Abstract
Accumulation of the microtubule-associated protein tau is associated with Alzheimer's disease (AD). In AD brain, tau is abnormally phosphorylated at many sites, and phosphorylation at Ser-262 and Ser-356 plays critical roles in tau accumulation and toxicity. Microtubule affinity–regulating kinase 4 (MARK4) phosphorylates tau at those sites, and a double de novo mutation in the linker region of MARK4, ΔG316E317D, is associated with an elevated risk of AD. However, it remains unclear how this mutation affects phosphorylation, aggregation, and accumulation of tau and tau-induced neurodegeneration. Here, we report that MARK4ΔG316E317D increases the abundance of highly phosphorylated, insoluble tau species and exacerbates neurodegeneration via Ser-262/356–dependent and –independent mechanisms. Using transgenic Drosophila expressing human MARK4 (MARK4wt) or a mutant version of MARK4 (MARK4ΔG316E317D), we found that coexpression of MARK4wt and MARK4ΔG316E317D increased total tau levels and enhanced tau-induced neurodegeneration and that MARK4ΔG316E317D had more potent effects than MARK4wt. Interestingly, the in vitro kinase activities of MARK4wt and MARK4ΔG316E317D were similar. When tau phosphorylation at Ser-262 and Ser-356 was blocked by alanine substitutions, MARK4wt did not promote tau accumulation or exacerbate neurodegeneration, whereas coexpression of MARK4ΔG316E317D did. Both MARK4wt and MARK4ΔG316E317D increased the levels of oligomeric forms of tau; however, only MARK4ΔG316E317D further increased the detergent insolubility of tau in vivo. Together, these findings suggest that MARK4ΔG316E317D increases tau levels and exacerbates tau toxicity via a novel gain-of-function mechanism and that modification in this region of MARK4 may affect disease pathogenesis.
Keywords: microtubule affinity–regulating kinase 4 (MARK4), Alzheimer's disease (AD), neurodegeneration, tau protein (tau), phosphorylation, Drosophila
Accumulation of the microtubule-associated protein tau is a pathological hallmark of AD and other neurodegenerative diseases (1–6). Tau is a natively unfolded protein, and its posttranslational modifications affect the processes of its oligomerization and aggregation (6). Tau proteins are phosphorylated at multiple sites by a number of kinases (3–5, 7). Among them, kinases that belong to the conserved Par-1/microtubule affinity–regulating kinase (MARK) family phosphorylate tau within the microtubule-binding repeats at Ser-262 and Ser-356. Phosphorylation of tau at these sites regulates its microtubule binding, intracellular localization, and protein–protein interactions (8–15). Tau phosphorylated at these sites are found in preneurofibrillary tangles and associated with higher potency of accumulation seeding, thus are believed to play an initiating role in tau abnormality (8, 11–14, 16–19).
Mammals have four Par-1/MARK family members, MARK1–4 (1). MARK4 dysregulation has been proposed to play a role in the pathogenesis of AD. MARK4 expression is elevated in the brains of AD patients, and its activity colocalizes with early pathological changes (20). MARK4 can potentiate tau aggregation in vitro (21), and a significant SNP has been mapped to MARK4 in a regional Bayesian genome-wide association study of AD (22). Importantly, a de novo mutation in MARK4, resulting in double amino acid change (ΔGly-316, E317D), has been linked to an elevated risk of early-onset AD (23). The level of tau phosphorylated at Ser-262 is higher when tau is coexpressed with mutant MARK4 than when tau is coexpressed with WT MARK4 (23), suggesting that this mutation increases the risk of AD by promoting the production of abnormally phosphorylated tau. However, it is not fully understood how this mutation alters the effects of MARK4 on tau metabolism and toxicity.
In this study, we used a Drosophila model to compare the effects of MARK4 carrying the ΔGly-316, E317D mutation (MARK4ΔG316E317D) on tau accumulation and toxicity with those of WT MARK4 (MARK4wt). The results revealed that coexpression of MARK4ΔG316E317D increases the abundance of highly phosphorylated and insoluble tau species, resulting in enhanced accumulation and toxicity of tau, via a novel gain-of-function mechanism.
Results
MARK4ΔG316E317D increases tau accumulation and promotes tau toxicity to a greater extent than MARK4wt
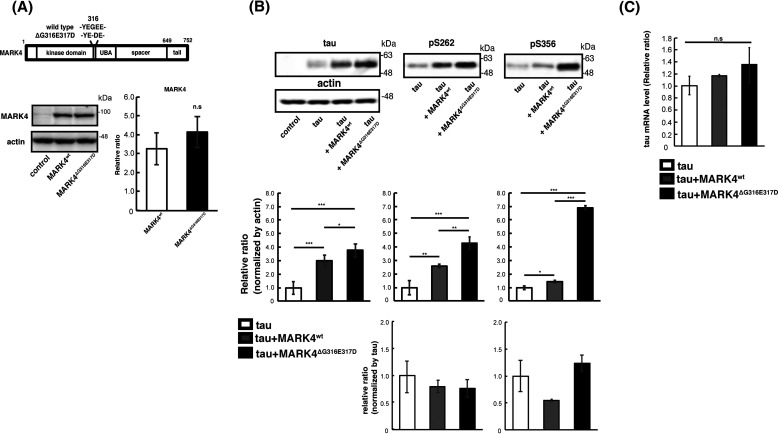
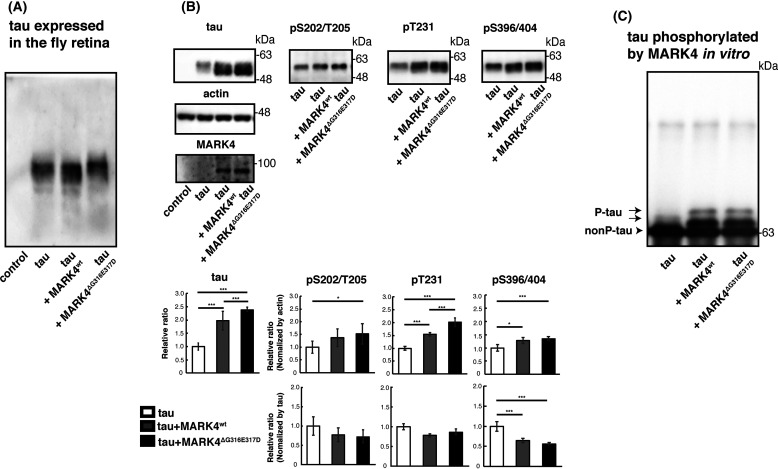
To assess the differences between the effects of MARK4wt and MARK4ΔG316E317D on metabolism and toxicity of tau in vivo, we established transgenic fly lines carrying human MARK4wt or MARK4ΔG316E317D under the control of a Gal4-responsive upstream activation sequence (UAS) sequence (Fig. 1A). MARK4wt or MARK4ΔG316E317D was coexpressed with human tau in the retina using the pan-retinal GMR-Gal4 driver. Western blotting revealed that these flies expressed MARK4wt and MARK4ΔG316E317D at similar levels (Fig. 1A). MARK4 phosphorylates tau at Ser-262 and Ser-356 (24), and we previously reported that tau phosphorylation at Ser-262 and Ser-356 by Par-1, a member of the PAR-1/MARK family, stabilizes tau and increases total tau levels (11, 12). Both MARK4wt and MARK4ΔG316E317D increased the levels of total tau and tau phosphorylated at Ser-262 and Ser-356; MARK4ΔG316E317D had stronger effects on both (Fig. 1B). The anti-phospho Ser-262 tau antibody and anti-phospho–Ser-356 tau antibody do not detect tau species that are not phosphorylated at corresponding sites ((11, 12) and Fig. S1). The prominent increase in tau phosphorylated at Ser-356 by MARK4ΔG316E317D is noteworthy because tau phosphorylation at Ser-356 in this model reflects abnormally elevated PAR-1/MARK activity (12). Coexpression of MARK4wt or MARK4ΔG316E317D did not affect the mRNA levels of tau (Fig. 1C), indicating that the increase in tau levels was not due to elevated transcription of the tau transgene.
