Patients with kidney failure are susceptible to coronavirus disease 2019 (COVID-19) and have a suboptimal seroconversion response to common vaccines (1). Whether patients on maintenance hemodialysis (HD) develop antibodies in response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus is not well documented. Additionally, the duration of SARS-CoV-2 viral RNA shedding among patients on maintenance HD with COVID-19 has not been extensively studied. We describe a case series of patients on maintenance HD at a single dialysis center in New York, in whom serial SARS-CoV-2 RNA and IgG antibody testing was performed.
COVID-19 was diagnosed in accordance with the Centers for Disease Control and Prevention guidelines (2). Surveillance swabs were performed once on all asymptomatic patients on HD. Patients with a positive SARS-CoV-2 RNA on nasal or nasopharyngeal specimen were considered to have confirmed COVID-19. We could not distinguish the location of the specimen sampling as this information was not included in the collection labels. Patients who presented to the HD center with signs or symptoms suggestive of COVID-19 were tested for SARS-CoV-2 RNA (Cepheid or Abbot SARS-CoV-2 RNA assay). Those with confirmed COVID-19 were subsequently retested longitudinally for SARS-CoV-2 RNA to document clearance of the virus. SARS-CoV-2 IgG antibody testing was performed on patients on HD with confirmed COVID-19 (Abbot IgG nucleocapsid antibody test, reference range for antibody positivity is >1.39). Serial SARS-CoV-2 IgG antibody tests were performed at 2-week intervals for a total of 4 weeks following the initial antibody test. The study was granted an exemption by the Institutional Review Board committee at the James J. Peters Veterans Affairs Medical Center.
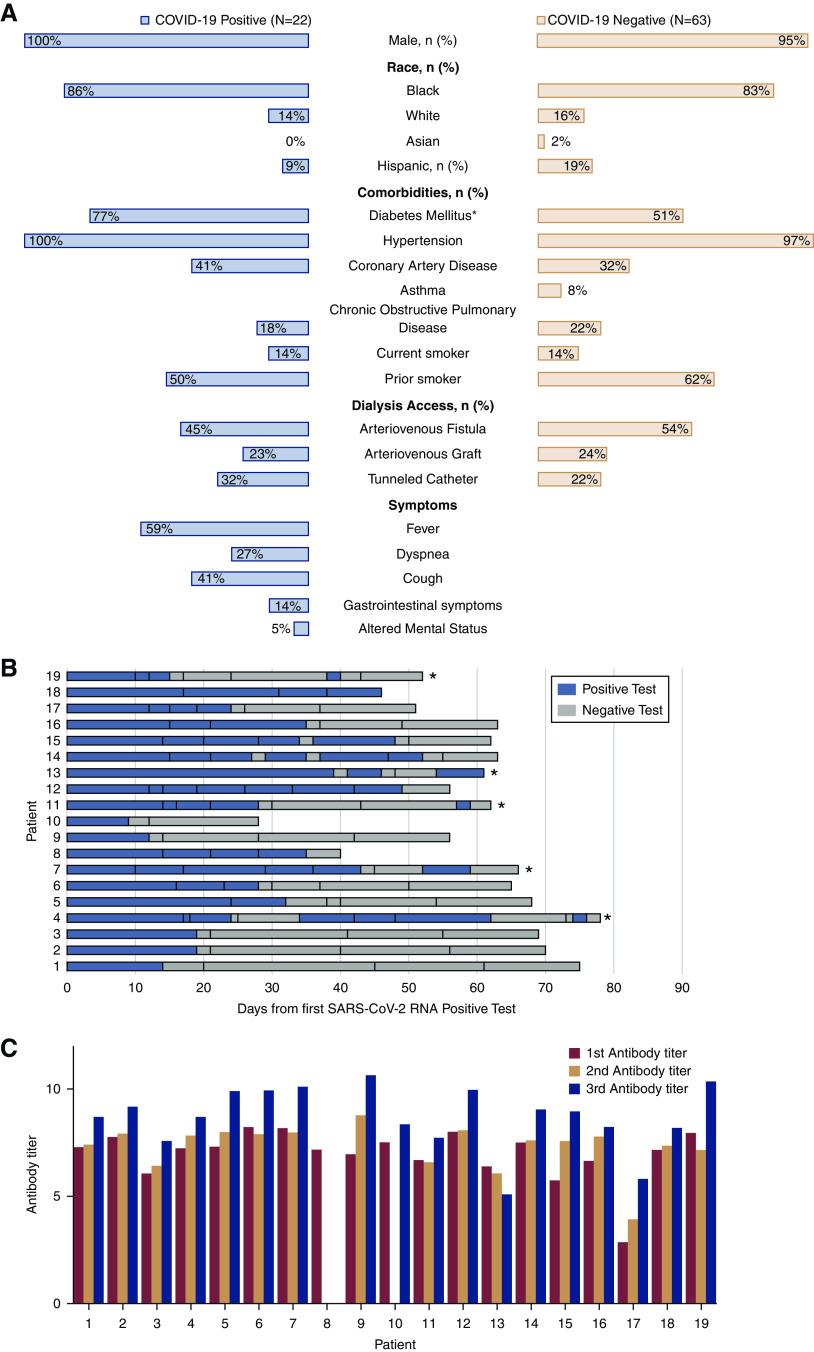
Of the 85 patients on maintenance HD at this center, 26% (22 of 85) were diagnosed with COVID-19. All patients, except one, presented to the HD center with signs or symptoms of COVID-19. One patient was asymptomatic and diagnosed with COVID-19 on surveillance SARS-CoV-2 RNA swab. Patient characteristics are presented in Figure 1A. Of the 22 patients diagnosed with COVID-19, 64% (14 of 22) required hospitalization, and five patients required admission to the intensive care unit; 27% (six of 22) of patients died, five deaths occurred during hospitalization, and one patient died following hospital discharge. Death occurred after a median of 31.5 days (range, 6–80) following the diagnosis of COVID-19. Three of the patients who were COVID-19 positive died before repeat PCR or initial antibody testing could be performed.
