Abstract
Objectives
To evaluate the ability of (1) a novel aEEG background evolution classification system and (2) specific hour of life (HOL) cut points when observation of aEEG normalization and development of cycling can predict adverse neurological outcomes in infants with HIE.
Study Design
Continuous aEEG data of term neonates with HIE were reviewed for background pattern and aEEG cycling from start of monitoring through rewarming. Infants were classified by overall background evolution pattern. Adverse outcomes were defined as death or severe MRI injury, as well as developmental outcomes in a subset of patients. aEEG characteristics were compared between outcome groups by multivariate regression models, likelihood ratios (LR), and receiver operating curve (ROC) analyses.
Results
Eighty infants receiving therapeutic hypothermia met inclusion criteria. Background evolution pattern seemed to distinguish outcome groups more reliably than background pattern at discrete intervals in time (LR 43.9, p-value <0.001). Infants who did not reach discontinuous background by 15.5 HOL, cycling by 45.5 HOL and normalization by 78 HOL were most likely to have adverse outcomes.
Conclusions
Evolution of aEEG in term neonates with HIE may be more useful at predicting outcome than evaluating aEEG at discrete intervals in time.
Keywords: hypoxic ischemic encephalopathy, monitoring, brain injury, magnetic resonance imaging, neurodevelopmental outcome
Background
While therapeutic hypothermia (TH) is now standard treatment for hypoxic ischemic encephalopathy (HIE) 1,2 a significant number of infants still suffer death or have long-term neurodevelopmental disability despite advances in neuroprotective treatments.3 aEEG has proven to be a good prognostic tool for neonates with HIE and can be used both to help evaluate therapeutic response as well as to aid with parental counseling and direction of care.4–7 However, recent studies have shown that treatment with hypothermia significantly lowers the predictive value of early aEEG.8–12 Since the predictive value of aEEG in neonates with HIE receiving TH improves over time, continuous aEEG throughout TH can aid in predicting outcome when compared to early aEEG alone.4,8,11,12 In particular, we and others have previously shown that severely abnormal aEEG background activity at greater than 36–48 HOL is predictive of adverse outcomes.4,8,11,12 Conversely, onset of sleep-wake cycle by 36 HOL is predictive of positive outcomes.4,8
Previous studies have described aEEG data analyzed in large 6–12 hour segments and used these interpretations at discrete time points to discriminate between favorable and adverse outcomes. No research has focused on describing how aEEG backgrounds evolve over time and related these overall evolution patterns to patient outcomes. This is potentially important as other studies have suggested that the trajectory of neonatal encephalopathy by clinical exam rather than a single assessment is a more reliable predictor of outcome in infants with HIE undergoing TH.13,14 Additionally, while previous studies have suggested important time intervals, no study to date has demonstrated specific HOL cut points that are useful to predict outcome. Assessment of the aEEG tracing in its entirety also allows for quantification of exact time to background improvement or normalization, as well as exact time to onset of aEEG cycling. Measuring the time to these positive prognostic features in the aEEG can provide practical cut points in time via a data-driven approach that can be used for clinical decision-making.
The objectives of this study are to (1) examine the predictive ability of a novel aEEG background evolution classification system to discriminate HIE infants with adverse neurological outcomes; and (2) establish cut points for latency to aEEG normalization and development of cycling that are most predictive of outcome in newborns with HIE.
Methods
Study Population
Neonates with HIE treated with whole-body hypothermia15 between May 2009 and November 2014 were enrolled in a prospective cohort study evaluating biomarkers of brain injury in HIE. Systemic hypothermia is provided to infants at our institution who meet the following criteria: ≥ 35 weeks gestation; ≥ 1800 grams birth weight; evidence of perinatal physiologic compromise (metabolic acidosis pH<7 or pH<7.15 with history of a perinatal sentinel event and low Apgar scores/ need for prolonged delivery room resuscitation); and moderate-severe encephalopathy15,16 on clinical exam. Infants with available and interpretable aEEG data initiated within 24 hours of life were included in this study. The Institutional Review Board at our hospital approved this study, and informed consent was obtained from the parents of each participant for data collection.
Data Collection
Clinical and demographic data were collected from the birth hospital and study site medical records. Continuous recordings of EEG data using a 10–20 international system neonatal montage are routinely performed in infants undergoing hypothermia from the time of admission through up to 12 hours post rewarming (Nihon Khoden, America, Inc., Irvine, CA). Biparietal (C3-P3, C4-P4) and cross-parietal (P3-P4) aEEG tracings were calculated off-line using Persyst v12 software (Persyst Development Corporation, AZ, USA). EEG is processed within Persyst with a time constant of 0.3sec, high pass filter at 70 Hz, notch filter at 60Hz. The aEEG tracing was derived using a proprietary filter developed by Persyst to mimic the standard 2 to 20 Hz asymmetric filter with a 50–60 Hz notch filter utilized to generate aEEG. 17 Reviewer display was set at an 8 hour window of aEEG tracing.
