Abstract
Frailty is a state of decreased physiologic reserve associated with poor outcomes before and after lung transplantation. Obesity, particularly central obesity characterized by excess proinflammatory visceral adipose tissue (VAT), is associated with incident frailty in middle-aged and older adults. The association between VAT and frailty in advanced lung disease, however, is unknown. In two, nonoverlapping multicenter cohorts of adults listed for lung transplantation, we measured VAT area on bioelectrical impedance assay (BIA) in one cohort and cross-sectional VAT and subcutaneous adipose tissue (SAT) areas on abdominal computed tomography (CT) in the other. We identified a nonlinear relationship between greater VAT by BIA and frailty. In fully adjusted piecewise regression models, every 20 cm2 increase in VAT area was associated with 50% increased odds of frailty in subjects with high VAT (95% CI 1.2–1.9, P < .001), and 10% decreased odds of frailty (95% CI 0.7–1.04, P = .12) in subjects with low VAT. Compared to frail subjects with low VAT, those with high VAT were more likely to have low grip strength and less likely to have weight loss, suggesting that mechanisms of frailty may differ by VAT. Further investigation of mechanisms linking VAT and frailty may identify new targets for prevention and treatment.
Keywords: clinical research/practice, lung transplantation/pulmonology, obesity, quality of life (QOL)
1 |. INTRODUCTION
Physical frailty is characterized by functional limitation and decreased physiologic reserve resulting from multiple physiologic deficits, affects up to 28% of adults with advanced lung disease, and up to 50% of adults with interstitial lung disease.1,2 Frailty is associated with decreased exercise capacity in lung transplant candidates,1 increased risk of unplanned re-hospitalization within 30 days of lung transplantation,3 and increased mortality both before and after lung transplantation.1,4 Despite consistent associations between frailty and adverse outcomes across populations, little work has focused on identifying risk factors for the development of frailty in advanced lung disease. Identifying risk factors for frailty is important to understanding its pathobiology and to elucidating novel targets for prevention and treatment, a research priority according to the American Society of Transplantation.5
Baseline obesity, particularly central obesity, is associated with an increased risk of frailty in large prospective cohorts of community-dwelling older adults independent of age, sex, physical activity, and comorbidities.6–8 Increased abdominal visceral adipose tissue (VAT) in particular is associated with frailty in middle-aged men.9 In obesity, VAT is characterized by a proinflammatory phenotype promoted by adipose tissue macrophages,10 increased CD8+ T cells,10 and interleukin-6 (IL-6) and tumor necrosis factor alpha production.10 Further, ectopic intra-muscular lipid deposition, which correlates closely with abdominal VAT,11 impairs muscle function.12 The association between VAT and frailty in candidates for lung transplantation is unknown.
We hypothesized that greater VAT and percent body fat measured by bioelectrical impedance assay (BIA) would be associated with frailty in adult lung transplant candidates. We secondarily hypothesized that greater abdominal VAT on computed tomography (CT) scan would also be associated with frailty.
2 |. METHODS
2.1 |. Study participants
We performed a cross-sectional analysis of the Lung Transplant Body Composition study, two non-overlapping National Heart, Blood, and Lung Institute-funded multi-center prospective cohort studies designed to evaluate associations between body composition, frailty, and outcomes in adults listed for lung transplantation at the University of California at San Francisco (UCSF), the University of Pennsylvania (Penn), and Columbia University Medical Center (CUMC). Enrollment and data acquisition occurred in two serial non-overlapping multi-center cohorts: (1) between 2011 and 2014 at Penn and CUMC, and (2) from 2017 to present at UCSF, Penn, and CUMC. Subjects enrolled in the cohort used for our primary analysis (recruited beginning in 2017) underwent BIA, while subjects in the secondary analysis cohort (recruited between 2011 and 2014) underwent abdominal CT scans for measurement of body composition. All studies were IRB approved at participating centers and participants provided written informed consent.
