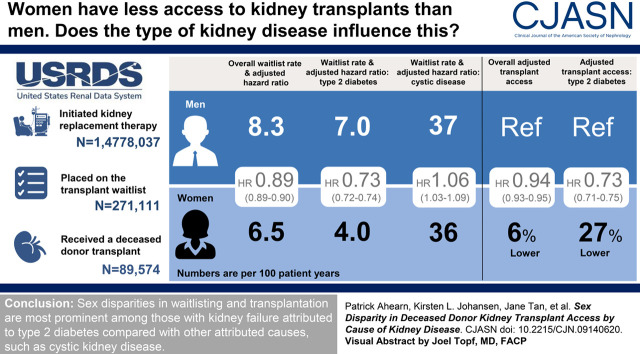
Visual Abstract
Keywords: diabetes, chronic dialysis, disparity, kidney transplantation, kidney failure
Abstract
Background and objectives
Women with kidney failure have lower access to kidney transplantation compared with men, but the magnitude of this disparity may not be uniform across all kidney diseases. We hypothesized that the attributed cause of kidney failure may modify the magnitude of the disparities in transplant access by sex.
Design, setting, participants, & measurements
We performed a retrospective cohort study of adults who developed kidney failure between 2005 and 2017 according to the United States Renal Data System. We used adjusted Cox models to examine the association between sex and either access to waitlist registration or deceased-donor kidney transplantation, and tested for interaction between sex and the attributed cause of kidney failure using adjusted models.
Results
Among a total of 1,478,037 patients, 271,111 were registered on the waitlist and 89,574 underwent deceased-donor transplantation. The rate of waitlisting was 6.5 per 100 person-years in women and 8.3 per 100 person-years for men. In adjusted analysis, women had lower access to the waitlist (hazard ratio, 0.89; 95% confidence interval, 0.89 to 0.90) and to deceased-donor transplantation after waitlisting (hazard ratio, 0.96; 95% confidence interval, 0.94 to 0.98). However, there was an interaction between sex and attributed cause of kidney disease in adjusted models (P<0.001). Women with kidney failure due to type 2 diabetes had 27% lower access to the kidney transplant waitlist (hazard ratio, 0.73; 95% confidence interval, 0.72 to 0.74) and 11% lower access to deceased-donor transplantation after waitlisting compared with men (hazard ratio, 0.89; 95% confidence interval, 0.86 to 0.92). In contrast, sex disparities in access to either the waitlist or transplantation were not observed in kidney failure secondary to cystic disease.
Conclusions
The disparity in transplant access by sex is not consistent across all causes of kidney failure. Lower deceased-donor transplantation rates in women compared with men are especially notable among patients with kidney failure attributed to diabetes.
Introduction
Kidney transplantation improves both survival and quality of life for patients who develop the need for maintenance KRT compared with dialysis (1–4), but women are less likely to receive kidney transplantation than men (5–12). Historically, women have had lower access to the transplant waitlist (5,7), but the reasons for this disparity have not been fully delineated. Although studies have examined differences in access to the transplant waitlist or transplantation itself on the basis of sex-related differences in body size (8), human leukocyte antibody sensitization (12,13), age (7), or other factors (7,14), few studies have determined whether there is variability in access to transplantation by sex depending on the attributed cause of kidney failure. Understanding whether the underlying attributed cause of kidney failure associates with differential access to transplantation by sex could enhance our understanding of potential reasons for these disparities.
Some conditions that cause kidney failure may not affect men and women equally and therefore partially contribute to the observed sex disparities in access to transplantation. In addition, some causes of kidney failure may elevate the risk for concurrent cardiovascular risk more than others. Because it is known in the general population that women with cardiovascular disease are less likely to receive and achieve aggressive risk reduction measures (such as aspirin) compared with men, differential cardiovascular risk profiles across different causes of kidney failure could also contribute to delayed or lower access to transplantation in women compared with men (15–17).
In this study, we examined whether the observed sex-based disparities in access to the waitlist and deceased-donor kidney transplantation differed by attributed cause of kidney failure using data from the United States Renal Data System (USRDS).
Materials and Methods
Study Population
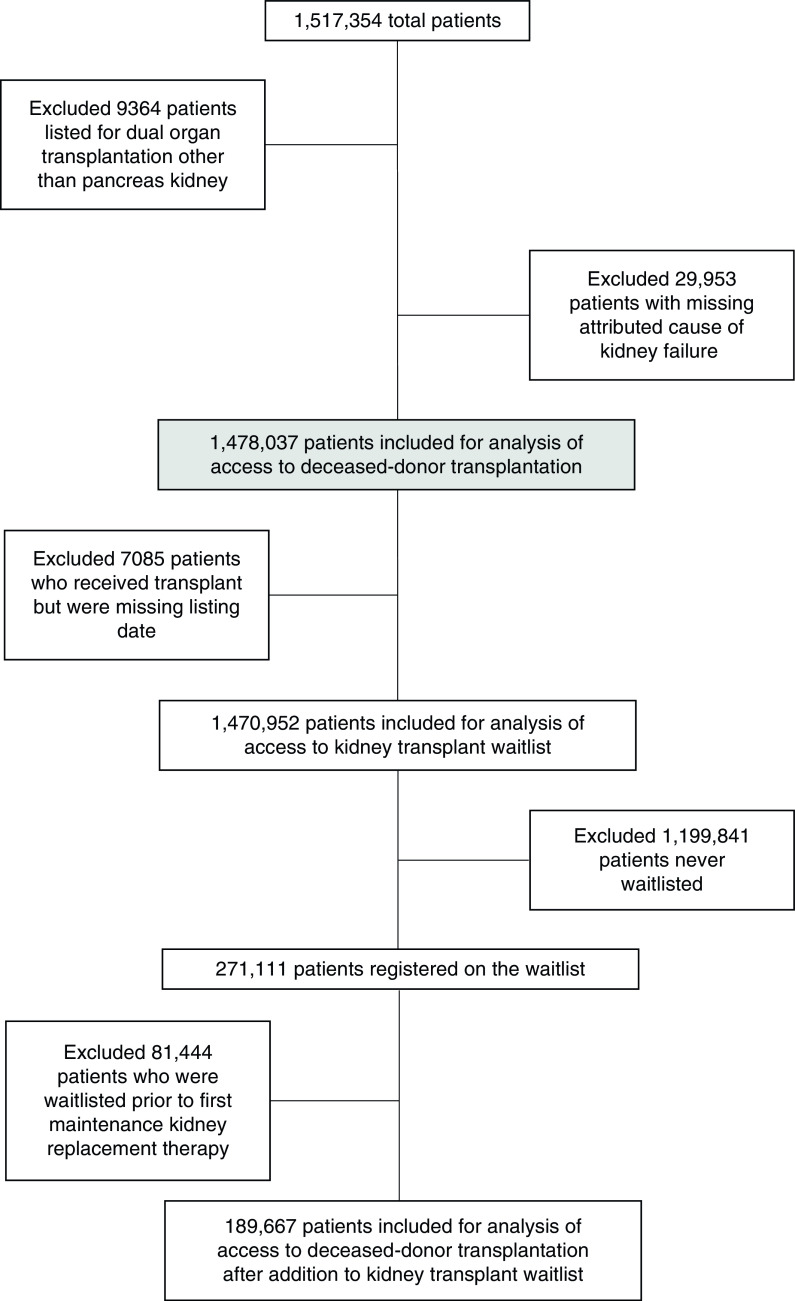
We included adults aged ≥18 years who started maintenance KRT between January 1, 2005 and December 31, 2017, according to the USRDS. We excluded (1) patients considered for multiorgan transplants (except simultaneous pancreas kidney transplants) and (2) patients with missing data on the cause of kidney failure (Figure 1).
