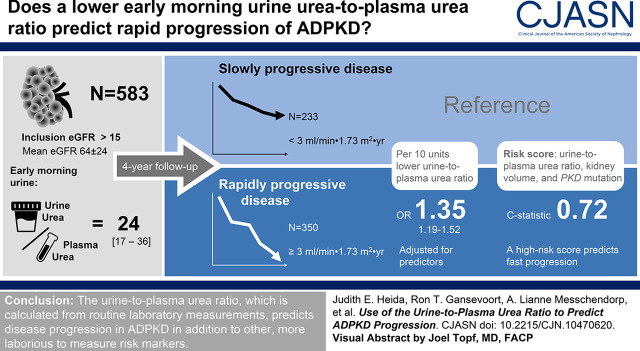
Visual Abstract
Keywords: ADPKD, urea, renal function decline, urine-to-plasma urea ratio, autosomal dominant polycystic kidney disease
Abstract
Background and objectives
Predicting disease progression in patients with autosomal dominant polycystic kidney disease (ADPKD) poses a challenge, especially in early-stage disease when kidney function is not yet affected. Ongoing growth of cysts causes maximal urine-concentrating capacity to decrease from early on. We therefore hypothesized that the urine-to-plasma urea ratio, as a reflection of the urine-concentrating capacity, can be used as a marker to predict ADPKD progression.
Design
The urine-to-plasma urea ratio was calculated by dividing concentrations of early morning fasting spot urine urea by plasma urea. First, this ratio was validated as surrogate marker in 30 patients with ADPKD who underwent a prolonged water deprivation test. Thereafter, association with kidney outcome was evaluated in 583 patients with ADPKD with a broad range of kidney function. Multivariable mixed-model regression was used to assess association with eGFR slope, and logarithmic regression to identify patients with rapidly progressive disease, using a cutoff of −3.0 ml/min per 1.73 m2 per year. The urine-to-plasma urea ratio was compared with established predictors, namely, sex, age, baseline eGFR, Mayo Clinic height-adjusted total kidney volume class, and PKD gene mutation.
Results
The maximal urine-concentrating capacity and urine-to-plasma urea ratio correlated strongly (R=0.90; P<0.001). Next, the urine-to-plasma urea ratio was significantly associated with rate of eGFR decline during a median follow-up of 4.0 (interquartile range, 2.6–5.0) years, both crude and after correction for established predictors (β=0.58; P=0.02). The odds ratio of rapidly progressive disease was 1.35 (95% confidence interval, 1.19 to 1.52; P<0.001) for every 10 units decrease in urine-to-plasma urea ratio, with adjustment for predictors. A combined risk score of the urine-to-plasma urea ratio, Mayo Clinic height-adjusted total kidney volume class, and PKD mutation predicted rapidly progressive disease better than each of the predictors separately.
Conclusions
The urine-to-plasma urea ratio, which is calculated from routine laboratory measurements, predicts disease progression in ADPKD in addition to other risk markers.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2021_01_27_CJN10470620_final.mp3
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by progressive kidney function loss caused by ongoing development and growth of cysts (1). Of these patients, 70% will develop kidney failure and require KRT at a mean age of 58 years (2). Determining which patients are at risk to develop kidney failure is done by assessment of several clinical parameters, most importantly, eGFR, total kidney volume (TKV) (3–5), and type of genetic mutation causing ADPKD (6,7). It is generally acknowledged that establishing prognosis is challenging, especially in the beginning of the disease (8,9). In early-stage disease, GFR has not yet declined and TKV is not severely increased. However, cyst formation in the kidneys has already led to changes in medullary architecture, resulting in a reduced maximal urine-concentrating capacity (10–15). A marker to reflect this early damage might therefore be valuable for prediction of future disease progression. Bankir and Bichet (16) suggested that the urine-to-plasma urea ratio could be used as a marker for urine-concentrating capacity. To our knowledge, we are the first to study the urine-to-plasma urea ratio as a marker for rate of disease progression in ADPKD.
In this study, we aimed to demonstrate the predictive value of the maximal urine-concentrating capacity in patients with ADPKD, and the suitability of the urine-to-plasma urea ratio as its surrogate marker. Also, we studied the association between urine-to-plasma urea ratio and rate of kidney function decline in ADPKD.
Materials and Methods
Study Population
For this study, we used two data sources. First, we performed a post hoc analysis of data from 30 patients with ADPKD who underwent a prolonged water deprivation test to determine maximal urine-concentrating capacity. These tests were performed between 2011 and 2014, and included patients aged 18–65 years without diabetes mellitus, active cardiovascular disease, or use of diuretics. A detailed study protocol can be found elsewhere (13,15).
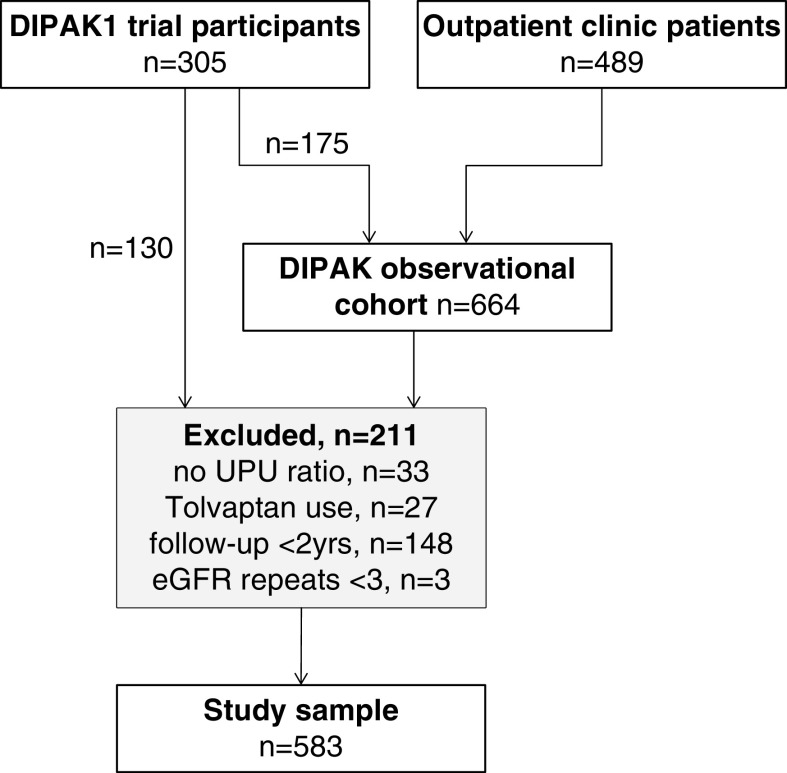
For the second part of our analyses, we used data from the Developing Interventions to Halt Progression of Autosomal Dominant Polycystic Kidney Disease (DIPAK)-1 trial, complemented with data from the subsequent DIPAK observational cohort study. The DIPAK-1 randomized controlled trial assessed the renoprotective effect of the somatostatin analog lanreotide in patients with ADPKD (ClinicalTrials.gov identifier NCT01616927). Design, methods, and the main outcomes have been published previously (17,18). In brief, patients with ADPKD (age between 18 and 60 years, with eGFR between 30 and 60 ml/min per 1.73 m2) were included between 2012 and 2015, and followed for 132 weeks. After end of this trial, a cohort study was initiated to investigate the natural course of the disease. Out of the 305 participants, 175 patients continued follow-up. Inclusion into this observational cohort was also open to patients with ADPKD who routinely visited outpatient clinics (n=489). Inclusion criteria for the observational cohort study were age ≥18 years and an eGFR ≥15 ml/min per 1.73 m2. Exclusion criteria for both studies were concomitant diseases or use of medication that may influence the natural course of ADPKD. For the present analyses, we included patients with ADPKD with a baseline urine-to-plasma urea ratio and at least three eGFR assessments during at least 2 years of follow-up to allow reliable calculation of slope of eGFR decline, leaving 583 patients (Figure 1).
