Abstract
Endozoochory, a mutualistic interaction between plants and frugivores, is one of the key processes responsible for maintenance of tropical biodiversity. Islands, which have a smaller subset of plants and frugivores when compared with mainland communities, offer an interesting setting to understand the organization of plant–frugivore communities vis‐a‐vis the mainland sites. We examined the relative influence of functional traits and phylogenetic relationships on the plant–seed disperser interactions on an island and a mainland site. The island site allowed us to investigate the organization of the plant–seed disperser community in the natural absence of key frugivore groups (bulbuls and barbets) of Asian tropics. The endemic Narcondam Hornbill was the most abundant frugivore on the island and played a central role in the community. Species strength of frugivores (a measure of relevance of frugivores for plants) was positively associated with their abundance. Among plants, figs had the highest species strength and played a central role in the community. Island‐mainland comparison revealed that the island plant–seed disperser community was more asymmetric, connected, and nested as compared to the mainland community. Neither phylogenetic relationships nor functional traits (after controlling for phylogenetic relationships) were able to explain the patterns of interactions between plants and frugivores on the island or the mainland pointing toward the diffused nature of plant–frugivore interactions. The diffused nature is a likely consequence of plasticity in foraging behavior and trait convergence that contribute to governing the interactions between plants and frugivores. This is one of the few studies to compare the plant–seed disperser communities between a tropical island and mainland and demonstrates key role played by a point‐endemic frugivore in seed dispersal on island.
Keywords: community phylogenetics, insular communities, Narcondam Hornbill, plant–frugivore interactions, seed dispersal
In this paper, we have examined the relative influence of functional traits and phylogenetic relationships on the plant–seed disperser interactions on an island and a mainland site. In the natural absence of key frugivore groups (bulbuls and barbets) on the island, we found the largest frugivore, a point‐endemic Endangered Hornbill played a pivotal role in seed dispersal on the island. Island‐mainland comparison revealed that in the island plant–seed disperser community neither phylogenetic relationships nor functional traits (after controlling for phylogenetic relationships) were able to explain the patterns of interactions between plants and frugivores on the island or the mainland, which is likely influenced by foraging behaviour plasticity and trait convergence.

1. INTRODUCTION
Endozoochory, a mutualistic interaction between plants and frugivorous animals, represents a critical stage in plant regeneration and contributes to the maintenance of tree diversity in tropics (Terborgh et al., 2002). The community of fleshy‐fruited plants and seed dispersers form a network of bipartite interactions. These networks could be influenced by different ecological (e.g., functional traits, phenological overlap) and evolutionary (e.g., co‐evolution) processes (Guimarães et al., 2011; Vázquez et al., 2009) whose relative roles are yet to be sufficiently investigated.
Shared evolutionary history can play a role in governing the interactions between plants and frugivores. Traits relevant to the seed dispersal such as beak width have been demonstrated to exhibit a phylogenetic signal (Rezende et al., 2007). On the other hand, trait convergence could result in unrelated species interacting with similar partners (Peralta, 2016). Thus, interactions could be influenced by trait complementarity—which itself could be modulated by trait conservatism—or by trait convergence (Bastazini et al., 2017; Rezende et al., 2007).
To assess the relative roles of functional traits and their phylogenetic history in determining patterns of ecological interactions, Bastazini et al. (2017) present an ecophylogenetic approach which outlines four scenarios based on the relationship between the degree of phylogenetic conservatism in species traits and their phylogenetic history. The four scenarios enable determining whether the interactions between plants and frugivores are (a) mediated by traits (with no trait conservatism), (b) mediated by phylogeny (with trait conservatism), (c) mediated by phylogeny (with no trait conservatism), or (d) mediated by both traits and phylogeny (with some trait conservatism) (see Figure 1 in Bastazini et al. (2017) for additional details).
FIGURE 1.

A pair of Narcondam Hornbill Rhyticeros narcondami on Caryota mitis palm with ripe fruits. Male has a red head while the female has a black head. Narcondam Hornbill is a point‐endemic hornbill restricted to the 6.8 km2 Narcondam Island in the Andaman Sea, India. Acrylic on canvas by Sartaj Ghuman
The influence of phylogenetic component would indicate the role of evolutionary processes like co‐evolution in the organization of the community, while the influence of functional component controlling for phylogenetic history would imply trait complementarity. An integrated approach that examines the role of evolutionary processes in tandem with ecological processes thus enables an understanding of the relative roles of these factors in patterns of community assembly. However, while the substantial phylogenetic data now available has improved our understanding of community assemblages, the complementary empirical data that would allow for an integrated approach have been lacking.
Despite zoochory being a pervasive interaction in the wet tropics of Asia, there is limited information on plant–disperser communities from the mainland sites in the region (Corlett, 2017) and very poor information from oceanic islands in the Indian Ocean (Escribano‐Avila et al., 2018). Most of the information on plant–disperser communities worldwide comes from mainland sites with few studies from oceanic islands and even few studies set in an island‐mainland comparative framework (Schleuning et al., 2014).
Given this background, we examined the organization of the plant–disperser community on a remote and small tropical island (Narcondam Island) in South Asia that is home to a point‐endemic hornbill (Figure 1) using the network and ecophylogenetic approaches (Figure 2a). The Narcondam island is characterized by a species‐poor frugivore assemblage, which also enabled us to examine the relative quantitative role of different species in seed dispersal.
FIGURE 2.
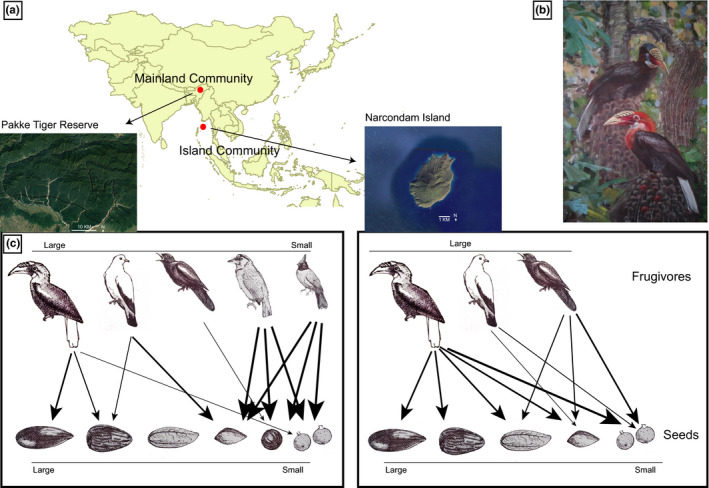
Schematic illustrating mainland and island plant and seed dispersal communities. (a) map of the South and Southeast Asia showing mainland and island site, (b) the island endemic large‐bodied frugivore Narcondam Hornbill Rhyticeros narcondami on Caryota mitis, (c) representative schematic showing organization of plant–seed disperser community on mainland and island. Mainland community is more diverse and symmetric as compared to the island community. On mainland, due to the presence of great diversity of small‐bodied frugivores which are more efficient in removal of small fruits, large‐bodied frugivores may predominantly feed on large‐seeded plants. On the island, in the absence of small‐bodied frugivores, large‐bodied frugivores will start feeding on small fruits resulting in dietary shifts and more dominant role in the community as indicated by the thickness of arrows. Dietary changes will in turn result in changes in interaction frequencies with corresponding changes in network structure. Painting and sketches by Sartaj Ghuman. Map source: Google Earth
In the Asian tropics, small‐bodied frugivores like bulbuls and barbets are known to play key roles in seed dispersal (Naniwadekar et al., 2019). However, the Narcondam Island does not have any bulbuls and barbets and most of the dominant frugivores on the island are medium‐ and large‐bodied avian frugivores (>100 g; Figure 2c). This offers an interesting system to understand the pattern of interactions in absence of key frugivore groups. We compared this island community with a mainland community from north‐east India with which it shares several frugivore and plant species.
The specific aims of the study were to (a) identify key seed dispersers and plant groups on an oceanic island community by comparing species‐level properties (degree, species strength and weighted betweenness) across different species, (b) examine the influence of abundance and morphological traits on species strength of plants and frugivore species, (c) compare the network‐level properties (web asymmetry, connectance, specialization, and weighted nestedness) of the island community with a mainland community, and (d) examine the influence of phylogenetic and functional component (fruit and seed size for plants and body size and degree of frugivory for frugivores) on the distribution of interactions between plants and frugivores on the island and mainland communities. This system allowed us to explicitly examine the change in the role of large‐bodied frugivores in plant–seed disperser communities in the absence of the important small‐bodied frugivores in Asian tropics (Figure 2c). We hypothesized that large‐bodied frugivores, unhindered by morphological constraints, will demonstrate dietary shifts on islands in the absence of small‐bodied frugivores and will feed on fruits which they typically do not feed on in the mainland site. This will alter the organization of the plant–seed disperser community on the island as compared to mainland.
