Hypercoagulability may be a key mechanism of death in patients with COVID-19. This cohort study evaluated the incidence of venous thromboembolism and major bleeding in critically ill patients with COVID-19 and examined the observational effect of early therapeutic anticoagulation on survival.
Visual Abstract. Early Anticoagulation in COVID-19.
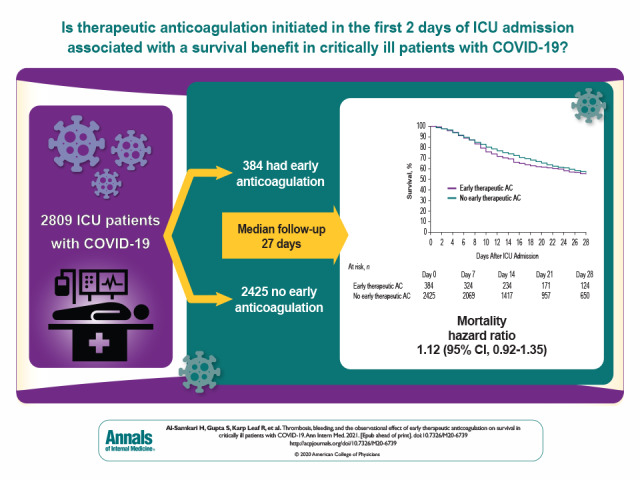
Hypercoagulability may be a key mechanism of death in patients with COVID-19. This cohort study evaluated the incidence of venous thromboembolism and major bleeding in critically ill patients with COVID-19 and examined the observational effect of early therapeutic anticoagulation on survival.
Abstract
Background:
Hypercoagulability may be a key mechanism of death in patients with coronavirus disease 2019 (COVID-19).
Objective:
To evaluate the incidence of venous thromboembolism (VTE) and major bleeding in critically ill patients with COVID-19 and examine the observational effect of early therapeutic anticoagulation on survival.
Design:
In a multicenter cohort study of 3239 critically ill adults with COVID-19, the incidence of VTE and major bleeding within 14 days after intensive care unit (ICU) admission was evaluated. A target trial emulation in which patients were categorized according to receipt or no receipt of therapeutic anticoagulation in the first 2 days of ICU admission was done to examine the observational effect of early therapeutic anticoagulation on survival. A Cox model with inverse probability weighting to adjust for confounding was used.
Setting:
67 hospitals in the United States.
Participants:
Adults with COVID-19 admitted to a participating ICU.
Measurements:
Time to death, censored at hospital discharge, or date of last follow-up.
Results:
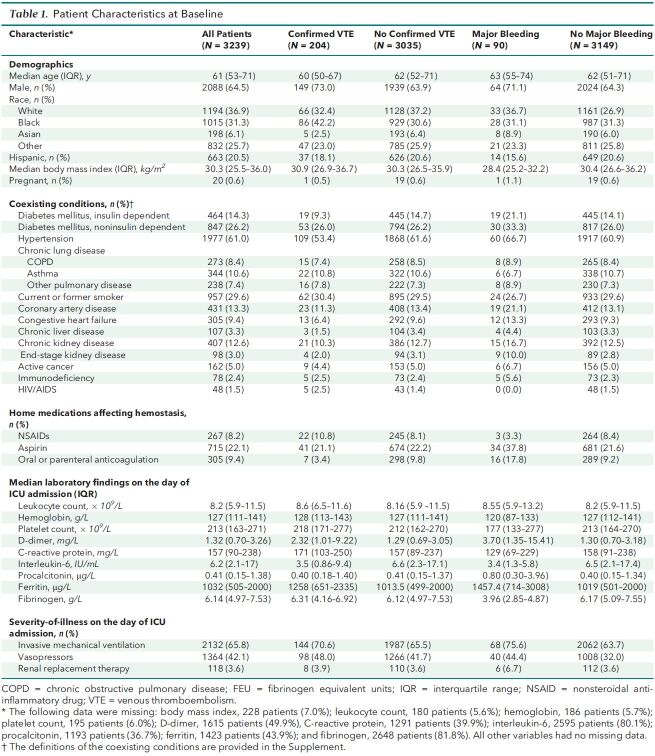
Among the 3239 patients included, the median age was 61 years (interquartile range, 53 to 71 years), and 2088 (64.5%) were men. A total of 204 patients (6.3%) developed VTE, and 90 patients (2.8%) developed a major bleeding event. Independent predictors of VTE were male sex and higher D-dimer level on ICU admission. Among the 2809 patients included in the target trial emulation, 384 (11.9%) received early therapeutic anticoagulation. In the primary analysis, during a median follow-up of 27 days, patients who received early therapeutic anticoagulation had a similar risk for death as those who did not (hazard ratio, 1.12 [95% CI, 0.92 to 1.35]).
Limitation:
Observational design.
Conclusion:
Among critically ill adults with COVID-19, early therapeutic anticoagulation did not affect survival in the target trial emulation.
Primary Funding Source:
None.
