Abstract
The particle size of a PEG-peptide DNA nanoparticle is key determinant of its biodistribution following i.v. dosing. To successfully cross the fenestrated endothelial cells of the liver, DNA nanoparticles should be less than 100 nm in diameter. In addition, DNA nanoparticles must be sufficiently charge-neutral to avoid recognition and binding to scavenger receptors found on Kupffer cells and endothelial cells in the liver. In the present study we demonstrate an approach to heat shrink DNA nanoparticles to reduce their size to less than 100 nm. Under optimal conditions, plasmid DNA was heated to 100°C for 10 min resulting in partial denaturation. The immediate addition of a polyacridine PEG-peptide followed by cooling to room temperature resulted in heat shrunken DNA nanoparticles that were approximately 70 nm in diameter, compared to 170 nm when heating was omitted. Heat shrinking resulted in the conversion of supercoiled DNA into open circular to remove strain during compaction. PEGylated polyacridine peptides threaded across double strand open circular DNA. Heat shrunken DNA nanoparticles of 70 nm diameter were stable to freeze drying and reconstitution in saline. Hydrodynamic dosing established that 70 nm heat shrunken DNA nanoparticles efficiently expressed luciferase in mouse liver. Biodistribution studies revealed that 70 nm DNA nanoparticles are rapidly and transiently taken up by liver whereas 170 nm DNA nanoparticles avoid liver uptake due to their larger size. The results provide a new approach to decrease the size of polyacridine PEG-peptide DNA nanoparticles to allow penetration of the fenestrated endothelium of the liver for the purpose of transfecting hepatocytes.
Introduction
The addition of a cationic polymer or peptide to plasmid DNA results in an immediate complexation, charge neutralization, and formation of a nanoparticle, often referred to as a polyplex1–4. There are many factors that contribute to the size of DNA nanoparticles which generally range from 30 to >500 nm in diameter as measured by dynamic light scattering5–8. The number of base pairs in plasmid DNA influences the particle size such that larger plasmids tend to produce moderately larger nanoparticles9. More importantly, the molecular weight and chemical composition of the cationic polymer strongly influence the size of DNA nanoparticle2, 10–11. In general, shorter polymers or peptides produce larger DNA nanoparticles2. In addition to these factors, the buffer, pH and concentration of reagents, all influence the size of DNA nanoparticles12–15.
DNA nanoparticle size is known to influence transfection efficiency13, 16. More efficient in vitro transfection is achieved with larger DNA nanoparticles due to increased sedimentation13. Alternatively, smaller DNA nanoparticles of 100 nm or less are required for intravenous delivery. Small DNA nanoparticles can cross the fenestrated endothelium in liver sinusoids to target hepatocytes17–18.
The size of a DNA nanoparticle can also increase significantly by freeze drying19, by including buffers containing mono-valent metal such as sodium19 and by binding to serum proteins such as albumin20. In particular, the binding of albumin, which is electronegative and the most abundant protein (50 mg/ml) in serum, results in rapid growth in the size of DNA nanoparticles20–21.
DNA nanoparticles are made biocompatible by incorporating a stealth layer of PEG on the surface to mask charge19, 22–23. This is commonly achieved by derivatizing the cationic polymer or peptide with polyethylene glycol (PEG) prior to complexation with DNA11. This modification blocks particle size increases resulting from freeze drying, the addition of sodium chloride, and when formulated at concentrations up to 5 mg/ml19. Most importantly, the masking of positive charge on a DNA nanoparticle with PEG effectively blocks albumin induced increases in particle size24–25. However, depending on the accessibility of remaining positive charge on a PEGylated DNA nanoparticle, albumin and other proteins coat the nanoparticle during circulation. When this happens, albumin-coated PEGylated DNA nanoparticles undergo charge-reversal to become electronegative. Recognition of albumin-coated PEGylated DNA nanoparticles by scavenger receptors found on liver Kupffer cells accounts for the rapid uptake of these by the liver25–26.
In an effort to develop biocompatible DNA nanoparticles that are stable in the circulation we designed and tested polyacridine PEG-peptides that reversibly bind DNA with high affinity 11, 22–23. A synthetic polylysine peptide was substituted with four optimally spaced acridine-lysine residues to increase DNA binding affinity by a combination of polyintercalation and ion pairing22. Covalent modification of a single Cys residue with a PEG results in a PEGylated polyacridine peptide that forms 170 nm DNA nanoparticles23. Despite demonstrating excellent biocompatibility and circulatory stability for up to 4 hours following i.v. administration, the 170 nm particles are too large to freely cross the fenestrated endothelium of the liver22–23.
There have been several prior studies that explored conditions to decrease the particle size of DNA nanoparticles13, 15, 27–31. The use of PEG10kDa-Cys-Lys30 as a trifluoro acetate salt resulted in ellipsoidal shaped DNA nanoparticles with diameters that ranged from 50–100 nm determined by number weighted-average dynamic light scattering15. The typical mean diameter for branched polyethylene amine (PEI) DNA nanoparticles prepared by bulk mixing ranges from 100–400 nm when determined by intensity-average light scattering13, 27, 29. An improved microfluidic mixing technique reduces the overall particle size of PEI DNA nanoparticles to <300 nm and narrows the particle size distribution, compared to 400 nm particles obtained using bulk mixing28. Alternative, high speed flash nanocomplexation of linear PEI and DNA resulted in very small nanoparticles with mean diameter of 30 nm when prepared at pH of 3.531. Alternatively, the pre-complexation of a PEGylated peptide nucleic acid was used to de-aggregate plasmid DNA prior to bulk mixing with PEI to form small nanoparticles of 30–50 nm27.
To decrease the particle size of polyacridine PEG-peptide DNA nanoparticles we explored the possibility of heating the DNA prior to the addition of PEG-peptide. The results establish that the heat shrinking protocol developed reliably decreased DNA nanoparticles to a size of less than 100 nm in diameter. The resulting 70 nm PEGylated DNA nanoparticles have distinct biodistribution properties suggesting their ability to cross the fenestrated endothelium of the liver. The results establish a practical solution to decrease DNA nanoparticle size, which currently limits the ability of many non-viral gene delivery DNA nanoparticles to reach their intended target cell.
Materials and Methods
Fmoc-amino acids, Fmoc-Lys (Boc)-Wang Resin, N-hydroxybenzotriazole (HOBt), and 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate, N-[(Dimethylamino)-1H-1,2,3-triazolo-[4,5-b]pyridin-1-ylmethylene]-N-methylmethanaminium hexafluorophosphate N-oxide (HATU) were from AAPPTec (Louisville, KY, U.S.A.). Trifluoroacetic acid (TFA), acetonitrile, diisoproplyethylamine (DIPEA), 1,2-Ethane dithiol and piperidine were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.). ACS grade N,N dimethylformamide (DMF) and Dichloromethane (DCM) were supplied by BDH Chemicals ( PA, USA). Maleimide-mPEG (5kD) was from Laysan Bio (Arab, AL, U.S.A.). pGL3 control vector, a 5.3-kbp luciferase plasmid containing a SV40 promoter and enhancer, was obtained from Promega (Madison, WI, U.S.A.). Trypsin was from Worthington (Lakewood, NJ). Tris (1 M pH 8.0) phenol/chloroform/isoamyl alcohol was purchased from Fisher Scientific (Pittsburgh, PA, U.S.A.). Lithium borate electrophoresis buffer was from Faster Better Media (Hunt Valley, MD, USA).
Synthesis of Polyacridine PEG-Peptide
A polyacridine PEG-peptide was prepared using methods described previously22–23. FMOC-Lys(Acr), where acridine is attached to ε-amine of Lys was synthesized as previously described11. Two polyacridine peptides (N-terminal Cys) Cys-Lys(Acr)-Lys4-Lys(Acr)-Lys4-Lys(Acr)-Lys4-Lys(Acr)-Lys and (C-terminal Cys) Lys(Acr)-Lys4-Lys(Acr)-Lys4-Lys(Acr)-Lys4-Lys(Acr)-Lys-Cys were prepared by solid phase peptide synthesis on a 30 μmol scale using FMOC-Lys(Boc)-Wang or FMOC-Cys(Trt)-Wang (0.5 μmol/mg). Peptide synthesis was achieved by double coupling of 2 mol equivalent of FMOC amino acids with HATU in DMF. Peptides were cleaved from the resin with 95/2.5/2.5 vol% TFA/ethanedithiol/water, and precipitated with cold ether. Crude peptides were dissolved in 0.1% TFA and chromatographed (1 μmol) on a XB-C18 semi-preparative RP-HPLC column (2.5 × 2.1 cm) (Phenomenex, Torrance, CA) eluted at 10 ml/min with 0.1% TFA and a 15–25% gradient of acetonitrile over 30 min. The major peak for each peptide eluting at 22 min was detected at Abs409nm, collected from multiple chromatograms, dried, reconstituted in water and quantified by Abs409nm (Lys(Acr), four per peptide, ɛ = 37064 M−1cm−1). Polyacridine peptides were characterized by LC-MS analysis by injecting 1 nmol on an analytical (0.47 × 25 cm) XB-C18 RP-HPLC column eluted at 0.7 ml/min with 0.1% TFA and a 10–30% acetonitrile gradient over 30 min and detected by positive mode ESI-MS ion trap.
