Abstract
There has been much recent interest in the protein “corona,” the nonspecific adsorption of proteins on the surface of nanoparticles used in biological applications. This research investigates an analogous DNA corona. We find that particles (200 nm and 1 μm) incubated with DNA form a DNA corona, with a higher concentration of DNA adsorbed on the surface of cationic nanoparticles. With protein present, a combined DNA and protein corona is formed although DNA in solution displaces protein from the nanoparticle surface. Displacement of protein from the nanoparticle surface is dependent on the concentration of DNA in solution and was also observed for planar surfaces. Overall, we expect this investigation of the DNA corona to be important for nanomedicine applications, as well as disease states, especially systemic lupus erythematosus, in which biological particles with bound DNA are important mediators of inflammation and thrombosis.
I. INTRODUCTION
Nanoparticles (NPs) used in biological applications such as drug and gene delivery, diagnostics, and sensing are exposed to extracellular proteins. These proteins adsorb on the surface of NPs to form a structure known as a protein “corona.”1–13 However, the in vivo milieu consists of many different types of biomolecules, not just proteins. For example, recent work has examined the formation of lipid and metabolite coronas on NPs.14–17 The interaction of these nonprotein biomolecules with NPs can influence the size and properties of NPs both directly, through the formation of their own corona, and indirectly, as they influence the protein corona.
To the best of our knowledge, no previous studies have examined the formation of coronas composed of DNA. DNA is an important biomolecule, not only intracellularly as the carrier of genetic information, but also extracellularly, with DNA released during cell death present in the blood.18,19 This extracellular DNA has potent biological activities and can promote inflammation and thrombosis in many diseases, especially systemic lupus erythematosus (SLE).20–23 In SLE, extracellular DNA released during cell death can act as a damage-associated molecular pattern to induce inflammation.18,24,25 Extracellular DNA can be free or can decorate the surface of naturally occurring extracellular vesicles (EVs).26–28 The work described below provides a model to examine the interaction of DNA with synthetic NPs, as well as the combined DNA-protein-NP interaction.
The formation of a corona is often described as a nonspecific, unintended, and often undesired, consequence of NPs used in applications with biomolecules present. In comparison, targeted DNA-NP interactions are well-studied, especially for gold NPs, for use of DNA-functionalized NPs in diagnostic and nanomedicine applications.29–31 It is important to note that these studies typically use thiol-modified DNA for gold NP functionalization rather than nonspecific DNA adsorption on the NP surface. Similarly, DNA-carbon nanotube interactions are well-studied with the use of single-stranded DNA on the carbon nanotube surface for dispersion in solution.32–37 In comparison, our experiments are designed to investigate the nonspecific adsorption of unmodified, double-stranded DNA on the surface of NPs. Previous studies in this area were application driven. For example, the adsorption of plasmid DNA on small (2–4 nm) gold NPs was used for gene delivery.38,39 In all cases, electrostatics of the highly negatively charged DNA dominate the interaction with the NP. Molecular-level studies of DNA and gold NPs (14 nm) showed that the interaction is also DNA sequence-dependent.40
Even for thiol-modified DNA and gold NPs, previous work is important in showing that DNA on the surface of NPs can influence subsequent cellular interaction. For example, it has been shown that the concentration of DNA on gold NPs influences protein corona formation and the subsequent cellular interaction.41–43 We expect that nonspecific DNA adsorption will also be important for protein corona formation and cellular responses. In addition, DNA adsorbed on the surface of particles has important implications for autoimmune diseases, such as SLE, where the presence of DNA-coated biological particles is important for forming immune complexes that promote inflammation by stimulating the nucleic acid sensors of the innate immune system.22,26–28,44–46 Immune complexes can also deposit in the tissue, especially the kidney, to activate complement and incite local inflammation.28 We expect that mechanistic studies of DNA coronas on synthetic NPs will inform future studies of DNA interactions with EVs, a biological particle.
Our results show that DNA forms a corona on the surface of both cationic and anionic NPs, but with a greater concentration of DNA on the cationic NPs. DNA decreases the protein corona by inhibiting the adsorption of protein and displacing protein pre-adsorbed on the NP surface. These results are not specific to NPs. Experiments with protein-coated planar surfaces show that DNA also displaces protein from the surface. Overall, we hope that this study of the DNA corona and the combined DNA and protein corona lays the groundwork for subsequent studies of DNA-protein-particle interactions, including the biological particles relevant to SLE.