Figure 1.
MARK4ΔG316E317D increases the level of tau to a greater extent than MARK4wt. A, (top) schematic representation of MARK4 and ΔG316E317D mutation. (bottom) MARK4wt and MARK4ΔG316E317D are expressed at similar levels in fly eyes. MARK4 expression was driven by the pan-retinal driver GMR-Gal4. Levels of MARK4wt or MARK4ΔG316E317D in fly head lysates were examined by Western blotting. Actin was used as a loading control. Representative blots (left panels) and quantification (right panels) are shown. B, coexpression of MARK4wt increased the levels of phosphorylated tau and total tau, and coexpression of MARK4ΔG316E317D increased the levels of total tau, pSer-262–tau, and pSer-356–tau in the eyes to a significantly greater extent than MARK4wt. Western blotting was performed with anti-tau antibody T46 (tau), anti-phospho–Ser-262 tau antibody (pSer-262), or anti-phospho–Ser-356 antibody (pSer-356). Representative blots (top) and quantification normalized to actin, or to total tau (bottom panels), are shown. C, coexpression of MARK4wt or MARK4ΔG316E317D does not affect tau mRNA levels. mRNA levels of tau expressed alone (tau), tau coexpressed with MARK4wt (tau+MARK4wt), and tau coexpressed with MARK4ΔG316E317D (tau+MARK4ΔG316E317D) were measured by quantitative PCR. Means ± S.D. (error bars); n = 4. n.s. (not significant), p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.005 (one-way ANOVA and Tukey's post-hoc test).
Next, we analyzed the effect of coexpression of MARK4 on tau toxicity. Expression of human tau in the eyes causes age-dependent and progressive neurodegeneration in the lamina, the first synaptic neuropil of the optic lobe containing photoreceptor axons (17). We found that flies coexpressing tau and MARK4wt or MARK4ΔG316E317D exhibited more neurodegeneration in the lamina than those expressing tau alone. Moreover, coexpression of MARK4ΔG316E317D caused more prominent neurodegeneration than the coexpression of MARK4wt (Fig. 2, A and B). By contrast, expression of MARK4wt or MARK4ΔG316E317D alone, without tau, did not cause neurodegeneration at the same age (Fig. 2, A and B). Together, these results suggest that MARK4ΔG316E317D promotes tau accumulation and exacerbates tau toxicity to a greater extent than MARK4wt.
Figure 2.
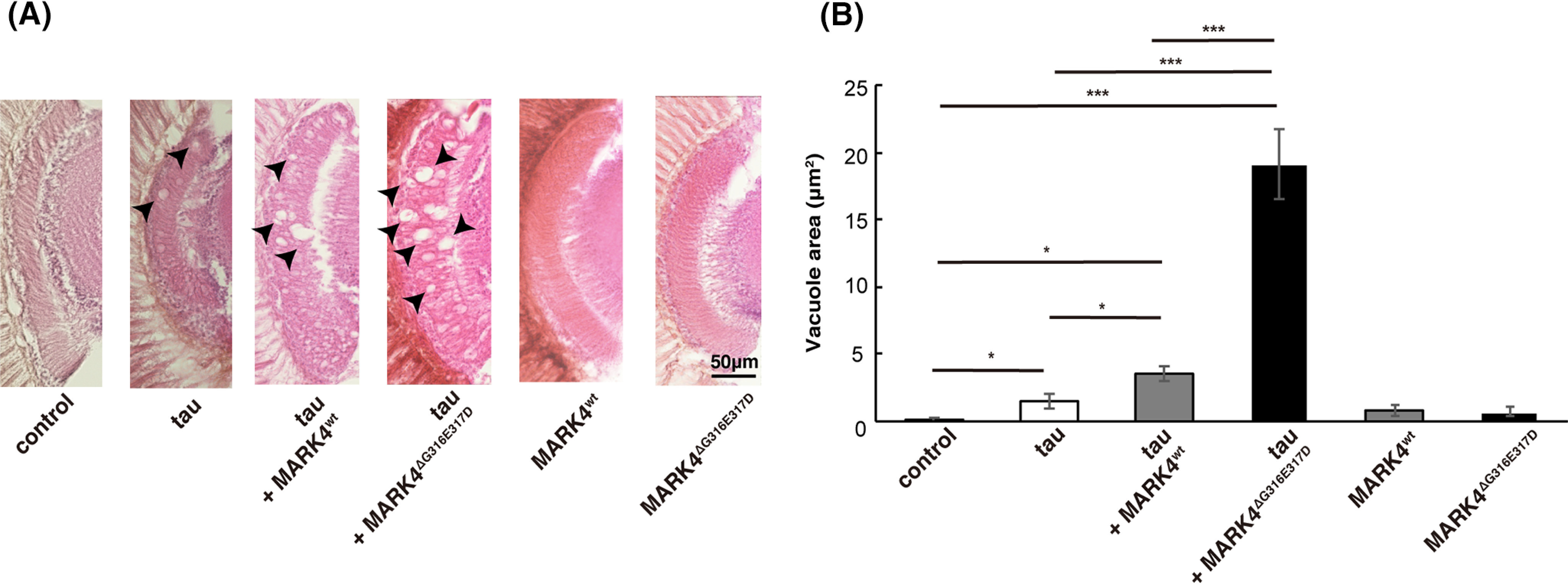
MARK4ΔG316E317D increases tau toxicity to a greater extent than MARK4wt. A, coexpression of MARK4 increased tau-induced neurodegeneration, and MARK4ΔG316E317D exerted a stronger effect than MARK4wt. Shown are lamina of flies carrying GMR-Gal4 driver alone (control), flies expressing tau (tau), flies coexpressing tau and MARK4wt (tau+MARK4wt), or flies coexpressing tau and MARK4ΔG316E317D (tau+MARK4ΔG316E317D). Expression of MARK4wt (MARK4wt) or MARK4ΔG316E317D (MARK4ΔG316E317D) alone did not cause neurodegeneration. Neurodegeneration is observed as vacuoles, indicated by arrows. B, quantification of the vacuole area. Means ± S.E. (error bars). n = 5. n.s. (not significant), p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.005 (one-way ANOVA and Tukey's post-hoc test).
MARK4wt and MARK4ΔG316E317D have similar kinase activities in vitro
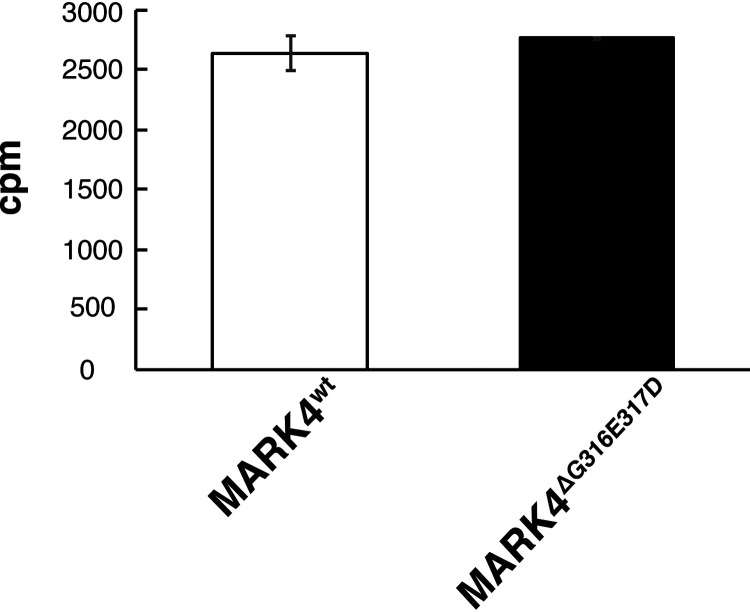
A previous study reported that, when HEK293 cells were cotransfected with tau and either MARK4wt or MARK4ΔG316E317D, cells transfected with MARK4ΔG316E317D exhibited a significant increase in tau Ser-262 phosphorylation relative to those transfected with MARK4wt (23). It was interpreted as evidence that the de novo mutation in MARK4 increases the ability of MARK4 to phosphorylate tau on Ser-262 more efficiently (23). However, it has not yet been tested whether MARK4ΔG316E317D has higher kinase activity than MARK4wt. To explore this possibility, we carried out an in vitro kinase assay. Specifically, we expressed MARK4wt or MARK4ΔG316E317D in HEK293 cells, immunoprecipitated MARK4 proteins from cell lysates, and measured their kinase activities using recombinant tau as a substrate. MARK4wt or MARK4ΔG316E317D had similar kinase activities (Fig. 3), indicating that the higher level of tau accumulation in cells expressing MARK4ΔG316E317D was not due to a difference in kinase activity.
