Abstract
Femoroacetabular impingement syndrome (FAIS) is characterized by premature contact of the femur and acetabulum during hip motion. Morphologic variations of FAIS present as either aspherical femoral deformity (cam femoroacetabular impingement) or overcoverage (pincer femoroacetabular impingement) or both. Patients with FAIS often describe discomfort with hip flexion, adduction, and internal rotation. The use of hip arthroscopy to treat FAIS has risen substantially over the last 15 years. Given that one practice domain of the athletic training profession involves injury prevention and wellness protection, optimal FAIS treatment and management strategies warrant discussion. Sports medicine professionals often help patients with FAIS explore nonoperative exercise strategies and direct rehabilitation exercises for those who pursue surgery. Both approaches demonstrate key pillars of exercise program design, which include postural control, core stabilization, hip strength and motor control, and mobility. The purpose of this article is 2-fold: to present an overview of FAIS, including common diagnostic strategies, and commonalities in therapeutic approaches between nonoperative and postoperative rehabilitation for the treatment and management of patients with FAIS.
Keywords: hip rehabilitation, hip physical examination, cam impingement, pincer deformity, hip arthroscopy, hip rehabilitation
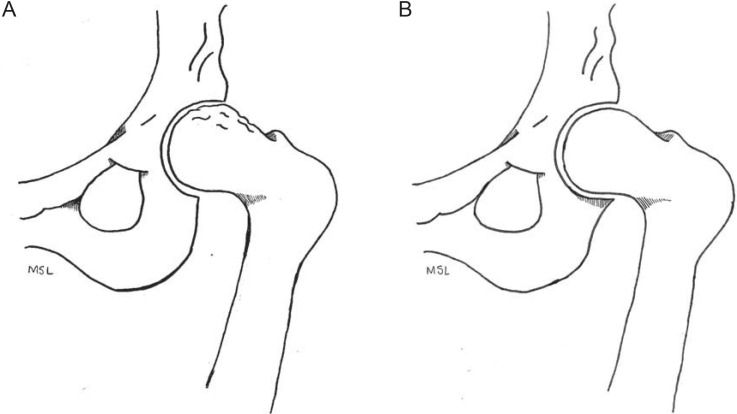
Femoroacetabular impingement syndrome (FAIS) is caused by premature contact of the femur and acetabulum during hip motion.1,2 The 2 classifications of FAIS are cam and pincer impingement (Figure 1). Aspherical deformation of the femoral head occurs with cam deformity, whereas pincer deformity presents with excessive prominence of the outer rim of the acetabulum.3
Figure 1.
Femoroacetabular impingement. A, Cam impingement. B, Pincer impingement.
Repetitive abutment of hip structures may damage the labrum and contribute to the early onset of osteoarthritis.4 Cam deformity in adolescent athletes increases the risk of early degenerative arthritis5 (Strength of Recommendation [SOR] Taxonomy: B; Centre for Evidence-Based Medicine [CEBM] rating: 3). Researchers5,6 have suggested a relationship between cam deformity and the volume and intensity of exercise during youth and adolescent growth. The source of pincer development remains elusive.
Surgeons perform arthroscopic hip surgery to target the deformity by reshaping the femur and socket and possibly reducing the risk of hip osteoarthritis.7,8 The use of hip arthroscopy to treat FAIS has risen substantially over the last 15 years.9–11 Reiman and Thorborg11 and Reiman et al12 found that current evidence may not support the recent rise in arthroscopic treatment of FAIS and that standardized reporting of outcomes is needed. Contrasting results from the UK FASHIoN randomized controlled trial1 indicated that patients with FAIS who underwent hip arthroscopy had better outcomes than patients who received nonoperative treatment (SOR: B; CEBM: 3).
Athletic trainers assist patients with FAIS using nonoperative or postoperative exercise strategies. Both approaches demonstrate key exercise pillars: postural control (also known as postural positioning), core stabilization (also known as core strength), hip strength (also known as hip strength and motor control), and mobility (also known as functional range of motion [ROM]). The purpose of our current concepts review is 2-fold: to present (1) an overview of FAIS and (2) both nonoperative and postoperative exercise protocols for the management of patients with FAIS.
DIAGNOSTIC CRITERIA
A 2016 international consensus statement2 described a multidisciplinary agreement on the diagnosis and management of patients with FAIS. In this statement, FAIS was defined as a motion-related clinical disorder with pain symptoms presenting in the hip, groin, back, and buttocks. The recommended evaluation of FAIS included a 3-pronged approach: symptoms, clinical signs, and diagnostic imaging.2
Patient-reported symptoms of FAIS are detailed in Table 1. Pain may be briefly relieved with the “C” sign palpation strategy (Figure 2). Questionnaires, such as the modified Harris Hip Score (mHHS) and various International Hip Outcome Tools (iHOT-33, iHOT-12), are available to quantify a patient's history, but no assessment tool has been cited as the criterion standard in the literature (Table 2).13,14
Table 1.
Common Femoroacetabular Impingement Syndrome Symptoms2
| Reported Patient Symptoms |
| Clicking |
| Catching |
| Locking |
| Restricting |
| Stiffening of the hip with movement |
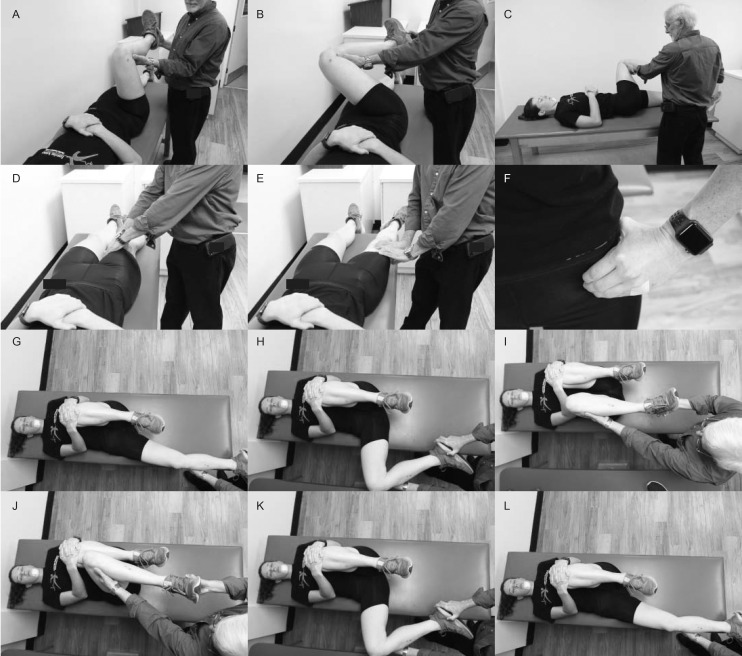
Figure 2.
Examples of clinical tests. A–C, Flexion, adduction, internal-rotation test. D and E, Supine log-roll test. F, “C” sign palpation. G–I, Dynamic internal-rotatory impingement test. J–L, Dynamic external-rotatory impingement test.