aEEG Scoring and Classification
Continuous aEEG data were reviewed by two investigators blinded to clinical and outcome data. For each infant, sequential 4-hour aEEG segments were scored for background pattern and cycling according to Hellstrom-Westas Classification.6 aEEG background patterns were classified as: continuous (C), discontinuous (DC), burst suppression (BS), low voltage (LV), flat (FT), or uninterpretable due to status epilepticus (SE). Cross parietal P3-P4 recordings were evaluated unless prevented by artifact or impedance in which case C3-P3 or C4-P4 recordings were used. If background pattern varied over the course of the segment, then the pattern that comprised the majority of the segment was used. Areas of suspected artifact (i.e. from patient movement, poor impedance or less commonly ECG) were verified by reviewing the raw EEG trace and aEEG tracings where artifact made aEEG uninterpretable were excluded. Discrepancies in scoring were resolved by consensus.
The background pattern score of the first and last segment for each aEEG were recorded and used to classify the aEEG into one of the six background pattern evolution categories (Table 1). If the background pattern did not evolve in a linear pattern, this was noted and discussed separately. Additionally, three latency factors for aEEG improvement were defined: the HOL that an infant achieved either a discontinuous (TTDC – time to discontinuous) or continuous normal voltage (TTN – time to normalization) background pattern, and onset of state changes (TTC – time to cycling). If TTDC/TTN/TTC did not occur before the end of the recording, a score of 99 was given.
Table 1:
aEEG background evolution categories
| Category | Description |
|---|---|
| 1. Persistently Normal (PN) | Continuous background at start and end of tracing |
| 2. Complete Normalization (CN) | Not continuous at start of tracing but Improved to continuous background and remained continuous by end of tracing |
| 3. Persistent Mild Abnormality (PMA) | Continuous or discontinuous at start and discontinuous at end of tracing |
| 4. Progression to Severe (PTS) | Continuous or discontinuous at start but progressed to abnormal background (burst suppression, low voltage, flat, or status epilepticus) at end of tracing |
| 5. Improved Without Normalization (IWN) | Abnormal background pattern at start (burst suppression, low voltage, flat, or status epilepticus) that improved at end of tracing but never reached continuous background |
| 6. Persistently Severe (PS) | Abnormal background pattern (burst suppression, low voltage, flat, or status epilepticus) at start and end of tracing |
MRI Scoring
MRI scans were performed per clinical protocol after completion of TH, typically in the second week of life (or prior to discharge). Scans were either performed on a 1.5T GE Signa (prior to 2010) or 3T Discovery 750 scanner (GE Healthcare, Milwaukee, WI, UA). Some infants underwent two MRIs due to a change in our imaging protocol during the study period. In these cases, the scan occurring in the second week of life was scored for this study. Standard anatomical sequences including T1, T2, and diffusion weighted images were read by an independent neuroradiologist blinded to aEEG and clinical data and scored according to Barkovich.18 Adverse outcome included death or severe MRI brain injury defined as BG score >2 or WS score >3.
Neurodevelopmental Outcomes
Surviving HIE infants are routinely followed in the Developmental Clinic through early childhood and developmental assessment scores at ~18 months of age were collected for this study. The Bayley Scales of Infant Development-II (BSID-II) 19 or Development-III (BSID-III) 20 were used per clinic protocol, 2009–2012 and 2013-present respectively, to assess developmental progress. The BSID-II and BSID-III are standardized tools that assess a child’s cognitive and motor skills. Given reports that the BSID-III overestimates developmental performance, 21–23 for the purposes of this study significant developmental delay was defined as a BSID-II Mental Developmental Index (MDI) or Psychomotor Developmental Index (PDI)<70 or BSID-III Cognitive Composite Score <85 or BDIS-III Motor Composite Score <80. Adverse outcome included death or severe developmental delay, as defined above.
Statistical Analyses
Descriptive data are presented as mean (± SD), median (Q1:Q3), or percent as appropriate. Univariate analyses with independent samples t-tests or Mann-Whitney U tests and chi square tests were used to evaluate differences between outcome groups. Logistic regression was used to adjust for covariates. Liklihood ratios were generated to compare the predictive ability of background evolution pattern and background patterns at discrete time intervals. Using the HOL that TTDC, TTN, and TTC were reached, receiver operating curves were generated to determine cut points for individual latency factors to distinguish outcome groups. These cut points were then combined to evaluate their ability to predict adverse outcomes. Analyses were performed using SPSS Statistics v23 (IBM Analytics, Armonk, New York, USA).
Results
Study Population
A total of 137 infants born during the study period were evaluated for inclusion in the study, of whom 80 met inclusion criteria and 57 were excluded (aEEG unavailable [n=36], uninterpretable due to artifact [n=17], or not initiated within 24 hours of life [n=2]). Of the 80 infants who were included in the study, 56 had favorable outcomes and 24 had adverse outcomes as defined by death (n= 9) or severe MRI brain injury (n=15). Characteristics of the study population are summarized in Table 2. Infants with adverse outcomes had lower 5-minute apgar scores (p=0.023), higher frequency of severe encephalopathy (p<0.001), and higher frequency of EEG seizures (p<0.001). Other demographic and clinical variables were similar between groups.