2.2 |. Measurements of adipose tissue
Bioelectrical impedance assay (BIA) was performed using the InBodyS10 (InBody USA, Cerritos, CA) to measure visceral adipose tissue (VAT) area, percent body fat, and skeletal muscle mass.13,14 VAT area and skeletal muscle mass are mathematically derived quantities, the calculations for which are proprietary and not available for review. VAT area by BIA has previously been closely correlated with single-slice VAT area as measured by abdominal CT (correlations of 0.83–0.90).13,15 Skeletal muscle mass has been correlated with fat free mass on dual-energy x-ray absorptiometry (correlations of 0.899 to 0.97).14,16,17 Single slice cross-sectional abdominal CT scans on GE or Siemens scanners were performed at the L4/L5 level on consenting subjects.18 Abdominal VAT, subcutaneous adipose tissue area (SAT), and total skeletal muscle cross-sectional areas were measured manually using published techniques by trained personnel blinded to frailty status (Supporting Information Figure S1).19,20
2.3 |. Statistical analysis
The primary outcome was the Fried Frailty Phenotype (FFP) as measured at the time of body composition assessment. The FFP assesses five domains: weight loss, exhaustion, walk time, grip strength, and physical activity.1,21 Frailty was defined as deficiency in three or more domains using previously described methods.1,21 Subjects with at least three measured frailty domains were included in all analyses.21
We operationalized VAT area (cm2) and percent body fat (%) by BIA as absolute values, and CT measures of VAT relative to SAT (VAT/SAT ratio) to account for differences in fat distribution consistent with prior approaches.20 We log2 transformed the VAT/SAT ratio to normalize the distribution and allow for estimation of the association with frailty per doubling of the VAT/SAT ratio. We examined nonlinear associations using generalized additive models with the “GAM” package in R adjusted for age and sex. After identifying a nonlinear relationship between VAT area by BIA and frailty, we investigated the use of quadratic terms for VAT and used the “U-test” package in STATA to evaluate the significance of the quadratic association. We subsequently performed piecewise regression analysis to investigate the association between VAT and frailty. In primary analyses, we used logistic regression with center as a random effect adjusting for generalized propensity scores calculated using the CBPS package in R. Propensity scores included age, sex, diagnosis, forced vital capacity (FVC), body mass index (BMI), and skeletal muscle mass. We also used the estimated inflection point of our curve to identify three distinct groups of VAT: low, moderate, and high VAT. We evaluated the relative odds of frailty for subjects with low and high VAT compared to those with moderate VAT (reference group). In secondary analyses, we used logistic regression models adjusting for the individual covariates. In post-hoc analyses, we investigated effect modification by diagnosis and age. Among frail subjects, we investigated frailty components satisfied by VAT below and above the breakpoint.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
3 |. RESULTS
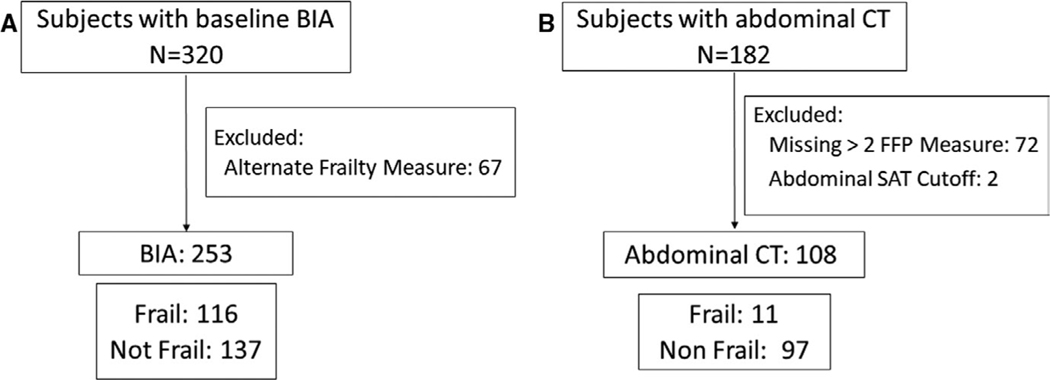