Figure 1.
Flow diagram showing inclusion of 583 participants into the study cohort. DIPAK, Developing Interventions to Halt Progression of Autosomal Dominant Polycystic Kidney Disease; UPU ratio, urine-to-plasma urea ratio.
All studies were approved by the Institutional Review Board of the University Medical Center Groningen and were conducted in adherence to the International Conference on Harmonization Good Clinical Practice Guidelines. Written informed consent was obtained from all participants.
Laboratory Measurements and Imaging
Urine osmolality was measured by freezing point depression using an Osmometer (Akray, Kyoto, Japan). Creatinine was measured using an isotope-dilution mass spectrometry-traceable enzymatic method, and urea was measured with an enzyme kinetic assay. eGFR was calculated with the CKD Epidemiology Collaboration equation (19). Copeptin was measured by a sandwich ELISA (Thermo Fisher Scientific, Henningsdorf/Berlin, Germany) (20). Mutations were screened using locus-specific, long-range amplification, followed by direct Sanger sequencing of exonic and flanking intronic regions of PKD1 and PKD2, HNF1B, PKHD1, and GANAB, combined with multiplex ligation-dependent probe amplification for larger deletions/duplications (18,21). Alternatively, a gene panel-based, next-generation sequencing approach was used, analyzing 137 genes; or a capture was performed with the Agilent SureSelectXT Inherited Disease Panel kit, followed by analysis of 78 genes using an in-house pipeline of the University of Leiden (22). Details are available upon request. TKV was assessed by manual tracing of the polycystic kidneys on T2-weighted coronal magnetic resonance images using Analyze direct 9.0 software (AnalyzeDirect, Inc., Overland Park, KS), as described previously (23). TKV was corrected for height and age to determine a Mayo height-adjusted total kidney volume (htTKV) class score for risk prediction (24).
Calculations
Urine-to-plasma urea ratio was calculated by dividing urine urea concentration by plasma urea concentration. Urine urea concentration was measured in the water deprivation studies in unstandardized spot urine samples and early morning fasting urine samples, and in the DIPAK cohort in early morning fasting urine samples.
Statistical Analyses
Normally distributed data are presented as means and SDs, nonparametric data are presented as medians and interquartile ranges (IQRs), and categorical data are presented as percentages. Urine-to-plasma urea ratio, copeptin concentration, and htTKV were ln transformed to attain a better fit. Univariable and multivariable linear regression analyses were performed to assess cross-sectional associations of the maximal urine-concentrating capacity and urine-to-plasma urea ratio. Thereafter, multivariable mixed modeling was used to assess associations of the urine-to-plasma urea ratio with slope of eGFR decline, our primary outcome measure. Patients with V2 receptor antagonist use were excluded from the longitudinal analysis. Patients using a somatostatin analog were not excluded. Slopes calculated with mixed models were used to identify patients with rapidly progressive disease, using a clinically valid cutoff of a decline of 3.0 ml/min per 1.73 m2 per year, or more logistic regression was performed to evaluate the additional value of the urine-to-plasma urea ratio as a marker for disease progression, using the Harrell C-statistic calculated with the Somer D package in STATA. Finally, we classified patients into clinically meaningful risk groups, combining genetic information, Mayo Clinic htTKV class, and urine-to-plasma urea ratio. Each of these predictors was given equal weight in this score, given that the relative strength of these predictors has never been established. PKD2 or nonclassified counted for one point, PKD1 nontruncating for two points, and PKD1 truncating for three points. Likewise, Mayo Clinic htTKV classes 1A and 2, classes 1B and 1C, and classes 1D and 1E counted for one, two, and three points, respectively; and the upper, middle, and lower tertile of urine-to-plasma urea ratio counted for one, two, and three points, respectively. Adding these points resulted in a total risk score. We examined if this total score was able to select patients with rapidly progressive disease more accurately than the predictors individually, including the 538 patients for whom data were complete.
As secondary analyses, we investigated the incidence of a combined kidney end point in our patient population with use of survival analysis, using a different set of selection criteria than for the above described analyses. All patients who had a least one update on survival state, a baseline urine-to-plasma urea ratio, and did not use tolvaptan were included (n=706). The combined kidney end point was defined as start of KRT (dialysis or transplantation), reaching an eGFR <15 ml/min per 1.73 m2, or the incidence of an eGFR decrease >40% during follow-up (25). The predictive value of the urine-to-plasma urea ratio was evaluated after adjustment for other predictors of disease progression using Cox regression.
Analyses were performed using SPSS (version 22; IBM Statistics), STATA (version SE 14), and GraphPad Prism (version 7.02).
Results
Urine-to-Plasma Urea Ratio as a Marker for Urine-Concentrating Capacity
Baseline characteristics of 30 patients with ADPKD who participated in water deprivation tests are presented in Table 1 and show a mean age of 42±13 years, mean eGFR of 74±32 ml/min per 1.73 m2, and median htTKV of 578 (IQR, 497–1140) ml/m. Medians of urine-to-plasma urea ratios, calculated with early morning fasting spot urine samples and unstandardized spot urine, were 37 (IQR, 23–48) and 25 (IQR, 16–40), respectively.
Table 1.
