040. [DOI] [PubMed] [Google Scholar]
- 10.Bansal S, Gaurang A, Sinha K, Singh Sandhu J. Shoulder impingement syndrome among competitive swimmers in India—prevalence, evaluation and risk factors. J Exerc Sci Fitness. 2007;5(2):102–108. [Google Scholar]
- 11.Walker H, Gabbe B, Wajswelner H, Blanch P, Bennell K. Shoulder pain in swimmers: a 12-month prospective cohort study of incidence and risk factors. Phys Ther Sport. 2012;13(4):243–249. doi: 10.1016/j.ptsp.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Bak K, Magnusson SP. Shoulder strength and range of motion in symptomatic and pain-free elite swimmers. Am J Sports Med. 1997;25(4):454–459. doi: 10.1177/036354659702500407. [DOI] [PubMed] [Google Scholar]
- 13.Beach ML, Whitney SL, Dickoff-Hoffman SA. Relationship of shoulder flexibility, strength, and endurance to shoulder pain in competitive swimmers. J Orthop Sports Phys Ther. 1992;16(6):262–268. doi: 10.2519/jospt.1992.16.6.262. [DOI] [PubMed] [Google Scholar]
- 14.Myers JB, Lephart SM. The role of the sensorimotor system in the athletic shoulder. J Athl Train. 2000;35(3):351–363. [PMC free article] [PubMed] [Google Scholar]
- 15.Blanch P. Conservative management of shoulder pain in swimming. Phys Ther Sport. 2004;5(3):109–124. doi: 10.1016/j.ptsp.2004.05.002. [DOI] [Google Scholar]
- 16.Horsley I, Herrington L, Hoyle R, Prescott E, Bellamy N. Do changes in hand grip strength correlate with shoulder rotator cuff function? Shoulder Elbow. 2016;8(2):124–129. doi: 10.1177/1758573215626103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higson E, Herrington L, Butler C, Horsley I. The short-term effect of swimming training load on shoulder rotational range of motion, shoulder joint position sense and pectoralis minor length. Shoulder Elbow. 2018;10(4):285–291. doi: 10.1177/1758573218773539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews MJ, Green D, Matthews H, Swanwick E. The effects of swimming fatigue on shoulder strength, range of motion, joint control, and performance in swimmers. Phys Ther Sport. 2017;23:118–122. doi: 10.1016/j.ptsp.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Werner BC, Holzgrefe RE, Griffin JW, et al. Validation of an innovative method of shoulder range-of-motion measurement using a smartphone clinometer application. J Shoulder Elbow Surg. 2014;23(11):e275–e282. doi: 10.1016/j.jse.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 20.Wilk KE, Reinold MM, Macrina LC, et al. Glenohumeral internal rotation measurements differ depending on stabilization techniques. Sports Health. 2009;1(2):131–136. doi: 10.1177/1941738108331201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furness J, Johnstone S, Hing W, Abbott A, Climstein M. Assessment of shoulder active range of motion in prone versus supine: a reliability and concurrent validity study. Physiother Theory Pract. 2015;31(7):489–495. doi: 10.3109/09593985.2015.1027070. [DOI] [PubMed] [Google Scholar]
- 22.Herrington L, Horsley I. Effects of latissimus dorsi length on shoulder flexion in canoeists, swimmers, rugby players, and controls. J Sport Health Sci. 2014;3(1):60–63. doi: org/10.1016/j.jshs.2013.01.004. [Google Scholar]
- 23.Herrington L, Horsley I, Rolf C. Evaluation of shoulder joint position sense in both asymptomatic and rehabilitated professional rugby players and matched controls. Phys Ther Sport. 2010;11(1):18–22. doi: 10.1016/j.ptsp.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Stark T, Walker B, Phillips J, Fejer R, Beck R. Hand-held dynamometry correlation with the gold standard isokinetic dynamometry: a systematic review. PM R. 2011;3(5):472–479. doi: 10.1016/j.pmrj.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 25.McLaine SJ, Ginn KA, Fell JW, Bird ML. Isometric shoulder strength in young swimmers. J Sci Med Sport. 2018;21(1):35–39. doi: 10.1016/j.jsams.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Cronin J, Lawton T, Harris N, Kilding A, McMaster DT. A brief review of handgrip strength and sport performance. J Strength Cond Res. 2017;31(11):3187–3217. doi: 10.1519/JSC.0000000000002149. [DOI] [PubMed] [Google Scholar]
- 27.Bourdon PC, Cardinale M, Murray A, et al. Monitoring athlete training loads: consensus statement. Int J Sports Physiol Perform. 2017;12(suppl 2):S2161–S2170. doi: 10.1123/IJSPP.2017-0208. [DOI] [PubMed] [Google Scholar]
- 28.Foster C, Florhaug JA, Franklin J, et al. A new approach to monitoring exercise training. J Strength Cond Res. 2001;15(1):109–115. [PubMed] [Google Scholar]
- 29.Borresen J, Lambert MI. Quantifying training load: a comparison of subjective and objective methods. Int J Sports Physiol Perform. 2008;3(1):16–30. doi: 10.1123/ijspp.3.1.16. [DOI] [PubMed] [Google Scholar]
- 30.Cohen J. Statistical Power Analysis for the Behavioral Sciences 2nd ed. Hillsdale, NJ: L Erlbaum Associates;; 1988. pp. 184–185. [Google Scholar]
- 31.Riemann BL, Lininger MR. Principles of statistics: what the sports medicine professional needs to know. Clin Sports Med. 2018;37(3):375–386. doi: 10.1016/j.csm.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Abe D, Yoshida T, Ueoka H, Sugiyama K, Fukuoka Y. Relationship between perceived exertion and blood lactate concentrations during incremental running test in young females. BMC Sports Sci Med Rehabil. 2015;7:5. doi: 10.1186/2052-1847-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore-Reed SD, Kibler WB, Myers NL, Smith BJ. Acute changes in passive glenohumeral rotation following tennis play exposure in elite female players. Int J Sports Phys Ther. 2016;11(2):230–236. [PMC free article] [PubMed] [Google Scholar]
- 34.Reinold MM, Wilk KE, Macrina LC, et al. Changes in shoulder and elbow passive range of motion after pitching in professional baseball players. Am J Sports Med. 2008;36(3):523–527. doi: 10.1177/0363546507308935. [DOI] [PubMed] [Google Scholar]
- 35.Pink M, Perry J, Browne A, Scovazzo ML, Kerrigan J. The normal shoulder during freestyle swimming: an electromyographic and cinematographic analysis of twelve muscles. Am J Sports Med. 1991;19(6):569–576. doi: 10.1177/036354659101900603. [DOI] [PubMed] [Google Scholar]
- 36.Coe R. It's the effect size, stupid: what effect size is and why it is important. Annual Conference of the British Educational Research Association; September 12–14 2002; Exeter United Kingdom. Paper presented at.
- 37.Sais WME, Mohammad WS. Influence of different testing postures on hand grip strength. Eur Sci J. 2014;10(36):290–301. [Google Scholar]
- 38.Scovazzo ML, Browne A, Pink M, Jobe FW, Kerrigan J. The painful shoulder during freestyle swimming: an electromyographic cinematographic analysis of twelve muscles. Am J Sports Med. 1991;19(6):577–582. doi: 10.1177/036354659101900604. [DOI] [PubMed] [Google Scholar]
- 39.Labriola JE, Lee TQ, Debski RE, McMahon PJ. Stability and instability of the glenohumeral joint: the role of shoulder muscles. J Shoulder Elbow Surg. 2005;14(1 suppl S):S32–S38. doi: 10.1016/j.jse.2004.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