Of the 320 subjects with available BIA measures, 67 had frailty assessed by a measure other than the FFP (Figure 1). Of the 253 subjects with available FFP frailty measures, 20 were missing measurement of one FFP domain while 11 were missing measurements of two FFP domains. Subjects with frailty assessed by other measures were less likely to be from Center A but were otherwise similar to those with FFP (Supporting Information Table S1). Among the 253 subjects included in BIA analyses, 116 (46%) were frail. Those with low VAT area tended to be male, less likely to have ILD, more likely to have cystic fibrosis, have a lower weight and BMI, greater 6-minute walk distance, and tended to be less likely to come from Center C (Table 1). Median (IQR) for VAT area on BIA was 112.3 cm2 (71.4–154.9 cm2) and for percent body fat was 31.8% (24.1–37.9%). Of the 253 subjects, 208 had been actively for transplantation, of whom 117 (56%) underwent transplantation, 15 (7%) died or were delisted for being too ill for transplantation, and 76 (37%) remain actively listed. Of the 182 subjects with available abdominal CT scans, only 108 had completed at least three available frailty measures and had available measures of abdominal adipose tissue, of whom 11 (10%) were frail (Figure 1). The median (IQR) abdominal VAT area on CT was 80.1 cm2 (44.0–151.6 cm2). Subjects included in CT analyses were less likely to be male, and more likely to have chronic obstructive pulmonary disease (COPD) or come from Center B but were otherwise similar to excluded CT subjects (Supporting Information Table S1). Compared to subjects with BIA, subjects with abdominal CT were less likely to be male, and more likely to have COPD and greater 6-minute walk distance (Supporting Information Table S1). A total of 248 subjects with BIA measures and 107 subjects with CT measures had complete data for all covariates and were included in adjusted models.
FIGURE 1.
Flowcharts for inclusion in the (A) bioelectrical impedance cohort and the (B) abdominal computed tomography cohort. BIA, bioelectrical impedance assay; CT, computed tomography; FFP, Fried Frailty Phenotype; SPPB, Short Physical Performance Battery; SAT, subcutaneous adipose tissue
TABLE 1.
Baseline characteristics by VAT group as measured by bioelectrical impedance assay
| Low VAT area (≤123 cm2) |
High VAT area (>123 cm2) |
|
|---|---|---|
| N = 142 | N = 111 | |
| Age, y | 60 (51−66) | 62 (55−67) |
| Male gender | 83 (58) | 49 (44) |
| LAS at evaluationa | 41.1 (35.6–46.9) | 39.6 (34.5–48.8) |
| Diagnosisa | ||
| COPD | 25 (18) | 22 (20) |
| Interstitial lung disease | 71 (50) | 74 (67) |
| Sarcoidosis | 4 (3) | 4 (4) |
| Cystic fibrosis | 22 (15) | 1 (1) |
| PAH | 11 (8) | 4 (4) |
| Other | 9 (6) | 2 (2) |
| Racea | ||
| White | 103 (73) | 85 (77) |
| African American | 9 (6) | 10 (9) |
| Other | 15 (14) | 15 (14) |
| Height, cm | 169.3 (160.0–176.0) | 167.6 (160.0–175.3) |
| Weight, kg | 67.6 (54.9–79.8) | 83.4 (72.6–91.6) |
| BMI, kg/m2 | 23.5 (20.6–26.8) | 29.9 (27.2–31.4) |
| BMI Category | ||
| <18.5 | 9 (6) | 0 (0) |
| 18.5–25 | 81 (57) | 12 (11) |
| 25–30 | 45 (32) | 47 (42) |
| 30–35 | 7 (5) | 46 (41) |
| >35 | 0 (0) | 6 (5) |
| Forced Vital Capacity (L)a | 1.9 (1.5–2.4) | 1.9 (1.3–2.3) |
| DLCO (mL/min/mm Hg)a | 8.5 (6.0–11.7) | 8.1 (5.9–10.5) |
| 6-minute walk distance | 919 (636–1200) | 817 (490–1132) |
| Center | ||
| Center A | 42 (30) | 17 (15) |
| Center B | 35 (25) | 27 (24) |
| Center C | 65 (46) | 67 (60) |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; DLCO, diffusing capacity of the lungs for carbon monoxide; FVC, forced vital capacity; LAS, lung allocation score; PAH, pulmonary arterial hypertension; VAT, visceral adipose tissue area.
Race missing for three subjects. Diagnosis missing for four subjects, LAS missing on 66 subjects, FVC missing for one subject, DLCO missing for 94 subjects.