Hypercoagulability in patients with coronavirus disease 2019 (COVID-19) may be a key mechanistic pathway leading to multiorgan failure and death (1, 2). Patients with COVID-19 have been reported to experience high rates of venous thromboembolism (VTE) (3) and extracorporeal circuit thrombosis (4). Autopsies show extensive fibrin thrombi within small vessels and capillaries (5). These observations have prompted some experts to recommend empirical treatment with therapeutic anticoagulation for critically ill patients with COVID-19 (1, 2).
Data on the incidence, risk factors, and prognosis associated with VTE in patients with COVID-19 are largely limited to single-center reports (4, 6–8). Granular, nationally representative data on VTE, as well as major bleeding events, are urgently needed. Furthermore, until data from randomized trials on the effect of therapeutic anticoagulation on outcomes in COVID-19 become available, clinical care may be informed by robust estimates from observational data.
We used data from a multicenter cohort study of critically ill patients with COVID-19 admitted to intensive care units (ICUs) in the United States to evaluate the incidence of VTE and major bleeding, identify risk factors for VTE, and examine the observational effect of early initiation of therapeutic anticoagulation on survival.
Methods
Study Design, Oversight, and Patient Population
We used data from STOP-COVID (Study of the Treatment and Outcomes in Critically Ill Patients With COVID-19), a multicenter cohort study that enrolled consecutive adults (aged ≥18 years) with laboratory-confirmed COVID-19 admitted to participating ICUs at 67 geographically diverse hospitals in the United States (Supplement Table 1 and Supplement Figure 1) (9). We included patients admitted to ICUs between 4 March 2020 and 11 April 2020. We followed patients until the first of hospital discharge, death, or 8 May 2020. The study was approved with a waiver of informed consent by the institutional review board at each participating site.
Data Collection
Study personnel at each site collected data by manual chart review and used a standardized case report form to enter data into a secure online database. Patient-level data included baseline information on demographics, coexisting conditions, symptoms, medications before hospital admission, and vital signs; daily data for the 14 days after ICU admission on physiologic and laboratory values, medications, nonmedication treatments, organ support, and acute organ injury; and outcome data on vital status at discharge. We also collected site-specific data on routine screening protocols for VTE and anticoagulation prophylaxis. A complete list of variables is provided in the Case Report Form in the Supplement, and definitions of outcomes and events are listed in Supplement Table 2. All data were validated through a series of automated and manual verifications (Methods section of the Supplement).
Definitions
We collected data on the incidence, clinical features, and 28-day mortality in patients with radiographically confirmed VTE (including deep venous thrombosis and pulmonary embolism), ischemic stroke, and major bleeding. Data on radiographically confirmed versus clinically suspected VTE are reported separately. Ischemic stroke was confirmed radiographically in all cases. We defined major bleeding as occurring in a critical area or organ (for example, intracranial, retroperitoneal, pericardial, or intramuscular bleeding with compartment syndrome) or requiring a procedural intervention; the requirement for red blood cell transfusion alone was not sufficient to qualify as a major bleed. This definition is similar to or stricter than definitions used by landmark clinical trials of novel anticoagulant reversal agents (10) and the International Society for Thrombosis and Haemostasis (11). We also collected data on the incidence of disseminated intravascular coagulation, heparin-induced thrombocytopenia, and coagulopathy (definitions provided in Supplement Table 2).
Statistical Analysis
Continuous and categorical variables are expressed as median (interquartile range [IQR]) and count (percentage), respectively. We used multivariable logistic regression to identify predictors of radiographically confirmed VTE. We prespecified the following covariates, which were assessed on the day of ICU admission: age; sex; body mass index; current smoking status; active cancer; and leukocyte count, platelet count, D-dimer, shock, invasive mechanical ventilation, therapeutic anticoagulation, and aspirin (Methods section of the Supplement). Missing data were multiply imputed with 5 data sets, and results were pooled using Rubin's rules (12).
To assess interhospital variation in VTE incidence and use of therapeutic anticoagulation in the first 14 days after ICU admission, we employed multilevel logistic regression modeling with patients nested in hospitals. This approach addresses the poor reliability of estimates stemming from hospitals submitting few cases. We additionally excluded hospitals that submitted data on fewer than 30 patients (for VTE incidence) and fewer than 10 patients (for therapeutic anticoagulation) to further improve the reliability of our estimates. We adjusted for the same covariates as the aforementioned VTE model.
Target Trial Specification
To examine the observational effect of early initiation of therapeutic anticoagulation on in-hospital survival, we emulated a hypothetical target trial comparing patients who received therapeutic anticoagulation in the 2 days after ICU admission to those who did not. We used the target trial framework to make our research question explicit and to guide the design of the observational analysis (13–16). The time period of 2 days was chosen for treatment assignment to provide greater homogeneity between patients, to limit indication bias, to allow longer follow-up, and for biological plausibility, because therapies initiated early and before irreversible organ injury may be more likely to be beneficial (17). To emulate the eligibility criteria of a hypothetical clinical trial of therapeutic anticoagulation, we excluded patients if they were receiving anticoagulation before hospitalization or if they had any of the following within 2 days after ICU admission: confirmed or suspected VTE, extracorporeal membrane oxygenation, major bleeding, or a platelet count less than 50 × 109 cells/L.