Baseline characteristics
| Characteristics | Water Deprivation Test Participants | DIPAK Participants |
|---|---|---|
| No. | 30 | 583 |
| Age, yr | 42±13 | 47±11 |
| Sex, % male | 57 | 42 |
| Body mass index, kg/m2 | 27±3.9 | 26±4.6 |
| Systolic BP, mm Hg | 134±12 | 131±14 |
| Diastolic BP, mm Hg | 84±8 | 81±9 |
| Antihypertensive therapy, % yes | 73 | 77 |
| Diuretics, % yes | — | 28 |
| Thiazide diuretics, % yes | — | 25 |
| Loop diuretics, % yes | — | 2 |
| PKD mutation, % | ||
| PKD1 T | 37 | 42 |
| PKD1 NT | 50 | 26 |
| PKD2 | 7 | 21 |
| Other | 7 | 9 |
| htTKV, ml/m | 578 [497–1140] | 898 [549–1364] |
| Mayo Clinic htTKV class, % | ||
| 1A | 7 | 5 |
| 1B | 23 | 20 |
| 1C | 23 | 33 |
| 1D | 17 | 22 |
| 1E | 20 | 12 |
| 2 | 10 | 3 |
| Plasma creatinine, mg/dl | 1.11 [0.87–1.65] | 1.22 [0.97–1.57] |
| eGFR, ml/min per 1.73 m2 | 74±32 | 64±24 |
| Plasma urea, mg/dl | 22 [17–29] | 21 [16–27] |
| Baseline copeptin, pmol/L | 9.0 [4.9–20] | 7.7 [4.5–13.2] |
| 24-h urine volume, L | 2.4±0.9 | 2.3±0.8 |
| Urine urea, mg/dl | 785 [573–1011] | 549 [406–692] |
| Estimated protein intake, g/24 h | 88±22 | 85±25 |
| Estimated salt intake, g/24 h | 9.1 [7.0–13.3] | 8.5 [6.3–11.1] |
| Maximal urine-concentrating capacity, mOsm/kg | 633±171 | — |
| Urine-to-plasma urea ratio | 37 [23–48] | 24 [17–36] |
Data presented as mean ± SD, median [interquartile range], or proportion of total population (%) as appropriate. Diuretic use was an exclusion criterion for participation in the water deprivation tests. Maximal urine-concentrating capacity was defined as the plateau of highest urine osmolality that was reached after a prolonged water deprivation test. Urine urea was measured in an early morning fasting void. Protein intake was estimated in grams with the following equation: (urine urea excretion in 24 h×0.4667×0.06+[0.031× weight])×6.25 (37). Salt intake was estimated with the following equation: sodium excretion in moles×(sum of molecular mass of sodium and chloride in grams per mole). DIPAK, Developing Interventions to Halt Progression of Autosomal Dominant Polycystic Kidney Disease; —, not applicable; T, truncating; NT, nontruncating; htTKV, height-adjusted total kidney volume.
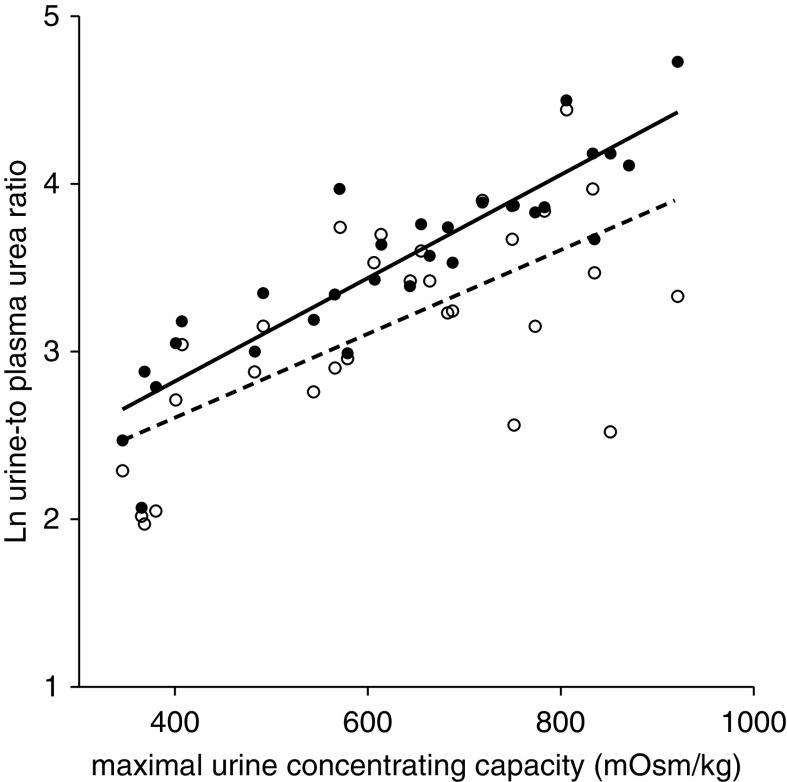
Maximal urine-concentrating capacity correlated positively with eGFR (standarized β=0.50; P<0.001) and negatively with htTKV (standarized β= −0.57; P=0.03); both analyses were adjusted for age and sex. Urine-to-plasma urea ratios in both early morning fasting spot and unstandardized spot urine samples were highly correlated with urine-concentrating capacity (Figure 2; R=0.90; P<0.001, and R=0.67; P<0.001, respectively) and remained significant when corrected for sex and age (standarized β=0.96; P<0.001, and standarized β=0.55; P=0.002, respectively). Fasting urine-to-plasma urea ratio also remained significantly associated when adjusting for eGFR (standarized β=0.81; P<0.001). Of note, when corrected for plasma creatinine, the urine-to-plasma urea ratio did not correlate as closely with the urine-concentrating capacity (fasting sample R=0.67; P<0.001, and unstandardized sample R=0.33; P=0.08). In conclusion, these analyses showed that early morning fasting spot urine-to-plasma urea ratio is the best representative of the urine-concentrating capacity, and we continued our analyses using this marker.
Figure 2.
Correlation of natural log (ln)-transformed urine-to-plasma urea ratio with maximal urine-concentrating capacity during prolonged water deprivation tests in 30 patients with ADPKD. Solid circles represent the urine-to-plasma urea ratio calculated from early morning fasting urine samples (R=0.90; P<0.001); open circles represent the urine-to-plasma urea ratio from unstandardized urine samples (R=0.67; P<0.001).
Maximal Urine-Concentrating Capacity and Urine-to-Plasma Urea Ratio Are associated with Disease Progression
In the years after the water deprivation test, participants showed a mean eGFR decline of 4.66±1.99 ml/min per 1.73 m2 per year, during a median follow-up period of 6.3 (IQR, 4.5–7.7) years, with an average of 15 eGFR assessments. Table 2 shows that measured maximal urine-concentrating capacity was significantly associated with subsequent rate of kidney function decline, after correction for age and sex (per 10 mOsm/kg, β=0.07; P=0.03). Early morning fasting spot urine-to-plasma urea ratio was likewise associated with subsequent rate of kidney function decline, unadjusted (per 1-natural log-transformed unit, β=1.66; P=0.05), which remained after correction for age, sex, and eGFR (β=5.56; P<0.001).
Table 2.
