Abstract
I. Purpose of review:
To provide an overview of current interventional treatment options for women with chronic pelvic pain (CPP).
II. Recent findings:
Accessibility of CT imaging, ultrasound, and fluoroscopy have assisted the development of novel interventional techniques. Similarly, neuromodulation techniques have improved with the development of novel stimulation patterns and device implants.
III. Summary:
Numerous small-scale studies report high success rates with injection intervention therapies in CPP but there are limited well designed large-scale studies that demonstrate superiority of treatment. Female pelvic pain is difficult to diagnose due to the multifactorial etiology and the variable presentation causing delay in accurate diagnosis and lack of response to conventional medical and initial interventional therapies. Despite the shortfalls of current studies, collectively our understanding of chronic pain conditions and helpful injection interventions are improving. Undoubtedly the breadth of current research will provide a rich foundation for future large-scale well-designed studies involving multiple disciplines with more uniform methods and criteria to produce reliable and reproducible results.
Keywords: Female pelvic pain, pelvic pain interventions, plexus blockade, ganglion impar block, pudendal nerve block, neuromodulation, pelvic pain injections
Introduction
The multifactorial etiology of female pelvic pain has necessitated an equally diverse body of research to better understand and treat these conditions. As research in pelvic pain has expanded, the literature has overlapped with various fields beyond pain medicine such as oncology, urology, gynecology and orthopedics. Each specialty contributes invaluable perspective in the management of pelvic pain but there remain several shortfalls in the collective research body. The main issue with current research is the lack of high power, randomized controlled trials or comparable well-designed studies. Nearly all interventional treatment studies have been conducted on small patient populations with distinctly varied inclusion criteria, data interpretation, procedural methods, and outcome measures. Though certain investigations have produced significant results, the heterogeneity among studies makes it difficult to interpret results and discern appropriate management for patients. This article aims to outline the current methods for female pelvic pain and provide vision to new emerging interventional treatments.
Non-interventional physicians are typically the first to evaluate patients with CPP and thus bear an enormous responsibility in proper referral to a pain specialist. While there are no strict guidelines for assessing patients with CPP, a comprehensive HPI, physical exam, diagnostic testing and imaging can help rule out specific etiologies of CPP [1]. After initial work up, practitioners should consider referral when pain symptoms disrupt daily activities/mood, pain is refractory to conservative treatment (medications/physical therapy), and or when no curative treatments are available. A common misconception is that pain clinics are “last resorts” for patients who have serially failed all other treatment modalities. In contrast, multidisciplinary pain clinics combine patient education, medication management, cognitive behavioral therapy (CBT)/psychological health, and physiotherapy used in parallel with targeted interventional treatments. Practitioners should strive to complete new patient assessment, work up, and trial of therapy (if indicated) within three months as delays in referral to multidisciplinary pain clinics risk poorer patient outcomes [2, 3].
1. Nerve Plexus blocks
1.1. Superior Hypogastric Plexus Block
The superior hypogastric plexus (SHP) is located within the retroperitoneum between vertebral levels L5 and S1. This nerve plexus is anterior and lateral to the abdominal aorta just below the aortic bifurcation. As part of autonomic abdominopelvic nervous system, the SHP contains afferent pain fibers responsible for visceral innervation of numerous pelvic structures such as the descending colon, rectum, internal sex organs, uterus, bladder, urethra, perineum and prostate in men [4].
A superior hypogastric plexus block (SHPB) is an intervention that provides sympathetic blockade of visceral pain fibers innervating these pelvic structures [5, 6]. The SHPB was first described in 1990 by Plancarte et al [7] as a treatment for chronic pelvic pain (CPP) secondary to malignancy which produced an overall 70% decrease in pelvic pain. In 1993, de Leon Cassola et al [5] reported an overall 69% success rate utilizing bilateral SHPB in 26 patients with cancer related pelvic pain. Prior to the study, all participants had failed opioid management and reported 10/10 excruciating pain. Following a successful diagnostic SHPB with bupivacaine, 18 patients underwent SHPB using 10% phenol solution which provided significant pain reduction (<4/10 visual analog pain scale) six months post procedure and a 67% reduction opioid usage two weeks post procedure. Notably, the eight patients who did not have satisfactory pain relief had extensive retroperitoneal tumor burden but were still able to reduce their previous opioid requirement.
Since its development, the SHPB has been used to treat common female pelvic pain syndromes including interstitial cystitis, urethral pain, endometriosis. Weschler et al [8] utilized this intervention for treatment of chronic endometriosis in six patients, five of whom reported improvement in pain without complications secondary to the intervention. Newer developments have shown the SHP can be targeted with pulsed radio frequency ablation which offers non-destructive neuromodulation to block pelvic visceral pain. A case report by Kim et al [9] described the use of pulsed RFA on the SHP for treatment of refractory interstitial cystitis in a 35-year-old female patient. Their findings reported complete pain and symptom relief lasting for two and a half years following two interventions. Clearly, this data is based on case reports and case series and cannot be extrapolated for use until prospective trials are published.
Figures 1 and 2 demonstrate lateral and anterior-posterior fluoroscopic contrast images obtained during a superior hypogastric block. These images obtained from Nagpal & Moody [10•] demonstrate how an experienced practitioner utilizes 2D images to perform a SHPB in the outpatient clinical setting. Please refer to this article for more information regarding procedural technique and image acquisition which cannot be comprehensively discussed due to the scope of this review article.
Figure 1.
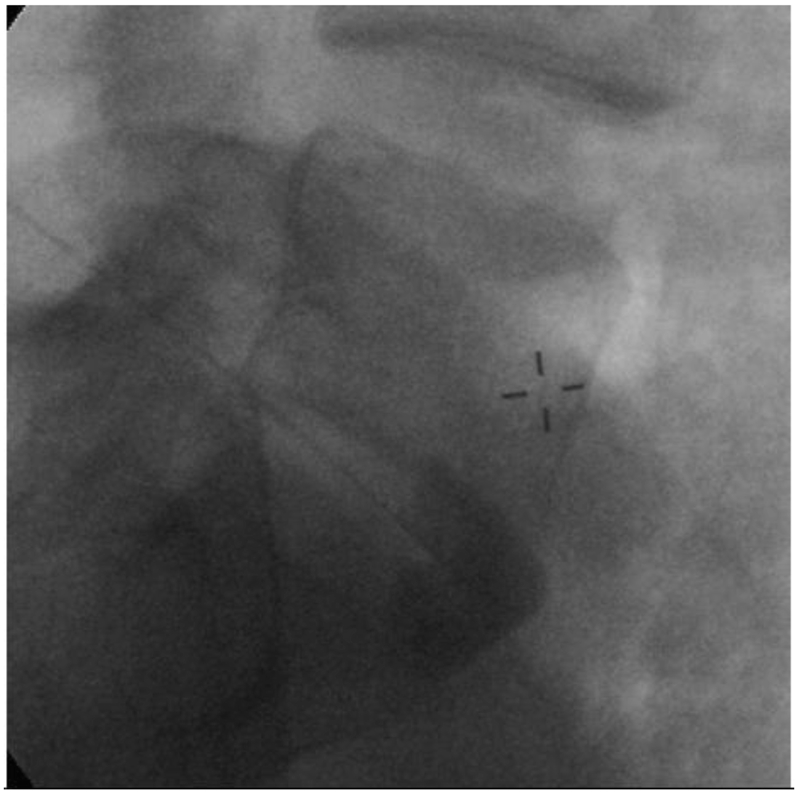
Superior hypogastric plexus block with contrast spread in a lateral view. Needle positioned at L5 vertebral body
Figure 2.
