Visual Abstract
Keywords: end stage kidney disease, kidney transplantation, epidemiology and outcomes, pediatric kidney transplantation, pediatric nephrology, pediatrics, transplantation, clinical epidemiology, children
Abstract
Background and objectives
Pre-emptive kidney transplantation is advocated as best practice for children with kidney failure who are transplant eligible; however, it is limited by late presentation. We aimed to determine whether socioeconomic deprivation and/or geographic location (distance to the center and rural/urban residence) are associated with late presentation, and to what degree these factors could explain differences in accessing pre-emptive transplantation.
Design, setting, participants, & measurements
A cohort study using prospectively collected United Kingdom Renal Registry and National Health Service Blood and Transplant data from January 1, 1996 to December 31, 2016 was performed. We included children aged >3 months to ≤16 years at the start of KRT. Multivariable logistic regression models were used to determine associations between the above exposures and our outcomes: late presentation (defined as starting KRT within 90 days of first nephrology review) and pre-emptive transplantation, with a priori specified covariates.
Results
Analysis was performed on 2160 children (41% females), with a median age of 3.8 years (interquartile range, 0.2–9.9 years) at first nephrology review. Excluding missing data, 478 were late presenters (24%); 565 (26%) underwent pre-emptive transplantation, none of whom were late presenting. No association was seen between distance or socioeconomic deprivation with late presentation, in crude or adjusted analyses. Excluding late presenters, greater area affluence was associated with higher odds of pre-emptive transplantation, (odds ratio, 1.20 per quintile greater affluence; 95% confidence interval, 1.10 to 1.31), with children of South Asian (odds ratio, 0.52; 95% confidence interval, 0.36 to 0.76) or Black ethnicity (odds ratio, 0.31; 95% confidence interval, 0.12 to 0.80) less likely to receive one. A longer distance to the center was associated with pre-emptive transplantation on crude analyses; however, this relationship was attenuated (odds ratio, 1.02 per 10 km; 95% confidence interval, 0.99 to 1.05) in the multivariable model.
Conclusions
Socioeconomic deprivation or geographic location are not associated with late presentation in children in the United Kingdom. Geographic location was not independently associated with pre-emptive transplantation; however, children from more affluent areas were more likely to receive a pre-emptive transplant.
Introduction
For children with kidney failure, transplantation is the preferred KRT modality, offering superior patient survival, growth, and quality of life compared with dialysis during a crucial period of development (1–3). Furthermore, there is evidence that pre-emptive kidney transplantation is associated with lower risk of graft loss and death (4,5). Supported by high-grade evidence, international guidance advocates timely consideration of and preparation for pre-emptive transplantation for patients with CKD who are eligible for transplantation (6).
Despite recommendations, one in four children in the United Kingdom (UK) starting KRT currently receive a pre-emptive transplant. Patient characteristics associated with reduced access include female sex and non-White ethnicity (3,7,8). Timely transplant preparation is dependent on prompt detection of kidney disease; one common reason precluding pre-emptive transplantation is late presentation, defined as early requirement for dialysis after initial presentation to a nephrologist. In accordance with international and national guidance (9,10), UK children with CKD (eGFR, <60 ml/min per 1.73 m2) are predominantly managed under specialist kidney care. Those with suspected CKD can be referred directly or via general pediatricians, suggesting late presentation signifies detection at an advanced stage. Late presentation is associated with similar patient and disease variables including older age, non-White ethnicity, and glomerular/unknown diagnoses (11). Many of the risk factors associated with late presentation and reduced access to pre-emptive transplantation are nonmodifiable.
Identifying modifiable factors associated with access to care will enable development of targeted interventions. Key to our understanding are the social determinants of health, “the circumstances in which people live and work” (12), which frequently drive health disparities. These include socioeconomic deprivation, education, environment, and access to services (13). In the United Kingdom, health care is publicly funded, yet inequities in chronic disease outcomes are reported among children from deprived areas (14,15). In addition, adult patients from socioeconomically deprived areas have reduced access to the transplant waiting list, and are less likely to receive living kidney transplants (16,17).
Few pediatric studies have explored whether variations in social determinants of health affect access to specialist nephrology care. US studies demonstrate that inequalities in deprivation, independent of ethnicity, are associated with reduced waiting list access and transplantation in children (18,19). Conversely, studies using Australian and New Zealand Dialysis and Transplant Registry data showed no association between deprivation and pre-emptive transplantation, but noted geographic remoteness was more common among late-presenting children (20) and was associated with reduced pre-emptive transplantation (21). Given differences in health care infrastructure and geography, it is unclear whether these results are generalizable to UK children. We hypothesized that children living remotely relative to the center, in rural locations where care utilization may be negatively affected (22), or in deprived areas, may be disadvantaged in terms of timely detection and/or access to early transplantation.
The aim of this study therefore was to determine whether socioeconomic deprivation and geographic location were associated with late presentation to specialist kidney services and, in patients not presenting late, access to pre-emptive kidney transplantation.
Materials and Methods
Study Population
The study population included UK children aged >3 months to ≤16 years with kidney failure starting KRT (incident patients) for ≥90 days identified from the UK Renal Registry (UKRR) database between January 1, 1996 and December 31, 2016. The age range studied was chosen to reflect a complete cohort of patients managed under pediatric nephrology care: children aged 16–18 years may be managed in either adult or pediatric services. Children requiring KRT within the first 3 months of life were not included to avoid misclassification as late presenting. The UKRR collects, reports, and analyzes high-quality clinical data on all children in the United Kingdom receiving long-term KRT. In the United Kingdom, treatment of children with kidney failure is centralized at 13 centers (Scotland, Wales, and Northern Ireland have one center each; England has 10 centers). Ten centers perform kidney transplants. UKRR data returns for English children are mandated through National Service Framework recommendations (23). Although not enforced for Wales, Scotland, and Northern Ireland, collaboration with the British Association for Pediatric Nephrology ensures all patients are captured. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Baseline Data Collection
We extracted baseline data, including age, sex, ethnicity (self-/proxy-reported), primary kidney disease categorized according to European Renal Association–European Transplant and Dialysis Association 2012 registry groupings (24), date of initial nephrology review, date of KRT start, and initial KRT modality. Linkage with National Health Service (NHS) Blood and Transplant data were used to validate UKRR-obtained transplant data (where applicable) including date of waitlisting, transplant date, and donor type (living or deceased).