Predictors of future kidney function decline of patients with ADPKD participating in the water deprivation tests (n=28)
| Predictors | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||||
| β | P Value | β | P Value | β | P Value | ||
| eGFR, per 10 ml/min per 1.73 m2 | 0.056 | 0.69 | 0.31 | 0.22 | |||
| Age, per 10 yr | 0.67 | 0.22 | |||||
| Sex, female versus male | −0.27 | 0.78 | |||||
| Maximal urine-concentrating capacity, per 10 mOsm/kg | 0.041 | 0.13 | 0.068 | 0.03 | 0.09 | 0.04 | |
| Age, per 10 yr | 0.54 | 0.14 | 0.30 | 0.59 | |||
| Sex, female versus male | −0.25 | 0.78 | −0.03 | 0.97 | |||
| eGFR, per 10 ml/min per 1.73 m2 | −0.19 | 0.57 | |||||
| Urine-to-plasma urea ratio, per 1 U | 1.66 | 0.05 | 3.18 | 0.002 | 5.56 | <0.001 | |
| Age, per 10 yr | 0.83 | 0.03 | 0.12 | 0.81 | |||
| Sex, female versus male | −0.79 | 0.36 | −0.56 | 0.48 | |||
| eGFR, per 10 ml/min per 1.73 m2 | −0.71 | 0.04 | |||||
Associations of baseline eGFR, measured maximal urine-concentrating capacity, and early morning fasting spot urine-to-plasma urea ratio with subsequent rate of kidney function decline were assessed with use of mixed-model analysis. Urine-to-plasma urea ratio was natural log-transformed to attain normal distribution. Maximal urine-concentrating capacity was defined as the plateau of highest urine osmolality that was reached after a prolonged water deprivation test. In these analyses, two patients were excluded because of V2 receptor antagonist (tolvaptan) use longer than 6 months during follow-up. ADPKD, autosomal dominant polycystic kidney disease.
Urine-to-Plasma Urea Ratio Is associated with Disease Progression in a Larger Patient Cohort
Baseline characteristics of 583 patients with ADPKD participating in the DIPAK studies are presented in Table 1. Their mean age was 47±11 years, mean eGFR was 64±24 ml/min per 1.73 m2, and median htTKV was 898 (IQR, 549–1364) ml/m. Median urine-to-plasma urea ratio was 24 (IQR, 17–36). At baseline age, systolic and diastolic BP, use of antihypertensive medication and diuretics, htTKV, plasma copeptin (as a surrogate marker of vasopressin), and 24-hour urine volume all had a significant negative correlation with urine-to-plasma urea ratio, whereas eGFR and protein intake showed a positive correlation (Supplemental Table 1).
During a median follow-up of 4.0 (IQR, 2.6–5.0) years, with an average of ten eGFR measurements per individual, mean eGFR decline was 3.49±1.86 ml/min per 1.73 m2 per year. The association of baseline urine-to-plasma urea ratio with subsequent decline in kidney function was statistically significant even when corrected for all established disease prediction markers (sex, baseline age, eGFR, Mayo Clinic htTKV class, and PKD mutation) (β=0.57; P=0.02; Supplemental Table 2). A sensitivity analysis using creatinine corrected urine-to-plasma urea ratio rendered similar results (β=0.23; P=0.003; Supplemental Table 3). There are no significant interactions between the association of the urine-to-plasma urea ratio with rate of disease progression and age, sex, eGFR, htTKV, diuretic use, protein intake, urine volume, use of lanreotide, and plasma copeptin concentration. Stepwise, multivariable regression analysis with backward analysis was performed, eliminating covariates that had a P>0.1, resulting in a final model including urine-to-plasma urea ratio, systolic BP, PKD mutation, Mayo Clinic htTKV class, and salt intake (all P<0.05; Table 3).
Table 3.
Association of baseline parameters with rate of kidney function decline in patients with ADPKD from the DIPAK cohort (n=583), in a stepwise backward elimination analysis
| Baseline Parameters | Univariable | Final Multivariable Model | ||
|---|---|---|---|---|
| β | P Value | β | P Value | |
| Urine-to-plasma urea ratio, per 1 U | 0.74 | <0.001 | 0.71 | <0.001 |
| Age, per 10 yr | 0.12 | 0.22 | X | X |
| Sex, female versus male | 0.63 | 0.002 | X | X |
| Systolic BP, per 10 mm Hg | −0.24 | 0.001 | −0.18 | 0.02 |
| Diastolic BP, per 10 mm Hg | −0.34 | 0.002 | X | X |
| Antihypertensive therapy, yes versus no | −0.64 | 0.01 | X | X |
| Diuretics, yes versus no | −0.38 | 0.10 | X | X |
| eGFR, per 10 ml/min per 1.73 m2 | 0.13 | 0.004 | X | X |
| PKD mutation | ||||
| PKD1 T | −1.15 | <0.001 | −0.72 | 0.003 |
| PKD1 NT | −0.86 | 0.001 | −0.63 | 0.02 |
| Mayo Clinic htTKV class | ||||
| 1B+C | −0.93 | 0.01 | −0.69 | 0.07 |
| 1D+E | −2.41 | <0.001 | −1.83 | <0.001 |
| 24-h urine volume, L | −0.05 | 0.73 | X | X |
| Estimated protein intake, per 1 g/24 h | −0.01 | 0.02 | X | X |
| Estimated salt intake, g/24 h | −0.99 | <0.001 | −0.76 | 0.001 |
Associations tested with mixed-model analysis. The first column lists all variables that were considered. Covariates contributing with a P>0.1 were excluded until a final multivariable model was reached. Urine-to-plasma urea ratio and salt intake were natural log-transformed to attain normal distribution. Reference groups are PKD2 and others (non-PKD1 mutations) combined, and Mayo Clinic htTKV class 2 and 1A combined. Protein intake was estimated in grams with the following equation: (urine urea excretion in 24 h×0.4667×0.06+[0.031×weight])×6.25 (37). Salt intake was estimated with the following equation: sodium excretion in moles×(sum of molecular mass of sodium and chloride in grams per mole). ADPKD, autosomal dominant polycystic kidney disease; DIPAK cohort, Developing Interventions to Halt Progression of Autosomal Dominant Polycystic Kidney Disease cohort; T, truncating; NT, nontruncating; htTKV, height-adjusted total kidney volume.
Next, our study population was divided into two groups of patients with rapidly progressive (n=350) and slowly progressive (n=233) disease. The odds ratio of rapidly progressive disease was 1.33 (95% confidence interval [95% CI], 1.19 to 1.48; P<0.001) for every 10-U decrease in urine-to-plasma urea ratio when analyzed crude, and 1.35 (95% CI, 1.19 to 1.52; P<0.001) when adjusted for sex, PKD mutation, and Mayo Clinic htTKV class. The Harrell C-statistic for this final model changed from 0.69 to 0.72 when the urine-to-plasma urea ratio was added (P=0.006).