Figure 3.
MARK4wt or MARK4ΔG316E317D have similar kinase activity in vitro. MARK4wt and MARK4ΔG316E317D expressed in HEK293 cells were immunoprecipitated and subjected to in vitro kinase assays. Incorporation of 32P in recombinant tau protein is expressed as means ± S.D. (error bars). (n = 3, n.s. (not significant), p > 0.05 (Student's t test)).
MARK4ΔG316E317D, but not MARK4wt, increases tau levels and exacerbates tau toxicity in a Ser-262/356–independent manner
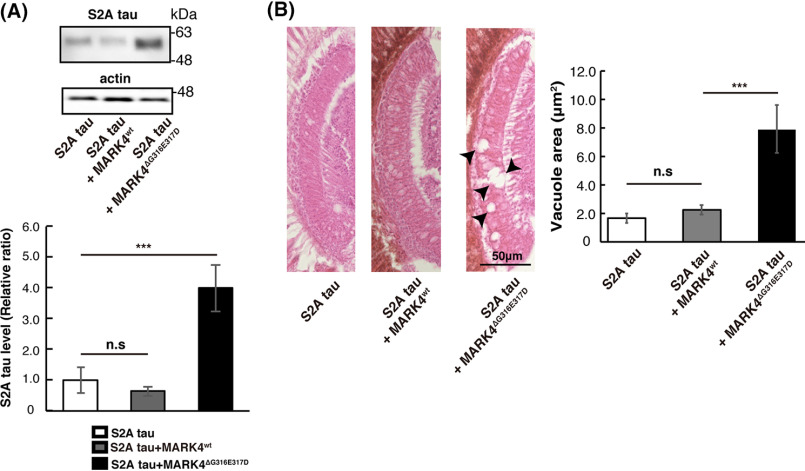
We previously reported that Par-1 overexpression causes tau accumulation via its phosphorylation at Ser-262 and Ser-356 and thus enhances neurodegeneration (11, 12). To determine whether the exacerbation of tau accumulation and toxicity by MARK4wt or MARK4ΔG316E317D is also mediated by tau phosphorylation at Ser-262 and Ser-356, we used a tau mutant in which both of those sites are replaced by alanines (S2A) (25). Similar to Par-1 (11, 12), the expression of MARK4wt did not increase the level of S2A tau (Fig. 4A). We also found that MARK4wt did not exacerbate neurodegeneration caused by S2A tau (Fig. 4B). These results suggest that MARK4wt increases tau levels and tau toxicity through tau phosphorylation at Ser-262 and Ser-356.
Figure 4.
MARK4ΔG316E317D increases tau levels and tau toxicity in a Ser-262/356–independent manner. A, MARK4ΔG316E317D positively regulated tau levels in a Ser-262/356–independent manner. Western blots of fly heads expressing S2A tau alone or coexpressing S2A tau with either MARK4wt (S2A tau+MARK4wt) or MARK4ΔG316E317D (S2A tau+MARK4ΔG316E317D). Means ± S.D. (error bars), n = 4. n.s. (not significant), p > 0.05; ***p < 0.005 (one-way ANOVA and Tukey's post-hoc test). B, coexpression of S2A tau with MARK4ΔG316E317D, but not with MARK4wt, enhanced neurodegeneration. Shown are lamina of flies harboring expressing S2A tau (S2A tau), coexpressing S2A tau and MARK4wt (S2A tau+MARK4wt), or coexpressing S2A tau and MARK4ΔG316E317D (S2A tau+MARK4ΔG316E317D). Means ± S.E. (error bars); n = 5. n.s. (not significant), p > 0.05; ***p < 0.005 (one-way ANOVA and Tukey's post-hoc test).
By contrast, MARK4ΔG316E317D significantly increased the levels of S2A tau (Fig. 4A), and coexpression of MARK4ΔG316E317D with S2A tau exacerbated neurodegeneration (Fig. 4B). These results suggest that coexpression of MARK4ΔG316E317D increases tau levels and tau toxicity via a novel gain-of-function mechanism that does not involve phosphorylation at Ser-262 and Ser-356.
Expression of MARK4wt or MARK4ΔG316E317D does not disrupt overall protein degradation
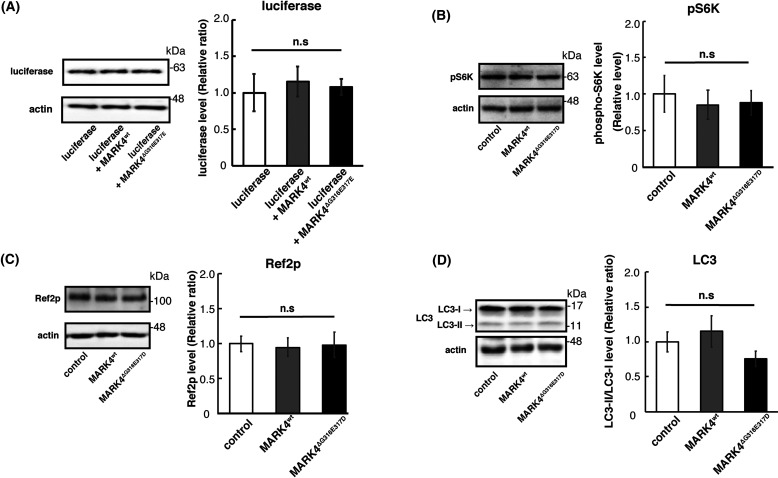
We found that MARK4ΔG316E317D increased tau protein levels without affecting the mRNA levels of tau (Fig. 1C). To investigate the mechanisms underlying the accumulation of tau caused by MARK4ΔG316E317D, we sought to determine whether this effect is specific to tau protein. When coexpressed with firefly luciferase, MARK4ΔG316E317D did not increase luciferase levels, indicating that the mutant protein does not cause nonspecific accumulation of exogenously expressed proteins (Fig. 5A).
Figure 5.
Neither MARK4wt nor MARK4ΔG316E317D inhibit the TOR pathway or increase autophagic activity. A, MARK4ΔG316E317D did not increase the levels of luciferase coexpressed in fly eyes. Western blots were performed on fly heads expressing luciferase alone (luciferase), coexpressing luciferase and MARK4wt (luciferase+MARK4wt), or coexpressing luciferase and MARK4ΔG316E317D (luciferase+MARK4ΔG316E317D). Actin was used as a loading control. Representative blots and quantitation are shown. Means ± S.D. (error bars); n = 4. n.s. (not significant), p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.005 (one-way ANOVA and Tukey's post-hoc test). B–D, expression of MARK4wt or MARK4ΔG316E317D did not promote autophagy. Western blots were performed on fly heads expressing control, MARK4wt, or MARK4ΔG316E317D. Blots were performed with anti-pS6K (pS6K) (B), anti-Ref2P (Ref2P) (C), and LC3 antibody (LC3) (D). Representative blots and quantitation are shown. Means ± S.D. (error bars); n = 3. n.s. (not significant), p > 0.05 (one-way ANOVA and Tukey post-hoc test). Actin was used as a loading control.