Purified C-terminal and N-terminal Cys polyacridine peptides were PEGylated by conjugation of a 5 kDa mPEG-maleimide to the N-terminal or C-terminal Cys residue. Peptides (1 μmol) were reacted with 1.2 equivalence of mPEG-mal in 1 ml of 50 mM ammonium acetate pH 7 for 2 hrs at RT. PEGylated peptides were purified by preparative RP-HPLC eluted at 10 ml/min with 0.1% TFA and a 10–65% acetonitrile gradient over 30 min. The purified PEG-peptides were concentrated by rotary evaporation and freeze dried twice with the addition of 0.5 ml of 0.1 vol% acetic acid to convert to the acetate salt. Final purity of >95% was determined by analytical RP-HPLC. Purified PEG-peptides were characterized by MALDI-TOF resulting in a calculated of mass of 8000 Da and observed average mass of 7994 Da for both N-terminal PEG and C-terminal PEG-peptides.
Heat Shrinking DNA Nanoparticles
Plasmid DNA (25 μg of pGL3) in 500 μl of 50 mM HEPES (pH 7.4) was heated 100°C for 10 min. PEG-peptide (20 nmols in 20 μl of water) was added, gently vortexed, and allowed to cool to RT. Experimental variables included plasmid DNA concentration (10–100 μg), temperature (4–100°C), time (0–15 min), buffer (HEPES, MOPS, Tris and PBS) and stoichiometry of PEG-peptide added to DNA (0.2–3.2 nmol/μg of DNA). The particle size and charge of DNA nanoparticles were determined by dynamic light scattering on a Brookhaven ZetaPlus (Holtsville, NY). The values reported are the intensity averaged unimodal distribution mean and standard error from 10 replicate measurements. Zeta potential measurements performed in 5 mM HEPES pH 7.4 are reported as the mean and standard deviation following ten measurements. The shape of heat shrunken DNA nanoparticles was determined using transmission electron microscopy. Heat shrunken DNA nanoparticles were prepared as described above and compared to larger DNA nanoparticles prepared by omitting heating. Both DNA nanoparticles (0.5 μg in 10 μl) were applied to a glow discharged copper coated carbon grid (400 mesh) for 10 min, then blotted dry, followed by the addition of 10 μl of uranyl acetate 2 wt/vol% for 20 sec which was removed by blotting. Images were captured using on a JEOL JEM-1230 Transmission Electron Microscope.
Agarose Gel Electrophoresis of Extracted DNA
Plasmid DNA (50 μg/mL in 50 mM HEPES pH 7.4) was incubated at temperatures ranging 4 to 100°C for 10 min, then cooled to RT. DNA (1 μg) was loaded onto a 0.8 wt% agarose gel prepared with 1X lithium borate buffer and 0.1 wt/vol% ethidium bromide, and electrophoresed at 150 V for 40 min. DNA bands were imaged using a UVP Biospectrum imaging system equipped with a UV transilluminator. The topography of plasmid DNA was analyzed following extraction from PEG-peptide nanoparticles by either phenol/chloroform/isoamyl alcohol extraction or tryptic digestion. PEG-peptide DNA nanoparticles (50 μg/ml in HEPES pH 7.4) prepared by heat shrinking of temperatures of 4, 25, 40, 60, 80 and 100°C for 10 min were combined with 250 μl of 1 M Tris buffered phenol/chloroform/isoamyl alcohol (pH 8.0) supplemented with 100 μl of 250 mM sodium chloride. Following centrifugation at 10,000 × G for 5 min, DNA recovered in the aqueous layer was quantified by absorbance and analyzed by agarose gel electrophoresis. Alternatively, PEG-peptide DNA nanoparticles (1μg) were digested at 37°C for 30 min with 10 U of trypsin in 100 μl of 50 mM HEPES, 250 mM sodium chloride pH 7.4 then analyze by gel electrophoresis.
Gel Filtration Chromatographic Analysis of DNA Nanoparticles
Gel filtration chromatography of the nanoparticles were carried out on ShodexTM OHPak SB-806MHQ HPLC column (0.8 × 30 cm) with an exclusion limit of 20 MDa. Heat shrunken PEG-peptide DNA nanoparticles prepared at 100°C for 0, 10, 20 and 35 min were injected onto the column eluted at 1 ml/min with 50 mM phosphate buffer pH 7.0, 500 mM sodium chloride while monitored absorbance 260 nm.
Biodistribution of PEG-Peptide DNA Nanoparticles and Dose escalation
Plasmid DNA (pGL3) was radio-iodinated as previously described32. Heat shrunken 70 nm PEG-peptide 125I-DNA nanoparticles were prepared by heating 25 μg of 125I-DNA in 500 μl of 50 mM HEPES pH 7.4 at 100°C for 10 min followed by the addition 10 nmol (10 μl) of PEG-peptide, and subsequent cooling to RT. Alternatively, 170 nm PEG-peptide 125I-DNA nanoparticles were prepared by omitting the heating step. Triplicate mice were dosed i.v. tail vein with 1 μg (0.6 μCi) of PEG-peptide DNA nanoparticle. At biodistribution times of 5, 30, 60, 120 and 240 min, mice were anesthetized by intraperitoneal injection of 100 μl ketamine (100 mg/kg) and xylazine (10 mg/kg) and sacrificed by cervical dislocation. The major organs (liver, lung, spleen, stomach, kidney, heart, thyroid, small intestine and large intestine) were harvested, rinsed with saline, and the radioactivity in each organ was determined by direct γ-counting and expressed as the percentage of dose in the organ. Dose escalation was performed by administering 100 μl containing 1, 5, 10, 50 and 100 μg of 70 nm heat shrunken DNA nanoparticles with constant 0.6 μCi of 125I-DNA via the tail vein to triplicate mice. The major organs were harvested and gamma counted at a biodistribution time of 5 min.
In Vivo Expression of Heat Shrunken DNA Nanoparticles
PEG-Peptide DNA (pGL3) nanoparticles (1 μg) possessing either 70 and 170 nm diameter were prepared in 1.8 mL of saline and delivery hydrodynamically via the tail vein to triplicate mice33. The circulatory stability of 70 and 170 nm DNA nanoparticles was determined by administering nanoparticles (1 μg) in 100 μl of saline via tail vein in triplicate mice, followed by a hydrodynamic stimulatory dose of 1.8 ml of saline administered at either 5, 30, 60, 120, or 240 min. Luciferase expression was determined by quantitative bioluminescence imaging in the liver at 24 hrs post-hydrodynamic delivery as previously described11, 22–23, 26, 34.
Statistical Analysis
The base-10 logarithm of the raw luminescent output from BLI results were analyzed for statistically significant differences by t-test or one-way analysis of variance (ANOVA) with Dunnet’s Multiple Comparisons Test on GraphPad Prism version 7.01 (GraphPad Software, La Jolla, CA, USA).
Results
Double stranded DNA (dsDNA) is a semi flexible polymer35–36. The rigidity of the dsDNA has been attributed to the electrostatic repulsion between phosphate ions in the DNA backbone and the π-stacking interactions between bases36. We reasoned that the semi flexible nature of dsDNA is one of the limiting factors that determines the size of DNA nanoparticles. Recently, it has been reported that at elevated temperatures, but below melting temperature (Tm), dsDNA exhibits higher flexibility and folds into more compact structures upon binding to genome architectural proteins36. Melting curve analysis of supercoiled DNA indicates that it remains double stranded even at elevated temperatures of 100°C35. Although supercoiled DNAs do not separate into single stranded DNAs at these temperatures, considerable denaturation of DNA happens at temperatures above Tm, plausibly in the form of discontinuous bubbles in local regions with low GC content. Since polyacridine PEG-peptides (Scheme 1) condense DNA into highly stable nanoparticles through a combination of electrostatic and polyintercalative binding, we hypothesized that at melting temperatures the increased flexibility of the DNA would result in PEG-peptide condensation of supercoiled DNA into smaller particles.