Target Trial Emulation
The primary analysis compares survival among patients who received or did not receive therapeutic anticoagulation in the first 2 days of ICU admission. Survival time was defined as the interval from ICU admission to death, censored at hospital discharge, or the end of follow-up, whichever occurred first. Hazard ratios and 95% CIs were estimated using a Cox model. Patients who initiated therapeutic anticoagulation on day 3 or later after ICU admission were categorized in the control group, in keeping with an intention-to-treat approach.
Inverse Probability Weighting
We adjusted for confounding using inverse probability weighting. We fit a logistic regression model with initiation of therapeutic anticoagulation in the first 2 days of ICU admission as the outcome, conditional on the following covariates that were prespecified based on clinical judgment: age; sex; race; ethnicity; body mass index; hypertension; diabetes mellitus; atrial fibrillation or atrial flutter; coronary artery disease; congestive heart failure; current smoking status; active cancer; duration of symptoms before ICU admission; severity-of-illness covariates assessed on ICU admission, including Pao 2:FIo 2, shock, the renal, liver, and coagulation components of the Sequential Organ Failure Assessment score (18), and inflammation (assessed by C-reactive protein, D-dimer, and ferritin); and concurrent therapies received on ICU admission, including aspirin, neuromuscular blockade, and prone positioning. Additional details are provided in the Methods section of the Supplement. We used the model's predicted probabilities to calculate stabilized inverse probability weights (19), which were then used to weight each individual's contribution to the survival curves and to the Cox model. We used a robust (sandwich) variance estimator to account for potential replications of patients induced by inverse probability weighting, which results in conservative 95% CIs.
Sensitivity Analyses
We conducted 5 sensitivity analyses of the target trial emulation. First, we included the aforementioned covariates in an unweighted Cox model. Second, to eliminate the potential for immortal time bias (20, 21), we categorized eligible patients into either the early therapeutic anticoagulation group or the no early therapeutic anticoagulation group on ICU day 1, and we repeated the process for eligible patients on ICU day 2. Our final estimates were obtained by pooling the data from the nested target trials on ICU days 1 and 2, using inverse probability weighting as previously described and in the Methods section of the Supplement. Third, we repeated the primary target trial but extended the exposure period for initiation of therapeutic anticoagulation from the first 2 days to the first 3 days after ICU admission. Fourth, we stratified the Cox model by site. Fifth, rather than censoring patients at hospital discharge, we kept them in the risk set until 8 May 2020, the date of last follow-up.
Subgroup Analyses
We used similar methods as the aforementioned primary analysis to examine the observational effect of early therapeutic anticoagulation on survival across the following prespecified subgroups: age (≤60 vs. >60 years); sex; body mass index (<40 vs. ≥40 kg/m2); days from symptom onset to ICU admission (≤3 vs. >3); receipt of invasive mechanical ventilation, shock, and Pao 2:FIo 2 ratio (<100, 100 to 199, and ≥200 or not mechanically ventilated) on ICU day 1; D-dimer level on ICU days 1 or 2 (≤1000, 1001 to 2500, and >2500 ng/mL); and number of ICU beds before COVID-19 (<50, 50 to 99, and ≥100). Analyses were done using SAS, version 9.4 (SAS Institute), and Stata 14.2 (StataCorp).
Role of the Funding Source
This study was not funded.
Results
Patient Characteristics
A total of 3239 patients from 67 sites were included in this analysis. The median age was 61 years (IQR, 53 to 71), 2088 (64%) were men, and 2132 (65.8%) received mechanical ventilation on ICU day 1. Additional characteristics overall and according to the presence or absence of VTE and major bleeding are shown in Table 1 and Supplement Table 3. Overall, 1273 patients (39.3%) died, 1404 (43.4%) were discharged from the hospital within 28 days, and 562 (17.4%) remained hospitalized at 28 days.
Table 1. Patient Characteristics at Baseline.
VTE, Major Bleeding, and Coagulation-Associated Complications
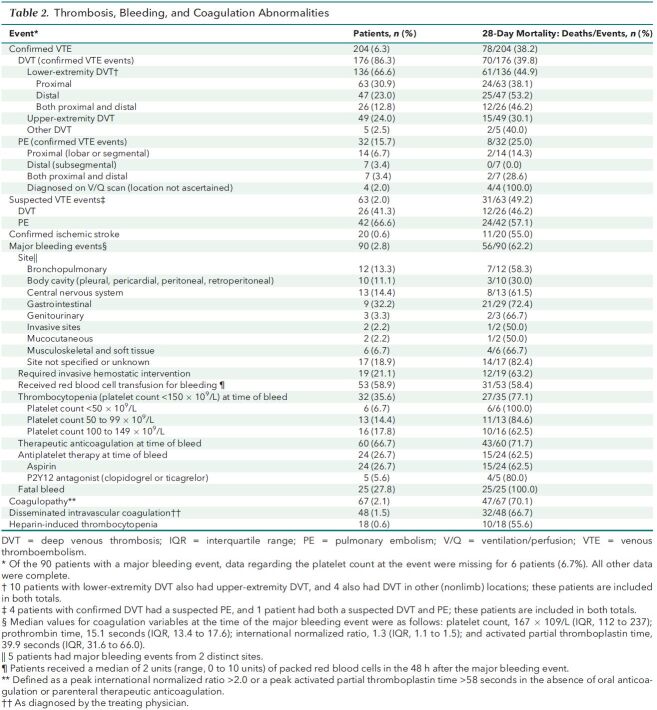
Routine screening for VTE in critically ill patients with COVID-19 occurred in 3 of the 67 hospitals (4.5%). A total of 204 patients (6.3%) had radiographically confirmed VTE during the first 14 days of ICU admission (Supplement Figure 2, panel A), including 176 patients (5.4%) with deep venous thrombosis and 32 patients (1.0%) with pulmonary embolism (Table 2). The 14-day radiographically confirmed VTE rate in hospitals using universal lower-extremity Doppler ultrasonography screening was 8.7%. The risk- and reliability-adjusted incidence of radiographically confirmed VTE across hospitals is shown in Supplement Figure 3, panel A, with a median incidence of 5.2% and a range of 1.1% at the lowest-incidence hospital to 17.7% at the highest. An additional 63 patients (2.0%) had a clinically suspected VTE event. A total of 20 patients (0.6%) had a radiographically confirmed ischemic stroke. Among patients with radiographically confirmed VTE or stroke, the 28-day mortality was 38.2% and 55%, respectively.
