To the Editor:
Our understanding of the impact of COVID-19 on liver transplant recipients has recently advanced significantly. In August 2020 Colmenero et al. published data on behalf of the Spanish Liver Transplant Society (SETH) in the Journal of Hepatology.1 The authors reported data on 111 LT recipients with SARS-CoV-2 infection and concluded that these patients were at no greater risk of severe COVID-19 than the general population. Furthermore, within LT recipients, comorbidity, male sex, and mycophenolate mofetil (MMF) use were reported as associated with severe disease. We congratulate our Spanish colleagues for rapidly conducting their comprehensive study in the midst of the pandemic.
Helpfully, Colmenero et al. provided an extract of their dataset: an important gesture in an era of rapid-fire reports. Having examined this, we feel a number of points would benefit from further exploration. We have also made comparisons with our own analysis of 151 LT recipients with SARS-CoV-2 infection (data extract supplied).2 A key difference is that Colmenero et al. used a composite endpoint of death, intensive care unit (ICU) admission, or mechanical ventilation, whereas we used death alone; neither showed a significant difference between LT and non-LT patients. This is in contrast to the high rates of mortality in patients with cirrhosis.3
The first point of note is that only 4/20 (20%) SETH patients who died were admitted to ICU, compared to 22/28 (79%) in our cohort; overall mortality was similar at 18% and 19%, respectively. Although the reasons for this are not apparent, those who died in the SETH cohort without ICU admission were older and had higher Charlson comorbidity index (CCI) scores, which may suggest that ICU admission was thought inappropriate. Within our cohort, 9% of LT recipients deemed in need of ICU due to severe enough disease were not admitted due to this being deemed inappropriate. ICU admission reflects a combination of patient and clinician factors and is therefore an imperfect marker of COVID-19 disease severity.
Second, although Colmenero et al. report a univariable association between age and severe COVID-19, their multivariable analysis shows no significant association with age in contrast to the general literature.4 , 5 On closer analysis, the authors have included age both alone and as a component of the CCI in their analysis, thus masking age as an independent variable. The same issue exists for diabetes and renal function, which are both components of the CCI.6
Third, Colmenero et al. reported a correlation between male sex and severe COVID-19 consistent with findings from other large non-LT datasets.7 However, comparing their Table 1, Table 2, and the raw dataset demonstrates that the association of poor outcome is in fact with female sex [12/79 (15%) men died vs. 8/32 (25%) women] and that there has been a transcription error.
Fourth, the multivariable analysis includes a number of variables that change over the disease course of COVID-19, such as immunosuppression withdrawal, and oxygen saturations at diagnosis. Patients were diagnosed with SARS-CoV-2 at varying time points, with time from diagnosis to ICU admission ranging from -1 to 11 days, with ICU admission not being universal as above. Furthermore, patients not hospitalized were excluded. This makes the use of a composite endpoint and time-dependent analysis (e.g. Cox regression) more difficult to interpret.
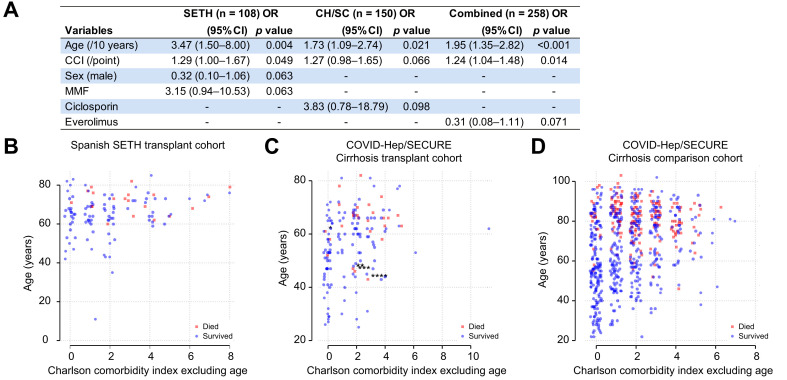
Considering the points above, we re-examined the SETH dataset in relation to the single endpoint of death. We adjusted reported CCI scores to remove age, and only considered baseline variables. To permit comparison, we retrospectively applied the same calculations to our own cohort, using diagnosis as the point-of-entry for both cohorts. We then performed a multivariable logistic regression analysis in each cohort and both cohorts combined, with death as the dependent variable and age, sex, CCI (without age), hypertension, and baseline tacrolimus, azathioprine, ciclosporin, MMF, everolimus, and corticosteroid use as independent variables (Fig. 1 A). When analyzed in this way, with backwards selection at p <0.1, age and CCI were significantly associated with death whereas no significant associations remained with immunosuppressive regimens (Fig. 1A). A limitation of our registry was that the precise duration from laboratory diagnosis to death was not known, thus preventing the performance of a time-dependent analysis. Our instructions to submitting physicians specified that patients should be followed until mortality or resolution of COVID-19 and both cohorts allowed inclusion of patients presenting at any time point.
Fig. 1.
Associations between age and comorbidity with death following SARS-CoV-2 infection in liver transplant patients.