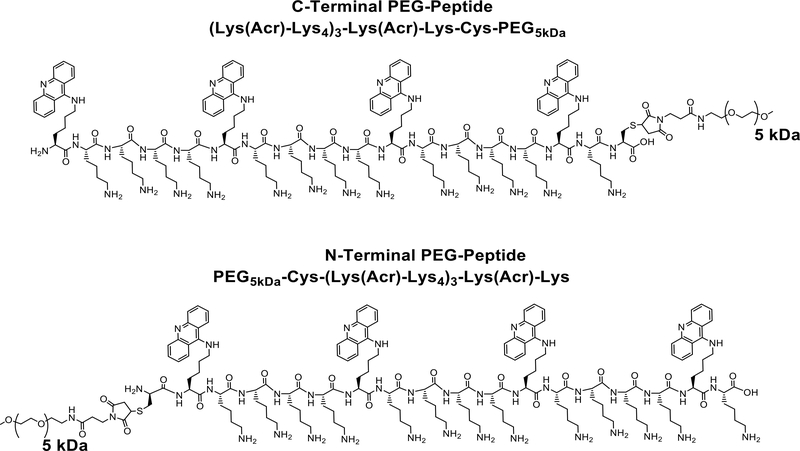
Scheme 1. Structure of PEGylated Polyacridine Peptides.
The structure of the C-terminal and N-terminal PEG-peptide used in this study are illustrated. Both PEG-peptides possess identical amino acid composition and mass.
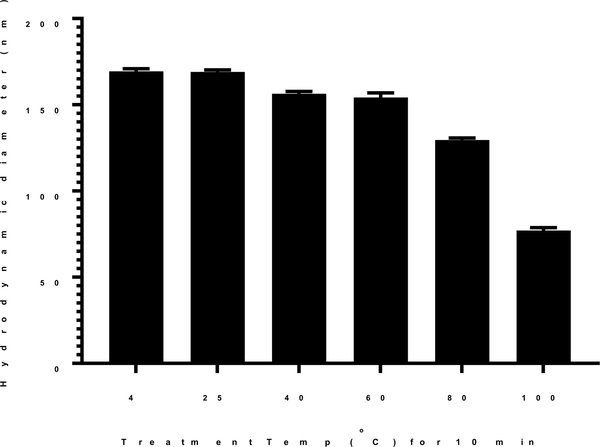
PEG-peptide DNA (pGL3) nanoparticles prepared at room temperature possess an average diameter of 170 nm (Fig. 1). The lowest temperature and time to heat DNA to effect heat shrinking was established by treating DNA at temperatures of 4, 25, 40, 60, 80 and 100 °C for 10 min prior to the addition of PEG-peptide. DNA incubated at 4, 25, 40 and 60°C resulted in nanoparticles with an average diameter of approximately 160–170 nm (Fig 1). However, heating DNA at 80°C for 10 min decreased the particle size to 130 nm, and heating at 100 °C resulted in nanoparticles with average diameter of approximately 70 nm (Fig. 1). An investigation of the rate of cooling time established no difference in particle size if DNA nanoparticles that were rapidly cooled on ice or allowed to cool to room temperature over time.
Figure 1. Effect of Temperature on Heat Shrinking DNA Nanoparticles.
Dynamic light scattering was used to measure the mean particle size of DNA nanoparticles prepared at a DNA concentration of 50 μg/ml in 50 mM HEPES pH 7.4 at a stoichiometry of 0.8 nmol of N-terminal PEG-peptide per μg of DNA. The DNA was heated for 10 min at the temperature indicated prior to the addition of N-terminal PEG-peptide. The mean particles size was determined by dynamic light scattering as the average of 10 measurements with error bars representing the standard error.
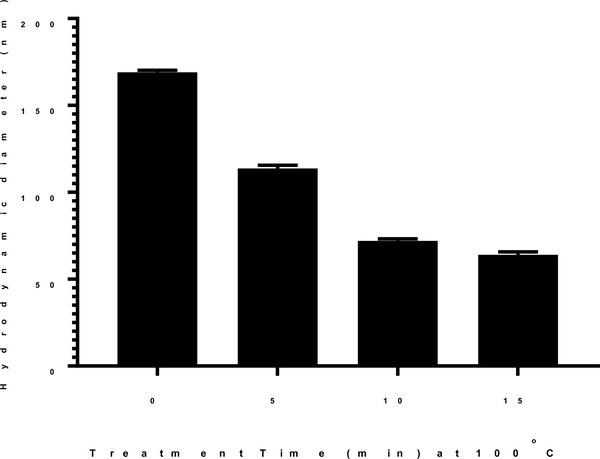
To determine the influence of heating time, plasmid DNA was prepared in 50 mM HEPES and heated at 100°C for varying time (Fig. 2). Pre-heated DNA was combined with polyacridine PEG-peptide and the mean particles size was determined by dynamic light scattering. Pre-heating DNA at 100°C for 5 min reduced the nanoparticle size to 113 nm, whereas pre-heating DNA for 10 min resulted in nanoparticles with average diameter of approximately 70 nm (Fig. 2). Pre-heating DNA for 15 min resulted in a further decrease to 60 nm nanoparticle size. Since the largest reduction in particle size was achieved by pre-heating DNA at 100°C for 10 min, these parameters were fixed throughout the rest of the study.
Figure 2. Effect of Heating Time on Heat Shrinking DNA Nanoparticles.
The particle size of heat shrunken DNA nanoparticles was determined by dynamic light scattering as a function of heating time. DNA (50 μg/ml in 50 mM HEPES pH 7.4) was heated at 100°C for times ranging from 0–15 min. Nanoparticles were formed by the addition of 0.8 nmols of N-terminal PEG-peptide per μg of DNA. The mean particles size was determined by dynamic light scattering as the average of 10 measurements with error bars representing the standard error.
The influence of different buffers on heat shrinking was determined by comparing sodium phosphate, Tris, MOPS and HEPES, each at 50 mM pH 7.4. Only the zwitter ionic buffers HEPES and MOPS resulted in heat shrinking to generate 70 nm DNA nanoparticles. Sodium phosphate resulted in no change and Tris resulted in an increase in particle size. These results are likely due to the presence of sodium. HEPES (pH 7.4) concentration was varied from 5 to 200 mM resulting in 70 nm DNA particles formed at each concentration. Dilute 5 mM HEPES resulted in the formation of minor amounts of linear DNA which was mitigated by increasing the concentration to 50 mM.
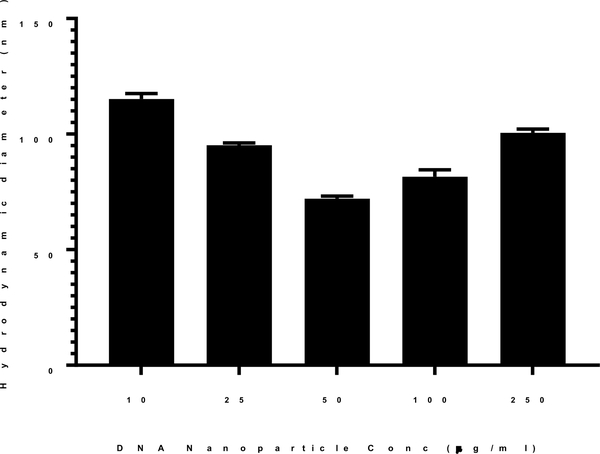
At fixed conditions of heating temperature of 100°C and time of 10 min, the concentration of DNA was varied from 10–250 μg/ml, prior to the addition of PEG-peptide. Each concentration resulted in a decrease in particle size compared to 170 nm DNA particles prepared at room temperature. However, the particle size decreased from 120 nm to a minimum of 70 nm when increasing concentration from 10 μg/ml up to 50 μg/ml (Fig. 3). Higher DNA concentrations of 100–250 μg/ml resulted in slightly larger particles of 90 nm (Fig. 3). The stability of heat shrunken 70 nm DNA nanoparticles was determined by the addition of sodium chloride, freeze drying and reconstitution19. Heat shrunken DNA nanoparticles were freeze-dried and reconstituted to the original concentration with either water or 150 mM sodium chloride. Particle size analysis demonstrated these manipulations had a negligible influence on particle size (not shown).
Figure 3. Effect of DNA Concentration on Heat Shrinking DNA Nanoparticles.
The size of heat shrunken DNA particles as a function of DNA concentration was determined. DNA was prepared at a concentration ranging from 10 to 200 μg/ml in 50 mM HEPES and heated at 100°C for 10 min. Nanoparticles were formed by the addition of 0.8 nmols of N-terminal PEG-peptide per μg of DNA. The mean particles size was determined by dynamic light scattering as the average of 10 measurements with error bars representing the standard error.