Table 2. Thrombosis, Bleeding, and Coagulation Abnormalities.
Major bleeding occurred in 90 patients (2.8%) (Supplement Figure 2, panel B), 56 of whom (62.2%) died within 28 days. The most common sites of bleeding were gastrointestinal (32.2%) and intracranial (14.4%). A total of 60 of the 90 patients (66.7%) with a major bleeding event were receiving therapeutic anticoagulation at the time of the event (Table 2). Other coagulation-associated complications included coagulopathy (2.1%), disseminated intravascular coagulation (1.5%), and heparin-induced thrombocytopenia (0.6%).
Predictors of VTE
In multivariable analysis, covariates associated with radiographically confirmed VTE were male sex (odds ratio [OR], 1.60 [CI, 1.13 to 2.27]) and higher D-dimer concentration on ICU day 1 (OR, 1.79 [CI, 1.14 to 2.81], for D-dimer of 1001 to 2500 vs. ≤1000 ng/mL; OR, 2.50 [CI, 1.40 to 4.50], for 2501 to 10 000 vs. ≤1000 ng/mL; and OR, 4.20 [CI, 2.17 to 8.14], for >10 000 vs. ≤1000 ng/mL). Other covariates examined were not associated with VTE (Supplement Figure 2, panel D). The increased risk for VTE in men was observed across multiple categories of body mass index and D-dimer (Supplement Figure 4).
Interhospital Variation in Use of Anticoagulation
All participating centers reported routinely administering, at a minimum, standard prophylactic doses of anticoagulation (enoxaparin, 40 mg subcutaneously once daily, or equivalent, or unfractionated heparin, 5000 units subcutaneously 2 to 3 times daily, with or without weight-based dose adjustment) for all critically ill patients with COVID-19. Eight centers (12%) transitioned to higher-than-standard doses for some or all patients with COVID-19 during the study period, using either a risk-adapted approach (based on such criteria as D-dimer) or empirical dose escalation (that is, enoxaparin, 30 to 40 mg twice daily, or equivalent) for all patients.
A total of 1412 patients (43.6%) received therapeutic anticoagulation within 14 days after ICU admission (Supplement Figure 2, panel C). The median time to initiation was 3 days (IQR, 1 to 7), and the most common reasons were concern for a hypercoagulable state (40.8%), atrial fibrillation or atrial flutter (18.8%), and suspected or confirmed VTE (16.9%). Most patients received unfractionated heparin (62.6%) or low-molecular-weight heparin (34.4%), with a minority receiving direct thrombin inhibitors and other agents (Supplement Table 4). The risk- and reliability-adjusted rate of therapeutic anticoagulation use varied widely across hospitals, with a median rate of 39.8% and a range of 15.1% to 78.5% at the lowest-use and highest-use hospitals, respectively (Supplement Figure 3, panel B).