Thereafter, a risk score combining PKD mutation, Mayo Clinic htTKV class, and urine-to-plasma urea ratio was calculated. The Harrell C-statistic for predicting fast progression was 0.68 for the risk score if the urine-to-plasma urea ratio was not included (Figure 3A), and increased to 0.72 for the total combined risk score (P=0.007; Figure 3B), which was also better than for each of the predictors separately (urine-to-plasma urea ratio score: 0.61; P<0.001; Mayo Clinic htTKV class score: 0.65; P=0.001; PKD mutation score: 0.63; P<0.001; Supplemental Figure 1). These predictor scores did not statistically differ from each other. In addition, including either the Mayo Clinic class score or the urine-to-plasma urea ratio score into a model adjusted for age, sex, baseline eGFR, and PKD mutation showed comparable predictive value (Harrell C-statistic of 0.70 and 0.69, respectively; P=0.65). Notably, use of the risk score in a subgroup of 122 patients with relatively early-stage disease (defined as age <40 years and eGFR>60 ml/min per 1.73 m2) also shows promise as predictor for future disease progression (Figure 3C; Harrell C-statistic of 0.71).
Figure 3.
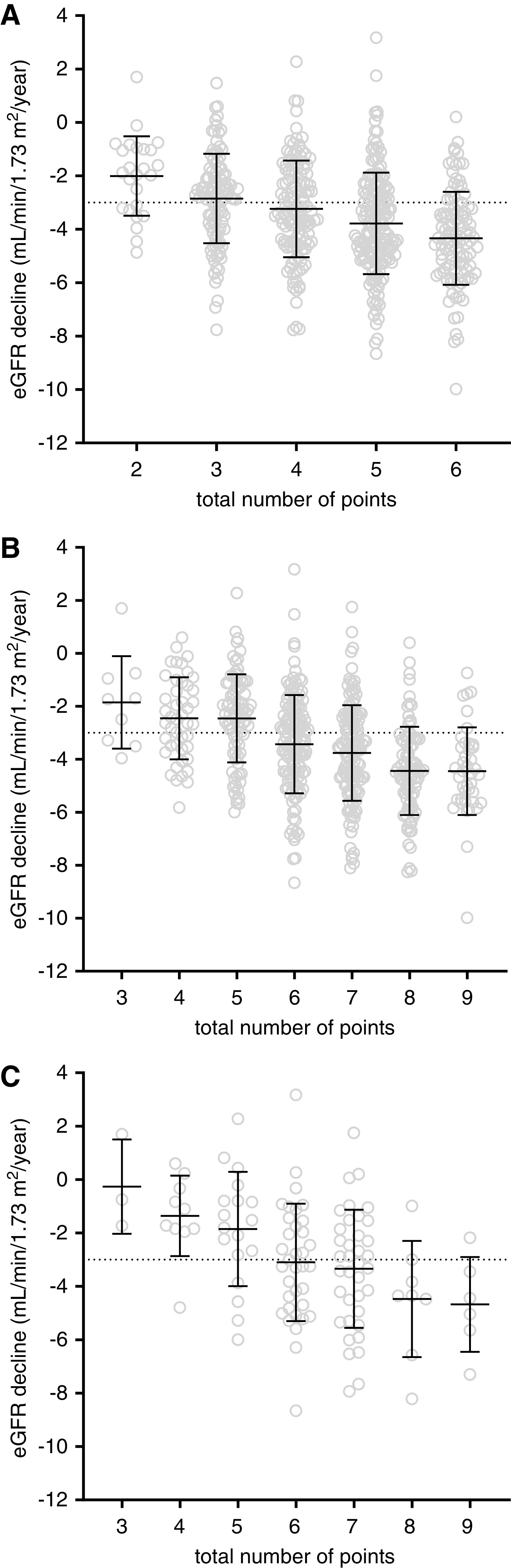
A risk score combining PKD mutation, Mayo Clinic htTKV class, and urine-to-plasma urea ratio was calculated. Rate of kidney function decline in DIPAK cohort participants (n=538), divided into risk groups according to the combined risk score of Mayo Clinic height-adjusted total kidney volume class and PKD mutation, without urine-to-plasma urea ratio (A) and the total risk score (B). Dotted line indicates division between rapidly progressive disease and moderately progressive disease (respectively below or above −3.0 ml/min per 1.73 m2 per year). Harrell C-statistics for the prediction of rapidly progressive disease was 0.68 for the risk score without the urine-to-plasma urea ratio, and 0.72 for the total risk score (P=0.007). (C) Use of the total risk score in patients with relatively early-stage disease (n=122, defined as age <40 years and eGFR>60 ml/min per 1.73 m2). The Harrell C-statistic was 0.71 in this subpopulation.
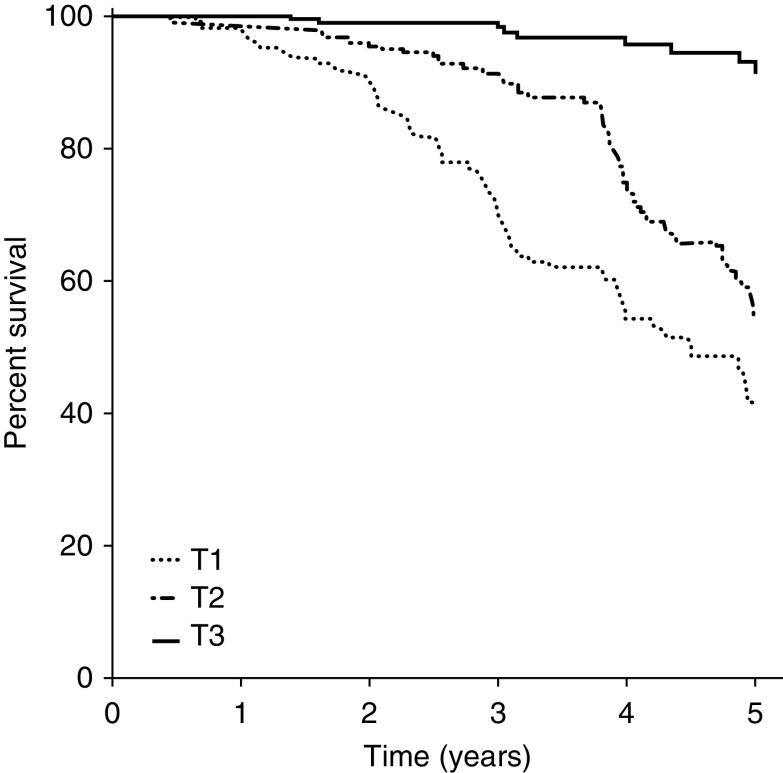
As secondary analyses, we examined survival in our population using a composite kidney end point. Baseline characteristics of the DIPAK participants included in these analyses can be found in Supplemental Table 4. Figure 4 shows that patients in the lower two tertiles of the urine-to-plasma urea ratio have a worse survival compared with the upper tertile (hazard ratio, 6.8; 95% CI, 3.3 to 13.7; and hazard ratio, 13.1; 95% CI, 6.5 to 26.1, respectively; both P<0.001). Lower baseline urine-to-plasma urea ratio was significantly associated with a higher risk of developing the combined kidney end point (adjusted hazard ratio, 1.43; 95% CI, 1.11 to 1.85; P=0.006) for 10 U. The Harrell C-statistic for this final model was 0.82, to which the urine-to-plasma urea ratio contributed significantly (P=0.03; Supplemental Table 5).