Outcomes
We examined two outcomes: (1) late presentation, defined as the need to start KRT within a 90-day period after initial nephrology review, and (2) pre-emptive transplantation, defined as receipt of a kidney transplant at the start of KRT. To account for patients planned for pre-emptive transplantation but who required a brief period of dialysis beforehand, individuals who received a kidney transplant within a week of their KRT start date were also deemed to be pre-emptively transplanted (n=14).
Exposure Variables
Our main variables of interest were socioeconomic deprivation and geography. The UKRR does not collect individual-level data, therefore, area-level deprivation data using the 2015 Indices of Multiple Deprivation (IMD) score were used as an ecologic proxy. This is a government tool derived from 2011 Census data and combines aspects of deprivation including average income, employment, health and disability, education, and crime to form an overall measure of “relative” deprivation (25) (see Supplemental File 1 for details). To account for differences in deprivation between the United Kingdom’s constituent countries, an adjusted, UK-wide IMD score, with England as the reference country, was used to align measures (26), as in other studies (14,27,28). We grouped IMD scores into five groups (quintiles), with one being the most deprived and five the least deprived. Geography was measured in two ways: (1) distance; the direct distance between the child’s recorded place of residence and the child's registered “base” nephrology center per 10 km, calculated as follows:
 |
Where Easting and Northing values represent the Ordnance Survey postcode grid reference to 1 m resolution. Due to information governance restrictions, we were unable to use third-party route-planning websites to calculate travel distance. (2) Urbanicity; output areas (England/Wales) or data zones (Scotland) linked to settlements with populations of ≥10,000 inhabitants are classified as “urban,” with remaining areas classified as “rural.” These units represent the smallest geographic area for which 2011 Census data are available and have an average population of 300 and 500–1000 people for England/Wales and Scotland, respectively (29,30). Data were not available for Northern Ireland children who were excluded from this analysis (n=63).
Statistical Analyses
Baseline characteristics of the study cohort were stratified by deprivation quintile and examined using a chi-squared test for categorical variables and Kruskal-Wallis test for nonparametric continuous variables. Multivariable logistic regression modeling was used to examine the independent effects of deprivation and geographic location on late presentation and pre-emptive transplantation, respectively. Associations are summarized with odds ratios (OR) and 95% confidence intervals (95% CIs). The choice of covariates for our multivariable model was determined a priori using directed acyclic graphs to draw a hypothetic causal pathway. Covariates in the late presentation analysis included sex, age group (at first nephrology review date), ethnicity (White, South Asian, Black, and Other), primary kidney disease, and whether a patient’s registered kidney unit was a transplanting center. The time period of KRT start was also included as the vintages 1996–2000, 2001–2005, 2006–2010, and 2011–2016, broadly representing changes in transplant legislation, notably, the Human Tissue Act and the UK Living Kidney Sharing Scheme, which came into effect in 2006 and 2012, respectively (31,32). Covariates examined in pre-emptive transplantation analyses were similar except for age, which was grouped according to KRT start. We postulated that geography may act to mediate any deprivation-outcome association, as socioeconomic position will determine choice of residence given housing and rental costs. We therefore present the total effect of socioeconomic deprivation, without adjustment for geography, and the direct effect, adjusting for geographic location covariates. Analyses were performed with deprivation as both a categorical and an ordinal variable. We compared the goodness of fit of both models to decide which parameter to use for the main results.
We postulated potential interactions between deprivation and distance to the center and/or ethnicity; we tested for interactions with ethnicity as both a categorical and binary (White/non-White) variable. Adult studies have shown that socioeconomic deprivation confounds and modifies the effect of ethnicity on dialysis outcomes (33), although this finding is not consistent (16,34). To explore this issue, the effect of deprivation on both outcomes was examined in a cohort of White-only patients.
We prespecified several sensitivity analyses. For both outcomes, analyses were repeated in patients with comorbidity data, which were available for 32% (n=694) of children. We tested our definition of late presentation was robust by repeating the analyses using thresholds of 180 and 365 days between first nephrology review and KRT start. As transplantation may be technically infeasible in younger children, effect estimates for pre-emptive transplantation were examined, excluding children ≤2 years at KRT start. All analyses were performed using Stata v15.0.
Results
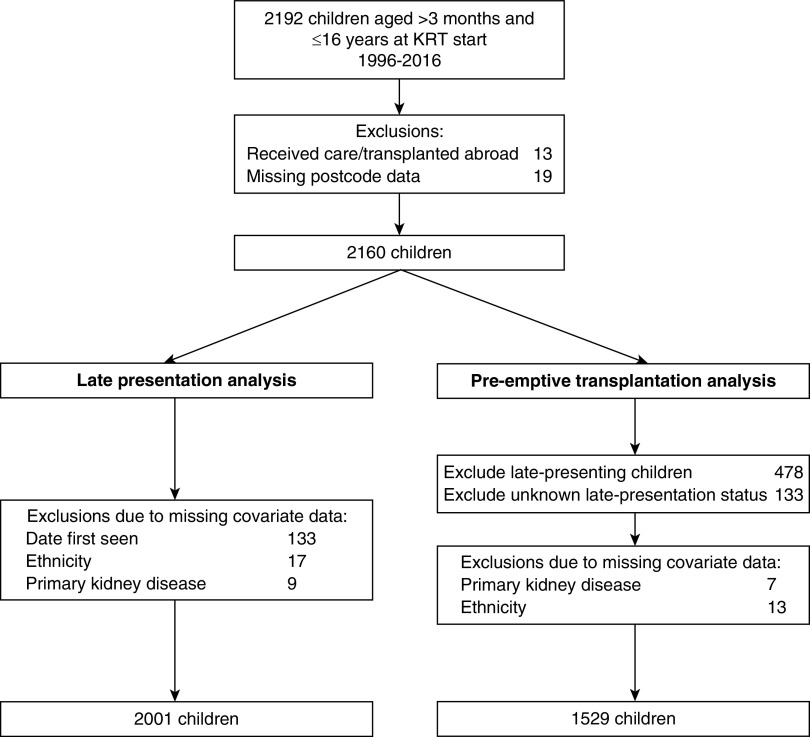
During the study period, 2192 children commenced long-term KRT for kidney failure in the United Kingdom. Children who were diagnosed and/or transplanted overseas (n=13) or with missing postcode data (n=19) were excluded, leaving 2160 children (Figure 1). Children with missing covariate data (n=159 for late presentation and n=31 for pre-emptive transplantation) were excluded (Supplemental Table 1). Late-presenting children (n=478) and those where late-presentation status could not be determined (n=133) were excluded from the pre-emptive transplantation analysis.