We next examined whether MARK4ΔG316E317D inhibits autophagic activity. MARK4 is known to negatively regulate the mTOR complex 1 (mTORC1) (26). mTORC1 is a conserved regulator of autophagy, which mediates the degradation of a variety of proteins, including tau (27). To test whether MARK4wt and MARK4ΔG316E317D had distinct effects on mTOR signaling, we first investigated whether expression of MARK4wt or MARK4ΔG316E317D in the fly retina inhibits TOR signaling. We found that neither MARK4wt nor MARK4ΔG316E317D affected phosphorylation of S6K, a target of TOR (Fig. 5B). In addition, neither MARK4wt nor MARK4ΔG316E317D affected LC3-II/LC3-I ratio or the levels of the autophagic substrate Ref2P (Fig. 5, C and D), suggesting that autophagic activity was not dampened. These results suggest that MARK4ΔG316E317D causes accumulation of tau via a mechanism other than overall suppression of protein degradation.
MARK4ΔG316E317D increases the levels of total and phosphorylated tau in vivo, although it does not phosphorylate tau directly at additional sites
Because hyperphosphorylation of tau is correlated with tau pathology (28, 29), we asked whether MARK4ΔG316E317D increases overall tau phosphorylation levels. We used Phos-tag analysis for comprehensive quantitative profiling of the phosphorylation status of tau (30). Drosophila expresses a single member of the Par-1/MARK family, Par-1; we previously reported that Par-1 phosphorylates human tau proteins at Ser-262 and Ser-356, stabilizing them and promoting their subsequent phosphorylation at other sites (13, 17). To highlight the difference between the effects of MARK4wt and MARK4ΔG316E317D on tau phosphorylation in this model, we carried out this experiment in a Par-1–knockdown background. Tau protein expressed in the fly retina separated into several bands, indicating that it was phosphorylated in multiple patterns (Fig. 6A). Interestingly, the phosphorylation forms of tau whose abundances increased differed depending on whether MARK4wt or MARK4ΔG316E317D was coexpressed. MARK4wt increased the intensity of a faster-migrating band, and coexpression of MARK4ΔG316E317D further increased the intensity of the slower-migrating band, indicating that MARK4ΔG316E317D increased tau species with additional phosphorylation in vivo (Fig. 6A).
Figure 6.
Coexpression of MARK4ΔG316E317D causes accumulation of total tau and tau-phosphorylated at sites other than Ser-262/356 in vivo. A, phosphorylation profile of tau expressed alone or coexpressed with MARK4wt or MARK4ΔG316E317D in vivo. Fly head extracts were separated by Phos-tag SDS-PAGE, followed by Western blotting with anti-tau antibody. Coexpression of MARK4wt increased the abundance of faster-migrating tau. By contrast, coexpression of MARK4ΔG316E317D increased the abundance of slower-migrating tau. B, Western blotting was performed using phospho-specific antibodies for SP/TP sites such as AT8 (pS202/T205), anti-pThr231 (pT231), and PHF1 (pS396/404), as well as pan-tau antibody T46 (tau). Actin was used as a loading control. Representative blots (top) and quantification (bottom panels, normalized either to actin or to total tau) are shown. Means ± S.D. (error bars); n = 4. n.s. (not significant), p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.005 (one-way ANOVA and Tukey's post-hoc test). C, MARK4wt and MARK4ΔG316E317D phosphorylated tau in the same pattern in vitro. MARK4wt and MARK4ΔG316E317D expressed in HEK293 cells were immunoprecipitated and incubated with recombinant tau. Tau proteins were separated by Phos-tag SDS-PAGE, followed by Western blotting with anti-tau antibody T46.
Tau has many serine and threonine sites followed by prolines, which are phosphorylated by proline-directed Ser/Thr kinases (SP/TP kinases) such as GSK3β, MAPK, and cyclin-dependent kinase 5 (Cdk5) (31–34). Although Par-1 does not directly phosphorylate those sites, Par-1 overexpression causes an overall increase in tau levels and proportional increases in tau phosphorylated at SP/TP sites (11). To determine whether MARK4ΔG316E317D promotes accumulation of tau phosphorylated at SP/TP sites to a greater extent than MARK4wt, we carried out Western blotting using phospho-specific antibodies against tau phosphorylated at the following SP/TP sites: AT8 (Ser-202, Thr-205), AT180 (Thr-231), and PHF1 (Ser-396, Ser-404). Coexpression of tau and MARK4wt increased the levels of tau phosphorylated at these sites (Fig. 6B), and coexpression of tau and MARK4ΔG316E317D further increased them, relative to expression of tau alone (Fig. 6B). These SP/TP sites are not expected to be direct substrates of MARK4 (13, 35), and the increases in the phospho-SP/TP tau species are proportional to those in total tau levels (Fig. 6B), suggesting that the elevated levels of the phospho-SP/TP tau caused by coexpression of MARK4wt or MARK4ΔG316E317D are likely a consequence of the accumulation of total tau.
However, we wondered whether the ΔG316E317D mutation might alter the kinases' substrate specificity and whether MARK4ΔG316E317D phosphorylates SP/TP sites. To determine whether MARK4ΔG316E317D directly phosphorylates those sites, we carried out an in vitro assay. Recombinant tau was incubated with MARK4wt or MARK4ΔG316E317D, and the phosphorylation pattern of tau was analyzed using the Phos-tag method. Tau exhibited the same phosphorylation pattern when it was incubated with MARK4wt or with MARK4ΔG316E317D (Fig. 6C), suggesting that MARK4ΔG316E317D increases accumulation of tau species phosphorylated at SP/TP sites via an indirect mechanism in vivo.
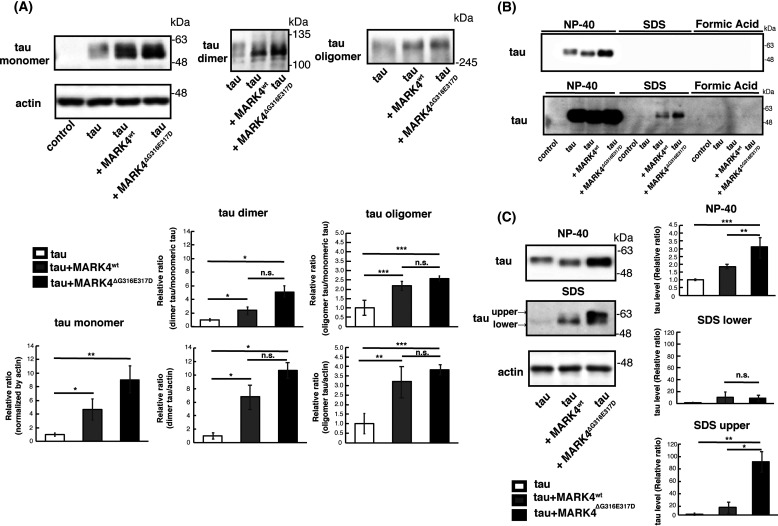
MARK4wt and MARK4ΔG316E317D both increase tau dimers and oligomers, and MARK4ΔG316E317D further promotes accumulation of insoluble forms of tau
Tau can aggregate into higher-order structures prone to accumulate, and tau phosphorylation at SP/TP sites promotes this process (29, 36). Hence, we asked whether coexpression of MARK4wt or MARK4ΔG316E317D would affect the formation of small aggregates such as dimers and oligomers. Tau proteins expressed in the Drosophila retina exist mostly in the monomeric form (18, 37). We found that both MARK4wt and MARK4ΔG316E317D increased the levels of dimers and oligomers to similar extents (Fig. 7A).