2. MATERIALS AND METHODS
2.1. Study area
The study was carried out on a small (area: 6.8 km2), volcanic island, the Narcondam Island (13°30′N, 94°38′E), in the Andaman Sea in India. The island is part of the Indo‐Myanmar Biodiversity Hotspot (Myers et al., 2000). Three distinct vegetation types on the island include the narrow strip of littoral forests, forests dominated by deciduous species in the north‐eastern part of the island and evergreen forests in most of the island (Page et al. in prep.). There is no published information on fruiting phenology of woody plants from the Narcondam Island. Ongoing long‐term phenology work from Andaman Islands indicates a peak in fruiting between November to February (LEMoN, 2019).
The Endangered Narcondam Hornbill Rhyticeros narcondami is found only on the Narcondam Island (BirdLife International, 2017). Around 50 species of birds have been reported from the island (Raman et al., 2013). There are two terrestrial mammals on the island, including a shrew (Corcidura sp.) and an invasive rat (Rattus sp.). There are at least four species of bats on the island including a fruit bat species (Pteropus hypomelanus). Narcondam Hornbill (body mass: 600–750 g) is the largest frugivore on the island. Additional details of study area are in ESM Online Resource 1.
2.2. Frugivore and plant abundance
We conducted the study between December 2019 and February 2020. We used variable‐width line‐transect surveys to estimate the density and abundance of birds on Narcondam Island (Thomas et al., 2010). We walked 20 unique trails on the island (ESM Online Resource 2: Figure S1). We divided the entire elevation gradient into three elevation zones: low (0–200 m), mid (200–400 m) and high (400–700 m) based on topography, vegetation structure, and composition. One to three observers walked trails in the mornings and afternoons. During the trail walks, we recorded species identity, the number of individuals, and the perpendicular distance of the sighting to the trail. The trail lengths varied between 0.22 and 1.26 km, and the total sampling effort was 51.4 km (ESM Online Resource 2: Table S1).
We laid 49 belt transects (50 m × 10 m) across the three different elevation zones (low: 18, mid: 14, high: 17) to estimate the richness and abundance of woody plants on the Narcondam Island. Within the belt, we enumerated all wood plants that were ≥10 cm GBH (girth at breast height) along with their girth and taxonomic identity. We measured girths of all woody plants ≥10 cm GBH within our plots. This allowed us to compare the abundance of hornbill food plants at Narcondam with different studies that have measured girths of woody plants at a cut‐off ≥10 cm GBH.
2.3. Plant–frugivore interactions
To systematically document plant–frugivore interactions, we performed spot sampling during our trail walks, and opportunistically outside our trail walks following Palacio et al. (2016). Whenever we encountered a frugivore on a fruiting tree, we recorded the species identity, number of frugivores, and frugivore foraging behavior (fruits swallowed, pecked or dropped) to ascertain whether the frugivore was a seed disperser or not. Plant–frugivore interaction information was systematically collected outside trail sampling whenever frugivore was detected on fruiting trees. To determine if we were missing documenting interactions of frugivore species with low detection probability through spot sampling, we systematically observed fruiting trees. We observed 40 fruiting individuals of 12 species following Naniwadekar et al. (2019) (ESM Online Resource 2: Table S2). Additional details of the tree watches are in ESM Online Resource 1. We measured widths of at least five fruits each of 23 species of fleshy‐fruited plants following Naniwadekar et al. (2019) (ESM Online Resource 2: Table S3). Plants were classified as small‐seeded (seed width: <5 mm), medium‐seeded (seed width: 5–15 mm), and large‐seeded (>15 mm) following Naniwadekar et al. (2019) (ESM Online Resource 2: Table S3).
2.4. Analysis
2.4.1. Frugivore and plant abundance
We estimated densities of perched birds with more than 40 visual detections using the R package “Distance” (Miller et al., 2019; Thomas et al., 2010). We estimated combined density of two Imperial‐pigeon species (Pied Imperial‐Pigeon Ducula bicolor and Green Imperial‐Pigeon Ducula aenea), since we had 45 detections overall. Additional details of the analysis are provided in the ESM Online Resource 1. We estimated the overall tree density and basal area (m2/ha), and density of 27 species of fleshy‐fruited plants that were fruiting during the study period.
2.4.2. Island plant–seed disperser community
We created a matrix of plants and frugivores with the frequency of interactions documented for each plant–frugivore combination through spot sampling. Frequency of interactions is the number of times a bird species was observed feeding on a plant species. Every time an individual bird was seen feeding on a plant, it was considered as one interaction irrespective of the number of fruits the bird ate during that interaction. We only considered those animals as seed dispersers which we had seen swallowing the seeds. We did not consider Alexandrine Parakeet Psittacula eupatria in the analysis as we documented the parakeets predating on seeds or feeding on unripe fruits as has also been documented elsewhere (Shanahan et al., 2001). We plotted the interaction accumulation curve using the “random” method and 1,000 permutations as implemented in the R package “vegan” to determine if we had adequately documented the diversity of unique plant–seed disperser interactions (Oksanen et al., 2019).
To determine the relative roles of species in the organization of plant–seed disperser communities, we examined species‐level properties like the degree, species strength, and weighted betweenness centrality. Degree is defined as number of mutualistic partners of the focal species, species strength is the sum of level of dependencies of all the partner species on the focal species, and betweenness centrality is a useful measure to identify connector species which connects multiple guilds in a community (Dormann et al., 2008; Martín González et al., 2010). We used the R package “bipartite” for this analysis (Dormann et al., 2008).
We used the general linear model to examine the influence of plant abundance (estimated using the vegetation plots) and fruit type (small‐seeded, figs, medium‐seeded and large‐seeded) on species strength of the 27 plant species that were fruiting during the study period. During exploratory analysis, we noticed that figs consistently had higher species strength values. Since certain fig groups are known to be keystone species in tropical forests and provide key nutrients (Shanahan et al., 2001), we performed the analysis with figs as a separate category. Species strength was log10 transformed to approximate normality. In the case of frugivores, since there were only seven species and species strength values were not normally distributed, we used Spearman's ρ to examine the correlation between abundance (encounter rate km−1) of frugivores and body mass with species strength. We obtained body mass information from Wilman et al. (2014). To determine if the island plant–seed disperser community was organized into distinct sub‐communities, we used the modularity analysis. Higher modularity may make the community more resilient to perturbations (Tylianakis et al., 2010). We calculated modularity (Q) using the QuanBiMo algorithm as implemented by the package “bipartite” in R following Dormann and Strauss (2014) . Significance of Q was determined by evaluating the standardized z‐scores obtained from null model expectations generated with r2dtable algorithm as implemented by package “bipartite” in R following Dormann and Strauss (2014).
2.5. Comparing the island and the mainland plant–seed disperser communities
To determine the differences in the organization of plant–seed disperser communities between island and mainland, we compared plant–disperser communities between the Narcondam Island with that of Pakke Tiger Reserve in north‐east India. Information on plant–seed disperser communities is limited from the Asian tropics. However, detailed information on interactions between 47 avian frugivores and 43 plant species is available from Pakke Tiger Reserve in north‐east India (Naniwadekar et al., 2019). Pakke is part of the Eastern Himalaya Biodiversity Hotspot. It is about 1,500 km straight line distance north from the Narcondam Island. Unlike the island community which is relatively young, the mainland site is several million years old. Representative species of all the frugivore genera found on the Narcondam Island are also found in Pakke with species like the Green Imperial‐Pigeon, Asian Koel Eudynamys scolopaceus and Common Hill Myna Gracula religiosa being reported from both sites. Several genera of fleshy‐fruited plants found on Narcondam Island are also found in Pakke. We examined network‐level properties like web asymmetry, connectance (proportion of realized interactions), specialization (H 2′) and nestedness (weighted NODF) on Narcondam Island and compared it with the mainland site (Pakke). Values of web asymmetry range between −1 to 1 with negative values indicating higher diversity of plant species as compared to animal species and vice versa (Blüthgen et al., 2007). Specialization is a measure of complementary specialization of the entire network with high values indicating that the species are more selective. Weighted NODF is a quantitative measure of nestedness with high values indicating higher nestedness. Significance of these metrics was determined by evaluating the standardized z‐scores obtained from null model expectations generated with r2dtable algorithm as implemented by package “bipartite” in R.