II. EXPERIMENT
A. NPs and characterization
Polystyrene NPs (200 nm and 1 μm) were used for all experiments. 200 nm NPs were either cationic, amine-modified (No. A37356, Thermo Fisher Scientific, Carlsbad, CA) or anionic, carboxylate-modified (No. C37486, Thermo Fisher), as noted in the text. Experiments with 1 μm NPs used only amine-modified NPs (No. A37362, Thermo Fisher). Prior to all experiments, the 200 nm amine-modified NPs were sonicated with a cup horn sonicator (10 s ON/10 s OFF/10 s ON, 20% amplitude; Q500, QSonica, Newtown, CT). The other NPs were vortexed briefly and then sonicated with a benchtop sonicator (5 min; Branson, Danbury, CT). Diameter, polydispersity index, and zeta potential of the NPs (8 and 0.8 pM for 200 nm and 1 μm, respectively, in water) were measured using dynamic light scattering (DLS; Malvern Zetasizer, Nano-Z, Malvern Instruments, Worcestershire, England). Measurements were carried out in triplicate with three distinct solutions. Each measurement consisted of 30 runs. Electrophoretic mobility was converted to a zeta potential using the Smoluchowski approximation. Transmission electron microscopy (TEM) was carried out with a FEI Tecnai G2 TWIN at the Shared Materials Instrumentation Facility at Duke University. Images were obtained at 160 kV with 17 kX magnification. NPs were drop cast on carbon-coated copper grids (No. FCF200-Cu, Electron Microscopy Sciences, Hatfield, PA) and dried at room temperature (RT). Diameters were measured with ImageJ.47 Average and standard deviation are reported for all measurements.
B. DNA corona formation and characterization
A DNA corona was formed by incubating NPs (880 pM) with calf thymus DNA (1 mg/ml, unless otherwise noted in the text, No. 15633019, Thermo Fisher) in Dulbecco's phosphate buffered saline (PBS; No. 21300025, Thermo Fisher), pH 7.3–7.5. The DNA is sheared by the manufacturer to ≤2000 base pairs. The DNA and NP mixture was briefly vortexed and then incubated at RT for 30 min. Unbound DNA was removed by a washing process of repeated centrifugation (18 000 rcf, 15 min) and resuspension in PBS (×3).
DNA concentration was measured with a double-stranded DNA assay (SpectraMax Quant AccuClear Nano, Molecular Devices, San Jose, CA) according to the manufacturer's instructions. Fluorescence intensity (Excite: 468 nm, Emit: 507 nm) was measured with a plate reader (SpectraMax iD3, Molecular Devices). The background signal from the reagent was subtracted from the signal. Average and standard deviation of three distinct samples are shown. Significance was determined by a two-tailed Student's t-test. DNA was imaged with gel electrophoresis [1% agarose (No. A9539, Sigma-Aldrich, St. Louis, MO) in Tris-borate-EDTA buffer; 150 V, 45 min] with ethidium bromide (EtBr; 0.5 μg/ml, No. E1510, Sigma-Aldrich) present in the gel. TrackIt Cyan Orange was used as the loading buffer (No. 10482028, Thermo Fisher) and a 1 kb DNA ladder was included (No. 10488085, Thermo Fisher). The gel was imaged with a PhotoDoc-It imager (Analytik Jena, Germany).
C. Bovine serum albumin corona formation and characterization
A bovine serum albumin (BSA) corona was formed by incubating amine-modified NPs (880 pM) with BSA (1.5 mg/ml, No. A2153, Sigma-Aldrich). The NP and BSA mixture was vortexed briefly and then incubated at RT for 30 min. Unbound and weakly bound protein was removed by centrifugation (18000 rcf, 15 min) and resuspension in PBS (×3).48 The final BSA-NP pellet was resuspended in 25 μl PBS for use in experiments.
Protein concentration was determined using a bicinchoninic acid assay (No. 23250, Thermo Fisher) according to the manufacturer's instructions with the NPs removed prior to measurement (18 000 rcf, 15 min). Average and standard deviation of three distinct samples are shown. Significance was determined by a two-tailed Student's t-test. Residual protein present in DNA was analyzed with gel electrophoresis. Samples (1 mg/ml) were heated for 5 min at 100 °C in the loading buffer (Laemmli loading buffer, No. BP-110R, Boston BioProducts, Ashland, MA) and then loaded onto a gel (Tris-glycine sodium dodecyl sulfate gel, No. 4561096, Bio-Rad, Hercules, CA) for gel electrophoresis (230 V, 35 min). A 10–250 kDa molecular weight marker (No. 1610374, Bio-Rad) was included. Gels were stained for 1 h (SimplyBlue Safe Stain, No. LC6060, Thermo Fisher), destained overnight, and then imaged (PhotoDoc-It). The purity of the DNA was determined by spectrophotometry (SpectraMax iD3) using the 260/280 ratio.
D. Sequential and simultaneous corona formation
To examine the effect of protein on a DNA corona or DNA on a protein corona, we first formed either a DNA or protein corona, as described above, and then incubated the biomolecule-NP complex with the other biomolecule (30 min, RT). We refer to this process as sequential corona formation. The resulting coronas are described as either DNA/BSA coronas, with the DNA corona formed first, or BSA/DNA coronas with the BSA corona formed first. We compared these coronas to a simultaneous corona formation with premixed solutions of BSA (1.5 mg/ml) and DNA (1 mg/ml) incubated with NPs (amine-modified, 200 nm) in PBS for 30 min at RT.