Figure 1.
Flow chart of study population and exclusions made.
Baseline characteristics of the 2160 children stratified by area-level deprivation quintile are shown in Table 1. There was strong evidence of an association between ethnicity and deprivation, with higher proportions of White children seen in less-deprived quintiles (P value for trend <0.001). There was strong evidence of increasing distance to the center with decreasing deprivation. Higher proportions of urban residence were also seen among more-deprived quintiles. Differences in the proportion of late presenters and median age and eGFR at first review by deprivation quintile were seen, although these associations were not linear. Differences too were noted in start modality: the proportion of children receiving a transplant was higher with lower deprivation, and therefore the proportion starting KRT with dialysis was lower. There were no differences noted in sex, primary kidney disease, time period, eGFR at KRT start, or the proportion of children waitlisted before KRT start date by deprivation quintile. Most children (85%) resided in England; few children from Wales and Northern Ireland contributed to the least-deprived quintiles.
Table 1.
Baseline characteristics of study cohort (n=2160) by socioeconomic deprivation quintile
| Variable | Socioeconomic Deprivation Quintile | ||||
|---|---|---|---|---|---|
| 1 (most), n=607 | 2, n=525 | 3, n=382 | 4, n=353 | 5 (least), n=293 | |
| Male gender, n, (%) | 336 (55) | 321 (61) | 230 (60) | 209 (59) | 174 (59) |
| Ethnic group, n (%)a | |||||
| White | 355 (59) | 361 (70) | 316 (83) | 307 (88) | 266 (91) |
| South Asian | 174 (29) | 95 (18) | 32 (8) | 27 (8) | 17 (6) |
| Black | 34 (6) | 27 (5) | 7 (2) | 6 (2) | 2 (0.7) |
| Mixed/other | 41 (7) | 31 (6) | 24 (6) | 10 (3) | 6 (2) |
| Distance from center, km, median (IQR) | 15 (7–41) | 24 (11–54) | 33 (16–68) | 40 (19–68) | 40 (21–67) |
| Urban residence, n (%)b | 572 (98) | 436 (87) | 255 (69) | 252 (72) | 233 (80) |
| Primary kidney disease, n (%)c | |||||
| Glomerular disease | 137 (23) | 116 (22) | 78 (21) | 78 (22) | 52 (18) |
| Systemic diseases affecting the kidney | 17 (3) | 18 (3) | 23 (6) | 15 (4) | 17 (6) |
| Familial/hereditary nephropathies | 113 (19) | 69 (13) | 69 (18) | 47 (13) | 50 (17) |
| Tubulointerstitial disease | 287 (48) | 271 (52) | 177 (47) | 176 (50) | 145 (50) |
| Miscellaneous kidney disorders | 50 (8) | 43 (8) | 32 (8) | 34 (10) | 28 (10) |
| Age at first nephrology review, yr, median (IQR)d | 5 (0.5–11) | 3 (0.2–9) | 5 (0.2–11) | 3 (0.2–9) | 3 (0.2–9) |
| eGFR at first nephrology review, ml/min per 1.73 m2, median (IQR)e | 18 (8–41) | 16 (9–33) | 14 (7–30) | 13 (8–32) | 15 (7–31) |
| Late presentation, n (%)f | 145 (25) | 93 (19) | 103 (29) | 72 (22) | 65 (24) |
| Country, n (%) | |||||
| England | 516 (85) | 427 (81) | 312 (82) | 308 (87) | 273 (93) |
| Scotland | 29 (5) | 46 (9) | 33 (9) | 28 (8) | 18 (6) |
| Wales | 37 (6) | 31 (6) | 23 (6) | 14 (4) | 2 (0.7) |
| Northern Ireland | 25 (4) | 21 (4) | 14 (4) | 3 (0.9) | 0 (0.0) |
| Age at KRT start, yr, median (IQR) | 10 (5–14) | 10 (5–13) | 10 (5–14) | 10 (4–14) | 9 (5–13) |
| Months from first nephrology review to KRT start, median (IQR)f | 26 (3–73) | 35 (7–88) | 24 (2–74) | 30 (5–85) | 27 (4–82) |
| Time period of KRT start, n (%) | |||||
| 1996–2000 | 139 (23) | 101 (19) | 81 (21) | 80 (23) | 56 (19) |
| 2001–2005 | 131 (22) | 119 (23) | 94 (25) | 82 (23) | 70 (24) |
| 2006–2010 | 149 (25) | 135 (26) | 92 (24) | 90 (26) | 81 (28) |
| 2011–2016 | 188 (31) | 170 (32) | 115 (30) | 101 (29) | 86 (29) |
| eGFR at KRT start, ml/min per 1.73 m2, median (IQR)g | 9 (7–12) | 9 (7–12) | 9 (7–12) | 9 (7–12) | 9.1 (7–12) |
| Waitlisted before KRT start, n (%)h | 183 (38) | 182 (43) | 120 (39) | 122 (40) | 103 (41) |
| Start modality, n (%)i | |||||
| Hemodialysis | 194 (37) | 141 (30) | 91 (27) | 80 (26) | 72 (28) |
| Peritoneal dialysis | 220 (41) | 193 (41) | 142 (42) | 118 (38) | 92 (36) |
| Transplant | 117 (22) | 137 (29) | 102 (30) | 110 (36) | 95 (37) |
IQR, interquartile range.
Ethnicity missing for n=22.
Urban residence data missing for n=63 (Northern Ireland residents).
Primary kidney disease data missing for n=18.
Age at first nephrology review missing for n=131.
eGFR at first nephrology review missing for n=700.
Late presentation and time to KRT data missing for n=133.
eGFR at KRT start data missing for n=362.
Waitlisting data unavailable for n=387.
Start modality data missing for n=256.