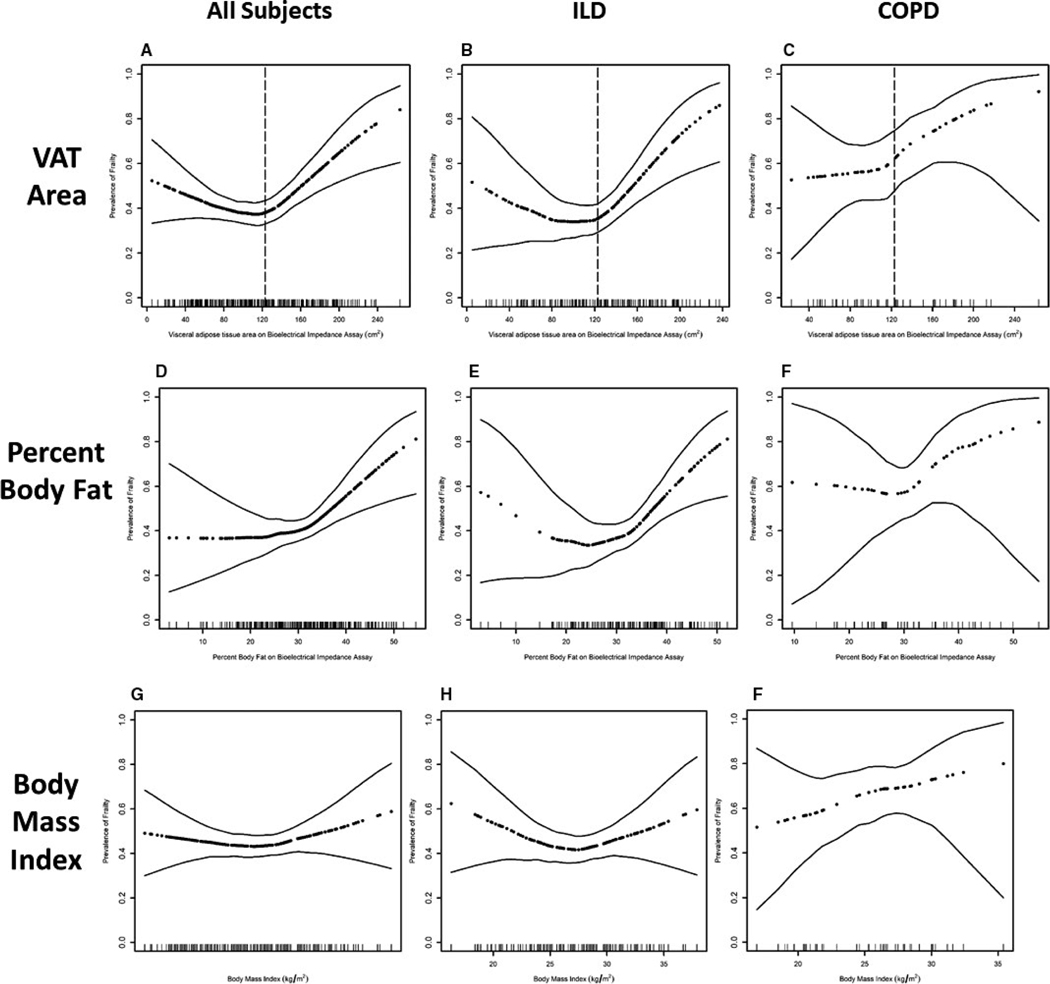
In generalized additive models adjusted for age, sex, and BMI, we identified a nonlinear relationship between VAT area on BIA and odds of frailty (Figure 2A). In unadjusted and fully adjusted models including a variable for VAT2, we observed a significant association between VAT2 and frailty as well as a significant quadratic relationship using the “U test” (Supporting Information Table S2).
FIGURE 2.