2.6. Community assembly on island and mainland
To determine the influence of phylogeny and functional traits on plant–frugivore interactions on the mainland and island site, we used the following approach. The plant phylogeny was pruned from the megaphylogeny (Qian & Jin, 2016) for both the island and mainland communities individually. This is a time‐calibrated phylogeny with largest representation from 98.6% of families and 51.6% of all genera of seed plants. Scientific names of plants were standardized by referring to “The Plant List” (www.theplantlist.org). However, some species from Pakke and Narcondam were missing from the megaphylogeny. In those cases, we chose the closest species from the Oriental region. When we failed to find a congener, we chose a genus close to the focal genus (ESM Online Resource 2: Table S3). We used fruit width and seed size class information for the analysis (ESM Online Resource 2: Table S3). In the case of the birds, we referred to the comprehensive and time‐calibrated bird phylogeny available at www.birdtree.org (Jetz et al., 2012). We found all the focal species for the two sites in the database except Pycnonotus flaviventris, Chrysocolaptes guttacristatus, and Sitta cinnamoventris. We used congeners from the Oriental region for these species for the analysis (ESM Online Resource 2: Table S4). For the frugivores, we used body mass and percentage fruits in the diet of frugivores (degree of frugivory) obtained from Wilman et al. (2014) (ESM Online Resource 2: Table S4). We obtained 100 trees from posterior distribution of 10,000 trees based on Ericson All Species backbone phylogeny with 9,9930 OTUs for seven bird species for Narcondam and 47 species for Pakke found in the network (Jetz et al., 2012). The maximum clade credibility tree was used for further analyses as these trees did not vary substantially in topology and branch lengths. We calculated the phylogenetic distances (pairwise distances between species using their branch lengths) using “cophenetic.phylo” in the R package “ape” (Paradis & Schliep, 2018). We used Gower method to estimate pairwise distances among traits of both plants and frugivores using “vegdist” in the R package “vegan.” We then followed the novel approach outlined by Bastazini et al. (2017) to determine whether phylogenetic proximity influenced the evolution of species trait and their interactions in the network or whether species traits influence the observed pattern of interactions after removing the potential confounding effect of the phylogeny. We used a weighted matrix of interactions between plants and frugivores. It was frequency of interactions for the island site and visitation rates for the mainland site. We applied this method for Narcondam and Pakke separately. We used the R packages “SYNCSA” for this analysis (Debastiani & Pillar, 2012; Oksanen et al., 2019). We used R (ver. 3.5.3) for all the analysis (R Core Team, 2019).
In addition, we assessed the phylogenetic signal separately in trait data for birds and plants in both sites, Narcondam and Pakke. We calculated the Blomberg's K for each trait using the R package “phytools” (Blomberg et al., 2003; Revell, 2012).
3. RESULTS
3.1. Frugivore and plant abundance on island
We detected six avian frugivores during the trail walks. We had 207 visual detections of Narcondam Hornbill, 71 detections of Asian Koel, and 45 detections of the two Imperial‐Pigeon species. We had only two detections each of the Common Hill Myna and Eye‐browed Thrush Turdus obscurus. Information on detection probability and mean flock size is summarized in ESM Online Resource 2: Table S5.
Narcondam Hornbill was the most common frugivore on the island and its abundance (mean (95% CI): 1,026 birds (751–1402)) was almost four times that of the Asian Koel (Figure 3a; ESM Online Resource 1: Table S5). The estimated overall mean (95% CI) density of the Narcondam Hornbill was 151 (110–206) birds/km2. The mean densities of the Narcondam Hornbill varied between 101–168 birds/km2 across the three elevation zones with the lowest mean densities in the highest elevation band (Figure 3b) (ESM Online Resource 2: Table S5).
FIGURE 3.
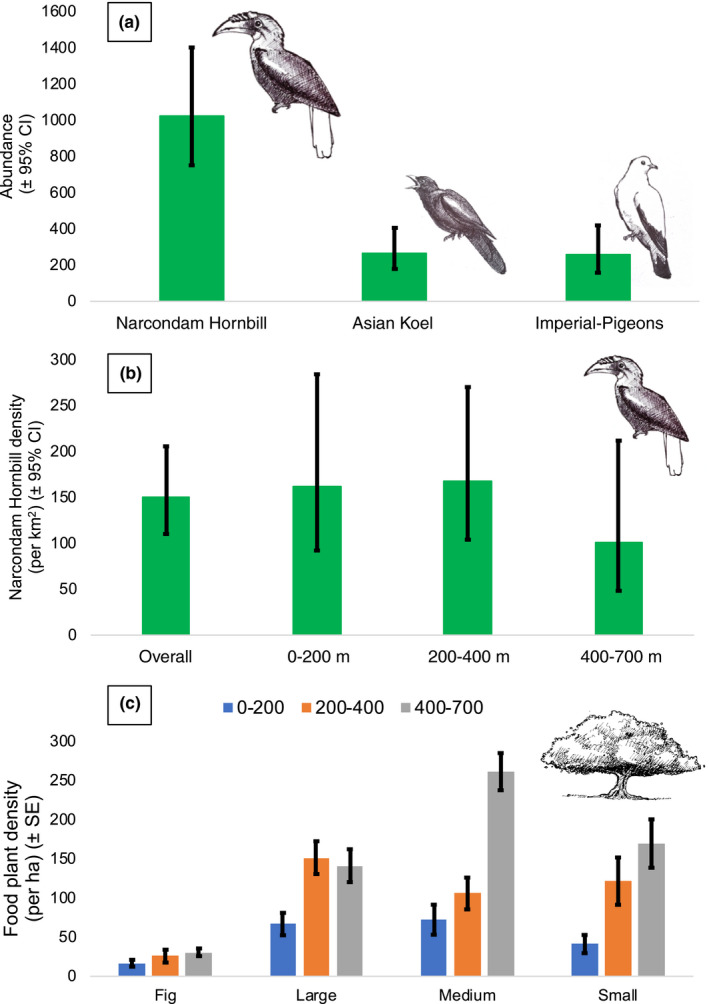
Abundance of Narcondam Hornbill Rhyticeros narcondami, Asian Koel Eudynamys scolopaceus and Imperial‐Pigeons (Pied Imperial‐Pigeon Ducula bicolor and Green Imperial‐Pigeon Ducula aenea) on Narcondam Island (a). Density (km−2) of Narcondam Hornbill on the island and across the three elevation zones on the island (b). Density (ha−1) of 27 species of fleshy‐fruited plants that were fruiting across the three elevation zones during the study period (c)
The mean (±SE) density and basal area of all the woody plants (≥10 cm GBH) was highest in the 0–200 m elevation band (density: 2091.1 ± 124.4 plants/ha; basal area (m2/ha): 58.3 ± 5.3), followed by 200–400 m elevation band (density: 1505.7 ± 101.1 plants/ha; basal area (m2/ha): 49.1 ± 3.0) and least in the 400–700 m elevation band (density: 1,311.8 ± 82.7 plants/ha; basal area (m2/ha): 39.2 ± 4.2). However, the mean abundance of the different categories of the frugivore food plant species (figs, small‐seeded nonfig plants, medium‐seeded plants, and large‐seeded plants), which were fruiting during the study period, was highest in the high elevation band (400–700 m), followed by middle elevation band and lowest in the low elevation band (0–200 m; Figure 3c).
3.2. Island plant–seed disperser community
We documented 752 interactions between seven frugivore species and 27 plant species during the spot scans performed on trail walks and opportunistically. We documented all the frugivore species (except Andaman Green Pigeon Treron chloropterus) previously reported from the island. We documented the migrant Daurian Starling Agropsar sturninus, for the first time. During spot scans on trail walks, we documented 262 interactions between six frugivore species and 19 plant species. During opportunistic spot scans, we documented 490 interactions between seven frugivore species and 26 plant species. Narcondam Hornbill was documented feeding on 22 plant species, followed by Asian Koel, which was documented feeding on 16 plant species (ESM Online Resource 2: Table S6). Of the 752 interactions, 533 were of the Narcondam Hornbill, 111 were of the Asian Koel, 36 of the Green Imperial‐Pigeon, 34 of the Pied Imperial‐Pigeon, 25 of the Common Hill Myna, 12 of the Eye‐browed Thrush and one of the Daurian Starling. Among the 27 plant species, eight species were represented by Ficus. We observed 282 interactions on Ficus rumphii, 80 on Ficus glaberrima, 54 on the small‐seeded Aidia densiflora and 50 on the large‐seeded Caryota mitis. We documented 64 unique interactions between plants and frugivores on the island during the study period. The interaction accumulation curve indicated that we had adequately documented interaction diversity (ESM Online Resource 2: Figure S2).
During tree watches, we documented 684 interactions (Narcondam Hornbill: 481, Asian Koel: 167, Pied Imperial‐Pigeon: 23, Green Imperial‐Pigeon: 7, Eye‐browed Thrush: 5, Common Hill Myna: 1), between 11 plant species and six frugivore species. We did not document any interaction during tree watches which we had not documented during the spot scans. For eight of the 11 tree species, we observed greater diversity of frugivores foraging on the particular plant species during spot sampling as compared to tree watches.