Table 2:
Study population characteristics.
| Survived with no/mild MRI Injury (n=56) | Death or Severe MRI Injury (n=24) | P value | |
|---|---|---|---|
| Gestational Age | 38.54 ± 1.67 | 39.04 ± 1.88 | 0.238 |
| Birth Weight | 3.20 ± 0.60 | 3.24 ± 0.66 | 0.793 |
| Gender (% male) | 62.5 | 50.0 | 0.330 |
| Race (%) | 0.654 | ||
| Caucasian | 42.9 | 41.7 | |
| Black | 50.0 | 50.0 | |
| Asian | 3.6 | 0.0 | |
| Other | 3.6 | 8.3 | |
| Ethnicity (% Hispanic) | 10.7 | 4.2 | 0.668 |
| Delivery (% cesarean) | 69.6 | 70.8 | 1.000 |
| Apgar 5 mina [median (range)] | 4 (0–9) | 3 (0–6) | 0.023 |
| Apgar 10 minb [median (range)] | 5 (0–8) | 4 (0–10) | 0.118 |
| Initial pHc | 6.97 ± 0.23 | 6.90 ± 0.20 | 0.193 |
| Initial Base Deficitd | 18.11 ± 5.00 | 20.48 ± 6.09 | 0.099 |
| Sarnat Stage III-Severe Encephalopathy (% Severe) | 5.4 | 45.8 | 0.000 |
| HOL at aEEG Start [median (range)] | 11.8 (4.9–22.1) | 9.1 (3.8–16.9) | 0.086 |
| Total EEG Recording Time | 78.58 ± 0.1.36 | 75.32 ± 3.45 | 0.817 |
| DOL at Time of MRI [median (range)] | 9 (4–16) | 10 (7–15) | 0.107 |
| Seizures (% electrographic) | 19.6 | 58.3 | 0.001 |
| Treatment for seizures (% treated) | 32.1 | 79.2 | 0.000 |
| Sedation (%) | 0.854 | ||
| None | 12.5 | 8.3 | |
| Intermittent | 50 | 54.2 | |
| Continuous | 37.5 | 37.5 | |
Data listed as mean ± SD unless otherwise specified.
Data available for 55/56 and 23/24 patients in the favorable and adverse outcome groups respectively.
Data available for 47/56 and 23/24 patients in the favorable and adverse outcome groups respectively.
Data available for 55/56 and 24/24 patients in the favorable and adverse outcome groups respectively.
Data available for 50/56 and 20/24 patients in the favorable and adverse outcome groups respectively.
HOL= hour of life; DOL= day of life
Evolution of aEEG Background Pattern
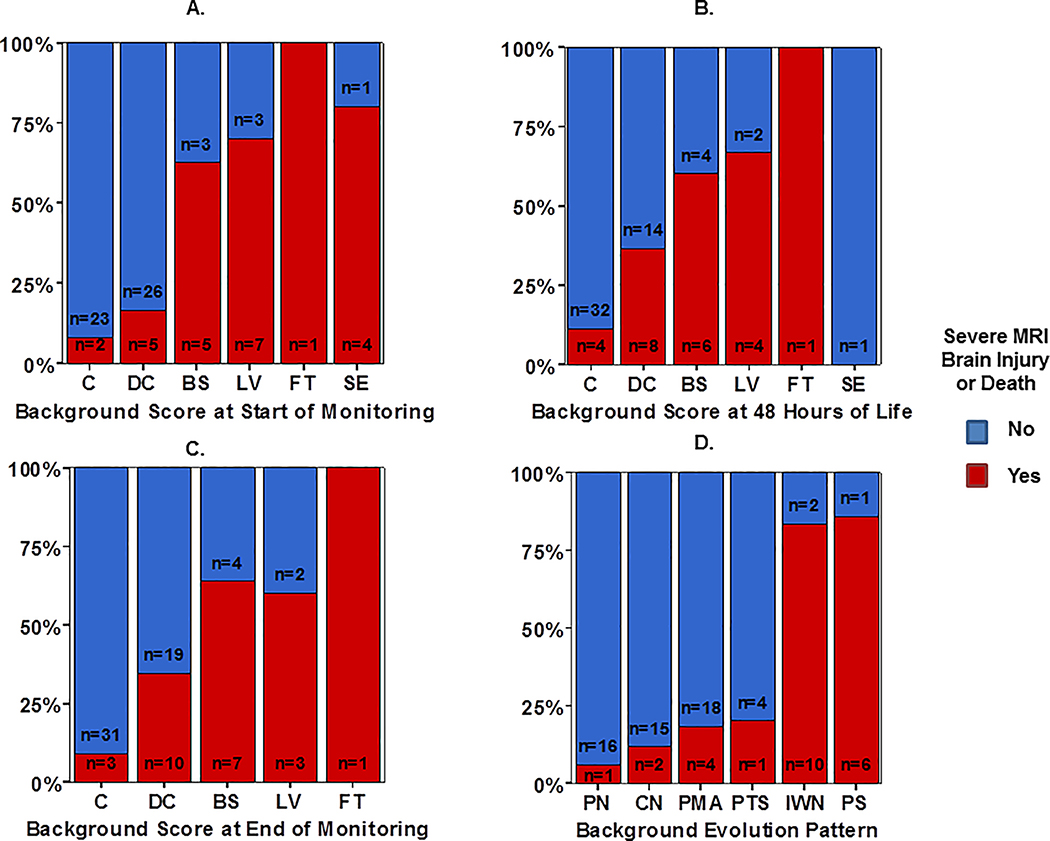
In general, distribution of aEEG background shifted from more to less severe patterns over the course of TH (Figure 1). As expected, aEEG background pattern was significantly predictive of outcome group at the start of monitoring (Figure 1a), 48 HOL (Figure 1b), and end of monitoring (Figure 1c). Background evolution classification seemed to distinguish outcome groups more reliably (Figure 1d), evidenced by a higher likelihood ratio (LR) compared to aEEG background assessment at the discrete time points assessed (Table 3). The background evolution classification score remained significantly associated with outcome (OR 1.1, p<0.001) even after controlling for gestational age, gender, and encephalopathy grade at presentation. Clinical encephalopathy stage (moderate versus severe15,16) was also independently associated with poor outcome (OR 2.8, p=0<001).