Association between VAT area on BIA and frailty in (A) all subjects, (B) subjects with ILD, and (C) subjects with COPD with demonstration of visceral fat area cut-points as presented in primary analyses. Association between percent body fat on BIA and prevalence of frailty in (D) all subjects, (E) subjects with ILD, and (F) subjects with COPD. Associations between body mass index and prevalence of frailty in (G) all subjects, (H), subjects with ILD, and (I) subjects with COPD. Dark dotted black line represents the effect estimates. Surrounding thin lines represent 95% confidence bands. Each vertical line in the rug plot along the x axis represents a single study subject. Models of visceral fat and percent body fat are adjusted for age, sex, and body mass index. Models of BMI are adjusted for age and sex. Only 47 subjects with COPD are included in this cohort resulting in wide confidence intervals, caution should be used in interpreting results. ILD, interstitial lung disease; COPD, chronic obstructive pulmonary disease
Using piecewise regression, we estimated an inflection point at 123 cm2 dividing the cohort into two groups: low VAT defined by VAT area less than or equal to 123 cm2 and high VAT defined by VAT area >123 cm2. The prevalence of frailty in the low VAT group was 40% while the prevalence of frailty in the high VAT group was 53%. In piecewise logistic regression models, subjects with high VAT had 50% increased odds of frailty (95% CI, 1.2–1.9, P < .001) for every 20 cm2 increase in VAT area in unadjusted and fully adjusted propensity score models (Table 2). In unadjusted analyses, subjects with low VAT had 10% decreased odds of frailty (95% CI, 0.7–1.0, P = .10) for every 20 cm2 increase in VAT area while in fully adjusted models, subjects with low VAT had 10% decreased odds of frailty (95% CI, 0.7–1.04, P = .12) for every 20 cm2 increase in VAT area. Results were similar in models adjusted for individual covariates (Supporting Information Table S3).
TABLE 2.
Association between VAT area and frailty in piecewise regression models adjusted for generalized propensity scores
| Low VAT area (≤123 cm2) N = 142 |
High VAT area (>123 cm2) N = 111 |
|||
|---|---|---|---|---|
| OR (95% CI) per 20 cm2 increase in VAT area | P | OR (95% CI) per 20 cm2 increase in VAT area | P | |
| Unadjusted | 0.9 (0.7−1.03) | .10 | 1.5 (1.2−1.9) | <.001 |
| Model 1a | 0.8 (0.6−1.1) | .20 | 1.6 (1.1−2.3) | .01 |
| Model 2b | 0.9 (0.7−1.1) | .24 | 1.5 (1.2−1.9) | .001 |
| Model 3c | 0.9 (0.7−1.04) | .12 | 1.5 (1.2−1.9) | <.001 |
CI, confidence interval; OR, odds ratio; VAT, visceral adipose tissue area on bioelectrical impedance assay.
Model 1: Adjusted for sex, age, diagnosis, forced vital capacity.
Model 2: Adjusted for Model 1 covariates + body mass index.
Model 3: Adjusted for Model 2 covariates + skeletal muscle mass.
Compared to frail subjects with low VAT, frail subjects with high VAT were more likely to have low grip strength and less likely to suffer from weight loss (Supporting Information Table S4).
Results of analyses evaluating three VAT groups, demonstrate increased odds of frailty among those at extremes of VAT area compared to moderate VAT (Supporting Information Table S5).
In subgroup analyses, there was no significant interaction between diagnosis or age and VAT group (Figure 2B–C, Supporting Information Table S6). Analyses of COPD included only 47 subjects, limiting interpretation.
Greater percent body fat seemed to be associated with increased odds of frailty in unadjusted (OR 1.05, 95% CI 0.9996–1.1, P = .052) and fully adjusted propensity score models (OR 1.06, 95% CI 1.003 to 1.0, P = .04, Figure 2D) although models with individual covariates were non-significant (Supporting Information Table S7). Among subjects with ILD there may have been a more “U-shaped” relationship between percent body fat and frailty (Figure 2E) although this did not appear in subjects with COPD (Figure 2F).
Unlike VAT or percent body fat, BMI did not appear to be associated with frailty in the full cohort, but may have a U-shaped relationship with frailty in subjects with ILD (Figure 2G–I).
Among patients who underwent abdominal CT, greater abdominal VAT relative to SAT seemed to be associated with increased odds of frailty in unadjusted and fully adjusted individual covariate models (OR 3.1, 95% CI, 0.99–9.8, P = .051) though this was less clear in fully adjusted propensity score models (OR 1.4, 95% CI, 0.7–2.9, P = .32) (Supporting Information Figure S2, Supporting Information Table S7).