Figure 4.
Kaplan–Meier curve of the incidence of a combined kidney end point (start of KRT, incidence of eGFR<15 ml/min per 1.73 m2 or eGFR decrease >40%) in the overall DIPAK cohort (n=706), according to tertiles of baseline urine-to-plasma urea ratio. The upper tertile (>31.6, tertile 3 [T3]) is depicted with a solid line, the middle tertile (19.7–31.6, tertile 2 [T2]) with a dashed line, and the lowest tertile (<19.7, tertile 1 [T1]) with a dotted line. Compared with the upper tertile, the second tertile had a hazard ratio of 6.76 (95% confidence interval, 3.35 to 13.7; P<0.001) and the first tertile had a hazard ratio of 13.1 (95% confidence interval, 6.55 to 26.1; P<0.001).
Discussion
In this study, we demonstrate that the extent to which the kidneys can maximally concentrate urine is associated with disease severity (eGFR and TKV) and disease progression (rate of kidney function decline) in patients with ADPKD, both when measured directly, with a prolonged water deprivation test, and when measured by its surrogate marker, the urine-to-plasma urea ratio. The association between urine-to-plasma urea ratio and rate of kidney function decline remained intact when correcting for all established ADPKD progression markers (24,26–28). Combining Mayo Clinic htTKV class, PKD mutation, and the urine-to-plasma urea ratio into a disease severity score predicted rapidly progressive disease better than these predictors individually.
First, we demonstrated that the actual, measured maximal urine-concentrating capacity can predict future kidney function decline. Unfortunately, the way to determine this urine-concentrating capacity is to subject a patient to an arduous evaluation: a prolonged water deprivation test. Implementation of this test in everyday clinical practice is far from attainable. A surrogate marker of the urine-concentrating capacity could therefore help the clinician considerably. Our data demonstrate that the urine-to-plasma urea ratio, calculated from an early morning fasting sample, could achieve this.
Use of the urine-to-plasma urea ratio as a marker of the maximal urine-concentrating capacity can be explained by reflecting on the central role of urea in the process of urine concentration. Urea cycling within the kidney is pivotal for building the osmolar gradient necessary to draw fluids from the lumen of tubules to the interstitial tissue (29). A high medullary urea concentration gradient is obtained by transport of urea from the lumen of the collecting duct into the medullary tissue, and thereafter back into the loop of Henle, from where it cycles back to the collecting duct, and so forth (30,31). Because this urea transport into medullary tissue is passive, concentration of urea in urine will be equal to concentration in the inner medulla. The gradient also depends on amount of urea filtered by the glomerulus, and thus of urea plasma concentration. Therefore, it was postulated that the ratio between urea concentration in plasma and urine is the best representation of this process (29,32). When concentrating urine is not possible because of an impaired medullar urea gradient that occurs during progression of ADPKD, the urine-to-plasma urea ratio will decrease concordantly with the severity of the disease. In previous clinical studies, the urine-to-plasma urea ratio was investigated to distinguish between transient kidney failure and longer-term function decline, with lower values also indicating worse prognosis (33–35).
A prior post hoc study of the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes (TEMPO 3:4) trial by Devuyst et al. (36) has shown that the early morning fasting spot urine osmolality, which may also be regarded as a marker of maximal urine-concentrating capacity, was associated with kidney function decline. To arrive at a certain urine osmolality, two factors are of importance: the abovementioned osmotic gradient and the vasopressin signal. An increase of vasopressin levels might compensate for the reduction in osmotic gradient that is caused by the presence of cysts, resulting in an adequate urine osmolality. The urine-to-plasma urea has a theoretical advantage of representing the underlying damage even when urine osmolality is within normal bounds. Unfortunately, early morning fasting urine osmolality was not measured in the DIPAK cohort. Therefore, a direct comparison of the predictive value of both measures could not be made.
When kidney function deteriorates, plasma urea concentration rises together with plasma creatinine concentration. Therefore, the urine-to-plasma urea ratio might also include information on GFR. It should be noted, however, that adjustment of the ratio for plasma creatinine as well as correcting for eGFR in our multivariable analysis did not eliminate the association of the urine-to-plasma urea ratio with rate of kidney function decline.
We consider as strengths of this study the availability of direct measurements of the maximal urine-concentrating capacity by a prolonged water deprivation test for validation of the urine-to-plasma urea ratio, and the relatively large size and intensive phenotyping of the DIPAK cohort. Limitations include the small study population of the water deprivation tests, thereby highlighting the importance of the subsequent analysis of the larger DIPAK cohort. Furthermore, our data demonstrate that prediction of future kidney function decline in patients with ADPKD is difficult. When established risk markers are used, there is a large dispersion in the rate of disease progression between patients in the same risk group, and overlap between groups. The combined risk score incorporating the urine-to-plasma urea ratio performs better, but is still not perfectly discriminative. We have included this ratio in a combined risk score to gauge its practical use; however, calibration of the weight of the components and external validation were beyond the scope of the present analyses. Future studies should be dedicated toward implementation of the urine-to-plasma urea ratio into clinical practice, not only as predictor for future kidney function decline but possibly also for tolvaptan efficacy.
In conclusion, our data show that the early morning fasting urine-to-plasma urea ratio is a good surrogate for maximal urine-concentrating capacity. This urine-to-plasma urea ratio holds promise as an easy to measure novel predictor for rapid disease progression in patients with ADPKD.
Disclosures
R.T. Gansevoort reports consultancy agreements with AstraZeneca, Bayer, Galapagos, Otsuka Pharmaceutical, and Sanofi-Genzyme; receiving research funding and honoraria from Bayer, Galapagos, Otsuka Pharmaceuticals (all funds paid directly to the institution); and serving as a scientific advisor or member of American Journal of Kidney Diseases, CJASN, Journal of Nephrology, Kidney360, Nephron Clinical Practice, and Nephrology Dialysis Transplantation. E. Meijer reports receiving research funding from Ipsen, Otsuka Pharmaceuticals, and Sanofi (all money was paid directly to the institution); and other interests/relationships with the Dutch Kidney Foundation, Health Holland, Nieren.nl, Nierpatiënten vereniging Nederland (Dutch patient association), and Working Group on Inherited Kidney Disorders of the European Renal Association - European Dialysis and Transplant Association. All remaining authors have nothing to disclose.