Figure 7.
MARK4ΔG316E317D promotes misfolding of tau. A, MARK4wt and MARK4ΔG316E317D promoted accumulation of tau oligomers and dimers at similar levels. Western blotting was performed on fly head lysates prepared under nonreducing conditions with oligomer-specific antibody T22 (oligomer) and pan-tau antibody Tau5 (dimer). Representative blots and quantification are shown. Actin was used as a loading control. Means ± S.D. (error bars); n = 3. n.s. (not significant), p > 0.05; *p < 0.05, **p < 0.01 (one-way ANOVA and Tukey's post-hoc test). B and C, tau in extracts from heads expressing tau alone (tau), coexpressing tau and MARK4wt (tau+MARK4wt), or coexpressing tau and MARK4ΔG316E317D (tau+MARK4ΔG316E317D) were detected by Western blotting with pan-tau antibody T46. Head homogenates were extracted first with RIPA buffer containing NP-40 (NP-40), next with 2% SDS (SDS), and finally with 70% formic acid (FA). B, representative Western blots are shown (shorter exposure (upper panel) and longer exposure (lower panel)). C, Western blotting was performed on fly heads extracted with RIPA buffer containing NP-40 (NP-40) and then with 2% SDS (SDS). Representative blots (left) and quantification (right) are shown.
Next, to assess whether coexpression of MARK4wt or MARK4ΔG316E317D would affect the detergent insolubility of tau, we performed sequential extraction followed by Western blotting with anti-tau antibody (38). As reported previously, tau proteins in the Drosophila retina exist mostly in a detergent-soluble form (18, 37) (Fig. 7B). When expressed alone or coexpressed with MARK4wt or MARK4ΔG316E317D, tau was mostly extracted with RIPA buffer containing Nonidet P-40 (NP-40) (Fig. 7B, NP-40). However, tau proteins were present in the SDS-soluble fraction when tau was coexpressed with MARK4wt or MARK4ΔG316E317D, and this effect was more prominent when MARK4ΔG316E317D was expressed (Fig. 7B, SDS). Tau was not detected in the detergent-insoluble fraction when coexpressed with either MARK4wt or MARK4ΔG316E317D (Fig. 7B, Formic Acid).
Tau proteins migrate into several bands with the Laemmli SDS-PAGE, and the migration speeds of these bands are related to their phosphorylation levels. We previously reported that tau in the slower-migrating band (tauupper) is more often phosphorylated at SP/TP sites than tau in the faster-migrating band (taulower) (11). Tau species in the NP-40 fraction were mostly taulower (Fig. 7C, NP-40), whereas tau proteins accumulating in the SDS fraction contained both tauupper and taulower. Consistent with the accumulation of phospho-SP/TP tau species (Fig. 6B), coexpression of MARK4ΔG316E317D, but not MARK4wt, increased the level of tauupper in the SDS-soluble fraction (Fig. 7C, SDS).
We further analyzed whether coexpression with MARK4wt or MARK4ΔG316E317D affects the solubility of S2A tau. In contrast to WT tau, dimers and NP-40–insoluble forms of S2A were undetectable with or without coexpression of either MARK4wt or MARK4ΔG316E317D (Fig. S2). S2A oligomers were detected, although either MARK4wt or MARK4ΔG316E317D did not increase S2A oligomers (Fig. S2). These results suggest that MARK4ΔG316E317D promotes the accumulation of S2A tau through mechanisms other than increasing its insolubility.
Taken together, these results indicate that MARK4wt increases the levels of Ser-262/356–phosphorylated tau, dimers, and oligomers. By contrast, MARK4ΔG316E317D promotes the accumulation of not only those tau species but also insoluble tau species, which may promote neurodegeneration.
Discussion
Accumulating evidence suggests that Par-1/MARK plays an initiating role in tau abnormalities leading to disease pathogenesis (11–14, 16–18). We previously reported that Par-1 overexpression increases, and knockdown decreases, tau phosphorylation at Ser-262 and Ser-356, which leads to accumulation of tau and exacerbation of neurodegeneration in a fly model of tau toxicity (11, 12). In this study, we found that human MARK4wt increased the levels of pTauSer-262, pTauSer-356, and total tau, and increased tau toxicity (Fig. 1 and Fig. 2). MARK4wt did not increase S2A tau levels or affect its toxicity (Fig. 4), suggesting that MARK4wt, similar to Par-1, affects tau metabolism and toxicity through tau phosphorylation at Ser-262 and Ser-356. On the other hand, we found that MARK4ΔG316E317D promoted tau accumulation via additional mechanisms. MARK4ΔG316E317D increased S2A tau levels (Fig. 4), indicating that MARK4ΔG316E317D can increase the abundance of tau that is not phosphorylated at Ser-262 or Ser-356. We also found that MARK4ΔG316E317D, but not MARK4wt, caused the accumulation of the aggregated forms of tau (Fig. 6 and Fig. 7). These results suggest that MARK4wt promotes accumulation of tau phosphorylated at Ser-262 and Ser-356 and that MARK4ΔG316E317D further increases tau accumulation via additional mechanisms. Although MARK4ΔG316E317D promotes accumulation of the detergent-insoluble form of tau (Fig. 7), considering that tau in this model exists mostly as a detergent-soluble form, MARK4ΔG316E317D is likely to increase total tau levels via not only enhancing tau misfolding but also other yet-to-be-determined mechanisms. Coexpression of MARK4ΔG316E317D increased S2A tau levels without affecting its solubility (Fig. S2), also suggesting that such mechanisms exist.
Rovelet-Lecrux et al. (23) reported that when HEK293 cells were cotransfected with tau and either MARK4wtor MARK4ΔG316E317D constructs, cells transfected with MARK4ΔG316E317D exhibited a significant increase in tau phosphorylation at Ser-262 relative to MARK4wt. The authors of that study concluded that this de novo mutation in MARK4 results in a gain-of-function in the ability of MARK4 to phosphorylate tau on Ser-262 (23). We also found that coexpression of MARK4ΔG316E317D increased the level of tau phosphorylated at Ser-262 to a greater extent than MARK4wt in fly eyes (Fig. 1). However, in vitro kinase assays revealed that MARK4wt and MARK4ΔG316E317D did not significantly differ in terms of kinase activity (Fig. 3), arguing against the idea that accumulation of tau is the result of more efficient phosphorylation of tau by MARK4ΔG316E317D.
The double mutation (ΔG316E317D) is located in the short linker that tethers the kinase to substrates or regulators (23). The predicted three-dimensional structure suggests that the double amino acid change (ΔG316E317D) increases the area of the substrate-binding region, facilitating a strong association of MARK4 with interacting proteins (23). MARK4 has been reported to interact with various proteins, including cytoskeletal proteins, kinases and phosphatases, ubiquitin-related enzymes, signaling molecules, mRNA binding proteins, and transcription mediators (26, 39–43). MARK4 mutation may change affinities to interacting proteins, thus creating the conditions that favor the accumulation of tau. The strengthened interaction of MARK4ΔG316E317D and substrates other than tau, or interaction with novel substrates, may indirectly stabilize tau.
In addition to AD, MARK4 has been suggested to play a critical role in other neurodegenerative diseases, including ischemic axonal injury (21) and synucleinopathies (44). Under pathological conditions, abnormalities in MARK4, such as additional posttranslational modifications in the linker region, might cause effects similar to those of the ΔG316E317D mutation on MARK4 and thus promote misfolding of tau and other aggregation-prone proteins. Identification of ΔG316E317D-induced changes in MARK4ΔG316E317D that promote tau accumulation may provide insight into the dysregulation of MARK4 in the pathogenesis of sporadic AD.
