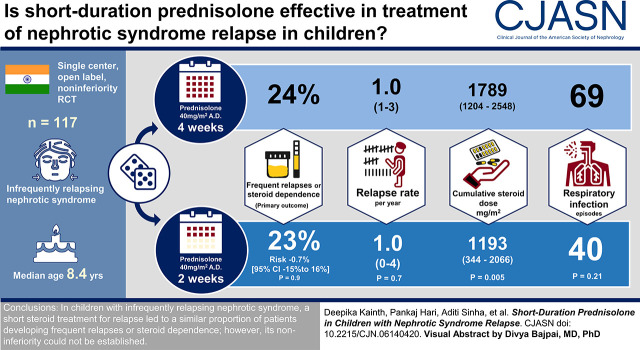
Visual Abstract
Keywords: infrequently relapsing nephrotic syndrome, short regimen, frequent relapses, prednisolone, nephrotic syndrome
Abstract
Background and objectives
In children with nephrotic syndrome, steroids are the cornerstone of therapy for relapse. The adequate duration and dosage of steroids, however, have not been an active area of research, especially in children with infrequently relapsing nephrotic syndrome. This study investigated the efficacy of an abbreviated regimen for treatment of a relapse in this population.
Design, setting, participants, & measurements
In a single-center, open-label, randomized controlled trial, we evaluated the efficacy of prednisolone as a “short regimen” (40 mg/m2 on alternate days for 2 weeks) compared with “standard regimen” (40 mg/m2 on alternate days for 4 weeks) for children aged 1–16 years who achieved remission of a relapse. The primary outcome was the proportion of children developing frequent relapses or steroid dependence at 12 months.
Results
A total of 117 patients were enrolled and randomized to short (55) or standard (62) regimen. Fourteen (24%) patients in standard regimen and 12 (23%) in short regimen developed frequent relapses or steroid dependence over a period of 1 year (risk difference, −1%; 95% confidence interval, −15 to 16; P=0.90). A large 95% confidence interval crossed the proposed noninferiority margin. In a time to event analysis, there was no significant difference in the proportion of children developing frequent relapses or steroid dependence and time to outcome between the two groups (hazard ratio, 1.01; 95% confidence interval, 0.83 to 1.23; P=0.98). Time to relapse, relapse rate, and steroid-related adverse events were similar in both groups. Cumulative steroid exposure was significantly lower in the short regimen (risk difference, −541 mg/m2; 95% confidence interval, −917 to −164 mg/m2; P<0.001).
Conclusions
In children with infrequently relapsing nephrotic syndrome, a short steroid treatment for relapse resulted in a similar proportion of patients developing frequent relapses or steroid dependence; however, noninferiority of a short regimen was not established.
Clinical Trial registry name and registration number:
Introduction
Nephrotic syndrome is characterized by proteinuria, hypoalbuminemia, hyperlipidemia, and edema with a course punctuated by relapses. Eighty percent of children have a steroid-responsive disease (1). The remainder are steroid resistant, and they have a higher risk of progression to CKD. Although extensive research has been undertaken to evaluate and establish the appropriate dose and duration of steroid therapy for the initial episode (2,3), the treatment of relapse remains empirical. A consensus recommendation for relapse in nephrotic syndrome is daily prednisolone (2 mg/kg) until remission, followed by 1.5 mg/kg every other day for 4 weeks (4). Owing to the relapsing disease course, repeated therapy with prednisolone can lead to lifelong steroid-related complications. Considering the toxic potential of steroids and a dearth of research into the therapy for relapse, evaluating a short (2-week) regimen as compared with the standard (4-week) regimen seems rational. We hypothesized that the intervention would prove noninferior in terms of development of frequent relapses or steroid dependence. A decrease in cumulative steroid dose would possibly reduce steroid toxicity.
Therefore, we conducted a single-center, open-label, randomized controlled trial between August 2015 and July 2017 to examine the efficacy of a short regimen versus standard regimen in children with steroid-sensitive nephrotic syndrome.
Materials and Methods
This open-label, prospective, randomized controlled trial was conducted to study the efficacy of a short regimen for relapse in children with steroid-sensitive nephrotic syndrome (http://ctri.nic.in; CTRI/2015/11/006345). The institute ethics committee approved the project, and the trial was conducted in accordance with principles of the Declaration of Helsinki. Patients were enrolled after obtaining informed written consent. Random numbers were computer generated for random (1:1) assignment using variable block size (four and six). Participants were stratified for presence of frequent relapses or steroid dependence prior to onset of infrequently relapsing course and age <4 years. Owing to use of stratification and block randomization, the maximum expected imbalance between two groups would be 12: strata (K) × block size (B)/2 (5). Sequentially numbered, opaque, sealed envelopes were designed to ensure allocation concealment. Preparation and safekeeping of the randomization list were ensured by staff not involved in study. Patients and investigators were not blinded.