4 |. DISCUSSION
In this study of adult lung transplant candidates, we found a strong relationship between visceral adipose tissue and frailty. Notably, the relationship does not appear linear. Rather, patients with either low VAT or high VAT are at increased risk of frailty after accounting for age, diagnosis, sex, forced vital capacity, BMI, and skeletal muscle mass. While the biomedical literature has tended to focus on the association between sarcopenia and frailty,22 our findings are consistent with a smaller body of work demonstrating an association between obesity and frailty in the general population.6–9 Notably, we are the first to identify a nonlinear relationship between VAT and frailty. While the reasons for this variable relationship are not known, defining the mechanisms driving this relationship may help develop strategies to prevent and even treat frailty.
Obesity, as measured by BMI, is associated with an increased risk of primary graft dysfunction (PGD) and death after lung transplantation.23 Leptin, a cytokine released by adipose tissue, is associated with risk of PGD after lung transplantation.23 However BMI is a poor measure of body composition and provides no information about adipose tissue or muscle mass thus prohibiting additional evaluation of mechanistic links between body composition and outcomes.23 Physical frailty, a state in which multiple accumulated physiologic deficits results in increased susceptibility to severe manifestations of acute insults, is associated with unplanned 30-day readmission after lung transplantation, and with an increased risk of death before and after lung transplantation.1,3,4 Prior work has focused on the role of muscle mass in the pathogenesis of frailty22; adipose tissue has not been studied in frailty in advanced lung disease. In multiple prospective cohort studies of community-dwelling adults, baseline obesity, particularly central obesity of which VAT is the primary component, is associated with increased risk of frailty.6–9 Given the growing prevalence of obesity in the United States,24 VAT may be an increasingly important risk factor for frailty in advanced lung disease.
The possible mechanisms for the association between greater VAT area and frailty are numerous. VAT may contribute to frailty by promoting inflammation, cellular senescence, and sarcopenia. We identified that frail subjects with high VAT are more likely to have low grip strength. This is consistent with prior work demonstrating that increased obesity duration is associated with greater decrements in grip strength8 possibly due to increased intra-muscular lipid and impaired muscle function.11,12 In the obese, VAT is associated with higher IL-6 levels, also a common finding in frailty.1,10 Senescent pre-adipocytes produce a senescence-associated secretory phenotype characterized by increased production of proinflammatory cytokines IL-6, chemokine ligand 1, monocyte chemoattractant protein-1, and tumor necrosis factor alpha, inducing an inflammatory phenotype locally and systemically.25 Cellular senescence in obese and aged adipose tissue, decreases grip strength and maximal walking speed in both young and old mice,25 while inhibition of cellular senescence improves 6-minute walk distance and 4-meter gait speed in patients with idiopathic pulmonary fibrosis.26 Greater VAT and greater VAT-to-SAT ratio have previously been associated with decreased survival after lung transplantation, suggesting that adipose tissue may play a role in the association between frailty and survival after lung transplantation.4,27
Since the underlying pathobiologic mechanisms of frailty are unknown, current treatments are limited to rehabilitation programs which are both underutilized and may have limited sustained benefits in advanced lung disease.28 Targeted therapies do not exist. Understanding the role of body composition in frailty has been identified as a research focus by the American Society of Transplantation5 and may identify unique endotypes with distinct pathobiology, outcome risks, and treatment targets. Further investigation of the role of intramuscular lipid deposition in muscle dysfunction, and adipose tissue in systemic inflammation and cellular senescence may identify mechanisms linking high adiposity and frailty. Identifying the mechanisms driving this association may identify more precise therapeutic targets for frailty, a major cause of morbidity and mortality in advanced lung disease and lung transplantation.