Figure 1:
Distribution of aEEG background pattern at the (A) start of monitoring, (B) 48 HOL, and (C) end of monitoring compared with overall aEEG background evolution classification (D), stratified by outcome group defined as severe MRI injury or death (red bar) or survived with normal/mild MRI (blue bar).
Table 3.
Comparison of aEEG Prediction of Outcomes
| Death or Severe MRI Injury | Death or Neurodevelopmental Delay | |||
|---|---|---|---|---|
| LR+ | P value | LR+ | P value | |
| aEEG at Start | 28.6 | <0.001 | 16.6 | 0.005 |
| aEEG at 48 hours | 18.1 | 0.002 | 30.2 | <0.001 |
| aEEG at End | 18.9 | 0.001 | 21.3 | <0.001 |
| aEEG Evolution | 35.4 | <0.001 | 30.1 | <0.001 |
LR+: likelihood ratio positive
Latency Factors
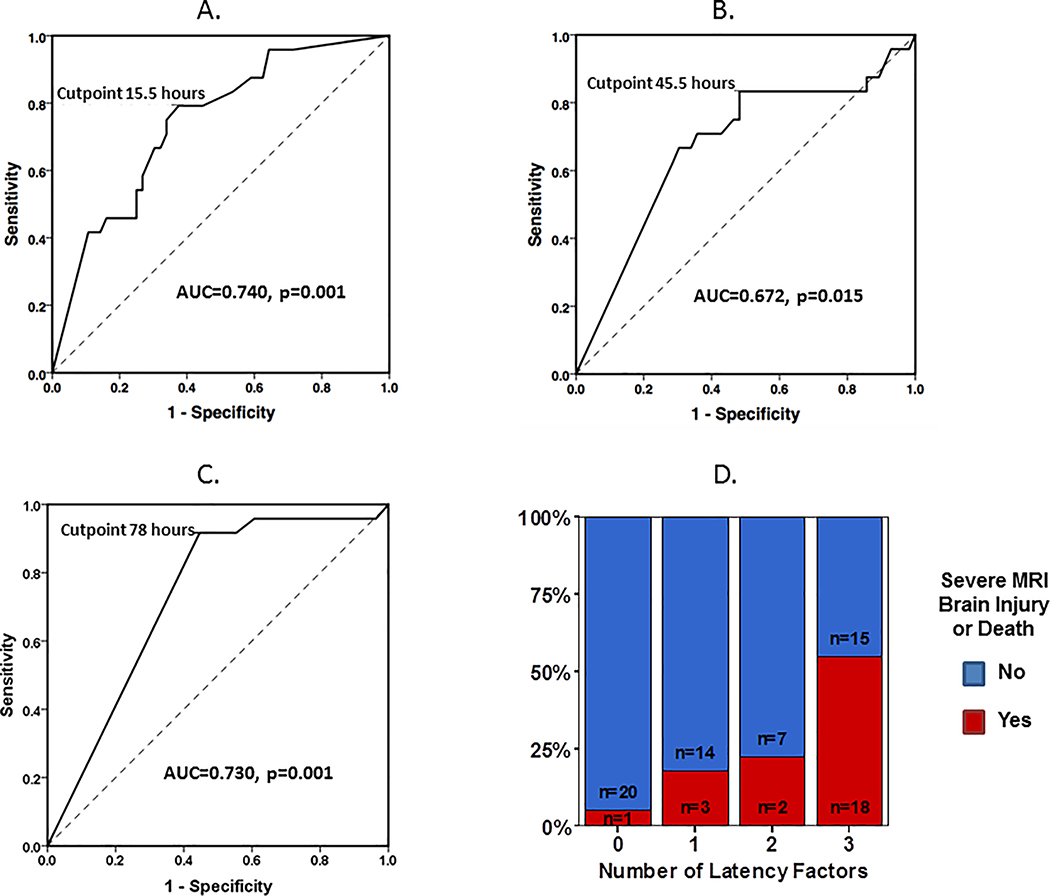
By the end of post-rewarming monitoring, aEEG improved to discontinuous background in 80% of patients, developed cycling in 61% of patients, and normalized to continuous background in 41% of patients. ROC analyses for TTDC, TTC, and TTN as a predictor of death or severe MRI brain injury are presented in Figure 2. AUC was 0.740 (p=0.001) for TTDC, 0.672 (p=0.015) for TTC, and 0.730 (p=0.001) for TTN. The optimal HOL cut points were 15.5 HOL for TTDC (0.792 sensitivity, 0.625 specificity), 45.5 HOL for TTC (0.833 sensitivity, 0.518 specificity) and 78 HOL for TTN (0.917 sensitivity, 0.554 specificity). Combining TTDC, TTC and TTN improved the specificity of the latency factors taken individually (Figure 2D). Infants who did not reach a discontinuous background pattern by 15.5 hours, demonstrate cycling by 45.5 hours and normalize by 78 hours of life (completion of rewarming) were likely to have adverse outcomes (sensitivity 0.750, specificity 0.732).