Table 2.
| Questionnaire |
Description |
| Modified Harris Hip Score | This questionnaire was modified from the original Harris Hip Score. The questionnaire assesses the following functional areas: gait (limp, assistive devices, distance); stair climbing, squatting, and sitting with lower extremities crossed; ability to use public transportation; hip range of motion; and overall pain. Total score ranges from 0 to 100, with <70 indicating a poor result and >90 indicating an excellent result. |
| International Hip Outcome Tool-33 | This 33-item questionnaire is used to assess health-related quality of life and was developed predominantly for research purposes. Questions relate to symptoms, functional limitations, sport and recreational activities, job-related concerns, and lifestyle concerns. Visual analog scale scores are summed, and the total score ranges from 0 to 100, with 100 representing the best score. |
| International Hip Outcome Tool-12 | This 12-item questionnaire was modified from the 33-item International Hip Outcome Tool that is used to assess the following domains: symptoms; functional limitations; sport and recreational activities; job-related concerns; and social, emotional, and lifestyle concerns. Each item is scored on a visual analog scale ranging from 0 to 100: 100 indicates the best function and fewest symptoms. |
Physical examination of the hip is well described but focuses on hip pain in general. Most reports on the diagnosis of FAIS have addressed either history or imaging. A limited number of strong studies focused on the clinical accuracy of physical examination tests for FAIS. The available research is impaired by low numbers of participants, differences in examination techniques, and assessments that were not limited to FAIS.15 Information concerning the statistical value of these physical examination maneuvers was absent or suggested the tests were inadequate as single diagnostic tools.
Conceptually, a complete hip examination considers 4 distinct anatomical layers: osteochondral, capsulolabral, musculotendinous, and neurovascular (Table 3).14,16 In a practical sense, a hip examination assesses the patient in the standing, seated, supine, lateral, and prone positions.14,17 Hip internal-rotation and hip-flexion ROM are important measures (Table 4). Side-to-side differences may reflect a pathologic hip condition. The seated position stabilizes both the pelvis and the hip-flexion angle for evaluation of internal and external rotation of the hip.14,17 Performing hip flexion, adduction, and internal rotation (FADIR) in the supine patient is another common clinical procedure used to diagnose FAIS (Video 1, available at http://dx.doi.org/10.4085/1062-6050-0488.19.S1).14,17,18,20 Loss of internal-rotation ROM unilaterally suggests FAIS. Researchers21 demonstrated that FADIR had a sensitivity of 94% and a specificity of 8%. It is currently the only physical examination sign recommended to help rule out hip disease in young and middle-aged active adults.15 Several other tests have been discussed in the literature, but the statistical analysis of their utility is either unacceptable or absent. The supine log-roll test (Video 2), Drehmann sign, dynamic internal rotatory impingement test (Video 3), and dynamic external rotatory impingement test (Video 4) fall into this category. An overview of common tests for determining pathologic hip conditions and the available sensitivity and specificity values are provided in Table 4 and Figure 2. No single clinical test is available for diagnosing FAIS with adequate sensitivity or specificity or both.
Table 3.
| Hip Layer |
Associated Structure |
| Osteochondral | Femur |
| Acetabulum | |
| Pelvis | |
| Capsulolabral | Labrum |
| Joint capsule | |
| Ligamentous complex | |
| Ligamentum teres | |
| Musculotendinous | Muscles of hemipelvis |
| Lumbosacral muscles | |
| Pelvic floor | |
| Neurovascular | Thoracolumbosacral plexus |
| Lumbopelvic tissue | |
| Lower extremity structures |
Table 4.
| Assessment |
Description |
Positive Sign |
Sensitivity |
Specificity |
Positive Predictive Value |
Negative Predictive Value |
| Flexion, adduction, internal- rotation test | With the patient supine, the clinician places the symptomatic hip in 90° of flexion, adducts the hip across the midline, and maximally internally rotates the hip. | Reproduction of pain and limited internal rotation | 0.2–0.94 | 0.08–0.8 | 0.16–0.67 | 0.44–0.89 |
| Supine log-roll test | With the patient supine, the clinician gently rolls the thigh internally and externally, moving the articular surface of the femoral head in relation to the acetabulum without stressing the surrounding extra-articular structures. | Reproduction of pain | 0.3 | NA | NA | NA |
| Drehmann sign | The examiner performs a passive hip-flexion maneuver on the supine patient. | Unavoidable passive external rotation of the hip | 0.44 | NA | NA | NA |
| C-palpation sign | While standing, the patient forms a “C” with 1 hand and places it above the greater trochanter, with the thumb posterior to the trochanter and fingers extending into the groin. Pressure is applied. | If the pressure attenuates symptoms temporarily, it may indicate an intra-articular pathologic condition. | NA | NA | NA | NA |
| Dynamic internal-rotatory impingement test | The test assesses anterior femoroacetabular congruence. The patient holds the contralateral extremity in flexion to achieve the 0-set point of the pelvis. The hip is dynamically moved in a wide arc from abduction or external rotation to flexion, adduction, and internal rotation. | Reproduction of pain; the degree of flexion causing impingement must be noted to determine the degree, type, and location of anterior impingement | NA | NA | NA | NA |
| Dynamic external-rotatory impingement test | The patient flexes the contralateral extremity to establish the 0-set point of the pelvis. The hip is dynamically moved from 90° of flexion or beyond through a wide abduction and external-rotation arc into extension. The test evaluates superolateral and posterior femoroacetabular impingement. | Reproduction of pain or feeling of instability | NA | NA | NA | NA |
| Hip scouring | The hip is abducted to 45° and flexed to 90°. Axial pressure is placed along the length of the femur while the thigh is rotated internally and externally. | Reproduction of pain with rotation | NA | NA | NA | NA |
| Normal hip range of motion | Internal rotation: 35°–45°; flexion: 120°–130° External rotation: 40°–50°; extension: 10°–20° |
Abbreviation: NA, not applicable.
Imaging
Anteroposterior and cross-table lateral radiographs of the pelvis help to determine morphology,23 but computed tomography or magnetic resonance imaging may provide better information, especially if the clinician uses arthrography.2 Radiographic measures of cam deformities are often assessed via α angles; the most common criterion for abnormality is an α angle of 55° or greater (Figure 3).2,18,23,24 The femoral head-neck offset is another measure used; an offset of less than 10 mm strongly suggests cam deformity.25 Abnormal morphology does not always reflect the presence of a pathologic lesion. However, collating the patient's symptoms with physical examination and imaging offers a holistic approach for determining the existence of FAIS.2 (SOR: B; CEBM: 2).
Figure 3.