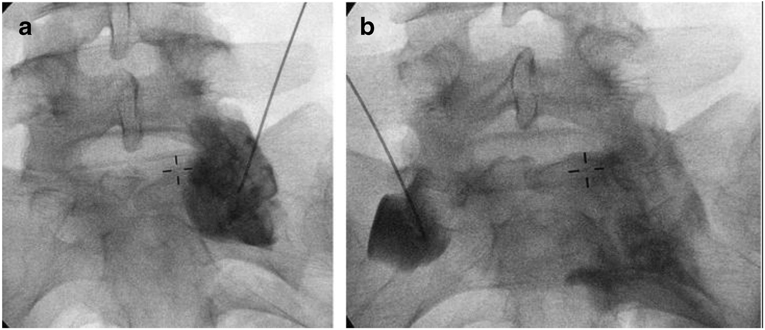
A & B) Superior hypogastric plexus block with contrast spread seen in an anterior-posterior view. Needle positioned at L5 S1 vertebral body junction.
1.2. Inferior Hypogastric Block
The inferior hypogastric plexus (IHP) is a caudal continuation of the superior hypogastric plexus located anterior to the sacrum, on either side of the rectum, and ventral to the S2, S3 and S4 spinal segments. The IHP is composed of efferent sympathetic fibers from the hypogastric and pelvic splanchnic nerves, preganglionic parasympathetic fibers from pelvic splanchnic nerves, and visceral afferent fibers from the pelvic viscera [11]. The IHP provides innervation to various plexus’ including the uterovaginal plexus, prostatic plexus, visceral plexus, and middle rectal plexus [12]. In practice, inferior hypogastric plexus blocks (IHPB) have been used sparingly for the diagnosis and treatment of pain syndromes involving the lower pelvic visceral structures such as the bladder, penis, vagina, rectum, anus, and perineum. However, because the IHPB is not commonly performed in clinical practice, there are limited case reports and case series describing the use of this procedure [11, 13].
Shultz [11] was the first to describe fluoroscopy guided trans sacral IHPB on 11 female patients suffering from CPP. Shultz reported statistically significant pain reduction without complications in 73% of the 15 interventions performed. These results demonstrated IHPB and SHPB were equally effective, and that IHPG might be a safer alternative to SHPB. More recently, in 2015 Amin et al [13•] published a randomized clinical trial compared IHPB vs acupuncture for treatment of idiopathic CPP in women. Results demonstrated IHPB had an overall 72.6% success rate and showed a significantly higher effect on reducing pain intensity when compared to acupuncture. Notably, patients in this study did not have structural pathology detected by radiographic imaging yet the IHGP alleviated pain in >70% of patients without procedural complications. Collectively, though there are few studies on IHPG, the two studies by Shultz [11] and Amin [13] demonstrate some degree of reproducibility as both studies have similar success rates.
2. Ganglion Impar Block [Fig 3]
Figure 3.
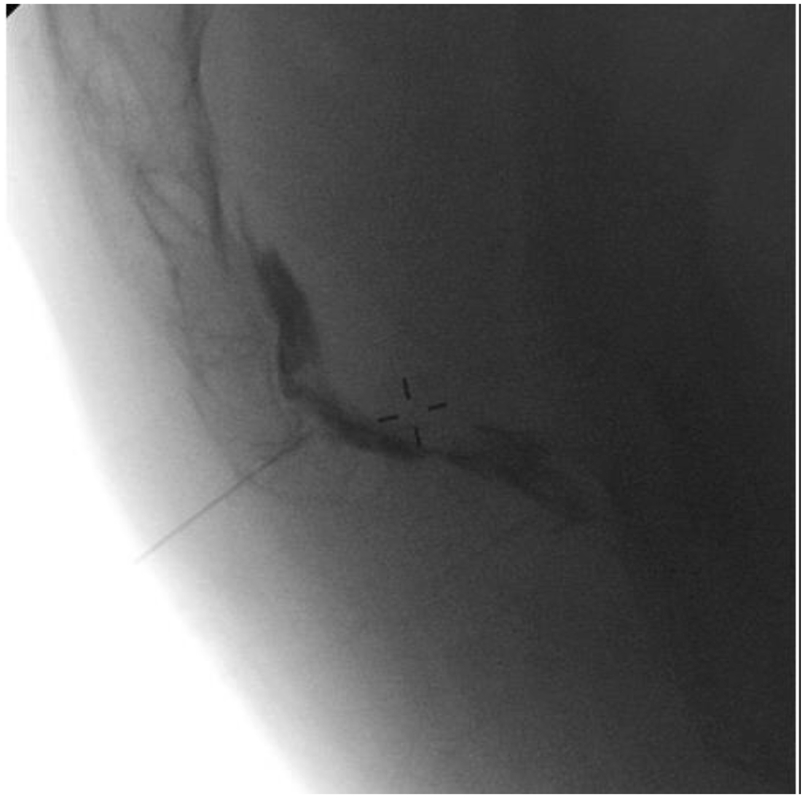
Ganglion impar block in a lateral view demonstrating "reverse comma" dye spread of contrast [10]
The ganglion impar (also known as the Ganglion of Walther) is the final pelvic ganglion of the efferent sympathetic trunk formed by the convergence of bilateral paravertebral sympathetic chains terminating anteriorly as a single midline ganglion [14, 15]. Classically located anterior to the anterior sacrococcygeal ligament, this ganglion can have variable anatomy within the pre-coccygeal space. The ganglion impar provides nociceptive and sympathetic innervation to the pelvic visceral, perineal region, distal rectum, distal urethra, distal third of the vagina, and vulva/scrotum [16, 17].
First described by Plancarte et al [18], targeted blockade of the ganglion impar can provide relief for rectal, anal, perineal, and genital pain. A 2018 retrospective single center study by Sousa et al [19•] reported on ganglion impar block (GIB) for treatment of uncontrolled oncologic pelvic pain due to various malignancies including rectal, prostatic, vulvar, and vaginal cancer. Neurolytic blockade was performed on 14 patients via sacrococcygeal approach under fluoroscopic guidance. Three months post procedure 79% of patients reported pain reduction, 43% reported improvement of greater than 50%, and 36% reported improvement between 30% and 50%.
Other applications of the GIB include treatment of coccygodynia (or coccydynia); a condition five times as prevalent in women that presents at a median age of 40. A 2015 retrospective study by Gunduz et al [20••] used fluoroscopy-guided trans sacrococcygeal GIB in 22 patients suffering from chronic coccygodynia (20 of whom were female). Results demonstrated 82 % of patients reported at least 50% pain reduction that lasted for median duration of six months. Thereafter, nine patients that underwent a second injection all achieved relief for median duration of 17 months. A similar 2014 study by Malec- Milewska et al [21] described use of GIB performed on nine women with chronic pelvic and perineal pain which reported permanent pain relief in four of the nine patients.