E. Detection of BSA and DNA on planar surfaces by ELISA
Briefly, 96-well plates (Immulon 2HB; Thermo Fisher Scientific) were coated with 100 μl/well of solutions of 1× ELISA coating buffer (0.1M sodium phosphate buffer, pH 9.0) with or without 100 ng/ml of BSA (No. A2934, Sigma-Aldrich). The plates were incubated at RT (19–23 °C) for 60 min. On completion of incubation, the plates were washed 3× with 1× PBS-free (Ca2+ free, Mg2+ free), pH 7.4. Next, 100 μl/well of native, double-stranded calf thymus DNA (Worthington Biochemical Corp., Lakewood, NJ) at concentrations from 0 to 5000 ng/ml was distributed to the ELISA plates in wells with or without BSA precoating. The format was established so that each combination of BSA and DNA could be assessed in subsequent immunoassays. All assays were performed with duplicate wells. The plates were incubated overnight at 4 °C. The plates were then washed 3× with 1× PBS-free and, as appropriate, incubated with 100 μl/well of a mouse antibovine serum albumin antibody (anti-BSA; 1:30 000; Abcam, Cambridge, MA), a human anti-DNA (VAL-1205; 200 ng/ml; a kind gift from Valerion Therapeutics, Concord, MA), or no antibody. All antibody solutions were prepared in 1× ELISA dilution buffer (EDB; 0.1% BSA, 0.05% Tween-20 in 1× PBS-free). Wells with no antibody received 100 μl/well of EDB alone. Plates were incubated at RT for 60 min. After 1 h, the wells were washed 3× with 1× PBS-free. The wells were then incubated, as appropriate, with 100 μl/well of either a 1:1000 dilution of antihuman IgG (γ-chain specific)-HRP conjugated or a 1:500 dilution of antimouse IgG (γ-chain specific)-HRP conjugated (both Sigma-Aldrich); antibodies were diluted with EDB. Wells without antibody also received antimouse and antihuman detection antibodies to investigate background binding levels. Plates were incubated at RT for 60 min. Following this incubation, the wells were washed 3× with 1× PBS-free and then incubated with 100 μl/well of TMB substrate solution [0.01% H2O2, 0.015% 3,3′,5,5′-tetramethylbenzidine dihydrochloride (both from Sigma-Aldrich) in 0.1M citrate buffer, pH 4.0]. The plates were incubated in the dark for 30 min at RT. The reaction was stopped by the addition of 100 μl/well of 2M H2SO4. The plates were then analyzed for absorbance levels at 450 nm using a spectrophotometer (Molecular Devices, San Jose, CA).
III. RESULTS AND DISCUSSION
A. NP characterization: DLS and TEM
Polystyrene NPs were selected for experiments as highly dispersed, homogeneous, NPs used in extensive previous protein corona experiments (Table I and Fig. S1).48–53 DLS and TEM values are generally in agreement with supplier-provided values, which are used to describe the NPs in the text. It is worth noting that the zeta potential of the 1 μm amine-modified NPs (+9 ± 3 mV) is significantly lower than the zeta potential of the 200 nm amine-modified NPs (+35 ± 2 mV).
TABLE I.
NP characterization; hydrodynamic diameter (dh), polydispersity index (PDI), zeta potential (ZP), and TEM diameter (Fig. S1).53
| NPs | dh (nm) | PDI | ZP (mV) | TEM (nm), n = 50 |
|---|---|---|---|---|
| 200 nm Amine | 273 ± 23 | 0.13 ± 0.02 | +35 ± 2 | 223 ± 8 |
| 200 nm Carboxylate | 227 ± 3 | 0.03 ± 0.01 | −46 ± 1 | 189 ± 12 |
| 1 μm Amine | 1132 ± 23 | 0.12 ± 0.06 | +9 ± 3 | 1055 ± 39 |
B. DNA corona forms on NPs with a greater DNA concentration on cationic NPs
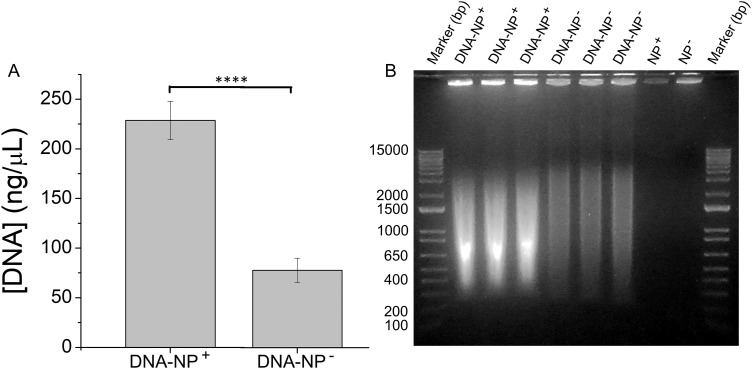
We first tested the formation of a DNA corona by incubating DNA (1 mg/ml) with either cationic (NP+) or anionic (NP−) polystyrene NPs (200 nm). This concentration of DNA was chosen based on previous measurements of DNA in mouse plasma.54 Two methods were used to probe DNA corona formation (Fig. 1). A fluorescence assay (AccuClear Nano) using a reagent that binds to dsDNA was used to measure DNA concentration [Fig. 1(a)]. Gel electrophoresis and EtBr staining were used to visualize DNA [Fig. 1(b)]. The NPs and DNA were incubated in PBS (30 min, RT) and then “washed,” as described in the Experimental section, to remove unbound DNA (Fig. S2),53 forming what is referred to as a “hard” corona in the protein corona literature.55–57
FIG. 1.