Participants
Patients with nephrotic syndrome were eligible during relapse and randomized at remission. Children (1–16 years) were included if they had infrequently relapsing course (fewer than two relapses in 6 months or fewer than four relapses in 12 months). Relapse was defined as proteinuria (dipstick) >3+ for at least 3 consecutive days. Presence of nil or trace proteinuria on 3 consecutive days was defined as remission. Children failing to remit in current relapse despite 4 weeks of adequate steroid therapy (steroid resistance), those with known secondary cause of nephrotic syndrome, those with an inadequately treated initial episode within 12 months of enrollment, those who received alternate immunosuppression within 6 months of enrollment, or those who did not receive adequate prednisolone (60 mg/m2) for at least 7 days for current relapse were excluded.
Intervention
The daily steroid therapy was given at 60 mg/m2 until disease remission. Following remission, eligible participants were randomized to receive alternate day prednisolone (40 mg/m2) as either short (2-week) or standard (4-week) regimen, which was started on the day of randomization. The maximum doses for daily and alternate day therapy were 60 and 40 mg, respectively.
Baseline characteristics were recorded. At each visit, data from urine protein diary (maintained by the patient) were assessed, and relapses were evaluated. Proteinuria was confirmed by dipstick at the site along with a complete clinical examination. Signs and symptoms of infection, if any, were recorded. Infection-associated relapse was defined as presence of >3+ proteinuria on 3 consecutive days precipitated by clinically diagnosed infection (respiratory tract, gastrointestinal, peritoneal, urinary tract, or skin infection). To assess for steroid-related adverse effects, BP was recorded at each visit. Patients with at least two readings >95th centile for age, sex, and height were prescribed antihypertensives (6). Hemoglobin A1c levels (baseline and end of study) were estimated for glucose intolerance. Ophthalmologic evaluation was done for cataract and glaucoma. Weight, height, and body mass index were recorded at each visit. Tuberculin skin testing was performed to rule out tuberculosis. Those testing positive (>10-mm induration) without clinical signs/symptoms received prophylactic isoniazid (10 mg/kg per day) for 6 months as per national guidelines (7).
Follow-Up
Patients were followed at predefined intervals (1, 3, 6, 9, and 12 months) and assessed for occurrence of relapse, clinical evidence of infection, and steroid-related adverse events. All subsequent relapses were treated according to allocated group. Protocol deviation was defined as patients in short regimen who received treatment as per standard regimen, and vice versa. Follow-up was censored if the patient developed steroid resistance.
Outcomes
The primary outcome was to compare the proportion of children developing either frequent relapses or steroid dependence during 1-year follow-up. Occurrence of two or more relapses in 6 months or four or more relapses in a 12-month duration was defined as frequent relapses. Two consecutive relapses while on alternate day steroids or within 14 days of discontinuation were termed as steroid dependence. Secondary outcomes were time to relapse, relapse rate, cumulative steroid dose, and steroid-related adverse effects during 1-year follow-up at a prespecified schedule.
Sample Size
The study aimed to test the noninferiority of the short regimen in terms of development of frequent relapses or steroid dependence, compared with the standard regimen. In view of nonavailability of a similar study, the sample size estimation was on the basis of a study testing an extended regimen for relapse. The proportion of children developing frequent relapses in the standard regimen (control arm) was 40% (8). The noninferiority was defined by the 95% confidence interval (95% CI) of the effect estimate excluding 10% higher incidence of the primary outcome. Assuming the proportion of children developing frequent relapses or steroid dependence to be 28% in the short regimen, 80% power, and 0.05 α-error, a total of 114 children (57 in each group) were required (www.sealedenvelope.com).
Statistical Analyses
Data were analyzed using Stata12.0 (StataCorp. 2011. Stata Statistical Software: Release 12; StataCorp LP, College Station, TX). Categorical data were analyzed using Fisher exact test or chi-squared test; continuous data were analyzed using t test (normal distribution) or Wilcoxon rank sum test (skewed distribution). The former was expressed as mean (SD), and the latter was expressed as median (interquartile range). Median number of relapses, relapse rates, and times to relapse were compared. Relapse incidence density (per 1000 patient-days) was calculated to adjust for variable duration of follow-up. The primary outcome was compared, and the difference in proportions was estimated using logistic regression. Kaplan–Meier curves for time to relapse and time to outcome were analyzed using log rank test. The growth parameters were assessed using SD scores at each visit, and their medians were compared. The outcomes were analyzed using the intention to treat principle. To account for the missing data in both primary and secondary outcomes, multiple imputation using chained equations was used. Categorical outcomes were imputed and analyzed using binary logistic regression. For continuous outcome, predictive mean matching was used to impute missing values. The method is used for skewed data. For each missing value in the outcome of interest, a predicted mean was calculated on the basis of the fitted linear regression model using available values. The missing value was then imputed by randomly choosing one of the observed values. For data “missing completely at random” for both primary and secondary variables, a total of five imputations were performed. A sensitivity analysis was not performed due to the small proportion of missing data.