Figure 1.
Cohort derivation and inclusion and exclusion criteria.
Factors of Interest
We abstracted the primary predictor, patient sex, from the Patients file in the USRDS. We abstracted the cause of kidney failure from data reported on the CMS-2728 Medical Evidence (MEDEVID) form (filed for all patients with kidney failure at maintenance KRT initiation) and categorized these causes as type 2 diabetes mellitus, type 1 diabetes mellitus, hypertension, cystic disease, GN, or other. The most common causes of kidney failure in the “other” category included unknown etiology (29%), tubular necrosis without recovery (18%), and multiple myeloma (7%). The attributed cause of kidney failure served as the effect modifier of interest in all primary analyses.
Covariates
We adjusted for other covariates that could be associated with a patient’s likelihood of waitlisting or transplantation (7,14,18–20). These include age at kidney failure onset (categorized as <35, 35 to <50, 50 to <65, and ≥65 years), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, and other), initial calendar year of maintenance KRT (to account for temporal trends), and zip code from the USRDS Patients file. For analyses from time of waitlist to transplantation, age at waitlisting (categorized as above) and year of waitlisting were abstracted from the Waitlist file. We used zip codes to determine Organ Procurement and Transplantation Network (OPTN) region and median neighborhood household income as a continuous variable by matching the patient’s zip code to the 2006–2010 American Community Survey (21). We abstracted body mass index (BMI) (categorized as <18.5, 18.5 to <25, 25 to <30, 30 to <35, 35 to <40, and ≥40 kg/m2 according to Centers for Disease Control definitions) (22), insurance type (none, Medicaid, Medicare, or other), and comorbidities (diabetes, congestive heart failure, peripheral arterial disease, stroke, myocardial infarction, tobacco use, chronic obstructive pulmonary disease, cancer) at kidney failure onset from the MEDEVID file of the USRDS. Panel reactive antibody (PRA) was abstracted from the Waitlist file in the USRDS and categorized as 0, >0 to 20, >20–80, and >80%.
Missing Covariates
Because 3% of income data were missing from the total cohort (which we believed to be an important predictor), we used chained multiple imputation to account for missingness. We imputed missing income data using models that included age, sex, race/ethnicity, insurance type, calendar year of maintenance KRT, cause of disease, OPTN region, and transplantation as an outcome with 20 repetitions.
Outcomes
Outcomes of interest were (1) time to kidney transplant waitlisting from first maintenance KRT date; (2) time to first deceased-donor kidney transplant from first maintenance KRT date; and (3) time to first deceased-donor kidney transplant starting from waitlisting for patients registered on the waiting list on or after first maintenance KRT date. Follow-up for outcomes was censored when living-donor kidney transplantation occurred.
Dates of waitlist registration were abstracted from the Waitlist file. Dates of transplantation and first transplant donor source (living versus deceased) were determined using the USRDS Patients file. Follow-up for outcomes was administratively censored on December 31, 2017.
Statistical Analyses
We compared baseline characteristics by sex and attributed cause of kidney failure using t test, Mann–Whitney U test, and chi-squared test as appropriate. We also compared differences in the burden of comorbid conditions at time of maintenance KRT initiation by sex and attributed cause of kidney failure.
Association between Sex and Waitlisting
We assessed the association between sex and access to the waitlist using an unadjusted Cox proportional hazards model. Time to waitlisting (in years) was measured from first date of kidney failure until waitlist registration (either active or inactive status) and censored for death or end of study follow-up. For those whose waitlist date was equal to or preceded the first maintenance KRT date, time to waitlisting was set to 0.1 days after first KRT date.
Next, we added covariates to the model in stepwise fashion, first including a limited set of characteristics including age at kidney failure, race/ethnicity, BMI, and attributed cause of kidney disease (Model 1). We next included median neighborhood income by zip code, insurance status, calendar year of maintenance KRT initiation, OPTN region, and comorbid conditions (Model 2). To test whether disparities in waitlisting by sex were more pronounced in those with different attributed causes of kidney failure, we tested for interactions between sex and the attributed cause of kidney failure in our fully adjusted Cox models.
To account for changes to the Kidney Allocation System (KAS), which were implemented on December 4, 2014, we performed additional stratified analyses where we examined access to the waitlist before versus after this date.
Association between Sex and Deceased-Donor Kidney Transplantation among all Patients with Kidney Failure
We examined the association between sex and hazard of deceased-donor kidney transplantation among all patients who started maintenance KRT using an unadjusted Cox model and censoring for living-donor transplantation or death. We included covariates in stepwise fashion as described and tested for interaction between sex and attributed cause of kidney failure in the fully adjusted models. For those whose first kidney transplant date was the same as the first maintenance KRT date, time to transplantation was set to 0.1 days after first KRT date.