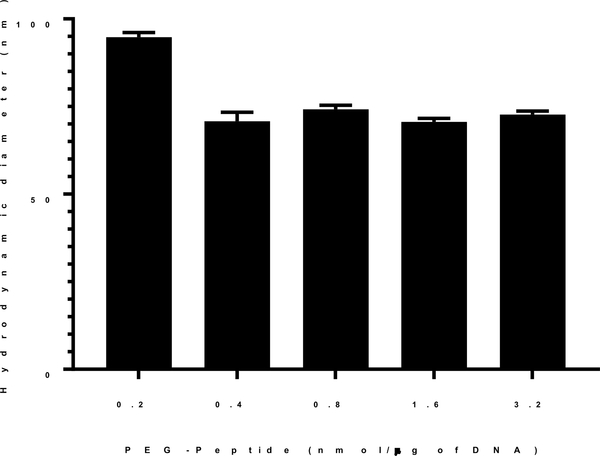
DNA nanoparticles prepared at a stoichiometry of 0.2–3.2 nmols of PEG-peptide per μg of DNA, corresponding to an N:P = 2–8, were consistently 170 nm in diameter. The particle size and zeta potential did not change at a higher stoichiometry of PEG-peptide, establishing complete compaction11. To determine the influence of PEG-peptide stoichiometry while heat shrinking DNA nanoparticles, a range of 0.2–3.2 nmols of PEG-peptide per μg of DNA was examined. The results established that 0.2 nmols of PEG-peptide per μg of DNA produced 90 nm nanoparticles, whereas increasing the stoichiometry to 0.4 or higher decreased the particle size to 70 nm (Fig. 4). The need for a higher stoichiometry of PEG-peptide to heat shrink DNA nanoparticles from 170 down to 70 nm is consistent with the increased surface area when reducing particle size.
Figure 4. Effect of PEG-Peptide Stoichiometry on Heat Shrinking DNA Nanoparticles.
DNA (50 μg/ml in 50 mM HEPES pH 7.4) was heated at 100°C for 10 min. The addition of N-terminal PEG-peptide was varied from 0.2–3.2 nmol per μg DNA. The mean particles size was determined by dynamic light scattering as the average of 10 measurements with error bars representing the standard error.
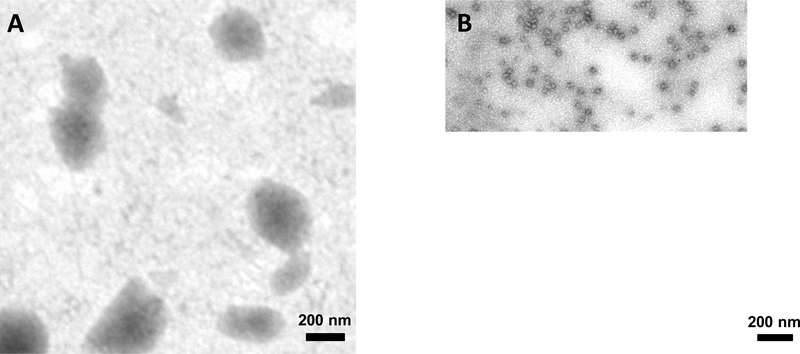
The size of PEG-peptide DNA nanoparticles was also determined by transmission electron microscopy. The results established that 170 nm DNA nanoparticles appear as roughly spherical with a broad size distribution ranging from 100–500 nm in diameter (Fig. 5A). Heat shrunken DNA nanoparticles appear nearly spherical and more uniform with significantly smaller size ranging from 40–50 nm (Fig. 5B).
Figure 5. DNA Nanoparticle Size Determined by Transmission Electron Microscopy.
The size of DNA nanoparticles as determine by TEM either before (A) or after (B) heat shrinking. DNA was prepared at 50 μg/ml DNA in HEPES pH 7.4 and kept at either RT or heated to 100°C for 10 min. Nanoparticles were formed by the addition of 0.4 nmol per μg of DNA. Each bar represents 200 nm.
Using the optimized protocol, heat shrinking of PEI, polylysine and chitosan DNA nanoparticles was attempted in an effort to decrease particle size. The results established that the particle size either remained unchanged or increased (Table 1). The importance of both the PEG and polyintercalator was examined by determining that PEG-CWK18 DNA nanoparticles did slightly decrease upon heat shrinking but much less than determined by for polyacridine PEG-peptide nanoparticles (Table 1). Both N-terminal PEG and C-terminal PEG polyacridine peptides resulted in heat shrunken particles of 70 nm. In contrast, a non-PEGylated alkylated-polyacridine peptide resulted in large 234 nm DNA nanoparticles which heat shrunk to 169 nm (Table 1). These data establish the importance of both the underlying polyacridine peptide and PEG to derive 70 nm DNA nanoparticles.
Table 1.
Particle Size of Polymer and Peptide DNA Nanoparticles Following Heat Shrinking.
| Polymer/Peptide | Particles Size (nm) at Room Temperature | Particle Size (nm) after Heat Shrinkinga |
|---|---|---|
| PEIb | 96 | 116 |
| Polylysinedp100c | 96 | 105 |
| Chitosanc | 380 | 379 |
| PEG5kDa-CWK18d | 125 | 83 |
| Alk-Cys-(Lys(Acr)-Lys4)3-Lys(Acr)-Lyse | 234 | 169 |
| PEG5kDa-Cys-(Lys(Acr)-Lys4)3-Lys(Acr)-Lyse | 170 | 70 |
| (Lys(Acr)-Lys4)3-Lys(Acr)-Lys-Cys-PEG5kDae | 170 | 70 |
Plasmid DNA (pGL3), 50 μg/ml in 50 mM HEPES pH 7.4, 100°C for 10 min, followed by the addition of polymer or peptide in 20 μl.
N/P:2
Weight ratio 3:1
0.8 nmol per μg of DNA
0.4 nmol per μg of DNA
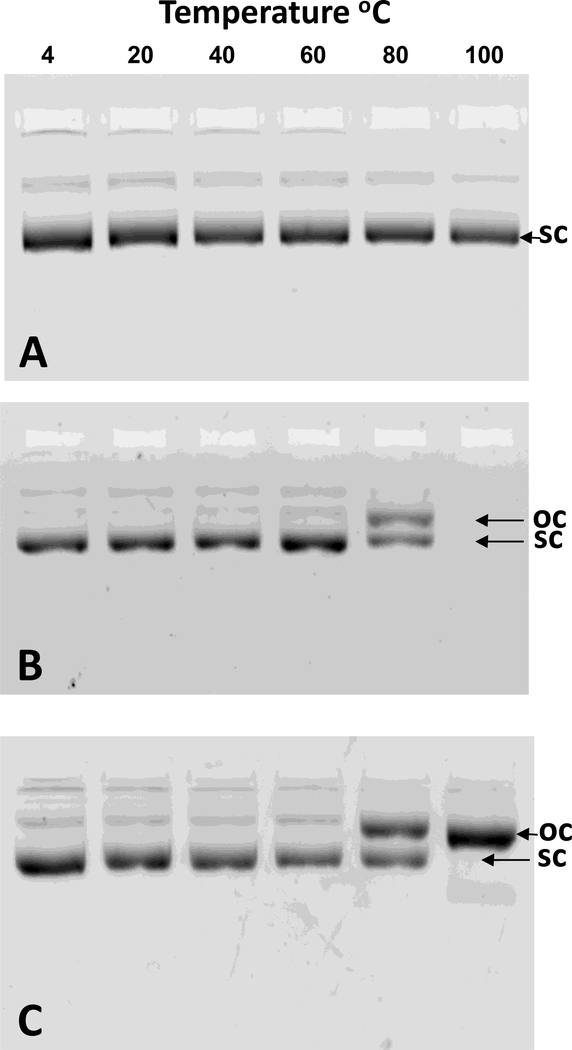
Gel electrophoresis was used to analyze the topography of DNA subjected to the heat shrinking. Heating plasmid DNA at temperatures up to 100°C for 10 min resulted in no strain breaks and full recovery of supercoiled DNA (Fig. 6A). Heating plasmid DNA at temperatures up to 60°C followed by the addition of PEG-peptide resulted in nanoparticles that were extracted by phenol:chloroform to remove PEG-peptide to recover supercoiled DNA as determined by gel electrophoresis (Fig. 6B). However, extraction of the PEG-peptide from nanoparticles prepared at 80°C resulted in two bands of equal intensity identified as supercoiled and open circular (Fig. 6B). Attempts to extract PEG-peptide from DNA nanoparticles prepared by heating DNA to 100°C failed, resulting in no bands detected by gel electrophoresis (Fig. 6B).
Figure 6. Effect of Heat Shrinking on DNA Topology.
The structure of plasmid DNA following heat shrinking was investigated by agarose gel electrophoresis. Panel A illustrates that plasmid DNA (50 μg/ml in 50 mM Hepes pH 7.4) remains supercoiled (sc) when heated for 10 min at temperatures up to 100°C. Panel B illustrates the influence of heating temperature on nanoparticle formation followed by phenol/chloroform/isoamyl alcohol extraction to remove PEG-peptide prior to gel electrophoresis. Supercoiled plasmid DNA was partially converted to open circular (oc) when heated for 10 min at 80°C, whereas no DNA bands were detected when heating to 100°C. Panel C illustrated the result of nanoparticle formation followed by trypsin digestion to remove PEG-peptide prior to gel electrophoresis. Partial conversion of supercoiled DNA to open circular was detected when heating at 80°C whereas complete conversion was detected when heating at 100°C.