Our finding of an association between low VAT and frailty was unexpected. Low VAT may reflect a state of protein-energy wasting and the absence of energy reserves, both of which are putative causes of frailty.21,29 Furthermore, unintentional weight loss—one of the FFP frailty domains—preferentially leads to the loss of the VAT, consistent with our observed association between low VAT and the weight loss frailty criterion.30
There are several limitations. Although we demonstrated a nonlinear relationship between VAT by BIA and frailty, the relationship appeared linear when VAT was quantified by abdominal CT. Given the very wide confidence bands at the extremes of the VAT/SAT ratio on abdominal CT, it is possible that the small sample size of the CT cohort and low frailty prevalence limited our power to detect a more complex relationship. Furthermore, the association between high VAT and greater frailty risk was demonstrated by both measures. BIA can vary by hydration status. How this potential variation may have impacted our findings is unknown. The reasons for differences in frailty prevalence between the BIA and CT cohorts are unclear. Reassuringly, while the BIA cohort was more likely to be male and less likely to have COPD, the two cohorts do not appear to vary by any other observable characteristics. Advanced lung disease represents a heterogeneous group of diseases and treatments. The modest sample size limited the number of covariates we could include in our models and resulted in under-powered subgroup analyses. Further, since this prospective cohort has not completed enrollment or follow-up, we are underpowered to evaluate clinical outcomes including the association between VAT and waiting list or transplant complications and mortality. While not a limitation, per se, a gold standard has yet to be established for the anatomic level used to quantify VAT on CT. As a result, levels other than L4/L5 have been used in other cohorts. It is possible that our effect estimates might have been different had another anatomic site been used. Finally, the VAT groups derived from piecewise regression allow for comparison of trends based on the distribution of VAT for subjects enrolled in our cohort. Thus, while not a limitation per se, these effect estimates should be interpreted with caution.
Our findings support a nuanced and complex relationship between body composition and frailty in advanced lung disease, and suggest that both high and low amounts of VAT are associated with increased frailty in adults listed for lung transplantation. This work highlights the importance of further validating and applying tissue-specific measures of body composition in place of crude measures like BMI. The novel association between VAT and frailty may help to define the pathobiology of frailty and identify a new target for prevention and treatment. Further work identifying mechanistic links between VAT and frailty in this population is warranted.
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge the contributions of our patients, co-ordinators and colleagues who made this research possible including: Allison Soong, MPH, Onumara Opara, Grace Gallagher, Wendy Gonzalez, MD, Michelle Oyster, MS, Andrew Courtwright, MD, PhD, Yubing Tong, PhD, and Matthew Baldwin, MD, MS.
Funding information
Stony Wold-Herbert Fund; National Heart, Lung, and Blood Institute, Grant/Award Number: K24 HL115354, K24 HL131937, R01 HL 114626, R01 HL 134851, R01 HL087115 and T32 HL105323
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations:
- BIA
bioelectrical impedance assay
- BMI
body mass index
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CT
computed tomography
- CUMC
Columbia University Medical Center
- FFP
Fried Frailty Phenotype
- FVC
forced vital capacity
- IL-6
interleukin-6
- IQR
interquartile range
- LTBC
Lung transplant body composition cohort
- OR
odds ratio
- Penn
University of Pennsylvania
- SAT
subcutaneous adipose tissue
- UCSF
University of California at San Francisco
- VAT
visceral adipose tissue
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
DISCLOSURES
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Singer JP, Diamond JM, Gries CJ, et al. Frailty phenotypes, disability, and outcomes in adult candidates for lung transplantation. Am J Respir Crit Care Med. 2015;192(11):1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milne KM, Kwan JM, Guler S, et al. Frailty is common and strongly associated with dyspnoea severity in fibrotic interstitial lung disease. Respirology. 2017;22(4):728–734. [DOI] [PubMed] [Google Scholar]