Association between Sex and Deceased-Donor Kidney Transplantation in Patients Waitlisted after Starting KRT
We also examined the association between sex and hazard of deceased-donor kidney transplantation among waitlisted patients who registered on the waitlist on or after first KRT using an unadjusted Cox model. Time in these models started from date of first waitlist registration (on day of or after KRT initiation) to deceased-donor kidney transplantation, and follow-up was censored for living-donor transplantation and death. We then serially adjusted models in the same fashion as above, except using age at waitlisting and year of waitlisting (instead of age at KRT and year of KRT initiation) and the addition of PRA to both Models 1 and 2. All covariates except age, year of waitlisting, and PRA were abstracted at the time of kidney failure onset. Finally, we tested for interaction between sex and attributed cause of kidney failure in the fully adjusted model.
We did not analyze transplantation as an outcome in stratified analysis by era of first maintenance KRT given that our post-KAS follow-up period was brief (ending in 2017) for this outcome, and fewer deceased-donor kidney transplants occurred during this short follow-up period.
For all interactions, we considered P<0.05 to be statistically significant. Plots of scaled Schoenfeld residuals were used to assess the assumption of proportional hazards.
Sensitivity Analysis
In sensitivity analysis, we performed competing risk analyses using Fine-Gray models adjusted for the same covariates as above but treating death as a competing risk for all outcomes. We also repeated Fine-Gray models for the outcome of deceased-donor kidney transplantation, treating living-donor transplantation as a competing risk. For these sensitivity analyses, we used a 5%–20% random data sampling approach and tested for interaction in this smaller subset due to infeasible run times.
Given a prior study that showed an interaction between age and sex for access to kidney transplantation (7), we tested for the presence of such interaction in our fully adjusted models and performed stratified analysis by age for outcomes in which a statistically significant interaction (P<0.05) was detected.
Finally, we performed additional analyses for access to the waitlist and to transplantation after first maintenance KRT, where we excluded those who were waitlisted before date of first maintenance KRT to understand whether sex disparities were present in those who did not have the advantage of pre-emptive waitlisting.
Data analyses were conducted using STATA 15 (College Station, TX) and SAS Software version 9.4 (Cary, NC). The University of California San Francisco Institutional Review Board considered this study nonhuman subject research, and the study was also approved by the Stanford University Institutional Review Board (Protocol #51697).
Results
Study Population
A total of 1,478,037 patients were included for analysis (Figure 1). After maintenance KRT initiation, a total of 271,111 (18%) were waitlisted (7.5 out of 100 person-years) during a median follow-up of 1.6 (interquartile range [IQR], 0.5–3.6) years, and 89,574 (6%) underwent deceased-donor kidney transplantation (2.1 out of 100 person-years) during a median follow-up of 2.1 (IQR 0.7–4.3) years. A total of 81,444 patients (30% of those waitlisted) were waitlisted before date of maintenance KRT initiation. Baseline characteristics differed between men and women in the overall cohort (Supplemental Table 1). More patients with kidney failure were men (57%) and, in general, men had a higher average number of cardiac comorbidities than women (Supplemental Table 1).
We examined characteristics of men versus women at time of kidney failure onset by attributed cause of kidney failure (Table 1). Women were older than men at time of kidney failure onset for all attributed causes of kidney failure except GN and type 1 diabetes in the overall cohort (Table 1). Those with kidney failure attributed to type 2 diabetes had the highest mean number of cardiovascular comorbidities at kidney failure onset. The mean number of cardiovascular comorbidities was statistically significantly higher in men compared for women for all attributed causes of kidney failure in the overall cohort except for type 1 diabetes. Characteristics of men and women who were ever registered on the waiting list are presented in Supplemental Table 2.
Table 1.
Characteristics of adults who started maintenance KRT in the United States between January 1, 2005 and December 31, 2017 by sex and attributed cause of kidney failure
| Characteristics | Type 2 Diabetes | Type 1 Diabetes | Hypertension | GN | Cystic Disease | Other |
|---|---|---|---|---|---|---|
| Total (N) | ||||||
| Men | 345,750 | 34,049 | 241,996 | 65,689 | 17,954 | 137,694 |
| Women | 275,519 | 27,022 | 171,240 | 53,469 | 15,314 | 92,341 |
| Median age at first kidney failure treatment, yr (IQR)a | ||||||
| Men | 64 (55–72) | 52 (40–64) | 67 (54–78) | 56 (41–68) | 54 (46–64) | 66 (54–76) |
| Women | 65 (57–73) | 52 (38–65) | 70 (58–79) | 53 (38–67) | 55 (47–64) | 66 (54–76) |
| Race/ethnicity, %b | ||||||
| Non-Hispanic White | ||||||
| Men | 50 | 59 | 51 | 61 | 75 | 70 |
| Women | 45 | 53 | 47 | 50 | 72 | 69 |
| Non-Hispanic Black | ||||||
| Men | 22 | 22 | 34 | 20 | 12 | 17 |