Several sociodemographic and clinical variables were associated with late presentation (Table 2). Being female, older age, and living in more deprived areas were associated with late presentation, although the association with deprivation was modest. Late presentation was also associated with lower eGFR values compared with timely presenters, both at presentation and KRT start. No late-presenting children had transplantation recorded as their KRT start modality. A lower proportion of children with tubulointerstitial disease, which predominantly represents congenital anomalies of the kidney and urinary tract, presented late. Distance from base center or living in an urban area were not associated with late presentation.
Table 2.
Patient and disease characteristics of children included in late presentation analysis, stratified by outcome (n=2001)
| Variable | Late Presentation (n=472) | Timely Presentation (n=1529) |
|---|---|---|
| Male gender, n (%) | 209 (44) | 964 (63) |
| Ethnic group, n (%) | ||
| White | 345 (73) | 1162 (76) |
| South Asian | 75 (16) | 249 (16) |
| Black | 22 (5) | 47 (3) |
| Mixed/other | 30 (6) | 71 (5) |
| Socioeconomic deprivation quintile, n (%) | ||
| 1 (most deprived) | 144 (31) | 432 (28) |
| 2 | 90 (19) | 386 (25) |
| 3 | 102 (22) | 250 (16) |
| 4 | 71 (15) | 254 (17) |
| 5 (least deprived) | 65 (14) | 207 (14) |
| Primary kidney disease, n (%) | ||
| Glomerular disease | 117 (25) | 312 (20) |
| Systemic diseases affecting the kidney | 28 (6) | 56 (4) |
| Familial/hereditary nephropathies | 94 (20) | 238 (16) |
| Tubulointerstitial disease | 153 (32) | 838 (55) |
| Miscellaneous kidney disorders | 80 (17) | 85 (6) |
| Age at first nephrology review, yr, median (IQR) | 11 (5–14) | 2 (0.1–8) |
| eGFR at first nephrology reviewa, ml/min per 1.73 m2, median (IQR) | 7 (5–12) | 22 (12–41) |
| Distance to base center, km, median (IQR) | 27 (14–55) | 26 (12–57) |
| Urban residenceb, n (%) | 379 (82) | 1234 (83) |
| Country, n (%) | ||
| England | 407 (86) | 1307 (86) |
| Scotland | 34 (7) | 94 (6) |
| Wales | 21 (4) | 79 (5) |
| Northern Ireland | 10 (2) | 49 (3) |
| Age at KRT start, yr, median (IQR) | 11 (5–14) | 10 (5–13) |
| Months from first nephrology review to KRT start, median (IQR) | 0.1 (0–1) | 48 (21–100) |
| eGFR at KRT start (ml/min per 1.73 m2)c, median (IQR) | 7 (5–10) | 10 (7–13) |
| Time period of KRT start, n (%) | ||
| 1996–2000 | 101 (21) | 326 (21) |
| 2001–2005 | 112 (24) | 329 (22) |
| 2006–2010 | 128 (27) | 393 (26) |
| 2011–2016 | 131 (28) | 481 (32) |
| Start modalityd, n (%) | ||
| Hemodialysis | 173 (44) | 366 (27) |
| Peritoneal dialysis | 220 (56) | 503 (36) |
| Transplant | 0 (0) | 512 (37) |
IQR, interquartile range.
eGFR at first seen date missing for n=557.
Urban residence data missing for n=59 (Northern Ireland residents).
eGFR at KRT start date missing for n=240.
Start modality data missing for n=227.
Being male, White ethnicity, greater affluence, primary kidney disease, younger age at first nephrology review, lower eGFR at first nephrology review, but higher eGFR at KRT start, further distance from base center, older age at KRT start, longer time (in months) from first nephrology review to KRT start, and more recent time period were all associated with higher probability of receiving a pre-emptive transplantation (Table 3). Living in an urban area was not associated with pre-emptive transplantation.
Table 3.
Patient and disease characteristics of children included in pre-emptive transplantation analysis, stratified by outcome (n=1529)
| Variable | Not Pre-Emptively Transplanted (n=1013) | Pre-Emptively Transplanted (n=516) |
|---|---|---|
| Male gender, n (%) | 613 (61) | 351 (68) |
| Ethnic group, n (%) | ||
| White | 725 (72) | 437 (85) |
| South Asian | 195 (19) | 54 (10) |
| Black | 41 (4) | 6 (1) |
| Mixed/other | 52 (5) | 19 (4) |
| Socioeconomic deprivation quintile, n (%) | ||
| 1 (most deprived) | 325 (32) | 107 (21) |
| 2 | 259 (26) | 127 (25) |
| 3 | 154 (15) | 96 (19) |
| 4 | 155 (15) | 99 (19) |
| 5 (least deprived) | 120 (12) | 87 (17) |
| Primary kidney disease group, n (%) | ||
| Glomerular disease | 293 (29) | 19 (4) |
| Systemic diseases affecting the kidney | 32 (3) | 24 (5) |
| Familial/hereditary nephropathies | 159 (16) | 79 (15) |
| Tubulointerstitial disease | 468 (46) | 370 (72) |
| Miscellaneous kidney disorders | 61 (6) | 24 (5) |
| Age at first nephrology review, yr, median (IQR) | 2 (0.1–8) | 1 (0.1–7) |
| eGFR at first nephrology review, ml/min per 1.73 m2, median (IQR)a | 25 (12–51) | 17 (11–30) |
| Distance to base center, km, median (IQR) | 23 (11–52) | 33 (15–66) |
| Urban residenceb | 823 (84) | 411 (82) |
| Country, n (%) | ||
| England | 863 (85) | 444 (86) |
| Scotland | 70 (7) | 24 (5) |
| Wales | 47 (5) | 32 (6) |
| Northern Ireland | 33 (3) | 16 (3) |
| Age at KRT start, yr, median (IQR) | 9 (3–13) | 11 (7–14) |
| Months from first nephrology review to KRT start, median (IQR) | 36 (14–80) | 76 (37–126) |
| Time period of KRT start, n (%) | ||
| 1996–2000 | 227 (22) | 99 (19) |
| 2001–2005 | 232 (23) | 97 (19) |
| 2006–2010 | 243 (24) | 150 (29) |
| 2011–2016 | 311 (31) | 170 (33) |
| eGFR at KRT start (ml/min per 1.73 m2)c, median (IQR) | 9 (7–11) | 11 (9–14) |
IQR, interquartile range.
eGFR at first seen date missing for n=461.