- 3.Courtwright AM, Zaleski D, Gardo L, et al. Causes, preventability, and cost of unplanned rehospitalizations within 30 days of discharge after lung transplantation. Transplantation. 2018;102(5):838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer JP, Diamond JM, Anderson MR, et al. Frailty phenotypes and mortality after lung transplantation: a prospective cohort study. Am J Transplant. 2018;18(8):1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobashigawa J, Dadhania D, Bhorade S, et al. Report from the American Society of Transplantation on frailty in solid organ transplantation. Am J Transplant. 2019;19(4):984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stenholm S, Strandberg TE, Pitkala K, Sainio P, Heliovaara M, Koskinen S. Midlife obesity and risk of frailty in old age during a 22-year follow-up in men and women: the Mini-Finland Follow-up Survey. J Gerontol A Biol Sci Med Sci. 2014;69(1):73–78. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Esquinas E, Jose Garcia-Garcia F, Leon-Munoz LM, et al. Obesity, fat distribution, and risk of frailty in two population-based cohorts of older adults in Spain. Obesity (Silver Spring). 2015;23(4):847–855. [DOI] [PubMed] [Google Scholar]
- 8.Stenholm S, Sallinen J, Koster A, et al. Association between obesity history and hand grip strength in older adults–exploring the roles of inflammation and insulin resistance as mediating factors. J Gerontol A Biol Sci Med Sci. 2011;66(3):341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawkins KL, Zhang L, Ng DK, et al. Abdominal obesity, sarcopenia, and osteoporosis are associated with frailty in men living with and without HIV. AIDS. 2018;32(10):1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–920. [DOI] [PubMed] [Google Scholar]
- 11.Taira S, Shimabukuro M, Higa M, et al. Lipid deposition in various sites of the skeletal muscles and liver exhibits a positive correlation with visceral fat accumulation in middle-aged Japanese men with metabolic syndrome. Intern Med. 2013;52(14):1561–1571. [DOI] [PubMed] [Google Scholar]
- 12.Choi SJ, Files DC, Zhang T, et al. Intramyocellular lipid and impaired myofiber contraction in normal weight and obese older adults. J Gerontol A Biol Sci Med Sci. 2016;71(4):557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagai M, Komiya H, Mori Y, Ohta T, Kasahara Y, Ikeda Y. Estimating visceral fat area by multifrequency bioelectrical impedance. Diabetes Care. 2010;33(5):1077–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling CH, de Craen AJ, Slagboom PE, et al. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin Nutr. 2011;30(5):610–615. [DOI] [PubMed] [Google Scholar]
- 15.Berker D, Koparal S, Isik S, et al. Compatibility of different methods for the measurement of visceral fat in different body mass index strata. Diagn Interv Radiol. 2010;16(2):99–105. [DOI] [PubMed] [Google Scholar]
- 16.Faria SL, Faria OP, Cardeal MD, Ito MK. Validation study of multifrequency bioelectrical impedance with dual-energy X-ray absorptiometry among obese patients. Obes Surg. 2014;24(9):1476–1480. [DOI] [PubMed] [Google Scholar]
- 17.Lee SY, Ahn S, Kim YJ, et al. Comparison between dual-energy X-ray absorptiometry and bioelectrical impedance analyses for accuracy in measuring whole body muscle mass and appendicular skeletal muscle mass. Nutrients. 2018;10(6):738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irlbeck T, Massaro JM, Bamberg F, O’Donnell CJ, Hoffmann U, Fox CS. Association between single-slice measurements of visceral and abdominal subcutaneous adipose tissue with volumetric measurements: the Framingham Heart Study. Int J Obes (Lond). 2010;34(4):781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derstine BA, Holcombe SA, Ross BE, Wang NC, Su GL, Wang SC. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep. 2018;8(1):11369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dan Lantsman C, Herman A, Verlaan JJ, Stern M, Mader R, Eshed I. Abdominal fat distribution in diffuse idiopathic skeletal hyperostosis and ankylosing spondylitis patients compared to controls. Clin Radiol. 2018;73(10):910–e15. [DOI] [PubMed] [Google Scholar]
- 21.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M1 46-M156. [DOI] [PubMed] [Google Scholar]
- 22.Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci. 2014;6:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer JP, Peterson ER, Snyder ME, et al. Body composition and mortality after adult lung transplantation in the United States. Am J Respir Crit Care Med. 2014;190(9):1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finkelstein EA, Khavjou OA, Thompson H, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42(6):563–570. [DOI] [PubMed] [Google Scholar]
- 25.Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Justice JN, Nambiar AM, Tchkonia T, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pienta MJ, Zhang P, Derstine BA, et al. Analytic morphomics predict outcomes after lung transplantation. Ann Thorac Surg. 2018;105(2):399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dowman L, Hill CJ, Holland AE. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev. 2014;(10):CD006322. [DOI] [PubMed] [Google Scholar]
- 29.Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–398. [DOI] [PubMed] [Google Scholar]
- 30.Chaston TB, Dixon JB. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes (Lond). 2008;32(4):619–628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.