| Women | 30 | 28 | 39 | 27 | 14 | 19 |
| Hispanic | ||||||
| Men | 20 | 15 | 11 | 13 | 10 | 9 |
| Women | 19 | 14 | 10 | 14 | 10 | 8 |
| Other | ||||||
| Men | 7 | 4 | 4 | 7 | 4 | 3 |
| Women | 7 | 4 | 5 | 8 | 4 | 4 |
| Median income, $1000s (IQR)b | ||||||
| Men | 47 (37–61) | 48 (38–61) | 47 (37–61) | 50 (40–66) | 53 (42–69) | 49 (39–65) |
| Women | 44 (36–57) | 46 (37–59) | 46 (36–59) | 49 (39–63) | 52 (41–68) | 49 (39–63) |
| Insurance coverage, %b | ||||||
| Medicare | ||||||
| Men | 62 | 45 | 62 | 40 | 33 | 61 |
| Women | 64 | 46 | 67 | 40 | 34 | 63 |
| Medicaid | ||||||
| Men | 11 | 16 | 10 | 12 | 8 | 10 |
| Women | 14 | 21 | 12 | 17 | 10 | 12 |
| Other | ||||||
| Men | 22 | 30 | 19 | 39 | 52 | 22 |
| Women | 16 | 26 | 15 | 36 | 50 | 20 |
| None | ||||||
| Men | 6 | 8 | 9 | 9 | 7 | 7 |
| Women | 5 | 6 | 6 | 7 | 5 | 5 |
| Median BMI, kg/m2 (IQR) b | ||||||
| Men | 28.8 (24.9–33.9) | 27.2 (23.5–32.0) | 26.4 (23.0–30.9) | 27.0 (23.5–31.7) | 27.0 (23.8–31.1) | 25.8 (22.4–30.1) |
| Women | 30.8 (25.7–37.1) | 28.2 (23.6–34.4) | 26.9 (22.5–32.8) | 26.6 (22.3–32.5) | 26.6 (22.7–32.3) | 26.6 (22.2–32.4) |
| Diabetes, %c | ||||||
| Men | 100 | 100 | 27 | 17 | 11 | 26 |
| Women | 100 | 100 | 31 | 15 | 10 | 28 |
| Mean number of cardiovascular comorbidities (SD)d,e | ||||||
| Men | 0.86 (0.98) | 0.64 (0.91) | 0.67 (0.89) | 0.38 (0.72) | 0.26 (0.60) | 0.66 (0.92) |
| Women | 0.84 (0.96) | 0.62 (0.89) | 0.64 (0.86) | 0.31 (0.64) | 0.16 (0.47) | 0.61 (0.88) |
| COPD, %f | ||||||
| Men | 9 | 6 | 10 | 7 | 4 | 11 |
| Women | 10 | 6 | 10 | 6 | 4 | 11 |
| Smoking, %b | ||||||
| Men | 6 | 8 | 8 | 8 | 7 | 8 |
| Women | 4 | 6 | 6 | 6 | 6 | 6 |
| Cancer, %g | ||||||
| Men | 5 | 3 | 8 | 6 | 4 | 19 |
| Women | 4 | 3 | 6 | 5 | 4 | 16 |
IQR, interquartile range; BMI, body mass index; COPD, chronic obstructive pulmonary disease.
P<0.05 for men versus women for all causes of kidney failure except type 1 diabetes (P=0.2).
P<0.05 for men versus women for all causes of kidney failure.
P<0.05 for men versus women for all nondiabetic causes of kidney failure.
P<0.05 for men versus women for all causes of kidney failure except type 1 diabetes (P=0.1).
Cardiovascular comorbidities defined as coronary artery disease, congestive heart failure, stroke or transient ischemic attack, and peripheral vascular disease.
P<0.05 for men versus women for all causes of kidney failure except hypertension (P=0.6), type 1 diabetes (P=0.4), and other (P=0.07).
P<0.05 for men versus women for all causes of kidney failure except type 1 diabetes (P=0.4).
Association between Sex and Waitlisting
In unadjusted analysis, the overall rate of waitlisting was 6.5 per 100 person-years in women versus 8.3 per 100 person-years for men. Women had lower hazard of kidney transplant waitlisting (hazard ratio [HR], 0.80; 95% confidence interval [95% CI], 0.79 to 0.81) compared with men (Table 2). In our fully adjusted model, access to the waitlist remained lower for women compared with men (HR, 0.89; 95% CI, 0.89 to 0.90, fully adjusted, Table 2).
Table 2.
Hazard of waitlisting and deceased-donor transplantation after first KRT (2005–2017) by sex
| Number of Events and Models | Access to Waitlist from Time of First Kidney Failure Treatment | Access to Deceased-Donor Transplantation from Time of First Kidney Failure Treatment |
|---|---|---|
| Outcomes occurring for men, N (% of outcomes occurring in men) | 167,519 (62) | 54,163 (60) |
| Outcomes occurring for women, N (% of outcomes occurring in women) | 103,592 (38) | 35,411 (40) |
| Overall models | Women (versus Men) Hazard Ratio (95% Confidence Interval) | |
| Unadjusted model | 0.80 (0.79 to 0.81) | 0.84 (0.83 to 0.86) |
| Minimally adjusted modela (Model 1) | 0.87 (0.86 to 0.88) | 0.93 (0.91 to 0.94) |
| Fully adjusted modelb (Model 2) | 0.89 (0.89 to 0.90) | 0.94 (0.93 to 0.95) |
Adjusted for attributed cause of kidney failure, age, race/ethnicity, and body mass index (BMI).
Adjusted for attributed cause of kidney failure, age, race/ethnicity, BMI, yr of first kidney failure treatment, median zip code household income, insurance coverage, Organ Procurement and Transplant Network region, smoking, chronic obstructive pulmonary disease, cancer, coronary artery disease, congestive heart failure, stroke or transient ischemic attack, and peripheral vascular disease.
We tested for and found a statistically significant interaction between sex and attributed cause of kidney failure in our fully adjusted model (P<0.001) for the outcome of waitlisting. In stratified analysis, we found that in patients with kidney failure attributed to type 2 diabetes mellitus, women were waitlisted at a rate of 4.0 per 100 person-years compared with 7.0 per 100 person-years in men. In the fully adjusted model, women with kidney failure attributed to type 2 diabetes had 27% lower access to the waitlist (HR, 0.73; 95% CI, 0.72 to 0.74) compared with men with kidney failure attributed to type 2 diabetes (Figure 2). In contrast, among those with cystic disease, women were waitlisted at a rate of 36.2 per 100 person-years compared with 36.9 per 100 person-years in men. In fully adjusted analysis, women had higher hazard (HR, 1.06; 95% CI, 1.03 to 1.09) of waitlist access compared with men (Figure 2). The observed sex disparities in access to waitlisting were similar in the pre- and post-KAS eras (Table 3).
Figure 2.