Trypsin digestion has been used previously to recover plasmid DNA from nanoparticles by removing PEG-peptides possessing Lys residues37. Trypsin digestion of heat shrunken DNA nanoparticles resulted in the recovery of supercoiled plasmid when DNA was heated up to 60°C (Fig. 6C). However, equal intensity bands of supercoiled and open circular DNA were recovered when heating DNA to 80°C and a single band corresponding to open circular was recovered when heating DNA to 100°C (Fig. 6C). These results establish that heat shrinking leads to single strand DNA breaks which is likely the result of strain caused by polyacridine PEG-peptide binding and compaction. The inability to extract PEG-peptides from heat shrunken DNA nanoparticles is potentially the result of PEG-peptide threading across the double stranded DNA during reannealing38.
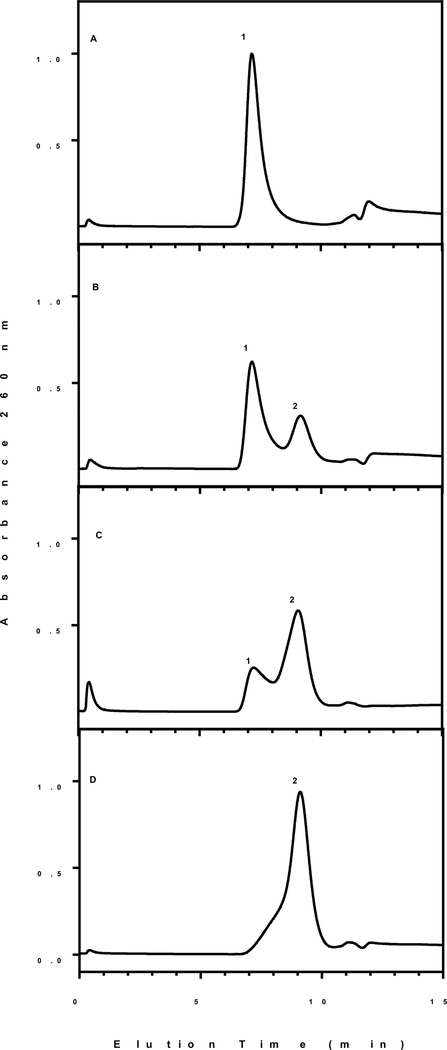
Heat shrunken DNA nanoparticles were analyzed by high-pressure gel filtration chromatography to determine the influence on prolonged heating. DNA nanoparticles formed at RT elute as a single peak at 6 min near the void of the column (Fig. 7A, peak 1). Heat shrinking DNA nanoparticles at 100°C for 10 min resulted in the formation of a second peak in the chromatogram eluting at 10 min (Fig. 7B, peak 2). Prolonged heating times of 20 and 35 min alter the peak ratio resulting in peak 2 eluting at 10 min to become the major product (Fig. 7C and D). The identity of peak 2 was determined by analyzing linearized DNA prepared by restriction digestion. PEG-peptide DNA nanoparticles generated with linear DNA resulted in a peak eluting coincident with peak 1. However, applying heat shrinking at 100°C for 10 min to linear DNA prior to the addition of PEG-peptide resulted in DNA nanoparticles that were mostly converted to peak 2. The data support a hypothesis that peak 2 is composed of single stranded DNA nanoparticles which is formed in smaller quantities when heating plasmid DNA at 100°C for 10 min. This hypothesis is further supported by the results of in vivo gene expression discussed below.
Figure 7. Gel Filtration Chromatographic Analysis of Heat Shrunken DNA Nanoparticles.
Plasmid DNA (50 μg/ml in 50 mM HEPES pH 7.4) was heated at 100 °C at varying times of 0 (A), 10 (B), 20 (C) and 35 min (D). Nanoparticles were formed by the addition of 0.4 nmol of N-terminal PEG-peptide per μg of DNA. Nanoparticles (2.4 μg) were injected onto a ShodexTM OHPak SB-806MHQ gel filtration column eluted at 1 ml/min with 50 mM phosphate buffer 0.5 M sodium chloride pH 7 while monitoring absorbance at 260 nm. Peak 1 was identified as double stranded DNA (supercoiled, circular, or linear) nanoparticles whereas, peak 2 was identified as single stranded DNA nanoparticles.
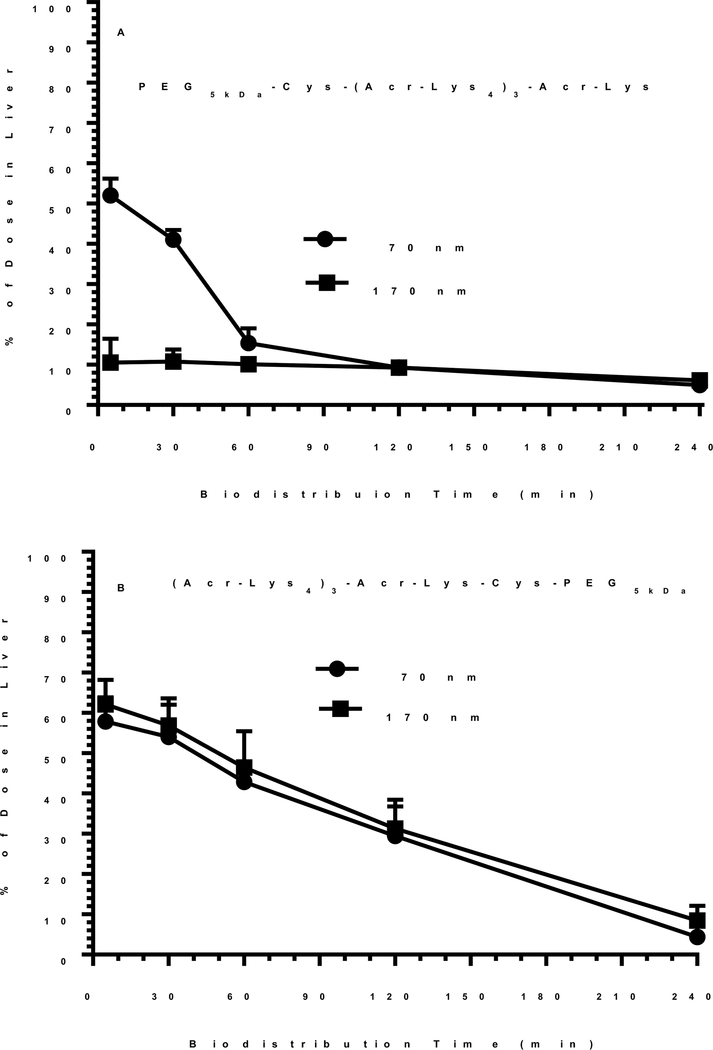
Biodistribution analysis of 170 nm DNA nanoparticles prepared with a polyacridine PEG-peptide possessing an N-terminal PEG (Scheme 1) revealed that only 10% of the dose was initially captured by liver in 5 min and decreased to 9% in 2 hours and 5% in 4 hours, without appreciable uptake by other organs (Fig. 8A). In contrast, 170 nm DNA nanoparticles prepared with a polyacridine peptide possessing a C-terminal PEG (Scheme 1) resulted in 65% of dose capture by the liver which decreased to 10% of dose in liver over 4 hours (Fig. 8B).
Figure 8. Biodistribution Analysis of Heat Shrunken DNA Nanoparticles.
The biodistribution of 170 nm and 70 nm DNA nanoparticles prepared with either a C-terminal or N-terminal PEG-peptide are compared. 125I-DNA nanoparticles (0.6 μCi) were dosed i.v. in mice. Triplicate mice were euthanized, dissected, and the major tissues gamma counted at times of 5, 30, 60, 120 and 240 min. The liver was the major site of biodistribution with other tissues possessing less than 2% of dose. Panel A illustrates the liver uptake resulting from 170 and 70 nm nanoparticles prepared with the C-terminal PEG-peptide. Panel B illustrates the liver uptake resulting from 170 and 70 nm nanoparticles prepared with the N-terminal PEG-peptide.