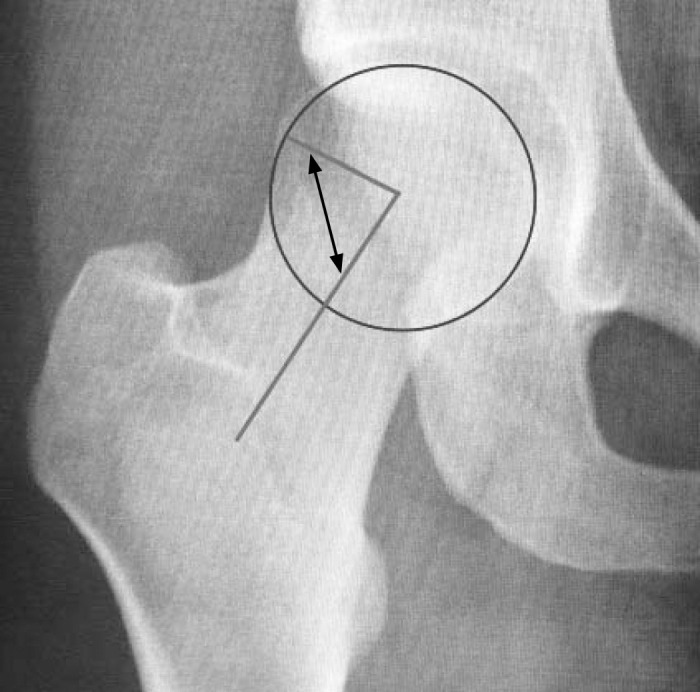
The α angle. This measure is used to locate the point of loss of concavity at the femoral head-neck junction. A line is drawn along the femoral neck axis through the center of the femoral head to form 1 ray of the α angle. A circle of best fit is then placed over the femoral head, and the point at which the femoral head-neck junction exits the circle is noted. A line is drawn from the center of the femoral head to this exit point to designate the other ray of the α angle.2,18,23,24
Treatment
Management of FAIS involves patient education, nonoperative treatment, or surgical approaches.2,3 In the acute phase, reducing painful activity is warranted. Patients should increase rest and use nonsteroidal anti-inflammatory medications or analgesics as needed for pain management. Patient education should encourage improved postural awareness during sitting, gait, sleeping, and physical activity. Avoiding a cross-legged seated position or static postures for extended periods may reduce exacerbation of FAIS.26 Patients should decrease combined movements of FADIR during activities of daily living and exercise.26 Common therapy patterns, such as full squats or pivoting on the affected side, may need to be reduced or eliminated completely, especially in the acute phase.26 Patients with FAIS may present with swayback posture and an anterior pelvic girdle tilt.26 Education increases patient awareness for facilitating the posterior pelvic girdle tilt to attenuate the anterior tilt, promoting better movement patterns.27
Formal nonoperative protocols to manage FAIS using high-evidence study designs are scarce.28 Patients who received 12 weeks of physical therapy that included hip and core strengthening, manual therapy, and lifestyle education reported improved outcomes (iHOT-33).29 An 8-week core strengthening program of pelvic-tilt (Video 5), bird-dog (Video 6), hip-extension (ie, bridging; Video 7), and isometric core-strength (planks) exercises (Video 8) and lifestyle management improved hip flexion and hip-adduction strength.30 Pennock et al31 explored the use of a nonoperative exercise protocol to manage FAIS in 76 adolescent and young adult athletes. Seventy percent were successfully treated using structured therapy, activity and sport-skill modification, and rest.31 In a recent meta-analysis of 5 randomized controlled trials, Hoit et al32 observed that nonoperative treatment was an effective initial option for managing patients with FAIS. Collectively, the nonoperative programs that were focused on hip and core strengthening in a supervised environment resulted in better patient-reported outcomes (PROs).32
More comparisons of nonoperative and operative approaches to treat FAIS are needed. Mansell et al33 examined the effectiveness of arthroscopic surgery and physical therapy for FAIS management in active-duty service members at multiple points up to 2 years. Exercise sessions included joint mobilizations, soft tissue mobility, stretching, and motor-control exercises. The authors noted improved Hip Outcome Score values in both groups and no difference between groups at 2 years. However, the high rate of crossover from physical therapy to arthroscopic surgery reduced group sizes, decreasing the ability to ascertain differences between treatments.33
In a large-scale randomized controlled trial, Griffin et al1 compared the effectiveness of nonoperative treatment and hip arthroscopy for FAIS. Participants were assigned to receive either hip arthroscopy or personalized physiotherapy. The nonoperative intervention was modeled on the study of Wall et al.8 Contact time with a physiotherapist over 12 to 24 weeks ranged from 6 to 10 visits. Both groups reported improved iHOT-33 scores at 12 months. The mean difference in iHOT-33 scores was 6.8 in favor of hip arthroscopy (P = .009) but the arthroscopic treatment group experienced more adverse effects (SOR: B; CEBM: 3).1
Comparing hip arthroscopy and nonoperative protocols presents challenges. That gap may preemptively influence a patient's decision toward surgery.34 Nonetheless, the ability to correct bony morphology, repair labral and cartilage integrity, and mitigate potential degenerative hip changes often supports the use of arthroscopy to treat patients with FAIS.8
The average time for return to sport is approximately 7 months.12 Elite-level athletes have displayed a return-to-sport success rate of 84% to 93% after arthroscopic surgery.35,36 Yet Ishøi et al37 found that only 57% of athletes who underwent arthroscopy for FAIS returned to sport at their preinjury level. They contended that this contrasting result was due to a stricter definition of return to sport. This aligns with other reports that PROs lack the standardization needed for informed decisions related to surgery.11,12,27,38 Returning to sport is different from returning to the preinjury level of activity, which increases the difficulty of determining timelines for returning to sport. Appropriate rehabilitation exercise progressions specific to the patient's goals and response to therapeutic interventions are needed.
Postoperative PROs depend on the preexisting level of hip degeneration.38,39 Patients with symptoms that lasted 12 to 24 months or longer had worse surgical outcomes.39,40 This suggests that surgical intervention may be needed if symptoms have not resolved with nonoperative treatment within 3 to 6 months40 (SOR: B; CEBM: 3). Generally, as patients age, the likelihood of successful outcomes after surgery declines, although adults over 40 years of age have described favorable outcomes when no substantial underlying degenerative changes were present.40 Professional athletes and younger athletes may be less willing to discontinue sport, increasing the likelihood of surgery.
Operative treatment of FAIS has risen substantially over the last 15 years.9–11 Physicians rely heavily on diagnostic imaging as the most important criterion for pursuing surgery to treat FAIS4; however, assuming that morphologic changes indicate pathologic lesions may create a “self-evident” philosophy that lowers the surgical threshold for FAIS.12 Surgical complications from hip arthroscopy may result in additional surgical intervention and patient costs.
EXERCISE PROTOCOLS
Nonoperative Exercise Protocol Goals
Modifying activity while implementing a well-constructed exercise program based on resistance training and focused stretching without aggravating symptoms is an appropriate way to begin nonoperative treatment8 (SOR: B; CEBM: 3). A nonoperative treatment plan involves 4 principles: improve postural alignment, increase core strength and endurance, increase hip-muscle strength and motor control, and increase lower body flexibility and the mobility of muscles with hip and pelvic attachments (Table 5).8,27,29–33 It is important to monitor the patient to ensure that the exercise is not eliciting pain. Similar to an acute postoperative time period, the practitioner must manage substantial tissue irritability and the presence of pain. Some improvement should be seen within 6 to 12 weeks (SOR: C; CEBM: 5).
Table 5.
Nonoperative Protocol for Femoroacetabular Impingement Syndrome: Exercise Focal Areas and Sample Exercise Progressionsa,27,29–31,41,42
| Focal Area (Videos) |
Sample Exercise |
Progression 1 |
Progression 2 |
Progression 3 |
| Posture (Videos 5, 9–11) | Supine anterior and posterior pelvic-floor tilts to achieve neutral pelvic alignment and improve awareness of pelvic tilt | Prone lumbar flexion and extension in a quadruped position to achieve neutral pelvic alignment and improve awareness of pelvic tilt | Series of seated anterior to posterior pelvic-girdle tilt oscillations to achieve neutral pelvic alignment and improve awareness of pelvic tilt (use chair or exercise ball) | Standing anterior to posterior pelvic-girdle tilt oscillations to achieve neutral alignment and awareness of pelvic tilt |
| Core stabilization (Videos 6, 8, 12–21) | Quadruped bird-dog variations | Plank variations | Dead-bug variations using Watkins-Randall progressions | Seated, kneeling, and standing rotational exercises |
| Hip strength and motor control (Videos 7, 22–35) | Side-lying open chain hip abduction, such as leg raises or clam shell | Supine hip-extension variations (ie, bridging) | Resisted side stepping with resistance band at various lower extremity locations | Unilateral stepping challenges, such as step-downs and multiplanar lunges |
| Flexibility and mobility (Videos 36–54) | Static stretching of hip in all 3 planes | Myofascial release of hip using a lacrosse ball or foam roller to target hip muscles in all 3 planes | Hip self-mobilization, such as long-axis distractions or lateral distractions in a nonweightbearing position | Dynamic mobility drills, such as pendulum swings at a wall, internal and external rotation (ie, open and close gate), lunge and reach, and toy soldier |
Not an all-inclusive exercise list.