Funding
The DIPAK Consortium is sponsored by IPSEN Farmaceutica BV, Dutch Kidney Foundation grants CP10.12 and CP15.01, and Dutch Government grant LSHM15018.
Supplementary Material
Acknowledgments
The DIPAK Consortium is an interuniversity collaboration in The Netherlands that is established to study ADPKD and to develop rational treatment strategies for this disease. Part of this work was presented in the form of a free communication during the 2020 fully virtual European Renal Association - European Dialysis and Transplant Association conference held June 6–9, 2020.
Ms. Judith E. Heida, Dr. Ron T. Gansevoort, Dr. Esther Meijer, Dr. Niek F. Casteleijn, Dr. Wendy E. Boertien, and Dr. Debbie Zittema conceived and designed the study. Dr. Ron T. Gansevoort, Dr. A. Lianne Messchendorp, Dr. Esther Meijer, Dr. Niek F. Casteleijn, Dr. Wendy E. Boertien, Dr. Debbie Zittema, and members of the DIPAK consortium were responsible for data acquisition. Ms. Judith E. Heida, Dr. Ron T. Gansevoort, and Dr. Debbie Zittema were responsible for data analysis/interpretation. Judith E. Heida conducted statistical analysis. Dr. Ron T. Gansevoort and Dr. Debbie Zittema provided supervision or mentorship. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author’s own contributions, and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Data Sharing Statement
Deidentified individual participant data collected during the trial will be made available, upon request, to researchers who provide a methodologically sound proposal and whose use of the data has been approved by the DIPAK Steering Committee. Proposals may be submitted to r.t.gansevoort@umcg.nl.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Contributor Information
Collaborators: DIPAK Consortium, Joost P.H. Drenth, Johan W. de Fijter, Maatje D.A. van Gastel, Monique Losekoot, Dorien J.M. Peters, Folkert W. Visser, Jack F. Wetzels, and Robert Zietse
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10470620/-/DCSupplemental.
Supplemental Table 1. Correlation of the urine-to-plasma urea ratio with clinical variables of ADPKD patients from the DIPAK cohort.
Supplemental Table 2. Associations of baseline urine-to-plasma urea ratio with rate of kidney function decline during follow-up in ADPKD patients from the DIPAK cohort (n=583).
Supplemental Table 3. Sensitivity analysis. Associations of baseline urine-to-plasma urea ratio corrected for plasma creatinine, with rate of kidney function decline during follow-up in ADPKD patients from the DIPAK cohort.
Supplemental Table 4. Baseline characteristics of the DIPAK cohort used for Cox regression analyses, both overall as well as divided into tertiles of the urine-to-plasma urea ratio.
Supplemental Table 5. Predictive value of the urine-to-plasma urea (UPU) ratio as continuous variable and divided into tertiles for end-stage kidney disease.
Supplemental Figure 1. Kidney function slopes of DIPAK cohort participants (n=583) divided into risk groups according to Mayo htTKV class score (panel A), PKD mutation score (panel B), and UPU ratio score (panel C).
References
- 1.Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Spithoven EM, Kramer A, Meijer E, Orskov B, Wanner C, Caskey F, Collart F, Finne P, Fogarty DG, Groothoff JW, Hoitsma A, Nogier MB, Postorino M, Ravani P, Zurriaga O, Jager KJ, Gansevoort RT; ERA-EDTA Registry; EuroCYST Consortium; WGIKD; EuroCYST Consortium; WGIKD: Analysis of data from the ERA-EDTA Registry indicates that conventional treatments for chronic kidney disease do not reduce the need for renal replacement therapy in autosomal dominant polycystic kidney disease. Kidney Int 86: 1244–1252, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, Li J, King BF, Martin D, Wetzel LH, Lockhart ME, Harris PC, Moxey-Mims M, Flessner M, Bennett WM, Grantham JJ: Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 7: 479–486, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrone RD, Mouksassi MS, Romero K, Czerwiec FS, Chapman AB, Gitomer BY, Torres VE, Miskulin DC, Broadbent S, Marier JF: Total kidney volume is a prognostic biomarker of renal function decline and progression to end-stage renal disease in patients with autosomal dominant polycystic kidney disease. Kidney Int Rep 2: 442–450, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu ASL, Shen C, Landsittel DP, Harris PC, Torres VE, Mrug M, Bae KT, Grantham JJ, Rahbari-Oskoui FF, Flessner MF, Bennett WM, Chapman AB; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP): Baseline total kidney volume and the rate of kidney growth are associated with chronic kidney disease progression in autosomal dominant polycystic kidney disease. Kidney Int 93: 691–699, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hateboer N, v Dijk MA, Bogdanova N, Coto E, Saggar-Malik AK, San Millan JL, Torra R, Breuning M, Ravine D: Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet 353: 103–107, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Cornec-Le Gall E, Audrézet MP, Chen JM, Hourmant M, Morin MP, Perrichot R, Charasse C, Whebe B, Renaudineau E, Jousset P, Guillodo MP, Grall-Jezequel A, Saliou P, Férec C, Le Meur Y: Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol 24: 1006–1013, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brosnahan GM, Abebe KZ, Moore CG, Rahbari-Oskoui FF, Bae KT, Grantham JJ, Schrier RW, Braun WE, Chapman AB, Flessner MF, Harris PC, Hogan MC, Perrone RD, Miskulin DC, Steinman TI, Torres VE; HALT-PKD Trial Investigators: Patterns of kidney function decline in autosomal dominant polycystic kidney disease: A post hoc analysis from the HALT-PKD trials. Am J Kidney Dis 71: 666–676, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu ASL, Shen C, Landsittel DP, Grantham JJ, Cook LT, Torres VE, Chapman AB, Bae KT, Mrug M, Harris PC, Rahbari-Oskoui FF, Shi T, Bennett WM; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP): Long-term trajectory of kidney function in autosomal-dominant polycystic kidney disease. Kidney Int 95: 1253–1261, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabow PA, Kaehny WD, Johnson AM, Duley IT, Manco-Johnson M, Lezotte DC, Schrier RW: The clinical utility of renal concentrating capacity in polycystic kidney disease. Kidney Int 35: 675–680, 1989 [DOI] [PubMed] [Google Scholar]
- 11.Fick GM, Duley IT, Johnson AM, Strain JD, Manco-Johnson ML, Gabow PA: The spectrum of autosomal dominant polycystic kidney disease in children. J Am Soc Nephrol 4: 1654–1660, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Seeman T, Dusek J, Vondrák K, Bláhová K, Simková E, Kreisinger J, Dvorák P, Kyncl M, Hríbal Z, Janda J: Renal concentrating capacity is linked to blood pressure in children with autosomal dominant polycystic kidney disease. Physiol Res 53: 629–634, 2004 [PubMed] [Google Scholar]