Le Clerc et al [14••] described a retrospective single center study on 83 patients (80.7% of which were women) that underwent serial CT guided ganglion impar blocks for treatment of CPP and perineal pain. In this study population, 26% suffered from refractory pudendal neuralgia and 29% suffered from isolated coccydynia. Three total blocks were performed, each spaced approximately 30 days apart. Pain scores were analyzed immediately post procedure and one month after intervention. Overall, 41% of patients demonstrated long term improvement, defined as greater than 50% reduction in pain, 50.6% reported no change, and 8.4 % reported worsened symptoms.
3. Pudendal Nerve Block [Fig 4]
Figure 4.
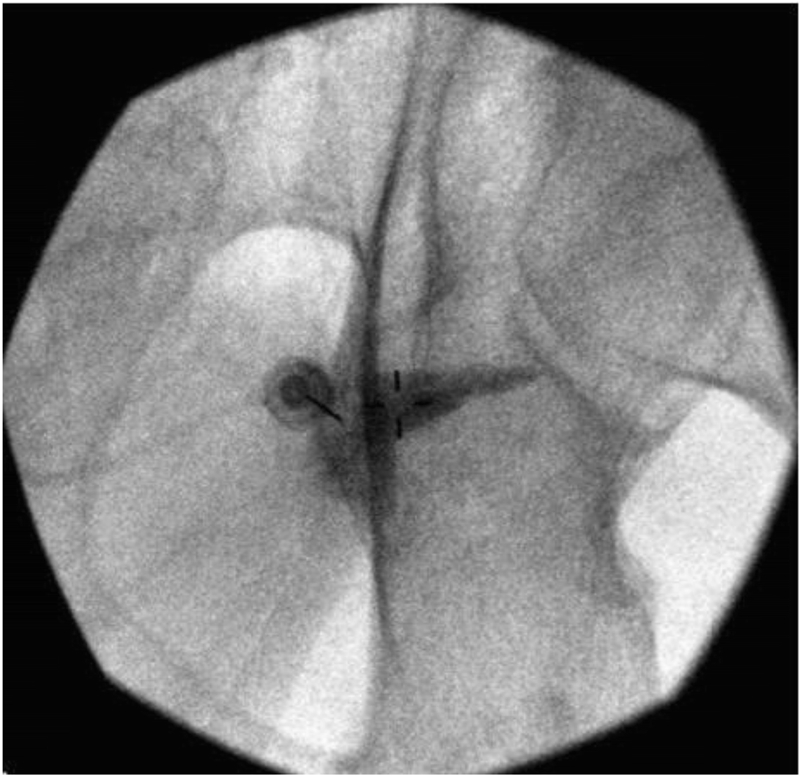
Pudendal block with needle visualized advancing to the base of ischial spine [10]
Pudendal neuralgia (PN) is a common chronic pelvic condition that presents in women as pain or paresthesia in the buttocks, vagina, vulva, labia, mons veneris, and clitoris, and in men as pain or paresthesia in the buttocks, perineum, and scrotum. PN is diagnosed clinically based on five essential criteria (Nantes criteria) which include: 1) pain in the anatomical distribution of the pudendal nerve 2) pain exacerbated by sitting 3) patient is not woken from sleep due to pain, 4) no objective sensory loss on physical exam and 5) positive response to pudendal nerve block [22]. Classically, PN presents as unilateral pain worsened throughout the day and exacerbated with sitting, however symptoms may extend to the groin, inner leg, buttocks, and abdomen [23, 24].
Patients with PN may be hesitant to proceed with the classical approaches which involve direct transvaginal or trans perineal injections to the pudendal nerve [25]. One alternative technique described by McDonald and Spigos [26] used CT guided nerve block via the posterior trans gluteal approach in 26 female patients with pudendal neuralgia. Patients underwent a total of five CT-guided pudendal blocks over a five-month period after which 62% of patients reported significant pain reduction. The trans sacral approach described by Cok et al [27] followed two female patients with pudendal nerve injury secondary to hysterectomy surgery who were then successfully treated via trans sacral S2-S4 pudendal nerve blocks. In the prone position using fluoroscopic guidance, the needle was advanced through the S2-S4 foramina to deliver a diagnostic block of 1% lidocaine. Those patients with a positive response to the diagnostic block subsequently underwent interventional block of 1% lidocaine and 80mg methylprednisolone. Six months following the procedure, both patients reported pain scores of 0 to 1 compared to 9-10 pre-procedure.
Other intervention techniques such as pulsed radiofrequency neuromodulation have been investigated for the treatment of PN. A prospective randomized controlled trial utilizing pulsed radiofrequency (PRF) for treatment of pudendal neuralgia evaluated 77 patients who were divided into PRF (n= 38) and pudendal nerve block (NB) (n=39) groups [28]. Immediately post procedure there was no difference between groups but PRF demonstrated statistically significant reduction in pain scores (VAS score assessments) across the two week, one month, and three month marks. Other studies investigating pulsed radiofrequency have also demonstrated positive results, but most of the evidence comes from low power studies or case reports [29, 30].
4. Sacroiliac Joint Injection
The sacroiliac joint (SIJ) is a highly specialized C-shaped or L-shaped joint located between the sacrum and the ilium bones of the pelvis. The SIJ is supported by several strong ligaments including ventral, dorsal, and interosseous ligaments. SIJ is formed within sacral segments S1, S2, and S3 and in 6% of American adults the fifth lumbar vertebra is assimilated (“sacralized”) into the body of the sacrum. Notably, it is uncommon for women to have complete inclusion of the S3 segment in the SIJ. For both sexes, SIJ mobility decreases from birth to puberty, however, in adult females SIJ mobility transiently increases and peaks at age 25 [31].
SIJ innervation arises from the posterior sacral network, which is comprised of the lateral branches from S1, S2, S3, S4/L5 posterior rami [32]. Targeted blockade is at the inferior portion of the joint, where the synovial part is located. The upper portion of the joint does not constitute a true joint as it is amphi arthrodial or fibrous. For sacroiliac joint pain, local anesthetics with or without corticosteroid can be injected into the posterior ligamentous structures to both the intraarticular space as well as the periarticular structures [31]. However, due to variable anatomy in this region, interventions targeting the SIJ have yielded inconsistent results. In addition, the SIJ as a pain generator is uncommon and physical examination findings with at least three positive provocative tests or radiographic findings in the absence of spondyloarthropathies have failed to predict positive response to diagnostic sacroiliac joint injections [33]. A systematic review by Kennedy et al reviewed all diagnostic and therapeutic injections performed for SIJ pain until 2015 and concluded that it is not clear that image guided diagnostic injections increases the predictability of positive response to therapeutic injections and overall the quality of the evidence is moderate [34].
Pulsed radiofrequency neuromodulation (PRN) of the SIJ has been studied to provide long term relief. A prospective randomized study by Dutta et al [35•] compared the effects of intraarticular steroid injection with PRN of the L4 and L5 primary dorsal rami and S1-S1lateral branch blocks in 30 diagnostic block positive patients who were randomly assigned. They reported that there was significant reduction in pain scores in the PRN group at 6 months and global perceived effect was higher in the PRN group that maintained at 6 months while the intraarticular steroid group showed a gradual decline in global perceived effect and increase in pain score after 3 months.