Posture
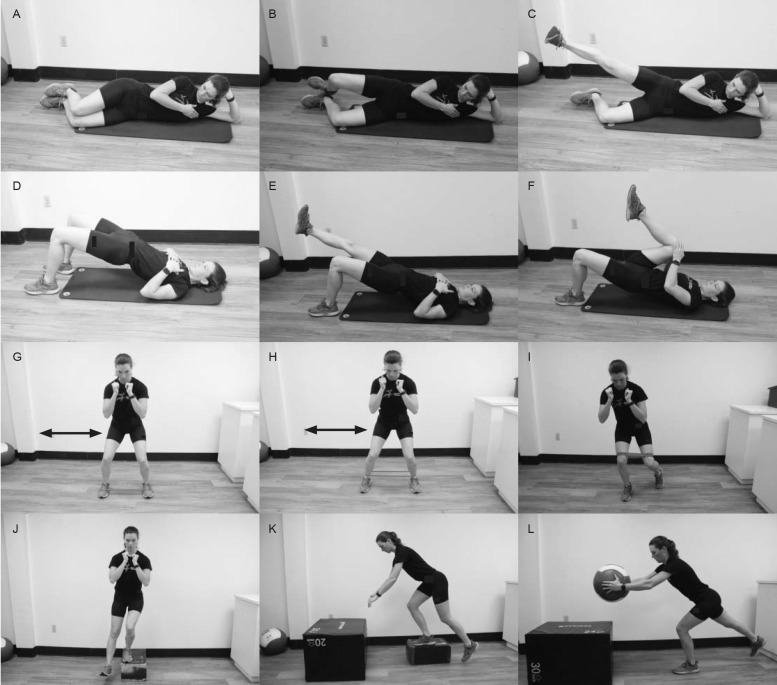
Postural exercises are used to teach the patient to maintain a neutral spine and improve body awareness (Figure 4; Videos 5, 9–1131; SOR: C; CEBM: 4). Neutral posture will reduce compensation patterns when the patient is loading asymmetrically at the hip. If swayback posture is present with anterior pelvic tilt, it might further contribute to abutment and aggravate symptoms.26 Patients can begin postural exercises in floor-based positions, such as supine abdominal drawing or hollowing coupled with anterior-to-posterior pelvic tilts, to achieve neutral alignment (Video 5). The same process can be advanced using cyclical lumbar flexion and extension in a quadruped position before moving to seated and standing positions (Videos 9–11).27,30 Practitioners must cue the patient to consistently check posture in order to increase the carryover of proper postural alignment when not at a treatment session. Postural improvement can be chronicled through video or photographs. Inclinometers, goniometers, and smartphone applications are cost-effective tools for quantifying improved posture.
Figure 4.
Examples of postural exercise. Pelvic girdle tilts: A, start position (inhale), B, end position (exhale), and C, repeat sequence. Cat/cow series: D, start position (cat), E, middle position (cow), and F, final position (neutral). Repeat sequence. Seated series: G, start position (posterior tilt), H, middle position (anterior tilt), and I, final position (neutral). Repeat sequence. Standing series: J, start position (posterior tilt), K, middle position (anterior tilt), and L, final position (neutral). Repeat sequence.
Core Stabilization
Teaching patients to improve core stabilization is another key intervention, as it is the fulcrum of the functional kinetic chain (Figure 5; Videos 6, 8, 12–1843; SOR: C; CEBM: 4). Recruitment of the transversus abdominus, multifidus, diaphragm, and pelvic floor muscles stabilizes the abdomen and lumbar spine and facilitates movement of the extremities and spine.27 Early stages should involve work in the supine position to coordinate breathing with abdominal drawing or hollowing, similar to the postural exercise focus. Progression can include hip extension (ie, bridge work), starting with bilateral and moving to unilateral lower extremity involvement (Videos 19–21). Quadruped bird-dog (Video 6) and supine dead-bug (Video 12) exercises offer challenges to core stabilization and should be advanced to focus on the upper or lower extremity before proceeding to contralateral upper and lower body movement patterns (Videos 13 and 14). The Watkins-Randall exercise progression provides a continuum for the dead-bug exercise.42 Beginning variations may require the practitioner to place a hand at the patient's lumbar spine to cue the patient to push into this hand while maintaining a neutral and painfree spine. After the patient has learned to maintain this force through the abdominal and trunk musculature, upper or lower extremity motion can be sequentially added before advanced variations of simultaneous upper and lower extremity motion are implemented.42
Figure 5.
Examples of core training exercises. Bird dog: A, start position, B, lift opposite upper and lower extremities and hold, and C, lower to start. Repeat other combination. Prone plank: D, start position, E, add hip extension (sample variation), and F, side plank (sample variation). Dead bug: G, start position, H, lower opposite upper and lower extremities and hold, and I, repeat other combination (with or without ball). Half-knee rotation: J, start position (high), K, middle position, and L, end position. Repeat sequence.
If patients can maintain neutral alignment throughout the activity, prone and side-plank variations challenge core stabilization (Videos 8, 15–17).30 As core stabilization improves, they can progress to rotational exercises from seated, kneeling, and standing postures (Video 1831; SOR: B; CEBM: 4). Using a nonoperative protocol, patients demonstrated improved core strength and endurance when they achieved a score of 4/5 on the double straight-legged raise test and maintained neutral alignment for 60 seconds while in a prone plank.31 A timed side plank has also been used to assess patient progress. During this 60-second test, the patient must hold a plank position with at least 50% of the pelvic width in the anteroposterior and vertical directions.30
Hip Strength and Motor Control
Nonoperative exercise protocols need to include exercises to address hip strength and motor control. Hip-abductor weakness is often present in patients with FAIS. Weakness in the 3 primary hip abductors (gluteus maximus, minimus, and medius muscles) is perpetuated by compensatory overactivity of the tensor fascia lata muscle.44 Although the tensor fascia lata functions as an abductor, it has strong internal-rotation capabilities. More internal rotation tends to increase the symptoms of FAIS.44 Restoring gluteal strength can start with floor exercises, such as side-lying hip abduction, clamshells, and bridging variations (Videos 22–24).27 Patients can progress to standing and dynamic exercises that increase both strength and motor control (Figure 6; Video 25). Side stepping with a resistance band positioned at the metatarsals effectively activates the gluteal muscles by increasing the lever arm and band torque without eliciting additional tensor fascia lata muscle activity (Videos 26–31).44
Figure 6.
Examples of hip-strength and motor-control exercises. Clam shell: A, start position, B, rotate top lower extremity open and hold, and C, variation (add knee extension), lower, and repeat. Bridging: D, start position, E, variation (extend 1 lower extremity and hold), and F, variation (add pull to chest and lower). Repeat. Resisted lateral band work: G, metatarsal placement, H, ankle placement, and I, above knee placement. Change planes. J, Step-down with heel taps. K, Lunge and reach. L, Weighted lunge and reach.
Progression to unilateral tasks, such as step-downs in multiple planes, challenges strength and neuromuscular control of the hip (Videos 32–34).45 Women typically demonstrate greater hip flexion in both resisted side stepping with an elastic resistance band and the forward step-down,44,45 which could further exaggerate symptoms, especially if anterior pelvic tilt is also increased.44 Clinicians should monitor pelvic control during the advance to dynamic activities. Variations, such as reverse lunges with front tap, ipsilateral Romanian deadlift with a dowel rod, and lateral step-down with heel hovers, help the patient achieve strength and motor control.33,41 Completing 3 sets of 10 repetitions, 3 to 4 days per week, can result in favorable outcomes.33,41 Medicine balls, kettlebells, or dumbbells can be added to promote hip strength and motor control (Video 35). Hip strength can be assessed using manual muscle testing; normal (100%) strength is achieved when the patient completes ROM against gravity with maximal resistance.31 Isometric strength can be recorded with a handheld dynamometer,30 which provides useful clinical information.