DNA adsorbs on the surface of NPs. (a) A fluorescence assay (AccuClear Nano) was used to measure the concentration of DNA adsorbed on amine- (NP+) and carboxylate- (NP−) modified NPs (200 nm). Experiments were carried out in triplicate and error bars show standard deviation. ****p < 0.0001. (b) Gel electrophoresis shows the DNA adsorbed on the NP surface. NPs are visible in the wells due to staining of the NPs by EtBr. Equal concentrations of NP+ and NP− were loaded for all experiments.
Both types of measurements show that DNA adsorbs on cationic (229 ± 19 ng/μl) and anionic (78 ± 12 ng/μl) NPs, with a greater concentration of DNA on the cationic NPs (Fig. 1). This is expected for a highly negatively charged molecule such as DNA. The DNA adsorbed on the anionic NPs is likely due to a combination of non-electrostatic interactions such as hydrogen bonding and van der Waals forces, as well as a small amount of histone proteins present in the DNA (Fig. S3).53 A similar interaction is observed between anionic NPs and anionic proteins.48,51 These results show that DNA forms a corona on synthetic NPs, analogous to a protein corona. All subsequent experiments used cationic, amine-modified, NPs (200 nm and 1 μm). The DNA corona results in a large increase in the hydrodynamic diameter of the NPs and a shift to a negative zeta potential (Table II), reflecting the negative charge of the DNA. The concentration of the DNA corona is also reported as a function of NP surface area (Table II), which shows no significant difference in the ratios of DNA (ng) to particle surface area (mm2, calculated using the TEM diameters) for the 200 nm and 1 μm particles. A wider range of particle diameters would need to be examined to determine whether this ratio is general.
TABLE II.
DNA-NP characterization.
| DNA-NPs | dh (nm) | Δdh (nm) | PDI | ZP (mV) | ΔZP (mV) | DNA/NP (ng/mm2) |
|---|---|---|---|---|---|---|
| 200 nm Amine | 1185 ± 10 | 912 | 0.25 ± 0.03 | −34 ± 2 | −69 | 0.49 ± 0.2 |
| 1 μm Amine | 1745 ± 56 | 613 | 0.17 ± 0.04 | −23 ± 5 | −32 | 0.37 ± 0.1 |
A specific interest in our study is the nature of the corona that results from the presence of both DNA and protein: DNA will not be the sole biomolecule in any biological environment. To address this question, we developed a protocol to first form either a DNA or protein corona and then incubated the biomolecule-NP complex with other biomolecule. We refer to this process as sequential corona formation. BSA was selected as a representative serum protein as the main (55%) protein present in serum.58 The resulting coronas are described as either DNA/BSA coronas, with the DNA corona formed first, or BSA/DNA coronas with the BSA corona formed first. In comparison, a corona formed simultaneously from a mixture of DNA and BSA is referred to as a DNA + BSA corona.
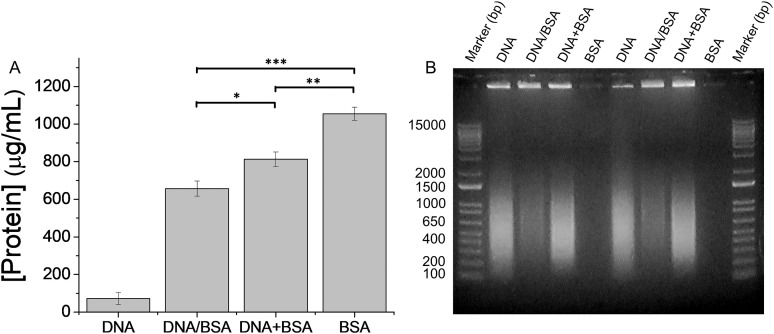
Incubation of NPs with DNA results in a DNA corona (Fig. 1 and Table II). A protein assay shows that this DNA corona contains a small amount of protein (72 ± 32 μg/ml) due to histones that are not fully removed during the DNA preparation (Fig. S3;53 260/280 = 1.9). Incubation of the DNA-NP complexes with BSA (1.5 mg/ml) results in the adsorption of protein (657 ± 40 μg/ml) forming a combined DNA and protein corona (Fig. 2). DNA quantification with AccuClear Nano is not compatible with the presence of BSA. Gel electrophoresis is used to provide a qualitative measure of DNA concentration. Similarly, a corona formed from a mixture of DNA and BSA results in protein (813 ± 39 μg/ml) and DNA adsorption on the NP surface (Fig. 2). In both cases, the combined DNA and protein corona has less protein adsorbed on the NP surface than a pure BSA corona (1055 ± 34 μg/ml). In the case of DNA/BSA-NPs, the initial DNA adsorption results in NPs with a negative effective surface charge (ZP = −34 ± 2 mV, Table II) that will adsorb less negatively charged BSA than the bare cationic NPs. In the case of a corona formed from a mixture of DNA and BSA, the presence of DNA in solution, rather than pre-adsorbed on the NP surface, results in greater BSA adsorption than the sequential corona formation (DNA/BSA), but still less BSA adsorption than a corona formed from BSA alone.
FIG. 2.