Ficus rumphii (among plants) and Narcondam Hornbill (among frugivores) had the highest degree, species strength and weighted betweenness centrality highlighting the central role they played in the community during the study period (ESM Online Resource 2: Table S6). General linear model indicated that the species strength in plants was influenced by plant type (figs and nonfig small‐, medium‐ and large‐seeded plants) and not by plant abundance (Adj. R2 = 0.40, F 4,22 = 5.322, p = .004) (Figure 4a; ESM Online Resource 2: Table S7). Ficus were different from other small‐seeded plants and had higher species strength values (mean (± SE) = 0.739 (± 0.101), n = 8 species) as compared to small‐seeded (mean (± SE) = 0.0816 (± 0.01), n = 9 species), medium‐seeded (mean (± SE) = 0.0376 (± 0.01), n = 6 species) and large‐seeded plant species (mean (± SE) = 0.0328 (± 0.01); n = 4 species) (Figure 4a). Among frugivores, species strength was significantly and positively correlated to their abundance (Spearman's ρ = 0.89, p = .0123, n = 7 species; Figure 4b) and not significantly correlated to their body mass (Spearman's ρ = 0.71, p = .088, n = 7).
FIGURE 4.
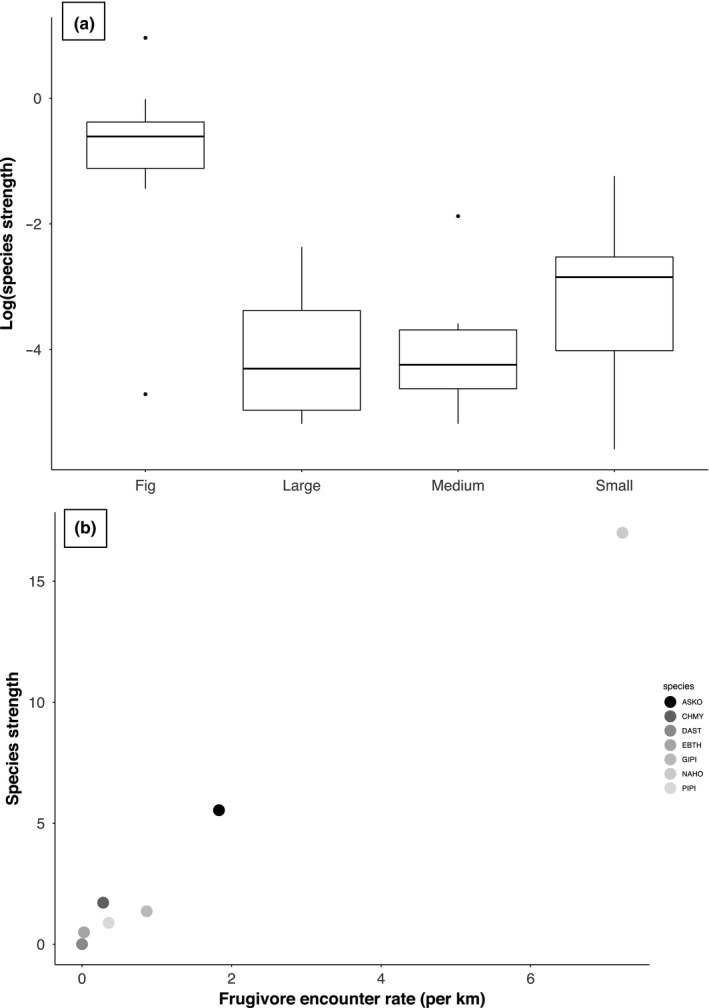
Relationship between species strength (log10 transformed in the case of plants) and plant type (a) and frugivore abundance (b). We separated figs and small‐seeded plants for they consistently had higher species strength values. Frugivore codes—ASKO, Asian Koel; CHMY, Common Hill Myna; DAST, Daurian Starling; EBTH, Eye‐browed Thrush; GIPI, Green Imperial‐Pigeon; NAHO, Narcondam Hornbill; PIPI, Pied Imperial‐Pigeon
The observed community was significantly more modular than expected by random (Q = 0.17, z = 13.3). The observed community was subdivided into three modules (Figure S4). One of the four modules comprised of the Narcondam Hornbill and different small‐ and medium‐ and all the four large‐seeded plants including Endocomia macrocoma, Caryota mitis, Pycnarrhena lucida, and Planchonella longipetiolata highlighting hornbill's importance for large‐seeded plants (Figure S4).
3.3. Comparing the island and the mainland plant–seed disperser communities
The island network was more asymmetric (−0.588) as compared to the mainland site (0.044). The observed values of connectance and nestedness on Narcondam were almost twice that of the mainland site and specialization (H 2′) was almost half that of the mainland site (Table 1). The observed network on Narcondam was less connected and less weighted than the null model predictions but it was more specialized than expected by random (Table 1).
TABLE 1.
Network‐level properties (observed and null model values) for the Narcondam Island and observed values for the mainland site, Pakke Tiger Reserve
| Observed value: Narcondam | Null mean: Narcondam | Null SD: Narcondam | Observed value: Mainland | |
|---|---|---|---|---|
| Connectance | 0.339 | 0.463 | 0.018 | 0.154 |
| Specialization (H 2′) | 0.233 | 0.144 | 0.009 | 0.500 |
| Weighted NODF | 45.178 | 59.76 | 3.834 | 24.885 |
Null model values for the mainland site can be found in Naniwadekar et al. (2019).
3.4. Community assembly on island and mainland
Among woody plants, we detected a strong phylogenetic signal for seed size and fruit width in Pakke Tiger Reserve (seed size: Blomberg's K = 0.73, p = .001; fruit width: Blomberg's K = 0.43, p = .005) and Narcondam (seed size: Blomberg's K = 1.48, p = .001; fruit width: Blomberg's K = 0.45, p = .027). Among frugivores, we detected a phylogenetic signal for the body mass of frugivores in Pakke (Blomberg's K = 1.22, p = .012) and Narcondam Island (Blomberg's K = 1.28, p = .043). We did not detect phylogenetic signal in the percentage of fruits in the diet of the frugivores of Pakke (Blomberg's K = 0.23, p = .38) and Narcondam (Blomberg's K = 0.90, p = .08). We did not find any significant correlation for the four models indicating that neither the functional nor the phylogenetic component was able to explain the observed interaction patterns between plants and frugivores in both island and mainland sites (Table 2 and Figure 5).
TABLE 2.
Correlation coefficients for the two models that evaluate the influence of the functional (after removing phylogenetic signal) and phylogenetic components on the observed patterns of interactions between plants and frugivores for the mainland site (Pakke Tiger Reserve) and the island site (Narcondam Wildlife Sanctuary)
| Parameter | ρ (Mainland) | ρ (Island) |
|---|---|---|
| Functional component (after removing phylogenetic signal) | 0.04 | 0.03 |
| Phylogenetic component | 0.023 | −0.17 |
None of the values were significant at p = .05.
FIGURE 5.