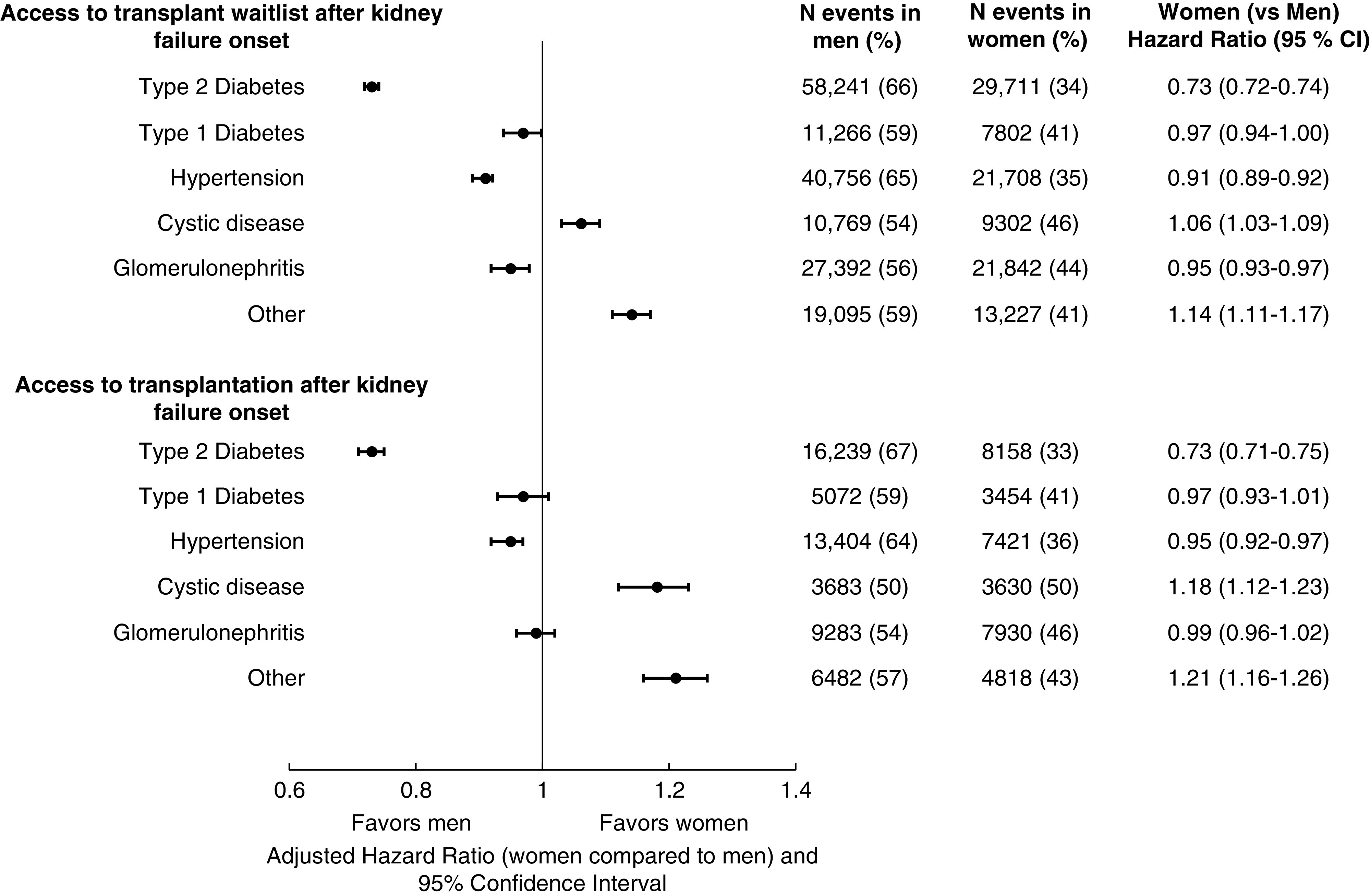
Access to kidney transplant waitlist and deceased-donor transplantation from kidney failure onset by attributed cause of kidney failure and sex. 95% CI, 95% confidence interval.
Table 3.
Hazard of waitlisting before and after 2014 Kidney Allocation System policies among patients with first onset of kidney failure (2005–2017) by sex
| Models | Women (versus Men) Hazard Ratio (95% Confidence Interval) | |
|---|---|---|
| Access to Waitlisting | Before 2014 Kidney Allocation System | After 2014 Kidney Allocation System |
| Unadjusted model | 0.79 (0.78 to 0.80) | 0.83 (0.82 to 0.85) |
| Minimally adjusted modela (Model 1) | 0.86 (0.85 to 0.87) | 0.90 (0.89 to 0.92) |
| Fully adjusted modelb (Model 2) | 0.89 (0.88 to 0.89) | 0.94 (0.92 to 0.96) |
| Fully adjusted model (Model 2) stratified by cause of kidney failure | ||
| Type 2 diabetes | 0.72 (0.71 to 0.73) | 0.77 (0.74 to 0.79) |
| Type 1 diabetes | 0.96 (0.93 to 0.99) | 1.03 (0.96 to 1.11) |
| Hypertension | 0.90 (0.88 to 0.92) | 0.94 (0.90 to 0.98) |
| Cystic disease | 1.04 (1.00 to 1.07) | 1.18 (1.11 to 1.25) |
| GN | 0.95 (0.93 to 0.97) | 0.98 (0.94 to 1.02) |
| Other | 1.13 (1.11 to 1.16) | 1.18 (1.11 to 1.24) |
Adjusted for attributed cause of kidney failure, age, race/ethnicity, and body mass index (BMI).
Adjusted for attributed cause of kidney failure, age, race/ethnicity, BMI, yr of first kidney failure treatment, median zip code household income, insurance coverage, Organ Procurement and Transplant Network region, smoking, chronic obstructive pulmonary disease, cancer, coronary artery disease, congestive heart failure, stroke or transient ischemic attack, peripheral vascular disease.
Association between Sex and Deceased-Donor Kidney Transplantation among all Patients with Kidney Failure
Overall access to deceased-donor kidney transplantation from date of first maintenance KRT was lower for women compared with men in unadjusted and adjusted models (Table 2). Women were transplanted at a rate of 1.9 per 100 person-years compared with 2.2 per100 person-years in men. There was a statistically significant interaction (P<0.001) between sex and attributed cause of disease for deceased-donor kidney transplantation as an outcome. Among those with kidney failure attributed to type 2 diabetes, women had 27% lower access compared with men (HR, 0.73; 95% CI, 0.71 to 0.75, Figure 2), but these sex disparities were not observed in those with cystic disease.