Urban residence data missing for n=49 (Northern Ireland residents).
eGFR at KRT start date missing for n=172.
There was no association between area-level deprivation, distance from the center, or urban location with late presentation in univariable or multivariable models (Supplemental Table 2, Table 4). Both deprivation and distance from the base center were associated with pre-emptive transplantation. After adjustment for other covariates, greater affluence was associated with a 21% higher odds per quintile as a total effect (95% CI, 1.10 to 1.32). This was hardly changed in the direct model, suggesting this association is not mediated by geographic location. Although residing farther from the center was associated with greater odds of pre-emptive transplantation, after adjustment for deprivation, this effect was consistent with chance. No association was seen between urban location and access to pre-emptive transplantation, in univariable or multivariable models.
Table 4.
Odds of late presentation or receiving a pre-emptive kidney transplant by socioeconomic deprivation status and geography
| Exposure Variable | Univariable Model Odds Ratio (95% Confidence Interval) | Multivariable Total Effect Modela Odds Ratio (95% Confidence Interval) | Multivariable Direct Effect Modelb Odds Ratio (95% Confidence Interval) |
|---|---|---|---|
| Late presentation (n=2001) | |||
| Socioeconomic deprivation (per quintile higher)c | 1.00 (0.93 to 1.08) | 1.05 (0.96 to 1.15) | 1.04 (0.95 to 1.14) |
| Distance to center (per 10 km) | 1.01 (0.98 to 1.03) | — | 1.00 (0.98 to 1.03) |
| Urban locationd,e | 0.91 (0.69 to 1.20) | — | 1.04 (0.75 to 1.45) |
| Pre-emptive transplantation (n=1529) | |||
| Socioeconomic deprivation (per quintile higher)c | 1.21 (1.12 to 1.31) | 1.21 (1.10 to 1.32) | 1.20 (1.10 to 1.31) |
| Distance to center (per 10 km) | 1.04 (1.01 to 1.06) | — | 1.02 (0.99 to 1.05) |
| Urban locationd,f | 0.88 (0.66 to 1.17) | — | 1.09 (0.78 to 1.53) |
Model represents total effect of socioeconomic deprivation on outcome; because distance to center and whether base nephrology unit is a transplanting center are thought to mediate any deprivation-outcome association, these are omitted from the multivariable model.
Multivariable model looks at direct effect of exposure on outcome: variables included in model are distance (per 10 km) or rural/urban location, socioeconomic deprivation, sex, age group, ethnic group, primary kidney disease, period of KRT start, and whether base nephrology unit is a transplanting center.
Socioeconomic deprivation is parameterized as an ordinal variable because this offered the best goodness of fit. Odds ratios represent unit change in odds for each higher quintile of deprivation (or higher area affluence).
Distance is omitted in the urban location multivariable model.
Analysis excludes children from Northern Ireland (n=1942).
Analysis excludes children from Northern Ireland (n=1480).
After adjustment for deprivation, children of Black (OR 0.31; 95% CI, 0.12 to 0.80) or South Asian ethnicity (OR 0.52; 95% CI, 0.36, 0.76) were less likely to receive a pre-emptive transplant compared with White children (Supplemental Table 3). There was no evidence of multiplicative or additive (biologic) interactions between socioeconomic deprivation and distance from the center or deprivation and ethnicity (White/non-White) for either outcome.
Sensitivity Analyses
Our results for late presentation were essentially unchanged when we restricted the sample to White children, adjusted for comorbidity, and extended our definitions of late presentation to 180 and 365 days (Supplemental Table 4). Similarly, the association of socioeconomic deprivation with pre-emptive transplantation remained robust after excluding children ≤2 years, examining a cohort of White children only and adjusting for comorbidity (Supplemental Table 5). Children with coexisting conditions had 47% lower odds of receiving a pre-emptive transplant (adjusted OR, 0.53; 95% CI, 0.33 to 0.85). As procedural data were not available, we excluded children that may require bilateral nephrectomies to examine the effect on pre-emptive transplantation access. This included children with autosomal recessive polycystic kidney disease, congenital nephrotic syndrome, Wilms’ tumor, and primary FSGS. Exclusion did not significantly alter effect estimates.
We performed a post hoc analysis to determine whether the association between deprivation and pre-emptive transplantation was due to greater access to living-donor transplantation. After inclusion of donor type (living, yes/no) in the full multivariable model, children from more affluent areas had greater odds of pre-emptive transplantation, although the effect estimate was attenuated (OR 1.13; 95% CI, 1.03, 1.23).
Discussion
This is the first study to explore associations between social determinants of health and access to nephrology care for UK children using patient-level data. No associations were found between area-level deprivation and geography for late presentation. Living in more affluent areas was associated with 20% greater likelihood of pre-emptive transplantation per quintile of deprivation, whereas South Asian and Black children were less likely to receive one. Although increasing the distance from home to the center was associated with a higher odds of pre-emptive transplantation on crude analysis, this relationship was lost in the fully adjusted model and did not mediate the association with deprivation. Urbanicity was not associated with either outcome.
Several studies have explored geographic location and late presentation with conflicting results. In European adults, a greater odds of late presentation was seen among patients attending large, metropolitan centers, compared with smaller, regional, or private centers, thought to be due to better access to, and communication with, specialist nephrology care within smaller hospital settings (35). Although no association was seen between direct measures of distance to the center on timing of diagnosis in this study, an Australian study reported higher proportions of late presentation among children classified as living in “remote” regions, although this was not in reference to a point of specialist care (20). We failed to find any relationship of distance on late presentation, which may reflect the smaller geographic area and a paucity of truly remote locations within the United Kingdom. Adult data have shown the proportion of long travel times to nephrology centers in the United Kingdom is similar to larger nations (36); however, this measure is unlikely to capture extreme differences in distance and infrastructure, which may act in combination to hinder health care access.
In addition, the association between socioeconomic deprivation and distance differs across countries. In the United Kingdom, higher proportions of affluent children lived remotely, whereas for Australian children, the inverse is true (21). Living in an urban location was not associated with either late presentation or pre-emptive transplantation. Although we were sufficiently powered to explore these associations, as the majority of our population resided in dense urban areas, this may have missed more subtle geographic variations.