Stout et al [36•] conducted a fluoroscopy centered anatomic study to improve on current SIJ radiofrequency neurotomy (RFN) lateral branch (LB) nerve targets. 20 cadavers were dissected with LBs marked to generate fluoroscopic images used to calculate miss rates of the procedure. Their research estimated current LB targets produced miss rates of 9.4% at S1, 0.99% at S2, and 35% at S3 which collectively denervated only 60% of all SIJs. Using this data, her study developed new targets and miss rates were reduced to: 2.8% at S1, 0.99% at S2, and 0% for S3 which improved SIJ denervation rates from 60% to 95%. In summary, sacroiliac joint injection is helpful for both diagnostic and therapeutic purposes, as the diagnostic injection confirms the location of pain and the injection of corticosteroid can provide pain relief. Radiofrequency ablation or pulsed radiofrequency neuromodulation should be considered for refractory SI joint pain as it may be superior compared to the traditional intraarticular depo-steroid injection.. Treatments using cooled radiofrequency should be considered for management of large sized lesions.
5. Myofascial injections
5.1. Trigger Point Injections
Myofascial trigger point (MTrP) is a hypersensitive palpable nodule in a taut band of skeletal muscle that can be spontaneously painful or painful only on compression. Myofascial pain syndrome (MPS) has been studied for decades, however, the diagnosis and relative importance has evolved over time. Though the etiology for myofascial pain is incompletely understood, theories suggest the pain is caused by metabolic derangements, central nervous system changes, or impairments in anatomy. Symptoms are dependent upon patient’s own perception of its characteristic qualities, intensity, distribution, and duration. As such, variability in symptom presentations has presented a challenge for standardization of diagnostic criteria. An important feature in clinical examination indicative of an active MTrP is the local twitch response (LTR), which is defined as a quick contraction or dimpling in the muscle fiber after a snapping pressure is applied in a taut band [37]. However, there is currently no standardized physical examination protocol to assess myofascial pelvic pain. For patients with CPP and pelvic floor disorder symptoms, myofascial pain commonly arises from the pelvic (levator ani) and internal hip (obturator internus) muscles, piriformis, deep gluteal muscles as well as connective tissues [38]. Sikdar et al [39] used an ultrasound to visualize and characterize myofascial trigger points and found the lesions appeared as focal, hypoechoic with an elliptical shape. In a study by Kim et al [40]. ultrasound-guidance and visualization of local twitch response on ultrasonography was incorporated while performing myofascial trigger point injections in men with chronic prostatitis and chronic pelvic pain syndrome, which yielded good outcomes. Fluoroscopy guided piriformis injection with local anesthetic and steroid has shown to help posterior hip and pelvic girdle pain in case series [41]. Among the invasive therapies, dry needling, anesthetic injections, steroids, and botulinum toxin-A (BTA) have been shown to provide pain relief [34]. A retrospective study by Bartley et al [42•] reviewed pelvic floor muscle injections performed over greater than a 3-year period. The study showed significant improvement in levator pain score in both unilateral or bilateral injections.
5.2. Botulinum Toxin Injections
In the recent years, botulinum neurotoxins (BoNT) has demonstrated effectiveness in the treatment of pelvic pain. Its mechanism of action for pain relief is multifold. It is thought to be due to eliminating tonic muscle contraction, blunting nociceptive responses, and inhibiting central glutamate release and thereby decreasing excitatory amino acid receptors used for perception of pain. In a prospective study by Morissey et al [43•], onabotulinumtoxinA was injected in 21 women with high-tone pelvic floor dysfunction using electromyography-guidance. Overall, the intervention with botulinum toxin resulted in improved Global Response Assessment, less dyspareunia, less sexual dysfunction, higher score on Quality of Life assessments, decreased resting pressure on vaginal manometry, and less tenderness on digital assessments. Jarvis et al [44••] conducted a prospective cohort study to investigate the effectiveness of BTA injection into the levator ani muscles in decreasing pain symptoms. In the 12 week follow-up, patients reported significant subjective improvement in pain from baseline and there was also a reduction in the pelvic floor muscle manometry measurements at resting pressure. However, a recent multicenter, randomized double blind study on 80 patients showed no difference in using Botox in myofascial pain in CPP but found overall decrease in global pain in both groups suggesting that Botox does not have an advantage over local anesthetic alone in this patient population [45•].
6. Neuromodulation
Neuromodulation is typically reserved for patients that have failed conservative management. Given the purpose of this article, neuromodulation will be discussed entirely as a treatment modality rather than by each unique pathology. While there is growing research documenting the efficacy of neuromodulation in chronic pain, the definitive mechanism of action is still debated. The most accepted explanation dubbed “gate control theory” was first proposed by Melzack and Wall [46]. Gate control theory claims that purposeful electrical activation of large myelinated A-beta afferent nerve fibers at the dorsal horn inhibits transmission of surrounding afferent nociceptive fibers. Effectively, by stimulating large myelinated afferent nerve fibers the central nervous system cannot process additional peripheral pain transmission [46, 47]. Other proposed mechanisms suggest neuromodulation activates intrinsic inhibitory pathways within the central nervous system which produce changes in various neurotransmitters associated with pain sensation and pain conduction. These neurotransmitters include GABA, glutamate, substance P, adenosine, bradykinin among others. Additional theories suggest that activation of ascending dorsal column fibers may induce the brain stem to generate descending inhibitory serotonergic pathways that modulate pain transmission [48].
6.1. Spinal cord stimulation
Traditional spinal cord stimulation (SCS) has been used since the 1960s for treatment of chronic pain. Common SCS programs generate tonic electrical stimulation via the lateral thalamic pathway to deliver continuous pulses at fixed amplitudes and frequencies. Leads are placed into the dorsal epidural space either by small laminotomy/laminectomy or via a percutaneous route where they provide constant electrical stimulation. While most of the research on SCS has been conducted on back pain and complex regional pain syndrome (CRPS), new studies on CPP appear promising. Two persistent challenges for successful SCS therapy are selecting appropriate patients and proper lead placement [17].
Conventionally, patients are considered for SCS when conservative medical management and prior interventions have failed. However aside from refractory symptoms, when selecting patients for SCS practitioners should consider how the underlying diagnosis will respond to neuromodulation. A 2011 article by Atkinson et al [3] provides recommendations for SCS patient selection by categorizing pain conditions into one of three groups (likely to respond, may respond, rarely respond) based on the condition’s indication for SCS [49, 50]. Atkinson reports the four conditions most likely to respond to SCS include failed back surgery syndrome, refractory angina pectoris, neuropathic pain secondary to peripheral nerve lesion, and radicular pain following cervical spine surgery. Other considerations for selection of SCS candidates include in depth assessment of patients’ health literacy, medical comorbidities, psychological heath, and socio-economic support [2]. The importance of these factors cannot be overstated but will not be extensively discussed given the scope of this review article.