Association between Sex and Deceased-Donor Kidney Transplantation in Patients Waitlisted after KRT
A total of 189,667 patients (70% of all waitlisted patients) were waitlisted after maintenance KRT initiation between January 1, 2005 and December 31, 2017 (Figure 1). Of these eligible patients, 61,507 received deceased-donor kidney transplantation during the study period. In unadjusted and adjusted models, women had a lower hazard for deceased-donor kidney transplantation compared with men (Supplemental Table 3).
However, there was a statistically significant interaction (P<0.001) between sex and attributed cause of kidney failure for the outcome of deceased-donor kidney transplantation after waitlisting. Among those with kidney failure attributed to type 2 diabetes, women were transplanted at a rate of 8.4 out of 100 person-years compared with men, who were transplanted at a rate of 9.6 out of 100 person-years. In adjusted analysis of those with kidney failure attributed to type 2 diabetes, women had 11% lower access to deceased-donor kidney transplantation compared with men (HR, 0.89; 95% CI, 0.86 to 0.92) (Figure 3). Conversely, among those with kidney failure attributed to cystic disease, the absolute rate of deceased-donor kidney transplantation after waitlisting for women was 14.9 out of 100-person years compared with 14.1 out of 100 person-years for men. In fully adjusted analysis, women and men with cystic disease had similar access to deceased-donor kidney transplantation after waitlisting (Figure 3).
Figure 3.
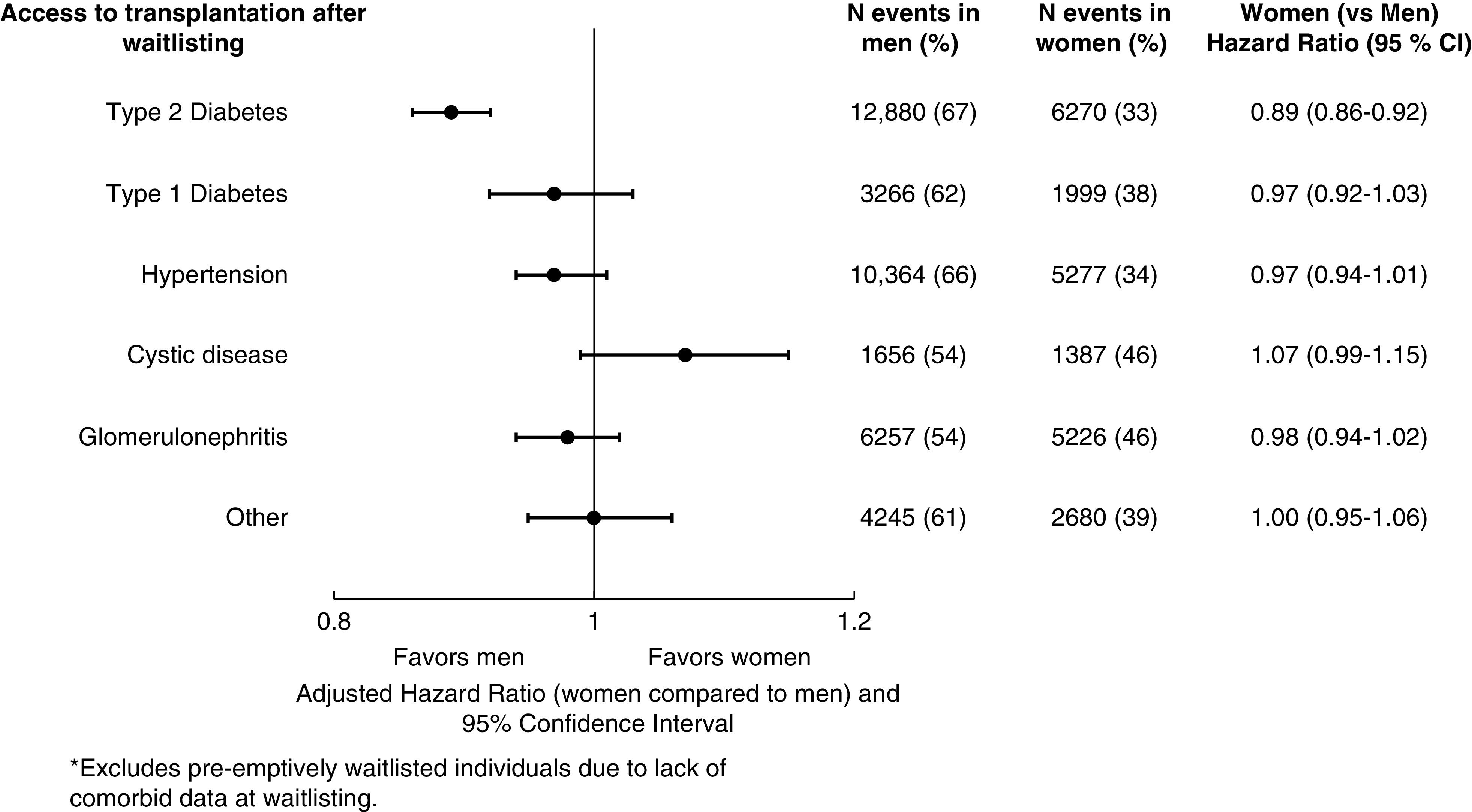
Access to deceased-donor kidney transplantation from waitlisting by attributed cause of kidney failure and sex.
Sensitivity Analysis
Results were similar after accounting for the competing risks of death and living-donor transplantation (Supplemental Tables 4 and 5).
Additionally, we found an interaction between age and sex in our adjusted models (P<0.001), such that the disparities in waitlisting and deceased-donor kidney transplantation after first KRT were most pronounced for older women who had type 2 diabetes as the attributed cause of kidney failure (Supplemental Table 6).
When we excluded those listed for transplantation before the date of first maintenance KRT, the disparity in waitlisting and transplantation after first kidney failure onset by sex remained most pronounced among those with type 2 diabetes (Supplemental Table 7).