Target Trial Emulation
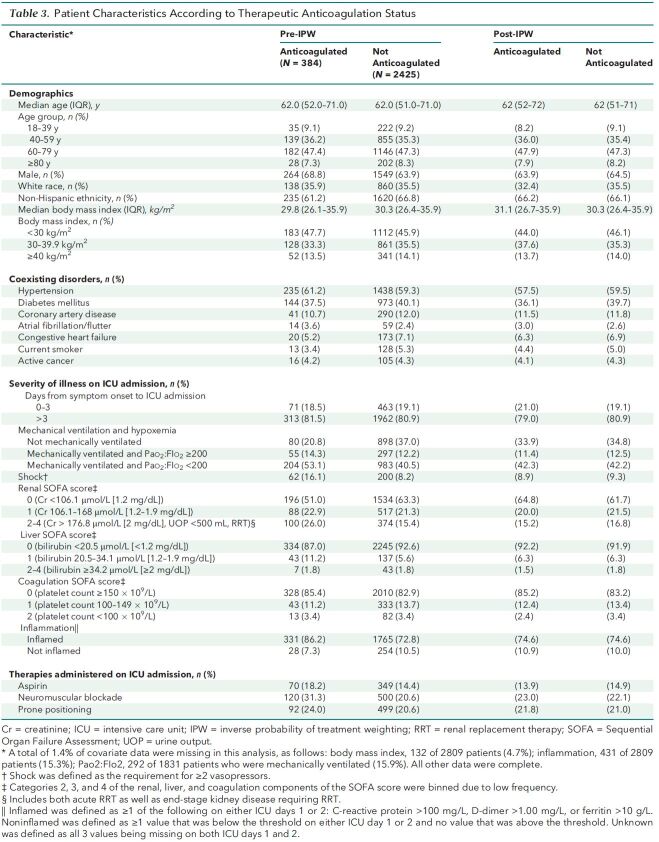
Among the 3239 patients enrolled, 2809 (86.7%) were included in the target trial (Figure 1). A total of 384 patients (11.9%) were treated with early therapeutic anticoagulation. The characteristics of patients before and after applying inverse probability of treatment weighting are shown in Table 3, and indications for therapeutic anticoagulation in the first 2 days of ICU admission in patients included in the target trial emulation are listed in Supplement Table 5). Before applying the weighting, patients treated with early therapeutic anticoagulation were more likely to have shock and greater severity of hypoxemia and renal dysfunction on ICU admission than were patients not treated with early therapeutic anticoagulation. Other characteristics were similar between groups (Table 3). After weighting was applied, baseline and acute severity-of-illness characteristics were well balanced between groups (Table 3 and Supplement Figure 5), with standardized differences below 0.1 for all 22 covariates.
Figure 1. Flow diagram for target trial emulation of therapeutic anticoagulation.
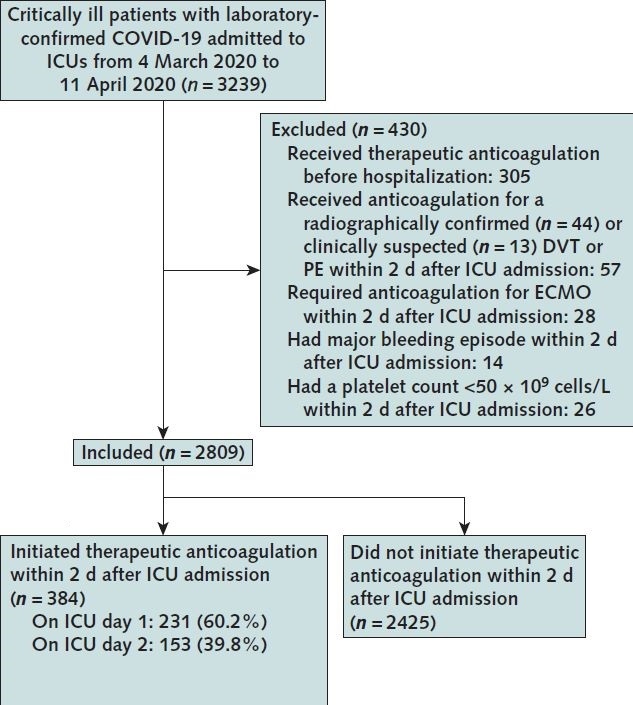
COVID-19 = coronavirus disease 2019; DVT = deep venous thrombosis; ECMO = extracorporeal membrane oxygenation; ICU = intensive care unit; PE = pulmonary embolism.
Table 3. Patient Characteristics According to Therapeutic Anticoagulation Status.
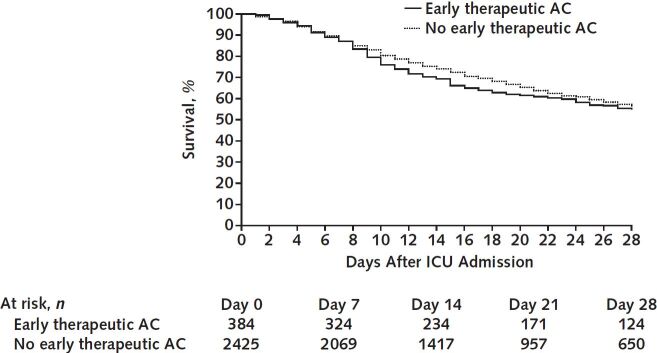
During a median follow-up of 27 days (IQR, 15 to 35), 1066 of the 2809 patients (38.0%) died, including 179 (46.6%) treated with early therapeutic anticoagulation and 887 (36.6%) not treated with early therapeutic anticoagulation. In the primary analysis, patients treated with early therapeutic anticoagulation had a similar survival to those not treated with early therapeutic anticoagulation (hazard ratio, 1.12 [CI, 0.92 to 1.35]) (Figures 2 and 3). Results were similar across all sensitivity and subgroup analyses (Figure 3).
Figure 2. Target trial emulation.
Survival in patients who received therapeutic anticoagulation in the first 2 days of ICU admission compared with those who did not. AC = anticoagulation; ICU = intensive care unit.
Figure 3. Results of sensitivity and subgroup analyses.