- 13.Zittema D, Boertien WE, van Beek AP, Dullaart RP, Franssen CF, de Jong PE, Meijer E, Gansevoort RT: Vasopressin, copeptin, and renal concentrating capacity in patients with autosomal dominant polycystic kidney disease without renal impairment. Clin J Am Soc Nephrol 7: 906–913, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Ho TA, Godefroid N, Gruzon D, Haymann JP, Maréchal C, Wang X, Serra A, Pirson Y, Devuyst O: Autosomal dominant polycystic kidney disease is associated with central and nephrogenic defects in osmoregulation. Kidney Int 82: 1121–1129, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Zittema D, Casteleijn NF, Bakker SJ, Boesten LS, Duit AA, Franssen CF, Gaillard CA, Gansevoort RT: Urine concentrating capacity, vasopressin and copeptin in ADPKD and IgA nephropathy patients with renal impairment. PLoS One 12: e0169263, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bankir L, Bichet DG: Polycystic kidney disease: An early urea-selective urine-concentrating defect in ADPKD. Nat Rev Nephrol 8: 437–439, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Meijer E, Drenth JP, d’Agnolo H, Casteleijn NF, de Fijter JW, Gevers TJ, Kappert P, Peters DJ, Salih M, Soonawala D, Spithoven EM, Torres VE, Visser FW, Wetzels JF, Zietse R, Gansevoort RT; DIPAK Consortium: Rationale and design of the DIPAK 1 study: A randomized controlled clinical trial assessing the efficacy of lanreotide to halt disease progression in autosomal dominant polycystic kidney disease. Am J Kidney Dis 63: 446–455, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meijer E, Visser FW, van Aerts RMM, Blijdorp CJ, Casteleijn NF, D’Agnolo HMA, Dekker SEI, Drenth JPH, de Fijter JW, van Gastel MDA, Gevers TJ, Lantinga MA, Losekoot M, Messchendorp AL, Neijenhuis MK, Pena MJ, Peters DJM, Salih M, Soonawala D, Spithoven EM, Wetzels JF, Zietse R, Gansevoort RT; DIPAK-1 Investigators: Effect of lanreotide on kidney function in patients with autosomal dominant polycystic kidney disease: The DIPAK 1 randomized clinical trial. JAMA 320: 2010–2019, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgenthaler NG, Struck J, Alonso C, Bergmann A: Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 52: 112–119, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Rossetti S, Consugar MB, Chapman AB, Torres VE, Guay-Woodford LM, Grantham JJ, Bennett WM, Meyers CM, Walker DL, Bae K, Zhang QJ, Thompson PA, Miller JP, Harris PC; CRISP Consortium: Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 18: 2143–2160, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Hopp K, Cornec-Le Gall E, Senum SR, Te Paske IBAW, Raj S, Lavu S, Baheti S, Edwards ME, Madsen CD, Heyer CM, Ong ACM, Bae KT, Fatica R, Steinman TI, Chapman AB, Gitomer B, Perrone RD, Rahbari-Oskoui FF, Torres VE, Harris PC; HALT Progression of Polycystic Kidney Disease Group, the ADPKD Modifier Study: Detection and characterization of mosaicism in autosomal dominant polycystic kidney disease. Kidney Int 97: 370–382, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Gastel MDA, Messchendorp AL, Kappert P, Kaatee MA, de Jong M, Renken RJ, Ter Horst GJ, Mahesh SVK, Gansevoort RT; DIPAK Consortium: T1 vs. T2 weighted magnetic resonance imaging to assess total kidney volume in patients with autosomal dominant polycystic kidney disease. Abdom Radiol (NY) 43: 1215–1222, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, Bae KT, Chapman AB, Grantham JJ, Mrug M, Hogan MC, El-Zoghby ZM, Harris PC, Erickson BJ, King BF, Torres VE; CRISP Investigators: Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J Am Soc Nephrol 26: 160–172, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Inker LA, Matsushita K, Greene T, Willis K, Lewis E, de Zeeuw D, Cheung AK, Coresh J: GFR decline as an end point for clinical trials in CKD: A scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 64: 821–835, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Schrier RW, Brosnahan G, Cadnapaphornchai MA, Chonchol M, Friend K, Gitomer B, Rossetti S: Predictors of autosomal dominant polycystic kidney disease progression. J Am Soc Nephrol 25: 2399–2418, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woon C, Bielinski-Bradbury A, O'Reilly K, Robinson P: A systematic review of the predictors of disease progression in patients with autosomal dominant polycystic kidney disease. BMC Nephrol 16: 140, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grantham JJ, Torres VE: The importance of total kidney volume in evaluating progression of polycystic kidney disease. Nat Rev Nephrol 12: 667–677, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bankir L, Yang B: New insights into urea and glucose handling by the kidney, and the urine concentrating mechanism. Kidney Int 81: 1179–1198, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Bankir LT, Trinh-Trang-Tan MM: Renal urea transporters. Direct and indirect regulation by vasopressin. Exp Physiol 85: 243S–252S, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Fenton RA: Essential role of vasopressin-regulated urea transport processes in the mammalian kidney. Pflugers Arch 458: 169–177, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Zittema D, van den Berg E, Meijer E, Boertien WE, Muller Kobold AC, Franssen CF, de Jong PE, Bakker SJ, Navis G, Gansevoort RT: Kidney function and plasma copeptin levels in healthy kidney donors and autosomal dominant polycystic kidney disease patients. Clin J Am Soc Nephrol 9: 1553–1562, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perlmutter M, Grossman SL, Rothenberg S, Dobkin G: Urine-serum urea nitrogen ratio: Simple test of renal function in acute azotemia and oliguria. J Am Med Assoc 170: 1533–1537, 1959 [DOI] [PubMed] [Google Scholar]
- 34.Miller TR, Anderson RJ, Linas SL, Henrich WL, Berns AS, Gabow PA, Schrier RW: Urinary diagnostic indices in acute renal failure: A prospective study. Ann Intern Med 89: 47–50, 1978 [DOI] [PubMed] [Google Scholar]
- 35.Pons B, Lautrette A, Oziel J, Dellamonica J, Vermesch R, Ezingeard E, Mariat C, Bernardin G, Zeni F, Cohen Y, Tardy B, Souweine B, Vincent F, Darmon M: Diagnostic accuracy of early urinary index changes in differentiating transient from persistent acute kidney injury in critically ill patients: Multicenter cohort study. Crit Care 17: R56, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devuyst O, Chapman AB, Gansevoort RT, Higashihara E, Perrone RD, Torres VE, Blais JD, Zhou W, Ouyang J, Czerwiec FS: Urine osmolality, response to tolvaptan, and outcome in autosomal dominant polycystic kidney disease: Results from the TEMPO 3:4 trial. J Am Soc Nephrol 28: 1592–1602, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maroni BJ, Steinman TI, Mitch WE: A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int 27: 58–65, 1985 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.