Changes in the DNA corona in response to protein (BSA) in solution. (a) Concentration of protein present on the surface of amine-modified NPs (200 nm) following sequential corona formation, with the DNA corona formed first (DNA/BSA) and simultaneous DNA and protein corona (DNA + BSA) formation. Experiments were carried out in triplicate and error bars show standard deviation. *p < 0.05, **p < 0.01, and ***p < 0.001. (b) Gel electrophoresis shows the DNA adsorbed on the NP surface. Equal concentrations of NPs (cationic, amine-modified, 200 nm) were loaded for all experiments. Control experiments show that DNA does not affect the protein quantification and that BSA does not affect the DNA gel (Fig. S4).53 DNA quantification with AccuClear Nano is not compatible with the presence of BSA. Gel electrophoresis is used to provide a qualitative measure of DNA concentration.
C. DNA in solution displaces the protein corona
Similar to the process described above for DNA-NPs (Fig. 2), we examined the response of BSA-NPs to DNA in solution (Fig. 3). Amine-modified NPs (200 nm) incubated with BSA form a protein corona [1055 ± 34 μg/ml, Fig. 3(a)]. When this BSA-NP complex is incubated with DNA (1 mg/ml, 30 min), the amount of BSA present on the NP surface decreases significantly (418 ± 38 μg/ml). This decrease in the protein corona is dependent on the concentration of DNA in solution [Fig. 3(b)]. We propose that this decreased protein corona is due to displacement of the BSA from the NP surface by DNA in solution. Control experiments show that DNA does not interfere with the measurement of protein concentration and that the number of wash steps, 3 for BSA-NPs and 6 for BSA/DNA-NPs, does not affect BSA adsorption (Fig. S4).53 After adding the DNA to the BSA-NPs, we measured the protein present in each wash step to trace the decrease in the protein corona [Fig. 3(c)]. Protein is detected in the first two wash steps (W1 = 224 ± 20 μg/ml, W2 = 80 ± 12 μg/ml), although not at a level that fully accounts for the difference between the BSA-NPs and BSA/DNA NPs (Δ = 637 μg/ml), suggesting some protein is lost during this process. A corona formed from a solution of DNA and BSA also results in a lower protein concentration on the NPs (813 ± 39 μg/ml) but not to the extent of sequential corona formation [Fig. 3(a)]. For both the sequential (BSA/DNA) and simultaneous (BSA + DNA) corona formation, DNA is visible in the corona [Fig. 3(d)].
FIG. 3.
Changes in the protein corona in response to DNA in solution. (a). Concentration of protein present on the surface of amine-modified NPs (200 nm) following sequential corona formation, with the BSA corona formed first (BSA/DNA), and simultaneous DNA and protein corona (DNA + BSA) formation. BSA, DNA + BSA, and DNA values are replotted from Fig. 2(a). NP concentrations are identical for all experiments and the DNA concentration in solution was 1 mg/ml. (b) Concentration of the protein present on the surface of BSA-NPs (200 nm) following incubation with increasing concentrations of DNA present in the solution. The value for 1 mg/ml DNA is replotted from (a). (c) Concentration of the protein present in the supernatant (Wash 1–3) after addition of DNA to the BSA-NPs for the formation of BSA/DNA-NPs shown in (a). (d) Gel electrophoresis shows the DNA adsorbed on the NP surface for the NPs shown in (a). Equal concentrations of NPs (amine-modified, 200 nm) were loaded for all experiments. Experiments in (a)–(c) were carried out in triplicate and error bars show standard deviation. **p < 0.01, ***p < 0.001, and ****p < 0.0001.
D. DNA displaces BSA from planar surfaces
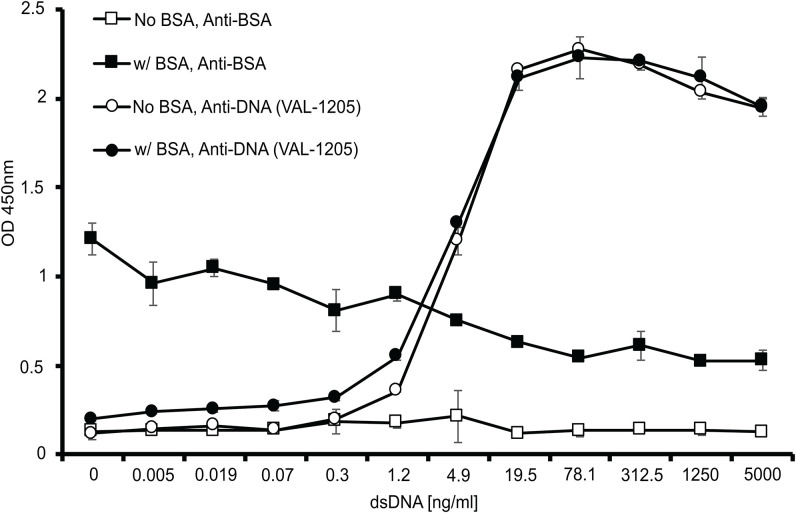
To determine whether the displacement of BSA from the NP surface was specific to NPs or a more general surface phenomenon, we carried out similar experiments using an ELISA-based format with planar polystyrene surfaces in place of polystyrene NPs. Plates were precoated with BSA to form the equivalent of a corona. The BSA-coated plates were then incubated with DNA. The adsorption of DNA on the planar surface and any change in the amount of adsorbed BSA is measured using antibodies against BSA and DNA (Fig. 4). The amount of DNA adsorbed on the plate surface was similar with or without the BSA precoating. Increasing the amount of DNA added to the plate led to a decrease in the amount of BSA present, similar to the DNA-mediated displacement of BSA from NPs [Fig. 3(b)].