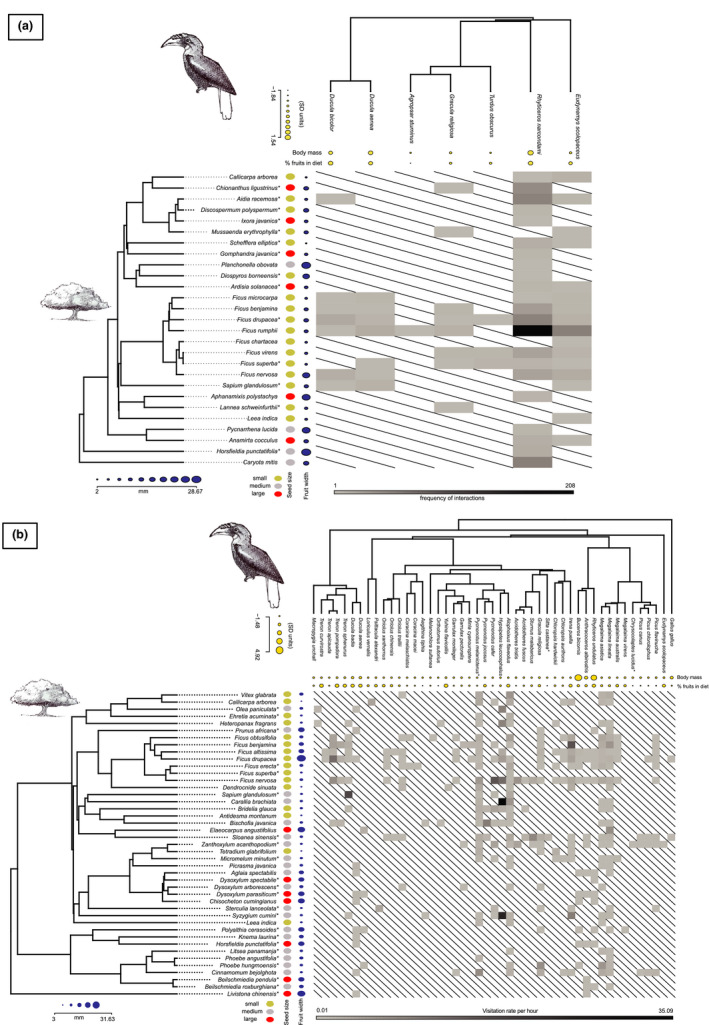
Seed dispersal interactions in Narcondam Island (a) and the mainland site, Pakke Tiger Reserve (b). Also shown is the pruned phylogeny for the plants and the frugivores and respective traits for plants (seed size and fruit width) and frugivores (body mass and percentage diet that is comprised of fruits). The matrix is weighted in both the island and mainland case and it has frequency of interactions for the island site and visitation rate of frugivores (h−1) for the mainland site shown by the gray bar below the matrix. *Species for which a congener or allied genus was used in the constructing the phylogenetic tree
4. DISCUSSION
In this study, we demonstrate the role of large‐bodied frugivores, especially the endemic Narcondam Hornbill, in contributing disproportionately to seed dispersal in the absence of key small‐bodied frugivore groups on the island. Narcondam Hornbill plays a central role in the plant–dispersal community as it is super‐abundant, has very high species strength and betweenness centrality values and is critical for the select large‐seeded plants fruiting. This study also demonstrates the key role played by figs in the plant–seed disperser community as demonstrated by its very high species strength values as compared to other plant types. In comparison to the mainland community, the island community was more asymmetric, connected, nested and less‐specialized. We have documented trait convergence and dietary shifts in frugivores which potentially contribute to the diffused nature of plant–seed disperser interactions. This also likely contributes to the absence of the role of phylogenetic or functional component in the assembly of plant–seed disperser communities on mainland or the island. This is one of the few studies from oceanic islands of the Indian Ocean and demonstrates how the largest frugivore, which is a point‐endemic, plays a critical quantitative role in seed dispersal. This is also one of the few studies that performed a paired comparison between an island and mainland community.
4.1. Narcondam for the hornbill and by the hornbill
There is no other place in the world where hornbills are reported to occur in such high densities as on Narcondam Island (151 birds/km2). On Sulawesi, the Knobbed Hornbills Rhyticeros cassidix occur in very high densities of up to 84 birds/km2 (Kinnaird et al., 1996). In north‐east India, Wreathed Hornbill Rhyticeros undulatus, a close relative of the Narcondam Hornbill, seasonally occur in densities of up to 68 birds/km2 (Naniwadekar & Datta, 2013). Our density estimates are similar to densities estimated by previous studies (Manchi, 2017; Raman et al., 2013). Reduction in hunting pressures may have resulted in the recovery of Narcondam Hornbill population from around 300–400 birds in early 2000s following the suggestions by Sankaran (2000). Among other frugivores, there is limited information on the density of the Asian Koel from other sites, but comparison with existing studies show that Narcondam has very high densities of as compared to other sites (Riley, 2001). The mean Imperial‐Pigeon densities are slightly lower than those reported from a mainland site in north‐east India (Dasgupta & Hilaluddin, 2012).
In this study, multiple measures (abundance and different network metrics—species strength, degree, betweenness, and modularity) indicate that Narcondam Hornbills are keystone seed dispersers on the island. Generally, as the body mass increases, the abundance of the frugivore and the frequency of visitation is found to decline (Godínez‐Alvarez et al., 2020). Interestingly, the Narcondam Island is unique where the largest frugivore, the Narcondam Hornbill, is also the most abundant frugivore on the island. Abundant species have been reported to influence their partners strongly and Narcondam Hornbill had the highest species strength values highlighting its importance for plants (Vázquez et al., 2007). Larger frugivores, like hornbills, are known to remove more fruits per visit as compared to other smaller frugivores (Naniwadekar et al., 2019). Highest number of interactions of the Narcondam Hornbill coupled with potentially higher fruit removal rates point toward the key quantitative role played by the hornbill on the island. Modularity analysis revealed that the hornbill was the key seed disperser for the large‐seeded plants as has been demonstrated in previous studies (Gopal et al., 2020; Naniwadekar et al., 2019). The hornbill was the only frugivore that has high betweenness centrality value pointing toward its vital role as a connector between different guilds within the community. This high reliance on a frugivore and the potentially low redundancy highlights the fragility of the island ecosystem because of strong reliance on a single frugivore.
Narcondam has very high densities of hornbill food plants, which likely explains the high hornbill densities. The overall fig density on Narcondam Island was 27 trees/ha (≥30 cm GBH). This is very high as compared to other sites in the world where the densities of hemi‐epiphytic figs are typically <1 tree/ha (Harrison, 2005). Ficus (overall) densities were 1.4 trees/ha at a site in the southern Western Ghats (Page & Shanker, 2020). Ficus densities of up to 10 trees/ha have been reported in Sulawesi (Kinnaird et al., 1996). Consistently across the mainland site and the island site, certain species of Ficus attracted the highest diversity of frugivores. On the island, Ficus represented the top six species in species strength, highlighting the key role they potentially played in the plant–disperser community at least during the study period. Association between canopy, hemi‐epiphytic figs, and hornbills has been documented elsewhere (Harrison et al., 2003). Most dominant fig species during our study on Narcondam Island, which are important hornbill food plants are mostly canopy, hemi‐epiphytic figs. Being small‐seeded, they often play the pioneering role in attracting frugivores on oceanic islands and accelerating plant colonization on the island (Harrison, 2005). Figs and hornbills may have played a critical role in setting up the plant community on the island which needs further investigation.
Based on published literature on hornbill food plants from north‐east (Datta, 2001; Naniwadekar et al., 2019; Naniwadekar et al., 2015) and Thailand (Chaisuriyanun et al., 2011; Kanwatanakid‐Savini et al., 2009; Poonswad et al., 1998), we identified at least 35 species of Narcondam Hornbill food plants. Overall density of the 35 hornbill food plant species (GBH ≥ 30 cm) on Narcondam Island is 327 trees/ha. In Pakke Tiger Reserve, the density of 45 hornbill food plant species was 163 trees/ha (Datta, 2001), highlighting the hyper abundance of hornbill food plants on Narcondam Island. Interestingly, the abundance of food plants is consistently distributed across the entire elevation gradient. The hyper abundance of frugivores and their food plants makes this island a unique ecosystem.
4.2. Plant–seed disperser communities on island and mainland
Island communities are more asymmetric as compared to mainland systems due to higher immigration rates of plants (as compared to frugivores) or human‐caused frugivore extinctions on the island (Nogales et al., 2016; Schleuning et al., 2014). There are no known human‐driven extinctions on the Narcondam island. We are unlikely to find additional species of frugivores that can play an important functional role in seed dispersal on the island as our list of frugivores is comparable to the frugivores reported by the previous studies (Raman et al., 2013). We feel that dispersal limitation of frugivores could also be a key factor in the web asymmetry. Andaman and Nicobar archipelago, of which Narcondam Island is part of, is an interesting example of this. Bulbuls and barbets, which are the key frugivores on the mainland (Naniwadekar et al., 2019), are absent on the remote and tiny Narcondam Island. Endemic species of bulbuls are found on other islands in the Andaman and Nicobar archipelago, but barbets are absent from the archipelago. Thus, the variation in the dispersal ability of different frugivore groups might also play an important role in contributing toward the web asymmetry on oceanic islands. Among other network properties, higher connectance on islands has been reported elsewhere (González‐Castro et al., 2012). Forbidden links are thought to contribute toward lower connectance (Olesen et al., 2011). Additionally, connectance and nestedness are negatively correlated (Rezende et al., 2007). In our case, higher connectance is associated with higher nestedness on the island site as compared to the mainland site. Given that most of the important frugivores on the island are medium (100–500 g) or large‐bodied (>500 g) with ability to handle small and large fruits (thereby less likelihood of forbidden links), it has likely resulted in higher connectance and nestedness. Given the small assemblage of frugivores on the island, low competitive pressures and low differentiation on the foraging niche axis of the different frugivores may also result in higher connectance and nestedness.