(A) Outcomes of multivariable logistic regression with backwards stepwise selection for retention in the model at p <0.1 with death as the dependent variable and the following independent variables: age, sex, Charlson comorbidity index (CCI; without age), hypertension, and baseline tacrolimus, ciclosporin, mycophenolate mofetil (MMF), everolimus, and corticosteroid use. SETH = cohort described by Colmenero et al; CH/SC = COVID-Hep/SECURE-Cirrhosis; combined represents the 2 cohorts combined. Within the SETH cohort, factors remaining significantly associated with death were age (odds ratio [OR] 3.47/10 years; 95% CI 1.50–8.00; p = 0.004) and CCI without age (1.29/point; 95% CI 1.00–1.67; p = 0.049). Within the COVID-Hep/SECURE-Cirrhosis cohort only age (OR 1.73/10 years; 95% CI 1.09–2.74; p = 0.02) remained significant. Within the combined cohort, age (1.95/10 years; 1.35–2.82; p <0.001) and CCI (OR 1.24; 95% CI 1.04–1.48; p = 0.014) remained significant. (B–D) Plots of age in years against CCI adjusted to exclude age and split by whether patients survived (blue circles) or died (red squares) following SARS-CoV-2 infection in (B) Spanish SETH cohort (n = 108 with complete data); (C) COVID-Hep/SECURE-Cirrhosis international cohort (n = 150); (D) COVID-Hep/SECURE-Cirrhosis comparison non-LT cohort from a single UK hospital network (n = 627). A single Spanish patient from the COVID-Hep/SECURE-Cirrhosis registries who could potentially have been included in the Spanish SETH registry was not included in the analysis. Asterisks for patients who died with CCI <3 (including points for age) in panel B denote additional at-risk cofactors: ∗concurrent influenza; ∗∗second liver transplant; ∗∗∗recurrent primary sclerosing cholangitis with jaundice at baseline; ∗∗∗∗baseline jaundice of unknown cause. Horizontal jitter has been added to the X axis. (This figure appears in color on the web.)
To explore the relationship between age, co-morbidity and death we plotted age against CCI according for both LT cohorts (Fig. 1B,C). Notably, no patient from either cohort with a CCI of 0 died. Conversely, patients who died were older with higher CCI scores. For the SETH cohort, the CCI threshold for death was <3. In our cohort, 6 patients died with a CCI <3 however in 4 cases an additional important cofactor not captured by CCI was identified (Fig. 1C). The pattern of increasing mortality with age and CCI in both cohorts was similar to that observed in our non-LT comparison cohort (Fig. 1D).
Considering the above, age and comorbidity appear key in determining outcome from SARS-CoV-2 infection in the LT population. The fact that both published datasets have similar findings supports their generalizability. Although immunosuppressive regimens may be of additional importance, it appears unlikely to confer more risk than 10 years of additional age. Further formal work with larger/combined datasets is required before medication changes can be recommended.
Financial support
The COVID-Hep.net registry was supported by the European Association for the Study of the Liver (EASL; grant number 2020RG03). SECURE-cirrhosis was also supported by the National Institutes of Health grant T32 DK007634 (AMM), North Carolina Translational and Clinical Sciences Institute (CTSA grant number UL1TR002489) and National Institutes of Health, through Grant Award Number UL1TR002489. TM is supported by the Wellcome Trust as a Clinical Research Fellow. EB is supported by the Oxford NIHR Biomedical Research Centre and is an NIHR Senior Investigator. The views expressed in this article are those of the authors and not necessarily those of EASL, the NHS, the NIHR, or the Departments of Health.
Authors’ contributions
All authors contributed equally.
Data availability statement
An extract from the COVID-Hep.net and SECURE-Cirrhosis registries representing the data used to produce this analysis is provided alongside the manuscript. Additional data may be available on request to the corresponding author.
Conflict of interest
The authors have no other conflicts of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2021.01.036.
Supplementary data
The following is the supplementary data to this article:
References
- 1.Colmenero J., Rodríguez-Perálvarez M., Salcedo M., Arias-Milla A., Muñoz-Serrano A., Graus J., et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021 Jan;74(1):148–155. doi: 10.1016/j.jhep.2020.07.040. Epub 2020 Aug 1. PMID: 32750442; PMCID: PMC7395653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Webb G.J., Marjot T., Cook J.A., Aloman C., Armstrong M.J., Brenner E.J., et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020 doi: 10.1016/S2468-1253(20)30271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marjot T., Moon A.M., Cook J.A., Abd-Elsalam S., Aloman C., Armstrong M.J., et al. J Hepatol. 2020:1–42. doi: 10.1016/j.jhep.2020.09.024. [DOI] [Google Scholar]
- 4.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin A.T., Cochran K.B., Walsh S.P. National Bureau of Economic Research; 2020. Assessing the age specificity of infection fatality rates for COVID-19: meta-analysis & public policy implications. Report No.: 0898-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 7.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
An extract from the COVID-Hep.net and SECURE-Cirrhosis registries representing the data used to produce this analysis is provided alongside the manuscript. Additional data may be available on request to the corresponding author.