The association between social disadvantage and child health is well established. Children from socioeconomically deprived backgrounds in high-income countries, including from the United Kingdom, are more likely to experience worse health outcomes, including chronic conditions and higher mortality (37). There is also evidence that health care utilization differs by socioeconomic status, with UK children from less-affluent areas having higher rates of primary care and emergency department use (38,39), longer hospital stays (40), and fewer preventative health care consultations (38). For children with kidney failure, poorer outcomes such as greater risk of graft loss are seen among children from deprived areas (41,42). The association between access to specialist kidney care is, however, inconsistent. Our study found no association between socioeconomic deprivation and late presentation, which correlates with unadjusted findings from another study (21). As UK health care coverage is universal and free at the point of delivery, this finding is reassuring and suggests deprivation is not implicated in the pathway to diagnosis: from identification and appraisal of symptoms, seeking health care review to subsequent diagnosis.
Strong evidence was, however, noted for reduced access to pre-emptive transplantation with increasing socioeconomic deprivation, signifying potential inequities in the management of UK children once diagnosed. Access to a living-donor transplant partly mediated this relationship; however, a strong association remained, suggesting other factors at play. Increasing wealth is independently associated with access to living- but not deceased-donor pre-emptive transplantation for US children (19); no such correlation was found in an Australian study (21). The reasons for our observed association between socioeconomic deprivation and pre-emptive transplantation, independent of donor type, are likely to be multifaceted and complex. In adults, perceived social support, knowledge of the transplant process, and levels of patient activation appear to mediate the association between income and access to living-donor transplant (43). Furthermore, qualitative research suggests that adults with kidney failure from socioeconomically deprived backgrounds are less confident and engaged in discussions about their treatment (44). How parents perceive transplant preparation, approach these conversations, and make decisions on behalf of their child, and whether this differs by socioeconomic status, requires future exploration. The possibility of clinician bias in the transplant preparation process must also be considered. Although there was no difference in the proportion of children pre-emptively waitlisted by deprivation quintile, there may be other unmeasured biases influencing access to transplant that have not been captured within this dataset.
Acknowledging the interplay between ethnicity and deprivation in access to kidney care is crucial. In the United Kingdom, people of Asian and Black ethnicity are most likely to live in the most-deprived neighborhoods; people of White ethnicity are least likely (45). Barriers to early transplantation for Hispanic and Black American children include socioeconomic deprivation, inadequate health insurance, and access to timely nephrology care (46). In adults, comorbidity and deprivation mediate the effect of ethnicity on transplant access (47). After adjustment for deprivation, timely presenting children of South Asian and Black ethnicity were less likely to be pre-emptively transplanted. This could imply residual confounding by deprivation, or that factors, including sociocultural, are implicated. Inadequate patient knowledge, misconceptions and concerns regarding transplantation, and restrictive social influences are identified as barriers to transplant access for ethnic minority patients lacking suitable donors. Interventions that consider the influence of cultural factors in treatment decision making (48) are urgently needed to address these inequities.
The strengths of our study include the use of prospectively collected, linked data from two national registries. High levels of data completeness enabled a detailed analysis. Comorbidity data are often not considered part of the “core dataset” for registries; however, we were sufficiently powered to discern a difference in access to pre-emptive transplantation for children with coexisting disease. Use of an adjusted UK-wide deprivation scoring system enabled a standardized comparison across devolved nations where the degree of deprivation is known to vary (26). Our main limitations include the use of an area-level ecologic, rather than individual measure of deprivation, which limits comparison across studies. Data collection only occurred from children receiving KRT for >90 days, which may introduce survivor bias, although this methodology is similar to other international registries. Distance to the center was approximated using direct distance, rather than by road travel, which may underestimate difficulties in accessing a tertiary center for families living remotely.
In conclusion, neither socioeconomic deprivation nor geographic location are associated with late presentation of CKD in UK children. Geographic location was not independently associated with pre-emptive transplantation; however, children from less-deprived backgrounds are more likely to receive a pre-emptive transplant. Further work is needed to understand why disparities in care exist for these children, and how they may be mitigated. Whether they reflect inequitable access to health care or other clinical or sociocultural factors requires investigation to enable clinicians to provide appropriate support to disadvantaged families in the predialysis phase.
Disclosures
A. Casula reports employment with The Renal Association. C.D. Inward reports employment with Bristol Royal Hospital for Children. F.J. Caskey reports receiving grants from Kidney Research UK, grants from National Institute for Health Research, and personal fees from Baxter outside the submitted work. L. Plumb reports employment with The Renal Association and receiving grants from Kidney Research UK, outside the submitted work. M.D. Sinha reports serving as a scientific advisor or member of BMC Nephrology, British and Irish Hypertension Society, PKD Charity RAB, and scientific committee for International Congress of Hypertension in Children and Adolescents and acknowledges financial support from the Department of Health via the NIHR comprehensive Biomedical Research Centre and Clinical Research Facilities awards to Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust. S. Marks reports employment with Great Ormond Street Hospital for Children NHS Foundation Trust, and the hospital receives funding for immunosuppressive drug studies by Astellas and Novartis; and reports serving as associate editor for pediatric transplantation for British Journal for Renal Medicine, Pediatric Nephrology, Pediatric Transplantation, and Transplantation. Y. Ben-Shlomo reports employment with Population Health Sciences, University of Bristol; receiving honorarium for speaking at Movement Disorders Course 2018; and serving as a member of the faculty of Public Health Academic Research Committee. Y. Ben-Shlomo is partly funded by National Institute for Health Research Applied Research Collaboration West (NIHR ARC West) at University Hospitals Bristol and Weston NHS Foundation Trust.
Funding
L. Plumb is supported by the NIHR Research Trainees Coordinating Centre (DRF-2016-09-055).
Supplementary Material
Acknowledgments
This publication presents independent research funded by the NIHR. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. Findings from this part of this study were presented in abstract form at the American Society of Nephrology Kidney Week 2019.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Mind the Gap: Acknowledging Deprivation Is Key to Narrowing Kidney Health Disparities in Both Children and Adults,” on pages 185–187.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11020720/-/DCSupplemental.
Supplemental Table 1. Comparison of clinical and demographic features of children included in study analyses and those excluded due to missing data.
Supplemental Table 2. Crude and multivariable models for associations between distance and socioeconomic deprivation with late presentation (n=2001).