Figure 2:
ROC analyses for (A) time to discontinuous aEEG background pattern (TTDC), (B) time to aEEG cycling (TTC), and (C) time to normalization (TTN) as a predictor of death or severe MRI brain injury. (D) Comparison of favorable versus adverse outcome frequency based on number of latency factors (0=TTDC, TTC, TTN all below cut point, 1–2 factors beyond cut point, or 3= TTDC, TTC, TTN all beyond cut point).
Secondary Analyses with Developmental Outcomes
Of the 71 surviving infants, 40 (56%) had available developmental outcome data. The infants lost to follow up had higher median 5- and 10-minute Apgar scores (4.5 vs 3.5, p=0.021 and 6 vs 5, p=0.005 respectively). Of the 49 infants included in the secondary analysis, 32 infants had positive outcomes (survival with developmental scores within limits) and 17 infants had adverse outcomes as defined by death (n=9) or significant developmental delay (n=8).
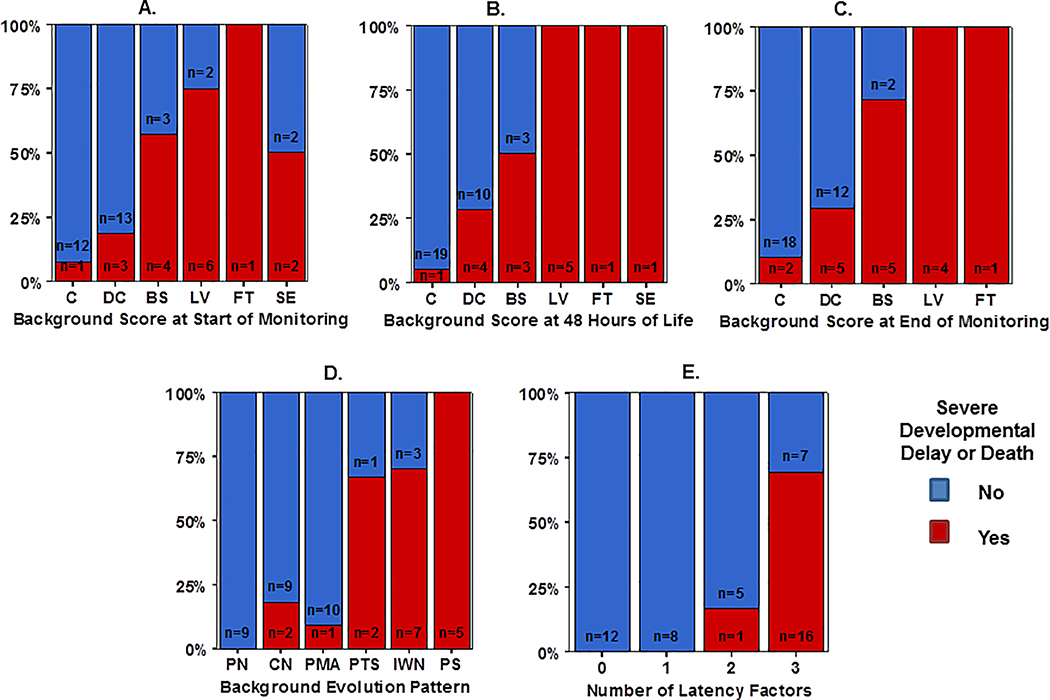
Background evolution classification better distinguished outcome groups than background patterns at the start and end of monitoring and performed equally to background assessment at 48 hours of life (Figure 3A–D, Table 3). Likewise, latency factors were highly predictive of adverse outcomes. Infants with TTDC >15.5, TTC >45.5 and TTN >78 were highly likely to have adverse outcomes (sensitivity 0.944, specificity 0.852) (Figure 3E).