Subgroup and adjusted analyses were performed for sex, age at enrollment, prior frequently relapsing or steroid-dependent course, and prior exposure to alternate immunosuppressive drugs. To adjust for multiple outcomes and subgroup analysis, Bonferroni correction was applied for each outcome if the P value was significant (P=0.05).
Results
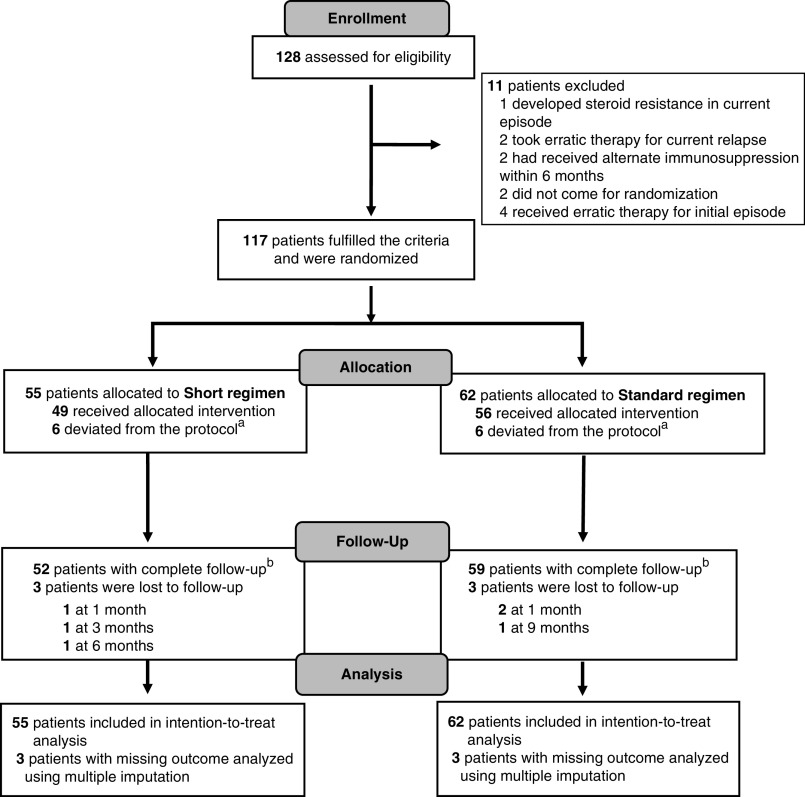
We enrolled 117 children; 55 were randomized to the short regimen, and 62 were randomized to the standard regimen (Figure 1). The median (interquartile range [IQR]) age of patients enrolled in the study was 8 (6–11) years, and 79% were boys. Baseline characteristics, including prior frequently relapsing or steroid dependence, age at onset and enrollment, steroid-related adverse events (abnormal eye examination, glucose intolerance, and hypertension), biochemical parameters at remission, and tuberculin skin test positivity, were similar in both groups (Table 1). Growth indices were comparable, with median SD scores between zero and −1.5 SD in both groups.
Figure 1.
Study flow. Patients were included in an intention-to-treat analysis. a Includes patients who received treatment regimen similar to other limb in at least one relapse after enrollment. b Patients with complete follow-up included in complete case analysis for primary and secondary outcomes.
Table 1.