Discussion
In this study, we found that sex disparities in access to waitlisting and to deceased-donor kidney transplantation varied by attributed cause of kidney failure. Although we found disparities by sex in access to the waitlist for multiple causes of kidney failure, disparities in access to deceased-donor kidney transplantation after waitlisting were present only in women whose kidney failure was attributed to type 2 diabetes. Conversely, access to waitlisting or deceased-donor kidney transplantation after waitlisting was similar or higher for women than men with cystic or “other” causes of kidney failure. We believe these data add to our prior knowledge surrounding sex disparities in access to kidney transplantation and identify women with kidney failure attributed to type 2 diabetes as a particularly at-risk population.
Patients with type 2 diabetes are at elevated cardiovascular risk (23), and those with diabetes that is severe enough to be the attributed cause of kidney failure are likely at the highest cardiovascular risk of any subgroup of patients with kidney failure. This is supported by our observation that those with kidney failure attributed to type 2 diabetes had the most cardiovascular comorbidities and the highest BMI at the time of kidney failure onset. However, we did not observe a higher prevalence of cardiovascular comorbidities in women compared with men. We also did not find a large attenuation in the observed sex disparities in access to deceased-donor kidney transplantation after accounting for known cardiovascular comorbidities. Thus, we speculate that women with conditions associated with high burden of cardiovascular disease (such as those with type 2 diabetes) could be perceived to be less eligible for transplantation compared with men, even when the actual burden of cardiovascular disease is similar to, or lower than, that observed in men. For comparison, women with kidney failure attributed to cystic causes also had lower prevalence of cardiovascular comorbidities compared with men, but these women had better access to the waitlist and deceased-donor kidney transplantation than men, supporting the possibility that perceived risk could play a role in the sex disparities that we observed for women with diabetes.
Newman et al. (24) also reported that women and patients with diabetes were at higher risk for hospitalizations after waitlist registration. We speculate that women with kidney failure attributed to diabetes and older women with other noncystic causes of kidney failure may be at higher risk of having delays in their access to transplantation due to acute illnesses or deconditioning following these hospitalizations. At kidney failure onset, women were older than men for all attributed causes of kidney failure except for type 1 diabetes and GN. Given the older age of women at time of onset of kidney failure and the established association between age and frailty in patients with kidney disease (25), frailty may also be an important contributory factor to sex disparities in access to deceased-donor kidney transplantation. Frailty is known to be more prevalent among women and previously associated with lower likelihood of waitlisting (26–31). A prior study also showed that the sex-related disparities in access to transplantation are especially prominent in older women (7), which we confirmed in our study. We hypothesize that differences in perceived or actual frailty in older women versus older men may contribute to our findings (28). However, our study is limited in the lack of frailty assessment in the USRDS.
Obesity in women with type 2 diabetes and kidney failure may be another factor contributing to the disparity in transplantation. In the overall cohort and those who were waitlisted, women with type 2 diabetes had the highest BMI at the time of initiation of KRT, which may be a barrier to transplant access (8,18), even after waitlisting. However, our analyses did adjust for BMI at the time of kidney failure onset, and thus body size likely does not fully explain our observations.
The strengths of our study include the use of a nationally representative cohort with sufficient power to detect the presence of effect modification. Limitations include the use of administrative data and lack of more granular clinical data surrounding the severity of comorbid conditions, the potential for residual confounding, and the potential for differential misclassification of comorbid conditions and other covariates in our study by sex. We do not have data on timing of referrals for kidney transplant evaluation and eligibility determination. In addition, there is a potential for small biases to be present given our construction of a cohort of patients with incident KRT for study (and not a cohort of waitlisted individuals). Lastly, we acknowledge that disparities by sex to deceased-donor kidney transplantation may be affected by the 2014 KAS, and further analyses limited to the post-KAS era will be valuable.
In conclusion, sex disparities in waitlisting and transplantation are most prominent among those with kidney failure attributed to type 2 diabetes compared with other attributed causes of kidney failure, such as cystic kidney disease. These findings have important implications for providers caring for patients with CKD and type 2 diabetes, as women with type 2 diabetes may benefit from earlier transplant referral and other risk factor modifications to improve their eligibility for and access to transplantation. Further studies are needed to determine how to best mitigate barriers to transplantation among women with kidney failure due to type 2 diabetes.
Disclosures
K.L. Johansen reports employment with Hennepin Healthcare; serving as a member of Steering Committee for GlaxoSmithKline prolyl hydroxylase inhibitor clinical trials program; and serving as an associate editor of JASN. E. Ku reports receiving grant funding from CareDX; and serving as a consultant to Tricida, outside of the submitted work. J.C. Tan reports that her spouse is employed by and has ownership interest in Genentech/Roche. J.C. Tan also reports receiving honoraria from University of Chicago Center for Continuing Medical Education; serving as an associate editor of Clinical Transplantation; serving on the Scientific Review Board of the American Society of Transplanation; and serving as a scientific advisor or member of the American Journal of Kidney Diseases. All remaining authors have nothing to disclose.
Funding
This work was funded by National Institute of Diabetes and Digestive and Kidney Diseases grants F32DK118869-01 (to P. Ahearn), R01DK115629 (to E. Ku and K.L. Johansen), and 5K24DK085153 (to K.L. Johansen), and National Heart, Lung, and Blood Institute grant K23HL131023 (to E. Ku). This publication was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health through Clinical and Translational Science Institute, University of California, San Francisco grant UL1 TR001872.
Supplementary Material
Acknowledgments
An abstract of this work was presented at the 2018 American Society of Nephrology Annual Meeting in San Diego, CA. The data reported here have been supplied by the United States Renal Data System. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the US government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09140620/-/DCSupplemental.
Supplemental Table 1. Characteristics of patients with incident kidney failure (2005–2017) by sex.
Supplemental Table 2. Characteristics of all waitlisted patients by sex and attributed cause of kidney failure.
Supplemental Table 3. Hazard of deceased-donor transplantation from waitlisting by sex.
Supplemental Table 4. Access to waitlist and deceased-donor transplantation by sex after first KRT with competing risk of death and living-donor kidney transplantation.
Supplemental Table 5. Access to deceased-donor transplantation after waitlisting by sex with competing risk of death and living-donor kidney transplantation.