To investigate the reason for this large difference in biodistribution from two very similar polyacridine PEG-peptide DNA nanoparticles, the size and charge was analyzed in the presence of bovine albumin. In the absence of albumin, the particle size and zeta potential of both DNA nanoparticles were indistinguishable at 170 nm and +12 mV. Upon addition of albumin the zeta potential of the C-terminal PEG-peptide DNA nanoparticle decreased to −1 mV due to albumin binding. In contrast, the zeta potential of the N-terminal PEG-peptide DNA particle was unaltered, suggesting it resists albumin binding. The lack of a sub-terminal Lys residue on the N-terminal PEG-peptide (Scheme 1), but not the C-terminal PEG-peptide, appears to influence albumin binding to these otherwise very similar DNA nanoparticles. The lack of albumin binding is consistent with the N-terminal PEG-peptide DNA nanoparticle avoiding rapid liver uptake by escaping capture by scavenger receptors on Kupffer cells in the liver24.
Heat shrunken 70 nm DNA nanoparticles prepared with either the N-terminal or C-terminal PEG-peptide also demonstrate the same selective binding of albumin as determined by zeta potential analysis. Biodistribution analysis of 70 nm heat shrunken DNA nanoparticles prepared with the N-terminal PEG-peptide established that 52% of the dose was rapidly taken up by the liver with only minor (<2%) distribution to other tissues (Fig. 8A). However, the percent of dose in liver decreased rapidly to 15% in 1 hour and 9% in 2 hours. Alternatively, biodistribution analysis of 70 nm DNA nanoparticles prepared with the C-terminal PEG-peptide were indistinguishable from that of 170 nm DNA nanoparticles (Fig. 8B), apparently due to the binding of albumin and rapid uptake by Kupffer cells.
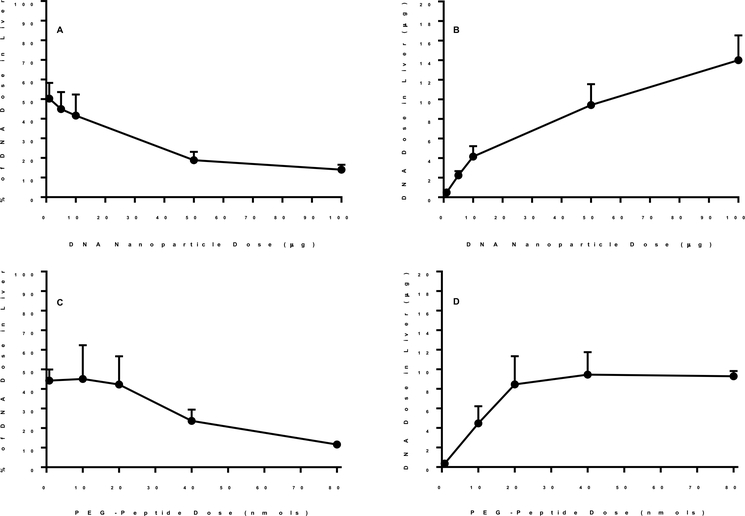
Further biodistribution analysis of N-terminal PEG-peptide 70 nm DNA nanoparticles conducted under dose escalation established that the liver uptake was partially saturable (Fig. 9A and B). Likewise, at a constant DNA dose, escalation of the PEG-peptide dose, resulting in the in situ formation of albumin nanoparticles24, also proved to saturate liver uptake (Fig. 9C and D). Taken together these results suggest, that despite no detectable albumin binding, N-terminal PEG-peptide 70 nm DNA nanoparticles likely bind to scavenger receptors in the liver.
Figure 9. Dose Escalation of Heat Shrunken DNA Nanoparticles.
The biodistribution of 70 nm 125I-DNA nanoparticles at 5 min was analyzed as a function of dose escalation of DNA nanoparticle (Panel A and B) or N-terminal PEG-peptide (Panel C and D). The percent of dose in liver is illustrated in panels A and C and compared to the amount of DNA recovered in liver in panels B and D. The results represent the mean and standard deviation of three determination.
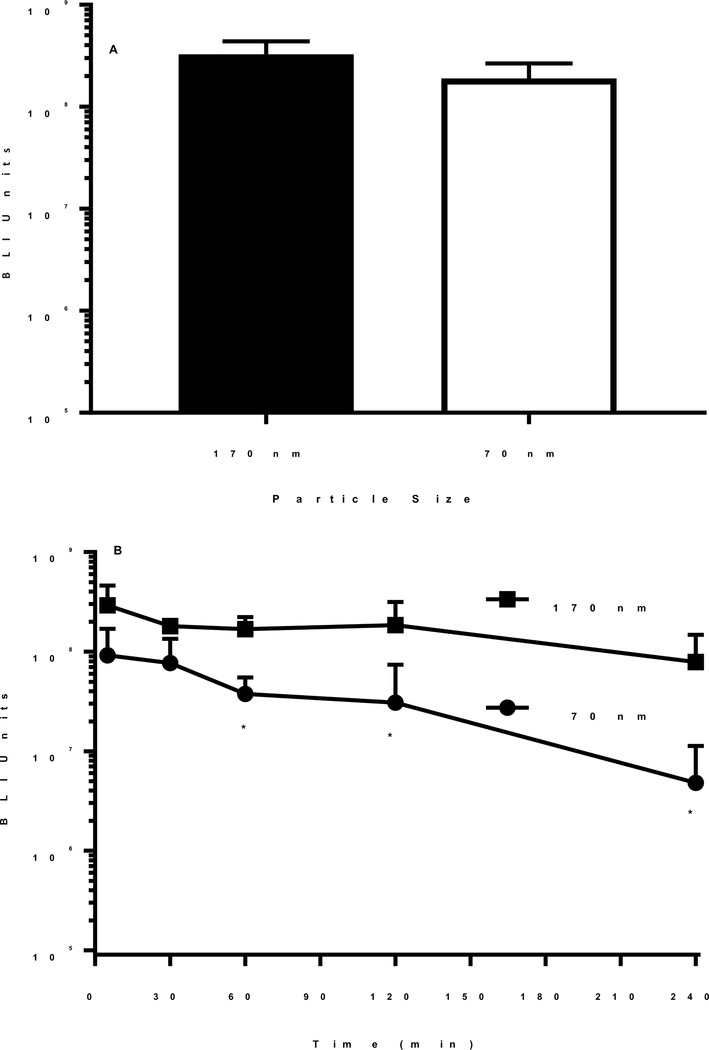
The transfection competency of 170 and 70 nm DNA nanoparticles prepared with the N-terminal PEG-peptide were compared by direct hydrodynamic dosing of a 1 μg dose of DNA. Both DNA nanoparticles produced nearly equivalent luciferase expression at 24 hours post hydrodynamic delivery, suggesting that the conversion of plasmid DNA to open circular DNA during the heat shrinking process had a negligible effect on DNA nanoparticle potency (Fig. 10A). In contrast, heating DNA at 100°C for 35 min resulted in nanoparticles (Fig. 7D) that were inactive when dosed hydrodynamically, in agreement with the hypothesis that this leads to single stranded DNA nanoparticles.
Figure 10. In Vivo Gene Expression of Heat Shrunken DNA Nanoparticles.
The luciferase expression in the liver of mice following the administration of 170 and 70 nm DNA nanoparticles by direct hydrodynamic dosing and hydrodynamic-stimulation is illustrated. Panel A illustrates the result of direct hydrodynamic dosing of 1 μg of 170 and 70 nm DNA nanoparticles prepared with N-terminal PEG-peptide into triplicate mice. Panel B illustrates the result of delayed hydrodynamic-stimulation at times of 5, 30, 60, 120 and 240 min following tail vein injection of 1 μg of 170 and 70 nm DNA nanoparticle prepared with N-terminal PEG-peptide into triplicate mice. The luciferase expression in both panel A and B was quantified by bioluminescence imaging at 24 hours post DNA administration. The expression of 70 nm DNA nanoparticles was statistically decreased (* p < 0.05) at the times indicate relative to 170 nm DNA nanoparticles.
The circulatory stability of 170 and 70 nm DNA nanoparticles were analyzed by administering a delayed hydrodynamic stimulatory dose of saline at varying times after the primary DNA dose. Using the approach, we have previously reported that 170 nm DNA nanoparticles prepared with either the C or N-terminal PEG peptide are stable in the circulation for up to 4 hours11, 22–23. DNA nanoparticles (1 μg) were dosed i.v. and then hydrodynamically stimulated with saline at times ranging from 5 min to 4 hours (Fig. 10). The luciferase expression measured in the liver at 24 hours remained constant indicating 170 nm DNA nanoparticles resist metabolism in the liver for up to 4 hours (Fig. 10B). Alternatively, when heat shrunken 70 nm DNA nanoparticles were dosed i.v. (1 μg) and hydrodynamically stimulated (Fig 10B), the expression decreased by 15-fold over four hours (Fig. 10B). This result suggests the rapid transient uptake of 70 nm DNA nanoparticles by the liver leads to accelerated metabolism of the DNA relative to 170 nm DNA nanoparticles that avoid binding to Kupffer cells.