4.3. Organization of plant–seed disperser communities
We detected contrasting phylogenetic signals in bird body mass, and seed and fruit traits on both the island and the mainland. While frugivore traits were more likely to be similar (than Brownian motion model) for closely related species, plant traits were less likely to be similar for closely related species. However, functional and phylogenetic component did not influence the network interactions in the plant–seed disperser communities on the island and mainland. The role of evolutionary history in shaping local networks of interacting species is thought to be limited (Segar et al., 2020). Unlike the plant–pollinator networks, the seed dispersal networks can be expected to be less‐specialized and diffused as unrelated species may exhibit trait convergence and forage on similar plant species (Jordano, 2000). We found that distantly related groups of frugivores dispersed seeds of a common assemblage of plants, many of which are distantly related to each other. A diverse array of frugivores disperse the super‐generalist Ficus, and even the large‐seeded plants, which otherwise have a smaller disperser assemblage, were predominantly dispersed by distant frugivore clades, like hornbills and Ducula pigeons, on the mainland site. Unlike Bastazini et al. (2017), we did not detect signal in the functional component of the plant–seed disperser communities. This is a likely consequence of the absence of trait complementarity among interacting frugivores and plants. While the small‐bodied frugivores may not be able to disperse large seeds due to morphological constraints, large‐bodied frugivores like hornbills, fed on plants that were large‐ and small‐seeded resulting in lack of trait complementarity. This finding is unlikely to be influenced by relatively short temporal window of sampling of this study given that relatively unrelated frugivores (e.g., Asian Koel and Narcondam Hornbill) fed on similar but relatively unrelated plant species and the largest frugivore fed on the large‐ and small‐seeded fruits.
Another important reason could be the behavioral plasticity shown by frugivorous birds while foraging. In the mainland site, where there are almost 50 frugivore species with a higher preponderance of small‐bodied frugivores, we rarely encountered Ducula pigeons on figs that attracted more than half of the frugivore species found in the area. On the mainland site, Ducula pigeons were mostly observed foraging on medium‐seeded and large‐seeded plants. On the island, where there are hardly any small frugivores, we mostly saw the Ducula pigeons foraging on Ficus. This behavior of Ducula pigeons feeding on figs on the island but less frequently on the mainland demonstrates phenotypic plasticity in Ducula pigeons, likely as a response to lack of competitive interactions, an aspect that has been seldom documented elsewhere. This also likely contributes to lower specialization on the island. Additionally, on Narcondam Island, we documented hornbills feeding on Callicarpa arborea, a small‐seeded species which is mostly dispersed by bulbuls and barbets on the mainland site (Naniwadekar et al., 2019). Asian Koel, which is a rare frugivore in the mainland site, was seen only once feeding on fruit there. However, on the island, it is the second‐most important frugivore. It remains to be determined, whether exploitative competition on the mainland site drives larger frugivores to feed on different resources in the mainland forested system. While habitat fragmentation and other anthropogenic threats often results in loss of large‐bodied frugivores thereby affecting large‐seeded plants, it will be interesting to investigate the implications of the absence of small‐bodied frugivore on the structuring of plant communities.
Bats are known to play an important role in seed dispersal on oceanic islands (McConkey & Drake, 2015). We recorded spat out wads of pulp of three Ficus spp., Balakata, and Planchonella. We had direct detections of foraging bats on fruiting Manilkara zapota (planted), Terminalia catappa, Ficus rumphii and Ardisia. We failed to detect foraging bats during the six night walks that lasted for at least 90 min each.
One may argue about the lack of phenological completeness of the study and its influence on the inferences drawn. The abundances of the frugivores are unlikely to change since it is a small and remote island in the middle of the Andaman Sea. Narcondam Hornbill is likely to remain the most abundant frugivore on the island year‐round and thereby play a key role in seed dispersal throughout the year. Additionally, the frugivore species composition on the island is unlikely to change as previous studies have documented similar assemblage of frugivores. We have a diverse representation of plant families in our study with varying fruit (figs, drupes, and arillate dehiscent capsular fruits) and seed traits thereby capturing the functional diversity in traits of fruits and seeds making the data amenable for ecophylogenetic analysis. Additionally, this is a remote island making it extremely challenging to conduct long bouts of fieldwork, thereby posing challenges to collect long‐term data. However, long‐term studies are required to document interactions within and between years to determine relative changes in species roles for frugivores and plants. Additionally, we collected data on plant–animal interactions on the island using a different method than the mainland site. However, results of data collected with spot scans and fruit tree watches were similar. Evidence suggests that weighted interaction data collected either as frequency of interactions or abundance data provide comparable results for species roles in the plant–animal mutualistic community (Miranda et al., 2019). Given this, we believe that methodical differences are unlikely to alter the findings of the study.
Only those plants and animals that are able to cross the oceanic barriers and establish contribute to structuring of communities on islands. Degree of isolation, area, and age of islands thereby offers an interesting gradient to understand the relative importance of processes in structuring of communities. While we did not detect a phylogenetic or functional signal across mainland and island communities, it will be interesting to investigate these patterns as the diversity of frugivores increases further. Stronger signals can be expected in a niche packing scenario. As demonstrated in this study, large‐bodied frugivores, particularly the Narcondam Hornbill, play a pivotal role on Narcondam Island in the absence of small frugivores on a relatively young and moderately isolated island. Several threatened hornbill species are found on oceanic islands (IUCN, 2019). Narcondam Island and its endemic hornbill give insights into the potential role of hornbills, which are among the largest frugivores on islands, in shaping and potentially maintaining the island tree communities by playing a key role as a seed disperser. The ecological role of hornbills and their contribution to mutualism and maintenance of plant species diversity is poorly understood from other oceanic islands. Narcondam Island through the hyper abundance of hornbills and its food plants and the asymmetric nature of the plant–disperser community with heavy reliance on a single frugivore demonstrates the unique ecological and evolutionary setting of oceanic islands. The disproportionate and singular role that species can play on oceanic islands points toward the extreme vulnerability of oceanic islands to any external perturbations. The perturbations can disrupt the delicate and intricate relationships between plants and seed dispersers, with dramatic cascading impacts on the entire community. Unfortunately, studies like this might not be possible in many other oceanic islands that harbor hornbills as they have been already modified extensively due to anthropogenic activities. It is indeed fortunate that Narcondam has been preserved as a Wildlife Sanctuary thereby safeguarding not only a unique hornbill species but also a unique ecosystem.
CONFLICT OF INTEREST
Authors have no competing interests to declare.
AUTHOR CONTRIBUTION
Rohit Naniwadekar: Conceptualization (lead); Data curation (equal); Formal analysis (lead); Funding acquisition (lead); Methodology (lead); Project administration (lead); Resources (lead); Visualization (equal); Writing‐original draft (lead). Abhishek Gopal: Conceptualization (supporting); Data curation (equal); Formal analysis (supporting); Methodology (supporting); Project administration (supporting); Writing‐review & editing (supporting). Navendu Page: Conceptualization (supporting); Data curation (equal); Formal analysis (supporting); Methodology (supporting); Project administration (supporting); Resources (supporting); Writing‐review & editing (supporting). Sartaj Ghuman: Conceptualization (supporting); Data curation (equal); Project administration (supporting); Supervision (supporting); Writing‐review & editing (supporting). Vivek Ramachandran: Conceptualization (supporting); Formal analysis (supporting); Resources (supporting); Writing‐review & editing (supporting). Jahnavi Joshi: Conceptualization (supporting); Data curation (supporting); Formal analysis (equal); Methodology (equal); Visualization (equal); Writing‐original draft (supporting).
ETHICAL APPROVAL
This study was an observational study and did not involve handling of animals. Ethics clearance for the observational study was obtained from Nature Conservation Foundation (NCF‐EC‐16/11/19‐(43)). We obtained the necessary permissions from the Forest Department (No: WII/NVP/NARCONDAM/2019), Andaman and Nicobar Police Department (DGP/Genl/107/20/2015/5552) and the Office of the Deputy Commissioner (South Andamans) (F.No.5‐5/LS/TP/2014/7554) to conduct the study.
Supporting information
Appendix S1
Appendix S2
ACKNOWLEDGMENTS
We thank Wildlife Conservation Trust, IDEAWILD, Nature Conservation Foundation, Mr. Uday Kumar, MMMRF, Mr. Rohit and Deepa Sobti, and Mr. Aravind Datar for providing funding support. We thank Mr. D. M. Shukla (PCCF, Wildlife), Mr. A. K. Paul and Mr. Soundra Pandian for giving us the necessary permissions and support. We thank Mr. Dependra Pathak, DGP (A&N) for giving us the necessary permissions. We thank Commandant A. K. Bhama and Captain Kundan Singh from the Indian Coast Guard for giving us permission and support. We thank Mr. Abhishek Dey, DC (South Andamans) for giving us permission. We thank Kulbhushansingh Suryawanshi, Divya Mudappa and T. R. Shankar Raman for providing us field equipment and for valuable discussions. We are grateful to the then Dean and Director WII, Dr. G. S. Rawat for supporting the project. We are indebted to the Special Armed Police unit led by Ms. Usha Rangnani (SP) for providing us logistic support at Narcondam Island. We thank Elrika D'Souza, Evan Nazareth, Rachana Rao and Rohan Arthur for providing us logistic support in Port Blair. We thank Prasenjeet Yadav, Adarsh Raju, Suri Venkatachalam, Shashank Dalvi, Anand Osuri, Narayan Sharma and Aparajita Datta for valuable discussions. We thank Hari Sridhar, Risa Sargent and Jennifer Lau for valuable comments on a previous version of this manuscript. We thank two anonymous reviewers for their comments that have helped improve the manuscript.