Flexibility and Mobility
Flexibility and mobility exercises should not elicit pain and should be performed at least 1 to 2 times per day.41 Static stretches should be held for 15 to 30 seconds (Video 36). If a static supine piriformis muscle figure-4 stretch triggers pain in the knee-crossed position, modification should include the use of a high flat surface (Video 37).41 Static stretching, myofascial release using lacrosse balls (Video 38) and foam rollers (Videos 39–42), and self-mobilization techniques (eg, banded distraction from the supine, prone, kneeling, half-kneeling, and standing positions; Videos 43 and 44) will improve flexibility and mobility in all of the hip and lower extremity muscles.27,41 Dynamic drills, such as internal and external hip rotation (ie, open and close gate; Videos 45–49), pendulum swings (Video 50), kickers (Videos 51 and 52), and traveling lunges (Videos 53 and 54), should be performed within a painfree ROM with proper posture (Figure 7; SOR: C; CEBM: 4). Flexibility of the lower extremity muscles with attachments at the hip or pelvis can be evaluated using the Thomas and Ober tests.31 Hamstrings flexibility can be determined by passively flexing the hip to 90° with concurrent knee extension; the goal is to achieve more than 20° of knee extension.31 With the patient lying supine, the examiner can assess the piriformis muscle by passively flexing the hip to 90° and externally rotating it, with the goal of achieving more than 40° of external rotation.31
Figure 7.
Examples of hip-flexibility and -mobility exercises. Sample static stretches: A, hip rotators, B, anterior: hip flexors, and C, posterior: hamstrings. Self-myofascial release with, D, lacrosse ball, E, foam rolling. Banded distraction: F, lateral, G, posterior. Example of dynamic rotation: hip rotation to, H, close, I, open gate. Examples of dynamic exercise: J and K, pendulum swings at a wall, L, kickers march.
The clinician should note that this nonoperative exercise protocol for FAIS does not differ substantially from regimens used to manage an injury with substantial acute tissue irritability and pain. Patients who pursue nonoperative approaches often have the same goals as patients who choose surgery: to return to the preinjury or sport-performance level after an intervention. In 6 weeks, the central goals should be to reduce pain in the affected hip to 0 to 2/10 on a numeric pain scale with repetitive transitions from supine to sitting and sitting to standing.31 Patients should be able to walk on varied terrain; jog for at least 30 minutes; and complete sport-specific tasks that involve cutting, jumping, and pivoting.31
Postoperative Exercise Protocol Goals
Nonoperative exercise protocols have been used to successfully manage FAIS,31 but patients often pursue surgery with the goal of returning to recreational and sporting activities as soon as possible. Postoperative hip rehabilitation should prioritize painfree motion and optimal hip-joint function.7 Participants in a 5-phase rehabilitation program after hip arthroscopy reported mHHSs of 80.1 ± 19.9 (good = 80–89) 12 months postoperatively.46 Return to function after hip arthroscopy aligns with the goals of nonoperative protocols: progressive exercises to challenge core stabilization and lower body neuromotor control with improved mobility in the lower extremity (Videos 5–54).7 Patient education is critical to facilitate appropriate healing and recovery. Progression through rehabilitation varies extensively based on the surgical procedure.47 A patient whose arthroscopic surgery included loose-body removal and labral debridement may advance more quickly to weightbearing in the acute recovery phase than a patient whose procedure included labral repair and refixation or microfracture.46,47 Phase timelines are fluid and based on the individual patient's response, but during the acute postoperative phase, careful attention must be paid to protecting the soft tissue and reducing joint inflammation.7,46,47 Phase goals, precautions, sample exercises, and common assessments in a 5-phase postoperative approach modeled from the literature are detailed in Tables 6 and 77,41,46–48 (SOR: B; CEBM: 3).
Table 6.
Postoperative Protocol for Femoroacetabular Impingement Syndrome: Goals, Precautions, and Sample Exercises and Benchmarks for Progressiona,7,19,40,46–48 Continued on Next Page
| Variable |
Description |
| Acute (1–7 d) | |
| Goals | Reduce swelling and inflammation and protect soft tissue repair Establish ROM within painfree limits Advance from using crutches to weightbearing if painfree and demonstrate noncompensatory ambulation Reduce side effects of immobilization |
| Precautions | Weightbearing too soon Improper gait patterning Extreme ROM |
| Sample exercises | Passive internal- and external-rotation ROM within painfree limits Isometric strengthening of gluteal, hamstrings, quadriceps, and transversus abdominis muscles with precaution in hip flexion Upright bicycling without resistance and without reaching 90° of hip flexion |
| Benchmark | Adequate pain control Appropriate gait with prescribed gait aid |
| Weeks 2–4 | |
| Goals | Reduce swelling and inflammation and protect soft tissue Restore normal mobility Improve ROM and hip and core muscle strength Normalize gait mechanics Continue low-level cardiovascular activity |
| Precautions | Too much weightbearing beyond patient strength and endurance Improper gait patterning |
| Sample exercises | Self-directed mobility, such as quadruped rocking exercise Therapist-assisted mobility, such as manual mobilization and distraction along long axis of femur Isotonic hip strength in all 3 planes with caution in hip flexion Focused hip-extension drills to improve gait Advancement from partial to full weightbearing positions Core stability exercises Increase bicycling duration, introduce interval training, or both with continued caution against excessive hip flexion |
| Benchmark | In the initial stages (acute through wk 3–4), prioritize passive ROM with restrictions in flexion (90°), extension (0°), abduction (25°–30°), internal rotation at 90° of hip flexion (0°), internal rotation in prone position limited by comfort, external rotation at 90° of hip flexion (30°), and external rotation in prone position (20°) After 3 wk, ROM progression within painfree range Achieve full weightbearing by wk 4 |
| Weeks 5–8 | |
| Goals | Restore normal mobility and ROM Increase lumbopelvic-hip complex Improve balance, proprioception, and cardiovascular endurance |
| Precautions | Avoid contact activities and forced stretching that elicits pain Caution against advancing exercise volume and intensity too quickly |
| Sample exercises | Lower body stretching program Open and closed chain lower body strength exercises focused on lower weights and more repetitions targeting gluteus medius and maximus muscles Progress balance challenges using unstable surfaces, single-legged lateral stepping, slide board, and step-downs with heel hover to improve motor control Involve core strength in prone (plank), supine (bridging), and kneeling (chop) positions Increase bicycling duration, introduce interval training, or both with continued caution against excessive hip flexion |
| Benchmark | Full, painfree hip active ROM in all planes Painfree, normal gait; hip-flexor strength of 4/5 on manual muscle testing; and hip abduction, adduction, extension, and internal- and external-rotation strength of 4/5 on manual muscle testing |
| Weeks 9–12 | |
| Goals | Achieve full ROM, Increased amplitude, increased speed, and demonstrated force generation and attenuation ability in functional positions Include cross-training |
| Precautions | Caution in advancing exercise volume and intensity too quickly Avoidance of contact activities, aggressive hip-flexor strengthening, and forced or aggressive stretching that elicits pain |
| Sample exercises | Lower body stretching program with advancement to some dynamic drills and end-range stretching of hip-flexor group Closed kinetic chain lower body strength exercises, such as miniband work in lateral stepping and minisquats Multiplanar stepping drills from elevated surface to improve motor control Involve core strength in prone (plank), supine (bridging), and kneeling (chop) positions with advancement to unstable surfaces Introduce cross-training, such as elliptical trainer, bicycling, stair stepping for up to 30 min of continuous exercise with heightened focus on achieving moderate intensity (ie, rating of perceived exertion of 5–7/10) |
| Benchmark | Criteria for progression to sport-specific training includes hip-flexor muscle strength of 4+/5 and 5/5 in all other lower extremity musculature |
| Weeks 13–16 | |
| Goals | Full ROM Increased force amplitude with demonstrated force-attenuation ability Higher-level speed and power drills and improved readiness for full reentry into the multiplicity of sport demands |
| Precautions | Lack of adherence to maintenance program to ensure consistent training for hip and core strength |
| Sample exercises | Lower body stretching program with advancement to dynamic mobility drills Closed kinetic chain lower body strength exercises, such as squats, deadlifts, and lunges, with advancement to single-legged positions Multiplanar agility drills Advanced core-strengthening exercises Plyometric drills as applicable to sport demands |
| Benchmark | Full ROM in all hip motion and cardiovascular endurance consistent with sport, work, or both demands must be demonstrated Balance, strength, and motor-control benchmarks include proficiency in the Y-balance test with a limb-to-limb comparison in the anterior-reach direction within 4 cm and in the posteromedial- and posterolateral-reach directions within 6 cm Performing single hop for distance, triple hop for distance, and triple-crossover hop for distance with at least 90% of limb symmetry Careful attention to alignment at takeoff or landing and demonstration of good control without hip internal rotation or valgus on the plant limb |
Abbreviation: ROM, range of motion.