Baseline characteristics of patients enrolled in a randomized trial of short versus standard steroid regimen for relapse in nephrotic syndrome
| Baseline Characteristics | Short Regimen | Standard Regimen |
|---|---|---|
| N | 55 | 62 |
| Age at enrollment,a yr | 8 (6–11) | 9 (6–12) |
| <4 | 2 (4) | 4 (6) |
| Age at onset,a yr | 3 (2–5) | 3 (2–5) |
| <4 | 32 (58) | 33 (53) |
| Boys | 44 (80) | 49 (79) |
| Adequate initial treatment | 39 (71) | 44 (71) |
| Initial remission, mo | 9.7±7.5 | 10.5±8.2 |
| Prior frequent relapses or steroid dependence | 20 (36) | 30 (48) |
| Prior immunosuppression | ||
| Levamisole | 6 (11) | 14 (23) |
| Cyclophosphamide | 7 (13) | 14 (23) |
| Long-term alternate-d steroids | 8 (14) | 8 (13) |
| Mycophenolate mofetil | 4 (7) | 3 (5) |
| Calcineurin inhibitors | 0 | 2 (3) |
| Rituximab | 0 | 1 (2) |
| Time to onset of infrequent relapsing course,a mo | 25 (14–39) | 23 (14–37) |
| <12 | 18 (32) | 16 (26) |
| Weight SD scorea | −0.2 (−0.9–0.4) | −0.3 (−0.8–0.4) |
| Height SD scorea | −0.7 (−1.3 to −0.06) | −0.7 (−1.6 to −0.02) |
| Body mass index SD scorea | 0.1 (−0.4–0.8) | 0.13 (−0.3–0.8) |
| Edema | 6 (11) | 9 (14) |
| Ascites | 3 (5) | 3 (5) |
| Hypertension (systolic or diastolic BP or both) | ||
| 95th to 99th centile + 5 mm Hg | 11 (20) | 9 (14) |
| >99th centile + 5 mm Hg | 3 (5) | 3 (5) |
| Eye toxicity | ||
| Cataract | 4 (7) | 6 (10) |
| Glaucoma | 2 (4) | 0 |
| Cushingoid facies | 10 (18) | 14 (23) |
| Urea, mg/dl | 24±9 | 24±9 |
| Creatinine, mg/dl | 0.4±0.2 | 0.4±0.2 |
| eGFR,a ml/min per 1.73 m2 | 138 (98–193) | 131 (102–167) |
| Albumin, g/dl | 3.8±0.7 | 3.8±0.8 |
| Cholesterol, mg/dl | 195±85 | 175±62 |
| Tuberculin skin test positivity | 4 (8%) | 4 (7%) |
| HbA1c, % | 5.3±0.3 | 5.1±0.6 |
Continuous parameters are reported as mean ± SD for normally distributed variables unless noted. Categorical parameters are reported as n (%). P value of 0.05 was considered as significant. HbA1c, hemoglobin A1c.
Continuous parameters are reported as median (interquartile range) for variables with skewed distribution.
Patients in the standard regimen received alternate day therapy for a median (IQR) duration of 44.5 (IQR, 34.5–63.5) days compared with 28.5 (IQR, 8–46) days in the short regimen group. Three patients (one in the short regimen and two in the standard regimen) did not have follow-up for at least one visit subsequent to randomization. Three patients (two in the short regimen and one in the standard regimen) were lost to follow-up at a later visit (Figure 1). Twelve patients (six in each group) deviated from the protocol as per the allocated group (Supplemental Table 1), with none deviating for more than one relapse.
Primary Outcome
Twenty-six patients developed frequent relapses or steroid dependence during the study: 14 (24%) in the standard regimen group and 12 (23%) in the short regimen group. The risk difference using complete patient analysis was −1% (95% CI, −15% to 16%; P=0.90) (Table 2), which was not different following multiple imputation for missing values (risk difference, −3%; 95% CI, −19% to 13%; P=0.70) (Supplemental Tables 2 and 3).
Table 2.
Study outcomes
| Outcome | Short Regimen, n=52 | Standard Regimen, n=59 | Risk or Mean Difference (95% Confidence Interval) | P Value |
|---|---|---|---|---|
| Primary outcomea | ||||
| Proportion of frequent relapses or steroid dependence, n (%) | 12/52 (23) | 14/59 (24) | −1% (−15% to 16%) | 0.90 |
| Secondary outcomesb | ||||
| Time to relapse, d | 78 (0–179) | 104 (54–198) | −26 (−65 to 13) | 0.20 |
| Relapse rate, per yr | 1 (0–4) | 1 (1–3) | −0.3 (−1 to 1.5) | 0.70 |
| Cumulative prednisolone dose, mg/m2 | 1193 (344–2066) | 1789 (1204–2548) | −541 (−917 to −164) | 0.005 |
| Infection-associated relapses | 0 (0–1) | 0 (0–1) | 0.1 (−0.1 to 0.4) | 0.35 |
| Other outcomesc | ||||
| Relapse incidence density per 1000 patient-d | 2.8 (2.7–9.7) | 2.8 (2.7–8.3) | −0.4 (−2.1 to 2.8) | 0.77 |
| Duration of treatment, d | 29 (8–46) | 45 (35–64) | −19 (−26 to −14) | <0.001 |
| Time to occurrence of frequent relapses or steroid dependence | 206 (117–345) | 156 (146–211) | −50 (−67 to 17) | 0.83 |
The outcomes are depicted for complete case analyses of the patients with complete follow-up.
Assessed during 1-year follow-up.
Outcomes are expressed as median (interquartile range) due to non-normal distribution. Outcomes were assessed at each follow-up visit (1, 3, 6, 9, and 12 months).
Outcomes are expressed as median (interquartile range) due to non-normal distribution.