Stimulation as cephalad as T11 is theorized to provide coverage for CPP due to stimulation of the dorsal columns that capture fibers exiting to the sacrum. This is supported by Kapural et al [51•] who reported on 6 patients with CPP who experienced significant pain relief and reduction in opioid requirements following lead placement at the T11 to L1 level. Other studies have cited success with lead placement as high as T6 down to L2 [52]. While effective, patients often complain about their SCS causing unpleasant paresthesias that worsen at increased settings [51]. The Senza ® system by Nevro which uses high frequency stimulation, and the Abbott BurstDR™ pattern are two unique alternative programs for SCS that minimize or eliminate unwanted paresthesia [53••]. Available research shows reasonable short- and long-term support of spinal cord stimulation for CPP. A published case series for three women (ages 65-72) treated with 10 kHz spinal cord stimulation for CPP revealed 50%+ improvement in numeric pain ratings over 9-12 months after implant [54]. An open-label, prospective study of spinal cord and dorsal nerve root stimulation for chronic pelvic and abdominal pain (most in patients with a reported post-surgical injury) revealed a 50% decrease in numeric pain ratings that endured up to 12 months after implant with a 69% response rate [55]. Approximately 10% of patients reported complications after implant including: superficial skin infection, lead migration, and headache from cerebrospinal fluid leak.
6.2. Dorsal root ganglion stimulation
Initially approved for CRPS treatment in 2016, dorsal root ganglion stimulation (DRGS) is a novel neurostimulation technique that is gaining traction for CPP treatment. Anatomy suggests that DRGS can modulate both sympathetic afferent transmission and somatic sensory innervation. There is evidence to suggest DRGS has upstream and downstream effects from point of stimulation that allows more broad coverage area and results in improved pain relief [56•]. Compared to traditional SCS, DRGS is more energy efficient, less prone to positional changes with lower incidence of lead migration and covers larger regions of the nervous system [12]. The ACCURATE study demonstrated that dorsal root ganglion stimulation is superior to traditional SCS for treatment of CRPS [57••]. DRGS is now being investigated for CPP treatment as some researchers hypothesize CPP could be a type of CRPS. This idea was proposed by Janicki [58] in 2003 when he reported both CRPS and CPP patients experience allodynia and or hyperesthesia; hallmark symptoms of the CRPS Budapest Criteria.
In 2017 a multicenter registry analyzed the efficacy of DRGS in treatment of 16 different pain syndromes, one of which was CPP. Of the six patients treated with DRGS who had CPP, both mean relief percentage (self-reported pain reduction) and numerical rating scale reduction percentage (collected from pre and post trials) demonstrated pain reductions of 76.6% and 76.8% respectively [59]. A recent case series by Hunter and Yang [60] involving seven patients with CPP reported successful treatment using DRGS with leads placed at bilateral L1 and S2. Among these seven patients there were considerable differences both in demographics and characteristics of pain conditions they experienced. Three out of seven were male, ages ranged from age 36 to 63, and duration of pain symptoms amongst patients ranged from 7 months to greater than 20 years. Similarly, etiology of pelvic pain encompassed acute genital trauma, post-surgical complications, chronic endometriosis, and CRPS secondary to infected implant device. Despite differences among patients all reported significant pain relief with the use of DRGS. This evidence supported the author's selection of the bilateral L1 and S2 DRG lead configuration which they claimed best intercepted pain transmission from the lower abdomen (L1–2), groin (L1–2) and pelvis (S2, S3, S4). Furthermore, in all patient’s pain relief persisted over one year since device implant; a testament to the durability of therapy and effective selection of L1 and S2 levels as targets for lead placement in DRGS.
6.3. Sacral Stimulation
Sacral neuromodulation (SNM) consists of implanting a neuroelectrode to provide stimulation to the sacral roots (typically S3) whereby pain is modulated by activating inhibitory pathways [61]. The intended and on-label purpose of SNM is treatment of urinary symptoms such as frequency and urgency. Currently, treatment for pelvic pain remains an off-label use below the S2 level [62]. Regardless, various low level studies demonstrate SNM has some efficacy in the treatment of generalized CPP, interstitial cystitis, vulvodynia, pudendal neuralgia and SI joint pain. Gajewski et al [63] conducted a case series from 1994-2008 that analyzed 78 patients with interstitial cystitis/painful bladder syndrome refractory to conservative treatment. A trial of sacral stimulation was performed, and those with at least 50% improvement were considered for permanent device implant. In total 46 patients underwent device implant and 72% of patients reported at least 50% improvement over an average follow up interval of 61.5 months. There was however, an explantation rate of 28%, mostly due to failed outcomes. A 2019 systematic review by Cottell et al [64••] reported on sacral neuromodulation for treatment of CPP. The ten studies analyzed, comprised of six prospective cohort studies and four retrospective case series, all demonstrated decrease pain scores following SNM implantation. Collectively, overall mean reduction in pain was reported as 4.4/10 (ranging from 3.1 to 6.5) using a numeric rating system.
Conclusion
The purpose of this review was to demonstrate the multitude of interventions currently available [Table 1] and elucidate new areas of research. The various interventional treatment options for female pelvic pain parallel the multiple potential etiologies. Review of literature outlines the variety of interventional therapies, which range from intramuscular myofascial trigger point injections, to intricate deep nerve plexus blocks. The accessibility of CT imaging, ultrasound, and fluoroscopy has expanded on procedural technique for nerve plexus blocks, but so far, no approach has demonstrated superiority. While traditional SCS remains widely used, recent advancements in neuromodulation have reported significant improvement in treatment of pelvic pain. Consequently, innovative devices with novel stimulation programs and implants such as DRGS and SNM are gaining traction for CPP treatment. In summary, despite the recent advancement in research and technology, female pelvic pain remains difficult to treat. Many low powered studies and low volume case reports have produced encouraging outcomes but lack sufficient evidence to guide the standard of care. Future research will necessitate high powered, well-constructed, research studies to improve evaluation and treatment of patients with pelvic pain.
Table 1.
Interventional options for pelvic pain conditions
| Intervent ions |
Distribution/ Location | Common pain conditions |
|---|---|---|
| Plexus blockade | + Generalized chronic pelvic pain | |
| Superior Hypogastric | Pelvic distribution: descending colon, internal sex organs, uterine, prostatic, urethral, rectal, perineal and bladder Location: Anterior to L5 and S1 | Oncologic pelvic pain due to cervical, prostatic, and testicular cancer, interstitial cystitis, urethral pain, endometriosis, post-surgical pain. |
| Inferior Hypogastric | Lower pelvic distribution: cervical, ovarian, prostatic, penile, vaginal, rectal, anal, perineal, and bladder Location: Sacrum | Oncologic pelvic pain due to gynecologic, colorectal, and genitourinary causes. |
| Ganglion Impar | Pelvic viscera, perineal area, distal rectum, distal urethra, distal third of vagina, vulva/scrotum Location: Lower sacrum, anterior to sacrococcygeal junction | Oncologic pelvic pain due to rectal, anal, perineal, prostatic, and vulvar cancer, Chronic Coccydynia, pudendal neuralgia, Vestibulodynia |
| Pudendal nerve block | Pudendal nerve distribution: S2- S4 | Most commonly pudendal neuralgia, interstitial cystitis |
| Sacro-iliac Joint injection | Inferior aspect of the Sacro-iliac joint | Most commonly, SIJ pain chronic back pain, ankylosing spondylitis |
| SIJ radiofrequency ablation | L4, L5 primary dorsal rami, S1, S2, S3 lateral branches | Long term relief of SIJ pain |
| Myofascial injections | Variable- depending on affected muscles | Variable muscle/fascia, pelvic floor dysfunction, vaginismus |
| Trigger point inj. | Muscles typically involved are Levator ani, Obturator internus, piriformis | |
| Botox inj. | ||
| Neuromodulation | ||
| SCS | Variable dependent on implant: T6 to L2 distribution | CRPS, chronic back pain, neuropathic pain |
| DRGS | Variable dependent on implant: L1-S2 distribution | CRPS, S1 neuritis, endometriosis, pudendal neuralgia, orchialgia, varicocele, anal trauma, rectal fistula, coccydynia, foot/leg pain, post-surgical pain pelvic pain |
| SNM | Variable depending on implant: S1- S5 sacral distribution | Urinary urgency, frequency, interstitial cystitis, vulvodynia, pudendal neuralgia, SI joint pain, coccydynia |
Grant Acknowledgements:
Donald McGeary, PhD, ABPP
NCCIH; PI: McGeary: R01 AT008422
Footnotes
Conflict of Interest
Donald McGreary reports grants from the National Center for Complementary and Integrative Health during the conduct of the study. Joseph Torres, Ameet Nagpal, Alice Iya and Malathy Srinivasan declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Contributor Information
Joseph E. Torres, UT Health San Antonio, Department of Anesthesiology.