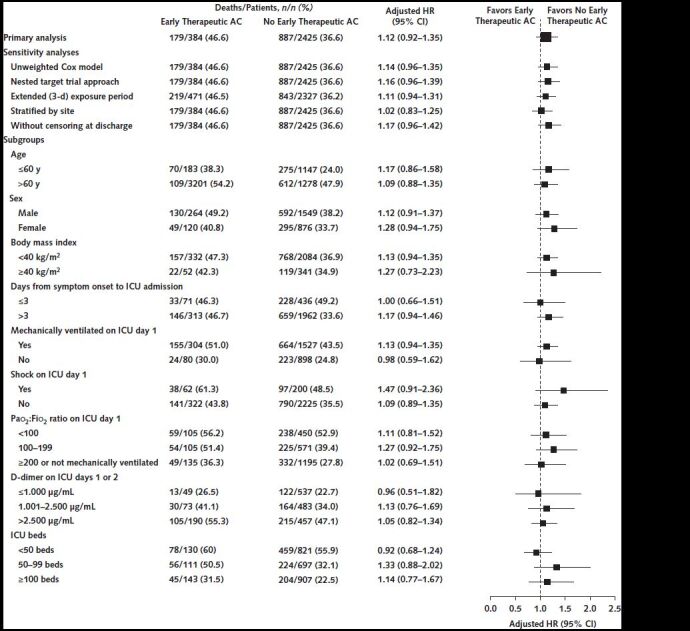
Shown are the hazard ratios for survival according to early initiation of therapeutic AC versus no early initiation of therapeutic AC in the primary analysis, the sensitivity analyses, and across subgroups. AC = anticoagulation; HR = hazard ratio; ICU = intensive care unit.
Discussion
This multicenter cohort study of 3239 critically ill adults with COVID-19 found that 6.3% of patients developed a radiographically confirmed VTE and 2.8% had a major bleeding event in the 14 days after ICU admission. Male sex and higher D-dimer levels at the time of ICU admission were each independently associated with a higher risk for VTE. Patients who received early therapeutic anticoagulation had similar in-hospital survival compared with those who did not. These data suggest that rates of VTE in critically ill patients with COVID-19 may be considerably lower in the United States than in other countries, and that initiation of early therapeutic anticoagulation may not have a survival benefit.
Single-center and regional studies, primarily from Europe, reported VTE rates ranging from 5% to 42% in critically ill patients with COVID-19 (4, 6–8, 22, 23), and a recent meta-analysis that included 1981 patients found a rate of 23.9% (24). The 6.3% incidence of VTE that we observed is similar to that reported by a study conducted in 2 New York City hospitals, which found a 7.7% incidence of VTE among patients who were mechanically ventilated (25). Our findings are also consistent with prior reports in critically ill patients without COVID-19 receiving standard doses of heparin-based thromboprophylaxis (26). Notably, standard doses of low-molecular-weight heparin thromboprophylaxis used by some of the European cohorts (for example, nadroparin, 2850 IU daily) (4, 7) are lower than the standard prophylactic dose used by most centers in this study. In addition, the high rate of initiation of therapeutic anticoagulation in our cohort (43.6% of all patients by day 14) may have contributed to our lower rates of VTE. Of importance, our VTE risk prediction model only included those with radiographically confirmed VTE (6.3%) and not those with suspected VTE (2.0%), as many patients with suspected VTE may have had alternative explanations for cardiopulmonary decompensation, including worsening of COVID-19 lung and myocardial disease.
We identified 2 predictors of VTE in critically ill patients with COVID-19: male sex and higher D-dimer level on ICU day 1. Both of these factors have been associated with adverse outcomes in COVID-19 (27–30) but have not been rigorously studied in the context of VTE. Elevated D-dimer on ICU day 1 may be indicative of elevated coagulation activation and therefore truly predictive of elevated VTE risk or may be representative of an existing thrombus. It is tempting to speculate that early, empirical therapeutic anticoagulation could be beneficial in select patients, such as men with elevated D-dimer on ICU admission. However, in our target trial emulation, we found no benefit of early therapeutic anticoagulation overall or in any subgroup.
We also examined rates of major bleeding. Although we found the rate to be approximately half as frequent as VTE, the associated 28-day mortality was much higher in patients with major bleeding (62.2% vs. 38.2%). Furthermore, two thirds of the major bleeding events occurred in patients receiving therapeutic anticoagulation. The high mortality associated with major bleeding in critically ill patients with COVID-19 highlights the need to identify which patients, if any, may benefit from therapeutic anticoagulation in the absence of standard indications. This need is further underscored by the considerable interhospital variation we detected in the use of therapeutic anticoagulation, which may reflect the lack of available evidence to guide decision making.
We also sought to examine the observational effect of early therapeutic anticoagulation on survival. When data from randomized trials are unavailable, analyzing observational data using a target trial emulation framework may provide more robust estimates on which to guide practice than traditional approaches (31–33). Traditional observational studies that do not explicitly emulate a target trial have resulted in erroneous findings, such as the conclusion that postmenopausal hormone therapy was associated with a 30% lower risk for coronary heart disease (34). These findings become null when the observational analysis is designed to explicitly emulate the corresponding target trial (35), a practice that helps minimize some of the biases that commonly affect observational studies. Our target trial emulation found that early initiation of therapeutic anticoagulation, compared with prophylactic-dose anticoagulation, did not affect survival. One potential explanation for our findings is that therapeutic anticoagulation may have reduced the risk for VTE but at the cost of an increased risk for major bleeding. Alternatively, other pathways of injury, such as inflammation (36), may be more important determinants of survival in critically ill patients with COVID-19 and may not have been affected by anticoagulation dose. Of note, our findings are consistent with the recommendations endorsed by several professional societies against broad empirical institution of therapeutic anticoagulation in patients with COVID-19 (37, 38).