Figure 3:
Distribution of aEEG background pattern at the (A) start of monitoring, (B) 48 HOL, and (C) end of monitoring compared with overall aEEG background evolution classification (D), stratified by outcome group defined as death or significant developmental delay (red bar) or survived with normal development (blue bar). (E) Comparison of favorable versus adverse outcome frequency based on number of latency factors (0=TTDC, TTC, TTN all below cut point, 1–2 factors beyond cut point, or 3= TTDC, TTC, TTN all beyond cut point).
Discussion
While prior studies have demonstrated that aEEG background and cycling at discrete points in time can predict outcome in term neonates with HIE,4,8,11,12 our current study is the first to focus on evaluating aEEG evolution over time as a continuum, defining overall evolution pattern during the course of hypothermia and rewarming as a predictor of outcome. This novel classification system that defined specific patterns of change in aEEG over the course of hypothermia might better predict outcomes than aEEG background pattern at specific time intervals during hypothermia treatment. Infants who started with mild aEEG abnormalities but progressed to severe (“Progression to Severe”), or had severe aEEG abnormalities that only partially improved (“Improved Without Normalization”), or those who had persistently severe aEEG background patterns (“Persistently Severe”) had increased risk for adverse outcomes. We also defined data-driven cutpoints in time when common aEEG features are most meaningful to assess. These data provide bedside clinicians with an additional or alternative approach to evaluate prognostic features of continuous aEEG monitoring during hypothermia.
A recent systematic review by del Rio et al evaluated aEEG background pattern in infants receiving therapeutic hypothermia using background pattern to predict neurodevelopmental outcomes at 12+ months of life. They demonstrated a maximum predictive reliability at 72 hours of life, with a post-test probability of 95.70% and +LR of 24.24 Our classification system using aEEG background evolution had comparable to slightly higher predictive reliabilities (post-test probability of 96.5%, +LR 30.1 for developmental outcomes, post-test probability of 97.1% and +LR 35.4 for MRI outcomes). This study also highlighted the importance of establishing predictive abilities in the setting of hypothermia, as studies from the pre-cooling era have demonstrated better prognostic reliability of aEEG in the first 24–48 hours of life in normothermic infants with HIE 8,24. It is reassuring that predictive abilities from our study are consistent with the pooled results for aEEG at 72 hours of life reported by del Rio and colleagues. Our study additionally provides important timepoints for achievement of specified milestones in aEEG evolution, which can provide useful data to clinicians to initiate prognostic discussion with families before outcome prediction can be more certain towards the end of hypothermia.
Several reports have systematically evaluated the evolution of clinical encephalopathy in infants with HIE. Gunn et al, in a secondary analysis of the CoolCap trial, demonstrated that there are variable trajectories of clinical encephalopathy in newborns receiving TH and that the predictive ability of clinical encephalopathy changes with hypothermia.13 Similarly, evaluation of clinical exam data from NICHD trial participants showed that the value of serial evaluation of clinical encephalopathy was more predictive of outcome than initial assessment alone.14 Our data mirror these observations, as ongoing assessment of evolving encephalopathy on aEEG may offer additional insights into the progression of disease when compared to aEEG assessment at a discrete interval in time. aEEG offers advantages over serial clinical exam, in that trajectories of aEEG background pattern may be more readily assessed in a continuous manner during bedside assessment, allowing for real-time assessment or later review of a preceding period of monitoring. That aEEG background evolution pattern offered significant, albeit modest, independent information over initial clinical encephalopathy grade in our adjusted models provides evidence for the additive information that monitoring encephalopathy over time can provide.
Of note, while background evolution patterns were categorized based on overall start and end points, we recognize that not all infants necessarily have linear trajectories of disease evolution. Upon more detailed assessment, 12 cases in our study had non-linear trajectories where aEEG background patterns either transiently improved then worsened or transiently worsened then improved over time. Unfortunately, sample size precluded further analysis or secondary mixed-modeling of these non-linear trajectories to assess their importance in determining outcomes. Other clinical circumstances may be associated with transient worsening of aEEG patterns and the impact of this on outcome risk is an area for future research. Additionally, aEEG background at the start of monitoring was more predictive of outcome than at the end of monitoring, which is different than previous studies.4,8,12 This may in part be due to the fact that there was a wide range in start times of aEEG (4–22 HOL) as we are an outborn children’s hospital with patients transferred for therapeutic hypothermia. Likewise, the end of monitoring in our study was up to 24 hours of completion of rewarming which is a later timepoint than reported in most studies. For this reason, we included a reference timepoint at 48 hours of life for comparison to other studies.
In addition to developing a classification system for aEEG background evolution, we also used a data driven approach to establish specific cut points that can guide the timing of assessment of aEEG to aid with clinical decision-making. Specifically, infants who fail to improve to a discontinuous background pattern by 15.5 HOL, do not demonstrate aEEG cycling by 45.5 HOL, and do not completely normalize to a continuous normal voltage by 78 HOL are highly likely to have adverse outcomes. These practical and recognizable aEEG features may be useful for clinicians as aEEG becomes increasingly incorporated into bedside care.