Ameet S. Nagpal, UT Health San Antonio, Department of Anesthesiology
Alice Iya, UT Health San Antonio, Department of Anesthesiology.
Donald McGeary, Rehabilitation Medicine; Associate Professor, Psychiatry, UT Health San Antonio.
Malathy Srinivasan, Attending physician, Rothman Orthopedics Institute, Clinical Assistant Professor/Clinical Educator track, Department of Physical Medicine and Rehabilitation, Sidney Kimmel Medical College at Thomas Jefferson University.
Reference list
Papers of particular interest, published recently, have been highlighted as:
•Of importance
••Of major importance
- 1.Baranowski AP, Lee J, Price C, Hughes J. Pelvic pain: a pathway for care developed for both men and women by the British Pain Society. Br J Anaesth. 2014;112(3):452–9 [DOI] [PubMed] [Google Scholar]
- 2.Nicholas MK. When to refer to a pain clinic. Best Pract Res Clin Rheumatol 2004;18:613–29 [DOI] [PubMed] [Google Scholar]
- 3.Atkinson L, Sundaraj SR, Brooker C, O’Callaghan J, Teddy P, Salmon J, et al. Recommendations for patient selection in spinal cord stimulation. Journal of Clinical Neuroscience. 2011;18(10):1295–302. [DOI] [PubMed] [Google Scholar]
- 4.Willard FH, Schuenke MD. The neuroanatomy of female pelvic pain In: Bailey A, Bernstein C, editors. Pain in women: a clinical guide. New York: Springer Science 1 Business Media; 2013. p. 17–55. [Google Scholar]
- 5.de Leon-Casasola OA, Kent E, Lema MJ. Neurolytic superior hypogastric plexus block for chronic pelvic pain associated with cancer. Pain. 1993. August;54(2):145–51. [DOI] [PubMed] [Google Scholar]
- 6.Plancarte R, de Leon-Casasola OA, El-Helaly M, Allende S, Lema MJ. Neurolytic superior hypogastric plexus block for chronic pelvic pain associated with cancer. Reg Anesth. 1997. December;22(6):562–8. [PubMed] [Google Scholar]
- 7.Plancarte R, Amescua C, Patt RB, Aldrete JA. Superior hypogastric plexus block for pelvic cancer pain. Anesthesiology. 1990. August;73(2):236–9. [DOI] [PubMed] [Google Scholar]
- 8.Wechsler RJ, Maurer PM, Halpern EJ, Frank ED. Superior hypogastric plexus block for chronic pelvic pain in the presence of endometriosis: CT techniques and results. Radiology. 1995. July;196(1):103–6. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Kim E, Kim BI. Pulsed radiofrequency treatment of the superior hypogastric plexus in an interstitial cystitis patient with chronic pain and symptoms refractory to oral and intravesical medications and bladder hydrodistension: A case report. Medicine. 2016. December;95(49):e5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. •.Nagpal AS, Moody EL. Interventional Management for Pelvic Pain. Phys Med Rehabil Clin N Am. 2017;28(3):621–46.This review article describes the various interventional options for pelvic pain.
- 11.Schultz DM. Inferior hypogastric plexus blockade: a trans sacral approach. Pain Physician. 2007. November;10(6):757–63. [PubMed] [Google Scholar]
- 12.Hunter CW, Stovall B, Chen G, Carlson J, Levy R. Anatomy, Pathophysiology and Interventional Therapies for Chronic Pelvic Pain: A Review. Pain Physician. 2018;21(2):147–67. [PubMed] [Google Scholar]
- 13. •.Amin MM, Ait-Allah AS, Ali AE-SA, Salem RA, Ahmed SR, Alsammani MA. Inferior hypogastric plexus blockade versus acupuncture for the management of idiopathic chronic pelvic pain: A randomized clinical trial. Biomed J. 2015. August;38(4):317–22.RCT involving 117 patients with CPP compared the inferior hypogastric block with Acupuncture and showed 72.6% success rate and significantly reduced pain intensity in the IHPB group.
- 14. ••.Le Clerc Q-C, Riant T, Levesque A, Labat J-J, Ploteau S, Robert R, et al. Repeated Ganglion Impar Block in a Cohort of 83 Patients with Chronic Pelvic and Perineal Pain. Pain Physician. 2017;20(6):E823–8.This retrospective review of 3 repeated ganglion impar blocks with chronic pelvic and perineal pain showed complete but temporary pain relief in 119 of the 220 blocks performed concluding that repeated blocks results in short term reduction in pain intensity with a moderate intermediate term effect.
- 15.Oh C-S, Chung I-H, Ji H-J, Yoon D-M. Clinical implications of topographic anatomy on the ganglion impar. Anesthesiology. 2004. July;101(1):249–50. [DOI] [PubMed] [Google Scholar]
- 16.Scott-Warren JT, Hill V, Rajasekaran A. Ganglion impar blockade: a review. Curr Pain Headache Rep. 2013. January;17(1):306. [DOI] [PubMed] [Google Scholar]
- 17.Benzon HT, Rathmell JP, Wu CL, Turk DC, Argoff CE, Hurley RW. Practical management of pain. 5th edition. Philadelphia: Elsevier Mosby; 2014. p. 683–800. [Google Scholar]
- 18.Plancarte D, Amescua C, Patt R, Allende S. A751 Presacral blockade of the ganglion of Walther (GANGLION IMPAR). Anesthesiology. 1990;73:3A [Google Scholar]
- 19. •.Sousa Correia J, Silva M, Castro C, Miranda L, Agrelo A. The efficacy of the ganglion impar block in perineal and pelvic cancer pain. Support Care Cancer. 2019. November;27(11):4327–30.This retrospective single center study showed significant pain reduction in oncological pelvic pain with ganglion impar injection under fluoroscopic guidance.
- 20. ••.Gunduz OH, Sencan S, Kenis-Coskun O. Pain Relief due to Trans sacrococcygeal Ganglion Impar Block in Chronic Coccygodynia: A Pilot Study: Ganglion Impar Block in Chronic Coccygodynia. Pain Med. 2015. July;16(7):1278–81.This retrospective study on 22 patients with chronic coccydynia showed that 82% of patients received >50% pain reduction and the remaining patients had complete relief after second injection for median duration of 17 months.