Secondary Outcomes
The median (IQR) times (days) to relapse were 78 (IQR, 0–179) and 104 (IQR, 54–198) in short and standard regimens, respectively (P=0.20) (Figure 2B). The median (IQR) number of relapses was one (IQR, 0–4) in the short regimen compared with one in the standard regimen (P=0.70). Also, there was no difference in the median (IQR) time to occurrence of primary outcome in the two groups (P=0.80) (Figure 2A). Median (IQR) cumulative steroid doses (milligrams per meter2) were 1193 (IQR, 344–2066) and 1789 (IQR, 1204–2548) in short and standard regimens, respectively (P<0.001) (Table 2). The imputation of missing data did not result in significant differences in the two groups (Supplemental Tables 2 and 3). No significant differences were observed in infections and infection-associated relapses. Further, the occurrence of cataract, glaucoma, hypertension, and glucose intolerance was similar. Two patients (standard regimen) without a prior frequently relapsing or steroid-dependent course had glaucoma (one developed at the end of study) compared with none in the short regimen. One patient had cataract both at baseline and at the end of the study. At last follow-up, three patients in the short regimen (two stage 1 and one stage 2) and five patients in the standard regimen (three stage 1 and two stage 2) had hypertension (P=0.54). Two patients in each group developed hypertension, with no evidence of the same at baseline. The median SD scores (height, weight, and body mass index) compared at the end of the study from baseline did not differ in the two groups (Supplemental Table 4). Four patients (one, short regimen; three, standard regimen) developed steroid resistance.
Figure 2.
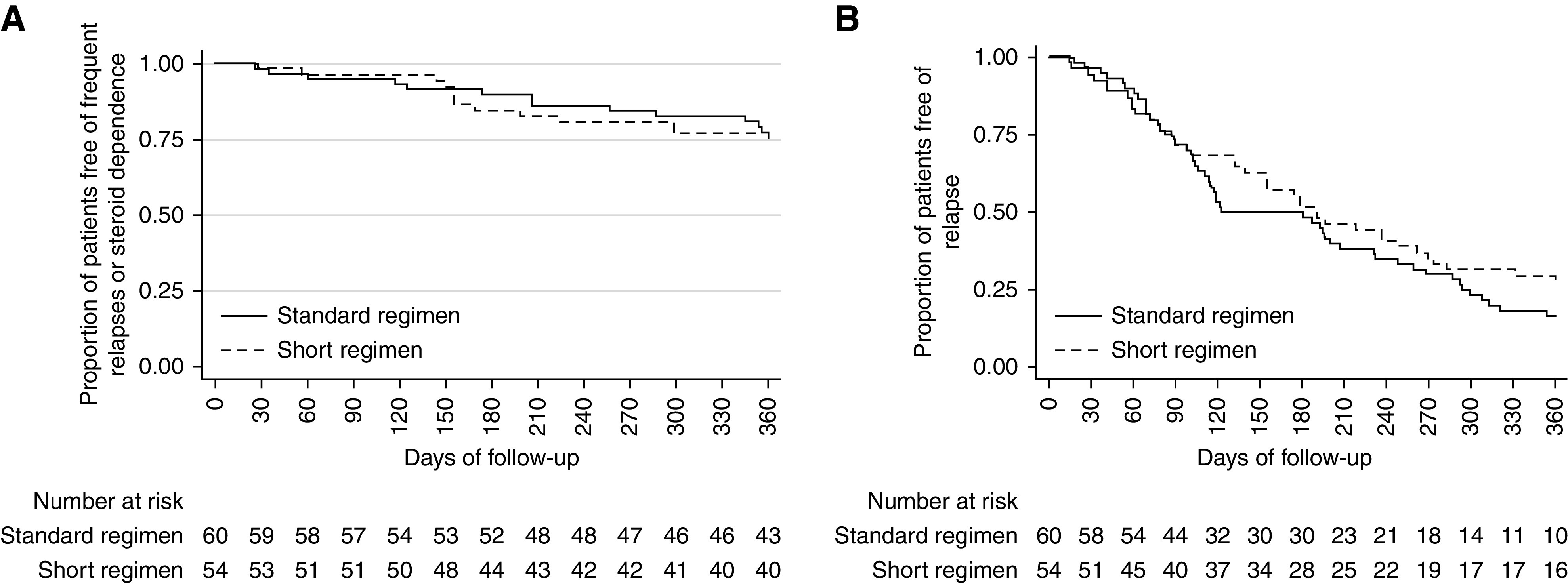
Times to study outcomes. (A) Time to occurrence of frequent relapses or steroid dependence and (B) time to first relapse in patients treated with short regimen (dotted lines) compared with standard regimen (solid lines).
Adverse Events
Sixty adverse events in the short regimen and 91 events in the standard regimen were observed; 40 (of 60) and 69 (of 91) events, respectively, were upper respiratory tract infections in each group (P=0.21) (Table 3). Five patients were hospitalized during the study: three in the short regimen (one each for acute febrile illness, severe edema, and hypovolemia) versus two in the standard regimen (both for edema control). There were no deaths.