FIG. 4.
Interaction of DNA with planar polystyrene surfaces coated with BSA. Wells of polystyrene microtiter plates were coated with BSA (100 ng/ml) as described in the Experimental section and, after washing, incubated overnight with DNA ranging in concentration from 0 to 5000 ng/ml. The amounts of BSA and DNA bound to the surface were assessed by ELISA using anti-BSA and anti-DNA (VAL-1205) antibodies, respectively. Error bars show the standard deviations from duplicate wells.
In the context of the interaction of DNA with an existing corona, these results suggest two possibilities. First, DNA could interact with the BSA so that very similar amounts of DNA bind to the plate whether or not the BSA is present. In this scenario, the interaction of DNA with the BSA would be comparable to that with polystyrene. Alternatively, DNA could displace BSA from the plate and have direct interaction with the polystyrene surface. Our data are consistent with the latter possibility since the amount of BSA detected is reduced as the concentration of DNA is increased (Fig. 4). It is nevertheless possible that the interaction of DNA with BSA can reduce the binding of the anti-BSA detection antibody.
IV. CONCLUSION
These experiments show that DNA can form a corona on NPs, analogous to the protein corona (Fig. 1 and Table II). In the presence of protein, DNA forms a combined DNA and protein corona (Figs. 2 and 3). For NPs with a pre-adsorbed protein corona, DNA in solution both displaces protein from the NP surface and adsorbs onto the protein-NP complexes (Fig. 3). A similar DNA concentration-dependent displacement of proteins from planar surfaces was also observed (Fig. 4). Much recent work has shown that a protein corona alters the in vivo transport and targeting of NPs designed as nanomedicines.59–63 Similarly, the presence of DNA on NPs is expected to affect the biological properties of NPs since DNA can induce production of cytokines and other proinflammatory mediators following uptake into cells and stimulation of internal nucleic acid sensors. In many diseases with nanomedicine applications, such as cancer, inflammation, and infection, levels of extracellular DNA are increased. It is possible that coronas formed in this disease environment would have higher concentrations of DNA leading to the stimulation of inflammation. The potential effects of DNA on the immunological properties of NPs could be further affected if DNA in the corona is resistant to nucleases, a future area of investigation. Taken together, these results suggest the DNA corona, and combined DNA and protein corona, could have important implications for nanomedicine as well as diseases such as SLE that are mediated by biological particles associated with DNA.
AUTHORS’ CONTRIBUTIONS
D.M.G. and D.T.J. contributed equally to this work.
ACKNOWLEDGMENTS
The authors thank Susan Thomas for the introduction of Payne and Pisetsky and Nathan Rayens for assistance with TEM and Duke University, a VA Merit Review Grant, and NIH (No. 1RO1AR073935) for funding.
Note: This paper is part of the Biointerphases Special Topic Collection on Protein Corona at Nanointerfaces.
REFERENCES
- 1.Fleischer C. C. and Payne C. K., Acc. Chem. Res. 47, 2651 (2014). 10.1021/ar500190q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Runa S., Hussey M., and Payne C. K., J. Phys. Chem. B 122, 1009 (2017). 10.1021/acs.jpcb.7b08650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Payne C. K., J. Chem. Phys. 151, 130901 (2019). 10.1063/1.5120178 [DOI] [PubMed] [Google Scholar]
- 4.Walczyk D., Bombelli F. B., Monopoli M. P., Lynch I., and Dawson K. A., J. Am. Chem. Soc. 132, 5761 (2010). 10.1021/ja910675v [DOI] [PubMed] [Google Scholar]
- 5.Del Pino P., Pelaz B., Zhang Q., Maffre P., Nienhaus G. U., and Parak W. J., Mater. Horiz. 1, 301 (2014). 10.1039/C3MH00106G [DOI] [Google Scholar]
- 6.Nienhaus K. and Nienhaus G. U., Curr. Opin. Biomed. Eng. 10, 11 (2019). 10.1016/j.cobme.2019.01.002 [DOI] [Google Scholar]
- 7.Park S. and Hamad-Schifferli K., Curr. Opin. Chem. Biol. 14, 616 (2010). 10.1016/j.cbpa.2010.06.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lynch I., Cedervall T., Lundqvist M., Cabaleiro-Lago C., Linse S., and Dawson K. A., Adv. Colloid Interfaces 134–135, 167 (2007). 10.1016/j.cis.2007.04.021 [DOI] [PubMed] [Google Scholar]
- 9.Walkey C. D. and Chan W. C. W., Chem. Soc. Rev. 41, 2780 (2012). 10.1039/C1CS15233E [DOI] [PubMed] [Google Scholar]