Supplemental Table 3. Crude and multivariable models for associations between distance and socioeconomic deprivation with pre-emptive transplantation (n=1529).
Supplemental Table 4. Sensitivity analyses: late presentation outcome.
Supplemental Table 5. Sensitivity analyses: pre-emptive transplantation outcome.
Supplemental File 1. Description of the index of multiple deprivation.
References
- 1.McDonald SP, Craig JC; Australian and New Zealand Paediatric Nephrology Association: Long-term survival of children with end-stage renal disease. N Engl J Med 350: 2654–2662, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Goldstein SL, Graham N, Burwinkle T, Warady B, Farrah R, Varni JW: Health-related quality of life in pediatric patients with ESRD. Pediatr Nephrol 21: 846–850, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Plumb L, Wong E, Casula A, Braddon F, Lewis M, Marks SD, Shenoy M, Sinha MD, Maxwell H: Chapter 4 demography of the UK paediatric renal replacement therapy population in 2016. Nephron 139[Suppl 1]: 105–116, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Amaral S, Sayed BA, Kutner N, Patzer RE: Preemptive kidney transplantation is associated with survival benefits among pediatric patients with end-stage renal disease. Kidney Int 90: 1100–1108, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reydit M, Salomon R, Macher MA, Ranchin B, Roussey G, Garaix F, Lahoche A, Decramer S, Fila M, Dunand O, Cloarec S: Pre-emptive kidney transplantation is associated with improved graft survival in children: Data from the French renal replacement therapy registry. Arch Pediatr 24: 1328–1329, 2017 [Google Scholar]
- 6.Chadban SJ, Ahn C, Axelrod DA, Foster BJ, Kasiske BL, Kher V, Kumar D, Oberbauer R, Pascual J, Pilmore HL, Rodrigue JR, Segev DL, Sheerin NS, Tinckam KJ, Wong G, Knoll GA. KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation 104[Suppl 1]: S11–S103, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Hogan J, Couchoud C, Bonthuis M, Groothoff JW, Jager KJ, Schaefer F, Van Stralen KJ; ESPN/ERA-EDTA Registry: Gender disparities in access to pediatric renal transplantation in Europe: Data from the ESPN/ERA-EDTA registry. Am J Transplant 16: 2097–2105, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Tjaden LA, Noordzij M, van Stralen KJ, Kuehni CE, Raes A, Cornelissen EA, O’Brien C, Papachristou F, Schaefer F, Groothoff JW, Jager KJ; ESPN/ERA-EDTA Registry Study Group: Racial disparities in access to and outcomes of kidney transplantation in children, adolescents, and young adults: Results from the ESPN/ERA-EDTA (European Society of Pediatric Nephrology/European Renal Association-European Dialysis and Transplant Association) registry. Am J Kidney Dis 67: 293–301, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Hogg RJ, Furth S, Lemley KV, Portman R, Schwartz GJ, Coresh J, Balk E, Lau J, Levin A, Kausz AT, Eknoyan G, Levey AS; National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative: National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: Evaluation, classification, and stratification. Pediatrics 111: 1416–1421, 2003 [DOI] [PubMed] [Google Scholar]
- 10.British Association for Paediatric Nephrology: Improving the Standard of Care in Children with Kidney Disease through Paediatric Nephrology Networks, London, Royal College of Paediatrics & Child Health, 2011 [Google Scholar]
- 11.Pruthi R, Casula A, Inward C, Roderick P, Sinha MD; British Association for Paediatric Nephrology: Early requirement for RRT in children at presentation in the United Kingdom: Association with transplantation and survival. Clin J Am Soc Nephrol 11: 795–802, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marmot M, Wilkinson R: Social Determinants of Health, Oxford, Oxford University Press, 2005 [Google Scholar]
- 13.Pearce A, Dundas R, Whitehead M, Taylor-Robinson D: Pathways to inequalities in child health. Arch Dis Child 104: 998–1003, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khanolkar AR, Amin R, Taylor-Robinson D, Viner RM, Warner JT, Stephenson T: Young people with type 1 diabetes of non-White ethnicity and lower socio-economic status have poorer glycaemic control in England and Wales. Diabet Med 33: 1508–1515, 2016. [DOI] [PubMed] [Google Scholar]
- 15.Taylor-Robinson DC, Smyth RL, Diggle PJ, Whitehead M: The effect of social deprivation on clinical outcomes and the use of treatments in the UK cystic fibrosis population: A longitudinal study. Lancet Respir Med 1: 121–128, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Udayaraj U, Ben-Shlomo Y, Roderick P, Casula A, Dudley C, Johnson R, Collett D, Ansell D, Tomson C, Caskey F: Social deprivation, ethnicity, and access to the deceased donor kidney transplant waiting list in England and Wales. Transplantation 90: 279–285, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Udayaraj U, Ben-Shlomo Y, Roderick P, Casula A, Dudley C, Collett D, Ansell D, Tomson C, Caskey F: Social deprivation, ethnicity, and uptake of living kidney donor transplantation in the United Kingdom. Transplantation 93: 610–616, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Patzer RE, Amaral S, Klein M, Kutner N, Perryman JP, Gazmararian JA, McClellan WM: Racial disparities in pediatric access to kidney transplantation: Does socioeconomic status play a role? Am J Transplant 12: 369–378, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patzer RE, Sayed BA, Kutner N, McClellan WM, Amaral S: Racial and ethnic differences in pediatric access to preemptive kidney transplantation in the United States. Am J Transplant 13: 1769–1781, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy SE, Bailey R, Kainer G: Causes and outcome of late referral of children who develop end-stage kidney disease. J Paediatr Child Health 48: 253–258, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Francis A, Didsbury M, Lim WH, Kim S, White S, Craig JC, Wong G: The impact of socioeconomic status and geographic remoteness on access to pre-emptive kidney transplantation and transplant outcomes among children. Pediatr Nephrol 31: 1011–1019, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Palmer B, Appleby J, Spencer J: Rural Health Care: A Rapid Review of the Impact of Rurality on the Costs of Delivering Health Care, London, Nuffield Trust, 2019 [Google Scholar]
- 23.The National Service Framework for Renal Services: Part One: Dialysis and Transplantation, London, Department of Health, 2004 [Google Scholar]