Our study is the first to define specific HOL cut points that can be used to guide clinical management. Prior studies have looked at the predictive ability of aEEG using pre-determined time intervals over the course of hypothermia. Thoresen et al evaluated 6-hour aEEG segments and described increasing positive predictive value over time, with positive outcomes associated with achieving a discontinuous or continuous background pattern by 48 hours of life.8 Similarly, Cseko et al evaluated the predictive value of 6–12 hour segments of aEEG in infants with HIE and reported that the PPV of both background pattern and aEEG cycling increased with time, with reliable prediction of outcome by 48 HOL.12 Likewise, our previous study of a separate cohort of 75 HIE infants evaluated 12-hour aEEG segments and found aEEG background pattern and cycling to have higher prognostic value after the first 2 days of life (the pre-rewarming period).4 The predictive value of aEEG background pattern at 48 hours in our current study (Sensitivity 0.48, Specificity 0.87, PPV 0.61, NPV 0.79) was lower than reported in these prior studies. This may be attributed to a number of infants classified as having discontinuous background patterns at 48 hours who went on to have poor outcomes. While discontinuous aEEG background has been traditionally grouped as a favorable aEEG pattern, conventional EEG classification schemes differentiate degrees of discontinuity,25,26 with increasing levels of discontinuity indicative of adverse outcomes. Our data suggest that evaluating background evolution pattern and latency factors may help to further differentiate infants with indeterminate risk based on point-in-time assessments of aEEG background alone.
Our study has several limitations. Our study spanned a 5-year period of enrollment, encompassing changes in clinical protocols for MRI and developmental assessments over the course of the study. Given our primary outcome was qualitative scoring of conventional MRI abnormalities, the transition from 1.5T to 3T during the study period affected this assessment less than if we had relied on quantitative MRI techniques. We also transitioned from a single MRI (day 7–10) to 2 MRIs (day 3–5, day 10–12) performed post-cooling. While timing of MRI was largely confined to scans performed in the second week of life, a few patients (n=7) underwent MRI only in the first week of life which may have affected classification of injury by MRI. Our secondary outcome of developmental delay at 18 months was likewise affected by the transition from BSID-II to BSID-III in our clinical follow-up program. While we defined a composite outcome using cut-offs supported by the literature21, the reliability of this approach remains controversial. Our developmental outcome data were also subject to significant loss to follow up. While this introduced possible selection bias, the fact that infants retained in the study appeared to have more severe disease (i.e. lower apgars, pH) supports the notion that infants with adverse outcomes were represented in the cohort evaluated. We recognize that this higher proportion of infants with severe outcomes may affect the generalizability of predictive values reported. Given these acknowledged limitations, it is reassuring that aEEG evolution was predictive of both MRI and developmental outcomes, as qualitative MRI performed in the newborn period has been demonstrated to be predictive of longer-term outcomes in cooled infants with HIE 27,28. Further work is needed to confirm these findings in infants prospectively monitored with more comprehensive longitudinal follow-up.
Other limitations include that we had to exclude 57 infants from our original cohort because their aEEG was uninterpretable leading to potential selection bias. However, we were able to analyze a relatively large sample size of 80 infants with continuous aEEG data, one of the largest to date. Our aEEGs were initiated at a median age around 10 hours of life, which limited our ability to truly assess the ‘starting point’ of aEEG background for an individual patient. This limitation was due to the fact that monitoring was initiated after transport and admission to our NICU since our study was conducted at an outborn referral center. Additionally, trained personnel for EEG/aEEG placement are not available in our hospital 24 hours a day. However, the variation in age at initiation of monitoring was relatively narrow and thus classification of evolution pattern was performed over a comparable period of observation across subjects. Future studies will need to confirm these findings when monitoring is initiated shortly after birth. Finally, as our focus was on the use of aEEG background assessment to predict outcomes in HIE, we did not evaluate the impact of seizures apart from identifying infants with status epilepticus. The interplay between seizure occurrence and evolution of aEEG background in HIE warrants further study.
Conclusions
Classification of aEEG background evolution over the course of hypothermia has prognostic value and might better distinguish HIE infants with adverse outcomes than aEEG background assessment at isolated points in time during monitoring. Infants who did not reach a discontinuous background by 15.5 HOL, achieve cycling by 45.5 HOL and normalize by 78 HOL are at particular risk for adverse outcomes. These recognizable features of aEEG can be used for bedside assessment of outcome risk in newborns with HIE undergoing hypothermia. Future studies are needed to further evaluate aEEG background evolution pattern classifications and examine how evolution pattern and cut points for latency factors might be used to guide clinical management at the bedside.
Acknowledgements
We thank Amanda Hastings and Maguire Brinkley for their efforts in coordinating developmental follow-up for this study. We also thank Nadia Kadri for her assistance with data management.