- 21.Malec-Milewska M, Horosz B, Kolęda I, Sękowska A, Kucia H, Kosson D, et al. Neurolytic block of ganglion of Walther for the management of chronic pelvic pain, wiitm. 2014;3:458–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labat J-J, Riant T, Robert R, Amarenco G, Lefaucheur J-P, Rigaud J. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). Neurourology and Urodynamics. 2008;27(4):306–10. [DOI] [PubMed] [Google Scholar]
- 23.Hibner M, Desai N, Robertson LJ, Nour M. Pudendal Neuralgia. Journal of Minimally Invasive Gynecology. 2010. March 1;17(2):148–53. [DOI] [PubMed] [Google Scholar]
- 24.Stav K, Dwyer PL, Roberts L. Pudendal Neuralgia: Fact or Fiction? Obstetrical & Gynecological Survey. 2009. March;64(3):190–9. [DOI] [PubMed] [Google Scholar]
- 25.Abdi S, Shenouda P, Patel N, Saini B, Bharat Y, Calvillo O. A Novel Technique for Pudendal Nerve Block. 2004;7(3):4. [PubMed] [Google Scholar]
- 26.McDonald JS, Spigos DG. Computed tomography-guided pudendal block for treatment of pelvic pain due to pudendal neuropathy. Obstet Gynecol. 2000. February;95(2):306–9. [DOI] [PubMed] [Google Scholar]
- 27.Cok OY, Eker HE, Cok T, Akin S, Aribogan A, Arslan G. Transsacral S2-S4 nerve block for vaginal pain due to pudendal neuralgia. J Minim Invasive Gynecol. 2011. June;18(3):401–4. [DOI] [PubMed] [Google Scholar]
- 28.Fang H, Zhang J, Yang Y, Ye L, Wang X. Clinical effect and safety of pulsed radiofrequency treatment for pudendal neuralgia: a prospective, randomized controlled clinical trial. J Pain Res. 2018. October 16;11:2367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhame EE, Levey KA, Gharibo CG. Successful treatment of refractory pudendal neuralgia with pulsed radiofrequency. Pain Physician. 2009. June;12(3):633–8. [PubMed] [Google Scholar]
- 30.Ozkan D, Akkaya T, Yildiz S, Comert A. Ultrasound-guided pulsed radiofrequency treatment of the pudendal nerve in chronic pelvic pain. Anaesthesist. 2016. February 1;65(2):134–6. [DOI] [PubMed] [Google Scholar]
- 31.Vleeming A, Schuenke MD, Masi AT, Carreiro JE, Danneels L, Willard FH. The sacroiliac joint: an overview of its anatomy, function and potential clinical implications. Journal of Anatomy. 2012;221(6):537–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts SL, Burnham RS, Ravichandiran K, Agur AM, Loh EY. Cadaveric study of sacroiliac joint innervation: Implications for diagnostic blocks and radiofrequency ablation. Reg Anesth Pain Med 2014;39(6):456–64. [DOI] [PubMed] [Google Scholar]
- 33.Laslett M, Aprill CN, McDonald B, Young SB. Diagnosis of sacroiliac joint pain: Validity of individual provocation tests and composites of tests. ManTher 2005;10:207–18 [DOI] [PubMed] [Google Scholar]
- 34.Kennedy DJ, Engel A, Kreiner DS, Nampiaparampil D et al. Fluoroscopically Guided Diagnostic and Therapeutic Intra-Articular Sacroiliac Joint Injections: A Systematic Review. Pain Med. 2015. August;16(8):1500–18. doi: 10.1111/pme.12833. Epub 2015 Jul 14. [DOI] [PubMed] [Google Scholar]
- 35. •.Dutta K, Dey S, Bhattacharyya P, Agarwal S, Dev P. Comparison of Efficacy of Lateral Branch Pulsed Radiofrequency Denervation and Intraarticular Depot Methylprednisolone Injection for Sacroiliac Joint Pain. Pain Physician. 2018. September;21(5):489–496.This randomized control study on 30 patients who were L4 medial branch, L5 DR and S1-3 lateral branch block positive and underwent radiofrequency ablation showed significant reduction in pain and global perceived effect at 6 months compared to intra-articular steroid group.
- 36.•.Stout A, Dreyfuss P, Swain N, Roberts S, Loh E, Agur A. Proposed Optimal Fluoroscopic Targets for Cooled Radiofrequency Neurotomy of the Sacral Lateral Branches to Improve Clinical Outcomes: An Anatomical Study. Pain Medicine. 2018. October 1;19(10):1916–23.This anatomical study used fluoroscopy to compare current targets for lateral branch blocks for SIJ denervation using radiofrequency ablation and identified new targets that decreased miss rates and improved SIJ denervation success rated from 60% to 95%.
- 37.Shah JP, Thaker N, Heimur J, Aredo JV, Sikdar S, Gerber LH. Myofascial Trigger Points Then and Now: A Historical and Scientific Perspective. PM R. 2015. July;7(7):746–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meister MR, Sutcliffe S, Ghetti C, Chu CM, Spitznagle T, Warren DK, et al. Development of a standardized, reproducible screening examination for assessment of pelvic floor myofascial pain. American Journal of Obstetrics and Gynecology. 2019. March 1;220(3):255.e1–255.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sikdar S, Shah JP, Gebreab T, Yen R-H, Gilliams E, Danoff J, et al. Novel Applications of Ultrasound Technology to Visualize and Characterize Myofascial Trigger Points and Surrounding Soft Tissue. Archives of Physical Medicine and Rehabilitation. 2009. November;90(11):1829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim DS, Jeong TY, Kim Y-K, Chang WH, Yoon J-G, Lee SC. Usefulness of a Myofascial Trigger Point Injection for Groin Pain in Patients With Chronic Prostatitis/Chronic Pelvic Pain Syndrome: A Pilot Study. Archives of Physical Medicine and Rehabilitation. 2013. May;94(5):930–6. [DOI] [PubMed] [Google Scholar]
- 41.Albayrak A, Ozcafer R, Balioglu MB, Kargin D et al. Piriformis syndrome: treatment of a rare cause of posterior hip pain with fluoroscopic-guided injection. Hip Int. 2015. Mar-Apr;25(2):172–5. doi: 10.5301/hipint.5000219. Epub 2015 Feb 12 [DOI] [PubMed] [Google Scholar]
- 42.Bartley J, Han E, Gupta P, Gaines N, Killinger KA, Boura JA, et al. Transvaginal Trigger Point Injections Improve Pain Scores in Women with Pelvic Floor Hypertonicity and Pelvic Pain Conditions: Female Pelvic Medicine & Reconstructive Surgery. 2019;25(5):392–6. [DOI] [PubMed] [Google Scholar]
- 43. •.Morrissey D, El-Khawand D, Ginzburg N, Wehbe S, O’Hare P, Whitmore K. Botulinum Toxin A Injections into Pelvic Floor Muscles Under Electromyographic Guidance for Women with Refractory High-Tone Pelvic Floor Dysfunction: A 6-Month Prospective Pilot Study. Female Pelvic Medicine & Reconstructive Surgery. 2015;21(5):277–82.This prospective open label study on 21 women with high tone pelvic floor dysfunction who had Botox as intervention using EMG guidance showed significant improvement in global perceived effect, reduction in dyspareunia, decreased resting pressure and pelvic floor tenderness and less sexual dysfunction.