Experimental procedures
Fly stocks and husbandry
Flies were maintained in standard cornmeal media at 25 °C under light-dark cycles of 12:12 h. The transgenic fly line carrying the human 0N4R tau, which has four tubulin-binding domains (R) at the C-terminal region and no N-terminal insert (N), is a kind gift from Dr. M. B. Feany (Harvard Medical School) (45). UAS-PAR-1 RNAi is a kind gift from Dr. Bingwei Lu (Stanford University) (13). UAS-S2A tau, human 0N4R tau carrying alanine substitutions at Ser-262 and Ser-356 (S2A), were reported previously (12, 17). To establish transgenic fly lines carrying UAS-MARK4wt and UAS-MARK4ΔG316E317D, cDNA coding human MARK4 was obtained from DNASU Plasmid Repository (clone HsCD00294885, The Biodesign Institute at Arizona State University). ΔG316E317D mutation was introduced by using PCR-based site-directed mutagenesis. cDNA was subcloned into pUASTattB and injected into the oocytes carrying into P{CaryP}attP2. Genotypes of the flies used in the experiments are described in Table S1.
Chemicals and antibodies
An anti-MYC antibody (4A6) (Merck Millipore), anti-tau (T46) (Thermo Fisher Scientific), anti-tau (Tau5) (Thermo Fisher Scientific), anti-phospho–Ser-262 tau antibody (Abcam), anti-phospho–Thr-231 tau antibody (AT180, Thermo Fisher Scientific), anti-phospho–Ser-356 tau antibody (Abcam), anti-oligomer antibody (T22) (Merck Millipore), anti-phospho-S6K (Cell Signaling Technology), anti-Ref2p (Abcam), anti-LC3 (Merck Millipore), and anti-actin antibody (Sigma-Aldrich) were purchased. AT8 antibody and PHF1 antibody are kind gifts from Dr. Peter Davis (Albert Einstein College of Medicine).
Cell culture and transfection
HEK293 cells were maintained in DMEM (Sigma-Aldrich) supplemented with 10% (v/v) fetal bovine serum, 100 units/ml penicillin, and 0.1 mg/ml streptomycin. Plasmids encoding MARK4 were transfected with Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer's protocol.
In vitro kinase assay of MARK4
MARK4 expressed in HEK293 cells was immunoprecipitated from the cell lysate with monoclonal anti-Myc antibody (4A6) and Dynabeads protein G (Thermo Fisher Scientific). Its kinase activity was measured using human 2N4R tau, which was expressed and purified from E. coli and [γ32P]ATP as substrates. Incorporation of 32P into tau was quantified using a liquid scintillation counter (Beckman).
SDS-PAGE, Phos-tag SDS-PAGE, and immunoblotting
SDS-PAGE for Western blotting of tau, MARK4, and actin was performed using 10% or 7.5% (w/v) polyacrylamide gels. Phos-tag SDS-PAGE was performed using 7.5% (w/v) polyacrylamide gels containing 25 μm or 50 μm Phos-tag acrylamide (Wako Chemicals) for tau. Proteins separated in the gel were transferred to a PVDF membrane (Merck Millipore) using a submerged blotting apparatus and then visualized using Immobilon Western Chemiluminescent HRP Substrate (Millipore). The chemiluminescent signal was detected by Fusion FX (Vilber), and intensity was quantified using ImageJ (National Institutes of Health). Western blots were repeated a minimum of three times with different animals, and representative blots are shown. Flies used for Western blotting were 2 days old after eclosion.
Histological analysis
Neurodegeneration in the fly retina was analyzed as previously reported (32). Fly heads were fixed in Bouin's fixative for 48 h at room temperature, incubated for 24 h in 50 mm Tris/150 mm NaCl, and embedded in paraffin. Serial sections (7-μm thickness) through the entire heads were stained with hematoxylin and eosin and examined by bright-field microscopy. Images of the sections that include the lamina were captured with Keyence microscope BZ-X700 (Keyence), and the vacuole area was measured using ImageJ (National Institutes of Health). Heads from more than three flies (more than five hemispheres) were analyzed for each genotype.
qRT-PCR
qRT-PCR was carried out as previously reported (32). More than 30 flies for each genotype were collected and frozen. Heads were mechanically isolated, and total RNA was extracted using Isogen Reagent (Nippon Gene) according to the manufacturer's protocol with an additional centrifugation step (11,000 × g for 5 min) to remove cuticle membranes prior to the addition of chloroform. Total RNA was reverse-transcribed using PrimeScript Master Mix (Takara Bio). qRT-PCR was performed using TOYOBO THUNDERBIRD SYBR qPCR Mix on a Thermal Cycler Dice Real Time System (Takara Bio). The average threshold cycle value (CT) was calculated from at least three replicates per sample. The expression of genes of interest was standardized relative to rp49. Relative expression values were determined by the ΔΔCT method. Primers were designed using Primer-Blast (National Institutes of Health). The following primers were used for RT-qPCR: htau for 5′-CAAGACAGACCACGGGGCGG-3′, htau rev 5′-CTGCTTGGCCAGGGAGGCAG-3′, rp49 for 5′-GCTAAGCTGTCGCACAAATG-3′, and rp49 rev 5′-GTTCGATCCGTAACCGATGT-3′.
Sequential extraction of tau protein
The following sequential series of solubilizing buffer are RIPA (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 20 mm EDTA, 1% NP-40, and 50 mm NaF), 2% SDS, and 70% formic acid (38). The sample was mixed with the indicated buffer, sonicated briefly, and centrifuged at 45,000 × g for 30 min at 4 °C. The pellet was subjected to extraction with the indicated buffer. The supernatant was collected and subjected to immunoblot analysis as described above. The same volume of each extract was loaded per lane.
Statistics
Statistics were done with Microsoft Excel and R (R Foundation for Statistical Computing, Vienna, Austria: RRID:SCR_001905 http://www.R-project.org/). Differences were assessed using the Student's t test or one-way analysis of variance (ANOVA) and Tukey's honest significance difference post-hoc test. p values < 0.05 were considered statistically significant.
Data availability
All data are available in this article and in the supporting information.
Supplementary Material
Acknowledgments
We thank Drs. Mel Feany and Bingwei Lu; TRiP at Harvard Medical School (National Institutes of Health/National Institute of General Medical Sciences R01-GM084947); the Bloomington Stock Center; and NIG Drosophila stock center, for fly stocks. We thank Dr. Peter Davis for AT8 and PHF1 antibody. We thank T. Miyashita for technical assistance. We thank Dr. S.-I. Hisanaga for critical comments.
This article contains supporting information.
Author contributions—T. O., T. S., A. A., S. S., and K. A. investigation; T. O. and K. M. I. methodology; T. O., K. M. I., and K. A. writing-original draft; T. O., T. S., A. A., K. M. I., and K. A. writing-review and editing; T. S. and A. A. formal analysis; T. S., K. M. I., and K. A. supervision; A. A. and K. A. data curation; K. M. I. and K. A. conceptualization; K. A. funding acquisition; K. A. project administration.
Funding and additional information—This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (Brain Protein Aging and Dementia Control) (JSPS KAKENHI Grant 17H05703) (to K. A.), a research award from the Hoan-sha Foundation (to K. A.), the Takeda Science Foundation (to K. A.), a research award from the Japan Foundation for Aging and Health (to K. A.), a Grant-in-Aid for Scientific Research on Challenging Research (Exploratory) (JSPS KAKENHI Grant number 19K21593) (to K. A.), and the Research Funding for Longevity Science 19-7 from the National Center for Geriatrics and Gerontology, Japan (to K. M. I.).
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
- AD
- Alzheimer's disease
- MARK
- microtubule affinity–regulating kinase
- SP/TP kinases
- proline-directed Ser/Thr kinases
- Cdk
- cyclin-dependent kinase
- NP-40
- Nonidet P-40
- ANOVA
- analysis of variance.