Our findings differ from those recently reported by a single-center study that found an association between therapeutic anticoagulation and decreased mortality in patients with COVID-19 who are mechanically ventilated (2). However, the latter study was limited by its inability to account for immortal time bias or severity-of-illness differences between groups. Furthermore, the reported short-term mortality of 62.7% in the patients who did not receive therapeutic anticoagulation is higher than in many cohorts and suggests the potential for residual confounding.
Our study has several strengths. We used granular data from many consecutive critically ill patients with laboratory-confirmed COVID-19, thereby minimizing selection or surveillance bias at each center. We included patients from 67 geographically diverse sites throughout the United States, thereby increasing the generalizability of our findings and allowing us to examine interhospital variation in the use of therapeutic anticoagulation. Furthermore, all data were obtained by detailed chart review rather than reliance on administrative or billing codes, which have well-described limitations in general (31–33, 39) and in the context of VTE (40). Very few data were missing for the covariates in our target trial emulation, which was the primary analysis in this study. Our target trial emulation included robust methods to account for immortal time bias and confounding by indication. Finally, results were similar across multiple sensitivity and subgroup analyses.
Our study has several limitations. First, as with all observational analyses, we cannot exclude the possibility of residual confounding. Second, we only captured data on VTE and major bleeding events for the first 14 days after ICU admission and thus likely underestimated their true incidence in this population. Third, VTE events (and pulmonary emboli, in particular) were likely underdiagnosed given the logistic challenges of imaging in critically ill patients with COVID-19 (41). Nevertheless, the considerably lower incidence of VTE in our study than in prior studies raises questions about the appropriateness of institutional policies advocating universal dose escalation of prophylactic anticoagulation in critically ill patients with COVID-19.
Using data from a nationally representative multicenter cohort study of critically ill adults with COVID-19 in the United States, we found the rates of radiographically confirmed VTE and major bleeding to be 6.3% and 2.8%, respectively. Male sex and higher D-dimer levels were independently associated with VTE. Patients who received therapeutic anticoagulation in the first 2 days of ICU admission had similar in-hospital survival compared with those who did not. Our findings do not support early empirical use of therapeutic anticoagulation in critically ill patients with COVID-19. These findings highlight the need for well-designed, adequately powered randomized clinical trials of therapeutic anticoagulation in critically ill patients with COVID-19.
Supplementary Material
Footnotes
This article was published at Annals.org on 26 January 2021
* Drs. Al-Samkari and Gupta contributed equally to this work.
† A full list of the STOP-COVID investigators is provided in the Supplement.
References
- 1. Tremblay D , van Gerwen M , Alsen M , et al. Impact of anticoagulation prior to COVID-19 infection: a propensity score-matched cohort study [Letter]. Blood. 2020;136:144-147. [PMID: ] doi: 10.1182/blood.2020006941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lala A , Johnson KW , Januzzi JL , et al; Mount Sinai COVID Informatics Center. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533-546. [PMID: ] doi: 10.1016/j.jacc.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cui S , Chen S , Li X , et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421-1424. [PMID: ] doi: 10.1111/jth.14830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klok FA , Kruip MJHA , van der Meer NJM , et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [PMID: ] doi: 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fox SE , Akmatbekov A , Harbert JL , et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681-686. [PMID: ] doi: 10.1016/S2213-2600(20)30243-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Helms J , Tacquard C , Severac F , et al; CRICS TRIGGERSEP Group. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089-1098. [PMID: ] doi: 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Middeldorp S , Coppens M , van Haaps TF , et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995-2002. [PMID: ] doi: 10.1111/jth.14888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lodigiani C , Iapichino G , Carenzo L , et al; Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9-14. [PMID: ] doi: 10.1016/j.thromres.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gupta S , Hayek SS , Wang W , et al; STOP-COVID Investigators. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020. [PMID: ] doi: 10.1001/jamainternmed.2020.3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Connolly SJ , Crowther M , Eikelboom JW , et al; ANNEXA-4 Investigators. Full study report of andexanet alfa for bleeding associated with factor xa inhibitors. N Engl J Med. 2019;380:1326-1335. [PMID: ] doi: 10.1056/NEJMoa1814051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schulman S , Kearon C ; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 12. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: J Wiley; 1987.