- 44. ••.Jarvis SK, Abbott JA, Lenart MB, Steensma A, Vancaillie TG. Pilot study of botulinum toxin type A in the treatment of chronic pelvic pain associated with spasm of the levator ani muscles. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2004;44(1):46–50.A prospective cohort study using Botox on 12 women with pelvic floor muscle spasm and hypertonicity showed improvement in manometry pressures and pelvic pain at 12week follow-up. These findings were further confirmed by an RCT by the same authors in 2006.
- 45.Levesque A, Ploteau S, Michel F, Siproudhis L et al. Botulinum toxin infiltrations versus local anaesthetic infiltrations in pelvic floor myofascial pain: multicentre, randomized, double-blind study. Ann Phys Rehabil Med. 2020. January 22. pii: S1877-0657(20)30029-4. doi: 10.1016/j.rehab.2019.12.009. [Epub ahead of print] PMID: 31981833 [DOI] [PubMed] [Google Scholar]
- 46.Melzack R, Wall PD. Pain Mechanisms: A New Theory. Science. 1965. November 19;150(3699):971–9. [DOI] [PubMed] [Google Scholar]
- 47.Rushton DN. Electrical stimulation in the treatment of pain. Disabil Rehabil. 2002. May 20;24(8):407–15. [DOI] [PubMed] [Google Scholar]
- 48.Miller JP, Eldabe S, Buchser E, Johanek LM, Guan Y, Linderoth B. Parameters of Spinal Cord Stimulation and Their Role in Electrical Charge Delivery: A Review. Neuromodulation. 2016. Jun;19(4):373–84. [DOI] [PubMed] [Google Scholar]
- 49.British Pain Society. Spinal cord stimulation for the management of pain: recommendations or best clinical practice; April 2009.
- 50.Raff M, Melvill R, Coetzee G, Smuts J. Spinal cord stimulation for the management of pain: Recommendations for best clinical practice. S Afr Med J. 2013. March 26;103(6):423. [DOI] [PubMed] [Google Scholar]
- 51. •.Kapural L, Narouze SN, Janicki TI, Mekhail N. Spinal Cord Stimulation Is an Effective Treatment for the Chronic Intractable Visceral Pelvic Pain. Pain Med. 2006. September;7(5):440–3.In this small case series report of 6 patients who had visceral pain and previous treatment with hypogastric blocks received SCS and had significant improvement with >50% pain reduction at 30 months and improved disability index and reduction in opioid usage.
- 52.Hunter C, Davé N, Diwan S, Deer T. Neuromodulation of Pelvic Visceral Pain: Review of the Literature and Case Series of Potential Novel Targets for Treatment. Pain Practice. 2013;13(1):3–17. [DOI] [PubMed] [Google Scholar]
- 53. ••.Deer T, Slavin KV, Amirdelfan K, North RB, Burton AW, Yearwood TL, et al. Success Using Neuromodulation with BURST (SUNBURST) Study: Results from a Prospective, Randomized Controlled Trial Using a Novel Burst Waveform. Neuromodulation. 2018. January;21(1):56–66.This study was conducted on 100 patients randomized and crossed over to receive burst stimulation and conventional tonic stimulation to determine the efficacy and showed that burst stimulation was not only noninferior to tonic stimulation but was also superior and 70.8% patients preferred burst stimulation.
- 54.Simopoulos T, Yong RJ, Gill JS. Treatment of chronic refractory neuropathic pelvic pain with high-frequency 10- kilohertz spinal cord stimulation. Pain Practice. 2018. July;18(6):805–9. [DOI] [PubMed] [Google Scholar]
- 55.Levine AB, Parrent AG, MacDougall KW. Stimulation of the spinal cord and dorsal nerve roots for chronic groin, pelvic, and abdominal pain. Pain physician. 2016. July;19(6):405–12. [PubMed] [Google Scholar]
- 56.Krames ES. The Dorsal Root Ganglion in Chronic Pain and as a Target for Neuromodulation: A Review. Neuromodulation: Technology at the Neural Interface. 2015;18(1):24–32. [DOI] [PubMed] [Google Scholar]
- 57. ••.Deer TR, Levy RM, Kramer J, Poree L, Amirdelfan K, Grigsby E, et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain. 2017. April;158(4):669–81.152 patients were studied in this prospective multicenter randomized comparative effectiveness trial and showed DRG stimulation was safe and effective and >50% pain relief was seen in 81% the DRG patients versus 55% of dorsal column SCS stimulation. DRG also provided improved psychological disposition and less unpleasant paresthesia.
- 58.Janicki TI. Chronic Pelvic Pain as a Form of Complex Regional Pain Syndrome. Clinical Obstetrics and Gynecology. 2003. December;46(4):797. [DOI] [PubMed] [Google Scholar]
- 59.Hunter CW, Sayed D, Lubenow T, Davis T, Carlson J, Rowe J, et al. DRG FOCUS: A Multicenter Study Evaluating Dorsal Root Ganglion Stimulation and Predictors for Trial Success. Neuromodulation: Technology at the Neural Interface. 2019;22(1):61–79. [DOI] [PubMed] [Google Scholar]
- 60.Hunter CW, Yang A. Dorsal Root Ganglion Stimulation for Chronic Pelvic Pain: A Case Series and Technical Report on a Novel Lead Configuration. Neuromodulation: Technology at the Neural Interface. 2019;22(1):87–95. [DOI] [PubMed] [Google Scholar]
- 61.Tam J, Loeb C, Grajower D, Kim J, Weissbart S. Neuromodulation for Chronic Pelvic Pain. Curr Urol Rep. 2018. May;19(5):32. [DOI] [PubMed] [Google Scholar]
- 62.Steven Siegel, Karen Noblett, Jeffrey Mangel, Jason Bennett, Griebling Tomas L., Sutherland Suzette E., et al. Five-Year Follow up Results of a Prospective, Multicenter Study of Patients with Overactive Bladder Treated with Sacral Neuromodulation. Journal of Urology. 2018. January l;199(1):229–36. [DOI] [PubMed] [Google Scholar]
- 63.Gajewski JB, Al- Zahrani AA. The long-term efficacy of sacral neuromodulation in the management of intractable cases of bladder pain syndrome: 14 years of experience in one centre. BJU International. 2011;107(8):1258–64. [DOI] [PubMed] [Google Scholar]
- 64. ••.Cottrell AM, Schneider MP, Goonewardene S, Yuan Y, Baranowski AP, Engeler DS, et al. Benefits and Harms of Electrical Neuromodulation for Chronic Pelvic Pain: A Systematic Review. Eur Urol Focus. 2019. October 19; a.This large systematic review aimed at identifying evidence for neuromodulation and studied 1099 patients who underwent electrical stimulation for chronic pelvic pain in various forms, 8 studies were RCT’s. This study found evidence in favor of percutaneous tibial nerve stimulation and transcutaneous electric nerve stimulation. However, only narrative synthesis was available for SCS, pudendal and sacral neuromodulation and studies had various confounding and bias concluding that neuromodulation may be useful in reducing pain and improving quality of life but larger controlled studies are needed.