Discussion
The development of DNA nanoparticles for i.v. dosed gene delivery to the liver requires careful control over particle size and charge. Biocompatible nanoparticles typically include covalently linked PEG to mask surface charge and eliminate nanoparticle aggregation in the circulation. Simultaneously controlling the nanoparticle size, charge and circulatory stability by carefully designed DNA binding molecules are important prerequisites to achieving targeted delivery of DNA into the nucleus of hepatocytes.
We have previously demonstrated that an optimized polyacridine PEG-peptide formed stable DNA nanoparticles of 170 nm possessing a long circulatory half-life that remained fully transfection competent for up to 4 hours when dosed i.v. in mice23. A key element in the development of a polyacridine PEG-peptide was determining the optimal number of polyintercalating acridine modified Lys residues Lys(Acr), and the optimal spacing of these with Lys residues, to achieve high affinity and compaction, while minimizing charge and maximizing circulatory stability with a short 18 amino acid peptide. A detailed analysis of PEG length, PEG location and linkage to a single Cys residue on the polyacridine peptide established that a maleimide-linked C or N-terminal 5 kDa PEG resulted in optimal circulatory stability and transfection competency in mice23.
Due to their compact size and defined chemical structure, polyacridine PEG-peptides are readily customizable into targeted carriers to prepare DNA nanoparticles for in vivo gene delivery. However, a major disadvantage for delivery to liver hepatocytes is the larger DNA nanoparticle size of 170 nm, which is too large to cross the liver fenestrae17. While the size of a DNA nanoparticle is dependent on the chemical structure of the PEG-peptide, and decreasing the number of Lys(Acr) or removing Lys residues leads to smaller DNA nanoparticles, this also leads to a loss in circulatory stability when administered i.v.11.
To maintain maximum circulatory stability while simultaneously decreasing DNA nanoparticle size to less 100 nm, the heat shrinking protocol was developed. There are likely multiple mechanisms that contribute to the observed DNA nanoparticle heat shrinking. Only under conditions of heating plasmid at 100°C for 10 min followed by the addition of PEG-peptide, is maximal heat shrinking to 70 nm realized (Fig. 1 and 2). Shorter heating times lead to an intermediate particle size between 170 nm and 70 nm. Similarly, a lower heating temperature of 80°C only partially heat shrinks DNA nanoparticles to 130 nm (Fig. 1). These results support a hypothesis that heating results in partially denatured DNA that folds into smaller nanoparticles36. The rate of PEG-peptide binding to DNA is rapid compared to the slower process of DNA annealing. Consequently, rapid binding of polyacridine PEG-peptide to double stranded regions would allow single stranded regions to fold into a small nanoparticle. In fact, upon prolonged heating at 100°C for up to 35 min, the resulting DNA particle elutes as major low molecular weight peak on GPC-HPLC (Fig. 7D), which upon direct hydrodynamic dosing was completely inactive (not shown). This result suggests that prolonged heating results in the formation of single stranded DNA nanoparticles, which are biologically inactive since transcription factor binding require a DNA duplex39.
Plasmid DNA heated to 100°C for 10 min is not physically transformed (Fig. 6A) and remains fully biologically active upon hydrodynamic dosing (not shown). Likewise, the addition of PEG-peptide to heat cycled DNA still results in 170 nm particles (not shown). However, the addition of PEG-peptide to DNA heated 80 or 100°C for 10 min alters the structure of supercoiled plasmid DNA (Fig. 6B). The formation of open circular DNA under partial heat shrinking at 80°C suggests that conformational strain arising from PEG-peptide binding leads to single strand breaks. A complete loss of extracted DNA bands occurs following heating to 100°C for 10 min and the addition of PEG-peptide (Fig. 6B). This result suggests that the PEG-peptide may have undergone threading during DNA duplex separation40, becoming trapped within the double strand during annealing. Other than the lack of phenol-chloroform extraction at 100°C, there is no direct evidence for threading, but there is precedent for this mechanism when polyintercalators are included within peptides40. Alternatively, removal of the PEG-peptide from heat shrunken DNA nanoparticles by trypsin digestion allows recover of the DNA which migrates as open circular (Fig. 6C). The finding that trypsin removes the PEG-peptide is also consistent with the threading hypothesis and complete conversion of the DNA to open circular is consistent with the need to relieve strain when undergoing maximal heat shrinking.
The finding that DNA concentration plays a role in heat shrinking into small DNA nanoparticles suggests a secondary mechanism (Fig. 3). A more concentrated DNA solution would favor entanglement of DNA molecules, such that the addition of PEG-peptide would result in the rapid capture of multiple plasmids into a single nanoparticle. Assuming equal packing density, a single 170 nm particle could convert into fourteen 70 nm DNA nanoparticles (Fig. 5). Heating DNA to 100°C could detangle plasmids such that the addition of PEG-peptide leads to the rapid capture of fewer plasmids resulting in 70 nm particles. This concept was previously proposed as a mechanism for generating smaller PEI DNA nanoparticle after the addition of a PNA-PEG to bind and detangle DNA41. The finding that heat shrinking to 70 nm requires a greater number of PEG-peptides relative to the formation of 170 nm DNA nanoparticles is consistent with the DNA detangle hypothesis (Fig. 4). An equivalent weight of 70 nm DNA nanoparticles possess 6-fold greater surface area compared to 170 nm particles. Assuming the PEG occupies the nanoparticle surface explains why PEG-peptide appears to be a limiting reagent to achieve maximal heat shrinking (Fig. 4).
The biodistribution profiles of 70 nm and 170 nm DNA nanoparticles are distinct. The 70 nm particles are rapidly and transiently taken up by liver resulting in 50% capture within 5 min. Alternatively, 170 nm nanoparticles nearly completely avoid uptake by liver, which is interpreted as the inability to cross the fenestrae (Fig. 8B). While both nanoparticles possess identical zeta potential of +12 mV, they uniquely avoid albumin binding, which we rationalize is due to an N-terminal PEG bound to Cys and a Lys-Acr as the sub-terminal residue on the polyacridine peptide. In contrast, 70 and 170 nm DNA nanoparticles prepared with the C-terminal PEG-peptide also possess +12 mV zeta potential, but are able to bind albumin due to Lys positioned as the sub-terminal residue. This subtle difference leads to albumin binding, charge reversal, and recognition by scavenger receptors on Kupffer cells, resulting in approximately 60% of both nanoparticles rapidly captured by the liver (Fig. 8B). The uptake of both nanoparticles by Kupffer cells masks the ability to observe smaller DNA nanoparticles crossing the liver fenestrae.
We have previously reported that both C-terminal and N-terminal PEG-peptide DNA nanoparticles are stable in the circulation of up to 4 hours23. In contrast, 70 nm particles lose 3-fold gene expression efficiency in 5 min and 15-fold in 4 hours (Fig. 9B). The loss in expression correlates with more rapid metabolism of 70 nm DNA nanoparticles. The circulatory stability afforded 170 nm particles is due to the inability to cross the fenestrae. Interestingly, although C-terminal PEG peptide 170 nm DNA nanoparticle are taken up by liver Kupffer cells, they are not appreciable metabolized, resulting in long circulatory stability23.
In conclusion, the ability to heat shrink polyacridine PEG-peptide DNA nanoparticles reveals new and interesting biophysical interactions between DNA and these delivery molecules. The small size of the DNA nanoparticles dramatically alters the circulatory properties following i.v. dosing in mice. The ability to control particle size and surface charge is fundamental to successful advancement of nanoparticle gene delivery systems designed to target liver hepatocytes.
Acknowledgement
The authors gratefully acknowledge support from NIH Grants GM117785 and T32 GM00865 and for technical support from the Central Microscopy Research Facility at the University of Iowa.
Data Availability
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.