Naniwadekar R, Gopal A, Page N, Ghuman S, Ramachandran V, Joshi J. Large frugivores matter more on an island: Insights from island‐mainland comparison of plant–frugivore communities. Ecol Evol. 2021;11:1399–1412. 10.1002/ece3.7151
DATA AVAILABILITY STATEMENT
Data used in this study are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.jsxksn080 (Naniwadekar et al., 2020).
REFERENCES
- Bastazini, V. A. G. , Ferreira, P. M. A. , Azambuja, B. O. , Casas, G. , Debastiani, V. J. , Guimarães, P. R. , & Pillar, V. D. (2017). Untangling the tangled bank: A novel method for partitioning the effects of phylogenies and traits on ecological networks. Evolutionary Biology, 44(3), 312–324. 10.1007/s11692-017-9409-8 [DOI] [Google Scholar]
- BirdLife International (2017). Rhyticeros narcondami (amended version of 2016 assessment). The IUCN Red List of Threatened Species 2017: e.T22682531A110038017. 2020, Retrieved from 10.2305/IUCN.UK.2018-2.RLTS.T22682528A132400385.en [DOI]
- Blomberg, S. P. , Garland, T. , & Ives, A. R. (2003). Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution, 57(4), 717–745. 10.1111/j.0014-3820.2003.tb00285.x [DOI] [PubMed] [Google Scholar]
- Blüthgen, N. , Menzel, F. , Hovestadt, T. , Fiala, B. , & Blüthgen, N. (2007). Specialization, constraints, and conflicting interests in mutualistic networks. Current Biology, 17(4), 341–346. 10.1016/j.cub.2006.12.039 [DOI] [PubMed] [Google Scholar]
- Chaisuriyanun, S. , Gale, G. , & Poonswad, P. (2011). Food consumed by Great Hornbill and Rhinoceros Hornbill in tropical rainforest, Budo Su‐Ngai Padi National Park, Thailand. Raffles Bulletin of Zoology, 24, 123–135. [Google Scholar]
- Corlett, R. T. (2017). Frugivory and seed dispersal by vertebrates in tropical and subtropical Asia: An update. Global Ecology and Conservation, 11, 1–22. 10.1016/j.gecco.2017.04.007 [DOI] [Google Scholar]
- Dasgupta, S. , & Hilaluddin (2012). Differential effects of hunting on populations of hornbills and imperial pigeons in the rainforests of the Eastern Indian Himalaya. Indian Forester, 138, 902–909. [Google Scholar]
- Datta, A. (2001). An ecological study of sympatric hornbills and fruiting patterns in a tropical forest in Arunachal Pradesh. PhD Dissertation. Saurashtra University. [Google Scholar]
- Debastiani, V. J. , & Pillar, V. D. (2012). SYNCSA: R tool for analysis of metacommunities based on functional traits and phylogeny of the community components. Bioinformatics, 28(15), 2067–2068. 10.1093/bioinformatics/bts325 [DOI] [PubMed] [Google Scholar]
- Dormann, C. F. , Gruber, B. , & Fründ, J. (2008). Introducing the bipartite package: Analysing ecological networks. R News, 8, 8–11. [Google Scholar]
- Dormann, C. F. , & Strauss, R. (2014). A method for detecting modules in quantitative bipartite networks. Methods in Ecology and Evolution, 5(1), 90–98. 10.1111/2041-210X.12139 [DOI] [Google Scholar]
- Escribano‐Avila, G. , Lara‐Romero, C. , & Heleno, R. (2018). Tropical seed dispersal networks: Emerging patterns, biases, and keystone species traits In Dattilo W., & Rico‐Gray V. (Eds.), Ecological networks in the tropics. Springer. [Google Scholar]
- Godínez‐Alvarez, H. , Ríos‐Casanova, L. , & Peco, B. (2020). Are large frugivorous birds better seed dispersers than medium‐ and small‐sized ones? Effect of body mass on seed dispersal effectiveness. Ecology and Evolution, 10(12), 6136–6143. 10.1002/ece3.6285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González‐Castro, A. , Traveset, A. , & Nogales, M. (2012). Seed dispersal interactions in the Mediterranean Region: Contrasting patterns between islands and mainland. Journal of Biogeography, 39(11), 1938–1947. 10.1111/j.1365-2699.2012.02693.x [DOI] [Google Scholar]
- Gopal, A. , Mudappa, D. , Raman, T. R. S. , & Naniwadekar, R. (2020). Forest cover and fruit crop size differentially influence frugivory of select rainforest tree species in Western Ghats, India. Biotropica, 52(5), 871–883. 10.1111/btp.12810 [DOI] [Google Scholar]
- Guimarães, P. R. Jr , Jordano, P. , & Thompson, J. N. (2011). Evolution and coevolution in mutualistic networks. Ecology Letters, 14(9), 877–885. 10.1111/j.1461-0248.2011.01649.x [DOI] [PubMed] [Google Scholar]
- Harrison, R. D. (2005). Figs and the diversity of tropical rainforests. BioScience, 55(12), 1053–1064. 10.1641/0006-3568(2005)055[1053:FATDOT]2.0.CO;2 [DOI] [Google Scholar]
- Harrison, R. D. , Hamid, A. A. , Kenta, T. , Lafrankie, J. , Lee, H.‐S. , Nagamasu, H. , Nakashizuka, T. , & Palmiotto, P. (2003). The diversity of hemi‐epiphytic figs (Ficus; Moraceae) in a Bornean lowland rain forest. Biological Journal of the Linnean Society, 78(4), 439–455. 10.1046/j.0024-4066.2002.00205.x [DOI] [Google Scholar]
- IUCN (2019). The IUCN Red List of threatened species. Version 2019–1. Retrieved from http://www.IUCN.redlist.org [Google Scholar]
- Jetz, W. , Thomas, G. H. , Joy, J. B. , Hartmann, K. , & Mooers, A. O. (2012). The global diversity of birds in space and time. Nature, 491(7424), 444–448. 10.1038/nature11631 [DOI] [PubMed] [Google Scholar]
- Jordano, P. (2000). Fruits and frugivory In Fener M. (Ed.), Seeds: The ecology of regeneration in plant communities (pp. 125–166). CABI Publishing. [Google Scholar]
- Kanwatanakid‐Savini, C. , Poonswad, P. , & Savini, T. (2009). An assessment of food overlap between gibbons and hornbills. The Raffles Bulletin of Zoology, 57(1), 189–198. [Google Scholar]
- Kinnaird, M. F. , O'Brien, T. G. , & Suryadi, S. (1996). Population fluctuation in Sulawesi Red‐Knobbed Hornbills: Tracking figs in space and time. The Auk, 113(2), 431–440. 10.2307/4088909 [DOI] [Google Scholar]
- LEMoN (2019). Long‐term Ecosystem Monitoring Network (LEMoN). National Centre for Biological Sciences, Interim report to the Andaman Forest Department. [Google Scholar]
- Manchi, S. (2017). Status, ecology and conservation of Narcondam Hornbill Aceros narcondami on the Narcondam Island, India. Salim Ali Centre for Ornithology and Natural History. [Google Scholar]
- Martín González, A. M. , Dalsgaard, B. , & Olesen, J. M. (2010). Centrality measures and the importance of generalist species in pollination networks. Ecological Complexity, 7(1), 36–43. 10.1016/j.ecocom.2009.03.008 [DOI] [Google Scholar]
- McConkey, K. R. , & Drake, D. R. (2015). Low redundancy in seed dispersal within an island frugivore community. AoB Plants, 7, plv088 10.1093/aobpla/plv088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, D. L. , Rexstad, E. , Thomas, L. , Marshall, L. , & Laake, J. L. (2019). Distance sampling in R. Journal of Statistical Software, 89(1), 1–28. 10.1101/063891 [DOI] [Google Scholar]
- Miranda, P. N. , Ribeiro, J. E. L. D. S. , Luna, P. , Brasil, I. , Delabie, J. H. C. , & Dáttilo, W. (2019). The dilemma of binary or weighted data in interaction networks. Ecological Complexity, 38, 1–10. 10.1016/j.ecocom.2018.12.006 [DOI] [Google Scholar]
- Myers, N. , Mittermeier, R. A. , Mittermeier, C. G. , da Fonseca, G. A. B. , & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403(6772), 853–858. 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Naniwadekar, R. , Gopal, A. , Page, N. , Ghuman, S. , Ramachandran, V. , & Joshi, J. (2020). Data from: Large frugivores matter more on an island: Insights from island‐mainland comparison of plant–frugivore communities Dryad, Dataset. 10.5061/dryad.jsxksn080 [DOI] [PMC free article] [PubMed]