References
- 1. Wang, Y., and Mandelkow, E. (2016) Tau in physiology and pathology. Nat. Rev. Neurosci. 17, 5–21 10.1038/nrn.2015.1 [DOI] [PubMed] [Google Scholar]
- 2. Grundke-Iqbal, I., Iqbal, K., Tung, Y. C., Quinlan, M., Wisniewski, H. M., and Binder, L. I. (1986) Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. U. S. A. 83, 4913–4917 10.1073/pnas.83.13.4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hasegawa, M., Morishima-Kawashima, M., Takio, K., Suzuki, M., Titani, K., and Ihara, Y. (1992) Protein sequence and mass spectrometric analyses of tau in the Alzheimer's disease brain. J. Biol. Chem. 267, 17047–17054 [PubMed] [Google Scholar]
- 4. Hanger, D. P., Betts, J. C., Loviny, T. L., Blackstock, W. P., and Anderton, B. H. (1998) New phosphorylation sites identified in hyperphosphorylated tau (paired helical filament-tau) from Alzheimer's disease brain using nanoelectrospray mass spectrometry. J. Neurochem. 71, 2465–2476 10.1046/j.1471-4159.1998.71062465.x [DOI] [PubMed] [Google Scholar]
- 5. Morishima-Kawashima, M., Hasegawa, M., Takio, K., Suzuki, M., Yoshida, H., Titani, K., and Ihara, Y. (1995) Proline-directed and non-proline-directed phosphorylation of PHF-tau. J. Biol. Chem. 270, 823–829 10.1074/jbc.270.2.823 [DOI] [PubMed] [Google Scholar]
- 6. Holtzman, D. M., Carrillo, M. C., Hendrix, J. A., Bain, L. J., Catafau, A. M., Gault, L. M., Goedert, M., Mandelkow, E., Mandelkow, E. M., Miller, D. S., and Ostrowitzki, S. (2016) Tau: From research to clinical development. Alzheimer's Dement. 12, 1033–1039 10.1016/j.jalz.2016.03.018 [DOI] [PubMed] [Google Scholar]
- 7. Ballatore, C., Lee, V. M., and Trojanowski, J. Q. (2007) Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat. Rev. Neurosci. 8, 663–672 10.1038/nrn2194 [DOI] [PubMed] [Google Scholar]
- 8. Augustinack, J. C., Schneider, A., Mandelkow, E. M., and Hyman, B. T. (2002) Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol. 103, 26–35 10.1007/s004010100423 [DOI] [PubMed] [Google Scholar]
- 9. Yu, W., Polepalli, J., Wagh, D., Rajadas, J., Malenka, R., and Lu, B. (2012) A critical role for the PAR-1/MARK-tau axis in mediating the toxic effects of Aβ on synapses and dendritic spines. Hum. Mol. Genet. 21, 1384–1390 10.1093/hmg/ddr576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zempel, H., Thies, E., Mandelkow, E., and Mandelkow, E. M. (2010) Aβ oligomers cause localized Ca2+ elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J. Neurosci. 30, 11938–11950 10.1523/JNEUROSCI.2357-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ando, K., Maruko-Otake, A., Ohtake, Y., Hayashishita, M., Sekiya, M., and Iijima, K. M. (2016) Stabilization of microtubule-unbound tau via tau phosphorylation at Ser-262/356 by Par-1/MARK contributes to augmentation of AD-related phosphorylation and Aβ42-induced tau toxicity. PLoS Genet. 12, e1005917 10.1371/journal.pgen.1005917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ando, K., Oka, M., Ohtake, Y., Hayashishita, M., Shimizu, S., Hisanaga, S., and Iijima, K. M. (2016) Tau phosphorylation at Alzheimer's disease-related Ser356 contributes to tau stabilization when PAR-1/MARK activity is elevated. Biochem. Biophys. Res. Commun. 478, 929–934 10.1016/j.bbrc.2016.08.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nishimura, I., Yang, Y., and Lu, B. (2004) PAR-1 kinase plays an initiator role in a temporally ordered phosphorylation process that confers tau toxicity in Drosophila. Cell 116, 671–682 10.1016/S0092-8674(04)00170-9 [DOI] [PubMed] [Google Scholar]
- 14. Lasagna-Reeves, C. A., de Haro, M., Hao, S., Park, J., Rousseaux, M. W. C., Al-Ramahi, I., Jafar-Nejad, P., Vilanova-Velez, L., See, L., De Maio, A., Nitschke, L., Wu, Z., Troncoso, J. C., Westbrook, T. F., Tang, J., et al. (2016) Reduction of Nuak1 decreases tau and reverses phenotypes in a tauopathy mouse model. Neuron 92, 407–418 10.1016/j.neuron.2016.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kang, H.-Y., Kim, H.-J., Kim, K., Oh, S.-I., Yoon, S., Kim, J., Park, S., Cheon, Y., Her, S., Lee, M., Lu, B., and Lee, S. (2020) Actin-microtubule crosslinker Pod-1 tunes PAR-1 signaling to control synaptic development and tau-mediated synaptic toxicity. Neurobiol. Aging 90, 93–98 10.1016/j.neurobiolaging.2020.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alonso, A. D., Di Clerico, J., Li, B., Corbo, C. P., Alaniz, M. E., Grundke-Iqbal, I., and Iqbal, K. (2010) Phosphorylation of tau at Thr212, Thr231, and Ser262 combined causes neurodegeneration. J. Biol. Chem. 285, 30851–30860 10.1074/jbc.M110.110957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iijima-Ando, K., Sekiya, M., Maruko-Otake, A., Ohtake, Y., Suzuki, E., Lu, B., and Iijima, K. M. (2012) Loss of axonal mitochondria promotes tau-mediated neurodegeneration and Alzheimer's disease-related tau phosphorylation via PAR-1. PLoS Genet. 8, e1002918 10.1371/journal.pgen.1002918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iijima, K., Gatt, A., and Iijima-Ando, K. (2010) Tau Ser262 phosphorylation is critical for Aβ42-induced tau toxicity in a transgenic Drosophila model of Alzheimer's disease. Hum. Mol. Genet. 19, 2947–2957 10.1093/hmg/ddq200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dujardin, S., Commins, C., Lathuiliere, A., Beerepoot, P., Fernandes, A. R., Kamath, T. V., De Los Santos, M. B., Klickstein, N., Corjuc, D. L., Corjuc, B. T., Dooley, P. M., Viode, A., Oakley, D. H., Moore, B. D., Mullin, K., et al. (2020) Tau molecular diversity contributes to clinical heterogeneity in Alzheimer's disease. Nat. Med. 26, 1256–1263 10.1038/s41591-020-0938-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lund, H., Gustafsson, E., Svensson, A., Nilsson, M., Berg, M., Sunnemark, D., and von Euler, G. (2014) MARK4 and MARK3 associate with early tau phosphorylation in Alzheimer's disease granulovacuolar degeneration bodies. Acta Neuropathol. Commun. 2, 22 10.1186/2051-5960-2-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hayden, E. Y., Putman, J., Nunez, S., Shin, W. S., Oberoi, M., Charreton, M., Dutta, S., Li, Z., Komuro, Y., Joy, M. T., Bitan, G., MacKenzie-Graham, A., Jiang, L., and Hinman, J. D. (2019) Ischemic axonal injury up-regulates MARK4 in cortical neurons and primes tau phosphorylation and aggregation. Acta Neuropathol. Commun. 7, 135 10.1186/s40478-019-0783-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pathak, G. A., Zhou, Z., Silzer, T. K., Barber, R. C., and Phillips, N. R, Alzheimer's Disease Neuroimaging Initiative, Breast and Prostate Cancer Cohort Consortium, and Alzheimer's Disease Genetics Consortium. (2020) Two-stage Bayesian GWAS of 9576 individuals identifies SNP regions that are targeted by miRNAs inversely expressed in Alzheimer's and cancer. Alzheimer's Dement. 16, 162–177 10.1002/alz.12003 [DOI] [PubMed] [Google Scholar]