References
- 1.Boussif O; Lezoualc’h F; Zanta MA; Mergny MD; Scherman D; Demeneix B, et al. , A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proceedings of the National Academy of Sciences of the United States of America 1995, 92, 7297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wadhwa MS; Collard WT; Adami RC; McKenzie DL; Rice KG, Peptide-mediated gene delivery: influence of peptide structure on gene expression. Bioconjug Chem 1997, 8, 81–8. [DOI] [PubMed] [Google Scholar]
- 3.Wagner E; Zenke M; Cotten M; Beug H; Birnstiel ML, Transferrin-polycation conjugates as carriers for DNA uptake into cells. Proc. Natl. Acad. Sci. USA 1990, 87, 3410–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kabanov AV; Kabanov VA, DNA Complexes with Polycations for the Delivery of Genetic Material into Cells. Bioconjug. Chem. 1995, 6, 7–20. [DOI] [PubMed] [Google Scholar]
- 5.Ogris M; Steinlein P; Kursa M; Mechtler K; Kircheis R; Wagner E, The size of DNA/transferrin-PEI complexes is an important factor for gene expression in cultured cells. Gene Therapy 1998, 5, 1425–1433. [DOI] [PubMed] [Google Scholar]
- 6.Bettinger T; Remy JS; Erbacher P, Size reduction of galactosylated PEI/DNA complexes improves lectin-mediated gene transfer into hepatocytes. Bioconjug Chem 1999, 10, 558–61. [DOI] [PubMed] [Google Scholar]
- 7.Park SY; Harriers D; Gelbart WM, Topological defects and optimum size of DNA condensates. Biophys. J. 1998, 75, 714–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erbacher P; Roche AC; Monsigny M; Midoux P, Glycosylated polylysine/DNA complexes: gene transfer efficiency in relation with the size and the sugar substitution level of glycosylated polylysines and with the plasmid size. Bioconjugate Chemistry 1995, 6, 401–410. [DOI] [PubMed] [Google Scholar]
- 9.Fink TL; Klepcyk PJ; Oette SM; Gedeon CR; Hyatt SL; Kowalczyk TH, et al. , Plasmid size up to 20 kbp does not limit effective in vivo lung gene transfer using compacted DNA nanoparticles. Gene Ther 2006, 13, 1048–51. [DOI] [PubMed] [Google Scholar]
- 10.Kaminskas LM; Boyd BJ; Karellas P; Krippner GY; Lessene R; Kelly B, et al. , The impact of molecular weight and PEG chain length on the systemic pharmacokinetics of PEGylated poly l-lysine dendrimers. Mol Pharm 2008, 5, 449–63. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez CA; Baumhover NJ; Duskey JT; Khargharia S; Kizzire K; Ericson MD, et al. , Metabolically stabilized long-circulating PEGylated polyacridine peptide polyplexes mediate hydrodynamically stimulated gene expression in liver. Gene Ther 2011, 18, 23–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwok KY; McKenzie DL; Evers DL; Rice KG, Formulation of highly soluble poly(ethylene glycol)-peptide DNA condensates. J Pharm Sci 1999, 88, 996–1003. [DOI] [PubMed] [Google Scholar]
- 13.Pezzoli D; Giupponi E; Mantovani D; Candiani G, Size matters for in vitro gene delivery: investigating the relationships among complexation protocol, transfection medium, size and sedimentation. Sci Rep 2017, 7, 44134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hickey JW; Santos JL; Williford JM; Mao HQ, Control of polymeric nanoparticle size to improve therapeutic delivery. J Control Release 2015, 219, 536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu G; Li D; Pasumarthy MK; Kowalczyk TH; Gedeon CR; Hyatt SL, et al. , Nanoparticles of Compacted DNA Transfect Postmitotic Cells. J. Biol. Chem 2003, 278, 32578–32586. [DOI] [PubMed] [Google Scholar]
- 16.Prabha S; Zhou W; Panyam J; Labhasetwar V, Size-dependency of nanoparticle-mediated gene transfection: studies with fractionated nanoparticles. Int J Pharm 2002, 244, 105–115. [DOI] [PubMed] [Google Scholar]
- 17.Wisse E; Jacobs F; Topal B; Frederik P; De Geest B, The size of endothelial fenestrae in human liver sinusoids: implications for hepatocyte-directed gene transfer. Gene Ther 2008, 15, 1193–9. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs F; Wisse E; De Geest B, The Role of Liver Sinusoidal Cells in Hepatocyte-Directed Gene Transfer. The American Journal of Pathology 2010, 176, 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwok KY; Adami RC; Hester KC; Park Y; Thomas S; Rice KG, Strategies for maintaining the particle size of peptide DNA condensates following freeze-drying. Int J Pharm 2000, 203, 81–8. [DOI] [PubMed] [Google Scholar]
- 20.Oupický D; Koňák Č; Dash PR; Seymour LW; Ulbrich K, Effect of Albumin and Polyanion on the Structure of DNA Complexes with Polycation Containing Hydrophilic Nonionic Block. Bioconjugate Chemistry 1999, 10, 764–772. [DOI] [PubMed] [Google Scholar]
- 21.Dash PR; Read ML; Barrett LB; Wolfert MA; Seymour LW, Factors affecting blood clearance and in vivo distribution of polyelectrolyte complexes for gene delivery. Gene Therapy 1999, 6, 643–650. [DOI] [PubMed] [Google Scholar]
- 22.Kizzire K; Khargharia S; Rice KG, High-affinity PEGylated polyacridine peptide polyplexes mediate potent in vivo gene expression. Gene Ther 2013, 20, 407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khargharia S; Kizzire K; Ericson MD; Baumhover NJ; Rice KG, PEG length and chemical linkage controls polyacridine peptide DNA polyplex pharmacokinetics, biodistribution, metabolic stability and in vivo gene expression. J Control Release 2013, 170, 325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen RJ; Mathew B; Rice KG, PEG-Peptide Inhibition of Scavenger Receptor Uptake of Nanoparticles by the Liver. Mol Pharm 2018, 15, 3881–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumhover NJ; Duskey JT; Khargharia S; White CW; Crowley ST; Allen RJ, et al. , Structure–Activity Relationship of PEGylated Polylysine Peptides as Scavenger Receptor Inhibitors for Non-Viral Gene Delivery. Molecular Pharmaceutics 2015, 12, 4321–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khargharia S; Baumhover NJ; Crowley ST; Duskey J; Rice KG, The Uptake Mechanism of PEGylated DNA Polyplexes by the Liver Influences Gene Expression. Gene Therapy 2014, 21, 1021–1028. [DOI] [PubMed] [Google Scholar]
- 27.Millili PG; H. YD; H. F; Naik UP; Sullivan MO, Formulation of Peptide Nucleic Acid Based Nucleic Acid Delivery Construct. Bioconjugate Chemistry 2010, 21, 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho YP; Grigsby CL; Zhao F; Leong KW, Tuning physical properties of nanocomplexes through microfluidics-assisted confinement. Nano Lett 2011, 11, 2178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu M; Ho YP; Grigsby CL; Nawaz AA; Leong KW; Huang TJ, Three-dimensional hydrodynamic focusing method for polyplex synthesis. ACS Nano 2014, 8, 332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grigsby CL; Ho YP; Lin C; Engbersen JF; Leong KW, Microfluidic preparation of polymer-nucleic acid nanocomplexes improves nonviral gene transfer. Sci Rep 2013, 3, 3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santos JL; Ren Y; Vandermark J; Archang MM; Williford JM; Liu HW, et al. , Continuous Production of Discrete Plasmid DNA-Polycation Nanoparticles Using Flash Nanocomplexation. Small 2016, 12, 6214–6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terebesi J; Kwok KY; Rice KG, Iodinated plasmid DNA as a tool for studying gene delivery. Anal Biochem 1998, 263, 120–3. [DOI] [PubMed] [Google Scholar]
- 33.Liu F; Song Y; Liu D, Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther 1999, 6, 1258–66. [DOI] [PubMed] [Google Scholar]
- 34.Rettig GR; Rice KG, Quantitative In Vivo Imaging of Non-viral-Mediated Gene Expression and RNAi-Mediated Knockdown. In Bioluminescence, 2009; pp 155–171. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y; Berrido AM; Hua ZC; Tse-Dinh YC; Leng F, Biochemical and biophysical properties of positively supercoiled DNA. Biophys Chem 2017, 230, 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Driessen RP; Sitters G; Laurens N; Moolenaar GF; Wuite GJ; Goosen N, et al. , Effect of temperature on the intrinsic flexibility of DNA and its interaction with architectural proteins. Biochemistry 2014, 53, 6430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adami RC; Collard WT; Gupta SA; Kwok KY; Bonadio J; Rice KG, Stability of Peptide-Condensed Plasmid DNA Formulations. Journal of Pharmaceutical Sciences 1998, 87, 678–683. [DOI] [PubMed] [Google Scholar]
- 38.Wakelin LP; Bu X; Eleftheriou A; Parmar A; Hayek C; Stewart BW, Bisintercalating threading diacridines: relationships between DNA binding, cytotoxicity, and cell cycle arrest. J Med Chem 2003, 46, 5790–802. [DOI] [PubMed] [Google Scholar]
- 39.Brady J; Radonovich M; Thoren M; Das G; Salzman NP, Simian virus 40 major late promoter: an upstream DNA sequence required for efficient in vitro transcription. Mol Cell Biol 1984, 4, 133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holman GG; Zewail-Foote M; Smith AR; Johnson KA; Iverson BL, A sequence-specific threading tetra-intercalator with an extremely slow dissociation rate constant. Nat Chem 2011, 3, 875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Millili PG; Selekman JA; Blocker KM; Johnson DA; Naik UP; Sullivan MO, Structural and functional consequences of poly(ethylene glycol) inclusion on DNA condensation for gene delivery. Microsc Res Tech 2010, 73, 866–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.