Not an all-inclusive exercise list.
Table 7.
Common Assessments Recommended for Progression Through the Femoroacetabuar Impingement Syndrome Exercise and Rehabilitative Protocolsa,19,30,31
| Focal Area |
Sample Assessment to Determine Patient Progress |
Description |
| Posture | Open | Qualitative analysis using technology, such as smartphone applications, photographs, and video; implementation of inclinometer and goniometry |
| Core stabilization | Double straight-legged test | The supine patient's hips are flexed to 90°. A blood pressure cuff is placed under the lumbar spine at L4–5 and inflated to 40 mm Hg. The clinician raises the patient's lower limbs until noticeable posterior rotation of the pelvis occurs. The patient performs an abdominal-bracing procedure to prevent more pelvic motion and then attempts to slowly lower the limbs to the table, maintaining abdominal contraction. When the cuff measures a fluctuation in pressure or anterior pelvic rotation is noticeable, the test is concluded. The clinician measures the amount of hip motion from the table before pelvic tilting:
|
| Isometric plank test hold | Patient lies in prone forearm plank position; the goal is to sustain isometric plank hold ≥60 s. | |
| Hip strength and motor control | Manual muscle testing | Manual muscle testing of gluteal muscles graded on 6-point ± scale, ranging from 0 (no contraction palpated) to 5 (normal [100%], complete range of motion against gravity with maximal resistance). Positions:
|
| Handheld dynamometer | Objective measure of lower body strength: the force generated and transmitted is sent through a transducer that quantifies it and presents the data in a digital format (which may reduce variability) | |
| Flexibility and mobility | Goniometry measurements of the hip in multiple planes | Hip goniometry measurements in multiple planes:
|
| Thomas test | Detects hip flexion by extending the affected hip while the contralateral hip is held flexed; a positive test results in excessive lordosis or the inability to keep the ipsilateral thigh on the table | |
| Ober test | Identifies iliotibial band tightness; the patient lies on the side of the unaffected extremity with the shoulder and pelvis in line; the lower hip and knee are flexed to remove any lumbar spine lordosis |
Not an all-inclusive list.
Phase 1: Postoperative Week 1
Immediately postoperatively, controlling pain, reducing swelling, and protecting the repaired tissues are critical.7,40,46,47 Using crutches reduces weightbearing on the operative extremity. If microfracture surgery or labral tear repair was performed, limited weightbearing may be required for up to 8 weeks.7 Otherwise, partial weightbearing with foot-flat walking is allowed, and crutches may be discontinued after week 1.7 Recommended exercises include isometric strengthening of the gluteal, hamstrings, quadriceps, and transversus abdominis muscles with caution in hip flexion; passive internal- and external-rotation ROM may be started within painfree limits. Caution during hip flexion in the first postoperative week can prevent irritation of the iliopsoas muscle group, protect the tissue, and diminish pain and inflammation.7
Phase 2: Postoperative Weeks 2–4
In phase 2, protecting the repaired tissue while concomitantly improving ROM, hip strength, and core strength are priorities. Performing ROM exercises to restore capsular extensibility reduces adhesions in the joint: quadruped rocking is an example that should be conducted within patient tolerance.7 Therapist-assisted mobilizations and distractions can be helpful. Achieving proper gait mechanics is critical.40 With improved core stabilization and hip strength, the patient will be able to efficiently distribute weight and transfer compressive forces.7 Closed kinetic chain exercises with proprioceptive challenges may be introduced to achieve neuromuscular control.7 Exercise volume and intensity should be progressed via increased sets, repetitions, and external loading.46 If the surgical incision has healed, pool activities may be initiated to promote normal gait.36 Stationary bicycling can be started and gradually increase to 5 to 30 minutes with a focus on ROM and endurance and less focus on performance metrics, such as cadence and resistance. Range of motion is prioritized in the advancing protocol.48 At 3 weeks, ROM may progress within the painfree range. Full weightbearing should be achieved by week 4 (Table 6).48
Phase 3: Postoperative Weeks 5–8
Priorities during phase 3 are advancing through therapy protocols with functional ROM, normalized gait, and mastery of activity and achieving hip and core strength to promote control in functional positions, such as quadruped, kneeling, and standing.46,47 Improving hip-flexor strength is also a main goal. Balance and proprioception exercises should proceed from stable to unstable surfaces.7 Patients should adhere to a daily lower body stretching program. Lower body strengthening exercises include both open and closed kinetic chain exercises using lower weights and more repetitions. Single-legged lateral stepping, slide board, and step-downs with heel hovers will improve motor control. A stable pelvic girdle position is pursued via core exercises in the prone, supine, and kneeling positions. The cardiovascular conditioning progression includes longer-duration bicycling or the introduction of interval training with continued caution against excessive hip flexion. To continue advancing, the patient should achieve full, painfree hip active ROM in all planes.48 Also important are a painfree normalized gait; hip-flexor strength of 4/5 on manual muscle testing; and hip abduction, adduction, extension, and internal- and external-rotation strength of 4/5 on manual muscle testing.48
Phase 4: Postoperative Weeks 9–12
Similar to the previous phases, the goal of phase 4 is to advance through the protocol of functional ROM. Increasing the amplitude and speed of exercises while maintaining motor control in functional positions enables the patient to approach a return to sport activities.36,37 Restoration of hip-flexor strength and improved balance, proprioception, and cardiovascular endurance are also prioritized.7 Miniband work along with multiplanar stepping drills and cross-training help the patient progress to near return-to-play status.36 Cardiovascular conditioning can proceed to cross-training with different modalities, such as an elliptical trainer or stair stepper, at moderate intensity (ie, rating of perceived exertion of 5 to 7/10).36 Precautions include avoiding contact activities, aggressive hip-flexor strengthening, and any forced stretching that elicits pain. Criteria for progression to sport-specific training include a manual muscle testing score of 4+/5 for hip-flexor strength and 5/5 for all other lower extremity muscles.48
Phase 5: Postoperative Weeks 13–16
Force production and control while advancing from rehabilitation to performance are emphasized in phase 5.37 Patients who are considered for a return to play or the job must have full hip ROM and cardiovascular endurance that correspond to the demands of sport or work.48 Lower body strengthening exercises should be accomplished in both bilateral and unilateral positions. Squatting should not reveal any lateral deviation of the hip or lower extremity away from the operative side.48 A benchmark for single-legged squatting is the demonstration of minimal hip internal rotation or valgus such that the ipsilateral patella does not cross the plane of the great toe at full squat.48 Balance, strength, and motor-control benchmarks include proficiency on the Y-balance test, with a limb-to-limb comparison in the anterior-reach direction within 4 cm and in the posteromedial- and posterolateral-reach directions within 6 cm.48 Video analysis of the lower extremity while athletes perform high-level maneuvers, such as cutting, pivoting, single-legged hops, box landings, and sport-specific plyometrics, is recommended.12 Executing a single hop for distance, triple hop for distance, and triple crossover hop for distance with at least 90% limb symmetry provides a benchmark for clearance to play.48 Similar to the single-legged squat, careful attention should be given to alignment at takeoff and landing and displaying good control without hip internal rotation or valgus on the plant limb.48 The timeline for return to play depends on the procedure performed and varies from patient to patient.46,48
Key Points
Nonoperative and postoperative rehabilitation protocols for femoracetabular impingement syndrome align in 4 central exercise goals: postural positioning, core strength, hip strength and motor control, and functional range of motion.