- 10.Ke P. C., Lin S., Parak W. J., Davis T. P., and Caruso F., ACS Nano 11, 11773 (2017). 10.1021/acsnano.7b08008 [DOI] [PubMed] [Google Scholar]
- 11.Mahmoudi M., Bertrand N., Zope H., and Farokhzad O. C., Nano Today 11, 817 (2016). 10.1016/j.nantod.2016.10.005 [DOI] [Google Scholar]
- 12.Yang S. T., Liu Y., Wang Y. W., and Cao A. N., Small 9, 1635 (2013). 10.1002/smll.201201492 [DOI] [PubMed] [Google Scholar]
- 13.Kobos L. and Shannahan J., WIRES Nanomed. Nanobiol. 12, e1608 (2020). 10.1002/wnan.1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hellstrand E., Lynch I., Andersson A., Drakenberg T., Dahlbäck B., Dawson K. A., Linse S., and Cedervall T., FEBS J. 276, 3372 (2009). 10.1111/j.1742-4658.2009.07062.x [DOI] [PubMed] [Google Scholar]
- 15.Olenick L. L. et al. , Chem 4, 2709 (2018). 10.1016/j.chempr.2018.09.018 [DOI] [Google Scholar]
- 16.Pink M., Verma N., Kersch C., and Schmitz-Spanke S., Environ. Sci. Nano 5, 1420 (2018). 10.1039/C8EN00161H [DOI] [Google Scholar]
- 17.Lima T., Bernfur K., Vilanova M., and Cedervall T., Sci. Rep. 10, 1 (2020). 10.1038/s41598-019-56847-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pisetsky D. S., Clin. Immunol. 144, 32 (2012). 10.1016/j.clim.2012.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nandi K., Verma R., Dawar R., and Goswami B., Horm. Mol. Biol. Clin. Investig. 41, 20190012 (2020). 10.1515/hmbci-2019-0012 [DOI] [PubMed] [Google Scholar]
- 20.Tan E. M., Schur P. H., Carr R. I., and Kunkel H. G., J. Clin. Invest. 45, 1732 (1966). 10.1172/JCI105479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pisetsky D. S., Rovin B. H., and Lipsky P. E., Arthritis Rheumatol. 69, 487 (2017). 10.1002/art.40008 [DOI] [PubMed] [Google Scholar]
- 22.Pisetsky D. S., F1000Research 8, 368 (2019). 10.12688/f1000research.17959.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pisetsky D. S., J. Autoimmun. 110, 102356 (2020). 10.1016/j.jaut.2019.102356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roh J. S. and Sohn D. H., Immune Netw. 18, e27 (2018). 10.4110/in.2018.18.e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderton H., Wicks I. P., and Silke J., Nat. Rev. Rheumatol. 16, 496–513 (2020). 10.1038/s41584-020-0455-8 [DOI] [PubMed] [Google Scholar]
- 26.Ullal A. J., Reich C. F. III, Clowse M., Criscione-Schreiber L. G., Tochacek M., Monestier M., and Pisetsky D. S., J. Autoimmun. 36, 173 (2011). 10.1016/j.jaut.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 27.Mobarrez F., Vikerfors A., Gustafsson J. T., Gunnarsson I., Zickert A., Larsson A., Pisetsky D. S., Wallén H., and Svenungsson E., Sci. Rep. 6, 36025 (2016). 10.1038/srep36025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mobarrez F., Svenungsson E., and Pisetsky D. S., Eur. J. Clin. Invest. 48, e13010 (2018). 10.1111/eci.13010 [DOI] [PubMed] [Google Scholar]
- 29.Giljohann D. A., Seferos D. S., Daniel W. L., Massich M. D., Patel P. C., and Mirkin C. A., Angew. Chem. Int. Ed. 49, 3280 (2010). 10.1002/anie.200904359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bardhan R., Lal S., Joshi A., and Halas N. J., Acc. Chem. Res. 44, 936 (2011). 10.1021/ar200023x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding Y., Jiang Z. W., Saha K., Kim C. S., Kim S. T., Landis R. F., and Rotello V. M., Mol. Therapy 22, 1075 (2014). 10.1038/mt.2014.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng M., Jagota A., Semke E. D., Diner B. A., McLean R. S., Lustig S. R., Richardson R. E., and Tassi N. G., Nat. Mater. 2, 338 (2003). 10.1038/nmat877 [DOI] [PubMed] [Google Scholar]
- 33.Johnson R. R., Johnson A. T. C., and Klein M. L., Nano Lett. 8, 69 (2008). 10.1021/nl071909j [DOI] [PubMed] [Google Scholar]
- 34.Manohar S., Tang T., and Jagota A., J. Phys. Chem. C 111, 17835 (2007). 10.1021/jp071316x [DOI] [Google Scholar]
- 35.Albertorio F., Hughes M. E., Golovchenko J. A., and Branton D., Nanotechnology 20, 395101 (2009). 10.1088/0957-4484/20/39/395101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tu X. M., Manohar S., Jagota A., and Zheng M., Nature 460, 250 (2009). 10.1038/nature08116 [DOI] [PubMed] [Google Scholar]
- 37.Salem D. P., Gong X., Liu A. T. X., Koman V. B., Dong J. Y., and Strano M. S., J. Am. Chem. Soc. 139, 16791 (2017). 10.1021/jacs.7b09258 [DOI] [PubMed] [Google Scholar]