Table 3.
Adverse events
| Parameter | Short Regimen, n=54 | Standard Regimen, n=60 | P Value |
|---|---|---|---|
| Infectionsa | 0.21 | ||
| Respiratoryb | 40 | 69 | |
| Othersc | 17 | 20 | |
| Hospitalizationsd | 3 | 2 | |
| Steroid-related toxicitye | |||
| Eye toxicity, n (%) | |||
| Cataract | 0 | 1 (2) | |
| Glaucoma | 0 | 2 (3) | |
| Hypertension (systolic or diastolic BP or both), n (%) | 0.54 | ||
| 95th to 99th centile + 5 mm Hg | 2 (4) | 3 (5) | |
| More than 99th centile + 5 mm Hg | 1 (2) | 2 (3) | |
| Cushingoid facies, n (%) | |||
| Mild | 4 (7) | 4 (7) | 0.81 |
| Severe | 1 (2) | 0 | |
| HbA1c, %, mean ± SD | 5.4±0.3 | 5.3±0.7 | 0.59 |
| Cholesterol, mg/dl, mean ± SD | 162±42 | 156±43 | 0.56 |
Two patients in standard regimen and one patient in short regimen could not be assessed for adverse events due to loss to follow-up after randomization. HbA1c, hemoglobin A1c.
Infections expressed as number of episodes in each group.
Includes both upper and lower respiratory tract infections.
Gastroenteritis, peritonitis, urinary tract infections, skin infections, fever without focus, dengue, and chikungunya.
Hospitalization indicated for edema control or hypovolemia.
Assessed at the end of study.
Other Outcomes
To correct for variable follow-up duration, the median (IQR) relapse incidence density (relapses per 1000 patient-days) was computed; difference was NS (short regimen: 2.8 [IQR, 2.7–9.7]; standard regimen: 2.8 [IQR, 2.7–8.3]; P=0.77).
Exploratory Subgroup and Adjusted Analyses
In an unadjusted post hoc analysis, occurrence of relapse was similar in the subgroups categorized by sex, age, prior history of frequently relapse or steroid dependence, and previous exposure to alternate immunosuppression. There was no interaction among the subgroups (Table 4). Further, the adjusted analysis did not establish an association of the outcome with these variables (Supplemental Table 5).
Table 4.
Subgroup analyses of the primary outcome
| Subgroup | Short Regimen, n=52a | Standard Regimen, n=59 | Risk Difference, % (95% Confidence Interval) | Pinteraction Valueb |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Boys | 10/43 (23) | 11/46 (24) | −1 (−18 to 17) | 0.89 |
| Girls | 2/9 (22) | 3/13 (23) | −3 (−37 to 31) | |
| Prior frequent relapses or steroid dependence, n (%) | ||||
| Yes | 3/20 (15) | 5/29 (17) | −2 (22 to 18) | 0.12 |
| No | 9/32 (28) | 9/30 (30) | −4 (−25 to 18) | |
| Age, yr, n (%) | ||||
| <4 | 1/2 (50) | 1/4 (25) | 25 (−56 to 106) | 0.63 |
| >4 | 11/50 (22) | 13/55 (24) | −2 (−18 to 14) | |
| Prior immunosuppression,c n (%) | ||||
| Yes, n (%) | 2/8 (25) | 3/16 (19) | −6 (−35 to 38) | 0.87 |
| No, n (%) | 10/44 (23) | 11/44 (25) | −1 (−19 to 16) |
Proportion of children developing frequent relapses or steroid dependence over 12-month follow-up duration.
P values were obtained from an interaction term between primary outcome and different levels for each subgroup.
Therapy with at least one of the following drugs prior to onset of infrequently relapsing disease: mycophenolate mofetil, calcineurin inhibitors, cyclophosphamide, and rituximab.
Discussion
In a single-center, randomized controlled trial, we examined efficacy of a short steroid regimen for disease relapse. We found that short regimen (compared with standard regimen) for relapse in children with infrequently relapsing nephrotic syndrome resulted in similar outcomes in terms of development of frequent relapses or steroid dependence over a period of 1 year, although noninferiority of short regimen could not be proven statistically.
The development of frequent relapses or steroid dependence is an important clinical outcome as it influences the choice of treatment and prognosis. It is determined by factors like young age (<4 years) at onset (9). Subjects were stratified according to age (<4 years) and a frequently relapsing or steroid-dependent course prior to development of infrequent relapses at randomization. Because children with only infrequent relapses were eligible, those <4 years accounted for only 5% of the study population. There was no difference in primary outcome in these subgroups.