- 24.Venkat-Raman G, Tomson CR, Gao Y, Cornet R, Stengel B, Gronhagen-Riska C, Reid C, Jacquelinet C, Schaeffner E, Boeschoten E, Casino F, Collart F, De Meester J, Zurriaga O, Kramar R, Jager KJ, Simpson K; ERA-EDTA Registry: New primary renal diagnosis codes for the ERA-EDTA. Nephrol Dial Transplant 27: 4414–4419, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith T, Noble M, Noble S, Wright G, McLennan D, Plunkett E: The English Indices of Deprivation 2015, London, Department for Communities and Local Government, 2015 [Google Scholar]
- 26.Abel GA, Barclay ME, Payne RA: Adjusted indices of multiple deprivation to enable comparisons within and between constituent countries of the UK including an illustration using mortality rates. BMJ Open 6: e012750, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey PK, Tomson CR, Ben-Shlomo Y: Study of living kidney donor-recipient relationships: Variation with socioeconomic deprivation in the White population of England. Clin Transplant 27: E327–E331, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton AJ, Caskey FJ, Casula A, Ben-Shlomo Y, Inward CD: Psychosocial health and lifestyle behaviors in young adults receiving renal replacement therapy compared to the general population: Findings from the SPEAK study. Am J Kidney Dis 73: 194–205, 2019 [DOI] [PubMed] [Google Scholar]
- 29.Department for Environment , Food and Rural Affairs: Guide to applying the rural urban classification to data. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/539241/Guide_to_applying_the_rural_urban_classification_to_data.pdf. Accessed December 9, 2020
- 30.Scottish Neighbourhood Statistics: SNS data zones 2011. Scotland’s Census, 2011. Available at: https://www.scotlandscensus.gov.uk/variables-classification/sns-data-zone-2011. Accessed May 1, 2020
- 31.Weale AR, Lear PA: Organ transplantation and the Human Tissue Act. Postgrad Med J 83: 141–142, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.NHS Blood and Transplant: Organ Donation and Transplant (2020). UK Living Kidney Sharing Scheme. Available at: https://www.odt.nhs.uk/living-donation/uk-living-kidney-sharing-scheme/. Accessed December 9, 2020
- 33.Port FK, Wolfe RA, Levin NW, Guire KE, Ferguson CW: Income and survival in chronic dialysis patients. ASAIO Trans 36: M154–M157, 1990 [PubMed] [Google Scholar]
- 34.Caskey FJ: Renal replacement therapy: Can we separate the effects of social deprivation and ethnicity? Kidney Int Suppl (2011) 3: 246–249, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wauters J-P, Bosson J-L, Forneris G, Turc-Baron C, Golshayan D, Paternoster G, Martina G, Hurot JM, von Albertini B, Forêt M, Cordonnier D, Piccoli G; Diamant Alpin Collaborative Dialysis Study Group: Patient referral is influenced by dialysis centre structure in the Diamant Alpin Dialysis cohort study. Nephrol Dial Transplant 19: 2341–2346, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Moist LM, Bragg-Gresham JL, Pisoni RL, Saran R, Akiba T, Jacobson SH, Fukuhara S, Mapes DL, Rayner HC, Saito A, Port FK: Travel time to dialysis as a predictor of health-related quality of life, adherence, and mortality: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 51: 641–650, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Spencer NJ, Blackburn CM, Read JM: Disabling chronic conditions in childhood and socioeconomic disadvantage: A systematic review and meta-analyses of observational studies. BMJ Open 5: e007062, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saxena S, Majeed A, Jones M: Socioeconomic differences in childhood consultation rates in general practice in England and Wales: Prospective cohort study. BMJ 318: 642–646, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kyle RG, Kukanova M, Campbell M, Wolfe I, Powell P, Callery P: Childhood disadvantage and emergency admission rates for common presentations in London: An exploratory analysis. Arch Dis Child 96: 221–226, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Petrou S, Kupek E: Socioeconomic differences in childhood hospital inpatient service utilisation and costs: Prospective cohort study. J Epidemiol Community Health 59: 591–597, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muneeruddin S, Chandar J, Abitbol CL, Seeherunvong W, Freundlich M, Ciancio G, Burke GW, Zilleruelo G: Two decades of pediatric kidney transplantation in a multi-ethnic cohort. Pediatr Transplant 14: 667–674, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Driollet B, Bayer F, Chatelet V, Macher MA, Salomon R, Ranchin B, Roussey G, Lahoche A, Garaix F, Decramer S, Mérieau E, Fila M, Zaloszyc A, Deschênes G, Valeri L, Launay L, Couchoud C, Leffondré K, Harambat J: Social deprivation is associated with poor kidney transplantation outcome in children. Kidney Int 96: 769–776, 2019 [DOI] [PubMed] [Google Scholar]
- 43.Bailey PK, Caskey FJ, MacNeill S, Tomson CRV, Dor FJMF, Ben-Shlomo Y: Mediators of socioeconomic inequity in living-donor kidney transplantation: Results from a UK multicenter case-control study. Transplant Direct 6: e540, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bailey PK, Ben-Shlomo Y, Tomson CR, Owen-Smith A: Socioeconomic deprivation and barriers to live-donor kidney transplantation: A qualitative study of deceased-donor kidney transplant recipients. BMJ Open 6: e010605, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ministry of Housing , Communities and Local Government: UK population by ethnicity: People living in deprived neighbourhoods. Available at: https://www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity/demographics/people-living-in-deprived-neighbourhoods/latest#full-page-history. Accessed December 9, 2020
- 46.Amaral S, Patzer R: Disparities, race/ethnicity and access to pediatric kidney transplantation [published correction appears in Curr Opin Nephrol Hypertens 22: 502, 2013]. Curr Opin Nephrol Hypertens 22: 336–343, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy KA, Jackson JW, Purnell TS, Shaffer AA, Haugen CE, Chu NM, Crews DC, Norman SP, Segev DL, McAdams-DeMarco MA: Association of socioeconomic status and comorbidities with racial disparities during kidney transplant evaluation. Clin J Am Soc Nephrol 15: 843–851, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ismail SY, Claassens L, Luchtenburg AE, Roodnat JI, Zuidema WC, Weimar W, Busschbach JJ, Massey EK: Living donor kidney transplantation among ethnic minorities in The Netherlands: A model for breaking the hurdles. Patient Educ Couns 90: 118–124, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.