- 13. García-Albéniz X , Hernán MA , Logan RW , et al. Continuation of annual screening mammography and breast cancer mortality in women older than 70 years. Ann Intern Med. 2020;172:381-389. doi: 10.7326/M18-1199 [DOI] [PubMed] [Google Scholar]
- 14. Emilsson L , García-Albéniz X , Logan RW , et al. Examining bias in studies of statin treatment and survival in patients with cancer. JAMA Oncol. 2018;4:63-70. [PMID: ] doi: 10.1001/jamaoncol.2017.2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gupta S , Wang W , Hayek SS , et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181:41-51. [PMID: ] doi: 10.1001/jamainternmed.2020.6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shaefi S, Brenner SK, Gupta S, et al. Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Medicine. [Forthcoming]. [DOI] [PMC free article] [PubMed]
- 17. Giammaria D , Pajewski A . Can early treatment of patients with risk factors contribute to managing the COVID-19 pandemic. J Glob Health. 2020;10:010377. [PMID: ] doi: 10.7189/jogh.10.010377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vincent JL , Moreno R , Takala J , et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. on behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 19. Xu S , Ross C , Raebel MA , et al. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010 Mar-Apr;13:273-7. [PMID: ] doi: 10.1111/j.1524-4733.2009.00671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hernán MA , Robins JM . Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183:758-64. [PMID: ] doi: 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Danaei G , García Rodríguez LA , Cantero OF , et al. Electronic medical records can be used to emulate target trials of sustained treatment strategies. J Clin Epidemiol. 2018;96:12-22. [PMID: ] doi: 10.1016/j.jclinepi.2017.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Desborough MJR , Doyle AJ , Griffiths A , et al. Image-proven thromboembolism in patients with severe COVID-19 in a tertiary critical care unit in the United Kingdom [Letter]. Thromb Res. 2020;193:1-4. [PMID: ] doi: 10.1016/j.thromres.2020.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Al-Samkari H , Karp Leaf RS , Dzik WH , et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489-500. [PMID: ] doi: 10.1182/blood.2020006520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chi G , Lee JJ , Jamil A , et al. Venous thromboembolism among hospitalized patients with COVID-19 undergoing thromboprophylaxis: a systematic review and meta-analysis. J Clin Med. 2020;9. [PMID: ] doi: 10.3390/jcm9082489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goyal P , Choi JJ , Pinheiro LC , et al. Clinical characteristics of covid-19 in New York City [Letter]. N Engl J Med. 2020;382:2372-2374. [PMID: ] doi: 10.1056/NEJMc2010419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang C , Zhang Z , Mi J , et al. The cumulative venous thromboembolism incidence and risk factors in intensive care patients receiving the guideline-recommended thromboprophylaxis. Medicine (Baltimore). 2019;98:e15833. [PMID: ] doi: 10.1097/MD.0000000000015833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mo P , Xing Y , Xiao Y , et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020. [PMID: ] doi: 10.1093/cid/ciaa270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lighter J , Phillips M , Hochman S , et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission [Letter]. Clin Infect Dis. 2020;71:896-897. [PMID: ] doi: 10.1093/cid/ciaa415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simonnet A , Chetboun M , Poissy J , et al; LICORN and the Lille COVID-19 and Obesity study group. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020;28:1195-1199. [PMID: ] doi: 10.1002/oby.22831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Short S, Gupta S, Brenner S, et al. D-dimer and death in critically ill patients with COVID-19. Crit Care Med. [Forthcoming]. [DOI] [PMC free article] [PubMed]
- 31. Dickerman BA , García-Albéniz X , Logan RW , et al. Avoidable flaws in observational analyses: an application to statins and cancer. Nat Med. 2019;25:1601-1606. [PMID: ] doi: 10.1038/s41591-019-0597-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petito LC , García-Albéniz X , Logan RW , et al. Estimates of overall survival in patients with cancer receiving different treatment regimens: emulating hypothetical target trials in the surveillance, epidemiology, and end results (SEER)-medicare linked database. JAMA Netw Open. 2020;3:e200452. [PMID: ] doi: 10.1001/jamanetworkopen.2020.0452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Admon AJ , Donnelly JP , Casey JD , et al. Emulating a novel clinical trial using existing observational data. predicting results of the PreVent study. Ann Am Thorac Soc. 2019;16:998-1007. [PMID: ] doi: 10.1513/AnnalsATS.201903-241OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grodstein F , Stampfer MJ , Manson JE , et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 35. Hernán MA , Alonso A , Logan R , et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19:766-79. [PMID: ] doi: 10.1097/EDE.0b013e3181875e61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. RECOVERY Collaborative Group; Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 37. Bikdeli B , Madhavan MV , Jimenez D , et al; Global COVID-19 Thrombosis Collaborative Group. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950-2973. [PMID: ] doi: 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thachil J , Juffermans NP , Ranucci M , et al. ISTH DIC subcommittee communication on anticoagulation in COVID-19. J Thromb Haemost. 2020;18:2138-2144. [PMID: ] doi: 10.1111/jth.15004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Walraven C, Bennett C, Forster AJ.. Administrative database research infrequently used validated diagnostic or procedural codes. J Clin Epidemiol. 2011;64:1054-9. [PMID: ] doi: 10.1016/j.jclinepi.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 40. Fang MC , Fan D , Sung SH , et al. Validity of using inpatient and outpatient administrative codes to identify acute venous thromboembolism: the CVRN VTE study. Med Care. 2017;55:e137-e143. [PMID: ] doi: 10.1097/MLR.0000000000000524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wichmann D , Sperhake JP , Lütgehetmann M , et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268-277. doi: 10.7326/M20-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.