Supplemental Table 6. Hazard of waitlisting and deceased-donor transplantation after first KRT by sex, age, and attributed cause of kidney failure.
Supplemental Table 7. Hazard of waitlisting and deceased-donor transplantation by sex after first maintenance KRT excluding those placed on waitlist before first maintenance KRT.
References
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Jofre R, López-Gómez JM, Valderrábano F: Quality of life for patient groups. Kidney Int 57: S121–S130, 2000 [Google Scholar]
- 3.Evans RW, Manninen DL, Garrison LP Jr., Hart LG, Blagg CR, Gutman RA, Hull AR, Lowrie EG: The quality of life of patients with end-stage renal disease. N Engl J Med 312: 553–559, 1985 [DOI] [PubMed] [Google Scholar]
- 4.United States Renal Data System , 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Bethesda, MD 2018. Available at https://usrds.org/media/2283/2018_volume_2_esrd_in_the_us.pdf. Accessed June 1, 2020
- 5.Wolfe RA, Ashby VB, Milford EL, Bloembergen WE, Agodoa LY, Held PJ, Port FK: Differences in access to cadaveric renal transplantation in the United States. Am J Kidney Dis 36: 1025–1033, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Alexander GC, Sehgal AR: Barriers to cadaveric renal transplantation among Blacks, women, and the poor. JAMA 280: 1148–1152, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Segev DL, Kucirka LM, Oberai PC, Parekh RS, Boulware LE, Powe NR, Montgomery RA: Age and comorbidities are effect modifiers of gender disparities in renal transplantation. J Am Soc Nephrol 20: 621–628, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill JS, Hendren E, Dong J, Johnston O, Gill J: Differential association of body mass index with access to kidney transplantation in men and women. Clin J Am Soc Nephrol 9: 951–959, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg PP, Furth SL, Fivush BA, Powe NR: Impact of gender on access to the renal transplant waiting list for pediatric and adult patients. J Am Soc Nephrol 11: 958–964, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Schaubel DE, Stewart DE, Morrison HI, Zimmerman DL, Cameron JI, Jeffery JJ, Fenton SS: Sex inequality in kidney transplantation rates. Arch Intern Med 160: 2349–2354, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Ojo A, Port FK: Influence of race and gender on related donor renal transplantation rates. Am J Kidney Dis 22: 835–841, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Kjellstrand CM: Age, sex, and race inequality in renal transplantation. Arch Intern Med 148: 1305–1309, 1988 [PubMed] [Google Scholar]
- 13.Bromberger B, Spragan D, Hashmi S, Morrison A, Thomasson A, Nazarian S, Sawinski D, Porrett P: Pregnancy-induced sensitization promotes sex disparity in living donor kidney transplantation. J Am Soc Nephrol 28: 3025–3033, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monson RS, Kemerley P, Walczak D, Benedetti E, Oberholzer J, Danielson KK: Disparities in completion rates of the medical prerenal transplant evaluation by race or ethnicity and gender. Transplantation 99: 236–242, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Opotowsky AR, McWilliams JM, Cannon CP: Gender differences in aspirin use among adults with coronary heart disease in the United States. J Gen Intern Med 22: 55–61, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan NA, Daskalopoulou SS, Karp I, Eisenberg MJ, Pelletier R, Tsadok MA, Dasgupta K, Norris CM, Pilote L; GENESIS PRAXY Team: Sex differences in acute coronary syndrome symptom presentation in young patients. JAMA Intern Med 173: 1863–1871, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Nguyen JT, Berger AK, Duval S, Luepker RV: Gender disparity in cardiac procedures and medication use for acute myocardial infarction. Am Heart J 155: 862–868, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segev DL, Simpkins CE, Thompson RE, Locke JE, Warren DS, Montgomery RA: Obesity impacts access to kidney transplantation. J Am Soc Nephrol 19: 349–355, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cecka JM, Kucheryavaya AY, Reinsmoen NL, Leffell MS: Calculated PRA: Initial results show benefits for sensitized patients and a reduction in positive crossmatches. Am J Transplant 11: 719–724, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Johansen KL, Zhang R, Huang Y, Patzer RE, Kutner NG: Association of race and insurance type with delayed assessment for kidney transplantation among patients initiating dialysis in the United States. Clin J Am Soc Nephrol 7: 1490–1497, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michigan Population Studies Center : Zip code characteristics: Mean and median household income. Available at: http://www.psc.isr.umich.edu/dis/census/Features/tract2zip/index.html. Accessed December 21, 2014
- 22.Centers for Disease Control and Prevention: Defining adult overweight and obesity. Available at: https://www.cdc.gov/obesity/adult/defining.html. Accessed September 11, 2018
- 23.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H: Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia 44[Suppl 2]: S14–S21, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Newman KL, Lynch RJ, Adams AB, Zhang R, Pastan SO, Patzer RE: Hospitalization among individuals waitlisted for kidney transplant. Transplantation 101: 2913–2923, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kittiskulnam P, Sheshadri A, Johansen KL: Consequences of CKD on functioning. Semin Nephrol 36: 305–318, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheshadri A, Johansen KL: Prehabilitation for the frail patient approaching ESRD. Semin Nephrol 37: 159–172, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johansen KL, Delgado C, Bao Y, Kurella Tamura M: Frailty and dialysis initiation. Semin Dial 26: 690–696, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salter ML, Gupta N, Massie AB, McAdams-DeMarco MA, Law AH, Jacob RL, Gimenez LF, Jaar BG, Walston JD, Segev DL: Perceived frailty and measured frailty among adults undergoing hemodialysis: A cross-sectional analysis. BMC Geriatr 15: 52, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansen KL, Chertow GM, Jin C, Kutner NG: Significance of frailty among dialysis patients. J Am Soc Nephrol 18: 2960–2967, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Wilhelm-Leen ER, Hall YN, K Tamura M, Chertow GM: Frailty and chronic kidney disease: The Third National Health and Nutrition Evaluation Survey. Am J Med 122: 664–671.e2, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng XS, Lentine KL, Koraishy FM, Myers J, Tan JC: Implications of frailty for peritransplant outcomes in kidney transplant recipients. Curr Transplant Rep 6: 16–25, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.