Source of funding and conflict of interest statement: This work was supported by the Clinical and Translational Science Institute at Children’s National (UL1TR000075, 1KL2RR031987-01) and the Intellectual and Developmental Disabilities Research Consortium (NIH P30HD040677). The authors have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Kleinman ME, de Caen AR, Chameides L, et al. Pediatric basic and advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Pediatrics 2010;126(5):e1261–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higgins RD, Raju T, Edwards AD, et al. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr 2011;159(5):851–858 e851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2013;1:CD003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massaro AN, Tsuchida T, Kadom N, et al. aEEG evolution during therapeutic hypothermia and prediction of NICU outcome in encephalopathic neonates. Neonatology 2012;102(3):197–202 [DOI] [PubMed] [Google Scholar]
- 5.Bonifacio SL, deVries LS, Groenendaal F. Impact of hypothermia on predictors of poor outcome: how do we decide to redirect care? Seminars in fetal & neonatal medicine 2015;20(2):122–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellstrom-Westas L, Rosen I, Svenningsen NW. Predictive value of early continuous amplitude integrated EEG recordings on outcome after severe birth asphyxia in full term infants. Archives of disease in childhood Fetal and neonatal edition 1995;72(1):F34–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toet MC, Hellstrom-Westas L, Groenendaal F, Eken P, de Vries LS. Amplitude integrated EEG 3 and 6 hours after birth in full term neonates with hypoxic-ischaemic encephalopathy. Archives of disease in childhood Fetal and neonatal edition 1999;81(1):F19–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thoresen M, Hellstrom-Westas L, Liu X, de Vries LS. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics 2010;126(1):e131–139 [DOI] [PubMed] [Google Scholar]
- 9.Azzopardi D, group Ts. Predictive value of the amplitude integrated EEG in infants with hypoxic ischaemic encephalopathy: data from a randomised trial of therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed 2014;99(1):F80–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkar S, Barks JD, Donn SM. Should amplitude-integrated electroencephalography be used to identify infants suitable for hypothermic neuroprotection? Journal of perinatology : official journal of the California Perinatal Association 2008;28(2):117–122 [DOI] [PubMed] [Google Scholar]
- 11.Hallberg B, Grossmann K, Bartocci M, Blennow M. The prognostic value of early aEEG in asphyxiated infants undergoing systemic hypothermia treatment. Acta Paediatr 2010;99(4):531–536 [DOI] [PubMed] [Google Scholar]
- 12.Cseko AJ, Bango M, Lakatos P, et al. Accuracy of amplitude-integrated electroencephalography in the prediction of neurodevelopmental outcome in asphyxiated infants receiving hypothermia treatment. Acta Paediatr 2013;102(7):707–711 [DOI] [PubMed] [Google Scholar]
- 13.Gunn AJ, Wyatt JS, Whitelaw A, et al. Therapeutic hypothermia changes the prognostic value of clinical evaluation of neonatal encephalopathy. J Pediatr 2008;152(1):55–58, 58 e51 [DOI] [PubMed] [Google Scholar]
- 14.Shankaran S, Laptook AR, Tyson JE, et al. Evolution of encephalopathy during whole body hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr 2012;160(4):567–572 e563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. The New England journal of medicine 2005;353(15):1574–1584 [DOI] [PubMed] [Google Scholar]
- 16.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Archives of neurology 1976;33(10):696–705 [DOI] [PubMed] [Google Scholar]
- 17.Monitoring cerebral function: long term monitoring of EEG and evoked potentials. New York: Elsevier; 1986 [Google Scholar]
- 18.Barkovich AJ, Hajnal BL, Vigneron D, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol 1998;19(1):143–149 [PMC free article] [PubMed] [Google Scholar]
- 19.Black MM, Matula K. Essentials of Bayley scales of infant development--II assessment. New York: Wiley; 2000:xii, 162 p. [Google Scholar]
- 20.Bayley N Bayley Scales of Infant Development - Third Edition San Antonio, TX: Harcourt Assessment; 2006 [Google Scholar]
- 21.Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW. Underestimation of developmental delay by the new Bayley-III Scale. Archives of pediatrics & adolescent medicine 2010;164(4):352–356 [DOI] [PubMed] [Google Scholar]
- 22.Vohr BR, Stephens BE, Higgins RD, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. The Journal of pediatrics 2012;161(2):222–228 e223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalak LF, DuPont TL, Sanchez PJ, et al. Neurodevelopmental outcomes after hypothermia therapy in the era of Bayley-III. Journal of perinatology : official journal of the California Perinatal Association 2014;34(8):629–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Rio R, Ochoa C, Alarcon A, et al. Amplitude Integrated Electroencephalogram as a Prognostic Tool in Neonates with Hypoxic-Ischemic Encephalopathy: A Systematic Review. PloS one 2016;11(11):e0165744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuchida TN, Wusthoff CJ, Shellhaas RA, et al. American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society 2013;30(2):161–173 [DOI] [PubMed] [Google Scholar]
- 26.Tsuchida TN. EEG background patterns and prognostication of neonatal encephalopathy in the era of hypothermia. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society 2013;30(2):122–125 [DOI] [PubMed] [Google Scholar]
- 27.Shankaran S, Barnes PD, Hintz SR, et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol 2010;9(1):39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]