- Naniwadekar, R. , Chaplod, S. , Datta, A. , Rathore, A. , & Sridhar, H. (2019). Large frugivores matter: Insights from network and seed dispersal effectiveness approaches. Journal of Animal Ecology, 88(8), 1250–1262. 10.1111/1365-2656.13005 [DOI] [PubMed] [Google Scholar]
- Naniwadekar, R. , & Datta, A. (2013). Spatial and temporal variation in hornbill densities in Namdapha Tiger Reserve, Arunachal Pradesh, North‐East India. Tropical Conservation Science, 6, 734–748. 10.1177/194008291300600603 [DOI] [Google Scholar]
- Naniwadekar, R. , Mishra, C. , & Datta, A. (2015). Fruit resource tracking by hornbill species at multiple scales in a tropical forest in India. Journal of Tropical Ecology, 31(6), 477–490. 10.1017/S0266467415000449 [DOI] [Google Scholar]
- Nogales, M. , Heleno, R. , Rumeu, B. , González‐Castro, A., Traveset, A. , Vargas, P. , & Olesen, J. M. (2016). Seed‐dispersal networks on the Canaries and the Galápagos archipelagos: Interaction modules as biogeographical entities: Modularity of seed‐dispersal networks on oceanic islands. Global Ecology and Biogeography, 25(7), 912–922. 10.1111/geb.12315 [DOI] [Google Scholar]
- Oksanen, J. et al (2019). vegan: Community ecology package. Retrieved from https://CRAN.R‐project.org/package=vegan [Google Scholar]
- Olesen, J. M. , Bascompte, J. , Dupont, Y. L. , Elberling, H. , Rasmussen, C. , & Jordano, P. (2011). Missing and forbidden links in mutualistic networks. Proceedings of the Royal Society B: Biological Sciences, 278(1706), 725–732. 10.1098/rspb.2010.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, N. V. , & Shanker, K. (2020). Climatic stability drives latitudinal trends in range size and richness of woody plants in Western Ghats, India. PLoS One, 15(7), e0235733 10.1371/journal.pone.0235733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacio, R. D. , Valderrama‐Ardila, C. , & Kattan, G. H. (2016). Generalist species have a central role in a highly diverse plant–frugivore network. Biotropica, 48(3), 349–355. 10.1111/btp.12290 [DOI] [Google Scholar]
- Paradis, E. , & Schliep, K. (2018). ape 5.0: An environment for modern phylogenetics and evolutionary analyses in {R}. Bioinformatics, 35, 526–528. 10.1093/bioinformatics/bty633 [DOI] [PubMed] [Google Scholar]
- Peralta, G. (2016). Merging evolutionary history into species interaction networks. Functional Ecology, 30(12), 1917–1925. 10.1111/1365-2435.12669 [DOI] [Google Scholar]
- Poonswad, P. , Tsuji, A. , Jirawatkavl, N. , & Chimchome, V. (1998). Some aspects of food and feeding ecology of sympatric hornbill species in Khao Yai National Park, Thailand In Poonswad P. (Ed.) The Asian Hornbills: Ecology and conservation (pp. 137–157). BIOTEC, NSTDA; (Thai studies in biodiversity no. 2). [Google Scholar]
- Qian, H. , & Jin, Y. (2016). An updated megaphylogeny of plants, a tool for generating plant phylogenies and an analysis of phylogenetic community structure. Journal of Plant Ecology, 9(2), 233–239. 10.1093/jpe/rtv047 [DOI] [Google Scholar]
- R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing; Retrieved from https://www.R‐project.org/ [Google Scholar]
- Raman, T. R. S. , Mudappa, D. , Khan, T. , Mistry, U. , Saxena, A. , Varma, K. , Ekka, N. , Lenin, J. , & Whitaker, R. (2013). An expedition to the Narcondams: Observations of the marine and terrestrial fauna including the island‐endemic hornbill. Current Science, 105(3), 346–360. [Google Scholar]
- Revell, L. J. (2012). phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217–223. 10.1111/j.2041-210X.2011.00169.x [DOI] [Google Scholar]
- Rezende, E. L. , Jordano, P. , & Bascompte, J. (2007). Effects of phenotypic complementarity and phylogeny on the nested structure of mutualistic networks. Oikos, 116(11), 1919–1929. 10.1111/j.0030-1299.2007.16029.x [DOI] [Google Scholar]
- Riley, J. (2001). Population sizes and the status of endemic and restricted‐range bird species on Sangihe Island, Indonesia. Bird Conservation International, 12(1), 53–78. 10.1017/S0959270902002046 [DOI] [Google Scholar]
- Sankaran, R. (2000). Narcondam Hornbill Aceros narcondami In Vijayan L. et al (Eds.), A study on the ecology, status and conservation perspectives of certain rare endemic avifauna of the Andaman and Nicobar Islands. Report submitted to Salim Ali Centre for ornithology and natural History, Coimbatore, India. Coimbatore, India, pp. 57–66. [Google Scholar]
- Schleuning, M. , Böhning‐Gaese, K. , Dehling, D. M. , & Burns, K. C. (2014). At a loss for birds: Insularity increases asymmetry in seed‐dispersal networks. Global Ecology and Biogeography, 23(4), 385–394. 10.1111/geb.12134 [DOI] [Google Scholar]
- Segar, S. T. , Fayle, T. M. , Srivastava, D. S. , Lewinsohn, T. M. , Lewis, O. T. , Novotny, V. , Kitching, R. L. , & Maunsell, S. C. (2020). The role of evolution in shaping ecological networks. Trends in Ecology & Evolution, 35(5), 454–466. 10.1016/j.tree.2020.01.004 [DOI] [PubMed] [Google Scholar]
- Shanahan, M. , So, S. , Gompton, S. G. , & Gorlett, R. (2001). Fig‐eating by vertebrate frugivores: A global review. Biological Reviews, 76(4), 529–572. 10.1017/S1464793101005760 [DOI] [PubMed] [Google Scholar]
- Terborgh, J. , Pitman, N. , Silman, M. , Schichter, H. , & Núñez, V. P. (2002). Maintenance of tree diversity in tropical forests In Levey D. J., Silva W. R., & Galetti M. (Eds.), Seed dispersal and frugivory: Ecology, evolution and conservation. CABI Publishing. [Google Scholar]
- Thomas, L. , Buckland, S. T. , Rexstad, E. A. , Laake, J. L. , Strindberg, S. , Hedley, S. L. , Bishop, J. R. B. , Marques, T. A. , & Burnham, K. P. (2010). Distance software: Design and analysis of distance sampling surveys for estimating population size. Journal of Applied Ecology, 47, 5–14. 10.1111/j.1365-2664.2009.01737.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylianakis, J. M. , Laliberté, E. , Nielsen, A. , & Bascompte, J. (2010). Conservation of species interaction networks. Biological Conservation, 143(10), 2270–2279. 10.1016/j.biocon.2009.12.004 [DOI] [Google Scholar]
- Vázquez, D. P. , Blüthgen, N. , Cagnolo, L. , & Chacoff, N. P. (2009). Uniting pattern and process in plant–animal mutualistic networks: A review. Annals of Botany, 103(9), 1445–1457. 10.1093/aob/mcp057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez, D. P. , Melián, C. J. , Williams, N. M. , Blüthgen, N. , Krasnov, B. R. , & Poulin, R. (2007). Species abundance and asymmetric interaction strength in ecological networks. Oikos, 116(7), 1120–1127. 10.1111/j.0030-1299.2007.15828.x [DOI] [Google Scholar]
- Wilman, H. , Belmaker, J. , Simpson, J. , de la Rosa, C. , Rivadeneira, M. M. , & Jetz, W. (2014). EltonTraits 1.0: Species‐level foraging attributes of the world's birds and mammals. Ecology, 95(7), 2027 10.1890/13-1917.1 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Naniwadekar, R. , Gopal, A. , Page, N. , Ghuman, S. , Ramachandran, V. , & Joshi, J. (2020). Data from: Large frugivores matter more on an island: Insights from island‐mainland comparison of plant–frugivore communities Dryad, Dataset. 10.5061/dryad.jsxksn080 [DOI] [PMC free article] [PubMed]
Supplementary Materials
Appendix S1
Appendix S2
Data Availability Statement
Data used in this study are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.jsxksn080 (Naniwadekar et al., 2020).