- 23. Rovelet-Lecrux, A., Charbonnier, C., Wallon, D., Nicolas, G., Seaman, M. N. J., Pottier, C., Breusegem, S. Y., Mathur, P. P., Jenardhanan, P., Le Guennec, K., Mukadam, A. S., Quenez, O., Coutant, S., Rousseau, S., Richard, A.-C., et al. (2015) De novo deleterious genetic variations target a biological network centered on Aβ peptide in early-onset Alzheimer disease. Mol. Psychiatry 20, 1046–1056 10.1038/mp.2015.100 [DOI] [PubMed] [Google Scholar]
- 24. Trinczek, B., Brajenovic, M., Ebneth, A., and Drewes, G. (2004) MARK4 is a novel microtubule-associated proteins/microtubule affinity-regulating kinase that binds to the cellular microtubule network and to centrosomes. J. Biol. Chem. 279, 5915–5923 10.1074/jbc.M304528200 [DOI] [PubMed] [Google Scholar]
- 25. Iijima-Ando, K., Zhao, L., Gatt, A., Shenton, C., and Iijima, K. (2010) A DNA damage-activated checkpoint kinase phosphorylates tau and enhances tau-induced neurodegeneration. Hum. Mol. Genet. 19, 1930–1938 10.1093/hmg/ddq068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li, L., and Guan, K. L. (2013) Microtubule-associated protein/microtubule affinity-regulating kinase 4 (MARK4) is a negative regulator of the mammalian target of rapamycin complex 1 (mTORC1). J. Biol. Chem. 288, 703–708 10.1074/jbc.C112.396903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang, Y., and Mandelkow, E. (2012) Degradation of tau protein by autophagy and proteasomal pathways. Biochem. Soc. Trans. 40, 644–652 10.1042/BST20120071 [DOI] [PubMed] [Google Scholar]
- 28. Chatterjee, S., Sang, T. K., Lawless, G. M., and Jackson, G. R. (2009) Dissociation of tau toxicity and phosphorylation: role of GSK-3β, MARK and Cdk5 in a Drosophila model. Hum. Mol. Genet. 18, 164–177 10.1093/hmg/ddn326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jackson, G. R., Wiedau-Pazos, M., Sang, T. K., Wagle, N., Brown, C. A., Massachi, S., and Geschwind, D. H. (2002) Human wild-type tau interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron 34, 509–519 10.1016/S0896-6273(02)00706-7 [DOI] [PubMed] [Google Scholar]
- 30. Hosokawa, T., Saito, T., Asada, A., Fukunaga, K., and Hisanaga, S. (2010) Quantitative measurement of in vivo phosphorylation states of Cdk5 activator p35 by Phos-tag SDS-PAGE. Mol. Cell. Proteomics 9, 1133–1143 10.1074/mcp.M900578-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kimura, T., Ishiguro, K., and Hisanaga, S. (2014) Physiological and pathological phosphorylation of tau by Cdk5. Front. Mol. Neurosci. 7, 65 10.3389/fnmol.2014.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saito, T., Oba, T., Shimizu, S., Asada, A., Iijima, K. M., and Ando, K. (2019) Cdk5 increases MARK4 activity and augments pathological tau accumulation and toxicity through tau phosphorylation at Ser262. Hum. Mol. Genet. 28, 3062–3071 10.1093/hmg/ddz120 [DOI] [PubMed] [Google Scholar]
- 33. Takashima, A. (2006) GSK-3 is essential in the pathogenesis of Alzheimer's disease. J. Alzheimer's Dis. 9, 309–317 10.3233/jad-2006-9s335 [DOI] [PubMed] [Google Scholar]
- 34. Leugers, C. J., Koh, J. Y., Hong, W., and Lee, G. (2013) Tau in MAPK activation. Front. Neurol. 4, 161 10.3389/fneur.2013.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Drewes, G., Ebneth, A., Preuss, U., Mandelkow, E. M., and Mandelkow, E. (1997) MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell 89, 297–308 10.1016/S0092-8674(00)80208-1 [DOI] [PubMed] [Google Scholar]
- 36. Steinhilb, M. L., Dias-Santagata, D., Mulkearns, E. E., Shulman, J. M., Biernat, J., Mandelkow, E. M., and Feany, M. B. (2007) S/P and T/P phosphorylation is critical for tau neurotoxicity in Drosophila. J. Neurosci. Res. 85, 1271–1278 10.1002/jnr.21232 [DOI] [PubMed] [Google Scholar]
- 37. Feuillette, S., Miguel, L., Frébourg, T., Campion, D., and Lecourtois, M. (2010) Drosophila models of human tauopathies indicate that Tau protein toxicity in vivo is mediated by soluble cytosolic phosphorylated forms of the protein. J. Neurochem. 113, 895–903 10.1111/j.1471-4159.2010.06663.x [DOI] [PubMed] [Google Scholar]
- 38. Shahani, N., Subramaniam, S., Wolf, T., Tackenberg, C., and Brandt, R. (2006) Tau aggregation and progressive neuronal degeneration in the absence of changes in spine density and morphology after targeted expression of Alzheimer's disease-relevant tau constructs in organotypic hippocampal slices. J. Neurosci. 26, 6103–6114 10.1523/JNEUROSCI.4245-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brajenovic, M., Joberty, G., Küster, B., Bouwmeester, T., and Drewes, G. (2004) Comprehensive proteomic analysis of human Par protein complexes reveals an interconnected protein network. J. Biol. Chem. 279, 12804–12811 10.1074/jbc.M312171200 [DOI] [PubMed] [Google Scholar]
- 40. Naz, F., Islam, A., Ahmad, F., and Hassan, M. I. (2015) Atypical PKC phosphorylates microtubule affinity-regulating kinase 4 in vitro. Mol. Cell. Biochem. 410, 223–228 10.1007/s11010-015-2555-3 [DOI] [PubMed] [Google Scholar]
- 41. Al-Hakim, A. K., Zagorska, A., Chapman, L., Deak, M., Peggie, M., and Alessi, D. R. (2008) Control of AMPK-related kinases by USP9X and atypical Lys(29)/Lys(33)-linked polyubiquitin chains. Biochem. J. 411, 249–260 10.1042/BJ20080067 [DOI] [PubMed] [Google Scholar]
- 42. Sowa, M. E., Bennett, E. J., Gygi, S. P., and Harper, J. W. (2009) Defining the human deubiquitinating enzyme interaction landscape. Cell 138, 389–403 10.1016/j.cell.2009.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clement, M., Chen, X., Chenoweth, H. L., Teng, Z., Thome, S., Newland, S. A., Harrison, J., Yu, X., Finigan, A. J., Mallat, Z., and Li, X. (2019) MARK4 (microtubule affinity-regulating kinase 4)-dependent inflammasome activation promotes atherosclerosis-brief report. Arterioscler. Thromb. Vasc. Biol. 39, 1645–1651 10.1161/ATVBAHA.119.312478 [DOI] [PubMed] [Google Scholar]
- 44. Henderson, M. X., Chung, C. H., Riddle, D. M., Zhang, B., Gathagan, R. J., Seeholzer, S. H., Trojanowski, J. Q., and Lee, V. M. Y. (2017) Unbiased proteomics of early lewy body formation model implicates active microtubule affinity-regulating kinases (MARKs) in synucleinopathies. J. Neurosci. 37, 5870–5884 10.1523/JNEUROSCI.2705-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wittmann, C. W., Wszolek, M. F., Shulman, J. M., Salvaterra, P. M., Lewis, J., Hutton, M., and Feany, M. B. (2001) Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 293, 711–714 10.1126/science.1062382 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in this article and in the supporting information.