- 38.Thomas M. and Klibanov A. M., Proc. Natl. Acad. Sci. U.S.A. 100, 9138 (2003). 10.1073/pnas.1233634100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandhu K. K., McIntosh C. M., Simard J. M., Smith S. W., and Rotello V. M., Bioconj. Chem. 13, 3 (2002). 10.1021/bc015545c [DOI] [PubMed] [Google Scholar]
- 40.Gearheart L. A., Ploehn H. J., and Murphy C. J., J. Phys. Chem. B 105, 12609 (2001). 10.1021/jp0106606 [DOI] [Google Scholar]
- 41.Giljohann D. A., Seferos D. S., Patel P. C., Millstone J. E., Rosi N. L., and Mirkin C. A., Nano Lett. 7, 3818 (2007). 10.1021/nl072471q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel P. C., Giljohann D. A., Daniel W. L., Zheng D., Prigodich A. E., and Mirkin C. A., Bioconj. Chem. 21, 2250 (2010). 10.1021/bc1002423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi C. H. J., Hao L. L., Narayan S. P., Auyeung E., and Mirkin C. A., Proc. Natl. Acad. Sci. U.S.A. 110, 7625 (2013). 10.1073/pnas.1305804110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mobarrez F., Fuzzi E., Gunnarsson I., Larsson A., Eketjall S., Pisetsky D. S., and Svenungsson E., J. Autoimmun. 102, 142 (2019). 10.1016/j.jaut.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 45.Ullal A. J., Marion T. N., and Pisetsky D. S., Clin. Immunol. 154, 178 (2014). 10.1016/j.clim.2014.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pisetsky D. S., Spencer D. M., Mobarrez F., Fuzzi E., Gunnarsson I., and Svenungsson E., Clin. Immunol. 212, 108349 (2020). 10.1016/j.clim.2020.108349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneider C. A., Rasband W. S., and Eliceiri K. W., Nat. Met. 9, 671 (2012). 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fleischer C. C. and Payne C. K., J. Phys. Chem. B 116, 8901 (2012). 10.1021/jp304630q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fleischer C. C. and Payne C. K., J. Phys. Chem. B 118, 14017 (2014). 10.1021/jp502624n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jayaram D. T., Runa S., Kemp M. L., and Payne C. K., Nanoscale 9, 7595 (2017). 10.1039/C6NR09500C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jayaram D. T., Pustulka S. M., Mannino R. G., and Lam W. A., Biophysical J. 115, 209 (2018). 10.1016/j.bpj.2018.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jayaram D. T., Kumar A., Kippner L. E., Ho P.-Y., Kemp M. L., Fan Y., and Payne C. K., RSC Adv. 9, 25039 (2019). 10.1039/C9RA04037D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.See supplementary material at 10.1116/6.0000439#suppl for TEM images of particles, control experiments for DNA corona formation and quantification, and protein quantification and DNA gel electrophoresis with mixtures of DNA and BSA. [DOI]
- 54.Björkman L., Reich C., and Pisetsky D., Scand. J. Immunol. 57, 525 (2003). 10.1046/j.1365-3083.2003.01261.x [DOI] [PubMed] [Google Scholar]
- 55.Casals E., Pfaller T., Duschl A., Oostingh G. J., and Puntes V., ACS Nano 4, 3623 (2010). 10.1021/nn901372t [DOI] [PubMed] [Google Scholar]
- 56.Monopoli M. P., Walczyk D., Campbell A., Elia G., Lynch I., Bombelli F. B., and Dawson K. A., J. Am. Chem. Soc. 133, 2525 (2011). 10.1021/ja107583h [DOI] [PubMed] [Google Scholar]
- 57.Kihara S., Van Der Heijden N. J., Seal C. K., Mata J. P., Whitten A. E., Köper I., and McGillivray D. J., Bioconj. Chem. 30, 1067 (2019). 10.1021/acs.bioconjchem.9b00015 [DOI] [PubMed] [Google Scholar]
- 58.Anderson N. L. and Anderson A. G., Mol. Cell. Proteomics 1, 845 (2002). 10.1074/mcp.R200007-MCP200 [DOI] [PubMed] [Google Scholar]
- 59.Aggarwal P., Hall J. B., McLeland C. B., Dobrovolskaia M. A., and McNeil S. E., Adv. Drug Deliver. Rev. 61, 428 (2009). 10.1016/j.addr.2009.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khlebtsov N. and Dykman L., Chem. Soc. Rev. 40, 1647 (2011). 10.1039/C0CS00018C [DOI] [PubMed] [Google Scholar]
- 61.Duan X. P. and Li Y. P., Small 9, 1521 (2013). 10.1002/smll.201201390 [DOI] [PubMed] [Google Scholar]
- 62.Chinen A. B., Guan C. M., Ko C. H., and Mirkin C. A., Small 13, 1603847 (2017). 10.1002/smll.201603847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilhelm S., Tavares A. J., Dai Q., Ohta S., Audet J., Dvorak H. F., and Chan W. C., Nat. Rev. Mater. 1, 16014 (2016). 10.1038/natrevmats.2016.14 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- See supplementary material at 10.1116/6.0000439#suppl for TEM images of particles, control experiments for DNA corona formation and quantification, and protein quantification and DNA gel electrophoresis with mixtures of DNA and BSA. [DOI]