The study used for sample size calculation was the only trial done on relapse and had 40% of children developing frequent relapses on standard therapy (8). It included patients who relapsed within 6 months (early) of initial episode, thus representing a population with severe disease as compared with our study. We found that the short regimen had a similar proportion of patients developing the primary outcome as compared with standard regimen; however, the event rate (23%) was lower than the study used for sample size calculation. The assumption of 12% higher survival free of frequent relapses for the short regimen as compared with the standard regimen was probably too optimistic, rendering the study underpowered to detect noninferiority. The upper limit of the 95% CI (16%) crossed the proposed noninferiority margin (10%). These results are, therefore, hypothesis generating, and to conclusively establish the noninferiority of an experimental regimen with almost similar proportion compared with standard regimen, a much larger study will be required. Nevertheless, the results obtained in our study consolidate our hypothesis of similar clinical outcomes with a short regimen for relapse treatment in steroid-sensitive disease.
The numbers of participants in short and standard regimens were not balanced. This difference can be explained owing to the design of randomization, which included both stratification and blocking (variable blocks of four and six) (5). Further, there was an imbalance in terms of previous exposure to alternate immunosuppression, the difference not reaching statistical significance. Because we did not stratify for these variables at the stage of study design, it was possible by chance that children with severe disease were randomized to the standard regimen. However, the adjusted analysis did not reveal any significant differences in the outcome.
The time to primary outcome, time to relapse, number of infections, infection-associated relapses, and hospitalizations were similar in two groups. There was no difference in growth trends over the study duration. The majority of relapses were preceded by upper respiratory tract infections. These findings are consistent with other studies and establish respiratory tract infection as the most prominent trigger for relapse (10).
The cumulative dose of steroids was significantly lower in the short regimen in accordance with the study design. No difference in steroid-related toxicity was observed in the two groups, despite a significantly lower steroid exposure in the short regimen group. This was perhaps due to fewer relapses per year in both groups. It is possible that children treated with the short regimen over prolonged follow-up may show significantly fewer adverse effects, like growth retardation and ocular, cardiovascular, and metabolic complications.
The hallmark of nephrotic syndrome is proteinuria mediated by inflammatory mediators, like IL-2, -4, and -13 and various other cytokines (11). An imbalance between T helper 17 cells and regulatory T cells has been directly linked to proteinuria, which reverts back to normal after steroid therapy (12,13). During a relapse, a significant surge in circulating memory T cells occurs, with a concomitant decrease in regulatory T cells suggesting a complex immunologic interplay, which is attenuated by corticosteroids (14). After steroid therapy is instituted, the innate hypothalamic-pituitary-adrenal (HPA) axis is suppressed. Leisti et al. (15) reported a higher likelihood of early relapse in children developing severe HPA axis suppression. It is possible that a longer duration of treatment, either for initial episode or for relapse, leads to prolonged HPA axis suppression. An immunologic trigger (infection) during this period, therefore, results in inadequate glucocorticoid response, culminating in relapse. It is therefore possible, at least theoretically, that a shorter regimen may lead to a decreased number of relapses owing to its shorter HPA axis suppression.
A major limitation of the study is that it could not establish noninferiority of the short regimen despite a similar occurrence of primary outcome. The observed event rates of the primary outcome and their difference between the two regimens were lower than those assumed during sample size calculation, which rendered the study underpowered. The study was open label, harboring the risk of detection bias. Being a single-center study, generalizability is limited. Further, the duration of follow-up was insufficient to assess differences in long-term effects of steroids.
Corticosteroid therapy with its toxic potential is a double-edged sword. The ideal duration and dose that minimize toxicity without increasing the occurrence of relapses need to be conclusively established. To our best knowledge, despite its shortcomings, this is the first randomized controlled trial conducted to examine a short regimen for relapse in nephrotic syndrome. The study was well designed with a low risk of bias owing to adequate implementation of allocation concealment, stratification, regular follow-up, and reporting. Our study was limited to infrequently relapsing patients, and it would be clinically relevant to study the efficacy of short regimen for treatment of any relapse subsequent to initial episode. Moreover, worldwide researchers are now inclined toward evaluating the effects of decreasing steroid exposure by reduction in either dose or duration. An ongoing noninferiority, randomized controlled trial, Reducing Steroids in Relapsing Nephrotic syndrome (RESTERN) hypothesizes that a decreased (2-week) versus standard (6-week) alternate day therapy following remission results in reduction of steroid exposure by 35% with similar clinical outcomes (16). Compared with our study, their population is more heterogenous, also including patients on immunosuppression, and the primary outcome is time to first relapse. If the study succeeds to show noninferiority of the abbreviated regimen, in conjunction with the results from our study, a change in the current treatment of relapse in nephrotic syndrome can be expected.
To conclude, a short regimen compared with the standard regimen for relapse resulted in a similar proportion developing frequent relapses or steroid dependence in steroid-sensitive nephrotic syndrome. The noninferiority of the short regimen, however, could not be established.
Disclosures
All authors have nothing to disclose.