Figure 1.
Hemodialysis patients with coronavirus disease 2019 (COVID-19) have prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral RNA shedding and mount an IgG antibody response. (A) Butterfly plot of patient characteristics in patients on hemodialysis who tested positive and tested negative for COVID-19. *Statistical significance with P=0.05. (B) Results of serial SARS-CoV-2 RNA testing. Vertical lines within the bars indicate when each test was performed. Blue bars indicate the test was positive, whereas gray bars indicate the test was negative. *Patients who tested positive again following two consecutive negative swabs. (C) Serial antibody titer data by patient.
Additionally, 86% (19 of 22) had repeat SARS-CoV-2 RNA testing after a median of 14 days (range, 9–39) following the initial positive PCR test (Figure 1B); 68% (13 of 19) of patients continued to test positive for SARS-CoV-2 RNA on repeat testing after 20 days, and 32% (six of 19) of patients continued to test positive 40 days after the first SARS-CoV-2–positive test. Two patients died with positive SARS-CoV-2 RNA tests, and the remaining 17 patients had two consecutive negative swabs at a median of 30 days (range, 12–56). Five patients tested positive for SARS-CoV-2 RNA on repeat testing despite being negative on two prior consecutive nasal or nasopharyngeal specimens after a median of 52 days (range, 38–74) following the COVID-19 diagnosis. Four of five of these patients were asymptomatic at the time of the repeat positive test, and one patient was hospitalized and ventilator dependent.
IgG antibody test was performed on 86% (19 of 22) of patients after a median of 35 days (range, 17–48) following the diagnosis of COVID-19. All 19 patients tested positive for IgG antibody on the initial test, with a median antibody titer of 7.2 (interquartile range [IQR], 6.6–7.8). Serial IgG antibody testing was performed at 2- and 4-week intervals following the initial antibody test in 18 of 19 surviving patients, and the median antibody titers were 7.6 (IQR, 7.15–7.92) and 8.95 (IQR, 8.22–9.93), respectively (Figure 1C). One patient died before serial antibody testing could be performed. Antibodies were positive after a median of 63 days (range, 46–81) following COVID-19 diagnosis in all patients. There was no significant difference between median antibody titers of patients who required hospitalization and those who did not: 7.4 (IQR, 6.41–7.95) versus 7.86 (IQR, 7.59–8.15; P=0.18). In 37% (seven of 19) of patients, the SARS-CoV-2 RNA was detectable despite the presence of IgG antibody.
The significance of prolonged viral RNA shedding among patients on HD with COVID-19 who have symptomatically recovered and have developed SARS-CoV-2 IgG antibody remains unknown (3) (van Kampen JJA, van de Vijver DAMC, Fraaij PLA, Haagmans BL, Lamers MM, Okba N, van den Akker JPC, Endeman H, Gommers DAMPJ, Cornelissen JJ, Hoek RAS, van der Eerden MM, Hesselink DA, Metselaar HJ, Verbon A, de Steenwinkel JEM, Aron GI, van Gorp ECM, van Boheemen S, Voermans JC, Boucher CAB, Molenkamp R, Koopmans MPG, Geurtsvankessel C, van der Eijk AA: Shedding of infectious virus in hospitalized patients with coronavirus disease-2019 (COVID-19): Duration and key determinants. medRxiv, 2020 10.1101/2020.06.08.20125310). Prolonged viral RNA shedding could have a substantial effect on the workflow of the HD center. Defining when the shed viral particles are no longer infectious is, therefore, a crucial unmet need. A recent study reported IgG antibody seroconversion in 100% (six of six) patients on HD with COVID-19 (4). This is consistent with our findings where all patients on maintenance HD with COVID-19 developed SARS-CoV-2 IgG antibody. The development of IgG antibody by all of the patients on HD in response to COVID-19 is encouraging, but whether the IgG antibodies persist or if they confer immunity, protecting patients against future SARS-CoV-2 infection, remains to be seen.
Disclosures
All authors have nothing to disclose.
Funding
None.
Acknowledgments
We acknowledge the hemodialysis staff at the James J. Peters Veterans Affairs Medical Center who were involved in the care of patients during the COVID-19 pandemic.
K. Campbell, L. Chan, A. Shaikh, and E. Zeldis designed the study, analyzed the data, and drafted the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Soni R, Horowitz B, Unruh M: Immunization in end-stage renal disease: Opportunity to improve outcomes. Semin Dial 26: 416–426, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention : Coronavirus Disease 2019 (COVID-19), 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed August 1, 2020
- 3.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C: Virological assessment of hospitalized patients with COVID-2019. Nature 581: 465–469, 2020. [DOI] [PubMed] [Google Scholar]
- 4.De Vriese AS, Reynders M: IgG antibody response to SARS-CoV-2 infection and viral RNA persistence in patients on maintenance hemodialysis. Am J Kidney Dis 76: 440–441, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]