The ability to stabilize the pelvis ensures hip alignment within the framework of the acetabulum.
Patient care for both nonoperative and postoperative femoroacetabular impingement syndrome relies on the practitioner's ability to individualize programming to specific desired outcomes.
The goal of management should be to restore painfree movement and correct functional deficits.
SUMMARY
For patients with FAIS, the nonoperative and postoperative rehabilitation protocols align on 4 central exercise goals: postural positioning, core strength, hip strength and motor control, and functional ROM. The ability to stabilize the pelvis ensures hip alignment within the framework of the acetabulum. Both nonoperative and postoperative FAIS management rely on the practitioner's ability to individualize the rehabilitation program to the patient's desired outcomes. A standard documentation of benchmarks and goals is not available in the current literature. We believe that the measures we described are useful for the clinician caring for patients with FAIS. In either scenario, the goal should be to restore painfree movement and correct functional deficits.7
SUPPLEMENTAL MATERIAL
Supplemental Videos
Found at DOI: http://dx.doi.org/10.4085/1062-6050-0488.19.S1
Supplementary Material
ACKNOWLEDGMENTS
We thank Alexis Mace and Micaela Lynch for providing the photographs and artwork to support this article.
REFERENCES
- 1.Griffin D, Dickenson E, Wall P, et al. Hip arthroscopy versus best conservative care for the treatment of femoroacetabular impingement syndrome (UK FASHIoN): a multicenter randomized controlled trial. Lancet. 2018;391(10136):2225–2235. doi: 10.1016/S0140-6736(18)31202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffin D, Dickenson E, O'Donnell J, et al. The Warwick Agreement on femoroacetabular impingement syndrome (FAI syndrome): an international consensus statement. Br J Sports Med. 2016;50(19):1169–1176. doi: 10.1136/bjsports-2016-096743. [DOI] [PubMed] [Google Scholar]
- 3.Byrd JW. Femoroacetabular impingement in athletes: current concepts. Am J Sports Med. 2014;42(3):737–751. doi: 10.1177/0363546513499136. [DOI] [PubMed] [Google Scholar]
- 4.Peters S, Laing A, Emerson C, et al. Surgical criteria for femoroacetabular impingement syndrome: a scoping review. Br J Sports Med. 2017;51:1605–1610. doi: 10.1136/bjsports-2016-096936. [DOI] [PubMed] [Google Scholar]
- 5.Wyles C, Norambuena G, Howe B, et al. Cam deformities and limited hip range of motion are associated with early osteoarthritic changes in adolescent athletes: a prospective matched cohort study. Am J Sports Med. 2017;45(13):3036–3043. doi: 10.1177/0363546517719460. [DOI] [PubMed] [Google Scholar]
- 6.Terrell SL, Lynch JM. Femoroacetabular impingement: why movement literacy matters. Strength Cond J. 2019;41(6):20–27. doi: 10.1519/SSC.0000000000000501. [DOI] [Google Scholar]
- 7.Voight M, Robinson K, Gill M, Griffin K. Postoperative rehabilitation guidelines for hip arthroscopy in an active population. Sports Health. 2010;2(3):222–230. doi: 10.1177/1941738110366383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wall P, Fernandez M, Griffin D, Foster N. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418–426. doi: 10.1016/j.pmrj.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Colvin A, Harrast J, Harner C. Trends in hip arthroscopy. J Bone Joint Surg Am. 2012;94(4):e23. doi: 10.2106/JBJS.J.01886. [DOI] [PubMed] [Google Scholar]
- 10.Cvetanovich G, Chalmers P, Levy D, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016;32(7):1286–1292. doi: 10.1016/j.arthro.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 11.Reiman M, Thorborg K. Femoroacetabular impingement surgery: are we moving too fast and too far beyond the evidence? Br J Sports Med. 2015;49:782–784. doi: 10.1136/bjsports-2014-093821. [DOI] [PubMed] [Google Scholar]
- 12.Reiman M, Peters S, Sylvain J, Hagymasi S, Mather R, Goode A. Femoroacetabular impingement surgery allows 74% of athletes to return to the same competitive level of sports participation but their level of performance remains unreported: a systematic review with meta-analysis. Br J Sports Med. 2018;52(15):972–981. doi: 10.1136/bjsports-2017-098696. [DOI] [PubMed] [Google Scholar]
- 13.Griffin DR, Parsons N, Mohtadi NGH, Safran MR. Multicenter Arthroscopy of the Hip Outcomes Research Network. A short version of the International Hip Outcome Tool (iHOT-12) for use in routine clinical practice. Arthroscopy. 2012;28(5):611–616. doi: 10.1016/j.arthro.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 14.Martin HD, Palmer IJ. History and physical examination of the hip: the basics. Curr Rev Musculoskelet Med. 2013;6(3):219–225. doi: 10.1007/s12178-013-9175-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reiman MP, Agricola R, Kemp JL, et al. Consensus recommendations of the classification, definition and diagnostic criteria of hip-related pain in young and middle-aged active adults from the International Hip-related Pain Research Network, Zurich 2018. Br J Sports Med. 2020;54(11):631–641. doi: 10.1136/bjsports-2019-101453. [DOI] [PubMed] [Google Scholar]
- 16.Draovitch P, Edelstein J, Kelly BT. The layer concept: utilization in determining the pain generators, pathology and how structure determines treatment. Curr Rev Musculoskelet Med. 2012;5(1):1–8. doi: 10.1007/s12178-011-9105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plante M, Wallace R, Busconi BD. Clinical diagnosis of hip pain. Clin Sports Med. 2011;30(2):225–238. doi: 10.1016/j.csm.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Morris W, Li R, Liu R, Salata M, Voos J. Origin of cam morphology in femoroacetabular impingement. Am J Sports Med. 2018;46(2):478–486. doi: 10.1177/0363546517697689. [DOI] [PubMed] [Google Scholar]
- 19.Daniels L, Worthingham C. Muscle Testing Techniques of Manual Examination 5th ed. Philadelphia, PA: WB Saunders;; 1986. [Google Scholar]
- 20.Casartelli NC, Brunner R, Maffiuletti NA, et al. The FADIR test accuracy for screening cam and pincer impingement morphology in youth ice hockey players. J Sci Med Sport. 2018;21(2):134–138. doi: 10.1016/j.jsams.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Tijssen M, van Cingel R, Willemsen L, de Visser E. Diagnostics of femoroacetabular impingement and labral pathology of the hip: a systematic review of the accuracy and validity of physical tests. Arthroscopy. 2012;28(6):860–871. doi: 10.1016/j.arthro.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Byrd JW. Evaluation of the hip: history and physical examination. N Am J Sports Phys Ther. 2007;2(4):231–240. [PMC free article] [PubMed] [Google Scholar]