Funding
Support was provided by Department of Biotechnology, Government of India grant BT/PR11030/MED/97/1644/2016.
Supplementary Material
Acknowledgments
The funding agency had no role in study design, collection and interpretation of data, and manuscript preparation.
Data Sharing Statement
Data collected for the study will be made available to others, including the complete deidentified patient dataset, the study protocol, and informed consent forms. The data will be available within 1 month of publication and available for 3 years at http://ctri.nic.in.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Steroid Regimen for Children with Nephrotic Syndrome Relapse,” on pages 179–181.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06140420/-/DCSupplemental.
Supplemental Table 1. Comparison of number of patients deviating from the allocated regimen at follow-up.
Supplemental Table 2. Comparison of study outcomes after multiple imputation for missing values with complete patient analyses.
Supplemental Table 3. Study outcomes after multiple imputation for missing values.
Supplemental Table 4. Changes in anthropometric measurements over the duration of the study.
Supplemental Table 5. Risk difference of proportion of children developing frequent relapses or steroid dependence at 12 months, unadjusted and adjusted for patient characteristics.
References
- 1.Churg J, Habib R, White RH: Pathology of the nephrotic syndrome in children: A report for the International Study of Kidney Disease in Children. Lancet 760: 1299–1302, 1970 [DOI] [PubMed] [Google Scholar]
- 2.Hodson EM, Knight JF, Willis NS, Craig JC: Corticosteroid therapy in nephrotic syndrome: A meta-analysis of randomised controlled trials. Arch Dis Child 83: 45–51, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha A, Saha A, Kumar M, Sharma S, Afzal K, Mehta A, Kalaivani M, Hari P, Bagga A: Extending initial prednisolone treatment in a randomized control trial from 3 to 6 months did not significantly influence the course of illness in children with steroid-sensitive nephrotic syndrome. Kidney Int 87: 217–224, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Ehrich JH, Brodehl J; Arbeitsgemeinschaft für Pädiatrische Nephrologie: Long versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Eur J Pediatr 152: 357–361, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Hallstrom A, Davis K: Imbalance in treatment assignments in stratified blocked randomization. Control Clin Trials 9: 375–382, 1988 [DOI] [PubMed] [Google Scholar]
- 6.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents: The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114[Suppl 4th Report]: 555–576, 2004 [PubMed] [Google Scholar]
- 7.World Health Organization : Index-TB guidelines: Guidelines on extra-pulmonary tuberculosis for India, 2016. Available at: https://tbcindia.gov.in/WriteReadData/l892s/5585665076Index-TB%20Guidelines.pdf. Accessed March 10, 2020
- 8.International Study of Kidney Disease in Children: Nephrotic syndrome in children: A randomized trial comparing two prednisone regimens in steroid-responsive patients who relapse early. Report of the International Study of Kidney Disease in Children. J Pediatr 95: 239–243, 1979 [PubMed] [Google Scholar]
- 9.Mishra K, Kanwal SK, Sajjan SV, Bhaskar V, Rath B: Predictors of poor outcome in children with steroid sensitive nephrotic syndrome. Nefrologia 38: 420–424, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Moorani KN: Infections are common cause of relapse in children with nephrotic syndrome. Pak Pediatr J 35: 213–219, 2011 [Google Scholar]
- 11.McCarthy ET, Sharma M, Savin VJ: Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 5: 2115–2121, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Shalhoub RJ: Pathogenesis of lipoid nephrosis: A disorder of T-cell function. Lancet 2: 556–560, 1974 [DOI] [PubMed] [Google Scholar]
- 13.Liu LL, Qin Y, Cai JF, Wang HY, Tao JL, Li H, Chen LM, Li MX, Li XM, Li XW: Th17/Treg imbalance in adult patients with minimal change nephrotic syndrome. Clin Immunol 139: 314–320, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Yan K, Nakahara K, Awa S, Nishibori Y, Nakajima N, Kataoka S, Maeda M, Watanabe T, Matsushima S, Watanabe N: The increase of memory T cell subsets in children with idiopathic nephrotic syndrome. Nephron 79: 274–278, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Leisti S, Hallman N, Koskimies O, Perheentupa J, Rapola J, Vilska J: Association of postmedication hypocortisolism with early first relapse of idiopathic nephrotic syndrome. Lancet 2: 795–796, 1977 [DOI] [PubMed] [Google Scholar]
- 16.Schijvens AM, Dorresteijn EM, Roeleveld N, Ter Heine R, van Wijk JAE, Bouts AHM, Keijzer-Veen MG, van de Kar NCAJ, van den Heuvel LPWJ, Schreuder MF: REducing STEroids in Relapsing Nephrotic syndrome: The RESTERN study- Protocol of a national, double-blind, randomised, placebo-controlled, non-inferiority intervention study. BMJ Open 7: e018148, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.