- 23.Nötzli H, Wyss T, Stoecklin C, Schmid M, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556–560. doi: 10.1302/0301-620x.84b4.12014. [DOI] [PubMed] [Google Scholar]
- 24.Sankar W, Nevitt M, Parvizi J, Felson D, Agricola R, Leunig M. Femoroacetabular impingement: defining the condition and its role in the pathophysiology of osteoarthritis. J Am Acad Orthop Surg. 2013;21(suppl 1):S7–S15. doi: 10.5435/JAAOS-21-07-S7. [DOI] [PubMed] [Google Scholar]
- 25.Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis–what the radiologist should know. Am J Roentgenol. 2007;188(6):1540–1552. doi: 10.2214/AJR.06.0921. [DOI] [PubMed] [Google Scholar]
- 26.Kolber M, Cheatham S, Hanney W, Kreymer B, Salamh P. Training considerations for individuals with femoral acetabular impingement. Strength Cond J. 2015;37(3):35–47. doi: 10.1519/SSC.0000000000000143. [DOI] [Google Scholar]
- 27.Terrell S, Lynch J. Exploring nonoperative exercise interventions for individuals with femoroacetebular impingement. ACSM's Health Fitness J. 2019;23(1):22–30. doi: 10.1249/FIT.0000000000000451. [DOI] [Google Scholar]
- 28.Casartelli N, Bizzini M, Kemp J, Naal F, Leunig M, Maffiuletti N. What treatment options exist for patients with femoroacetabular impingement syndrome but without surgical indication? Br J Sports Med. 2018;52(9):552–553. doi: 10.1136/bjsports-2017-098059. [DOI] [PubMed] [Google Scholar]
- 29.Kemp J, Coburn S, Jones D, Crossley K. The Physiotherapy for Femoroacetabular Impingement Rehabilitation STudy (physioFIRST): a pilot randomized controlled trial. J Orthop Sports Phys Ther. 2018;48(4):307–315. doi: 10.2519/jospt.2018.7941. [DOI] [PubMed] [Google Scholar]
- 30.Aoyama M, Ohnishi Y, Utsunomiya H, et al. A prospective, randomized, controlled trial comparing conservative treatment with trunk stabilization exercise to standard hip muscle exercise for treating femoroacetabular impingement: a pilot study. Clin J Sport Med. 2019;29(4):267–275. doi: 10.1097/JSM.0000000000000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pennock A, Bomar J, Johnson K, Randich K, Upasani V. Nonoperative management of femoroacetabular impingement: a prospective study. Am J Sports Med. 2018;46(14):3415–3422. doi: 10.1177/0363546518804805. [DOI] [PubMed] [Google Scholar]
- 32.Hoit G, Whelan D, Dwyer T, Ajrawat P, Chahal J. Physiotherapy as an initial treatment option for femoroacetabular impingement: a systematic review of the literature and meta-analysis of 5 randomized controlled trials. Am J Sports Med. 2020;48(8):2042–2050. doi: 10.1177/0363546519882668. [DOI] [PubMed] [Google Scholar]
- 33.Mansell N, Rhon D, Meyer J, Slevin J, Marchant B. Arthroscopic surgery or physical therapy for patients with femoroacetabular impingement syndrome: a randomized controlled trial with 2-year follow-up. Am J Sports Med. 2018;46(6):1306–1314. doi: 10.1177/0363546517751912. [DOI] [PubMed] [Google Scholar]
- 34.Young J, Wright A, Rhon D. Nonoperative management prior to hip arthroscopy for femoroacetabular impingement syndrome: an investigation into the utilization and content of physical therapy. J Orthop Sports Phys Ther. 2019;49(8):593–600. doi: 10.2519/jospt.2019.8581. [DOI] [PubMed] [Google Scholar]
- 35.Shibata K, Matsuda S, Safran M. Arthroscopic hip surgery in the elite athlete: comparison of female and male competitive athletes. Am J Sports Med. 2017;45(8):1730–1739. doi: 10.1177/0363546517697296. [DOI] [PubMed] [Google Scholar]
- 36.Philippon M, Schenker M, Briggs K, Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):908–914. doi: 10.1007/s00167-007-0332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishøi L, Thorborg K, Kraemer O, Hölmich P. Return to sport and performance after hip arthroscopy for femoroacetabular impingement in 18- to 30-year-old athletes: a cross-sectional cohort study of 189 athletes. Am J Sports Med. 2018;46(11):2578–2587. doi: 10.1177/0363546518789070. [DOI] [PubMed] [Google Scholar]
- 38.Griffin D, Kinnard M, Formby P, McCabe M, Anderson T. Outcomes of hip arthroscopy in the older adult: a systematic review of the literature. Am J Sports Med. 2017;45(8):1928–1936. doi: 10.1177/0363546516667915. [DOI] [PubMed] [Google Scholar]
- 39.Basques B, Waterman B, Ukwuani G, et al. Preoperative symptom duration is associated with outcomes after hip arthroscopy. Am J Sports Med. 2019;47(1):131–137. doi: 10.1177/0363546518808046. [DOI] [PubMed] [Google Scholar]
- 40.Kunze K, Beck E, Nwachukwu B, Ahn J, Nho S. Early hip arthroscopy for femoroacetabular impingement syndrome provides superior outcomes when compared with delaying surgical treatment beyond 6 months. Am J Sports Med. 2019;47(9):2038–2044. doi: 10.1177/0363546519837192. [DOI] [PubMed] [Google Scholar]
- 41.Mansell N, Rhon D, Marchant B, Slevin J, Meyer J. Two-year outcomes after arthroscopic surgery compared to physical therapy for femoroacetabular impingement: a protocol for a randomized clinical trial. BMC Musculoskelet Disord. 2016;17:60. doi: 10.1186/s12891-016-0914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pitt D. Trunk stabilization. Desert Institute for Spine Disorders Web site. 2020 https://www.azspinesurgeon.com/images/Trunk_Stabilization_Program.pdf Accessed June 12.
- 43.Borghuis J, Hof A, Lemmink K. The importance of sensory-motor control in providing core stability: implications for measurement and training. Sports Med. 2008;38(11):893–916. doi: 10.2165/00007256-200838110-00002. [DOI] [PubMed] [Google Scholar]
- 44.Lewis C, Foley H, Lee T, Berry J. Hip-muscle activity in men and women during resisted side stepping with different band positions. J Athl Train. 2018;53(11):1071–1081. doi: 10.4085/1062-6050-46-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis C, Loverro K, Khuu A. Kinematic differences during single-leg step-down between individuals with femoroacetabular impingement syndrome and individuals without hip pain. J Orthop Sports Phys Ther. 2018;48(4):270–279. doi: 10.2519/jospt.2018.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spencer-Gardner L, Eishen J, Levy B, Sierra R, Engasser W, Krych A. A comprehensive five-phase rehabilitation programme after hip arthroscopy for femoroacetabular impingement. Knee Surg Sports Traumtol Arthrosc. 2014;22(4):848–859. doi: 10.1007/s00167-013-2664-z. [DOI] [PubMed] [Google Scholar]
- 47.Campbell A, Voight M. Post-operative rehab following hip arthroscopy. Training & Conditioning Web site. 20192020 https://training-conditioning.com/article/post-operative-rehab-following-hip-arthroscopy-d33/ Published Aug 2. Accessed June 12.
- 48.Domb B, Sgroi T, VanDevender J. Physical therapy protocol after hip arthroscopy: clinical guidelines supported by 2-year outcomes. Sports Health. 2016;8(4):347–354. doi: 10.1177/1941738116647920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.